Abstract
Periodontitis is defined as an oral bacterial dysbiosis-induced persistent inflammation on dental supporting tissue resulting in periodontal tissue breakdown and alveolar bone destruction. The disease is initiated by the interaction between periodontopathogens and the host immune system. Its development and severity can be associated with several systemic diseases, such as cardiovascular disease (CVD), diabetes mellitus, and rheumatoid arthritis (RA). Moreover, the latest research has suggested that the oral and gut microbiome hypothesis lays the oral and systemic connection mechanism. Bacterial homeostasis and restoration in the oral cavity and intestine become therapeutics concepts. Concerning the treatment of periodontitis, a local inflammatory condition, prolonged systemic administration of antibiotics is no longer recommended due to bacterial resistance issues. Probiotics and several bioactive metabolites have been widely investigated to address the needs of host modulation therapy in periodontitis. Evidence suggests that the use of probiotics helps downregulate the inflammation process through the regulation of toll-like receptor 4 (TLR4) and the production of fatty acid, targeting reactive oxygen species (ROS). In brief, several herbals have anti-inflammatory properties by inhibiting pro-inflammatory cytokines and mediators, including mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB). Consistently, improvement of periodontal pocket depth (PPD) and gingival index (GI) was seen in a group given melatonin as an adjunct treatment. In all, this review will highlight host modulation agents regarding periodontitis therapy, plausible mechanisms on how probiotics and metabolites work on periodontal restoration, and their reported studies. Limitations given by published studies will be elaborated, while future directions will be proposed.
Keywords: Periodontitis, Probiotics, Metabolites, Host modulation agent, Microbiome
1. Introduction
Periodontitis is a highly prevalent chronic inflammatory disease of the periodontal tissue previously believed to be influenced by bacteria dysbiosis solely [1]. Pathogenic bacteria initiate the disease in the oral cavity. These gram-negative bacteria contain endotoxins, lipopolysaccharides (LPS), on the outer membrane and evoke the local immune response. When the bacterial LPS interacts with the gingival tissue, it initiates inflammation and tissue breakdown, progressing tooth mobility [2,3].
Over the years, researchers have been studying the potential correlation of periodontitis and or progression of several systemic diseases. Several systemic diseases such as cardiovascular disease [4], oral and colorectal cancer, gastrointestinal diseases, respiratory tract infection and pneumonia, adverse pregnancy outcomes, diabetes and insulin resistance, and Alzheimer's disease have been linked positively in relation to periodontitis [[5], [6], [7]].
In severe periodontitis patients, 108–1010 of the keystone periodontal pathogen Porphyromonas gingivalis can be swallowed each day, hence, interfering with the gut’s microbiota [8,9]. There are two hypotheses regarding the periodontitis-systemic health mechanism, the first being bacteremia and upregulation of inflammation mediators originating from periodontal pockets and the second one being the impairment of the gut barrier function and modulation induced by dysbiotic oral bacteria resulting in endotoxemia and systemic inflammation [8].
At present, most clinicians prescribe systemic antibiotic treatment using the empirical guideline solely, without guidance from a microbiologic standpoint of the subgingival bacterial biofilm populations [10]. However, periodontal pathogens have varied resistance and susceptibility to the antibiotics of choice, increasing the likelihood of a clinical treatment failure [11]. This information suggested a new approach and strategy to managing periodontal diseases.
Host modulation therapy (HMT), a term developed almost three decades ago, is an adjunct treatment strategy that aims to promote periodontal regeneration and restore the balance of pro-inflammatory mediators, as seen in healthy individuals [12]. While the use of sub antimicrobial-dose doxycycline (SDD) has been approved as an adjunct to conventional periodontitis treatment, studies have shown that non steroid anti-inflammatory drugs (NSAIDs) and bisphosphonate as HMT have raised some concerns. Prolonged use of these synthetic agents might cause unwanted effects, including medication-related osteonecrosis of the jaw (MRONJ), rebound effects upon stopping the medication, gastrointestinal, renal, and lastly, hemostatic problems [13]. Due to the minimal side effects, the current trend of investigation and therapy has shifted to alternative materials, which leads to the discovery of probiotics and other natural agents which will now be discussed.
2. Periodontitis
Periodontitis is a disease with a multifactorial aetiology involving microorganisms and host responses. Biofilm bacteria on the tooth surface are the main aetiology, while the host response will determine the development of the disease along with other local factors such as plaque and calculus, genetic factors, environmental factors, patient system health, and lifestyle [14,15].
A microbial dysbiosis and a susceptible host that forms the inflammatory response are required for the transition to periodontitis. Dental plaque alone is not sufficient to cause periodontitis, the host's inflammatory response to a microbial challenge that can lead to the destruction of the periodontium [15]. Specific organisms that are prominent and involved in the aetiology of periodontitis are red bacterial complexes, including P. gingivalis, Treponema denticola, and Tannerella forsythia [15,16]. P. gingivalis is a gram-negative bacterium and assumed to interfere with host defence by altering the growth and development of the microbial community which further modify and give destructive effect in homeostatic conditions [15].
When pathogenic bacteria induce immune and inflammatory processes, the body produces leukocytes, fibroblasts, or other inflammatory cells to secrete substances that can protect tissues from infection, including metalloproteinases, cytokines, transglutaminases, prostaglandins, and proteolytic enzymes. The main cause of tissue damage is an imbalance between the levels of matrix metalloproteinases (MMPs) with their endogenous inhibitors. Furthermore, alveolar bone and tissue damage occurs through stimulation of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-8, IL-12, IL-17, tumour necrosis factor (TNF)-α, and receptor activator of nuclear factor kappa B ligand (RANK-L) [17,18].
