ABSTRACT
Background:
Periodontitis, the second most common reason for tooth loss in adults, is a chronic inflammatory condition that increases the prevalence of cancer by inhibiting apoptosis and promoting tumor cell growth. However, it is still debatable if tooth loss is an important risk factor in oral cancer (OC). The aim of this systematic review is to analyze the relationship between tooth loss and the probability of developing head-and-neck cancer and also to see if there is an association between tooth loss, periodontitis, and the risk of OC.
Materials and Methods:
Studies that depicted a link between tooth loss and OC (till 2017) were searched from online databases accompanied by a thorough manual search of relevant journals. Data were collected from eligible studies, and meta-analysis was carried out using the Meta-Analysis software. The effect of various inclusions was assessed by sensitivity and subgroup analysis. Publication bias was also evaluated.
Results:
The meta-analysis consisted of 15 publications. When the number of teeth lost was counted, there was significant variability (I2 = 98.7%, P = 0.0001). When more than 15 teeth were missing in a subgroup analysis, there was a 2.4 times greater risk of OC (odds ratio: 2.496, 95% confidence interval [CI] = 2.067–3.015, P = 0.001) with no heterogeneity (I2 = 0.00%, 95% CI for I2 = 0.00–68.98). Subgroup analysis revealed that there was no evidence of publication bias.
Conclusion:
It was concluded that tooth loss can increase the OC risk by nearly 2 folds. However, large-scale population-based studies are needed to substantiate the findings.
Key Words: Missing teeth, oral cancer, oral malignancy, periodontal disease, tooth loss
INTRODUCTION
Periodontal disease is an inflammatory disorder of the supporting tissue of teeth caused by the microorganisms that cause progressive destruction of the periodontal ligament and alveolar bone leading to periodontal pocket formation, clinical attachment loss, and gingival recession. It is the most common chronic inflammatory disease affecting the tissue around the teeth.[1] The prevalence of periodontitis is over 50% among adults. Around 5%–15% of the global population has severe forms of periodontitis.[2] Eke et al.[3] stated that in the older population, the prevalence of severe periodontitis is as high as 60.8%. Periodontitis causes the loss of connective tissue and alveolar bone around the teeth and can eventually lead to tooth loss.
Tooth loss is a multifactorial process involving dental caries, periodontal disease, and a variety of socio-environmental factors such as socio-economic status, educational level, and general health status.[4] Periodontal diseases are the second leading reason for tooth loss having a prevalence of 24.6% and 11% in studies done by Oginni[5] and Shigli et al.[6] The furcation involvement, periodontal pocket, and tooth mobility are all clinical manifestations of periodontal disease that lead to tooth loss.[7] In numerous studies, the loss of attachment has been found to be correlating with dental mortality. Bouma et al.[8] while investigating loss of attachment in extracted teeth reported that 17% of advanced periodontitis patients accounted for 64% of all teeth having an attachment loss of over 50%.
Initial attachment loss, loss of bone height, and smoking significantly increase the incidence of tooth loss.[9] While inflammatory processes in the oral cavity occur locally, multiple studies have demonstrated that chronic inflammation caused by periodontal disease or the spread of bacterial components can lead to a variety of extraoral disorders. Periodontal disease has been linked to oro-digestive malignancies (oral, esophageal, gastric, colonic, and pancreatic) as well as other cancers such as breast, prostate, and bladder.[10,11] Oral cancer (OC) is a highly relevant problem of global public health, especially for dental surgeons. OC is the sixth most common cancer worldwide. By definition, OC is a malignant neoplasia which arises on lip and oral cavity.[12] OC seems to be the most frequent cancer in India, accounting for 50%–70% of total cancer mortality and the greatest incidence of cancer-related deaths.[13]
Carcinogenesis has been linked to persistent inflammation in the mouth cavity and the subsequent mobilization of inflammatory mediators to distant regions in the human body in some cancers. Another research has linked it to a direct carcinogen caused by periodontitis-associated bacterial species, either immediately in oral cells or by migrating from the mouth.[14] Cobe[15] was the first to report systemic transmission of mouth germs after ordinary activities or dental operations. However, with periodontal disease, germs are likely to migrate from oral cavity to certain other organs and systems via the bloodstream. Although the exact mechanism by which periodontal bacteria promote cancer has yet to be fully understood, local inflammatory responses induced by bacterial infection have been linked to cellular change.
