Abstract
Purpose
There is a lack of uniformity in the definition of normal ovary ultrasound parameters. Our aim was to summarize and meta-analyze the evidence on the topic. Full-text English articles published through December 31, 2020 were retrieved via MEDLINE and Embase. Data available for meta-analysis included: ovarian follicular count, ovarian volume, and ovarian Pulsatility Index (PI) assessed by Doppler ultrasound.
Methods
Cohort, cross-sectional, prospective studies with a single or double arm were considered eligible. Interventional studies were included when providing baseline data. Both studies on pre- and post-menopausal women were screened; however, data on menopausal women were not sufficient to perform a meta-analysis. Studies on pre-pubertal girls were considered separately. Eighty-one papers were included in the meta-analysis.
Results
The mean ovarian volume was 6.11 [5.81–6.42] ml in healthy women in reproductive age (5.81–6.42) and 1.67 ml [1.02–2.32] in pre-pubertal girls. In reproductive age, the mean follicular count was 8.04 [7.26–8.82] when calculated in the whole ovary and 5.88 [5.20–6.56] in an ovarian section, and the mean ovarian PI was 1.86 [1.35–2.37]. Age and the frequency of the transducers partly modulated these values. In particular, the 25–30-year group showed the higher mean follicular count (9.27 [7.71–10.82]), followed by a progressive age-related reduction (5.67 [2.23–9.12] in fertile women > 35 years). A significant difference in follicular count was also found according to the transducer’s upper MHz limit.
Conclusion
Our findings provide a significant input to improve the interpretation and diagnostic accuracy of ovarian ultrasound parameters in different physiological and pathological settings.
Keywords: Ovary, Ultrasound, Ovarian volume, Follicular count
Introduction
Ultrasound examination is the standard imaging method to analyze ovarian morphology, while providing also some important functional information or to identify patients with polycystic ovary morphology (PCOM) [1]. The existing Rotterdam guidelines define the ultrasound characteristics of PCOM by the presence of ovarian volume > 10 ml or the presence of 12 or more follicles measuring 2–9 mm in diameter in each ovary [1]. Other typical ultrasound features of PCOM have been widely studied including central stromal echogenicity [2], increased ovarian blood flow [reduced pulsatility index (PI) and reduced resistance index] [3], stromal index and stromal to ovarian area ratio [4, 5]. In addition, the Androgen Excess Society guidelines criteria have increased the threshold count of small ovarian follicles to 25 [6]. However, the accurate determination of numerous follicles can be obtained only with the new-generation US machines, not available in many centers.
The structure of the ovary is basically made up of an outer cortical and an inner medullary region. The cortex consists primarily of follicles in different stages of maturation, the medulla of stromal cells, lymphatics, blood vessels, and nerves. The sonographic features of the ovaries are highly variable, depending on the cyclic influence of the hypothalamic–pituitary hormonal axis, which determines ovarian hormone production, follicular maturation, and degeneration [7].
Ultrasound examination is also an excellent diagnostic tool to discriminate between benign and malignant ovarian masses in the hands of experienced examiners using subjective assessment [8]. A consensus opinion on terms, definitions, and measurements to describe the sonographic features of adnexal tumors was established by the International Ovarian Tumor Analysis (IOTA) Group [9]. The IOTA group created ultrasound-based models with similar accuracy to that of expert ultrasound examiners to characterize the ultrasound appearance of benign ovarian tumors (i.e., endometriomas, dermoid cysts, cystadenofibromas) [10–12], and to differentiate from the borderline [13, 14], and the malignant ones [15–17].
In contrast, the sonographic appearance of the ovary in physiological conditions has been poorly investigated. Only some prospective studies described ovarian characteristics in healthy pre- and post-menopausal women in terms of volume and vascularization patterns [18–20]. However, there is a lack of uniformity in the definition of normal ovary ultrasound parameters and no consensus statement has been established.
Two Italian societies—the Italian Society of Gynecology and Obstetrics (SIGO) and the Italian Society of Endocrinology (SIE)—agreed on the urgent need to produce a consensus statement to define normal ovary ultrasound parameters. To reach this goal, a joint commission of the 2 societies promoted a systematic review and meta-analysis of the existing evidence on ultrasound parameters of the normal ovary. Therefore, the aim of this review is to define the sonographic parameters of the normal ovary, including ovarian follicular count, ovarian volume, and vascular indices. This represents a fundamental and critical step for orientating clinicians not only in interpreting sonographic data, but also to substantiate future research in the field of pathological conditions such as Polycystic Ovary Syndrome (PCOS).
Even though majority of retrieved records enrolled pre-menopausal women, we did not exclude the few studies involving post-menopausal ones, and presented data accordingly.
Methods
Research question
This study aimed to answer this question: which are the normal ovarian follicular count, ovarian volume, ovarian stroma, and vascular indices assessed by ultrasound and Doppler ultrasound?
Study outcomes
The study outcomes were the mean values of the following parameters: mean ovarian follicular count (whole ovary; number), mean ovarian follicular count (ovarian section; number), mean ovarian volume (ml), and mean ovarian artery PI (number). We were not able to provide a meta-analysis of ovarian stroma volume or other vascular indices [i.e., PSV (peak systolic velocity) and RI (Resistance Index)] due to the lack of an adequate number of eligible studies reporting these measures.
Type of study design included
Studies that are considered eligible were cohort studies, cross-sectional studies, and prospective studies with a single arm or including two groups (i.e., healthy controls). We included interventional studies when they provided baseline data (obtained before any treatment, i.e., ovarian stimulation for Assisted Reproductive Technology, ART).
Study population
Both studies on pre-menopausal and post-menopausal women were considered eligible for the qualitative analysis; however, data on menopausal women were not sufficient to perform a meta-analysis. Studies on pre-pubertal girls were considered separately. Records were selected when presenting data obtained from healthy volunteers (including control arms) or from the general population (i.e., screening studies). Regarding data on women from infertile couples, they were included only when a male factor or a tubal factor was specified as the only identified infertility factor.
Search strategy
We performed a systematic review of the literature using methodological approaches previously published [21], and following a protocol written prior to starting the review (PROSPERO registration protocol: CRD42022300584). An extensive search was performed in the following databases: MEDLINE and Embase. Only articles in English and with full-text were included. The search was performed using the words “ovarian”, “Doppler” and “ultrasound” [All Fields], accruing all records on human beings published between January 2000 and December 31, 2020.
Study selection
All the team members independently screened records for inclusion, blinded to each other’s’ decisions. Two of the team members (IS and EM) checked decisions and resolved eventual conflicts. Selections were recorded in a dedicated Excel spreadsheet.
Data extraction
The following data were recorded: number of subjects, ovarian volume (ml; mean and standard deviation—SD), ovarian follicular fount (in the whole ovary or in the ovarian section, mean and SD), ovarian artery Pulsatility Index (PI) (mean and SD), reproductive stage of enrolled subjects, (pre-pubertal/reproductive age/menopausal), age, 2D (2 dimensional) or 3D ultrasound technique, use of transabdominal (TA) or transvaginal (TV) approach, menstrual phase in reproductive age women, description of the size of the follicles included in the follicular count, upper limit of the frequency of the transducer used in MHz, and localization of the artery sampled for Doppler analysis. All the team members independently extracted data and two of the team members (IS and EM) checked them. Disagreements between individual judgements was resolved by collective discussion during dedicated online meetings. Study investigators were contacted for unreported data or additional details. Data were recorded in a dedicated Excel spreadsheet.
Quality assessment
The quality of studies was assessed according to the Oxford Centre for Evidence-Based Medicine: Levels of Evidence [22]. As shown in Table 1, the vast majority of studies were judged as Level 3 (non-randomized or cohort studies).
Table 1.
