Abstract
Purpose:
Older survivors of leukemia and lymphoma often experience long-term effects of chemotherapy. We described common concerns related to their cancer and treatment in older survivors of leukemia and non-Hogkin lymphoma (NHL) and assessed correlates of these concerns.
Methods:
We utilized data from the Women’s Health Initiative (WHI) Life and Longevity After Cancer (LILAC) study that recruited post-menopausal women aged 50–79. Participants diagnosed with leukemia and NHL were included (n=420). They were asked about 14 areas of current concerns related to their cancer and treatment and to rate each from 0 (no concern) to 2 (major concern), with total scores ranging from 0–28. Linear regression was used to assess factors correlated with the concern score, and logistic regression for factors correlated with the three most common concerns.
Results:
Mean age at assessment was 81 years (range 69–99); 72% reported at least one concern, and median concern score among these survivors was 3.5 (Q1-Q3 2–5). Factors significantly correlated with concern scores were sadness, pain, distress, higher prior symptom count, and loneliness (all p<0.05). Significant factors correlated with common concerns were 1) Fatigue/sleep: sadness/depression, distress, higher prior symptom count, greater loneliness, and worse physical functioning; 2) Physical functioning/activity: older age, public insurance, higher body mass index, pain, worse QoL, and higher treatment-related comorbidities; 3) Memory/concentration: prior chemotherapy or radiation, worse QoL, higher prior symptom count, and greater loneliness (all p<0.05).
Conclusions and Implications for Cancer Surviors:
Almost three-quarters of older survivors of leukemia and lymphoma reported at least one concern; a multifaceted intervention may be needed to address these concerns.
Keywords: Older cancer survivors, leukemia and lymphoma, women, common concerns
Introduction
Leukemia and non-Hodgkin lymphoma (NHL) are two of the most common hematologic malignancies [1]. With increasingly effective therapeutic options, more adults with leukemia and NHL are living longer. Five-year survival rates for all types of leukemia and lymphoma are 64% and 73%, respectively [2]. The diagnosis of acute leukemia is often sudden, and leukemia-directed treatments can be intense (e.g., inpatient chemotherapy with or without stem cell transplantation), while chronic leukemias often require prolonged therapy [3, 4]. Prolonged and recurrent hospitalizations are common among patients with leukemia due to treatments and their associated complications (e.g., infections, bleeding) [5]. Similarly, treatments of NHL often result in hospitalizations [6], and survivors of NHL frequently experience long term and late effects of chemotherapy (e.g., secondary cancers, cardiac problems) [7, 8]. Among older cancer survivors (age ≥65), these long-term health consequences may be worsened by aging-related conditions such as functional and cognitive impairments that are more common in cancer survivors than in individuals without a history of cancer [9].
The increase in number of older cancer survivors underscores the importance of understanding their physical and psychosocial concerns (e.g., functional decline, cognitive decline) [10]. Cancer survivors with concerns that are not adequately addressed are more likely to report greater levels of psychological distress than those whose concerns are addressed [11]. Prior studies often included survivors with solid tumors or a mixed cancer population that included hematologic malignancies but did not focus on the specific concerns of survivors with leukemia and NHL [12–17]. Of studies that focused on survivors of hematologic malignancies, most had small to moderate sample sizes (N=20–250) and focused on concerns within five years of treatment, or they did not focus on older adults [18, 19]. A better understanding of the physical and psychosocial concerns of older cancer survivors and identification of those who are more likely to have concerns may inform support systems and guide interventions to address them, thereby improving outcomes in older survivors of leukemia and NHL.
In this study, we aimed to describe common concerns in older survivors of leukemia and NHL and to assess demographic and clinical factors associated with these concerns.
Methods
Study Design, Setting, and Sample
This analysis utilized data from the Women’s Health Initiative (WHI) and Life and Longevity After Cancer (LILAC) studies. Full details of WHI and LILAC have previously been described [20–22]. Briefly, the WHI recruited post-menopausal women aged between 50 and 79 years from 40 clinical sites (24 states and the District of Columbia) from 1993 to 1998 into one or more randomized trials (WHI-Clinical Trials, n=68,132) or into an observational study (WHI-Observational Study, n=93,676). The studies were closed in 2004–2005. Participants were subsequently invited to continue in WHI extension studies beyond 2005.
Starting in 2013, the LILAC study enrolled participants diagnosed with certain types of cancer (breast, endometrial, ovarian/fallopian tube/primary peritoneal, lung, colorectal, melanoma, NHL, and leukemia) during the WHI [23]. The LILAC survivorship cohort was developed to study cancer survivorship and issues pertinent to cancer survivors. Participants completed the baseline LILAC questionnaire and additional follow-up questionnaires at one (Form 370) and two years (Form 371) after LILAC enrollment. For the purpose of this analysis, we included participants who were 1) enrolled in the LILAC study and completed the baseline LILAC questionnaire (Form 340, 2013–2016); 2) had a diagnosis of leukemia or NHL after enrollment in WHI; 3) completed the Year 2 follow-up survey (Form 371); and 4) completed at least half of the questions on current concerns (Form 371). All participants provided informed consent, and WHI was approved by the institutional review board at each participating institution.
Dependent Variable
On the Year 2 follow-up survey, participants were asked about 14 areas of current concern related to their cancer and treatment and to rate each need from 0–2 [no concern or “I’m fine” (0), moderate concern or “I could use some help” (1), and major concern or “It’s a big problem for me” (2)]. These areas included bone health or falls, fatigue or sleep, emotional health, physical functioning or activity, memory or concentration, weight change or gain or loss, personal care (dressing, bathing, etc.), sexual functioning, shortness of breath or heart problems, coordinating care among healthcare providers, genetic counseling or testing, financial advice or assistance, numbers of tests to monitor your health, and end of life planning. These areas of concerns were selected because they are known to be prevalent in cancer survivors and are highlighted in the National Comprehensive Cancer Network guidelines [24, 25].
Measures
Demographic factors included age at the Year 2 follow-up survey (Form 371, i.e., age when they complete the questionnaire on current concern), race, marital status, education, and insurance status. Clinical factors included time since diagnosis and treatment received (chemotherapy, radiation, stem cell transplantation). We also included body mass index (BMI), number of comorbidities, sadness/depression, pain, anxiety, fatigue, depression, number of symptoms, activities of daily living, quality of life (QoL), social support, loneliness, social network size, and physical activity level, as outlined below:
Body mass index:
This was calculated based on self-reported weight on the LILAC baseline questionnaire (Form 340) and most recent height (WHI Form 80).
Comorbidities:
Participants self-reported 18 comorbidities (e.g., low blood counts, high blood pressure, kidney problems) on the LILAC baseline questionnaire (Form 340).