The goal of periodontitis treatment is to re-establish the homeostatic relationship between the periodontal tissues and the dental plaque polymicrobial community. Scaling and root planing (SRP) is the most effective and extensively used treatment for removing pathogenic biofilms [16,19]. Scaling and root planing has been known to improve clinical parameters such as clinical attachment level (CAL), bleeding on probing (BOP), and probing pocket depth (PPD) [19].
In conventional SRP, microorganisms in the dental biofilm can be eliminated incompletely. Cytokine levels were seen to decrease but remain higher than in healthy individuals [19]. Currently, adjunctive therapies, including local antibiotics or anti-inflammatory agents, are widely used to significantly improve the healing process [16,19]. Although the use of antibiotics can play a role in the treatment of periodontitis, their use is limited due to the development of antibiotic resistance. In recent years, target molecules that modulate microbial signalling mechanisms, host inflammatory substances, and bone immune responses have been widely researched [20]. Several studies in periodontitis treatment have focused on inflammatory pathways such as proinflammatory cytokines and complement, as well as tissue-destroying enzymes like MMPs [15,21]. Furthermore, antioxidant therapy can also be used to control the disease because the presence of reactive oxygen species (ROS) is associated with inflammatory conditions in periodontitis [22].
3. Oral and gut connection
Oral and gut are not only linked anatomically but also linked through the microbiomes that live in those organs [8,23]. While obligate anaerobic bacteria represent most of the microbial population in the gut, the oral cavity hosts the largest number of aerobic bacteria. The human gut hosts thousands of species of microorganisms including bacteria, archaea, and other eukaryotic microorganisms creating what's been referred as gut microbiome [24]. This large and diverse microbial community plays an important role that complements the activity of the mammalian digestive system. The gut microbiota contributes to human metabolism by producing enzymes that are not encoded by the human genome, like breaking down polysaccharides, polyphenols, and the synthesis of vitamins [25]. In a normal environment, dental plaque biofilm in the oral cavity represents a major component of the oral microbiome, participating in the regulation of the host's metabolism [26].
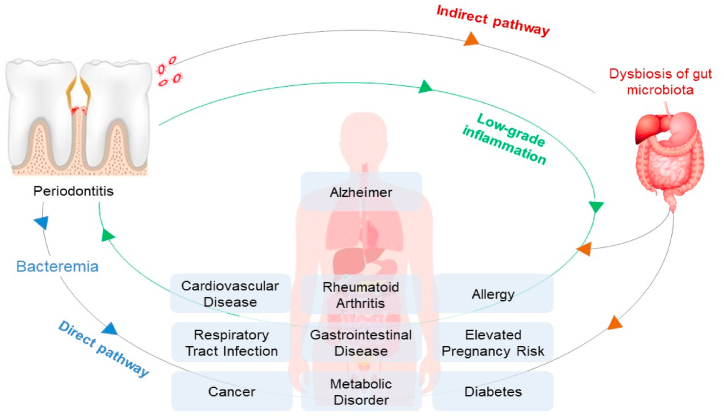
The periodontal disease does not only affect the oral environment, causing tooth loss if leave untreated, but also affect the overall systemic conditions of the host [6,7,9]. There are two pathways that periodontal disease and systemic disease can be linked (Fig. 1.). First, the direct pathway in which the pathogenic bacteria from periodontal pockets can cause bacteremia that induces systemic inflammation [8,27]. P.gingivalis is an acid-resistant bacteria that can tolerate the low pH of the stomach, colonise in the gut, and disturb the balance of gut microbiota [28]. Oral and oropharyngeal microbiota can reach the gut through saliva ingestion, mastication, and drinking. On a daily basis, periodontal patients can ingest around 108–1010 CFU/mL P.gingivalis, the keystone bacteria in periodontitis, through saliva [8,9].
Fig. 1.
The possible pathways that links periodontitis and systemic disease.
The second pathway is indirect, in which, the imbalance of oral microbiota leads to the imbalance of gut microbiota [8]. Meanwhile, the gut microbiota oversees many metabolic functions, including short-chain fatty acid production, amino acid synthesis, and the fermentation of indigestible substrates. Therefore, when the dysbiosis of the gut microbiota happens, it can induce the bacteremia condition causing allergies, diabetes, metabolic disorders, cancer, or even periodontitis [8,29]. Hence, from that process, it can be assumed that there is a reciprocal relationship between the gut microbiota and the oral microbiota.
Gut dysbiosis that occurs in the intestine due to an increase in periodontal pathogens will also cause the spread of bacteria to the connective tissue. Bacteria and their LPS components can act as toll-like receptor (TLR) ligands and will be recognized by neutrophils and macrophages in connective tissue through several receptors such as TLR2 and TLR4. This process can trigger the activation of pro-inflammatory cells such as IL-1β, IL-6, and TNF-α as well as release in ROS [[30], [31], [32]].
Inflammatory mediators such as cytokines can be transferred from one organ to another via the blood circulation [33]. This inflammatory mechanism is thought to be associated with oral inflammatory diseases, such as periodontitis, with systemic disease. Periodontitis is thought to contribute to the production of low levels of systemic inflammatory factors or low-grade inflammation (LGI) and conversely, LGI is also a risk factor for periodontitis [34,35].
Cytokines that are produced locally in periodontitis can move into the systemic circulation and change the inflammatory status so that it can eventually exacerbate existing diseases and even become risk factors for the development of systemic diseases, such as Alzheimer's disease, cardiovascular disease (CVD), rheumatoid arthritis, diabetes mellitus, and inflammatory bowel disease (IBD) [31,34]. When compared with healthy controls, patients with severe periodontitis had increased levels of pro-inflammatory mediators such as (IL-1, IL-6, C-reactive protein (CRP), fibrinogen) and the number of neutrophils in the blood. In contrast, periodontal treatment can improve levels of markers of systemic inflammation [36].