Description of the studies included in the meta-analysis
| Author [Ref.] | Year | LoE | Study design | No. of subjects | Age, years (mean) | Fertility status | Menstrual phase | Probe upper MhZ limit | Method | Follicles description | Follicles: section vs. whole ovary | Artery sampled for Doppler analysis (PI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adali et al. [24] | 2009 | 3 | Prospective | 42 | 24.3 | Reproductive age | Follicular | 6.5 | 2D TV | NA | NA | Inside ovarian stroma, not close to surface or follicles |
| Adams et al. [25] | 2004 | 2 | Retrospective | 29 | 28.4 | Reproductive age | Follicular | 5.0 | 2D TA and TV | 2–8 mm | NA | NA |
| Ajossa et al. [26] | 2002 | 3 | Prospective | 15 | 29.5 | Reproductive age | Follicular | 7.0 | 2D TV | 2–8 mm | NS | NA |
| Alebić et al. [27] | 2018 | 3 | Retrospective | 705 | 33.4 | Reproductive age | follicular | 7.0 | 2D TV | 2–9 mm | Whole | NA |
| Allemand et al. [28] | 2006 | 3 | Retrospective | 29 | 30.9 | Reproductive age | Follicular | 8.0 | 2D and 3D TV | < 10 mm | Whole/section | NA |
| Assens et al. [29] | 2020 | 3 | Prospective | 115 | 16 | Reproductive age | Follicular | 8.0 | 2D and 3D TA | 2–8 mm | Whole | NA |
| Aviram et al. [30] | 2008 | 3 | Retrospective | 77 | NR | Menopausal | NA | NR | 2D TV | NA | NA | NA |
| Badouraki et al. [31] | 2008 | 3 | Prospective | 99 | 6.9 | Prepubertal | NA | 7.5 | 2D TA | NA | NA | NA |
| Bancsi et al. [32] | 2002 | Prospective | 120 | 34.9 | Reproductive age | Follicular | 7.5 | 2D TV | < 5 mm | NA | NA | |
| Basir et al. [33] | 2001 | 3 | Prospective | 20 | 33 | Reproductive age | Follicular | 7.0 | 2D TV | NA | NA | Within ovarian parenchyma, close proximity to follicles or c.l |
| Bath et al. [34] | 2003 | 3 | Retrospective | 11 | 23 | Reproductive age | Follicular | 4.0 | 2D TV | 2–10 mm | Whole | NA |
| Battaglia et al. (a) [35] | 2002 | 3 | Prospective | 10 | 6.9 | Prepubertal | NA | 3.5 | 2D TA | Small subcapsular | Whole | In the ovarian stroma at the max distance from the surface |
| Battaglia et al. (b) [35] | 2002 | 3 | Prospective | 15 | 7.6 | Prepubertal | NA | 3.5 | 2D TA | Small subcapsular | Whole | “ |
| Battaglia et al. [36] | 2006 | 3 | Prospective | 14 | 23.2 | Reproductive age | NR | 6.5 | 2D TV | Small antral | Whole | “ |
| Battaglia et al. [37] | 2012 | 3 | Prospective | 52 | 25.8 | Reproductive age | NR | 9.0 | 2D TV | Small subcapsular | Whole | “ |
| Bentzen et al. [38] | 2013 | 3 | Retrospective | 366 | 33.7 | Reproductive age | Follicular | 9.0 | 2D TV | 2–10 mm | Whole | NA |
| Carmina et al. [3] | 2005 | 3 | Prospective | 50 | 25.9 | Reproductive age | Follicular | NR | 2D TV | NA | NA | NR |
| Carmina et al. [39] | 2018 | 3 | Retrospective | 28 | 23.4 | Reproductive age | Follicular | 10.0 | 2D TV | 2–10 mm | Whole | NA |
| Catteau-Jonard et al. [40] | 2012 | 3 | Prospective | 95 | 29.0 | Reproductive age | Follicular | 7.0 | 2D TV | 2–9 mm | Whole | NA |
| Chan et al. [41] | 2006 | 3 | Retrospective | 70 | 36 | Reproductive age | NR | 7.0 | 2D and 3D TV | < 10 mm | Whole | NA |
| Chen et al. (a) [42] | 2008 | 3 | Retrospective | 26 | 17.85 | Reproductive age | Follicular | 6.0 | 2D TV | NA | NA | NA |
| Chen et al. (b) [43] | 2008 | 3 | Prospective | 153 | 27.15 | Reproductive age | Follicular | 6.0 | 2D TV | All countable follicles | Whole | NA |
| Christ et al. [44] | 2014 | 3 | Retrospective | 60 | 27 | Reproductive age | Follicular | 12.0 | 2D TV | 2–10 mm | Whole | NA |
| Christiansen et al. [45] | 2016 | 2 | Cross-sectional | 148 | 36.2 | Reproductive age | Follicular | 7.5 | 2D TV | 2–9 mm | Whole | NA |
| Çil et al. [46] | 2009 | 3 | Prospective | 25 | 33.04 | Reproductive | Follicular | 9.0 | 2D TV | 2–10 mm | Whole | Small artery in the ovarian stroma not close to the surface |
| Codner et al. [47] | 2006 | 3 | Prospective | 38 | 26.3 | Reproductive age | NR | 7.5 | 2D TA and TV | 2–9 mm | Section | NA |
| Dao et al. (a) [48] | 2019 | 3 | Retrospective | 55 | 7.5 | Prepubertal | NA | NR | 2D TA | NA | NA | NA |
| Dao et al. (b) [48] | 2019 | 3 | Retrospective | 93 | 14.7 | Reproductive age | NR | NR | 2D TA | NA | NA | NA |
| De Guevara et al. [49] | 2013 | 3 | Prospective | 35 | 37 | Reproductive age | Follicular | 7.5 | 2D TV | 2–9 mm | Whole | NA |
| Deb et al. [50] | 2013 | 3 | Prospective | 36 | 28.12 | Reproductive age | Follicular | 9.0 | 2D and 3D TV | 2–10 mm | Whole | NA |
| Dewailly et al. [51] | 2011 | 3 | Retrospective | 66 | 30 | Reproductive age | Follicular | 9.0 | 2D TV | < 10 mm | Whole | NA |
| Dumesic et al. [52] | 2001 | 3 | Cross-sectional | 25 | 30.8 | Reproductive age | Follicular | 8.0 | 2D and 3D TV | < 10 mm | Whole | NA |
| Elgindy et al. [53] | 2008 | 3 | Prospective | 33 | 30.19 | Reproductive age | Follicular | 6.5 | 2D TV | 2–10 mm | Whole | NA |
| Erdem et al. [54] | 2003 | 3 | Retrospective | 62 | 37.6 | Reproductive age | Follicular | 5.0 | 2D TV | < 8 mm | Whole | NA |
| Fruzzetti et al. [55] | 2015 | 3 | Cross-sectional | 72 | 13.7 | Reproductive age | Follicular | 10.0 | 2D TA and TV | NA | NA | NA |
| Fulghesu et al. [5] | 2001 | 3 | Retrospective | 30 | NR | Reproductive age | Follicular | 6.5 | 2D TV | NA | NA | NA |
| Fulghesu et al. [56] | 2006 | 3 | Prospective | 10 | 24.4 | Reproductive age | Follicular | 6.5 | 2D TV | Total no. of follicles | Section | NA |
| Golestani et al. (a) [57] | 2008 | 3 | Retrospective | 20 | 13.5 | Reproductive age | NR | 3.5 | 2D TA | NA | NA | NR |
| Golestani et al. (b) [57] | 2008 | 3 | Retrospective | 40 | 9.6 | Prepubertal | NA | 3.5 | 2D TA | NA | NA | NR |
| Greenwood et al. [58] | 2017 | 2 | Prospective | 226 | 33.1 | Reproductive age | Follicular | 8.0 | 2D TV | 2–10 mm | Whole | NA |
| Herter et al. [59] | 2002 | 3 | Prospective | 139 | 6 | Prepubertal | NA | 5.0 | 2D TA | NA | NA | NA |
| Homer et al. [60] | 2019 | 2 | Retrospective | 20 | 25.7 | Reproductive age | Follicular | 9.0 | 3D TV | 2–9 mm | Whole | NA |
| Jarrett et al. [61] | 2020 | 2 | Prospective | 12 | 30 | Reproductive age | Follicular | 12.0 | 2D and 3D TV | NA | Whole | NA |
| Järvelä et al. (a) [62] | 2002 | 3 | Prospective | 28 | 35 | Reproductive age | Follicular | 7.0 | 3D TV | 2–8 mm | NA | NA |
| Järvelä et al. (b) [63] | 2003 | 3 | Prospective | 29 | NR | Reproductive age | Follicular | 7.0 | 3D TV | Tot no. of follicles | Whole | NA |
| Järvelä et al. (c) [64] | 2007 | 3 | Prospective | 11 | 30.8 | Reproductive age | Follicular | 7.0 | 3D TV | NA | NA | NA |
| Jokubkiene et al. [65] | 2006 | 3 | Prospective | 14 | 28 | Reproductive age | Follicular | 10.0 | 3D TV | NA | NA | NA |
| Jokubkiene et al. [66] | 2012 | 3 | Prospective | 214 | 30 | Reproductive age | Follicular | 12.0 | 3D TV | 2–10 mm | Whole | NA |
| Jonard et al. [67] | 2005 | 2 | RCT | 57 | 29 | Reproductive age | NR | 7.0 | 2D TV | 2–9 mm | Whole | NA |
| Kline et al. [68] | 2004 | 4 | Prospective | 65 | 35 | Reproductive age | NR | 10.0 | 2D TV | All countable follicles | Whole | NA |
| Köşüş et al. [69] | 2011 | 3 | Prospective | 65 | 26.7 | Reproductive age | Follicular | 6.5 | 2D TV | All countable follicles | Whole | NA |
| Lam et al. [70] | 2007 | 3 | Prospective | 40 | 32.5 | Reproductive age | Follicular | 7.5 | 3D TV | 2–9 mm | Section | One of the main vessels with the ovarian stroma |
| Łebkowska et al. [71] | 2016 | 2 | cross-sectional | 16 | 24 | Reproductive age | Follicular | 9.0 | 2D TV | 2–9 mm | Whole | NA |
| Lie Fong et al. [72] | 2017 | 3 | Retrospective | 297 | 28.3 | Reproductive age | NR | 7.5 | 2D TV | 2–9 mm | Whole | NA |
| Lujan et al. [4] | 2013 | 3 | Prospective | 70 | 27 | Reproductive age | Follicular | 12.0 | 2D TV | 2–9 mm | Whole/section | NA |
| Merino et al. (a) [73] | 2019 | 3 | Retrospective | 53 | 13.9 | Reproductive age | Follicular | 5.0 | 2D TA | 2–9 mm | Section | NA |
| Merino et al. (b) [73] | 2019 | 3 | Retrospective | 22 | 14.3 | Reproductive age | Follicular | 5.0 | 2D TA | 2–9 mm | Section | NA |
| Murphy et al. [74] | 2006 | 3 | Prospective | 17 | 29.7 | Reproductive age | Follicular | 5.0 | 2D TV and TA | 2–10 mm | Section | NA |
| Orbak et al. [75] | 2007 | 3 | Retrospective | 55 | 0.01 | Prepubertal | NA | 7.5 | 2D TA | NA | NA | NA |
| Özay et al. [76] | 2019 | 3 | Prospective | 90 | 21.54 | Reproductive age | NR | 9.0 | 2D TV | NA | NA | In the ovarian stroma at the max distance from the surface |