Furthermore, there is still limited evidence about carcinogenic pathways triggered by a handful of the subgingival species identified in tumorous tissue. Only studies that examined and quantified tooth loss or periodontitis as a possible risk element for carcinogen in humans were included as well as research that evaluated potential confounding factors are included in this review. Hence, the aim of our meta-analysis is to conduct a literature study and use meta-analytic tools to assess the link between tooth loss, periodontitis, and the risk of OC.
MATERIALS AND METHODS
Reporting format
This systematic review and meta-analysis were carried out in accordance with the Meta-analyses of Observational Studies in Epidemiology statement and were registered under the ID (ID-CRD42019123983) with the International Prospective Register of Systematic Reviews.
Focused question
On the basis of the Population, Intervention, Control, and Outcome principle, the following question was formulated. “Do the patients with tooth loss (population) when observed (intervention) in comparison to patients without tooth loss (control) exhibit risk of OC (outcome)?”
Search strategy
In order to find related papers online, databases MEDLINE (PubMed), Google Scholar, and Cochrane Library combined with a thorough handsearch of relevant journals and gray literature were searched from the year 1989-28/12/2018. A wide-ranging search strategy was undertaken to identify as many related studies as feasible. Bibliographies of published papers were also reviewed. For MEDLINE, the search strategy used the following keywords and “MeSH Term” (“Oral cancer” [MeSH Terms]) OR (“Oral malignancy” [MeSH Terms]) AND (“Tooth loss” [MeSH Terms] OR (“Missing teeth [MeSH Terms]”) AND (“Periodontal disease” [MeSH Terms]) OR (“Periodontitis” [MeSH Terms]) if the database search engine enabled this. The following key terms were used for Google Scholar and Cochrane library to identify the articles “oral cancer” OR “oral malignancy” AND “tooth loss” OR “loss of teeth” OR “missing teeth” AND “periodontal disease” OR “periodontitis.” A combined as well as individual search strategy was employed to screen all the relevant articles.
Screening and study selection
The results of the numerous database searches were aggregated, and duplicate articles were deleted. Additional papers were discovered by looking through the bibliographies of the retrieved articles. Two reviewers (NG and SR) independently selected references on the basis of titles and abstract on the association between tooth loss and OC risk. The differences in their opinion interpretation were evaluated by kappa statistics. To resolve disagreements at this stage, the third reviewer (AK) was consulted. The full text of the articles was then reviewed and the discrepancies at this point were resolved by the fourth reviewer (VL).
Inclusion criteria
Studies involving human participants.
Studies published in English language.
Case-control studies involving individuals diagnosed with OC.
Exclusion criteria
At the title phase, studies that included tooth loss with cancer other than oral cavity, in vitro studies, animal studies, case reports and case series, cohort studies, reviews, and meta-analyses were excluded.
After assessing each of the ten abstracts, the readers were standardized through discussion sessions.
Data extraction
Two independent researchers (NG and SR) extracted information from each eligible paper. The following are the data gathered from each publication: “author's name, publication year, country, number of cases (with OC) and controls (without OC), mean, standard deviation or range of age, follow-up period, and definition of reference group. The discrepancy was resolved through agreement by all the authors.”
Data analysis
We calculated a pooled odds ratio (OR) of tooth loss among individuals with OC in comparison to those without OC and relevant 95% confidence interval (CI) by using the Comprehensive Meta-Analysis software, OpenMeta[Analyst](OpenMetaAnalyst: Wallace, Byron C., Issa J. Dahabreh, Thomas A. Trikalinos, Joseph Lau, Paul Trow, and Christopher H. Schmid. “Closing the Gap between Methodologists and End-Users: R as a Computational Back-End.” Journal of Statistical Software 49 (2012): 5 to obtain the forest plots and to evaluate heterogeneity of the included studies.
Heterogeneity was measured as the percentage of variation across samples due to confounding variables. Cochrane's Q and I2 statistics were used to examine levels of heterogeneity. Q-tests for analysis of variance were employed to see if confounding variables accounted for variance within effect estimates for pooled effect sizes with significant heterogeneity. We determined the potential contribution of each study to the heterogeneity using sensitivity analyses. Effect on summary estimates was assessed from two models including and excluding such study.