In 2019, Iwauchi et al. studied the relationship between faecal microbiota and subgingival plaque microbiota in elderly patients. The study showed that there is a higher similarity in faecal and subgingival plaque microbiota found in elderly patients compared to those found in adult patients [9]. As such there is a higher prevalence of oral bacteria transitioning to the gut in the elderly than in adult patients. This could happen because of the declining function of the gastrointestinal tract due to ageing, making the oral bacteria easier to invade the gut and the systemic circulation [9].
Nowadays, there is more research focusing on systemic health improvement through gut microbiota regulation. One of the normal gut microbiotas, Akkermansia muciniphila, normally found in large numbers in the gut since the first year of the host’s life. There are some considerations of A. muciniphila being a potential probiotic characteristic and a significant key in homeostatic conditions. A. muciniphila can adhere to the mucus layer, mainly protecting epithelial cells from microbial attacks [[37], [38], [39]]. However, A. muciniphila will decrease through host ageing. A decreasing number of A. muciniphila will make the mucus layer thinner, which results in the microbial toxins being easier to disrupt the host [39]. A. muciniphila also contributes to preventing diabetes type 2, obesity, and other metabolic diseases as it can improve the glucose level and the metabolism of lipid [38,39]. Early data suggested that oral administration of A. muciniphila is safe to consume, but it still needed more studies to confirm [39]. Numbers of studies have investigated the efficacy of these bacteria.
4. Host modulation therapy
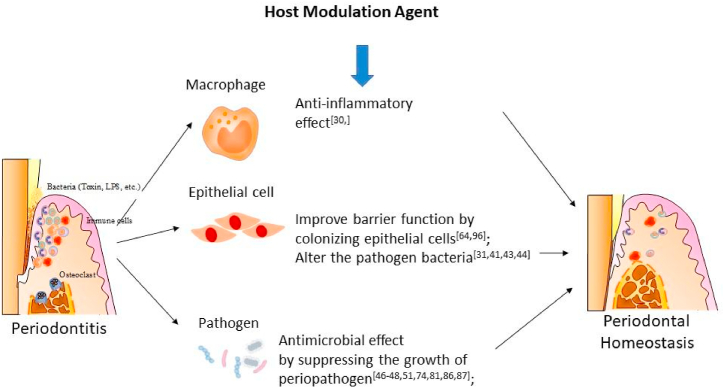
Not all individuals develop severe periodontitis, despite the bad oral hygiene. Bacteria is an essential key to periodontitis, but it is not sufficient to cause the disease alone. Inflammatory mediators have been recorded to play a key role in periodontal tissue damage, as it is a double-edged sword [12]. Host modulation therapy is a treatment concept that aims to modify the host’s response towards the inflammatory process of a disease, reducing its damage (Fig. 2). Specifically, in periodontitis, host modulation therapy is intended to prevent tissue and bone damage by creating a more favourable environment for tissue repair, hence, reducing the host’s susceptibility [40].
Fig. 2.
Properties of host modulation agent in periodontal homeostasis.
Several anti-inflammatory agents, both synthetic and naturally sourced are available and considered for use as an adjunct treatment along with conventional periodontal approaches. Doxycycline, a member of the tetracycline family, was identified to be superior in decreasing the pathological MMP level without interfering with the connective tissue’s turnover rate. Therefore, SDD was introduced to act as a host modulation agent and has been the only agent approved by the United States Food and Drug Administration (FDA) for periodontitis treatment. However, SDD should be avoided to patients with a history of allergy to tetracyclines, pregnant mothers, and lastly young children as studies have recorded that tetracyclines can discolour developing teeth permanently. Another concern regarding the use of SDD is the potential antibiotic resistance because patients were given low doses of doxycycline 2 times a day for over 3 months [41]. These concerns have shifted the current trend into naturally sourced agents with minimum side effects. In this paper, the authors will discuss three metabolites as an option of HMT, namely, herbal, melatonin, and probiotics. Specifically, probiotics will be the focus topic in this paper.
4.1. Probiotics
The World Health Organization (WHO) defined probiotics as the bacteria that provides numerous benefits to the host when used in adequate proportion. Probiotics in dentistry come in many forms, including liquid, paste, and solid form [42,43]. There are a couple mechanisms where probiotics can prevent periodontal disease. First, probiotics act as an anti-inflammatory agent. In gingival areas where a large amount of P. gingivalis detected showed an increase in the level of inflammatory cytokines. Probiotics treatment with lactobacilli will reduce the level of inflammatory cytokines in gingival areas infected with P. gingivalis [43]. Second, probiotics can adhere in oral mucosa by colonising the epithelial cells and alter the pathogen bacteria. Third, probiotic’s ability to produce substances such as bacteriocin, reuterin, and reutericyclin can suppress the growth in periopathogen [42,44]. Separately, probiotic strains also induce acidity from the production of lactic acid, which helped probiotic bacteria to grow and prevented pathogenic bacteria to develop [43]. Some of the most well researched species are Lactobacillus spp., Bifidobacterium spp., and Saccharomyces spp. [45]. These probiotics are also commonly found in the gastrointestinal tract.