| Ozkan et al. [77] | 2007 | 3 | Prospective | 43 | 20.8 | Reproductive age | Follicular | 6.0 | 2D TV | NA | NA | Ovarian stroma and in the wall of dominant follicle or c.l |
| Pan et al. [78] | 2002 | 3 | Prospective | 100 | 30.9 | Reproductive age | NR | NR | 3D TV | NA | NA | NA |
| Panidis et al. [79] | 2012 | 3 | Prospective | 254 | 31.3 | Reproductive | NR | NR | 2D TV | 2–9 mm | Whole | NA |
| Pascual et al. [80] | 2008 | 3 | Retrospective | 45 | 32.3 | Reproductive age | Follicular | 10.0 | 3D TV | 2–9 mm | Whole | NA |
| Peigné et al. [81] | 2018 | 3 | Case–control | 157 | 29 | Reproductive age | Follicular | 9.0 | 2D TV | 2–9 mm | Whole | NA |
| Pellizzari et al. [82] | 2002 | 3 | Case–control | 13 | 24.15 | Reproductive age | Follicular | 6.5 | 2D TV | NA | NA | Within the ovarian stroma |
| Petri Nahás et al. [83] | 2004 | 3 | Prospective | 30 | 36.66 | Reproductive age | NR | 7.5 | 2D TV | NA | NA | Ovarian artery in the pelvic infundibulum |
| Phy et al. [84] | 2004 | 4 | Prospective | 33 | 30.4 | Reproductive age | Follicular | 8.0 | 2D and 3D TV | 2–10 mm | Section | NA |
| Pirgon et al. [85] | 2015 | 3 | Case–control | 30 | 15.2 | Reproductive age | Follicular | 7.5 | 2D TV | 2–10 mm | Whole | NA |
| Pirwany et al. [86] | 2001 | 4 | cross-sectional | 14 | 31.2 | Reproductive age | Follicular | NR | NR | NA | NA | NA |
| Rosenfield et al. [87] | 2012 | 3 | Prospective | 19 | 24.5 | Reproductive age | Follicular | 9.0 | 2D TA and TV | 2–9 mm | NA | NA |
| Santoro et al. (a) [88] | 2003 | 4 | Prospective | 14 | NR (> 45) | Reproductive age | Follicular | 5.0 | 2D TV | < 10 mm | Whole | NA |
| Santoro et al. (b) [88] | 2003 | 4 | Prospective | 22 | NR | Reproductive age | Follicular | 5.0 | 2D TV | < 10 mm | Whole | NA |
| Sanverdi et al. [89] | 2018 | 4 | Cross-sectional | 139 | 31.04 | Reproductive age | Follicular | NR | 2D TV | AFC | Whole | NA |
| Sasaki et al. [90] | 2019 | 3 | Retrospective | 118 | 32.66 | Reproductive age | Follicular | 7.0 | NR | AFC | Whole | NA |
| Scheffer et al. (a) [91] | 2003 | 3 | Prospective | 49 | NR | Reproductive age | Follicular | 7.5 | 2D TV | 2–10 mm | Whole | NA |
| Scheffer et al. (b) [91] | 2003 | 3 | Prospective | 53 | NR | Reproductive age | Follicular | 7.5 | 2D TV | 2–10 mm | Whole | NA |
| Scheffer et al. (c) [91] | 2003 | 3 | Prospective | 60 | NR | Reproductive age | Follicular | 7.5 | 2D TV | 2–10 mm | Whole | NA |
| Shahrami et al. [92] | 2016 | 4 | Cross-sectional | 53 | 27.23 | Reproductive age | Follicular | NR | NR | NA | NA | NA |
| Shen et al. [93] | 2008 | 3 | Prospective | 23 | 31 | Reproductive age | Follicular | 9.0 | 2D TV | NA | NA | Large vessel at ovarian hilum |
| Singha et al. [94] | 2015 | 4 | Cross-sectional | 52 | 37 | Reproductive age | NR | 8.0 | 2D TV | NA | NA | NA |
| Su et al. [95] | 2008 | 4 | Cross-sectional | 18 | 45 | Reproductive age | Follicular | NR | 2D TV | 2–10 mm | Whole | NA |
| Taponen et al. [96] | 2004 | 2 | Retrospective | 58 | 31 | Reproductive age | NR | 6.0 | 2D TV | 2–8 mm | Section | NA |
| Tomioka et al. [97] | 2018 | 3 | Prospective | 11 | 30 | Reproductive age | Follicular | 10.0 | 2D TA and TV | 2–10 mm | Whole | NA |
| van Hooff et al. [98] | 2000 | 3 | Prospective | 58 | 16.4 | Reproductive age | Follicular | 5.0 | 2D TA | NA | NA | NA |
| Weerakiet et al. [99] | 2007 | 3 | Cross-sectional | 21 | 33.81 | Reproductive age | Follicular | 7.5 | 2D TV | < 10 mm | Whole | NA |
| Wongwananuruk et al. [100] | 2018 | 3 | Prospective | 63 | 29.7 | Reproductive age | Follicular | 8.0 | 2D TV or TR | 2–9 mm | Whole/section | NA |
| Younis et al. [101] | 2011 | 3 | Prospective | 101 | 28.76 | Reproductive age | Follicular | 9.0 | 2D TV | 2–9 mm | Whole | Stromal ovarian blood flow |
| Zhang et al. [102] | 2013 | 3 | Case–control | 685 | 26.56 | Reproductive age | NR | NR | 2D TV | 2–9 mm | Whole | NA |
LoE level of evidence according to the Oxford Centre for Evidence-Based Medicine, NA not applicable, NR not reported, PI pulsatilty index, TA transabdominal, TV transvaginal, 2D two-dimensional, 3D three-dimensional
Statistical analysis
Four separate meta-analyses were conducted, one for every outcome (ovarian volume, ovarian follicular count—whole ovary, ovarian follicular count—ovarian section, ovarian artery PI). Heterogeneity was assessed using I2 statistics and a random-effects model was applied for all analyses. The effect measures were expressed as mean [lower limit; upper limit]. When the mean was not provided, the closest approximation of mean and SD (standard deviation) from median and IQR (interquartile range) was calculated [23].
Subgroup analyses were performed: (a) according to 5 age bands in studies including reproductive-aged women: < 20 years old (1st group); ≥ 20 and < 25 years old (2nd group); ≥ 25 and < 30 years old (3rd group); ≥ 30 and < 35 years old (4th group); and ≥ 35 years old (5th group); (b) according to 4 groups identified with the upper limit of MHz reported for the transducer: ≤ 5 MHz (1st group), 5–7.5 MHz (2nd group), 8–9 MHz (3rd group), and 10–12 MHz (4th group). Meta-regression analyses were conducted in order to test the effects of age on the evaluated parameters.
All analyses were performed using Comprehensive Meta-analysis Version 2, Biostat (Englewood, NJ, USA).
Results
Analysis of available studies
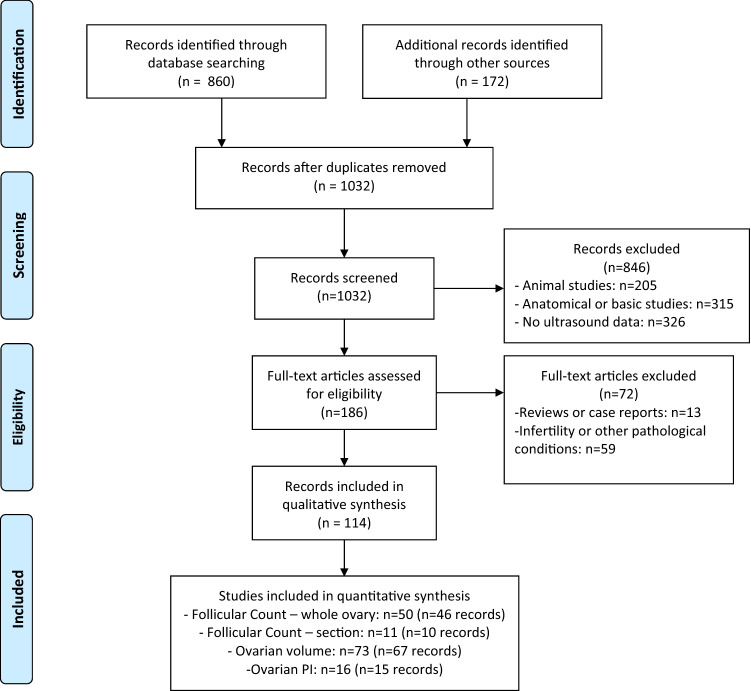
The initial literature search produced 1032 potentially relevant citations. After screening and detailed assessment (see flow chart—Fig. 1 for reasons for exclusion), 50 studies were included in the meta-analysis of follicular count in the whole ovary, 11 in the meta-analysis of follicular count per ovarian section, 73 in the meta-analysis of ovarian volume, and 16 in the meta-analysis of ovarian PI. Some papers provided data for more than one of the 4 meta-analyses. The flow chart of study selection is reported in Fig. 1 and the details of the retrieved studies are reported in Table 1.
Fig. 1.
Flow diagram for the studies included in the meta-analyses. From PRISMA 2009 flow diagram. From reference [103]
To determine the age-related differences in ovarian parameters, studies enrolling pre-pubertal girls or fertile women were considered separately. Data on menopausal women were not sufficient to perform a meta-analysis. Moreover, in the analysis of the studies including reproductive-aged women, when information on age was available, the results were stratified into 5 age bands: < 20 years old (1st group); ≥ 20 and < 25 years old (2nd group); ≥ 25 and < 30 years old (3rd group); ≥ 30 and < 35 years old (4th group); and ≥ 35 years old (5th group).
Ovarian follicular count: whole ovary
Studies reporting information on follicular count calculated on the whole ovary were included in a separate meta-analysis from those reporting the same data obtained within an ovarian section. In general, we included studies reporting information on all visible follicles measuring ≥ 2.0 mm, with an upper limit of 8.0–10.0 mm (see Table 1).
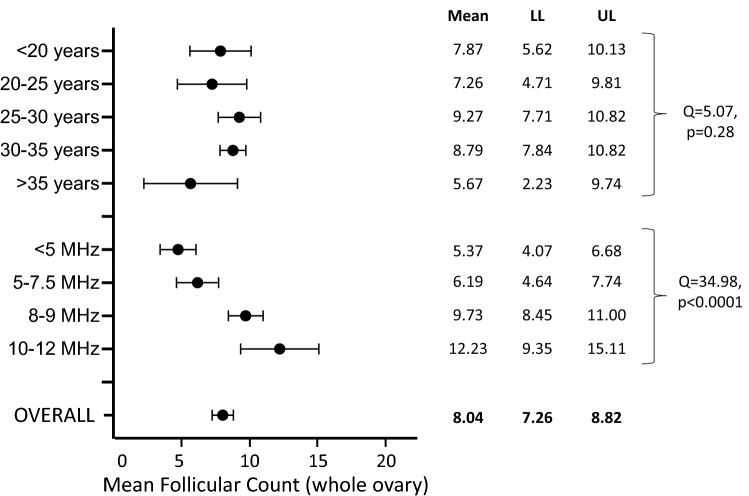
Fifty studies were identified including information on follicular count calculated on the whole ovary. After excluding the 2 studies enrolling pre-pubertal girls [35a and b], the overall mean follicular number was 8.04 [7.26–8.82] (n = 5013 subjects, mean age 29.66 years; Fig. 2).
Fig. 2.
Mean follicular count (whole ovary) at ultrasound in healthy women of reproductive age. LL lower limit, UL upper limit
After stratifying the population in reproductive years according to the above-mentioned age groups, a mean follicular count of 7.87 [5.62–10.13] was found in the 1st group (< 20 years; n = 2 studies) [29, 85], 7.26 [4.71–9.81] in the 2nd group (≥ 20 and < 25 years; n = 4 studies) [3, 36, 39, 71], 9.27 [7.71–10.82] in the 3rd group (≥ 25 and < 30 years; n = 14 studies) [4, 37, 40, 43, 44, 50, 67, 69, 72, 81, 100–102], 8.79 [7.84–9.74] in the 4th group (≥ 30 and < 35 years; n = 16 studies) [27, 28, 38, 46, 51–53, 58, 61, 66, 79, 80, 89, 90, 97, 99], and 5.67 [2.23–9.12] in the 5th group (≥ 35 years; n = 9 studies) [41, 45, 49, 54, 68, 88a, 91b and c, 95], respectively (Fig. 2). Overall, among the 5 groups, no statistically significant difference in ovarian follicular count appeared (Q = 5.07, p = 0.28) (Fig. 2). However, at meta-regression analysis, age showed a significant modulation effect in reproductive-aged women on follicular count calculated on the whole ovary (n = 42 studies) (s = 21.63, p < 0.0001; I = 6.93, p < 0.0001].