Sadness/depression:
Participants were asked, “Do you feel sad or depressed?” on the LILAC Year 2 follow-up survey (Form 371).
Pain:
Participants were asked to rate their pain at its worst in the last 24 hours from 0 (no pain) to 10 (pain as bad you can imagine) on the LILAC Year 2 follow-up survey (Form 371). Presence of pain was categorized into yes (1–10) vs. no (0).
Anxiety, fatigue, and distress:
Participants were asked to rate their overall level of anxiety, fatigue, and distress during the past week from 0 (none) to 10 (worst) on the LILAC Year 2 follow-up survey (Form 371). Anxiety, fatigue, and distress were individually categorized as yes (1–10) or no (0).
Symptoms:
Participants were asked about the presence of 24 physical and psychological symptoms (e.g., abdominal/pelvic pain, constipation, shortness of breath, feeling anxious, trouble concentrating) on the LILAC Year 1 follow-up survey (Form 370). The number of symptoms was summed [26].
Physical functioning:
This was assessed using the 10-item RAND-36 physical functioning subscale (Form 151/155) at the closest time prior to completion of the Year 2 follow-up survey [27]. Participants were asked if their health limited them in performing several activities (e.g., moderate activities, lifting or carrying groceries, walking certain distances). Response options for each activity were “no, not limited at all” (3 points), “yes, limited a little” (2 pounts), or “yes, limited a lot” (1 points). The scores were summed across all items and transformed to a 0–100 scale, with higher scores indicating greater physical functioning.
Global quality of life:
Participants were asked “Overall, how would you rate your quality of life?” (Form 151/155). Response options ranged from 0 to 10, with higher scores indicating better QoL [28].
Social support:
Participants were asked how often someone was available to take them to the doctor, have a good time with, hug them, prepare their meals, and understand their problems on the LILAC baseline questionnaire (Form 340) [29]. Response options included none of the time, a little of the time, some of the time, most of the time, and all of the time. Scores were transformed to 0–100, with higher scores indicating greater support.
Loneliness:
Participants were asked on the LILAC baseline questionnaire (Form 340) how often they felt they lack of companionship, how often they felt left out, and how often they felt isolated from others (3-item Loneliness Scale from the University of California, Los Angeles) [30]. Response options were “often”, “some of the time”, “hardly ever (or never)”, or no answer. Responses of “often” received 1 point, “some of the time” 2 points, and “hardly ever (or never)” 3 points, with the responses across the three questions being summed to a scale of 3–9, with lower scores indicating more feelings of loneliness.
Social network size:
Participants reported their number of children and first-degree relatives on enrollment to WHI (Form 32).
Physical activity level:
Participants reported the frequency (days per week) and duration (minutes) of mild, moderate, and strenuous recreational physical activity (Form 340). Physical activity summarized as energy expenditure was calculated as the product of metabolic equivalents (METs) of task intensity values for each physical activity [31] multiplied by the hours per week of reported participation (MET-h/week) [32].
Statistical Analyses
We used descriptive statistics to characterize the distribution of the demographic and clinical characteristics of the study sample. For the dependent variable, we described percentages of participants reporting no (0), moderate (1), and major concerns (2) for each of the 14 individual concerns. We compared differences in the demographic and clinical characteristics between those with any concerns in the 14 areas vs. no concerns. Categorical variables were tested using Fisher’s exact test, and continuous variables were tested using two-sample t-tests or Wilcoxon rank-sum tests depending on whether the distributions were normal or skewed. Normality of continuous variables was tested using the Kolmogorov-Smirnov test.
For multiple linear regression, we evaluated factors associated with the concern score. Summary concern scores were generated that ranged from 0–28, with higher scores indicating greater concerns. For participants with missing responses to concern questions, scores were pro-rated and scaled such that all participants were on the 0–28 scoring range. Participants with missing responses to more than half of the concern questions were excluded from the study. We used a backwards selection model and included potential variables that were significant at p<0.20 on bivariate analyses. Variables were removed during selection until all variables were significant at p<0.10. We included time from diagnosis in all models regardless of significance.
Because factors associated with overall and individual concerns may be different, we generated a separate multvariable logistic regression for the three most common concerns. Steps in generating the final models were similar to the above. We removed variables that were similar to the dependent variables (e.g., physical functioning was not included in the model for concern in physical functioning or activity). All p-values were from two-sided tests, and the results were considered statistically significant if p<0.05. As this was an exploratory analysis, correction for multiple testing was not performed. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Demographics
As reported previously, there were 161,808 participants in the WHI, and 30,306 had incident cancers.[23] Of these, 20,784 had one of the eight designated cancers in LILAC and 9,934 were eligible to participate in LILAC. A total of 7,760 participants consented to the LILAC study, and 7,650 completed the baseline LILAC questionnaire. After excluding those without leukemia or NHL (N=5,203) and those who did not respond to at least half of the current concern questions (N=13), our sample included a total of 420 participants.
Mean age at completion of the questionnaire on current concern was 80.2 (SD 5.5, range 69–99); 49% (204/420) and 14% (57/420) were aged 70–79 and ≥80 years, respectively (Table 1). The majority were White (397/420; 95%). Over half were married (206/420; 52%), and 40% (157/420) had attended post-graduate or professional school. The two most common types of lymphoma were chronic lymphocytic leukemia/small lymphocytic lymphoma (116/420; 28%) and diffuse large B-cell lymphoma (89/420; 22%). Median time since diagnosis was 8.8 years (range 2.5–20.8); 29%, 36%, and 36% were 2–5 years, 6–10 years, and ≥11 years, respectively. Figure 1 shows the distribution of time since diagnosis. In terms of treatment, 62% had received chemotherapy, 21% had received radiation, and 1% had received a stem cell transplantation.
