Summary
Background
Osteoporosis heavily affects postmenopausal women and is influenced by environmental exposures. Determining the impact of criteria air pollutants and their mixtures on bone mineral density (BMD) in postmenopausal women is an urgent priority.
Methods
We conducted a prospective observational study using data from the ethnically diverse Women's Health Initiative Study (WHI) (enrollment, September 1994–December 1998; data analysis, January 2020 to August 2022). We used log-normal, ordinary kriging to estimate daily mean concentrations of PM10, NO, NO2, and SO2 at participants' geocoded addresses (1-, 3-, and 5-year averages before BMD assessments). We measured whole-body, total hip, femoral neck, and lumbar spine BMD at enrollment and follow-up (Y1, Y3, Y6) via dual-energy X-ray absorptiometry. We estimated associations using multivariable linear and linear mixed-effects models and mixture effects using Bayesian kernel machine regression (BKMR) models.
Findings
In cross-sectional and longitudinal analyses, mean PM10, NO, NO2, and SO2 averaged over 1, 3, and 5 years before the visit were negatively associated with whole-body, total hip, femoral neck, and lumbar spine BMD. For example, lumbar spine BMD decreased 0.026 (95% CI: 0.016, 0.036) g/cm2/year per a 10% increase in 3-year mean NO2 concentration. BKMR suggested that nitrogen oxides exposure was inversely associated with whole-body and lumbar spine BMD.
Interpretation
In this cohort study, higher levels of air pollutants were associated with bone damage, particularly on lumbar spine, among postmenopausal women. These findings highlight nitrogen oxides exposure as a leading contributor to bone loss in postmenopausal women, expanding previous findings of air pollution-related bone damage.
Funding
US National Institutes of Health.
Keywords: Air pollution, Mixtures, Bone mineral density, Postmenopause, Bone damage
Research in context.
Evidence before this study
Previous epidemiological studies on individual pollutants have suggested adverse effects on bone mineral density, osteoporosis risk, and fractures in older individuals. Animal studies have also found evidence of this air pollution-related bone damage. However, no studies have been done specifically in postmenopausal women. Also, no mixture analyses of air pollutants on bone outcomes have been reported.
Added value of this study
We show for the first time that from air pollution mixtures, nitrogen oxides contribute the most to bone damage and that the lumbar spine is one of the most susceptible sites. Our findings, derived from the analysis of a large number of postmenopausal, ethnically diverse women, clarify the impact of air pollutants mixtures on bone mineral density, specifically in postmenopausal women.
Implications of all the available evidence
Our results suggest that public health policies should aim to reduce air pollution in general but should be stronger in reducing nitrogen oxides exposure. Improvements in air pollution exposure, particularly nitrogen oxides, will reduce bone damage in postmenopausal women, prevent bone fractures, and reduce the health cost burden associated with osteoporosis in this population. Further efforts should focus on detecting those at higher risk of air pollution-related bone damage.
Introduction
Osteoporotic fractures in the US are very common. Approximately ∼2.1 million osteoporosis-related bone fractures occur annually, resulting in up to $20.3 billion in annual direct health costs.1,2 Osteoporosis impacts women more than men, with 80% of the estimated 10 million Americans with osteoporosis being women. Postmenopausal women are at higher risk, with one in two women over 50 experiencing a bone fracture because of osteoporosis.3 Hence, the identification of novel, preventable risk factors for bone loss and fractures in older women is an urgent priority. Ambient concentrations of particulate matter air pollution are associated with cardiovascular4 and respiratory diseases,5 lung cancer,6 and impaired cognition.7 Our team recently showed that long-term air pollution exposure reduces bone mineral density (BMD) and increases bone fracture risk in later life.8 Our findings have now been confirmed in multiple human studies9, 10, 11, 12, 13, 14 and are also supported by animal studies.15 For example, recent reports from Zhang et al. suggested that long-term exposure to PM2.5 was associated with decreased BMD T-score and increased osteoporosis risk among participants from rural areas of China.16 However, to our knowledge, no studies have prospectively determined the impact of criteria air pollutants and their mixtures on bone mineral density (BMD) in postmenopausal women in the US. The goal of this study was to determine the influence of four criteria air pollutants and their mixtures on bone mineral density in a large, geographically, and ethnically diverse population of US postmenopausal women.
Methods
Population
The Woman's Health Initiative (WHI) recruited 161,808 postmenopausal women from 40 clinical centers nationwide between October 1, 1993 and December 21, 1998.17 All women were 50–79 years old when they were enrolled in at least one of three clinical trials (CT; N = 68,132) or an observational study (OS; N = 93,676). The three WHI CT were a randomized controlled clinical trial of menopausal hormone therapy, dietary modification, and calcium/vitamin D supplementation. The WHI bone mineral density substudy, in which BMD was measured at enrollment, year 1, year 3, 6, and year 9 clinic visits, included all participants at three clinical centers (Birmingham, AL; Pittsburgh, PA; and Tucson, AZ); and a satellite clinic (Phoenix, AZ) (N = 11,020) chosen to maximize racial diversity. At enrollment, 9041 women had available BMD and long-term air pollution data. Those participants without air pollution estimations were excluded.
Air pollution exposure data
WHI participant addresses from study inception to date have been accurately geocoded.18,19 Geocoded participant address-specific daily mean concentrations of particulate matter ≤10 μm (PM10) from 1993 to 2012 were spatially estimated using available US Environmental Protection Agency (EPA) Air Quality System data and national-scale, log-normal, ordinary kriging.20, 21, 22 Participants' addresses were reviewed at least once a year with participants as a part of cohort retention and follow-up activities; therefore, mean averages were based on the geocoded participant address-specific concentration on each day of the averaging period ending on the visit day. Analogous participant address-specific daily mean concentrations of gaseous air pollutants (nitrogen oxide [NO], nitrogen dioxide [NO2], and sulfur dioxide [SO2]) also were estimated using the same methods. In addition, monthly mean geocoded participant address-specific concentrations of PM10 were spatiotemporally estimated using generalized additive mixed models and geographic information system-based predictors.23 The spatial estimation of the daily mean exposure for the different pollutants used data from the U.S. EPA Air Quality System. Its validity was evaluated using standard cross-validation statistics, including mean prediction error (PE), standardized root mean squared error (SPE), standardized root mean square (RMSS), and standard error (SE). Observed values of PE and SPE close to zero, RMSS close to one, and RMS close to SE, providing evidence of model validity. Cross-validation for these models has been previously published.24 Pollutant-, duration- and model-specific estimates were averaged over one, three, and five years before (and ending on) dates of BMD assessment. As most air pollution predictions were made in the 1990s, and the US EPA Federal Reference Method network for PM2.5 was established in 1999,25 this fraction was not included in the analysis.
BMD assessments
A substudy measuring BMD at enrollment and at year 1, 3, and 6 clinic visits included all participants (N = 11,020) at three clinical centers (Birmingham, AL; Pittsburgh, PA; and Tucson, AZ) and a satellite clinic (Phoenix, AZ) chosen to maximize racial diversity. Participants without air pollution estimations were excluded. We thus analyzed data from 4202 CT participants and 4839 OS participants (total N = 9041). Participants underwent dual-energy X-ray absorptiometry scanning using Hologic machines (QDR2000, 2000+, or 4500). Quality assurance included cross-clinic calibration phantoms and review of a random sample of scans. When Hologic QDR 2000 machines were upgraded to QDR 4500 machines, in vivo cross-calibration was performed, and results were adjusted for correction factors and longitudinal changes in scanner performance. Certified technicians used dual-energy X-ray absorptiometry (DXA, QDR 2000, 2000+, or 4500 W; Hologic Inc, Bedford, MA, USA) to measure BMD in g/cm2 following standard protocols26 at screening and annual visits (1, 3, 6, and 9 years of follow-up). WHI quality assurance protocols included routine spine and hip phantoms and a random sample review. Among-site calibration phantoms close agreed (interscanner variability <1.5% for the spine, <4.8% for the hip, and <1.7% for linearity).27 We used measurements of absolute BMD at the total hip, lumbar spine, femoral neck, and total body from scans of each participant.