We also explored the differences in the follicular count according to the frequency of the transducer. Considering the upper limit of MHz reported for the transducer in each study, 4 groups were identified: ≤ 5 MHz (1st group), 5–7.5 MHz (2nd group), 8–9 MHz (3rd group), and 10–12 MHz (4th group). A mean follicular count of 5.37 [4.07–6.68] was found in the 1st group (4 studies) [34, 54, 88a and b], 6.19 [4.64–7.74] in the 2nd group (18 studies) [27, 36, 40, 41, 43, 45, 49, 53, 63, 67, 69, 72, 85, 90, 91a–c, 99], 9.73 [8.45–11.00] in the 3rd group (15 studies) [ 28, 29, 37, 38, 39, 46, 50–52, 58, 60, 71, 81, 100, 101], and 12.23 [9.35–15.11] in the 4th group (7 studies) [4, 44, 61, 66, 68, 80, 97] (Fig. 2). Four studies failed to provide information on this parameter. A significant difference was found when stratifying the studies according to the transducer’s MHz (Q = 34.98, p < 0.0001) (Fig. 2). Therefore, pairwise comparisons of studies in the 4 groups were performed, and a statistically significant difference was found in the follicular count:
between the 1st and the 3rd groups (≤ 5 vs. 8–9 MHz) [Q = 21.86; p < 0.0001] and between the 1st and the 4th groups (≤ 5 vs. 10–12 MHz) [Q = 18.07; p < 0.0001];
between the 2nd and the 3rd groups (5–7.5 vs. 8–9 MHz) [Q = 11.95; p = 0.001] and between the 2nd and the 4th groups (5–7.5 vs. 10–12 MHz) [Q = 13.11; p < 0.0001].
Ovarian follicular count: a section
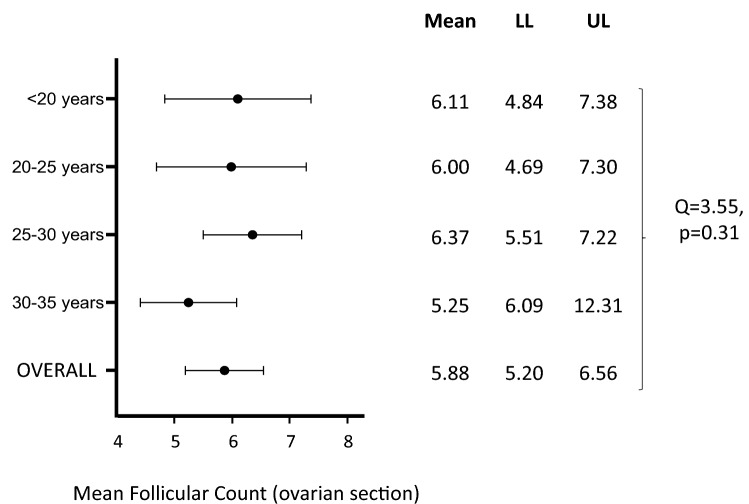
Eleven studies including information on ovarian follicular count calculated within an ovarian section (maximum length in sagittal section) were identified. All of them considered women in their reproductive years. The pooled ovarian follicular number was 5.88 [5.20–6.56] (Fig. 3).
Fig. 3.
Mean follicular count (within ovarian section) at ultrasound in healthy women of reproductive age. LL lower limit, UL upper limit
After stratifying the studies according to the previously identified age groups, no studies were found in the 5th one (≥ 35 years). Mean ovarian follicular counts of 6.11 [4.84–7.38], 6.00 [4.69–7.30], 6.37 [5.51–7.22] and 5.25 [6.09–12.31] ml were found in the 1st (< 20 years; n = 2 studies) [73a and b], 2nd (≥ 20 and < 25 years; n = 1 study) [56], 3rd (≥ 25 and < 30 years; n = 4 studies) [4, 47, 74, 100], and 4th group (≥ 30 and < 35 years; n = 4 studies) [28, 70, 84, 96], respectively, with no statistically significant difference between the four groups (Q = 3.55, p = 0.31) (Fig. 3). Similarly, no significant difference was found when stratifying the studies according to the transducer’s MHz (Q = 2.73, p = 0.43) (not shown).
Ovarian volume
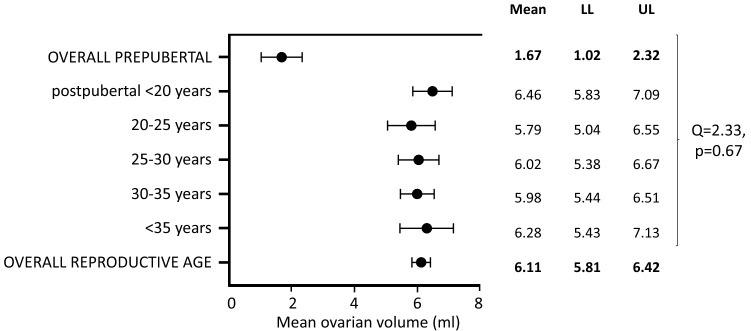
Seventy-three studies were identified, which reported information on ovarian volume calculated using the formula for a prolate ellipsoid. Among them, 65 studies enrolled women in their reproductive years, whereas 6 [31, 35a and b, 48a, 57b, 59] enrolled girls of pre-pubertal age, one newborn [75] and one post-menopausal woman [30]. When considering the 65 studies including women in their reproductive years (n = 4107 subjects, mean age 27.42 years), the overall mean ovarian volume was 6.11 [5.81–6.42] ml (Fig. 4). After excluding one study enrolling newborns [75], in the remaining six studies considering subjects of pre-pubertal age (n = 358 subjects, mean age 7.42 years), the mean ovarian volume was 1.67 [1.02–2.32] ml (Fig. 4), which was significantly lower than in women in reproductive age (Q = 147.05, p < 0.0001).
Fig. 4.
Mean ovarian volume (ml) at ultrasound, calculated using the formula for a prolate ellipsoid, in healthy pre-pubertal girls and women of reproductive age. LL lower limit, UL upper limit
Among the studies on women in their reproductive years, 62 provided information on the mean age or the age range of the sample. We analyzed the studies according to the above-mentioned 5 age sub-groups, and found a mean ovarian volume of 6.46 [5.83–7.09] ml in the 1st group (< 20 years; n = 9 studies) [29, 42, 48b, 55, 57a, 73a and b, 85, 98], 5.79 [5.04–6.55] ml in the 2nd group (≥ 20 and < 25 years; n = 9 studies) [24, 34, 36, 39, 56, 71, 77, 82, 87], 6.02 [5.38–6.67] ml in the 3rd group (≥ 25 and < 30 years; n = 17 studies) [3, 4, 25, 26, 37, 43, 44, 47, 50, 65, 67, 69, 74, 92, 100–102], 5.98 [5.44–6.51] ml in the 4th group (≥ 30 and < 35 years; n = 18 studies) [28, 38, 46, 51–53, 61, 64, 66, 70, 78–80, 84, 86, 96, 97, 99], and 6.28 [5.43–7.13] ml in the 5th group (≥ 35 years; n = 9 studies) [41, 49, 54, 62, 83, 91b and c, 94, 95], respectively (Fig. 4). Overall, among the 5 groups in reproductive age, no statistically significant difference in ovarian volume was found (Q = 2.33, p = 0.67) (Fig. 4). However, at meta-regression analysis, exploring the effect of age on ovarian volume in reproductive-aged women (n = 60 studies), a significance relationship was observed [slope (s) 0.01, p = 0.06; intercept (I) 5.04, p < 0.0001].
Ovarian pulsatility index (PI)
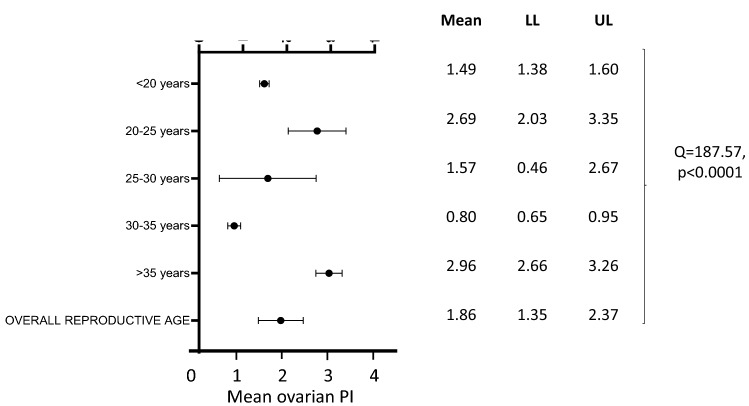
Sixteen studies including information on ovarian artery PI were identified (see Table 1). After excluding two studies enrolling pre-pubertal girls [35a, 57a], which used a transabdominal approach, the pooled mean PI was 1.86 [1.35–2.37] (Fig. 5). All studies except one [70] employed a two-dimensional (2D) technique.
Fig. 5.
Mean ovarian pulsatility index (PI) at Doppler ultrasound in healthy women of reproductive age. LL lower limit, UL upper limit
After stratifying the studies according to the previously identified age bands, a mean ovarian PI of 1.49 [1.38–1.60], 2.69 [2.03–3.35], 1.57 [0.46–2.67], 0.80 [0.65–0.95], and 2.96 [2.66–3.26] was found in the 1st (< 20 years; n = 1 study) [57b], 2nd (≥ 20 and < 25 years; n = 5 studies) [24, 36, 76, 77, 82], 3rd (≥ 25 and < 30 years; n = 3 studies) [3, 37, 101], 4th (≥ 30 and < 35 years; n = 4 studies) [33, 46, 70, 93], and 5th groups (≥ 35 years; n = 1 study) [83], respectively, with a statistically significant difference between the five groups (Q = 187.57, p < 0.0001) (Fig. 5). Therefore, pairwise comparisons of groups with different mean age bands were performed, and a statistically significant difference was found in ovarian PI:
between the 1st and the 2nd groups (< 20 vs. ≥ 20 and < 25 years) [Q = 12.42; p < 0.0001], between the 1st and the 4th groups (< 20 vs. ≥ 30 and < 35 years) [Q = 54.49; p < 0.0001], between the 1st and the 5th groups (< 20 vs. ≥ 35 years) [Q = 82.83; p < 0.0001];
between the 2nd and the 4th groups (≥ 20 and < 25 vs. ≥ 30 and < 35 years) [Q = 30.15, p < 0.00001];
between the 3rd and the 5th groups (≥ 25 and < 30 vs. ≥ 35 years) [Q = 5.73, p = 0.017];
between the 4th and the 5th groups (≥ 30 and < 35 vs. ≥ 35 years) [Q = 163.27, p < 0.0001] (Fig. 5).