Table 1:
Demographic and clinical characteristics of the study sample
| Variable | No concern (N=117) | Any concern (N=303) | Total (N=420) | P-value |
|---|---|---|---|---|
| Age at completion of form 371, mean (SD) | 80.2 (5.5) | 81.4 (5.8) | 81.8 (5.7) | 0.06 |
| Age group in years, n (%) | 0.68 | |||
| <75 | 17 (14.5) | 39 (12.9) | 56 (13.3) | |
| 75–79 | 36 (30.8) | 81 (26.7) | 117 (27.9) | |
| 80–84 | 36 (30.8) | 92 (30.4) | 128 (30.5) | |
| 85–89 | 19 (16.2) | 68 (22.4) | 87 (20.7) | |
| ≥90 | 9 (7.7) | 23 (7.6) | 32 (7.6) | |
| Race and ethnicity, n (%) | 0.52 | |||
| White | 108 (92.3) | 289 (95.4) | 397 (94.5) | |
| Black | 5 (4.3) | 8 (2.6) | 13 (3.1) | |
| Asian/Pacific Islander | 3 (2.6) | 3 (1.0) | 6 (1.4) | |
| Hispanic/Latino | 1 (0.9) | 2 (0.7) | 3 (0.7) | |
| Other | 0 (0) | 1 (0.3) | 1 (0.2) | |
| Marital status, n (%) | 0.31 | |||
| Married/Living as married | 64 (59.3) | 142 (49.7) | 206 (52.3) | |
| Widowed | 28 (25.9) | 99 (34.6) | 127 (32.2) | |
| Divorced/Separated | 12 (11.1) | 36 (12.6) | 48 (12.2) | |
| Single/Never married | 4 (3.7) | 9 (3.2) | 13 (3.3) | |
| Education, n (%) | 0.09 | |||
| ≤High school graduate | 11 (9.5) | 54 (17.9) | 65 (15.6) | |
| Some college/college graduate | 60 (51.7) | 136 (45.0) | 196 (46.9) | |
| Post-graduate/Professional school | 45 (38.8) | 112 (37.1) | 157 (37.6) | |
| Insurance, n (%) | 0.24 | |||
| Both public and private | 46 (50.6) | 109 (43.4) | 155 (45.3) | |
| Private | 9 (9.9) | 18 (7.2) | 27 (7.9) | |
| Public | 36 (39.6) | 124 (49.4) | 160 (46.8) | |
| Years since diagnosis, median [Q1-Q3] | 8.7 [5.5–13.4] | 8.8 [5.7–12.9] | 8.8 [5.6–13] | 0.92 |
| Years since diagnosis, n (%) | 0.81 | |||
| 2 to 5 years | 35 (29.9) | 85 (28.1) | 120 (28.6) | |
| 6 to 10 years | 39 (33.3) | 111 (36.6) | 150 (35.7) | |
| ≥11 years | 43 (36.8) | 107 (35.3) | 150 (35.7) | |
| Type of treatment received, n (%) | ||||
| Chemotherapy | 60 (51.3) | 199 (65.7) | 259 (61.7) | 0.007 |
| Radiation | 19 (16.2) | 67 (22.1) | 86 (20.5) | 0.22 |
| Stem cell transplantation | 0 (0) | 3 (1.0) | 3 (0.7) | 0.56 |
| Number of treatment-related comorbidities, median [Q1-Q3] | 1 [0–2] | 2 [0–4] | 1 [0–3] | <0.001 |
| BMI, mean (SD) | 25.0 (4.4) | 25.7 (4.7) | 25.5 (4.6) | 0.20 |
| Sadness or depression, n (%) | 4 (3.5) | 70 (23.3) | 74 (17.8) | <0.001 |
| Pain, n (%) | 38 (34.6) | 180 (61.0) | 218 (53.8) | <0.001 |
| Anxiety, n (%) | 71 (67.1) | 239 (80.5) | 310 (75.2) | <0.001 |
| Fatigue, n (%) | 91 (79.1) | 279 (93.9) | 370 (89.8) | <0.001 |
| Distress, n (%) | 55 (47.4) | 214 (72.8) | 269 (65.6) | <0.001 |
| Total number of symptoms, median [Q1-Q3] | 5 [2.5–8] | 9 [7–12] | 8 [5–11] | <0.001 |