Ethics statement
Written informed consent was obtained from all participants at randomization/enrollment after Institutional Review Board approval at each WHI clinical center.
Statistical analysis
Statistical analysis involved first visually examining all variables for normality and the presence of outliers. To determine normality, we used QQ plots by using the function qqnorm in R. qqline function adds a line to a “theoretical”, by default, normal. We also determined normality using Kolmogorov–Smirnov test (ks.test ()) and outliers using the Grubbs test (grubbs.test ()) in R. In the case of non-normal distributions of continuous variables, data transformation (e.g., log transformation) was used. Continuous variables were log-transformed if distributions were skewed (e.g., air pollutant levels). Where data were missing, multiple imputation protocols were established to maintain statistical power, except for excluded observations without air pollution data. Imputation involved replacing missing values with values based on patterns of non-missing exposures, outcomes, and covariates within participants and the relationships between them among participants.28 It included a combination of imputed datasets (e.g., using Rubin's rule) to account for the uncertainty of the imputation and ensure correct standard error estimation in tabulated summary statistics.29 Multiple imputation (five imputations per analysis) was performed in R using the ‘MICE’ package, an advanced, widely used protocol to handle missing values, which, unlike simple imputation, creates a data series by imputing missing values. This procedure replaces each missing value with several possible values, considering the uncertainty behind the estimate of the missing value.30 Although CT and OS participants were included in the final models, analyses also were stratified by study membership. Pearson's product moment correlation coefficient was denoted as r for the sample statistic. It was used after confirming that both variables (i.e., air pollutants) being studied were normally distributed. For a correlation between variables x and y, the formula for calculating the sample Pearson's correlation coefficient was given by:
where xi and yi are the values of x and y for the ith individual. Cross-sectional relationships between one-year mean air pollution exposures and BMD were assessed using linear regression. Longitudinal relationships across visits were assessed using generalized mixed models. Associations were conventionally expressed as change in absolute BMD per 10% increase of mean pollutant level across different averaging periods. All models were adjusted for demographic characteristics (i.e., age at BMD assessment, race/ethnicity, education, income, US Census region at randomization/enrollment clinical center), clinical characteristics (body mass index), behavioral characteristics (smoking, physical activity), hormone therapy randomization arm (hormone therapy or placebo), dietary modification trial arm (low fat or control), and calcium/vitamin D randomization arm (calcium/vitamin D or placebo). Models were also adjusted by study membership (i.e., CT [yes/no] and OS [yes/no]). Sensitivity to stratification by study membership (CT or OS) also was evaluated (see Appendix page 7). To help control for residual, center-level confounding, we also included the covariate of neighborhood socioeconomic status z-score, a key social determinant of health.31 We also explored the effect of solar irradiance, calcium/vitamin D (CaD) intervention, and aging (≤64 and >65 years old) in the association. Additionally, because air pollution is a complex mixture, we assessed associations between pollutants and each BMD anatomical site using Bayesian kernel machine regression (BKMR) in longitudinal data.32,33 BKMR uses a kernel function to flexibly model highly correlated exposures without prior specification of the exposure–response function.32,33 We ran 10,000 iterations of a Markov chain Monte Carlo algorithm with a hierarchical variable selection of exposures based on exposure correlation structures with the following model: Yi = h ([Group = (PM10, NO, SO2, NO2) + βTCi + εi. A Gaussian kernel function was used to model the outcome Yi on h() that allows for potential nonlinear and non-additive exposure–response functions; βT denotes coefficients for the vector of covariates Ci for the ith individual, and εi represents the error. Model convergence and fit were assessed by visual examination of trace plots for all parameters across 10,000 iterations. For univariate exposure-response plots, other pollutants were fixed at their median value. Posterior inclusion probabilities—representing the proportion of iterations where the exposure variable was included in the model—were estimated for each exposure and averaging period. Given the critical age dependence of bone damage, we also fit age as a linear, categorical, or spline variable to examine the fitness of the models (Akaike information criteria).
Role of the funding source
The funding sources did not play any role in access to datasets or the decisions for publication.
Results
We evaluated N = 9041 WHI participants (32,663 visits). Characteristics of the study population at enrollment are in Table 1. On average, WHI participants were aged 63.3 years (standard deviation [SD]: 7.4 years) at baseline. Most participants were White (72.3% of those aged <60 years; 79.8% of those 60–69 years; and 84.3% of those ≥70 years). The most common education level was college or vocational (48.3% of those aged <60 years; 44.2% of those 60–69 years; and 46.2% of those ≥70 years). In general, most participants had a modest income (<$49,999/year), consumed <7 servings of alcoholic drinks/week, were never smokers, and had low physical activity. We observed a reduction in mean [SD] BMD with age (e.g., total hip BMD: 0.90 [0.13]; 0.84 [0.79]; and 0.79 [0.13] g/cm2 at ages <60, 60–69, and ≥70 years, Table 1). Full descriptions of CT and OS participants are in the appendix (Appendix pp 2 and 3), respectively. Participants were exposed to slightly higher mean PM10, NO2, NO, and SO2 concentrations over longer averaging periods (Table 2).
Table 1.
Demographic characteristics of women in the Women's Health Initiative at enrollment (N = 9041) with available bone mineral density and long-term air pollution data.
| Variable | <60 years old |
60–69 years old |
≥70 years old |
|||
|---|---|---|---|---|---|---|
| N = 3132 |
N = 3992 |
N = 1917 |
||||
| Mean/n | SD/% | Mean/n | SD/% | Mean/n | SD/% | |
| Bone mineral density (corrected), g/cm2 | ||||||
| Total hip | 0.90 | 0.13 | 0.84 | 0.13 | 0.79 | 0.13 |
| Femoral neck | 0.77 | 0.12 | 0.71 | 0.12 | 0.67 | 0.11 |
| Lumbar spine | 1.01 | 0.15 | 0.97 | 0.17 | 0.97 | 0.18 |
| Whole body | 1.05 | 0.10 | 1.00 | 0.10 | 0.97 | 0.11 |
| Ethnicity | ||||||
| White (non-Hispanic) | 2265 | 25.05% | 3187 | 35.25% | 1616 | 17.87% |
| Black | 517 | 5.72% | 520 | 5.75% | 192 | 2.12% |
| Hispanic | 266 | 2.94% | 206 | 2.28% | 80 | 0.88% |
| Other race | 81 | 0.90% | 71 | 0.79% | 23 | 0.25% |
| Not available | 3 | 0.03% | 8 | 0.09% | 6 | 0.07% |
| Education level | ||||||
| High school or less | 815 | 9.01% | 1342 | 14.84% | 644 | 7.12% |
| College or vocational school | 1498 | 16.57% | 1763 | 19.50% | 886 | 9.80% |
| Grad school or higher | 769 | 8.51% | 862 | 9.53% | 375 | 4.15% |
| Not available | 23 | 0.25% | 25 | 0.28% | 12 | 0.13% |
| Household income | ||||||
| <$49,999 | 1752 | 19.38% | 2875 | 31.80% | 1500 | 16.59% |
| $50,000–$99,999 | 933 | 10.32% | 703 | 7.78% | 218 | 2.41% |
| >$100,000 | 253 | 2.80% | 141 | 1.56% | 39 | 0.43% |
| Not available | 194 | 2.15% | 273 | 3.02% | 160 | 1.77% |
| Alcohol consumption | ||||||
| Never | 445 | 4.92% | 716 | 7.92% | 346 | 3.83% |
| Former | 647 | 7.16% | 834 | 9.22% | 446 | 4.93% |
| <7 drinks per week | 1788 | 19.78% | 2101 | 23.24% | 937 | 10.36% |
| ≥7 drinks per week | 231 | 2.56% | 315 | 3.48% | 167 | 1.85% |
| Not available | 21 | 0.23% | 26 | 0.29% | 21 | 0.23% |
| Smoking | ||||||
| Never | 1669 | 18.46% | 2205 | 24.39% | 1114 | 12.32% |
| Former | 1112 | 12.30% | 1467 | 16.23% | 676 | 7.48% |
| Current | 323 | 3.57% | 285 | 3.15% | 80 | 0.88% |
| Not available | 28 | 0.31% | 35 | 0.39% | 47 | 0.52% |
| Physical activity, MET-hours/week | 0.32 | 0.18 | 0.04 | 0.20 | 0.05 | 0.23 |
| Body mass index, kg/m2 | ||||||
| ≤18.5 | 17 | 0.19% | 37 | 0.41% | 25 | 0.28% |
| 18.6–24.9 | 862 | 9.53% | 1189 | 13.15% | 694 | 7.68% |
| ≥25 kg/m2 | 2243 | 24.81% | 2751 | 30.43% | 1193 | 13.20% |
| Not available | 10 | 0.11% | 15 | 0.17% | 5 | 0.06% |
SD: standard deviation; CT: clinical trial; MET: metabolic equivalent.