Twelve studies on women in their reproductive years provided information on the transducer’s frequency; no studies were identified in the 4th group (10–12 MHz). No significant difference on ovarian artery PI emerged when stratifying the studies according to the different MHz (Q = 4.90, p = 0.09) (not shown).
Discussion
The present systematic and meta-analytic approach demonstrates for the first time that the overall mean ovarian volume was 6.11 ml in women in reproductive age and 1.67 ml in pre-pubertal girls, with a range of 5.81–6.42 and of 1.02–2.32, respectively. In reproductive age, the overall mean follicular count was 8.04 when calculated in the whole ovary and 5.88 when calculated in an ovarian section, with a range of 7.26–8.82 and of 5.20–6.56, respectively. However, age and the frequency of the transducers were found to significantly modulate these values. In contrast, the authors agreed that all the other sonographic parameters (i.e., ovarian stroma) could not be considered eligible for meta-analysis, mainly due to the paucity and heterogeneity of data.
The standardization of ultrasound parameters is of paramount clinical relevance since it contributes to the diagnostic workflow of several endocrine conditions, including Premature Ovarian Insufficiency, PCOS and poor ovarian responders (POR) in ART procedures (Bologna criteria: antral follicle count ≤ 5–7 follicles [104]. Noteworthy, we hereby demonstrated that the population of women with a normal ovarian function showed a follicular count ranging between 7.26 and 8.82, thus corroborating the proposed criteria for either PCOM (> 12 according to Rotterdam criteria) or POR (≤ 5–7 according to Bologna criteria).
Another relevant finding of the present study is the apparent age-dependent modulation of the total ovarian follicular count (as calculated in the whole ovary). Despite being not able to highlight a significant difference among the 5 age sub-groups, we noted that the age range 25–30 years was the one with the higher mean follicular count (9.27), followed by a progressive age-related reduction (5.67 in fertile women older than 35 years). In addition, pubertal girls younger than 20 showed a higher mean follicular count (7.87) than those in the 20–25-year group (7.26). In line with this trend, multifollicular ovaries are seen commonly in girls with a gynecological age of ˂ 8 years, and should be considered as a physiologic condition during early adolescence [105]. This is relevant to avoid a misdiagnosis of PCOS in this population. A similar age-modulated trend, although without significant differences between groups, was observed when the follicles were counted in an ovarian section. Our study, meta-analyzing data on follicular count obtained in different ultrasound settings, could be of relevance since the analysis of follicular count per ovarian section is commonly performed in clinical practice.
Another important parameter that we were able to meta-analyze was the ovarian volume. As expected, a significant difference was found when comparing pre-pubertal girls and women in their reproductive years (mean values 1.67 vs. 6.11, respectively), whereas our data did not reveal a clear modulation by age in fertile women. As observed for the follicular count, even when we considered the mean upper limit for normal ovarian volume (6.42 ml), this value fell well below the proposed PCOM threshold of 10 ml, thus supporting the appropriateness of this cut-off in defining PCOM.
Even though the ovarian PI is a Doppler ultrasound parameter with a more limited clinical application, we were able to meta-analyze available data while finding again an age-dependent modulation, which revealed two peaks: one pertaining the 20–25 years and one pertaining the > 35 years group. Intriguingly, PI of arterial blood vessels within the genital district has been previously reported to increase as a function of metabolic risk factors, including body mass index, waist circumference, and insulin-resistance biomarkers [106]. However, since data on cardiometabolic risk factors or relative treatments were not systemically available in the included studies, the potential contributions of these mediators could only be mentioned without any inference.
Regarding the role of ultrasound methodology, the TV approach has been proved as more sensitive and specific than the TA one, not only in the diagnosis of pelvic disease of gynecologic origin, but also in cases of ovarian follicle monitoring and evaluation for PCOM [107]. In the 2014 “task force report from the Androgen Excess and Polycystic Ovary Syndrome Society”, which proposed a threshold of ≥ 25 follicle number per ovary to define PCOM when using transducers with a frequency ≥ 8 MHz, the TA route was described as “not suitable for recording a precise follicle count” [6]. It should be noted that, in situations when the TA approach is the only possible, it can provide a reliable assessment of ovarian volume. As for the transrectal route, in adolescent patients, a 3D version combined with the TA technique has been showed to improve the precision of PCOM definition [108]. In the reviewed studies, all those performed in pre-pubertal girls used a TA-only approach, whereas none reported the use of the transrectal one. Regarding the studies on women in reproductive age, only 4 employed only TA ultrasound [48, 57, 73, 98], while a very few others [25, 29, 47, 55, 74, 87, 97] reported the use of both methods (TA and TV) (see Table 1). None of the 4 studies conducted with TA ultrasound provided data for the meta-analysis of Ovarian Follicular Count in the whole ovary, and only one [73] provided data for the meta-analysis of Ovarian Follicular Count per section. Therefore, no subgroup analysis was performed.
The main strength of our research is the standardized, meta-analytic approach, which, to our knowledge, is the first ever conducted on this topic. In addition, we decided to perform several sub-analyses according to potential modulating factors, including follicular count obtained per ovarian section, which is commonly performed in clinical practice. In addition, we refrained from interpreting our findings in a pathological setting. The key guidance of the present document was to provide informative and user-friendly data, that could be used and critically considered in future research on pathological ovarian conditions. It is advisable that the much-needed standardization of ovarian ultrasonography will allow the development of more evidence-based, universally accepted criteria for the diagnosis of ovarian disorders, especially PCOM.
Among the limitations, we would like to mention the lack of an indication pertaining the cycle phase for ovarian ultrasound; however, ad highlighted in the summarizing Table, the vast majority of records reported to have conducted the ultrasound study during the follicular phase.
Conclusions
In conclusion, our systematic review and meta-analysis provides a relevant clinical information for a more accurate assessment of physiological ultrasound ovarian parameters in pre-pubertal girls and women in reproductive age. Each center should standardize ovarian US according to the available machines, at least when analyzing those parameters which resulted to be significantly modulated by the quality of the transducer. However, we strongly believe that such guidance should improve the interpretation and diagnostic accuracy of ovarian ultrasound parameters in different physiological and pathological settings.
Acknowledgements
We thank members of the Women’s Endocrinology Group of the Italian Society of Endocrinology: Gambineri, Roberto Mioni, Paolo Moghetti, Costanzo Moretti, Luca Persani, and Linda Vignozzi.
Author contributions
Conceptualization: FM, IS, CBP, SF, AG, RM, PM, CM, LP, LV; Literature Search: FM, IS, EM, CBP, CB, FD, SFe, SF, LG, MV, AG, RM; Data Analysis: FM, IS, EM, GR, LV; Draft: FM, IS, EM, LV; Critical revision: FM, IS, EM, GR, CBP, CB, FD, SFe, SF, LG, MV, AG, RM, PM, CM, LP, GS, FG, LV.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. The authors did not receive support from any organization for the submitted work. SFe was supported by the program of the Postgraduate School of Endocrinology, University of Milan, Milan, Italy.
Declarations
Conflict interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
This article does not include research on human participants and/or animals.
Informed consent
Informed consent is not required.
Footnotes
Members of the Women’s Endocrinology Group of the Italian Society of Endocrinology are listed in the Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
F. Moro and I. Scavello have contributed equally.
Contributor Information
L. Vignozzi, Email: linda.vignozzi@unifi.it
the Women’s Endocrinology Group of the Italian Society of Endocrinology:
A. Gambineri, R. Mioni, P. Moghetti, P. Moretti, L. Persani, and L. Vignozzi
References
- 1.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Christ JP, Vanden Brink H, Brooks ED, Pierson RA, Chizen DR, Lujan ME. Ultrasound features of polycystic ovaries relate to degree of reproductive and metabolic disturbance in polycystic ovary syndrome. Fertil Steril. 2015;103:787–794. doi: 10.1016/j.fertnstert.2014.12.094. [DOI] [PubMed] [Google Scholar]
- 3.Carmina E, Orio F, Palomba S, Longo RA, Lombardi G, Lobo RA. Ovarian size and blood flow in women with polycystic ovary syndrome (PCOS) and their correlations with some endocrine parameters. Fertil Steril. 2005;84:413–419. doi: 10.1016/j.fertnstert.2004.12.061. [DOI] [PubMed] [Google Scholar]
- 4.Lujan ME, Jarrett BY, Brooks ED, Reines JK, Peppin AK, Muhn N, Haider E, Pierson RA, Chizen DR. Updated ultrasound criteria for polycystic ovary syndrome: reliable thresholds for elevated follicle population and ovarian volume. Hum Reprod. 2013;28(5):1361–1368. doi: 10.1093/humrep/det062. [DOI] [PubMed] [Google Scholar]