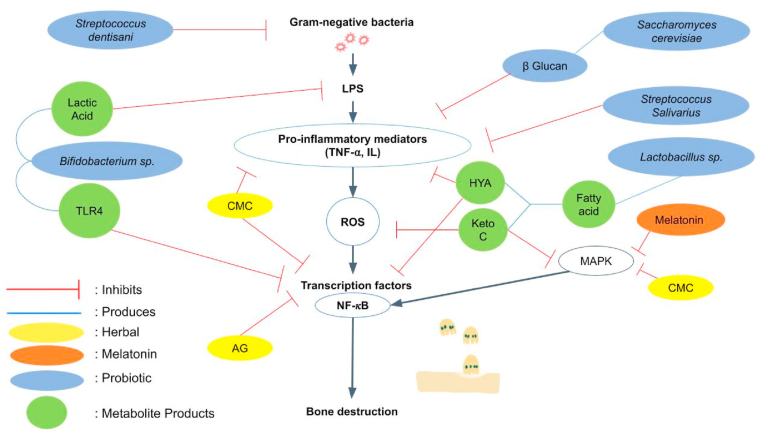
The first species is Bifidobacteria, which can be found naturally in the oral cavity and intestinal lumen. B. lactis HN019 is a Bifidobacterium strain that is widely used and studied as a probiotic due to its good effect on modulating the immune system [46]. Administration of B. lactis HN019 in rats with periodontitis can reduce IL-1β levels, the ratio of RANKL-osteoprotegerin (OPG), and regulate the expression of TNF-α and IL-6. A recent study evaluated B. lactis HN019’s effects toward periodontal parameters and reported its immunological and antibacterial properties by observing the BD-3, TLR4, and CD4 levels in gingival tissues [47]. The content of organic acids such as lactic acid produced by Bifidobacterium can disrupt the outer membrane of Gram-negative bacteria [48]. Bifidobacterium also known to reduce the adhesion P. gingivalis. [47] (Fig. 3) These studies are thought to cause B. lactis and B. infantis to be antagonistic against periodontal pathogen and can be used as adjunctive agents in periodontal therapy [48].
Fig. 3.
Different host modulation agents and their role in suppressing alveolar bone destruction.
Another note-worthy species is Saccharomyces cerevisiae. It is a yeast that has probiotic properties due to the beta glucans which consist in the cell wall. β-glucan is thought to have beneficial effects by modulating immunological parameters and affecting the microbiota. β-glucan can stimulate phagocytic activity and the production of inflammatory cytokines, thereby activating leukocytes (Fig. 3.). In addition, in a previous study β-glucan was found to increase the concentration of transforming growth factor (TGF)-β1 in the gingival crevicular fluid of patients with chronic periodontitis. This mechanism provides an anti-infective effect and increase its potential to accelerate periodontal healing [49]. Previous animal study also showed that S. cerevisiae as monotherapy or adjunctive therapy to SRP could be beneficial in controlling alveolar bone loss in periodontitis without adverse effect [43,49].
Minic et al. previous study pointed out that there is a significant reduction of BOP, Plaque Index (PI), and also PPD in the group of periodontitis patients that received SRP therapy combined with 5-days of applying local probiotics, compared to the group whom only received the SRP therapy [45]. In addition, previous systematic review reported that the combination of non-surgical periodontal therapy (NSPT) combined with probiotic preparation resulted in a better clinical outcome when compared to NSPT combined with placebo agent [13].
Lastly, Lactobacillus has been identified to produce metabolites such as 10-hydroxy-cis-12-octadecenoic (HYA) and 10-oxo-trans-11-octadecenoic acid (KetoC) through the polyunsaturated fatty acid process (PUFA) (Fig. 3.). Studies showed that KetoC continues to show promising results. In-vitro studies about KetoC confirmed its antioxidant properties by upregulating the nuclear erythroid 2-related factor 2-antioxidant response element (NRF2-ARE) pathway in HepG2 cells while countering oxidative stress in gingival epithelial cells through the G Protein coupled Receptor (GPR) 120-NRF2 ARE-MAPK pathway [50,51]. KetoC's hydrophobic nature and carbon-carbon double bond structure also play important role in its antibacterial properties as gram-negative bacteria are more susceptible to fatty acid due to its outer membrane causing KetoC to attach easily to the bacteria [50,52]. The action of ketoC depends on the presence of the free fatty acid receptor (FFAR) GPR120 in macrophages to further function in mitogen-activated protein kinase (MAPK) and inhibit NF-κB signalling in macrophages induced by bacterial LPS [53].
Apart from KetoC, HYA has been known to improve epithelial barrier function both in oral and gut by improving the expression of E-cadherin in the gingival tissue and regulating the GPR40-MEK-ERK respectively [54,55]. HYA modulates the inflammation process by decreasing the local inflammatory cytokines and reducing extracellular signal regulated kinase (ERK) phosphorylation and thus, suppressing alveolar bone loss. HYA also decr eases TNFR2 expression in colitis-induced mice which shares many similarities with human ulcerative colitis [56]. Ikeguchi et al. studied the effects of KetoC and HYA in the brain and found that KetoC and HYA inhibited LPS-induced nitric oxide (NO) production and suppressed the expression of inducible NO synthase in BV-2 cells, conforming their anti-inflammatory characteristics that is not only in the intestine but also in microglial cells in the brain [57]. HYA was also known for reducing The expression of tumor necrosis factor receptor type II (TNFR2) which can activate the NF-κB pathway [53,58]. In addition to that, previous studies found that ketoC and HYA can inhibit the production of cytokine IL-6, IL-1β, and TNF-α [53].
Several new species of Streptococcus have gained researchers’ interest due to their ability to colonise, biocompatibility, and easy dose determination experimentally [59,60]. Streptococcus is a gram-positive bacterium that is included in the normal flora of the human oral cavity and also intestine [61]. Streptococcus salivarius was isolated from the saliva of healthy children [59]. In an in vitro study, S. salivarius strains K12 and M18 were reported to have the ability to inhibit the activity of periodontopathogens such as P. gingivalis, P. intermedia, F. nucleatum, and Aggregatibacter actinomycetemcomitans. Previous studies have also shown that S. salivarius is able to maintain immune homeostasis by targeting host cells. S. salivarius strains K12 and M18 were also able to inhibit the release of IL-6 and IL-8 triggered by P. gingivalis, A. actinomycetemcomitans, and F. nucleatum. [61] (Fig. 3.)