Table 2.
Long-term air pollution exposure concentrations in women in Women's Health Initiative (N = 9401 at enrollment) with bone mineral density data (concentrations at 1-, 3-, and 5-year average before first examination).
| Air pollutant | 1-year average |
3-year average |
5-year average |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| PM10 (μg/m3) | 22.15 | 5.57 | 22.45 | 5.48 | 22.91 | 5.48 |
| NO2 (ppb) | 17.90 | 5.27 | 18.12 | 4.74 | 18.17 | 4.74 |
| NO (ppb) | 39.29 | 19.72 | 39.82 | 17.87 | 39.86 | 17.87 |
| SO2 (ppb) | 6.39 | 3.68 | 6.50 | 3.55 | 6.55 | 3.55 |
PM10: particulate matter <10 μm; SO2: sulfur dioxide; NO and NO2: nitrogen oxides; SD: standard deviation; ppb: parts per billion.
Summary statistics of air pollutants per BMD assessment site are shown in the appendix material (Appendix p 4). The highest correlations were observed between NO2 and NO for all averaging periods evaluated (r = 0.83, 0.85, and 0.86 for 1-, 3- and 5-year means; appendix material [Appendix p 8]). The lowest correlations were observed between SO2 and PM10 (r = −0.06, −0.06, and −0.1 for 1-, 3-, and 5-year means), as well as SO2 and NO (r = −0.12, −0.09, and −0.07 for 1-, 3-, and 5-year means).
One-, 3-, and 5-year average PM10 concentrations were cross-sectionally associated with whole-body, total hip, femoral neck, and lumbar spine BMD (Table 3). PM10 concentrations also were longitudinally associated with femoral neck and lumbar spine BMD (Table 4). For example, lumbar spine BMD decreased 0.064 (95% confidence interval [CI]: 0.052, 0.077) g/cm2/per year per 10% (2.21 μg/m3) increase in 5-year mean PM10 concentration. Results suggested a cumulative effect on lumbar spine with increasing effect size according to the duration of PM10 exposure.
Table 3.
Cross-sectional analysis of the effect of PM10, NO, NO2, and SO2 on bone mineral density in the Clinical Trials and Observational Study participants of the Women's Health Initiative (N = 32,663 visits).
| BMD | Average period | PM10 |
NO |
NO2 |
SO2 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Est∗ | 95% CI | Est∗ | 95% CI | Est∗ | 95% CI | Est∗ | 95% CI | ||
| Whole body | 1-yr average | −0.047 | (−0.054, −0.039) | −0.031 | (−0.034, −0.027) | −0.050 | (−0.055, −0.044) | −0.026 | (−0.029, −0.022) |
| 3-yr average | −0.051 | (−0.058, −0.043) | −0.029 | (−0.034, −0.023) | −0.049 | (−0.054, −0.043) | −0.017 | (−0.022, −0.011) | |
| 5-yr average | −0.053 | (−0.060, −0.045) | −0.026 | (−0.031, −0.020) | −0.055 | (−0.062, −0.047) | −0.018 | (−0.023, −0.012) | |
| Total Hip | 1-yr average | −0.022 | (−0.031, −0.012) | −0.020 | (−0.027, −0.012) | −0.018 | (−0.023, −0.012) | −0.025 | (−0.030, −0.019) |
| 3-yr average | −0.024 | (−0.033, −0.014) | −0.020 | (−0.025, −0.014) | −0.015 | (−0.022, −0.007) | −0.025 | (−0.032, −0.017) | |
| 5-yr average | −0.026 | (−0.035, −0.016) | −0.018 | (−0.023, −0.012) | −0.015 | (−0.024, −0.005) | −0.027 | (−0.041, −0.022) | |
| Femoral Neck | 1-yr average | −0.013 | (−0.020, −0.005) | −0.018 | (−0.021, −0.014) | −0.019 | (−0.083, −0.045) | −0.019 | (−0.024, −0.013) |
| 3-yr average | −0.015 | (−0.022, −0.007) | −0.020 | (−0.025, −0.014) | −0.021 | (−0.028, −0.013) | −0.018 | (−0.025, −0.010) | |
| 5-yr average | −0.017 | (−0.026, −0.007) | −0.021 | (−0.026, −0.015) | −0.024 | (−0.029, −0.018) | −0.021 | (−0.028, −0.013) | |
| Lumbar spine | 1-yr average | −0.014 | (−0.027, −0.0002) | −0.030 | (−0.035, −0.024) | −0.029 | (−0.038, −0.019) | −0.032 | (−0.041, −0.022) |
| 3-yr average | −0.015 | (−0.028, −0.0001) | −0.027 | (−0.034, −0.019) | −0.022 | (−0.033, −0.010) | −0.028 | (−0.037, −0.018) | |
| 5-yr average | −0.026 | (−0.035, −0.016) | −0.018 | (−0.023, −0.012) | −0.020 | (−0.033, −0.006) | −0.033 | (−0.044, −0.021) | |
Est∗: Estimates from multivariable adjusted models for demographic characteristics (i.e., age, race/ethnicity, education, income, neighborhood socioeconomic status, and US Census region), clinical characteristics (body mass index), behavioral characteristics (smoking, physical activity), study membership (clinical trials or observational study), hormone therapy randomization arm (hormone treatment or control), dietary modification trial arm, and calcium/vitamin D randomization arm (calcium/vitamin D or control). 95% CI: 95% confidence interval; PM10: particulate matter <10 μm; SO2: sulfur dioxide; NO and NO2: nitrogen oxides; BMD, bone mineral density.
Table 4.
Longitudinal associations of PM10, NO, NO2, and SO2 on bone mineral density in the Clinical Trials and Observational Study of participants in the Women's Health Initiative (N = 32,663 visits).