| Physical Functioning, mean (SD) | 90 [75–95] | 60 [35–80] | 75 [45–90] | <0.001 |
| Quality of life, mean (SD) | 9 [8–10] | 8 [6–8] | 8 [7–9] | <0.001 |
| Social support score, median [Q1-Q3] | 95 [75–100] | 80 [62.5–95] | 82.5 [65–100] | <0.001 |
| Loneliness score, median [Q1-Q3] | 9 [9–9] | 9 [7.5–9] | 9 [8–9] | <0.001 |
| Social network size at WHI enrollment, median [Q1-Q3] | 5 [4–7] | 5 [4–7] | 5 [4–7] | 0.80 |
| Physical activity level, MET-h/week, median [Q1-Q3] | 13.6 [4.3–28.6] | 6.7 [1.0–16.5] | 8.6 [1.6–19.3] | <0.001 |
Ranged from 0–28; the scores were pro-rated among those who had missing responses
Figure 1:
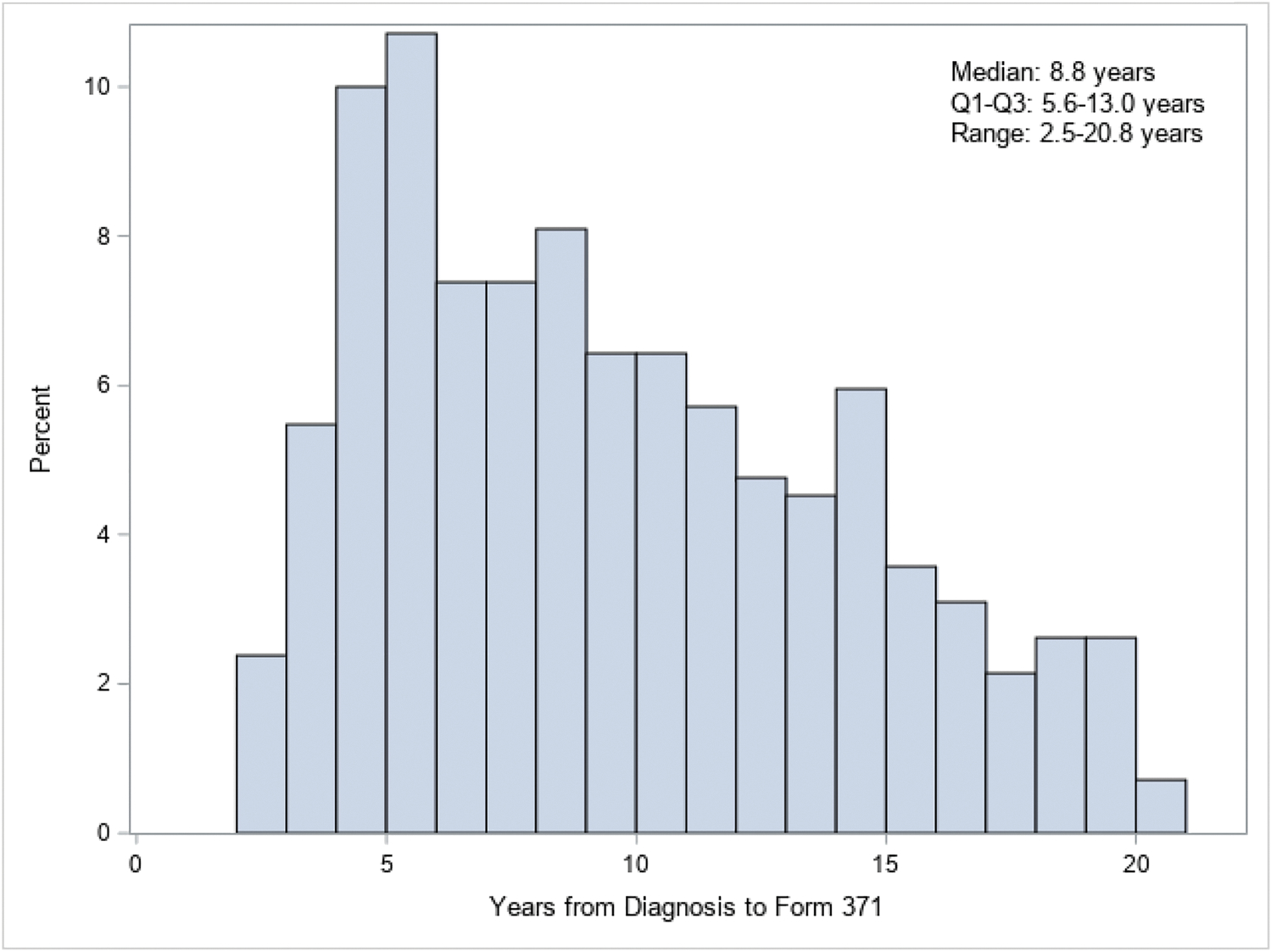
Distribution of time since leukemia or lymphoma diagnosis
Current concerns
Approximately 72% (303/420) reported at least one concern, with 13%, 14%, and 45% reporting 1, 2, and ≥3 concerns respectively. Table 2 shows the percentages of participants with no, moderate, and major concerns in the various areas. The most common concerns were fatigue or sleep (moderate or major concern: 41%; 170/415), physical functioning or activity (41%; 169/416), memory or concentration (34%; 129/413), bone health or falls (30%; 122/414), and shortness of breath or heart problems (26%; 106/413) (Figure 2).
Table 2:
Individual concerns
| Types of concern (N=420)* | No concern, n (%) | Moderate concern, n (%) | Major concern, n (%) |
|---|---|---|---|
| Bone health or falls | 292 (70.5) | 104 (25.1) | 18 (4.4) |
| Fatigue or sleep | 245 (59.0) | 132 (31.8) | 38 (9.2) |
| Emotional health | 330 (79.7) | 80 (19.3) | 4 (1.0) |
| Physical functioning or activity | 247 (59.4) | 137 (32.9) | 32 (7.7) |
| Memory or concentration | 274 (66.3) | 121 (29.3) | 18 (4.4) |
| Weight changes | 328 (78.9) | 80 (19.2) | 8 (1.9) |
| Personal care | 385 (93.0) | 23 (5.6) | 6 (1.5) |
| Sexual functioning | 334 (92.8) | 18 (5.0) | 8 (2.2) |
| Shortness of breath/heart problems | 303 (74.1) | 89 (21.8) | 17 (4.2) |
| Coordinating care between health care providers | 360 (87.8) | 41 (10.0) | 9 (2.2) |
| Genetic counseling or testing | 373 (95.6) | 15 (3.9) | 2 (0.5) |
| Financial advice or assistance | 368 (90.6) | 32 (7.9) | 6 (1.5) |
| Number of tests to monitor their health | 368 (90.9) | 35 (8.6) | 2 (0.5) |
| End of life planning | 365 (89.0) | 41 (10.0) | 4 (1.0) |
Totals for each concern may not sum to n=420 due to non-response
Figure 2:
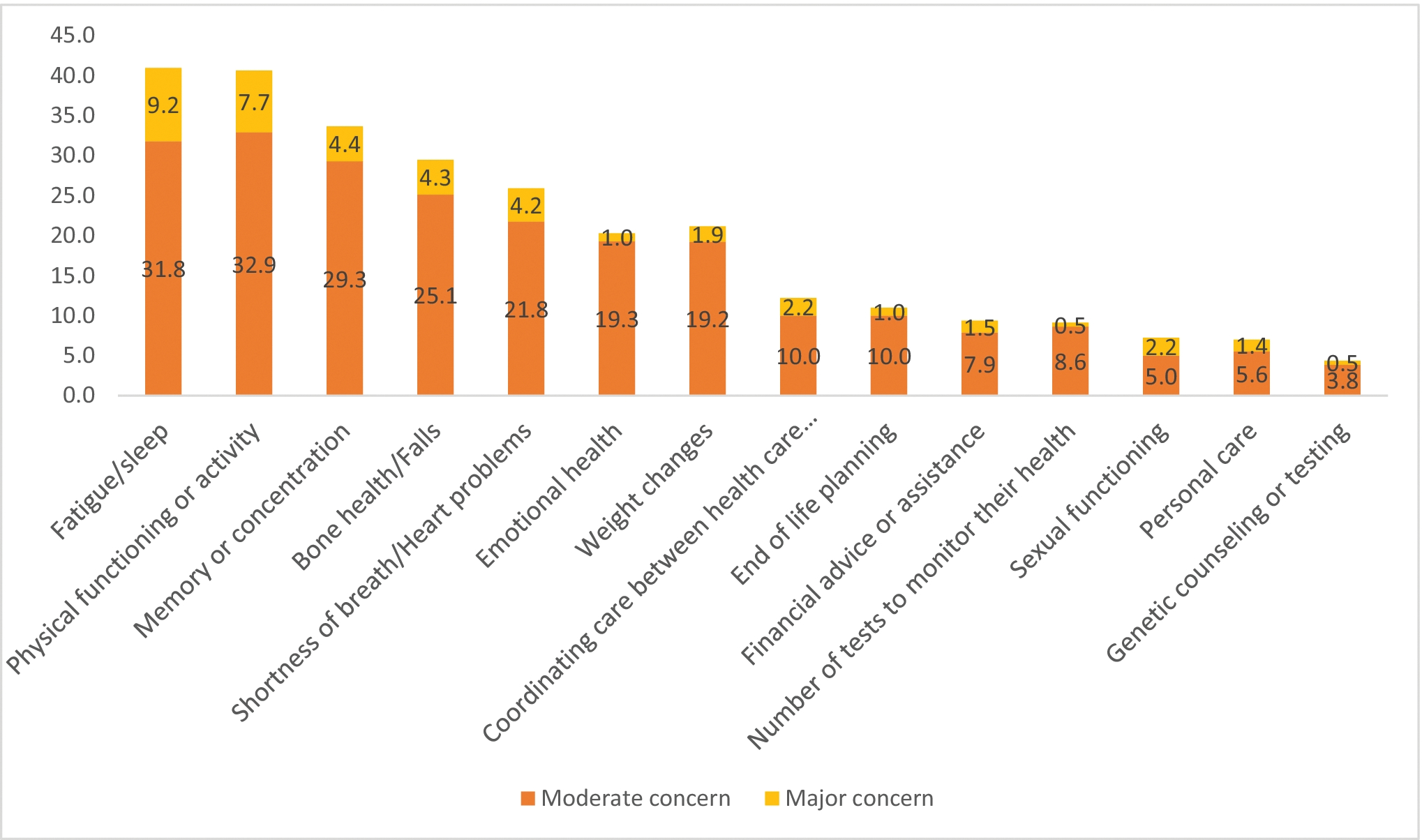
Percentages of leukemia and lymphoma survivors with unmet needs in the individual areas
The median concern score in the whole sample was 2.1 (Q1-Q3, 0–5; N=420), and 3.5 (Q1-Q3, 2–6; N=303) among those who reported any concern.