Another streptococcus species, Streptococcus dentisani, is a new bacterial species isolated from the oral cavity of healthy patients who have never had caries or periodontal disease [60,62]. These bacteria showed antibacterial activity against pathogenic bacteria such as S. mutans, S. sobrinus, P. intermedia, and F. nucleatum [60]. Another in vitro study by Esteban-Fernández et al. also showed the inhibitory action of S. dentisani against bacterial colonization of P. gingivalis and F. nucleatum on human gingival fibroblast-1 (HGF-1) (Fig. 3.). This is thought to occur due to the expression of bacteriocins that affect these pathogens. In addition, S. dentisani has anti-inflammatory properties, which can regulate the inflammatory response by reducing the production of pro-inflammatory cytokines triggered by exposure to P. gingivalis or F. nucleatum [62].
4.2. Herbal
Herbs and natural resources have been used in the medical field to treat various diseases for more than 3000 years, and the interest in using them in a periodontitis treatment keeps increasing [63,64]. The most important advantages of these resources are minimal side effects and lower costs compared to synthetic materials or medicine [63]. Different plant species showing anti-inflammatory effects and their potential to replace synthetic materials in periodontal treatment.
Curcumin, an Indian spice belonging to the ginger family, has been widely developed as a treatment for a variety of diseases including periodontitis [65,66]. Chemically modified curcumin (CMC) suggests beneficial effects in the reduction of inflammation [66]. Previous animal studies showed that CMC has been proven to reduce the activation of NF-κB, MAPK, MMPs, IL-1β, and also to inhibit alveolar bone loss [[67], [68], [69], [70]]. (Fig. 3)
Andrographis paniculata (Burm F.) has been used in South Asian countries for inflammatory diseases for its active phytochemical compound called Andrographolide (AG). AG has been proposed as a natural adjunct to mechanical therapy in controlling inflammation and bone resorption in periodontal treatments. Research has shown that there is an AG's inhibitory effect on NF-κB and signal transducer and activator of transcription 3 (STAT3), i.e., the cells' transcription factors and signaling pathways that are involved in the production of inflammatory mediators [49]. (Fig. 3)
Lamiaceae family plants from the Mediterranean area are extensively used in the medical field due to their essential oils (EOs) components [64,71]. Thyme EOs in a cellular model has proven to be able to reduce IL-1β, TNF-α, and IL-6, while increasing anti-inflammatory cytokines such as IL-10. Lavender EOs, along with other EOs such as Terpineols, Linalool, Eucalyptol, based on in-vitro studies also known to have anti-inflammatory effects. Their effects are decreasing LPS-induced TNF-α and IL-6, as well as inhibiting the activation of NF-κB and MAPK [72,73].
Another variant is Aglycone baicalein, a major bioactive flavonoid extracted from Scutellaria baicalensis Georgi (Huang-qin in Chinese). Baicalein is known for providing good anti-inflammatory and antioxidant effects by reducing inflammatory mediators such as IL-1β, TNF-α, monocyte chemoattractant protein-1 (MCP-1), MMP-1, and MMP-2 on periodontal ligament cells stimulated by bacterial LPS and inhibit MAPK signaling activation by LPS. Apart from that, it is also presumed that Baicalein may function as an activator of Wnt signaling-a promoter of osteogenesis of inflamed periodontal ligament cells, by activating Wnt target proteins (LEF1, Cyclin D, and beta-catenin). This study concludes that Baicalein may be used to treat periodontal disease as a host response modulator [50].
Lastly, an Ayurvedic blend of toothpaste, Sudantha, is clinically proven to improve gingival and periodontal health due to its ability to inhibit the expression of the pro-inflammatory cytokine IL-8 and host inflammatory mediators (IL-1β and TNF-α) in a dose-dependent manner [51]. However, further studies are still needed to understand the recommendations for these herbal ingredients utilization in modulating the host immune-inflammatory response without interfering with the cell intrinsic host inflammatory surveillance.
4.3. Melatonin
Melatonin (N-acetyl-5- methoxytryptamine) is a hormone well known for its effect on the circadian rhythm and sleep quality. Other than that, melatonin also possesses potent antioxidant, anti-inflammatory and immunomodulatory, anti-tumoral, neuroprotective [52]. And lastly, osteopromotion and bone loss inhibition properties [53]. Melatonin is physiologically present in saliva and gingival crevicular fluid. Melatonin works as a powerful antioxidant by removing various free radicals mentioned as “scavenging action” and an anti-inflammatory agent by inhibiting nuclear transcription factors such as NF-κB and MAPK, making it a potential biomarker & therapy [54,55]. (Fig. 3) Several in-vitro studies have also been done to evaluate melatonin's ability as an antimicrobial agent. An animal study also confirmed melatonin's abilities to modulate osteoblastic-osteoclastic activities and reduce oxidative damage in irradiated periodontal tissues [56]. On top of that, human trials were conducted to evaluate melatonin's ability as a locally delivered adjunctive treatment and a possible host modulation therapy with oral administration [74].
5. Conclusion
While there are multiple etiological factors that cause periodontitis, bacteria dysbiosis remains the primary inducing factor. Not only the imbalance of oral microbiota but also the imbalance of gut microbiota influences periodontal tissue health. Furthermore, those microbiotas also impact the host's overall systemic condition. Researchers have further studied natural agents for HMT as an adjunctive treatment, with probiotics being the most studied agent. In particular, the metabolites product of probiotics positively contributes to periodontal homeostasis. Future studies are required to investigate further metabolites products' role in periodontitis management and the long-term effect of probiotics.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Benso Sulijaya was supported by Direktorat Riset and Pengembangan, Universitas Indonesia (NKB-757/UN2.RST/HKP.05.00/2022).