| BMD | Average period | PM10 |
NO |
NO2 |
SO2 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Est∗ | 95% CI | Est∗ | 95% CI | Est∗ | 95% CI | Est∗ | 95% CI | ||
| Whole body | 1-yr average | 0.001 | (−0.005, 0.007) | −0.006 | (−0.010, −0.003) | 0.003 | (−0.002, 0.008) | −0.009 | (−0.013, −0.006) |
| 3-yr average | 0.006 | (−0.001, 0.0114) | −0.022 | (−0.027, −0.017) | −0.004 | (−0.010, 0.002) | −0.012 | (−0.017, −0.007) | |
| 5-yr average | 0.004 | (−0.004, 0.012) | −0.001 | (−0.006, 0.004) | 0.001 | (−0.007, 0.008) | −0.034 | (−0.010, 0.003) | |
| Total hip | 1-yr average | 0.014 | (0.006, 0.021) | 0.001 | (−0.003, 0.005) | 0.012 | (0.007, 0.018) | −0.004 | (−0.008, 0.001) |
| 3-yr average | 0.005 | (−0.003, 0.014) | −0.006 | (−0.011, −0.002) | 0.014 | (−0.006, 0.090) | −0.018 | (−0.023, −0.012) | |
| 5-yr average | −0.004 | (−0.014, 0.005) | 0.009 | (0.002, 0.015) | 0.024 | (0.015, 0.033) | −0.008 | (−0.015, −0.001) | |
| Femoral neck | 1-yr average | 0.005 | (−0.001, 0.011) | −0.001 | (−0.004, 0.002) | 0.001 | (−0.003, 0.006) | 0.001 | (−0.003, 0.003) |
| 3-yr average | 0.002 | (−0.005, 0.009) | −0.013 | (−0.017, −0.007) | −0.007 | (−0.013, −0.001) | −0.002 | (−0.008, 0.003) | |
| 5-yr average | −0.011 | (−0.018, −0.003) | −0.006 | (−0.011, 0.001) | −0.008 | (−0.016, −0.001) | −0.016 | (−0.022, −0.009) | |
| Lumbar spine | 1-yr average | −0.006 | (−0.016, 0.003) | −0.009 | (−0.014, −0.003) | −0.021 | (−0.028, −0.013) | −0.006 | (−0.012, −0.001) |
| 3-yr average | −0.038 | (−0.050, −0.026) | −0.023 | (−0.030, −0.016) | −0.026 | (−0.036, −0.016) | −0.012 | (−0.021, −0.004) | |
| 5-yr average | −0.064 | (−0.077, −0.052) | −0.022 | (−0.031, −0.013) | −0.011 | (−0.022, 0.001) | −0.030 | (−0.040, −0.020) | |
Est∗: Estimates from multivariable adjusted models for demographic characteristics (i.e., age, race/ethnicity, education, income, neighborhood socioeconomic status, and US Census region), clinical characteristics (body mass index), behavioral characteristics (smoking, physical activity), study membership (clinical trials or observational study), hormone therapy randomization arm (hormone treatment or control), dietary modification trial arm, and calcium/vitamin D randomization arm (calcium/vitamin D or control). 95% CI: 95% confidence interval; PM10: particulate matter <10 μm; SO2: sulfur dioxide; NO and NO2: nitrogen oxides; BMD, bone mineral density.
One-, 3-, and 5-year average NO concentrations also were cross-sectionally and negatively associated with whole-body, total hip, femoral neck, and lumbar spine BMD (Table 3). NO concentrations were also longitudinally associated with BMD at all anatomical sites at different time windows (Table 4). Over time, no cumulative effect of NO was observed for any anatomical site evaluated. One-, 3-, and 5-year average NO2 concentrations were cross-sectionally associated with whole-body, total hip, femoral neck, and lumbar spine BMD (Table 3). One-, 3-, and 5-year mean NO2 concentrations also were also longitudinally associated with femoral neck and lumbar spine BMD (Table 4). For example, lumbar spine BMD decreased 0.026 (95% CI: 0.016, 0.036) g/cm2/year per 10% increase in 3-year mean NO2 concentration.
One-, 3-, and 5-year average SO2 concentrations also were cross-sectionally associated with whole-body, total hip, lumbar spine, and femoral neck BMD (Table 3). SO2 concentrations also were longitudinally associated with whole-body, total hip, femoral neck, and lumbar spine BMD (Table 4). Although ozone (O3) was also explored, we observed a lack of consistent and non-significant associations with BMD (Appendix p 6) and was not included in the final analyses.
In stratified analyses, we did not observe effect differences between CT and OS participants (Appendix p 6). Our models included age as a linear, categorical, or spline variable to sufficiently control our results by age and showed a negative association with age for BMD at the most relevant anatomical site (i.e., lumbar spine) at all time windows evaluated (Appendix p 7). In sensitivity analysis, we explored the influence of two different measures of solar irradiance (i.e., solar irradiance in Watts, measuring the daily UVB flux reaching the earth, within the wavelength range necessary for vitamin D synthesis; solar irradiance in Langleys, related to the amount that reaches a given area of the earth's surface). Although we observed some reductions in the estimates, most significant associations remained unaffected (Appendix p 9), suggesting a potential influence of solar irradiance in the association. We also explored calcium/vitamin D (CaD) interaction in the association between air pollutants at the three averaged periods, finding significant interaction of this intervention in favor of the control group (Appendix p 10). In sensitivity analysis, we also explored the influence of aging in the association and found higher estimates in the oldest for all the pollutants and average periods (Appendix p 11).
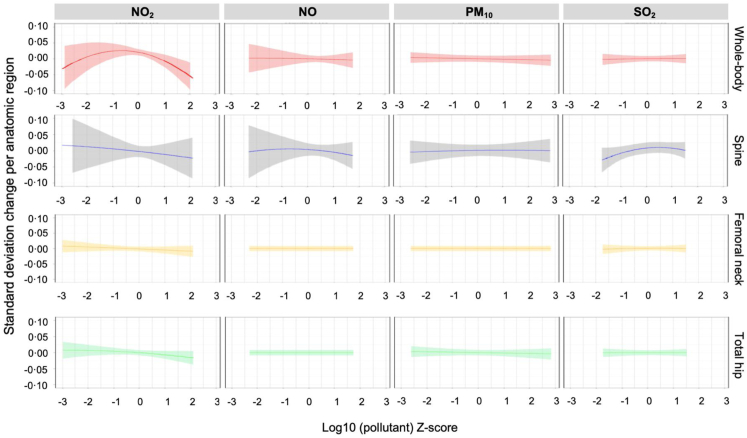
BKMR analysis of the associations between air pollutants and BMD at different anatomical sites revealed that only NO2 was negatively associated with whole-body and lumbar spine BMD (Fig. 1). Posterior inclusion probabilities were higher for NO on whole body BMD and for NO2 at the three time windows and for NO and SO2 on spine BMD at the 1-year time window (Appendix p 9). The 3- (Appendix p 10) and 5-year (Appendix p 11) average NO2–BMD associations were similar to the 1-year association, but this was not the case for the other pollutants at most of the anatomical sites. A slight tendency to a positive association with lumbar spine BMD was also observed for SO2.
Fig. 1.
Bayesian kernel machine regression univariate exposure-response plots with 95% credible intervals for the effect of each pollutant on the different bone mineral density sites evaluated.
Discussion
This study—with >9000 women participating in WHI and >30,000 visits—supports ours8 and other findings10,16,34, 35, 36 of an association between air pollution and bone damage in postmenopausal women. This association was independent of socioeconomic, geographic, and lifestyle factors. Mixture analyses showed significance for NO2, particularly affecting whole-body and spine BMD, although longitudinal models showed negative associations for all pollutants. Although we observed some stronger associations at longer average periods (e.g., SO2 and whole-body BMD (β at 1-year: −0.009; −0.012 at 3-year; −0.034 at 5-year), dose–response associations were not evident in most models. Our findings confirm that poor air quality may be a risk factor for bone loss, independent of socioeconomic or demographic factors, and expands previous findings to postmenopausal women. Indeed, to our knowledge, this is the first study of the impact of criteria air pollutants on bone health in postmenopausal women.
Our findings show that both particulate matter and gases may adversely impact BMD and that nitrogen oxides may play a critical role in bone damage and osteoporosis risk. The annual rate of postmenopausal bone loss has been calculated to be 1.3–1.5 percent at the lumbar spine and 1.4 percent at the femoral neck after menopause.37 Our analyses of the effects of age on these women showed different rates of reductions per anatomical site, with annual average reductions of 0.64% for total hip BMD, 0.59% for femoral neck, 0.35% for total body, and 0.18% for lumbar spine BMD. Therefore, the magnitude of the effects of nitrogen oxides on lumbar spine BMD would be about 1.22% annual reductions, almost double the annual effects of age on any of the anatomical sites evaluated. Implications for fracture risk of this BMD reduction are unknown, but further research is guaranteed. Our study did not include analyses of forearm BMD, which is a relevant anatomical site for osteoporosis-related bone damage. However and according to the International Society for Densitometry,38 osteoporosis may be diagnosed in postmenopausal women and in men aged 50 and older if the T-score of the lumbar spine, total hip, or femoral neck is −2.5 or less, without including forearm BMD. Our results using the most relevant anatomical sites showed consistent associations through all the models evaluated.