RESULTS
Study selection and characteristics
The design, criteria, and evaluation techniques of the studies included in this systematic review and meta-analysis differed substantially. To draw conclusions from the available studies, several criteria were taken into account. Search strategy identified 132 potential studies from different databases. An average of 132 articles were found using the search method with 132 coming from PubMed. Following the removal of duplicates, 51 articles were chosen for further screening, out of which 20 were excluded as they did not answer our focused question and 16 were excluded as they were published in other languages, and finally, 15 articles were chosen for qualitative and statistical analysis. The article published between the years 1989 and 2017 were considered and 15 articles were finally enrolled in the meta-analysis. The details of the identification, screening, and methodology of the selection according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines are presented in Figure 1. All the studies that were included were case–control studies. A complete reference list of studies included for meta-analysis is reported in Table 1.[16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]
Figure 1.
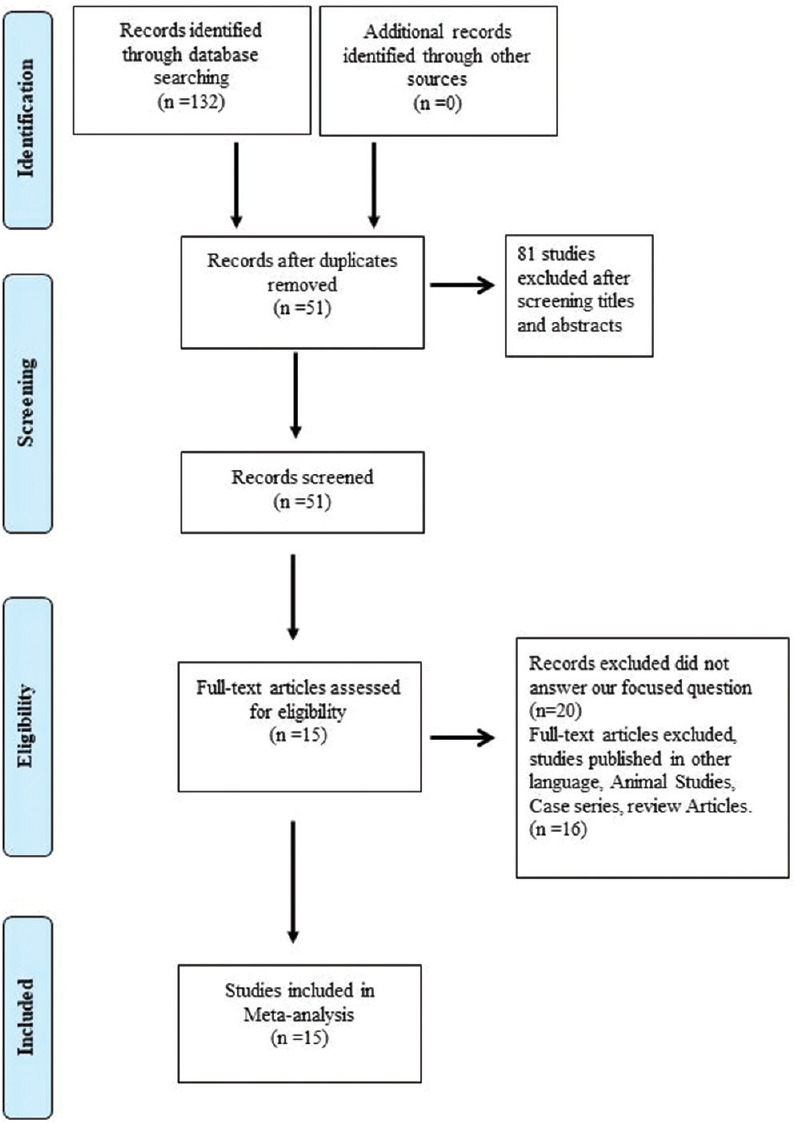
Flow diagram of study selection process according to PRISMA guideline. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Table 1.