Correlates of common concerns
Factors significantly correlated with higher concern scores were greater sadness/depression [β=0.68; 95% Confidence Interval (CI) 1.00, 2.35], greater pain (β=0.60; 95% CI 0.06, 1.14), greater distress (β=0.64; 95% CI 0.06, 1.22), and higher prior symptom count (β=0.07; 95% CI 0.01, 0.04), as well as greater loneliness (β=−0.46; 95% CI −0.65, −0.27), worse QoL (β=−0.34; 95% CI −0.52, −0.16), and lower physical functioning (β=−0.37; 95% CI −0.48, −0.27) (Table 3).
Table 3:
Multivariable linear regression evaluating factors associated with concern score (0–28 scale)
| Variable | Multivariable analyses β (95% CI) | P-value |
|---|---|---|
| Time from diagnosis to form 371 (1-year increase) | −0.03 (−0.09 to 0.02) | 0.20 |
| Chemotherapy treatment | 0.06 | |
| No chemotherapy | Reference | |
| Chemotherapy | 0.68 (0.19 to 1.18) | |
| Sadness or depression (0–10 scale) | <0.001 | |
| 0 | Reference | |
| >0 | 1.67 (1.00 to 2.35) | |
| Pain (0–10 scale) | 0.03 | |
| 0 | Reference | |
| >0 | 0.60 (0.06 to 1.14) | |
| Distress (0–10 scale) | 0.03 | |
| 0 | Reference | |
| >0 | 0.64 (0.06 to 1.22) | |
| Quality of life (1 point increase) | −0.34 (−0.52 to −0.16) | <0.001 |
| Total number of symptoms (1-unit increase) | 0.07 (0.01 to 0.14) | 0.03 |
| Loneliness score (1-point increase) | −0.46 (−0.65 to −0.27) | <0.001 |
| Physical Functioning (10-point increase) | −0.37 (−0.48 to −0.27) | <0.001 |
On multivariable logistic regression, factors correlated with moderate or major concerns relating to fatigue or sleep were the following: Any sadness/depression [Odds ratio (OR)=2.57; 95% CI 1.40–4.72), any distress (OR=1.84; 95% CI 1.07–3.17), higher prior symptom count (1 unit increase; OR=1.11; 95% CI 1.05–1.18), greater loneliness (1-point improvement in loneliness; OR=0.83, 95% CI 0.70–0.99), and worse physical functioning (10-point improvement in functioning; OR=0.87; 95% CI 0.80–0.95) (Table 4).
Table 4:
Multivariable logistic regression evaluating factors correlated with moderate/major concerns relating to fatigue.
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Time from diagnosis to form 371 (1-year increase) | 0.97 (0.92–1.02) | 0.18 |
| Sadness or depression (0–10 scale) | 0.002 | |
| 0 | Reference | |
| >0 | 2.57 (1.40–4.72) | |
| Pain (0–10 scale) | 0.06 | |
| 0 | Reference | |
| >0 | 1.50 (0.97–2.59) | |
| Distress (0–10 scale) | 0.03 | |
| 0 | Reference | |
| >0 | 1.84 (1.07–3.17) | |
| Total number of symptoms (1-unit increase) | 1.11 (1.05–1.18) | <0.001 |
| Loneliness score (1-point increase) | 0.83 (0.70–0.99) | 0.04 |
| Physical Functioning (10-point increase) | 0.87 (0.80–0.95) | 0.002 |
Factors correlated with moderate or major concerns relating to physical functioning and memory or concentration are shown in Tables 5 and 6, respectively. For physical functioning, these factors included older age, public insurance type (vs. private or combination insurance), higher BMI, presence of pain, worse QoL, and higher number of treatment-related comorbidities (Table 5). For memory or concentration, these factors included prior receipt of chemotherapy or radiation, worse QoL, higher prior symptom count, and greater loneliness (Table 6).
Table 5:
Multivariable logistic regression evaluating factors correlated with moderate/major concerns relating to physical function.
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Time from diagnosis to form 371 (1-year increase) | 0.95 (0.90–1.01) | 0.08 |
| Age at form 371 (1-year increase) | 1.08 (1.03–1.13) | 0.001 |
| Insurance type | 0.007 | |
| Public (Medicare/Medicaid) | 1.95 (1.20–3.18) | |
| Private or combination public/private | Reference | |
| BMI (1 kg/m2 increase) | 1.06 (1.01–1.12) | 0.03 |
| Pain (0–10 scale) | <0.001 | |
| 0 | Reference | |
| >0 | 2.94 (1.79–4.82) | |
| Fatigue (0–10 scale) | 0.06 | |
| 0 | Reference | |
| >0 | 2.93 (0.95–9.04) | |
| Quality of life (1-point increase) | 0.59 (0.50–0.70) | <0.001 |
| Number of treatment-related comorbidities (1-unit increase) | 1.17 (1.07–1.29) | 0.001 |
Table 6:
Multivariable logistic regression evaluating factors correlated with moderate/major concerns relating to memory or concentration.