Air pollution exposure may increase reactive oxygen and nitrogen species, which cause oxidative damage.39 Increased oxidative stress causes cell death through various signaling mechanisms such as mitochondrial impairment, cell-cycle arrest, DNA damage response, inflammation, protein inhibition, and lipid peroxidation.40
Nitrogen oxides, the air pollutants linked to bone damage in our analyses, are reactive nitrogen species. We observed a U-shaped dose–response function for NO2 and whole-body BMD, interpreted on the basis of a negative linear slope for bone health. NO2 is one of six air pollutants that have national air quality standards to limit levels in outdoor air.41 NO2 exposure has been associated with reduced lung function, increased asthma attacks, and greater likelihood of emergency department and hospital admissions.42 NO2 also is likely to be a cause of asthma in children.43 Eckel et al. found that people with lung cancer have shortened survival from NO2 and other outdoor air pollutants.44 Additionally, NO2 has been linked to cardiovascular damage,45 lower birth weight in newborns,46 and increased risk of premature death.47 Our results are among the first directly linking NO2 and osteoporosis risk. Similar tendencies were shown for NO in our BKMR analysis, which could be justified by similarities (e.g., sources) between these two pollutants and also the highest correlation we observed (r = 0.83–0.86). We decided to include both pollutants separately based on their clinical effects, which could be different. For example, exposure to NO induces respiratory ailments, hematologic side effects, metabolic disorders, low blood pressure, nausea, vomiting, and diarrhea. NO2 causes more respiratory effects (wheezing, coughing, colds, flu, and bronchitis).48 On the other hand, a slight tendency to a positive association with lumbar spine BMD was also observed for SO2; however, the significance of this finding is unknown and was not consistent with results from the regression models. This positive association could be justified by chance, as we assessed 16 associations; therefore, we expect ∼3 of them to be significant due to chance (multiple comparisons).
We previously published the first evidence of an association between air pollution and bone damage.8 In that study, we found adverse effects of particulate matter on the risk of hospitalizations for osteoporosis-related bone fractures in Medicare participants and of black carbon on reduced BMD in participants of the BACH/Bone study, a male-only cohort.8 Several studies have since confirmed this finding, including short-term effects on hip fractures,9 in different settings and populations (e.g., rural China),10 in nationwide time-series studies (China),49 and in young populations,11 but none examined postmenopausal women or used a mixture approach. Hun Sung et al.14 evaluated the association of air pollution with osteoporotic fracture risk among women >50 years of age, showing an increased risk (HR = 1.13, 95% CI: 1.02, 1.24) of osteoporotic bone fractures. A recent meta-analysis reported inconsistent associations between PM exposure and osteoporosis risk or fractures but attributed it to heterogeneous study designs, participant characteristics, and analyses.50 While air pollution studies are indeed heterogeneous, results from our studies are consistent and suggest that thousands of aging populations may be affected around the globe, particularly by gases like NO2 that were not included in previous analyses of particulate matter.
Since our first report of associations between air pollution and bone damage, several studies around the world have now shown that long-term exposure to air pollution is associated with decreased bone mass, but none of them have been able to study air pollution mixtures. Chen et al. showed that short-term exposure to PM2.5 resulted in an increase in the number of outpatient visits for knee osteoarthritis in Beijing, suggesting potential additional air pollution-related bone damage and suggested an exposure-response relationship.51 In studies from India, Ranzani et al. reported that PM2.5 also was associated with lower BMD in the spine (−0.57 g per 3 μg/m3 increase in PM2.5) and hip (−0.13 g per 3 μg/m3).11 Studies from Zhang et al. reported that PM10 and NO2, with concentrations 2–3 times higher than the average in US cities, also showed more deleterious effects in bones of men than women.49 Therefore, these results suggest that complexities of air pollution mixtures (origin, concentrations, geographic location, and concentration of pollutants) may have different influences on bone health.
Available evidence suggests that exposure to air pollutants is associated with increased levels of proinflammatory mediators and markers of oxidative stress as well as multiple other metabolic alterations52 that can alter bone remodeling.53 For example, exposure to high particulate concentrations significantly increases serum levels of monocytes, NK cells, and helper T cells,54 as well as proinflammatory cytokines such as TNFα, MCP-1, IL-8, MIP-1α, IL-6, IL-1β, and GM-CSF in vitro and in vivo models.55, 56, 57 The most-studied pollutants concerning intracellular formation of free radicals are O3,58 NOx, and heavy metals.59 Other studies on the effect of indoor air pollutants in older individuals with chronic obstructive pulmonary disease have also revealed a potential effect on purine metabolism.60 Additional studies from You et al. using proton nuclear magnetic resonance spectroscopy suggested that, among postmenopausal women, elevated glutamine was significantly associated with low BMD and that elevated lactate, acetone, lipids, and very low-density lipoprotein were associated with high BMD.61 Studies in postmenopausal Brazilian women with osteopenia or osteoporosis have shown reduced taurine levels in plasma compared to healthy volunteers.62 In this context, we therefore hypothesize that air pollution mixtures—especially those with high enough NOx concentrations—induce inflammation and oxidative damage. The resulting generation of reactive molecules and metabolites that interact with bone cells—particularly mononuclear pre-osteoclasts—may plausibly affect osteogenesis, bone resorption, physiology, and in turn, susceptibility to osteoporosis. Further research is nonetheless needed to confirm these hypotheses.
Vitamin D (VitD) is a fat-soluble vitamin. VitD and its metabolites have a significant clinical role because of their interrelationship with calcium homeostasis and bone metabolism. Subclinical vitamin D deficiency is very common, as the recent National Health and Nutrition Examination Survey (NHANES) showed it (41.6% of adult participants ≥20 years had 25(OH)D levels below 20 ng/mL,63 which may contribute to the development of osteoporosis and an increased risk of fractures and falls in older adults. Controlled trials have suggested that vitamin D and calcium supplementation can reduce the risk of falls and fractures in older adults.64, 65, 66 The vast amount of information available in WHI allowed us to explore the potential interaction of the Calcium/Vitamin D, which resulted in being significant, particularly for those pollutants more related to bone damage (NO and NO2). However, the direction of the associations was not consistent for reductions or increases in BMD, probably because of the design of these interventions. Further research is needed to clarify this influence in the association between air pollution and bone damage.
Bone damage, including osteoporosis, falls, and fractures, are among the commonest geriatric syndromes, and the risk of these conditions increases with age.67 In women, fracture risk increases from around menopause (45–55 years old), whereas in men fracture risk increases parallels that of women but only starts around age 75. At that time, fracture incidence also hastens in women.68 This suggests an enormous influence of age on bone strength in both men and women, which has also been evidenced for the association between air pollutants and BMD in the current study in postmenopausal women, with higher estimates for those older than 65. Our findings may suggest that additional prevention strategies should be taken into account for this susceptible population, which may derive in reductions in morbidity (e.g., bone fractures, hospitalizations) and also mortality.
The sources of air pollutants may play a critical role in the estimated association and in the potential interventions to reduce bone damage. In the U.S., most toxics in ambient air are anthropometric and generated by mobile or stationary sources, including automobiles, trucks, buses, factories, refineries, construction sites, and industries that use cleaning solvents. Car and truck exhaust is a major source of NOx, as are the emissions from electrical power generation plants. Automobile exhaust contains more NO than NO2, but it quickly combines with oxygen in the air to form NO2 in the atmosphere. As such, inhalation is its main source of uptake.30 Air pollution source apportionment studies have been performed to evaluate the contribution of different sources using receptor methods for particle source apportionment, including chemical mass balance, positive matrix factorization, factor analysis, Enrichment factor, isotopic tools, etc.69 Source apportionment in future studies of air pollution-induced bone damage may help clarify the focus of forthcoming efforts to reduce bone damage in aging societies.