Characteristics of the study included in the meta-analysis
| Author | Country | Study design | Sample size | Age/sex | Outcome | Duration |
|---|---|---|---|---|---|---|
| Kabatetal., 1989[16] | NewYork | Case-control study | Cases-125 Control-107 | - | Oral cancer | 4 years |
| Zhengetal.,1990[17] | Beijing, China | Case-control study | Cases-404 controls-404 | 18-80 years | Oral cancer | 1 year |
| Bundgaardetal., 1995[18] | Denmark | Case-control study | Cases-161 Controls-483 | Below 75 years | Oral cancer | 4 years |
| Garroteetal., 2001[19] | Cuba | Case-control study | Cases-200 Control-200 | 25-88 years | Oral cancer | 3 years |
| Balarametal., 2002[20] | India | Case-control study | Cases-590 Control-582 | 18-80 years | Oral cancer | 3 years |
| Lissowskaetal., 2003[21] | Poland | Case-control study | Cases-122 Control-124 | 23-80 years | Oral cancer | 3 years |
| Rosenquistetal., 2005[22] | Southern Sweden | Case-control study | Cases-132 Controls-320 | Male-36/87 years Female-33/87 years | Oral cancer | 4 years |
| Hirakietal., 2008[23] | Japan | Case-control study | Cases-5240 Control-10,480 | 1879 years | Oral cancer | 5 years |
| Tezaletal., 2009[24] | NewYork | Case-control study | Cases-266 Control-207 | - | Oral cancer | 6 years |
| Changetal., 2013[25] | Taiwan | Case-control study | Cases-212 Control-296 | 20-80 years | Oral cancer | 2 years |
| Narayanetal., 2014[26] | India | Case-control study | Cases-242 Controls-254 | - | Oral cancer | - |
| Chenetal., 2017[27] | Fujian China | Case-control study | Cases-250 Control-996 | Oral cancer | 5 years | |
| Hashimetal., 2016[28] | NewYork | Case-control study | Cases-8925 Control-12,527 | Above 40 years age | Oral cancer | - |
| Guptaetal., 2017[29] | India | Case-control study | Cases-187 Control-240 | 30-80 years | Oral cancer | 1 year |
| Kawakitaetal., 2017[30] | China | Case-control study | Cases-921 Control-806 | 18-80 years | Oral cancer | 5 years |
Tooth loss and risk of oral cancer
A total of 29303 participants (10439 cases and 18864 controls) were included in the 15 researches exploring the association between tooth loss and risk of OC. The pooled results indicated that patients with OC risk significantly increased in people who had lost teeth (fixed effect model OR = 1.35, 95% CI = 1.260–1.445, P < 0.001) with any number of teeth lost. However, substantial heterogeneity was observed when the number of teeth lost was varied (I2 = 98.7%, P < 0.0001).
Hence, the subgroup analysis was performed to check the stability of the primary outcome where we analyzed the studies that reported the minimum number of teeth lost to be 15. Six studies were finally selected. Consistent results were demonstrated in subgroup meta-analysis, and it demonstrated a 2.4 times increased risk of occurrence of OC when more than 15 teeth were missing (OR: 2.496, 95% CI = 2.067–3.015 and P < 0.001) with no heterogeneity (I2 = 0.00%, 95% CI for I2 = 0.00–68.98) [Tables 2 and 3].
Table 2.
Summary of the result
| Study | Cases | Controls | OR | 95% CI | Z | P | Weight (%) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Fixed | Random | |||||||
| Zhengetal., 1990[17] | 50/404 | 29/404 | 1.826 | 1.1302.952 | 15.46 | 15.46 | ||
| Bundgaardetal., 1995[18] | 88/161 | 167/483 | 2.281 | 1.5873.278 | 27.11 | 27.11 | ||
| Garroteetal., 2001[19] | 148/200 | 103/200 | 2.68 | 1.7604.081 | 20.16 | 20.16 | ||
| Lissowskaetal., 2003[21] | 89/122 | 65/124 | 2.448 | 1.4374.170 | 12.56 | 12.56 | ||
| Rosenquistetal., 2005[22] | 28/132 | 22/320 | 3.647 | 1.9996.654 | 9.85 | 9.85 | ||
| Changetal., 2013[25] | 68/317 | 25/296 | 2.96 | 1.8144.831 | 14.86 | 14.86 | ||
| Total (fixedeffects) | 471/1336 | 411/1827 | 2.496 | 2.0673.015 | 9.5 | <0.001 | 100 | 100 |
| Total (randomeffects) | 471/1336 | 411/1827 | 2.501 | 2.0713.020 | 9.518 | <0.001 | 100 | 100 |
OR: Odds ratio; CI: Confidence interval
Table 3.