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Time from diagnosis to form 371 (1-year increase) | 1.01 (0.96–1.06) | 0.70 |
| Chemotherapy ever | <0.001 | |
| Yes | 2.45 (1.48–4.06) | |
| No | Reference | |
| Radiation ever | 0.003 | |
| Yes | 2.31 (1.32–4.04) | |
| No | Reference | |
| Quality of life (1-point increase) | 0.74 (0.64–0.86) | <0.001 |
| Total number of symptoms (1-unit increase) | 1.10 (1.04–1.17) | 0.001 |
| Loneliness score (1-point increase) | 0.70 (0.58–0.83) | <0.001 |
Discussion
In a cohort of older survivors of leukemia and NHL, almost three-quarters reported some concerns, with the most prevalent being fatigue or sleep, physical functioning or activity, and memory or concentration (34–41%). Common correlates of overall and prevalent concerns included worse psychological health (e.g., sadness, distress), physical symptoms (e.g., pain, greater symptom count, worse physical functioning), worse QoL, and greater loneliness. Older age, public insurance type, higher BMI, and higher number of comorbidities were correlated with concern about physical functioning. Prior receipt of chemotherapy or radiation was correlated with concern about memory or concentration. Time since diagnosis was not a correlate of concerns.
Our finding that older survivors of leukemia and NHL had a substantial prevalence of concerns, with over 40% reporting concerns in at least three areas, is consistent with prior studies of survivors of hematologic malignancies, demonstrating multiple unmet needs up to almost six years from completion of treatment [33–35]. For example, in a cross-sectional survey of 477 leukemia and lymphoma survivors who were up to 48 months from completion of treatment, 41% reported at least one unmet need and at least one in four survivors had an unmet need in eight areas [34]. However, a key distinction of our study from prior work was its focus on an older population. The mean age of leukemia and lymphoma survivors in existing studies assessing unmet needs has ranged from 50 to 58 years [33–35], while the mean age of survivors in this analysis was 81 years. This substantial difference in age may account for the differences we observed in unmet needs compared to existing studies of younger cancer survivors. While concerns related to fatigue, physical functioning, and memory issues were most prevalent in our study, emotional concerns, care coordination, sexual issues, employment, and child care needs were more frequently reported in younger survivors (note that child care and employment were not included in our concern questionnaire given its focus on an older population) [33–35]. Our findings illustrate that the concerns and unmet needs of older survivors are distinct from those of younger populations. Understanding the concerns of older leukemia and lymphoma survivors as elucidated by our study is a critical step to ensure that survivorship interventions are appropriately targeted for this population.
We identified several correlates of concerns in this cohort of older survivors with leukemia and NHL, including worse psychological health (e.g., sadness, distress), physical symptoms (e.g., pain, physical functioning, symptom count), and worse QoL. These multilevel associations have previously been reported [36–38]. For example, a systematic review of 26 studies found that worse psychological health, symptoms, and QoL, among other demographic and clinical factors, were strongly associated with greater unmet needs among post-treatment cancer survivors [38]. These multilevel correlates demonstrate the need for a comprehensive, multidisplinary approach to the lingering concerns of older cancer survivors. Older age, public insurance type, higher BMI, and higher number of comorbidities were correlated with concerns in physical functioning, which has been observed previously as well. [39]. These findings may be explained by age-related physical changes including increased risk of comorbidities (not controlled in our study) and physical impairment from cancer treatments, all of which are associated with reduced physical functioning [40, 41]. Insurance type may also be a proxy for older age, as the public insurance was Medicare among the majority of our sample. It is important to understand the extent to which cancer exacerbates existing health conditions and/or creates new health conditions in older adults. Future prospective studies that examine the trajectory of decline in physical functioning in younger and older survivors over time are needed to disentangle the effects of cancer and aging [42].
Prior receipt of chemotherapy or radiation was correlated with concern about memory or concentration [43, 44]. Chemotherapy-related cognitive impairment (CRCI) consists of problems in memory, attention, concentration, and executive functioning associated with chemotherapy treatment in individuals with cancer [45]. Although research in this area is still emerging, it has been shown that a subset of individuals with hematologic malignancy experience CRCI [46, 47]. In addition, combined chemotherapy and central nervous system-directed radiation treatments have been shown to cause frequent and serious neurological damage. Cognitive assessments should be considered throughout the cancer treatment process, particularly during therapeutic protocols using radiotherapy and chemotherapy as well as in post-treatment survivorship visits [48]. In addition, the inclusion of cognitive screenings and endpoints in clinical trials is needed to expand our understanding and approach to cognitive dysfunction related to age and/or cancer therapies in older survivors with leukemia and NHL.
We identified a novel correlate of concern: greater loneliness. In addition to physical and psychological health, the social environment plays an important role in aging [49]. Loneliness refers to a subjective assessment that social relationships are lacking [50]. Loneliness is known to be associated with several negative outcomes such as worse psychological health, low self-ratings of health, and reduced survival in older adults [51–53]. Older adults are especially vulnerable to social loneliness due to various reasons such as loss of family members and friends, limited mobility due to development of comorbidities, and hearing loss. Clearly, better studies of loneliness in older survivors of leukemia and NHL are needed, examining the effects of loneliness on outcomes as well as uptake and adherence to interventions to address loneliness and other concerns.
Our study demonstrated that demographic, psychological, physical, and clinical factors that may be associated with an older cancer survivor’s lingering concerns should be assessed by healthcare providers. One strategy to measure and address these multifaceted correlates is through the use of a comprehensive geriatric assessment (CGA), which is an in-depth, multidimensional evaluation of an individual’s health used to identify issues that are potentially treatable to improve outcomes [54, 55]. CGA results can help elicit a multidisciplinary management approach to address these persistent concerns among older survivors of leukemia and NHL. CGA addresses social support and can guide strategies and interventions (e.g., peer support, support group, increased family involvement) to reduce social loneliness [56, 57]. Using CGA to guide survivorship care is currently being tested among older survivors of solid tumors (ClinicalTrials.gov Identifier: NCT05006482).
Our study has several limitations. First, it is important to acknowledge survivorship bias in the sample, and as such our findings may be most relevant to longer term older survivors of hematologic malignancies. Second, our sample consists of women only, and most of them were White and had at least some college education, thereby limiting the generalizability of our findings. Third, while we included leukemia and NHL survivors, <5% had acute leukemia. This subgroup is associated with short term suvival and likely has more concerns related to uncertainty and end-of life, although a prior study identified psychological health and physical symptoms as two prevalent concerns [35, 58–60]. Finally, correlates of concerns were either collected at the same time or prior to the questionnaires on concerns being complete, therefore causation cannot be established.
In conclusion, we found that almost three-quarters of older survivors of leukemia and lymphoma reported at least one concern. Prevalent concerns included fatigue or sleep, physical functioning or activity, and memory or concentration. Common correlates of overall and prevalent concerns included worse psychological health, physical symtoms, quality of life, and loneliness. A multifaceted and multicomponent intervention may be important in addressing common concerns in older cancer survivors in order to improve their QoL and survivorship experience.