- 5.Fulghesu AM, Ciampelli M, Belosi C, Apa R, Pavone V, Lanzone A. A new ultrasound criterion for the diagnosis of polycystic ovary syndrome: the ovarian stroma/total area ratio. Fertil Steril. 2001;76:326–331. doi: 10.1016/S0015-0282(01)01919-7. [DOI] [PubMed] [Google Scholar]
- 6.Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, Escobar-Morreale HF. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2014;20(3):334–352. doi: 10.1093/humupd/dmt061. [DOI] [PubMed] [Google Scholar]
- 7.Heilbrun ME, Olpin J, Shaaban A. Imaging of benign adnexal masses: characteristic presentations on ultrasound, computed tomography, and magnetic resonance imaging. Clin Obstet Gynecol. 2009;52(1):21–39. doi: 10.1097/GRF.0b013e318195834b. [DOI] [PubMed] [Google Scholar]
- 8.Timmerman D, Schwärzler P, Collins WP, Claerhout F, Coenen M, Amant F, Vergote I, Bourne TH. Subjective assessment of adnexal masses with the use of ultrasonography: an analysis of interobserver variability and experience. Ultrasound Obstet Gynecol. 1999;13(1):11–16. doi: 10.1046/j.1469-0705.1999.13010011.x. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman D, Valentin L, Bourne TH, Collins WP, Verrelst H, Vergote I, International Ovarian Tumor Analysis (IOTA) Group Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol. 2000;16(5):500–505. doi: 10.1046/j.1469-0705.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Holsbeke C, Van Calster B, Guerriero S, Savelli L, Leone F, Fischerova D, Czekierdowski A, Fruscio R, Veldman J, Van de Putte G, Testa AC, Bourne T, Valentin L, Timmerman D. Imaging in gynaecology: how good are we in identifying endometriomas? Facts Views Vis Obgyn. 2009;1(1):7–17. [PMC free article] [PubMed] [Google Scholar]
- 11.Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475–490. doi: 10.1148/radiographics.21.2.g01mr09475. [DOI] [PubMed] [Google Scholar]
- 12.Virgilio BA, De Blasis I, Sladkevicius P, Moro F, Zannoni GF, Arciuolo D, Mascilini F, Ciccarone F, Timmerman D, Kaijser J, Fruscio R, Van Holsbeke C, Franchi D, Epstein E, Leone FPG, Guerriero S, Czekierdowski A, Scambia G, Testa AC, Valentin L. Imaging in gynecological disease (16): clinical and ultrasound characteristics of serous cystadenofibromas in adnexa. Ultrasound Obstet Gynecol. 2019;54(6):823–830. doi: 10.1002/uog.20277. [DOI] [PubMed] [Google Scholar]
- 13.Franchi D, Boveri S, Fruscio R, Fischerova D, Guerriero S, Moruzzi MC, Colombo N, Timmerman D, Valentin L, Testa AC. Imaging in gynecological disease (8): ultrasound characteristics of recurrent borderline ovarian tumors. Ultrasound Obstet Gynecol. 2013;41(4):452–458. doi: 10.1002/uog.12276. [DOI] [PubMed] [Google Scholar]
- 14.Exacoustos C, Romanini ME, Rinaldo D, Amoroso C, Szabolcs B, Zupi E, Arduini D. Preoperative sonographic features of borderline ovarian tumors. Ultrasound Obstet Gynecol. 2005;25(1):50–59. doi: 10.1002/uog.1823. [DOI] [PubMed] [Google Scholar]
- 15.Van Calster B, Van Hoorde K, Valentin L, Testa AC, Fischerova D, Van Holsbeke C, Savelli L, Franchi D, Epstein E, Kaijser J, Van Belle V, Czekierdowski A, Guerriero S, Fruscio R, Lanzani C, Scala F, Bourne T, Timmerman D, International Ovarian Tumour Analysis Group Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014;349:g5920. doi: 10.1136/bmj.g5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timmerman D, Van Calster B, Testa A, Savelli L, Fischerova D, Froyman W, Wynants L, Van Holsbeke C, Epstein E, Franchi D, Kaijser J, Czekierdowski A, Guerriero S, Fruscio R, Leone FPG, Rossi A, Landolfo C, Vergote I, Bourne T, Valentin L. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am J Obstet Gynecol. 2016;214(4):424–437. doi: 10.1016/j.ajog.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Moro F, Esposito R, Landolfo C, Froyman W, Timmerman D, Bourne T, Scambia G, Valentin L, Testa AC. Ultrasound evaluation of ovarian masses and assessment of the extension of ovarian malignancy. Br J Radiol. 2021;94(1125):20201375. doi: 10.1259/bjr.20201375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sladkevicius P, Valentin L, Marsál K. Blood flow velocity in the uterine and ovarian arteries during the normal menstrual cycle. Ultrasound Obstet Gynecol. 1993;3(3):199–208. doi: 10.1046/j.1469-0705.1993.03030199.x. [DOI] [PubMed] [Google Scholar]
- 19.Sladkevicius P, Valentin L, Marsàl K. Transvaginal gray-scale and Doppler ultrasound examinations of the uterus and ovaries in healthy postmenopausal women. Ultrasound Obstet Gynecol. 1995;6(2):81–90. doi: 10.1046/j.1469-0705.1995.06020081.x. [DOI] [PubMed] [Google Scholar]
- 20.Merz E, Miric-Tesanic D, Bahlmann F, Weber G, Wellek S. Sonographic size of uterus and ovaries in pre- and postmenopausal women. Ultrasound Obstet Gynecol. 1996;7(1):38–42. doi: 10.1046/j.1469-0705.1996.07010038.x. [DOI] [PubMed] [Google Scholar]
- 21.Mueller M, D'Addario M, Egger M, Cevallos M, Dekkers O, Mugglin C, Scott P. Methods to systematically review and meta-analyse observational studies: a systematic scoping review of recommendations. BMC Med Res Methodol. 2018;18(1):44. doi: 10.1186/s12874-018-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.OCEBM Levels of Evidence Working Group. “The Oxford Levels of Evidence 2”. Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
- 23.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 24.Adali E, Kolusari A, Adali F, Yildizhan R, Kurdoglu M, Sahin HG. Doppler analysis of uterine perfusion and ovarian stromal blood flow in polycystic ovary syndrome. Int J Gynaecol Obstet. 2009;105(2):154–157. doi: 10.1016/j.ijgo.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Adams JM, Taylor AE, Crowley WF, Jr, Hall JE. Polycystic ovarian morphology with regular ovulatory cycles: insights into the pathophysiology of polycystic ovarian syndrome. J Clin Endocrinol Metab. 2004;89(9):4343–4350. doi: 10.1210/jc.2003-031600. [DOI] [PubMed] [Google Scholar]
- 26.Ajossa S, Guerriero S, Paoletti AM, Orrù M, Melis GB. Hyperinsulinemia and uterine perfusion in patients with polycystic ovary syndrome. Ultrasound Obstet Gynecol. 2002;20(3):276–280. doi: 10.1046/j.1469-0705.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- 27.Alebic MŠ, Stojanovic N, Dewailly D. Discordance between serum anti-Müllerian hormone concentrations and antral follicle counts: not only technical issues. Hum Reprod. 2018;33(6):1141–1148. doi: 10.1093/humrep/dey098. [DOI] [PubMed] [Google Scholar]
- 28.Allemand MC, Tummon IS, Phy JL, Foong SC, Dumesic DA, Session DR. Diagnosis of polycystic ovaries by three-dimensional transvaginal ultrasound. Fertil Steril. 2006;85(1):214–219. doi: 10.1016/j.fertnstert.2005.07.1279. [DOI] [PubMed] [Google Scholar]
- 29.Assens M, Dyre L, Henriksen LS, Brocks V, Sundberg K, Jensen LN, Pedersen AT, Main KM. Menstrual pattern, reproductive hormones, and transabdominal 3D ultrasound in 317 adolescent girls. J Clin Endocrinol Metab. 2020;105(9):dgaa355. doi: 10.1210/clinem/dgaa355. [DOI] [PubMed] [Google Scholar]
- 30.Aviram R, Gassner G, Markovitch O, Cohen I, Fishman A, Tepper R. Volumes of normal ovaries, ovaries with benign lesions, and ovaries with cancer in menopausal women: is there an optimal cut-off value to predict malignancy? J Clin Ultrasound. 2008;36(1):1–5. doi: 10.1002/jcu.20416. [DOI] [PubMed] [Google Scholar]
- 31.Badouraki M, Christoforidis A, Economou I, Dimitriadis AS, Katzos G. Sonographic assessment of uterine and ovarian development in normal girls aged 1 to 12 years. J Clin Ultrasound. 2008;36(9):539–544. doi: 10.1002/jcu.20522. [DOI] [PubMed] [Google Scholar]
- 32.Bancsi LF, Broekmans FJ, Eijkemans MJ, de Jong FH, Habbema JD, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77(2):328–336. doi: 10.1016/S0015-0282(01)02983-1. [DOI] [PubMed] [Google Scholar]
- 33.Basir GS, Lam TP, Wai-Sum O, Ho PC. Haemodynamic evaluation of tubal and male factors of infertility in natural and ovarian stimulation cycles. J Assist Reprod Genet. 2001;18(3):125–128. doi: 10.1023/a:1009489518468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bath LE, Wallace WH, Shaw MP, Fitzpatrick C, Anderson RA. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Müllerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18(11):2368–2374. doi: 10.1093/humrep/deg473. [DOI] [PubMed] [Google Scholar]
- 35.Battaglia C, Regnani G, Mancini F, Iughetti L, Flamigni C, Venturoli S. Polycystic ovaries in childhood: a common finding in daughters of PCOS patients. A pilot study. Hum Reprod. 2002;17(3):771–776. doi: 10.1093/humrep/17.3.771. [DOI] [PubMed] [Google Scholar]
- 36.Battaglia C, Pasini A, Mancini F, Persico N, Burnelli R, Cicognani A, de Aloysio D. Utero-ovarian ultrasonographic and Doppler flow analyses in female childhood cancer survivors with regular menstruation and normal circulating follicle-stimulating hormone levels. Fertil Steril. 2006;85(2):455–461. doi: 10.1016/j.fertnstert.2005.07.1299. [DOI] [PubMed] [Google Scholar]
- 37.Battaglia C, Battaglia B, Morotti E, Paradisi R, Zanetti I, Meriggiola MC, Venturoli S. Two- and three-dimensional sonographic and color Doppler techniques for diagnosis of polycystic ovary syndrome. The stromal/ovarian volume ratio as a new diagnostic criterion. J Ultrasound Med. 2012;31(7):1015–1024. doi: 10.7863/jum.2012.31.7.1015. [DOI] [PubMed] [Google Scholar]
- 38.Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Andersen AN. Ovarian antral follicle subclasses and anti-Mullerian hormone during normal reproductive aging. J Clin Endocrinol Metab. 2013;98(4):1602–1611. doi: 10.1210/jc.2012-1829. [DOI] [PubMed] [Google Scholar]
- 39.Carmina E, Fruzzetti F, Lobo RA. Features of polycystic ovary syndrome (PCOS) in women with functional hypothalamic amenorrhea (FHA) may be reversible with recovery of menstrual function. Gynecol Endocrinol. 2018;34(4):301–304. doi: 10.1080/09513590.2017.1395842. [DOI] [PubMed] [Google Scholar]