Test for heterogeneity for subgroup analysis (>15 teeth missing)
| Test of heterogeneity | Value |
|---|---|
| Q | 3.9727 |
| DF | 5 |
| Significancelevel | P=0.5534 |
| I2 (inconsistency) | 0.00% |
| 95% CI for I2 | 0.0068.98 |
CI: Confidence interval
Forest plot
The results on the impact of tooth loss as a consequence of periodontal disease in a risk of occurrence of OC in this meta-analysis were supported further by the forest plots in Figure 2. The majority of research within the forest plot link greater tooth loss to a higher risk of OC.
Figure 2.
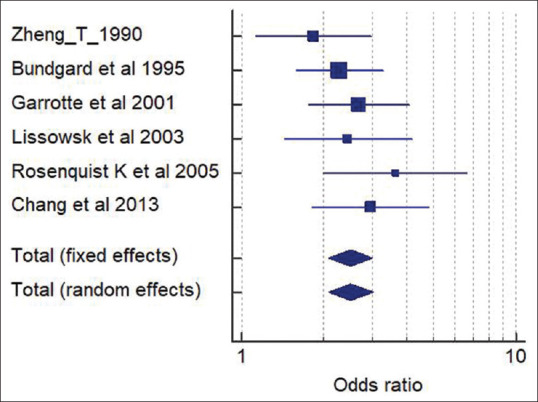
Forest plot of the studies showing association between tooth loss and OC risk. OC: oral cancer.
The horizontal axis (X-axis) of the forest plot represents the standardized mean differences, whereas the vertical line in the picture represents the “lines of null effect,” or no significant difference was found between tooth loss, periodontal disease, and mouth cancer risk. The average impact sizes for OC are represented by the square symbol inside the bottom-most row of the corresponding forest plots. The horizontal line that runs across the square symbol represents a 95% confidence interval for the average impact size. Each diamond symbol in the forest plot represents the total or combined effect size for all of the studies. It is worth noting that the two studies with significant effect sizes and possible outliers were excluded from the analysis, but no significant difference in the final result was found.
Publication bias
Begg's funnel plot and Egger's test were both used to assess publication bias for each of the articles included in this meta-analysis. The results of subgroup analysis show that there is no evidence of biasness in the link between missing teeth and the risk of mouth cancer [Figure 3].
Figure 3.
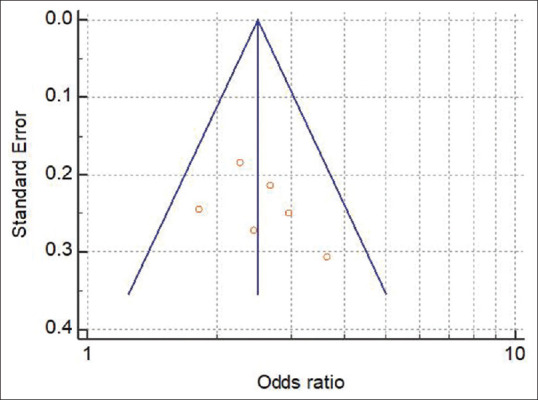
Funnel plot of the studies showing association between tooth loss and oral cancer risk.
DISCUSSION
OC is the 15th most diagnosed malignant carcinoma with an incidence rate of 3.9/100,000.[19] OC has become a major public health fear worldwide because of its higher occurrence and mortality, especially in the case of younger trends. It is one of the top three cancer types in the Indian subcontinent, making it a major public health concern.[31] The variation in incidence and pattern of OC may be due to the cumulative impact of aging on the population and some geographical variations in the prevalence of a particular risk factor.[32] Because of a broad exposure to risk factors such as the chewing of tobacco and inadequate exposure to newly diagnosed support, the low-income groups in India are most vulnerable to delayed reporting of OC.[33] Some risk factors for OC include poor oral hygiene, inflammation caused by excessive dentures and other rough surfaces on the teeth, poor diet, and certain persistent infections caused by fungi, bacteria, or viruses.[34]
More recently, several studies have indicated a correlation between periodontal disease, tooth loss, and OC. In our meta-analysis, the tooth loss proved to be the risk factor for OC (OR = 1.35, 95% CI = 1.260–1.445, P < 0.001).