Our work has several limitations. First, as this study was performed only in the US, these results may not be generalized to other populations. However, our population included White, Black, Hispanic, and other races/ethnicities, contributing to the potential generalizability of the results. As with any human population study, there remains the potential for unmeasured or residual confounding. However, we adjusted for many important confounders, including age, ethnicity, education, income, neighborhood socioeconomic status z-score, BMI, smoking, physical activity, study membership, and US Census region. We also recognize that BMD might not be the best measurement of bone health, but Z-score (which compares your bone density to the average values for a person of the same age and gender), unfortunately, is unavailable in WHI. Another limitation of our study was our inability to measure PM2.5, which may trigger bone damage. However, the availability of PM2.5 in the US during enrollment and initial follow-up visits (1993–1995) was sparse due to limited monitoring in those years in the corresponding sites. Our study derives from environmental determinations of air pollution and behavior risk factors that have been questioned to the participants but not directly assessed. These factors and how they were addressed may be influencing or biasing our results. However, despite these limitations, our study is consistent with similar studies done in other geographical regions, adding the value of clarifying pollutants with stronger effects on bone health. Additionally, we did not examine associations between air pollution and fractures or the interaction of common medications. However, these analyses are currently underway. Another important limitation of this study is the lack of information about individual covariates such as indoor air pollution, time spent outdoors, vitamin D levels, and other environmental and occupational factors, which may play a role and even confound the observed associations. However, we included analyses for the interaction of the CaD intervention, finding significant interaction of this intervention in favor of control groups. Further research is needed to clarify the potential interaction of CaD in the association between air pollution and bone damage. We also included sensitivity analyses for solar irradiance without observing important changes in the estimates for the association of air pollutants on BMD. Ongoing research is examining interactions of the pollutants examined in this study with hormone therapy, dietary modification, and other interventions available among WHI participants. We also acknowledge that estimating individual exposure to air pollutants using our approach may result in a considerable error and potential exposure misclassification.70,71 Inadequate characterization of personal exposure is known that can bias the magnitude of the effect estimates in time series epidemiological studies of ambient air pollution.72 However, exposure potential exposure misclassification is likely to be non-differential (i.e., independent of outcome status), which has been shown to result in measures of association consistently biased towards the null.73, 74, 75 However, our study was designed to investigate the association between living in a location with a certain level of air quality and bone health. This approach has been extensively used in air pollution research and is highly relevant to regulating local and nationwide ambient air quality standards.76 Previous studies in WHI77 and other cohorts78,79 have illustrated that substituting imputed personal exposure for ambient pollutant concentrations shifts associations with outcomes away from the null and decreases their precision.
On the other hand, our study has several strengths, including the longitudinal design with data over more than six years of follow-up, detailed information regarding important osteoporosis risk factors, high numbers of participants from various racial/ethnic groups and various sites in the US, comprehensive assessment of several air pollutants, application of advanced mixture analysis to data from an at-risk cohort of postmenopausal women, and high variability in pollutant exposures. WHI participant addresses, from study inception to the last visit, have been accurately geocoded; therefore, average estimates for air pollutants include potential changes in exposure. Other changes in habits (e.g., smoking) may impact bone metabolism. For example, it is known that osteocalcin and uncarboxylated osteocalcin significantly increased after successful smoking cessation, and it is associated with improvements in BMD.80 These types of changes were not included in our analyses. They may also influence our results and potentially may also compromise our ability to generalize our findings to other populations.
To our knowledge, this is the first analysis determining the impact of various criteria air pollutants, particularly mixtures, on bone damage in postmenopausal women. The inverse air pollutant–bone damage association we observed was most substantial over longer averaging periods for some pollutants, with the lumbar spine being the most affected site, and with effects observed at mean nitrogen oxides concentrations that fall below current EPA National Ambient Air Quality Standards. These results thus have implications for the health of aging women and public health.
Contributors
Prada, Whitsel, and Baccarelli contributed with the original idea, design, WHI proposals and approvals, discussions, funding, and manuscript writing. Crandall contributed to the design and discussions about bone health. Kupsco and Kioumourtzoglou contributed to BKMR modeling and discussions. Stewart contributed to WHI data management and discussions. Liao and Yanosky contributed to air pollution estimates. Prada, Shen, and Ramirez contributed with data analyses, tables, and figures. Wactawski-Wende, Miller, and Lonita-Laza contributed to data analysis approaches and discussions. Dr. Eric Whitsel has accessed and verified the data; Dr. Baccarelli and Prada are responsible for the decision to submit the manuscript.
Data sharing statement
WHI data are not publicly available. The principal investigators of each WHI contributing study are responsible for access to the data.
Declaration of interests
Most authors declare that they have no competing interest in any form. JWW declares funding from NHLBI/NIH to support the Women's Health Initiative (JWW is the PI of the northeast WHI regional center), which has occurred since 1993 and will go through 2027. WHI funding for JWW supports support travel to the annual WHI meeting. JWW is dean of the School of Public Health and Health Professions at the University at Buffalo and serves as a board member of several western New York community organizations (unpaid). GM receives royalties for his books (The exposome: a primer [2014]; The exposome: a new paradigm for health and disease [2020], published by Academic Press). GM also receives stipend for serving as Editor of Exposome, published by Oxford University Press. GM also has received honoraria from Kansas University Medical Center (speaker), Mayo Clinic (speaker), Foundation for Gender Specific Medicine (book chapter), AAAS (speaker), Vichy (speaker), University of Colorado (speaker), and the University of Arizona (speaker). When GM speaks at universities in the U.S. the travel is paid by them.
Acknowledgments
We thank all investigators in the Baccarelli Laboratory for data analysis discussions. We also acknowledge all WHI participants and investigators. AK was funded by R00 ES030749 from NIEHS. JDY was funded by Penn State College of Medicine. JW was funded by NHLBI/NIH. GM was funded by NIH grants U2C ES030163 and UL1-TR00187 to Columbia. EA was funded by R01-ES020836 from NIH/NIEHS. This study was supported by the NlH (R01ES025225; P30ES009089; R01AG069120; R01ES032242; R01ES027747; R35ES031688; R01AG058704; R01ES028805, R01ES030616, and R01ES029943). The WHI program is founded by through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101864.
Contributor Information
Diddier Prada, Email: dgp2114@cumc.columbia.edu.
Carolyn J. Crandall, Email: CCrandall@mednet.ucla.edu.
Allison Kupsco, Email: ak4181@cumc.columbia.edu.
Marianthi-Anna Kioumourtzoglou, Email: mk3961@cumc.columbia.edu.
James D. Stewart, Email: j.stewart@unc.edu.
Duanping Liao, Email: DLiao@phs.psu.edu.
Jeff D. Yanosky, Email: JYanosky@phs.psu.edu.
Andrea Ramirez, Email: andrea1703999@comunidad.unam.mx.
Jean Wactawski-Wende, Email: jww@buffalo.edu.
Yike Shen, Email: ys3419@cumc.columbia.edu.
Gary Miller, Email: gm2815@cumc.columbia.edu.
Iuliana Ionita-Laza, Email: ii2135@cumc.columbia.edu.
Eric A. Whitsel, Email: eric_whitsel@med.unc.edu.
Andrea A. Baccarelli, Email: ab4303@cumc.columbia.edu.