Data availability statement
The data that has been used is confidential.
Declaration of interest's statement
The authors declare no competing interests
References
- 1.Kwon T., Lamster I.B., Levin L. Current concepts in the management of periodontitis. Int. Dent. J. 2021;71(6):462–476. doi: 10.1111/idj.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AlQranei M.S., Chellaiah M.A. Osteoclastogenesis in periodontal diseases: possible mediators and mechanisms. J. Oral Biosci. 2020;62(2):123–130. doi: 10.1016/j.job.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ketherin K., Sandra F. Osteoclastogenesis in periodontitis: signaling pathway, synthetic and natural inhibitors. Mol. Cellular Biomed. Sci. 2018;2(1):11. doi: 10.21705/mcbs.v2i1.16. [DOI] [Google Scholar]
- 4.Carrizales-Sepúlveda E.F., Ordaz-Farías A., Vera-Pineda R., Flores-Ramírez R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. 2018;27(11):1327–1334. doi: 10.1016/j.hlc.2018.05.102. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., Sun C., Song E.J., et al. Is periodontitis a risk indicator for gastrointestinal cancers? A meta‐analysis of cohort studies. J. Clin. Periodontol. 2020;47(2):134–147. doi: 10.1111/jcpe.13217. [DOI] [PubMed] [Google Scholar]
- 6.Bui F.Q., Almeida-da-Silva C.L.C., Huynh B., et al. Association between periodontal pathogens and systemic disease. Biomed. J. 2019;42(1) doi: 10.1016/j.bj.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadrameli M., Bathini P., Alberi L. Linking mechanisms of periodontitis to Alzheimer's disease. Curr. Opin. Neurol. 2020;33(2):230–238. doi: 10.1097/WCO.0000000000000797. [DOI] [PubMed] [Google Scholar]
- 8.Olsen I., Yamazaki K. Can oral bacteria affect the microbiome of the gut? J. Oral Microbiol. 2019;11(1) doi: 10.1080/20002297.2019.1586422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwauchi M., Horigome A., Ishikawa K., et al. Relationship between oral and gut microbiota in elderly people. Immun Inflamm Dis. 2019;7(3) doi: 10.1002/iid3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nibali L., Koidou V.P., Hamborg T., Donos N. Empirical or microbiologically guided systemic antimicrobials as adjuncts to non‐surgical periodontal therapy? A systematic review. J. Clin. Periodontol. 2019;46(10):999–1012. doi: 10.1111/jcpe.13164. [DOI] [PubMed] [Google Scholar]
- 11.Rams T.E., Degener J.E., van Winkelhoff A.J. Antibiotic resistance in human chronic periodontitis microbiota. J. Periodontol. 2014;85(1) doi: 10.1902/jop.2013.130142. [DOI] [PubMed] [Google Scholar]
- 12.Sekar S., Murugan T., Elavarasu S. Host modulation by therapeutic agents. J. Pharm. BioAllied Sci. 2012;4(6):256. doi: 10.4103/0975-7406.100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donos N., Calciolari E., Brusselaers N., Goldoni M., Bostanci N., Belibasakis G.N. The adjunctive use of host modulators in non-surgical periodontal therapy. A systematic review of randomized, placebo-controlled clinical studies. J. Clin. Periodontol. 2020;47(S22):199–238. doi: 10.1111/jcpe.13232. [DOI] [PubMed] [Google Scholar]
- 14.Sulijaya B., Takahashi N., Yamazaki K. Host modulation therapy using anti-inflammatory and antioxidant agents in periodontitis: a review to a clinical translation. Arch. Oral Biol. 2019;105:72–80. doi: 10.1016/j.archoralbio.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35(1):3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darveau R.P. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8(7):481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-García M., Hernández-Lemus E. Periodontal inflammation and systemic diseases: an overview. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.709438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isola G., Polizzi A., Santonocito S., Dalessandri D., Migliorati M., Indelicato F. 2021. Scientia Pharmaceutica Review New Frontiers on Adjuvants Drug Strategies and Treatments in Periodontitis. Published online. [DOI] [Google Scholar]
- 19.Cheng R., Wu Z., Li M., Shao M., Hu T. Interleukin-1β is a potential therapeutic target for periodontitis: a narrative review. Int. J. Oral Sci. 2020;12(1) doi: 10.1038/s41368-019-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim W.J., Soh Y., Heo S.M. Recent advances of therapeutic targets for the treatment of periodontal disease. Biomol Ther (Seoul). 2021;29(3):263–267. doi: 10.4062/biomolther.2021.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sima C., van Dyke T.E. Therapeutic targets for management of periodontitis and diabetes. Curr. Pharmaceut. Des. 2016;22:2216–2237. doi: 10.2174/1381612822666160216150338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro M.M.L., Duarte N.N., Nascimento P.C., et al. Antioxidants as adjuvants in periodontitis treatment: a systematic review and meta-analysis. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/9187978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maki K.A., Kazmi N., Barb J.J., Ames N. The oral and gut bacterial microbiomes: similarities, differences, and connections. Biol. Res. Nurs. 2021;23(1):7–20. doi: 10.1177/1099800420941606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasmi Benahmed A., Gasmi A., Doşa A., et al. Association between the gut and oral microbiome with obesity. Anaerobe. 2021;70 doi: 10.1016/j.anaerobe.2020.102248. [DOI] [PubMed] [Google Scholar]