Supplementary Material
Acknowledgement:
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. The WHI Life and Longevity after Cancer (LILAC) study is funded by UM1 CA173642 and a grant to Dr. Paskett from the Breast Cancer Research Foundation. Dr. Loh is supported by the National Cancer Institute (R00CA237744), Wilmot Research Fellowship Award, and Conquer Cancer American Society of Clinical Oncology Career Development Award.
Footnotes
Conflict of Interest: Dr. Loh has served as a consultant to Pfizer and Seattle Genetics and has received honoraria from Pfizer. Dr. Paskett has received grant funding from the Merck Foundation and Pfizer. All other authors report no conflicts of interest.
Data sharing: Access to raw data will require approval from the WHI Publication and Presentations Committee.
Data availability:
The datasets generated and/or analyzed during the current study are available through request from the WHI.
References
- 1.Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer 2011; 105: 1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odejide OO, Uno H, Murillo A, Tulsky JA, Abel GA. Goals of care discussions for patients with blood cancers: Association of person, place, and time with end-of-life care utilization. Cancer 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quintás-Cardama A, Cortes JE. Chronic myeloid leukemia: diagnosis and treatment. Mayo Clin Proc 2006; 81: 973–988. [DOI] [PubMed] [Google Scholar]
- 4.Wall S, Woyach JA. Chronic Lymphocytic Leukemia and Other Lymphoproliferative Disorders. Clin Geriatr Med 2016; 32: 175–189. [DOI] [PubMed] [Google Scholar]
- 5.Kumar AJ, Henzer T, Rodday AM, Parsons SK. Risk factors for length of stay and charge per day differ between older and younger hospitalized patients with AML. Cancer Med 2018; 7: 2744–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyman GH, Delgado DJ. Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer 2003; 98: 2402–2409. [DOI] [PubMed] [Google Scholar]
- 7.Haddy TB, Adde MA, McCalla J, Domanski MJ, Datiles M 3rd, Meehan SC et al. Late effects in long-term survivors of high-grade non-Hodgkin’s lymphomas. J Clin Oncol 1998; 16: 2070–2079. [DOI] [PubMed] [Google Scholar]
- 8.Ng AK, LaCasce A, Travis LB. Long-term complications of lymphoma and its treatment. J Clin Oncol 2011; 29: 1885–1892. [DOI] [PubMed] [Google Scholar]
- 9.Mohile SG, Fan L, Reeve E, Jean-Pierre P, Mustian K, Peppone L et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol 2011; 29: 1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev 2011; 20: 1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyson GJ, Thompson K, Palmer S, Thomas DM, Schofield P. The relationship between unmet needs and distress amongst young people with cancer. Support Care Cancer 2012; 20: 75–85. [DOI] [PubMed] [Google Scholar]
- 12.Lisy K, Langdon L, Piper A, Jefford M. Identifying the most prevalent unmet needs of cancer survivors in Australia: A systematic review. Asia Pac J Clin Oncol 2019; 15: e68–e78. [DOI] [PubMed] [Google Scholar]
- 13.Shakeel S, Tung J, Rahal R, Finley C. Evaluation of Factors Associated With Unmet Needs in Adult Cancer Survivors in Canada. JAMA Netw Open 2020; 3: e200506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armes J, Crowe M, Colbourne L, Morgan H, Murrells T, Oakley C et al. Patients’ supportive care needs beyond the end of cancer treatment: a prospective, longitudinal survey. J Clin Oncol 2009; 27: 6172–6179. [DOI] [PubMed] [Google Scholar]
- 15.Burg MA, Adorno G, Lopez ED, Loerzel V, Stein K, Wallace C et al. Current unmet needs of cancer survivors: analysis of open-ended responses to the American Cancer Society Study of Cancer Survivors II. Cancer 2015; 121: 623–630. [DOI] [PubMed] [Google Scholar]
- 16.Beckjord EB, Reynolds KA, van Londen GJ, Burns R, Singh R, Arvey SR et al. Population-level trends in posttreatment cancer survivors’ concerns and associated receipt of care: results from the 2006 and 2010 LIVESTRONG surveys. J Psychosoc Oncol 2014; 32: 125–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molassiotis A, Yates P, Li Q, So WKW, Pongthavornkamol K, Pittayapan P et al. Mapping unmet supportive care needs, quality-of-life perceptions and current symptoms in cancer survivors across the Asia-Pacific region: results from the International STEP Study. Ann Oncol 2017; 28: 2552–2558. [DOI] [PubMed] [Google Scholar]
- 18.Hall A, Lynagh M, Bryant J, Sanson-Fisher R. Supportive care needs of hematological cancer survivors: a critical review of the literature. Crit Rev Oncol Hematol 2013; 88: 102–116. [DOI] [PubMed] [Google Scholar]
- 19.Konstantinidis TI, Spinthouri M, Ramoutsaki A, Marnelou A, Kritsotakis G, Govina O. Assessment of Unmet Supportive Care Needs in Haematological Cancer Survivors. Asian Pac J Cancer Prev 2019; 20: 1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Study TWsHI. Design of the Women’s Health Initiative clinical trial and observational study. Controlled clinical trials 1998; 19: 61–109. [DOI] [PubMed] [Google Scholar]
- 21.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol 2003; 13: S5–17. [DOI] [PubMed] [Google Scholar]
- 22.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol 2003; 13: S18–77. [DOI] [PubMed] [Google Scholar]
- 23.Paskett ED, Caan BJ, Johnson L, Bernardo BM, Young GS, Pennell ML et al. The Women’s Health Initiative (WHI) Life and Longevity After Cancer (LILAC) Study: Description and Baseline Characteristics of Participants. Cancer Epidemiol Biomarkers Prev 2018; 27: 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowland JH, Bellizzi KM. Cancer survivorship issues: life after treatment and implications for an aging population. J Clin Oncol 2014; 32: 2662–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tevaarwerk A, Denlinger CS, Sanft T, Ansbaugh SM, Armenian S, Baker KS et al. Survivorship, Version 1.2021. J Natl Compr Canc Netw 2021; 19: 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellizzi KM, Latini DM, Cowan JE, DuChane J, Carroll PR. Fear of recurrence, symptom burden, and health-related quality of life in men with prostate cancer. Urology 2008; 72: 1269–1273. [DOI] [PubMed] [Google Scholar]
- 27.Hays RD, Anderson R, Revicki D. Psychometric considerations in evaluating health-related quality of life measures. Qual Life Res 1993; 2: 441–449. [DOI] [PubMed] [Google Scholar]
- 28.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- 29.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991; 32: 705–714. [DOI] [PubMed] [Google Scholar]
- 30.Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess 1996; 66: 20–40. [DOI] [PubMed] [Google Scholar]