- 40.Catteau-Jonard S, Bancquart J, Poncelet E, Lefebvre-Maunoury C, Robin G, Dewailly D. Polycystic ovaries at ultrasound: normal variant or silent polycystic ovary syndrome? Ultrasound Obstet Gynecol. 2012;40(2):223–229. doi: 10.1002/uog.11202. [DOI] [PubMed] [Google Scholar]
- 41.Chan CC, Ng EH, Tang OS, Lee CP, Ho PC. The prevalence of polycystic ovaries in Chinese women with a history of gestational diabetes mellitus. Gynecol Endocrinol. 2006;22(9):516–520. doi: 10.1080/09513590600906649. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Yang D, Li L, Chen X. The role of ovarian volume as a diagnostic criterion for Chinese adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2008;21(6):347–350. doi: 10.1016/j.jpag.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Li L, Chen X, Zhang Q, Wang W, Li Y, Yang D. Ovarian volume and follicle number in the diagnosis of polycystic ovary syndrome in Chinese women. Ultrasound Obstet Gynecol. 2008;32(5):700–703. doi: 10.1002/uog.5393. [DOI] [PubMed] [Google Scholar]
- 44.Christ JP, Willis AD, Brooks ED, Vanden Brink H, Jarrett BY, Pierson RA, Chizen DR, Lujan ME. Follicle number, not assessments of the ovarian stroma, represents the best ultrasonographic marker of polycystic ovary syndrome. Fertil Steril. 2014;101(1):280–287.e1. doi: 10.1016/j.fertnstert.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christiansen SC, Eilertsen TB, Vanky E, Carlsen SM. Does AMH reflect follicle number similarly in women with and without PCOS? PLoS One. 2016;11(1):e0146739. doi: 10.1371/journal.pone.0146739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cil AP, Leventoğlu A, Sönmezer M, Soylukoç R, Oktay K. Assessment of ovarian reserve and Doppler characteristics in patients with multiple sclerosis using immunomodulating drugs. J Turk Ger Gynecol Assoc. 2009;10(4):213–219. [PMC free article] [PubMed] [Google Scholar]
- 47.Codner E, Soto N, Lopez P, Trejo L, Avila A, Eyzaguirre FC, Iniguez G, Cassorla F. Diagnostic criteria for polycystic ovary syndrome and ovarian morphology in women with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2006;91(6):2250–2256. doi: 10.1210/jc.2006-0108. [DOI] [PubMed] [Google Scholar]
- 48.Dao KA, Tadros S, Chan S, Poremski PM, Squires JH. Pediatric ovarian volumes measured at ultrasound after contralateral unilateral oophorectomy. Pediatr Radiol. 2019;49(5):632–637. doi: 10.1007/s00247-018-04336-7. [DOI] [PubMed] [Google Scholar]
- 49.de Guevara AL, Crisosto N, Echiburú B, Preisler J, Vantman N, Bollmann J, Pérez-Bravo F, Sir-Petermann T. Evaluation of ovarian function in 35–40-year-old women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):165–170. doi: 10.1016/j.ejogrb.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Deb S, Campbell BK, Clewes JS, Pincott-Allen C, Raine-Fenning NJ. Intracycle variation in number of antral follicles stratified by size and in endocrine markers of ovarian reserve in women with normal ovulatory menstrual cycles. Ultrasound Obstet Gynecol. 2013;41(2):216–222. doi: 10.1002/uog.11226.52. [DOI] [PubMed] [Google Scholar]
- 51.Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, Duhamel A, Catteau-Jonard S. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123–3129. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 52.Dumesic DA, Damario MA, Session DR, Famuyide A, Lesnick TG, Thornhill AR, McNeilly AS. Ovarian morphology and serum hormone markers as predictors of ovarian follicle recruitment by gonadotropins for in vitro fertilization. J Clin Endocrinol Metab. 2001;86(6):2538–2543. doi: 10.1210/jcem.86.6.7605. [DOI] [PubMed] [Google Scholar]
- 53.Elgindy EA, El-Haieg DO, El-Sebaey A. Anti-Müllerian hormone: correlation of early follicular, ovulatory and midluteal levels with ovarian response and cycle outcome in intracytoplasmic sperm injection patients. Fertil Steril. 2008;89(6):1670–1676. doi: 10.1016/j.fertnstert.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 54.Erdem M, Erdem A, Biberoglu K, Arslan M. Age-related changes in ovarian volume, antral follicle counts and basal follicle stimulating hormone levels: comparison between fertile and infertile women. Gynecol Endocrinol. 2003;17(3):199–205. doi: 10.1080/gye.17.3.199.205. [DOI] [PubMed] [Google Scholar]
- 55.Fruzzetti F, Campagna AM, Perini D, Carmina E. Ovarian volume in normal and hyperandrogenic adolescent women. Fertil Steril. 2015;104(1):196–199. doi: 10.1016/j.fertnstert.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 56.Fulghesu AM, Angioni S, Belosi C, Apa R, Selvaggi L, Ciampelli M, Iuculano A, Melis GB, Lanzone A. Pituitary-ovarian response to the gonadotrophin-releasing hormone-agonist test in anovulatory patients with polycystic ovary syndrome: predictive role of ovarian stroma. Clin Endocrinol (Oxf) 2006;65(3):396–401. doi: 10.1111/j.1365-2265.2006.02611.x. [DOI] [PubMed] [Google Scholar]
- 57.Golestani R, Sheikhvatan M, Behpour AM, Mehdizadeh M, Hajiashrafi AA. Relationship between uterine and ovarian arterial blood flow measured by Doppler sonography at different stages of puberty. Taiwan J Obstet Gynecol. 2008;47(1):62–65. doi: 10.1016/S1028-4559(08)60056-X. [DOI] [PubMed] [Google Scholar]
- 58.Greenwood EA, Cedars MI, Santoro N, Eisenberg E, Kao CN, Haisenleder DJ, Diamond MP, Huddleston HG, National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Cooperative Reproductive Medicine Network Antimüllerian hormone levels and antral follicle counts are not reduced compared with community controls in patients with rigorously defined unexplained infertility. Fertil Steril. 2017;108(6):1070–1077. doi: 10.1016/j.fertnstert.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 59.Herter LD, Golendziner E, Flores JA, Becker E, Jr, Spritzer PM. Ovarian and uterine sonography in healthy girls between 1 and 13 years old: correlation of findings with age and pubertal status. AJR Am J Roentgenol. 2002;178(6):1531–1536. doi: 10.2214/ajr.178.6.1781531. [DOI] [PubMed] [Google Scholar]
- 60.Homer MV, Toloubeydokhti T, Lawson MA, Garzo G, Duleba AJ, Chang RJ. Individual 17-hydroxyprogesterone responses to hCG are not correlated with follicle size in polycystic ovary syndrome. J Endocr Soc. 2019;3(4):687–698. doi: 10.1210/js.2018-00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jarrett BY, Vanden Brink H, Oldfield AL, Lujan ME. Ultrasound characterization of disordered antral follicle development in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2020;105(11):e3847–e3861. doi: 10.1210/clinem/dgaa515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Järvelä IY, Mason HD, Sladkevicius P, Kelly S, Ojha K, Campbell S, Nargund G. Characterization of normal and polycystic ovaries using three-dimensional power Doppler ultrasonography. J Assist Reprod Genet. 2002;19(12):582–590. doi: 10.1023/a:1021267200316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Järvelä IY, Sladkevicius P, Kelly S, Ojha K, Campbell S, Nargund G. Effect of pituitary down-regulation on the ovary before in vitro fertilization as measured using three-dimensional power Doppler ultrasound. Fertil Steril. 2003;79(5):1129–1135. doi: 10.1016/S0015-0282(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 64.Järvelä IY, Niinimäki M, Martikainen H, Ruokonen A, Tapanainen JS. Ovarian response to the human chorionic gonadotrophin stimulation test in normal ovulatory women: the impact of regressing corpus luteum. Fertil Steril. 2007;87(5):1122–1130. doi: 10.1016/j.fertnstert.2006.08.114. [DOI] [PubMed] [Google Scholar]
- 65.Jokubkiene L, Sladkevicius P, Rovas L, Valentin L. Assessment of changes in volume and vascularity of the ovaries during the normal menstrual cycle using three-dimensional power Doppler ultrasound. Hum Reprod. 2006;21(10):2661–2668. doi: 10.1093/humrep/del211. [DOI] [PubMed] [Google Scholar]
- 66.Jokubkiene L, Sladkevicius P, Valentin L. Number of antral follicles, ovarian volume, and vascular indices in asymptomatic women 20 to 39 years old as assessed by 3-dimensional sonography: a prospective cross-sectional study. J Ultrasound Med. 2012;31(10):1635–1649. doi: 10.7863/jum.2012.31.10.1635. [DOI] [PubMed] [Google Scholar]
- 67.Jonard S, Robert Y, Dewailly D. Revisiting the ovarian volume as a diagnostic criterion for polycystic ovaries. Hum Reprod. 2005;20(10):2893–2898. doi: 10.1093/humrep/dei159. [DOI] [PubMed] [Google Scholar]
- 68.Kline J, Kinney A, Reuss ML, Kelly A, Levin B, Ferin M, Warburton D. Trisomic pregnancy and the oocyte pool. Hum Reprod. 2004;19(7):1633–1643. doi: 10.1093/humrep/deh310. [DOI] [PubMed] [Google Scholar]
- 69.Köşüş N, Köşüş A, Turhan NÖ, Kamalak Z. Do threshold values of ovarian volume and follicle number for diagnosing polycystic ovarian syndrome in Turkish women differ from western countries? Eur J Obstet Gynecol Reprod Biol. 2011;154(2):177–181. doi: 10.1016/j.ejogrb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 70.Lam PM, Johnson IR, Raine-Fenning NJ. Three-dimensional ultrasound features of the polycystic ovary and the effect of different phenotypic expressions on these parameters. Hum Reprod. 2007;22(12):3116–3123. doi: 10.1093/humrep/dem218. [DOI] [PubMed] [Google Scholar]
- 71.Łebkowska A, Adamska A, Karczewska-Kupczewska M, Nikołajuk A, Otziomek E, Milewski R, Górska M, Wołczyński S, Kowalska I. Serum anti-Müllerian hormone concentration in women with polycystic ovary syndrome and type 1 diabetes mellitus. Metabolism. 2016;65(5):804–811. doi: 10.1016/j.metabol.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Lie Fong S, Laven JSE, Duhamel A, Dewailly D. Polycystic ovarian morphology and the diagnosis of polycystic ovary syndrome: redefining threshold levels for follicle count and serum anti-Müllerian hormone using cluster analysis. Hum Reprod. 2017;32(8):1723–1731. doi: 10.1093/humrep/dex226. [DOI] [PubMed] [Google Scholar]