- 25.Rowland I., Gibson G., Heinken A., et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57(1) doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamont R.J., Koo H., Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 2018;16(12):745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S.Y., Hwang B.O., Lim M., et al. Oral–gut microbiome Axis in gastrointestinal disease and cancer. Cancers. 2021;13(9):2124. doi: 10.3390/cancers13092124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saygun I., Nizam N., Keskiner I., et al. Salivary infectious agents and periodontal disease status. J. Periodontal. Res. 2011;46(2) doi: 10.1111/j.1600-0765.2010.01335.x. [DOI] [PubMed] [Google Scholar]
- 29.Kato T., Yamazaki K., Nakajima M., et al. Oral administration of Porphyromonas gingivalis alters the gut microbiome and serum metabolome. mSphere. 2018;3(5) doi: 10.1128/mSphere.00460-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sansores-España L.D., Melgar-Rodríguez S., Olivares-Sagredo K., et al. Oral-gut-brain Axis in experimental models of periodontitis: associating gut dysbiosis with neurodegenerative diseases. Frontiers in Aging. 2021;2 doi: 10.3389/fragi.2021.781582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajishengallis G., Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021;21(7):426–440. doi: 10.1038/s41577-020-00488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chassaing B., Gewirtz A.T. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol. Pathol. 2014;42(1):49–53. doi: 10.1177/0192623313508481. [DOI] [PubMed] [Google Scholar]
- 33.Cai Z., Zhu T., Liu F., Zhuang Z., Zhao L. Co-Pathogens in periodontitis and inflammatory bowel disease. Front. Med. 2021;8 doi: 10.3389/fmed.2021.723719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cecoro G., Annunziata M., Iuorio M.T., Nastri L., Guida L. Periodontitis, low-grade inflammation and systemic health: a scoping review. Medicina (B Aires) 2020;56(6):272. doi: 10.3390/medicina56060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao J., Li L., Zhang Y., et al. Periodontitis may induce gut microbiota dysbiosis via salivary microbiota. Int. J. Oral Sci. 2022;14(1) doi: 10.1038/s41368-022-00183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y., Liao Y., Luo B., Li L., Zhang Y., Yan F. Non-surgical periodontal treatment restored the gut microbiota and intestinal barrier in apolipoprotein E−/− mice with periodontitis. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods. 2017;33 doi: 10.1016/j.jff.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh H., Torralba M.G., Moncera K.J., et al. Gastro-intestinal and oral microbiome signatures associated with healthy aging. Geroscience. 2019;41(6):907–921. doi: 10.1007/s11357-019-00098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang T., Li Q., Cheng L., Buch H., Zhang F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019;12(6) doi: 10.1111/1751-7915.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajishengallis G., Chavakis T., Lambris J.D. Current understanding of periodontal disease pathogenesis and targets for host‐modulation therapy. Periodontol. 2020;(1):84. doi: 10.1111/prd.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preshaw P.M. Host modulation therapy with anti-inflammatory agents. Periodontol. 2018;(1):76. doi: 10.1111/prd.12148. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan S., Mahendra J., Kanakamedala A., Namasivayam A. 2020. A Review on Probiotics A New Paradigm in Periodontal Health.http://annalsofrscb.ro483 [Google Scholar]
- 43.Nguyen T., Brody H., Radaic A., Kapila Y. Probiotics for periodontal health—current molecular findings. Periodontol. 2021;87(1):254–267. doi: 10.1111/prd.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moraes R.M., Lescura C.M., Milhan N.V.M., Ribeiro J.L., Silva F.A., Anbinder A.L. Live and heat-killed Lactobacillus reuteri reduce alveolar bone loss on induced periodontitis in rats. Arch. Oral Biol. 2020;119 doi: 10.1016/j.archoralbio.2020.104894. [DOI] [PubMed] [Google Scholar]
- 45.Minić I., Pejčić A., Bradić-Vasić M. Effect of the local probiotics in the therapy of periodontitis A randomized prospective study. Int. J. Dent. Hyg. 2021 doi: 10.1111/idh.12509. Published online. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira L.F.F., Salvador S.L., Silva P.H.F., et al. Benefits of Bifidobacterium animalis subsp. lactis probiotic in experimental periodontitis. J. Periodontol. 2017;88(2) doi: 10.1902/jop.2016.160217. [DOI] [PubMed] [Google Scholar]
- 47.Invernici M.M., Furlaneto F.A.C., Salvador S.L., et al. Bifidobacterium animalis subsp lactis HN019 presents antimicrobial potential against periodontopathogens and modulates the immunological response of oral mucosa in periodontitis patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Argandoña Valdez R.M., Ximenez-Fyvie L ann, Caiaffa K.S., et al. Antagonist effect of probiotic bifidobacteria on biofilms of pathogens associated with periodontal disease. Microb. Pathog. 2021;150 doi: 10.1016/j.micpath.2020.104657. [DOI] [PubMed] [Google Scholar]
- 49.Garcia V.G., Knoll L.R., Longo M., et al. Effect of the probiotic Saccharomyces cerevisiae on ligature-induced periodontitis in rats. J. Periodontal. Res. 2016;51(1):26–37. doi: 10.1111/jre.12274. [DOI] [PubMed] [Google Scholar]
- 50.Furumoto H., Nanthirudjanar T., Kume T., et al. 10-Oxo-trans-11-octadecenoic acid generated from linoleic acid by a gut lactic acid bacterium Lactobacillus plantarum is cytoprotective against oxidative stress. Toxicol. Appl. Pharmacol. 2016;296:1–9. doi: 10.1016/j.taap.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Tomofuji T., Irie K., Sanbe T., et al. Periodontitis and increase in circulating oxidative stress. Jap. Dent. Sci. Rev. 2009;45(1):46–51. doi: 10.1016/j.jdsr.2008.12.002. [DOI] [Google Scholar]