It was first brought to the attention of dental practitioners by Seymour et al., 2010,[35] that the incidence of OC may be impaired by bad oral hygiene. Meisel et al., 2012,[36] reported a potential link between PD and premalignant oral lesions. Periodontitis, the most common cause of tooth loss in adults, is a chronic inflammatory condition that can increase the risk of cancer by inhibiting apoptosis and promoting tumor cell growth. Periodontitis refers to the persistent low degree of systemic inflammation that contributes to more inflammatory markers being released and circulated. Some of the known inflammatory indicators produced in the immunological response to periodontal disease include pro-inflammatory plasma cytokines, peripheral white blood cells, prostanoids, proteases, including matrix metalloproteinases, and acute-phase proteins.[37] Chronic inflammation caused by periodontal infections can also lead to a breakdown of normal cell function and possible carcinogenesis.[38] In the case of a person with chronic periodontal disorder, the immune system may be deficient in resolution of infection and therefore ineffective in tumor growth surveillance. As a result, periodontitis is regarded as a marker for a certain sort of immune activity that may influence cancer growth and progression. Increased synthesis of carcinogenic nitrosamines is another possible cause. Poor oral hygiene, periodontal disease, cigarette smoking, and some dietary factors increase the formation of endogenous carcinogenic nitrosamines in the oral cavity. Oral bacteria also cause more nitrosamine to be produced.[39] As a result, a link among missing teeth and head-and-neck cancer appears to be likely.
We searched the open published studies related to tooth loss, periodontal disease, and OC risk. We found total 15 case–control studies in our present meta-analysis. We found the presence of significant statistical heterogeneity across the selected studies in the present meta-analysis (I2 = 99.8%, P < 0.05) when we considered outcome for any number of teeth. The reason behind the heterogeneity is due to variations in the area or country of study being carried out, sample size, age, and gender difference. Hence, the subgroup analysis was carried out to substantiate the degree of risk of causing OC when more than 15 teeth were missing. Subsequently, we found no evidence of heterogeneity across the studies and no evidence of publication bias. Our results indicated that the increase in the number of tooth loss (i.e., more than 15 teeth missing) can further upsurge the OC risk by nearly 2.4 folds.
The main strengths of this meta-analysis are the absence of heterogeneity of risk estimates across the studies, the absence of evidence of publication bias, and the clear evidence demonstrating that there is an increased risk of occurrence of OC with increase in number of teeth lost.
Although we have done this meta-analysis with precaution, some limitations in our current meta-analysis need to be recognized. The relatively small number of published research, the use of varied study methods, and differing definitions of tooth loss and periodontal outcomes across the studies are the key drawbacks. The second limitation is that we have only considered more than 15 teeth lost in our subgroup analysis. A different number of teeth lost might have shown a difference in the risk estimates. In summary, available evidence from this meta-analysis points to an association between tooth loss, periodontal disease, and OC.
Implication: This study shows an increase in the incidence of OC in individuals with tooth loss. However, we cannot conclude in this meta-analysis that loss of tooth could be a risk factor for OC due to substantial variability between studies and mixed findings in between case–control studies.