- 73.Merino PM, Lopez P, Salinas A, Pastene C, Muñoz A, Cassorla F, Codner E. Ovarian function in adolescents conceived using assisted reproductive technologies. J Pediatr Adolesc Gynecol. 2019;32(2):117–121. doi: 10.1016/j.jpag.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Murphy MK, Hall JE, Adams JM, Lee H, Welt CK. Polycystic ovarian morphology in normal women does not predict the development of polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(10):3878–3884. doi: 10.1210/jc.2006-1085. [DOI] [PubMed] [Google Scholar]
- 75.Orbak Z, Kantarci M, Yildirim ZK, Karaca L, Doneray H. Ovarian volume and uterine length in neonatal girls. J Pediatr Endocrinol Metab. 2007;20(3):397–403. doi: 10.1515/JPEM.2007.20.3.397. [DOI] [PubMed] [Google Scholar]
- 76.Özay AC, EmekçiÖzay Ö, Okyay RE, Gülekli B. The effect of myoinositol on ovarian blood flows in women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(3):237–241. doi: 10.1080/09513590.2018.1520827. [DOI] [PubMed] [Google Scholar]
- 77.Ozkan S, Vural B, Calişkan E, Bodur H, Türköz E, Vural F. Color Doppler sonographic analysis of uterine and ovarian artery blood flow in women with polycystic ovary syndrome. J Clin Ultrasound. 2007;35(6):305–313. doi: 10.1002/jcu.20358. [DOI] [PubMed] [Google Scholar]
- 78.Pan HA, Wu MH, Cheng YC, Li CH, Chang FM. Quantification of Doppler signal in polycystic ovary syndrome using three-dimensional power Doppler ultrasonography: a possible new marker for diagnosis. Hum Reprod. 2002;17(1):201–206. doi: 10.1093/humrep/17.1.201. [DOI] [PubMed] [Google Scholar]
- 79.Panidis D, Tziomalos K, Misichronis G, Papadakis E, Betsas G, Katsikis I, Macut D. Insulin resistance and endocrine characteristics of the different phenotypes of polycystic ovary syndrome: a prospective study. Hum Reprod. 2012;27(2):541–549. doi: 10.1093/humrep/der418. [DOI] [PubMed] [Google Scholar]
- 80.Pascual MA, Graupera B, Hereter L, Tresserra F, Rodriguez I, Alcázar JL. Assessment of ovarian vascularization in the polycystic ovary by three-dimensional power Doppler ultrasonography. Gynecol Endocrinol. 2008;24(11):631–636. doi: 10.1080/09513590802308099. [DOI] [PubMed] [Google Scholar]
- 81.Peigné M, Catteau-Jonard S, Robin G, Dumont A, Pigny P, Dewailly D. The numbers of 2–5 and 6–9 mm ovarian follicles are inversely correlated in both normal women and in polycystic ovary syndrome patients: what is the missing link? Hum Reprod. 2018;33(4):706–714. doi: 10.1093/humrep/dey017. [DOI] [PubMed] [Google Scholar]
- 82.Pellizzari P, Esposito C, Siliotti F, Marchiori S, Gangemi M. Colour Doppler analysis of ovarian and uterine arteries in women with hypoestrogenic amenorrhoea. Hum Reprod. 2002;17(12):3208–3212. doi: 10.1093/humrep/17.12.3208. [DOI] [PubMed] [Google Scholar]
- 83.Petri Nahás EA, Pontes A, Nahas-Neto J, Borges VT, Dias R, Traiman P. Effect of total abdominal hysterectomy on ovarian blood supply in women of reproductive age. J Ultrasound Med. 2005;24(2):169–174. doi: 10.7863/jum.2005.24.2.169. [DOI] [PubMed] [Google Scholar]
- 84.Phy J, Foong S, Session D, Thornhill A, Tummon I, Dumesic D. Transvaginal ultrasound detection of multifollicular ovaries in non-hirsute ovulatory women. Ultrasound Obstet Gynecol. 2004;23(2):183–187. doi: 10.1002/uog.954. [DOI] [PubMed] [Google Scholar]
- 85.Pirgon O, Sivrice C, Demirtas H, Dundar B. Assessment of ovarian reserve in euthyroid adolescents with Hashimoto thyroiditis. Gynecol Endocrinol. 2016;32(4):306–310. doi: 10.3109/09513590.2015.1116510. [DOI] [PubMed] [Google Scholar]
- 86.Pirwany IR, Fleming R, Greer IA, Packard CJ, Sattar N. Lipids and lipoprotein subfractions in women with PCOS: relationship to metabolic and endocrine parameters. Clin Endocrinol (Oxf) 2001;54(4):447–453. doi: 10.1046/j.1365-2265.2001.01228.x. [DOI] [PubMed] [Google Scholar]
- 87.Rosenfield RL, Wroblewski K, Padmanabhan V, Littlejohn E, Mortensen M, Ehrmann DA. Antimüllerian hormone levels are independently related to ovarian hyperandrogenism and polycystic ovaries. Fertil Steril. 2012;98(1):242–249. doi: 10.1016/j.fertnstert.2012.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Santoro N, Isaac B, Neal-Perry G, Adel T, Weingart L, Nussbaum A, Thakur S, Jinnai H, Khosla N, Barad D. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88(11):5502–5509. doi: 10.1210/jc.2002-021839. [DOI] [PubMed] [Google Scholar]
- 89.Sanverdi I, Ozkaya E, Kucur SK, Bilen D, Eken MK, Bilgic BE. Antral follicle diameter variance within each ovary may be a predictor for poor response in cases with normal ovarian reserve. Exp Clin Endocrinol Diabetes. 2018;126(8):521–527. doi: 10.1055/s-0043-125404. [DOI] [PubMed] [Google Scholar]
- 90.Sasaki RSA, Approbato MS, Maia MCS, Ferreira EABFE, Zanluchi N. Ovulatory status of overweight women without polycystic ovary syndrome. JBRA Assist Reprod. 2019;23(1):2–6. doi: 10.5935/1518-0557.20180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scheffer GJ, Broekmans FJ, Looman CW, Blankenstein M, Fauser BC, teJong FH, teVelde ER. The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum Reprod. 2003;18(4):700–706. doi: 10.1093/humrep/deg135. [DOI] [PubMed] [Google Scholar]
- 92.Shahrami SH, Abbasi Ranjbar Z, Milani F, Kezem-Nejad E, Hassanzadeh Rad A, DalilHeirat SF. The relation between diverse phenotypes of PCOS with clinical manifestations, anthropometric indices and metabolic characteristics. Acta Med Iran. 2016;54(2):134–139. [PubMed] [Google Scholar]
- 93.Shen O, Ben Chetrit A, Margalioth EJ, Lavie O, Rabinowitz RR. Effects of a copper-medicated intrauterine device on ovarian artery, uterine artery, and intrauterine blood flow. Gynecol Obstet Invest. 2008;66(4):253–256. doi: 10.1159/000148656. [DOI] [PubMed] [Google Scholar]
- 94.Singha A, Saha S, Bhattacharjee R, Mondal S, Choudhuri S, Biswas D, Das SK, Ghosh S, Mukhopadhyay S, Chowdhury S. Deterioraron of ovarian function after total abdominal hysterectomy with preservaron of ovaries. Endocr Pract. 2016;22(12):1387–1392. doi: 10.4158/EP161215.OR. [DOI] [PubMed] [Google Scholar]
- 95.Su HI, Sammel MD, Freeman EW, Lin H, DeBlasis T, Gracia CR. Body size affects measures of ovarian reserve in late reproductive age women. Menopause. 2008;15(5):857–861. doi: 10.1097/gme.0b013e318165981e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taponen S, Ahonkallio S, Martikainen H, Koivunen R, Ruokonen A, Sovio U, Hartikainen AL, Pouta A, Laitinen J, King V, Franks S, McCarthy MI, Järvelin MR. Prevalence of polycystic ovaries in women with self-reported symptoms of oligomenorrhoea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Hum Reprod. 2004;19(5):1083–1088. doi: 10.1093/humrep/deh214. [DOI] [PubMed] [Google Scholar]
- 97.Tomioka RB, Ferreira GRV, Aikawa NE, Maciel GAR, Serafini PC, Sallum AM, Campos LMA, Goldestein-Schainberg C, Bonfá E, Silva CA. Non-steroidal anti-inflammatory drug induces luteinized unruptured follicle syndrome in young female juvenile idiopathic arthritis patients. Clin Rheumatol. 2018;37(10):2869–2873. doi: 10.1007/s10067-018-4208-x. [DOI] [PubMed] [Google Scholar]
- 98.van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Polycystic ovaries in adolescents and the relationship with menstrual cycle patterns, luteinizing hormone, androgens, and insulin. Fertil Steril. 2000;74(1):49–58. doi: 10.1016/S0015-0282(00)00584-7. [DOI] [PubMed] [Google Scholar]
- 99.Weerakiet S, Lertvikool S, Tingthanatikul Y, Wansumrith S, Leelaphiwat S, Jultanmas R. Ovarian reserve in women with polycystic ovary syndrome who underwent laparoscopic ovarian drilling. Gynecol Endocrinol. 2007;23(8):455–460. doi: 10.1080/09513590701485212. [DOI] [PubMed] [Google Scholar]
- 100.Wongwananuruk T, Panichyawat N, Indhavivadhana S, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Pratumvinit B, Sa-Nga-Areekul N. Accuracy of anti-Müllerian hormone and total follicles count to diagnose polycystic ovary syndrome in reproductive women. Taiwan J Obstet Gynecol. 2018;57(4):499–506. doi: 10.1016/j.tjog.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Younis JS, Jadaon JE, Haddad S, Izhaki I, Ben-Ami M. Prospective evaluation of basal stromal Doppler studies in women with good ovarian reserve and infertility undergoing in vitro fertilization-embryo transfer treatment: patients with polycystic ovary syndrome versus ovulatory patients. Fertil Steril. 2011;95(5):1754–1758. doi: 10.1016/j.fertnstert.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 102.Zhang HY, Guo CX, Zhu FF, Qu PP, Lin WJ, Xiong J. Clinical characteristics, metabolic features, and phenotype of Chinese women with polycystic ovary syndrome: a large-scale case-control study. Arch Gynecol Obstet. 2013;287(3):525–531. doi: 10.1007/s00404-012-2568-z. [DOI] [PubMed] [Google Scholar]
- 103.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, ESHRE working group on Poor Ovarian Response Definition ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 105.Codner E, Villarroel C, Eyzaguirre FC, López P, Merino PM, Pérez-Bravo F, Iñiguez G, Cassorla F. Polycystic ovarian morphology in postmenarchal adolescents. Fertil Steril. 2011;95(2):702–706.e1–2. doi: 10.1016/j.fertnstert.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 106.Scavello I, Maseroli E, Cipriani S, Di Stasi V, Verde N, Menafra D, Scannerini S, Marchiani S, Rastrelli G, Ricca V, Sorbi F, Fambrini M, Petraglia F, Maggi M, Vignozzi L. Cardiometabolic risk is unraveled by color Doppler ultrasound of the clitoral and uterine arteries in women consulting for sexual symptoms. Sci Rep. 2021;11(1):18899. doi: 10.1038/s41598-021-98336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qureshi IA, Ullah H, Akram MH, Ashfaq S, Nayyar S. Transvaginal versus transabdominal sonography in the evaluation of pelvic pathology. J Coll Physicians Surg Pak. 2004;14(7):390–393. [PubMed] [Google Scholar]
- 108.Sun L, Fu Q. Three-dimensional transrectal ultrasonography in adolescent patients with polycystic ovarian syndrome. Int J Gynaecol Obstet. 2007;98(1):34–38. doi: 10.1016/j.ijgo.2007.02.024. [DOI] [PubMed] [Google Scholar]