- 52.Sulijaya B., Yamada-Hara M., Yokoji-Takeuchi M., et al. Antimicrobial function of the polyunsaturated fatty acid KetoC in an experimental model of periodontitis. J. Periodontol. 2019;90(12):1470–1480. doi: 10.1002/JPER.19-0130. [DOI] [PubMed] [Google Scholar]
- 53.Sulijaya B., Takahashi N., Yamazaki K. Lactobacillus-derived bioactive metabolites for the regulation of periodontal health: evidences to clinical setting. Molecules. 2020;25(9) doi: 10.3390/molecules25092088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada M., Takahashi N., Matsuda Y., et al. A bacterial metabolite ameliorates periodontal pathogen-induced gingival epithelial barrier disruption via GPR40 signaling. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-27408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyamoto J., Mizukure T., Park S.B., et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J. Biol. Chem. 2015;290(5):2902–2918. doi: 10.1074/jbc.M114.610733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chassaing B., Aitken J.D., Malleshappa M., Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Im. 2014;104 doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikeguchi S., Izumi Y., Kitamura N., et al. Inhibitory effect of the gut microbial linoleic acid metabolites, 10-oxo-trans-11-octadecenoic acid and 10-hydroxy-cis-12-octadecenoic acid, on BV-2 microglial cell activation. J. Pharmacol. Sci. 2018;138(1):9–15. doi: 10.1016/j.jphs.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 58.Kitaura H., Marahleh A., Ohori F., et al. Role of the interaction of tumor necrosis factor-α and tumor necrosis factor receptors 1 and 2 in bone-related cells. Int. J. Mol. Sci. 2022;23(3) doi: 10.3390/ijms23031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burton J.P., Wescombe P.A., Moore C.J., Chilcott C.N., Tagg J.R. Safety assessment of the oral cavity probiotic Streptococcus salivarius K12. Appl. Environ. Microbiol. 2006;72(4):3050–3053. doi: 10.1128/AEM.72.4.3050-3053.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.López-López A., Camelo-Castillo A., Ferrer M.D., áurea Simon-Soro, Mira A. Health-associated niche inhabitants as oral probiotics: the case of Streptococcus dentisani. Front. Microbiol. 2017;8(MAR) doi: 10.3389/fmicb.2017.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y., Ding Y., Guo Q. Probiotic species in the management of periodontal diseases: an overview. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.806463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esteban-Fernández A., Ferrer M.D., Zorraquín-Peña I., López-López A., Moreno-Arribas V., Mira A. 2019. In Vitro Beneficial Effects of Streptococcus Dentisani as Potential Oral Probiotic for Periodontal Diseases. [DOI] [PubMed] [Google Scholar]
- 63.George P. Concerns regarding the safety and toxicity of medicinal plants-An overview. J. Appl. Pharmaceut. Sci. 2011;(6):40–44. [Google Scholar]
- 64.Castellino G., Mesa F., Cappello F., et al. Effects of essential oils and selected compounds from Lamiaceae family as adjutants on the treatment of subjects with periodontitis and cardiovascular risk. Appl. Sci. 2021;(20):11. doi: 10.3390/app11209563. [DOI] [Google Scholar]
- 65.Livada R., Shiloah J., Tipton D.A., Mkh Dabbous. The potential role of curcumin in periodontal therapy: a review of the literature. J. Int. Acad. Periodontol. 2017;19(3):70–79. [PubMed] [Google Scholar]
- 66.Molayem S. Current evidence on the use of curcumin in modern periodontology-A narrative review. J. Oral Med. Dent. Res. 2021;2(1):1–24. [Google Scholar]
- 67.Deng J., Golub L.M., Lee H.M., et al. Chemically-modified curcumin 2.24: a novel systemic therapy for natural periodontitis in dogs. J. Exp. Pharmacol. 2020;12:47–60. doi: 10.2147/JEP.S236792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yetkin Ay Z., Bakır B., Bozkurt Ş.B., Kayis S.A., Hakki S.S. Positive effect of curcumin on experimental peridontitis via suppression of IL-1-beta and IL-6 expression level. Int. J. Vitam. Nutr. Res. 2022;92(3–4):231–239. doi: 10.1024/0300-9831/a000672. [DOI] [PubMed] [Google Scholar]
- 69.Wang H.H., Lee H.M., Raja V., et al. Enhanced efficacy of chemically modified curcumin in experimental periodontitis: systemic implications. J. Exp. Pharmacol. 2019;11:1–14. doi: 10.2147/JEP.S171119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Curylofo-Zotti F.A., Elburki M.S., Oliveira P.A., et al. Differential effects of natural Curcumin and chemically modified curcumin on inflammation and bone resorption in model of experimental periodontitis. Arch. Oral Biol. 2018;91:42–50. doi: 10.1016/j.archoralbio.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Yilmaz H.G., Bayindir H. Clinical evaluation of chlorhexidine and essential oils for adjunctive effects in ultrasonic instrumentation of furcation involvements: a randomized controlled clinical trial. Int. J. Dent. Hyg. 2012;10(2):113–117. doi: 10.1111/j.1601-5037.2011.00538.x. [DOI] [PubMed] [Google Scholar]
- 72.Huo M., Gao R., Jiang L., et al. Suppression of LPS-induced inflammatory responses by gossypol in RAW 264.7 cells and mouse models. Int. Immunopharm. 2013;15(2):442–449. doi: 10.1016/j.intimp.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Peng L.Y., Shi H.T., Yuan M., et al. Madecassoside protects against LPS-induced acute lung injury via inhibiting TLR4/NF-κB activation and blood-air barrier permeability. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Sharkawy H., Elmeadawy S., Elshinnawi U., Anees M. Is dietary melatonin supplementation a viable adjunctive therapy for chronic periodontitis?—a randomized controlled clinical trial. J. Periodontal. Res. 2019;54(2):190–197. doi: 10.1111/jre.12619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.