Appendix A. Supplementary data
References
- 1.Clynes M.A., Harvey N.C., Curtis E.M., Fuggle N.R., Dennison E.M., Cooper C. The epidemiology of osteoporosis. Br Med Bull. 2020;133(1):105–117. doi: 10.1093/bmb/ldaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burge R., Dawson-Hughes B., Solomon D.H., Wong J.B., King A., Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.Holroyd C., Cooper C., Dennison E. Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008;22(5):671–685. doi: 10.1016/j.beem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Lelieveld J., Klingmuller K., Pozzer A., et al. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J. 2019;40(20):1590–1596. doi: 10.1093/eurheartj/ehz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo L., Zhang Y., Jiang J., et al. Short-term effects of ambient air pollution on hospitalization for respiratory disease in Taiyuan, China: a time-series analysis. Int J Environ Res Public Health. 2018;15(10) doi: 10.3390/ijerph15102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo H., Chang Z., Wu J., Li W. Air pollution and lung cancer incidence in China: who are faced with a greater effect? Environ Int. 2019;132 doi: 10.1016/j.envint.2019.105077. [DOI] [PubMed] [Google Scholar]
- 7.Schikowski T., Altug H. The role of air pollution in cognitive impairment and decline. Neurochem Int. 2020;136 doi: 10.1016/j.neuint.2020.104708. [DOI] [PubMed] [Google Scholar]
- 8.Prada D., Zhong J., Colicino E., et al. Association of air particulate pollution with bone loss over time and bone fracture risk: analysis of data from two independent studies. Lancet Planet Health. 2017;1(8):e337–e347. doi: 10.1016/S2542-5196(17)30136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzucchelli R., Crespi Villarias N., Perez Fernandez E., et al. Short-term association between outdoor air pollution and osteoporotic hip fracture. Osteoporos Int. 2018;29(10):2231–2241. doi: 10.1007/s00198-018-4605-7. [DOI] [PubMed] [Google Scholar]
- 10.Qiao D., Pan J., Chen G., et al. Long-term exposure to air pollution might increase prevalence of osteoporosis in Chinese rural population. Environ Res. 2020;183 doi: 10.1016/j.envres.2020.109264. [DOI] [PubMed] [Google Scholar]
- 11.Ranzani O.T., Mila C., Kulkarni B., Kinra S., Tonne C. Association of ambient and household air pollution with bone mineral content among adults in peri-urban south India. JAMA Netw Open. 2020;3(1):e1918504. doi: 10.1001/jamanetworkopen.2019.18504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y.H., Wang C.F., Chiu H., et al. Air pollutants interaction and gender difference on bone mineral density T-score in Taiwanese adults. Int J Environ Res Public Health. 2020;17(24):9165. doi: 10.3390/ijerph17249165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh T.K., Song I.A. Exposure to air pollution and risk of hip fracture: a population-based cohort study with a 6-year follow-up in South Korea. J Occup Environ Med. 2020;62(12):1034–1039. doi: 10.1097/JOM.0000000000002041. [DOI] [PubMed] [Google Scholar]
- 14.Sung J.H., Kim K., Cho Y., et al. Association of air pollution with osteoporotic fracture risk among women over 50 years of age. J Bone Miner Metab. 2020;38(6):839–847. doi: 10.1007/s00774-020-01117-x. [DOI] [PubMed] [Google Scholar]
- 15.Kheirouri S., Alizadeh M., Abad R.M.S., Barkabi-Zanjani S., Mesgari-Abbasi M. Effects of sulfur dioxide, ozone, and ambient air pollution on bone metabolism related biochemical parameters in a rat model. Environ Anal Health Toxicol. 2020;35(4) doi: 10.5620/eaht.2020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang F., Zhou F., Liu H., et al. Long-term exposure to air pollution might decrease bone mineral density T-score and increase the prevalence of osteoporosis in Hubei province: evidence from China Osteoporosis Prevalence Study. Osteoporos Int. 2022;33:2357. doi: 10.1007/s00198-022-06488-7. [DOI] [PubMed] [Google Scholar]
- 17.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative study group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 18.Whitsel E.A., Quibrera P.M., Smith R.L., et al. Accuracy of commercial geocoding: assessment and implications. Epidemiol Perspect Innov. 2006;3:8. doi: 10.1186/1742-5573-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitsel E.A., Rose K.M., Wood J.L., Henley A.C., Liao D., Heiss G. Accuracy and repeatability of commercial geocoding. Am J Epidemiol. 2004;160(10):1023–1029. doi: 10.1093/aje/kwh310. [DOI] [PubMed] [Google Scholar]
- 20.Liao D., Peuquet D.J., Duan Y., et al. GIS approaches for the estimation of residential-level ambient PM concentrations. Environ Health Perspect. 2006;114(9):1374–1380. doi: 10.1289/ehp.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cressie N. 1993. Spatial Prediction and Kriging. [Google Scholar]
- 22.Jian X., Yu Y.S. Semivariogram modeling by weighted least squares. Comput Geosci. 1996;22:387–397. [Google Scholar]
- 23.Yanosky J.D., Paciorek C.J., Laden F., et al. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health. 2014;13:63. doi: 10.1186/1476-069X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holliday K.M., Gondalia R., Baldassari A., et al. Gaseous air pollutants and DNA methylation in a methylome-wide association study of an ethnically and environmentally diverse population of U.S. adults. Environ Res. 2022;212(Pt C) doi: 10.1016/j.envres.2022.113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S.Y., Olives C., Sheppard L., et al. Historical prediction modeling approach for estimating long-term concentrations of PM2.5 in cohort studies before the 1999 implementation of widespread monitoring. Environ Health Perspect. 2017;125(1):38–46. doi: 10.1289/EHP131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson R.D., LaCroix A.Z., Cauley J.A., McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 Suppl):S98–S106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 27.Jackson R.D., LaCroix A.Z., Gass M., et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 28.Sterne J.A., White I.R., Carlin J.B., et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood A.M., White I.R., Royston P. How should variable selection be performed with multiply imputed data? Stat Med. 2008;27(17):3227–3246. doi: 10.1002/sim.3177. [DOI] [PubMed] [Google Scholar]
- 30.Agency USEP . Vol. 2022. 2022. Hazardous air pollutants: sources and exposure. [Google Scholar]
- 31.Diez Roux A.V., Merkin S.S., Arnett D., et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 32.Bobb J.F., Valeri L., Claus Henn B., et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. doi: 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valeri L., Mazumdar M.M., Bobb J.F., et al. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20–40 months of age: evidence from rural Bangladesh. Environ Health Perspect. 2017;125(6) doi: 10.1289/EHP614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adami G., Cattani G., Rossini M., et al. Association between exposure to fine particulate matter and osteoporosis: a population-based cohort study. Osteoporos Int. 2022;33(1):169–176. doi: 10.1007/s00198-021-06060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y., Li R., Cai M., et al. Ambient air pollution, bone mineral density and osteoporosis: results from a national population-based cohort study. Chemosphere. 2023;310 doi: 10.1016/j.chemosphere.2022.136871. [DOI] [PubMed] [Google Scholar]
- 36.Shin J., Kweon H.J., Kwon K.J., Han S.H. Incidence of osteoporosis and ambient air pollution in South Korea: a population-based retrospective cohort study. BMC Public Health. 2021;21(1):1794. doi: 10.1186/s12889-021-11866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pouilles J.M., Tremollieres F., Ribot C. Variability of vertebral and femoral postmenopausal bone loss: a longitudinal study. Osteoporos Int. 1996;6(4):320–324. doi: 10.1007/BF01623392. [DOI] [PubMed] [Google Scholar]
- 38.International Society for Clinical Densitometry . 2019. 2019 ISCD Official Positions.https://iscd.org/learn/official-positions/adult-positions/ [Google Scholar]
- 39.Tripathi R., Gupta R., Sahu M., et al. Free radical biology in neurological manifestations: mechanisms to therapeutics interventions. Environ Sci Pollut Res Int. 2021;29:62160. doi: 10.1007/s11356-021-16693-2. [DOI] [PubMed] [Google Scholar]