- 31.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr., Montoye HJ, Sallis JF et al. Compendium of physical activities: classification of energy costs of human physical activities. Medicine and science in sports and exercise 1993; 25: 71–80. [DOI] [PubMed] [Google Scholar]
- 32.LaMonte MJ, Manson JE, Chomistek AK, Larson JC, Lewis CE, Bea JW et al. Physical Activity and Incidence of Heart Failure in Postmenopausal Women. JACC Heart failure 2018; 6: 983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lobb EA, Joske D, Butow P, Kristjanson LJ, Cannell P, Cull G et al. When the safety net of treatment has been removed: patients’ unmet needs at the completion of treatment for haematological malignancies. Patient Educ Couns 2009; 77: 103–108. [DOI] [PubMed] [Google Scholar]
- 34.Parry C, Lomax JB, Morningstar EA, Fairclough DL. Identification and correlates of unmet service needs in adult leukemia and lymphoma survivors after treatment. J Oncol Pract 2012; 8: e135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaleta AK, Gardan P, McManus S, Miller MF, Clark K, Nicksic NE et al. Cancer-Related Distress and Unmet Needs Among Acute Myeloid Leukemia Survivors. Blood 2019; 134: 4787–4787. [Google Scholar]
- 36.Wang T, Molassiotis A, Chung BPM, Tan JY. Unmet care needs of advanced cancer patients and their informal caregivers: a systematic review. BMC Palliat Care 2018; 17: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrari M, Ripamonti CI, Hulbert-Williams NJ, Miccinesi G. Relationships among unmet needs, depression, and anxiety in non-advanced cancer patients. Tumori 2019; 105: 144–150. [DOI] [PubMed] [Google Scholar]
- 38.Miroševič Š, Prins JB, Selič P, Zaletel Kragelj L, Klemenc Ketiš Z. Prevalence and factors associated with unmet needs in post-treatment cancer survivors: A systematic review. Eur J Cancer Care (Engl) 2019; 28: e13060. [DOI] [PubMed] [Google Scholar]
- 39.Winters-Stone KM, Medysky ME, Savin MA. Patient-reported and objectively measured physical function in older breast cancer survivors and cancer-free controls. J Geriatr Oncol 2019; 10: 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018; 14: 513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev 2016; 25: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leach CR, Bellizzi KM, Hurria A, Reeve BB. Is it my cancer or am i just getting older?: Impact of cancer on age-related health conditions of older cancer survivors. Cancer 2016; 122: 1946–1953. [DOI] [PubMed] [Google Scholar]
- 43.van der Poel MW, Oerlemans S, Schouten HC, Mols F, Pruijt JF, Maas H et al. Quality of life more impaired in younger than in older diffuse large B cell lymphoma survivors compared to a normative population: a study from the population-based PROFILES registry. Ann Hematol 2014; 93: 811–819. [DOI] [PubMed] [Google Scholar]
- 44.Gutenkunst SL, Vardy JL, Dhillon HM, Bell ML. Correlates of cognitive impairment in adult cancer survivors who have received chemotherapy and report cognitive problems. Support Care Cancer 2021; 29: 1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loh KP, Janelsins MC, Mohile SG, Holmes HM, Hsu T, Inouye SK et al. Chemotherapy-related cognitive impairment in older patients with cancer. J Geriatr Oncol 2016; 7: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams AM, Zent CS, Janelsins MC. What is known and unknown about chemotherapy-related cognitive impairment in patients with haematological malignancies and areas of needed research. Br J Haematol 2016; 174: 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.La Carpia D, Liperoti R, Guglielmo M, Di Capua B, Devizzi LF, Matteucci P et al. Cognitive decline in older long-term survivors from Non-Hodgkin Lymphoma: a multicenter cross-sectional study. Journal of Geriatric Oncology 2020; 11: 790–795. [DOI] [PubMed] [Google Scholar]
- 48.Allegra A, Innao V, Basile G, Pugliese M, Allegra AG, Pulvirenti N et al. Post-chemotherapy cognitive impairment in hematological patients: current understanding of chemobrain in hematology. Expert Rev Hematol 2020; 13: 393–404. [DOI] [PubMed] [Google Scholar]
- 49.Adams RN, Mosher CE, Abonour R, Robertson MJ, Champion VL, Kroenke K. Cognitive and Situational Precipitants of Loneliness Among Patients With Cancer: A Qualitative Analysis. Oncol Nurs Forum 2016; 43: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kotwal AA, Cenzer IS, Waite LJ, Covinsky KE, Perissinotto CM, Boscardin WJ et al. The epidemiology of social isolation and loneliness among older adults during the last years of life. J Am Geriatr Soc 2021; 69: 3081–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czaja SJ, Moxley JH, Rogers WA. Social Support, Isolation, Loneliness, and Health Among Older Adults in the PRISM Randomized Controlled Trial. Front Psychol 2021; 12: 728658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powell VD, Kumar N, Galecki AT, Kabeto M, Clauw DJ, Williams DA et al. Bad company: Loneliness longitudinally predicts the symptom cluster of pain, fatigue, and depression in older adults. J Am Geriatr Soc 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward M, May P, Normand C, Kenny RA, Nolan A. Mortality risk associated with combinations of loneliness and social isolation. Findings from The Irish Longitudinal Study on Ageing (TILDA). Age Ageing 2021; 50: 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parker SG, McCue P, Phelps K, McCleod A, Arora S, Nockels K et al. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing 2018; 47: 149–155. [DOI] [PubMed] [Google Scholar]
- 55.Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol 2018; 19: e305–e316. [DOI] [PubMed] [Google Scholar]
- 56.Lai DWL, Li J, Ou X, Li CYP. Effectiveness of a peer-based intervention on loneliness and social isolation of older Chinese immigrants in Canada: a randomized controlled trial. BMC Geriatr 2020; 20: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kotwal AA, Fuller SM, Myers JJ, Hill D, Tha SH, Smith AK et al. A peer intervention reduces loneliness and improves social well-being in low-income older adults: A mixed-methods study. J Am Geriatr Soc 2021; 69: 3365–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica 2012; 97: 1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geyer MB, Hsu M, Devlin SM, Tallman MS, Douer D, Park JH. Overall survival among older US adults with ALL remains low despite modest improvement since 1980: SEER analysis. Blood 2017; 129: 1878–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boucher NA, Johnson KS, LeBlanc TW. Acute Leukemia Patients’ Needs: Qualitative Findings and Opportunities for Early Palliative Care. J Pain Symptom Manage 2018; 55: 433–439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available through request from the WHI.