CONCLUSION
Our meta-analysis shows that tooth loss is related independently to its harmful increase in OC risk. Our results highlight the fact that increasing the number of tooth loss in periodontitis can be detrimental to our health. Although current evidence suggests a link between tooth loss, periodontal disease, and OC, large-scale population-based association research is needed in future to determine whether tooth loss has a role in increasing the prevalence of OC.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
REFERENCES
- 1.Hirohata N, Aizawa S, Komine-Aizawa S. Periodontitis and systemic disorders. J Nihon Univ Med Assn. 2014;73:211–8. [Google Scholar]
- 2.Hussain M, Stover CM, Dupont A. P. gingivalis in periodontal disease and atherosclerosis – Scenes of action for antimicrobial peptides and complement. Front Immunol. 2015;6:45. doi: 10.3389/fimmu.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washin. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–20. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 4.Gerritsen AE, Allen PF, Witter DJ, Bronkhorst EM, Creugers NH. Tooth loss and oral health-related quality of life: A systematic review and meta-analysis. Health Qual Life Outcomes. 2010;8:126. doi: 10.1186/1477-7525-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oginni FO. Tooth loss in a sub-urban Nigerian population: Causes and pattern of mortality revisited. Int Dent J. 2005;55:17–23. doi: 10.1111/j.1875-595x.2005.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 6.Shigli K, Hebbal M, Angadi GS. Relative contribution of caries and periodontal disease in adult tooth loss among patients reporting to the institute of dental sciences, Belgaum, India. Gerodontology. 2009;26:214–8. doi: 10.1111/j.1741-2358.2008.00236.x. [DOI] [PubMed] [Google Scholar]
- 7.Ong G. Periodontal disease and tooth loss. Int Dent J. 1998;48:233–8. doi: 10.1111/j.1875-595x.1998.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 8.Bouma J, Schaub RM, van de Poel F. Periodontal status and total tooth extraction in a medium-sized city in the Netherlands. Community Dent Oral Epidemiol. 1985;13:323–7. doi: 10.1111/j.1600-0528.1985.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoare A, Soto C, Rojas-Celis V, Bravo D. Chronic inflammation as a link between periodontitis and carcinogenesis. Mediators Inflamm. 2019;2019:1029857. doi: 10.1155/2019/1029857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu T, Cen L, Kaplan C, Zhou X, Lux R, Shi W, et al. Cellular components mediating coadherence of candida albicans and fusobacterium nucleatum. J Dent Res. 2015;94:1432–8. doi: 10.1177/0022034515593706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbella S, Veronesi P, Galimberti V, Weinstein R, Del Fabbro M, Francetti L. Is periodontitis a risk indicator for cancer? A meta-analysis. PLoS One. 2018;13:e0195683. doi: 10.1371/journal.pone.0195683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44:10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boring CC, Squires TS, Tong T. Cancer statistics, 1992. CA Cancer J Clin. 1992;42:19–38. doi: 10.3322/canjclin.42.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Yao QW, Zhou DS, Peng HJ, Ji P, Liu DS. Association of periodontal disease with oral cancer: A meta-analysis. Tumour Biol. 2014;35:7073–7. doi: 10.1007/s13277-014-1951-8. [DOI] [PubMed] [Google Scholar]
- 15.Cobe HM. Transitory bacteremia. Oral Surg Oral Med Oral Pathol. 1954;7:609–15. doi: 10.1016/0030-4220(54)90071-7. [DOI] [PubMed] [Google Scholar]
- 16.Kabat GC, Hebert JR, Wynder EL. Risk factors for oral cancer in women. Cancer Res. 1989;49:2803–6. [PubMed] [Google Scholar]
- 17.Zheng TZ, Boyle P, Hu HF, Duan J, Jian PJ, Ma DQ, et al. Dentition, oral hygiene, and risk of oral cancer: A case-control study in Beijing, People's republic of China. Cancer Causes Control. 1990;1:235–41. doi: 10.1007/BF00117475. [DOI] [PubMed] [Google Scholar]
- 18.Bundgaard T, Wildt J, Frydenberg M, Elbrønd O, Nielsen JE. Case-control study of squamous cell cancer of the oral cavity in Denmark. Cancer Causes Control. 1995;6:57–67. doi: 10.1007/BF00051681. [DOI] [PubMed] [Google Scholar]