- 40.Filomeni G., De Zio D., Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manisalidis I., Stavropoulou E., Stavropoulos A., Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.U.S. EPA . U.S. Environmental Protection Agency; Washington, DC: 2016. Integrated science assessment (ISA) for oxides of nitrogen – health criteria (Final Report, Jan 2016)https://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=310879 EPA/600/R-15/068. [Google Scholar]
- 43.Gillespie-Bennett J., Pierse N., Wickens K., et al. The respiratory health effects of nitrogen dioxide in children with asthma. Eur Respir J. 2011;38(2):303–309. doi: 10.1183/09031936.00115409. [DOI] [PubMed] [Google Scholar]
- 44.Eckel S.P., Cockburn M., Shu Y.H., et al. Air pollution affects lung cancer survival. Thorax. 2016;71(10):891–898. doi: 10.1136/thoraxjnl-2015-207927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collart P., Dubourg D., Leveque A., Sierra N.B., Coppieters Y. Short-term effects of nitrogen dioxide on hospital admissions for cardiovascular disease in Wallonia, Belgium. Int J Cardiol. 2018;255:231–236. doi: 10.1016/j.ijcard.2017.12.058. [DOI] [PubMed] [Google Scholar]
- 46.Garshol B.F., Aamodt G., Madsen C., Vatn M.H., Bengtson M.B. The effect of nitrogen dioxide on low birth weight in women with inflammatory bowel disease: a Norwegian pregnancy cohort study (MoBa) Scand J Gastroenterol. 2020;55(3):272–278. doi: 10.1080/00365521.2020.1726446. [DOI] [PubMed] [Google Scholar]
- 47.Khomenko S., Cirach M., Pereira-Barboza E., et al. Premature mortality due to air pollution in European cities: a health impact assessment. Lancet Planet Health. 2021;5(3):e121–e134. doi: 10.1016/S2542-5196(20)30272-2. [DOI] [PubMed] [Google Scholar]
- 48.Wuebbles D., Sanyal S. Encyclopedia of biodiversity. Elsevier; 2017. Atmospheric gases. [Google Scholar]
- 49.Gu J., Shi Y., Zhu Y., et al. Ambient air pollution and cause-specific risk of hospital admission in China: a nationwide time-series study. PLoS Med. 2020;17(8):e1003188. doi: 10.1371/journal.pmed.1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pang K.L., Ekeuku S.O., Chin K.Y. Particulate air pollution and osteoporosis: a systematic review. Risk Manag Healthc Policy. 2021;14:2715–2732. doi: 10.2147/RMHP.S316429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H., Wu J., Wang M., et al. Impact of exposure to ambient fine particulate matter pollution on adults with knee osteoarthritis. Int J Environ Res Public Health. 2021;18(18):9644. doi: 10.3390/ijerph18189644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Araujo J.A. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual Atmos Health. 2010;4(1):79–93. doi: 10.1007/s11869-010-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Briot K., Geusens P., Em Bultink I., Lems W.F., Roux C. Inflammatory diseases and bone fragility. Osteoporos Int. 2017;28(12):3301–3314. doi: 10.1007/s00198-017-4189-7. [DOI] [PubMed] [Google Scholar]
- 54.Pope C.A., 3rd, Bhatnagar A., McCracken J.P., Abplanalp W., Conklin D.J., O'Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;119(11):1204–1214. doi: 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alfaro-Moreno E., Torres V., Miranda J., et al. Induction of IL-6 and inhibition of IL-8 secretion in the human airway cell line Calu-3 by urban particulate matter collected with a modified method of PM sampling. Environ Res. 2009;109(5):528–535. doi: 10.1016/j.envres.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 56.Calderon-Garciduenas L., Mora-Tiscareno A., Francolira M., et al. Exposure to urban air pollution and bone health in clinically healthy six-year-old children. Arh Hig Rada Toksikol. 2013;64(1):23–34. doi: 10.2478/10004-1254-64-2013-2219. [DOI] [PubMed] [Google Scholar]
- 57.van Eeden S.F., Tan W.C., Suwa T., et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am J Respir Crit Care Med. 2001;164(5):826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 58.Yang W., Omaye S.T. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674(1-2):45–54. doi: 10.1016/j.mrgentox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Solleiro-Villavicencio H., Rivas-Arancibia S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4(+)T cells in neurodegenerative diseases. Front Cell Neurosci. 2018;12:114. doi: 10.3389/fncel.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang Q., Hu D., Wang X., et al. The modification of indoor PM2.5 exposure to chronic obstructive pulmonary disease in Chinese elderly people: a meet-in-metabolite analysis. Environ Int. 2018;121(Pt 2):1243–1252. doi: 10.1016/j.envint.2018.10.046. [DOI] [PubMed] [Google Scholar]
- 61.You Y.S., Lin C.Y., Liang H.J., et al. Association between the metabolome and low bone mineral density in Taiwanese women determined by (1)H NMR spectroscopy. J Bone Miner Res. 2014;29(1):212–222. doi: 10.1002/jbmr.2018. [DOI] [PubMed] [Google Scholar]
- 62.Pontes T.A., Barbosa A.D., Silva R.D., Melo-Junior M.R., Silva R.O. Osteopenia-osteoporosis discrimination in postmenopausal women by 1H NMR-based metabonomics. PLoS One. 2019;14(5):e0217348. doi: 10.1371/journal.pone.0217348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forrest K.Y., Stuhldreher W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Bischoff H.A., Stahelin H.B., Dick W., et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 65.Latham N.K., Anderson C.S., Reid I.R. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J Am Geriatr Soc. 2003;51(9):1219–1226. doi: 10.1046/j.1532-5415.2003.51405.x. [DOI] [PubMed] [Google Scholar]
- 66.Bischoff-Ferrari H.A., Willett W.C., Wong J.B., Giovannucci E., Dietrich T., Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 67.Laurent M.R., Dedeyne L., Dupont J., Mellaerts B., Dejaeger M., Gielen E. Age-related bone loss and sarcopenia in men. Maturitas. 2019;122:51–56. doi: 10.1016/j.maturitas.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 68.Almeida M., Laurent M.R., Dubois V., et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97(1):135–187. doi: 10.1152/physrev.00033.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polissar A.V., Hopke P.K., Poirot R.L. Atmospheric aerosol over Vermont: chemical composition and sources. Environ Sci Technol. 2001;35(23):4604–4621. doi: 10.1021/es0105865. [DOI] [PubMed] [Google Scholar]
- 70.Gamble J.F., Lewis R.J. Health and respirable particulate (PM10) air pollution: a causal or statistical association? Environ Health Perspect. 1996;104(8):838–850. doi: 10.1289/ehp.96104838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gamble J.F. PM2.5 and mortality in long-term prospective cohort studies: cause-effect or statistical associations? Environ Health Perspect. 1998;106(9):535–549. doi: 10.1289/ehp.98106535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeger S.L., Thomas D., Dominici F., et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Armstrong B.G. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med. 1998;55(10):651–656. doi: 10.1136/oem.55.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mertens T.E. Estimating the effects of misclassification. Lancet. 1993;342(8868):418–421. doi: 10.1016/0140-6736(93)92820-j. [DOI] [PubMed] [Google Scholar]
- 75.Copeland K.T., Checkoway H., McMichael A.J., Holbrook R.H. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105(5):488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- 76.Turner M.C., Andersen Z.J., Baccarelli A., et al. Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA Cancer J Clin. 2020;70(6):460–479. doi: 10.3322/caac.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holliday K.M., Avery C.L., Poole C., et al. Estimating personal exposures from ambient air pollution measures: using meta-analysis to assess measurement error. Epidemiology. 2014;25(1):35–43. doi: 10.1097/EDE.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Avery C.L., Mills K.T., Williams R., et al. Estimating error in using ambient PM2.5 concentrations as proxies for personal exposures: a review. Epidemiology. 2010;21(2):215–223. doi: 10.1097/EDE.0b013e3181cb41f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Avery C.L., Mills K.T., Williams R., et al. Estimating error in using residential outdoor PM2.5 concentrations as proxies for personal exposures: a meta-analysis. Environ Health Perspect. 2010;118(5):673–678. doi: 10.1289/ehp.0901158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kiyota Y., Muramatsu H., Sato Y., et al. Smoking cessation increases levels of osteocalcin and uncarboxylated osteocalcin in human sera. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-73789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.