- 19.Garrote LF, Herrero R, Reyes RM, Vaccarella S, Anta JL, Ferbeye L, et al. Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer. 2001;85:46–54. doi: 10.1054/bjoc.2000.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balaram P, Sridhar H, Rajkumar T, Vaccarella S, Herrero R, Nandakumar A, et al. Oral cancer in southern India: The influence of smoking, drinking, paan-chewing and oral hygiene. Int J Cancer. 2002;98:440–5. doi: 10.1002/ijc.10200. [DOI] [PubMed] [Google Scholar]
- 21.Lissowska J, Pilarska A, Pilarski P, Samolczyk-Wanyura D, Piekarczyk J, Bardin-Mikolłajczak A, et al. Smoking, alcohol, diet, dentition and sexual practices in the epidemiology of oral cancer in Poland. Eur J Cancer Prev. 2003;12:25–33. doi: 10.1097/00008469-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Rosenquist K, Wennerberg J, Schildt EB, Bladström A, Göran Hansson B, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in Southern Sweden. Acta Otolaryngol. 2005;125:1327–36. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 23.Hiraki A, Matsuo K, Suzuki T, Kawase T, Tajima K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008;17:1222–7. doi: 10.1158/1055-9965.EPI-07-2761. [DOI] [PubMed] [Google Scholar]
- 24.Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, Reid ME, et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2406–12. doi: 10.1158/1055-9965.EPI-09-0334. [DOI] [PubMed] [Google Scholar]
- 25.Chang JS, Lo HI, Wong TY, Huang CC, Lee WT, Tsai ST, et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013;49:1010–7. doi: 10.1016/j.oraloncology.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Narayan TV, Revanna GM, Hallikeri U, Kuriakose MA. Dental caries and periodontal disease status in patients with oral squamous cell carcinoma: A screening study in urban and semiurban population of Karnataka. J Maxillofac Oral Surg. 2014;13:435–43. doi: 10.1007/s12663-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen F, He BC, Yan LJ, Qiu Y, Lin LS, Cai L. Influence of oral hygiene and its interaction with standard of education on the risk of oral cancer in women who neither smoked nor drank alcohol: A hospital-based, case-control study. Br J Oral Maxillofac Surg. 2017;55:260–5. doi: 10.1016/j.bjoms.2016.11.316. [DOI] [PubMed] [Google Scholar]
- 28.Hashim D, Sartori S, Brennan P, Curado MP, Wünsch-Filho V, Divaris K, et al. The role of oral hygiene in head and neck cancer: Results from international head and neck cancer epidemiology (INHANCE) consortium. Ann Oncol. 2016;27:1619–25. doi: 10.1093/annonc/mdw224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta B, Bray F, Kumar N, Johnson NW. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: A case-control study from India. Cancer Epidemiol. 2017;51:7–14. doi: 10.1016/j.canep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Kawakita D, Lee YA, Li Q, Chen Y, Chen CJ, Hsu WL, et al. Impact of oral hygiene on head and neck cancer risk in a Chinese population. Head Neck. 2017;39:2549–57. doi: 10.1002/hed.24929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B, et al. Effect of screening on oral cancer mortality in Kerala, India: A cluster-randomised controlled trial. Lancet. 2005;365:1927–33. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 32.Manoharan N, Tyagi BB, Raina V. Cancer incidences in rural Delhi – 2004-05. Asian Pac J Cancer Prev. 2010;11:73–7. [PubMed] [Google Scholar]
- 33.Khandekar SP, Bagdey PS, Tiwari RR. Oral cancer and some epidemiological factors: A hospital based study. Indian J Community Med. 2006;31:157–9. [Google Scholar]
- 34.Maisonneuve P, Amar S, Lowenfels AB. Periodontal disease, edentulism, and pancreatic cancer: A meta-analysis. Ann Oncol. 2017;28:985–95. doi: 10.1093/annonc/mdx019. [DOI] [PubMed] [Google Scholar]
- 35.Seymour RA. Is oral health a risk for malignant disease? Dent Update. 2010;37:279–80. doi: 10.12968/denu.2010.37.5.279. 282-3. [DOI] [PubMed] [Google Scholar]
- 36.Meisel P, Holtfreter B, Biffar R, Suemnig W, Kocher T. Association of periodontitis with the risk of oral leukoplakia. Oral Oncol. 2012;48:859–63. doi: 10.1016/j.oraloncology.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–35. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abnet CC, Kamangar F, Dawsey SM, Stolzenberg-Solomon RZ, Albanes D, Pietinen P, et al. Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of finnish smokers. Scand J Gastroenterol. 2005;40:681–7. doi: 10.1080/00365520510015430. [DOI] [PubMed] [Google Scholar]


