Abstract
Background
Doxorubicin resistance represents a major clinical challenge for treating patients with advanced breast cancer (BC). Exosomes, exchanging genetic cargo between heterogeneous populations of tumour cells, have been proposed to mediate drug resistance and cancer progression in other cancer types. However, their specific role in mediating doxorubicin resistance in BC remains unclear. Here, we demonstrate the important role of exosomal miR-181b-5p (exo-miR-181b-5p) in mediating doxorubicin resistance.
Methods
Small-RNA sequencing and bioinformatic analyses were used to screen miRNAs mediating doxorubicin resistance in BC, which were further verified by RT-qPCR. SA-β-gal staining assays allowed us to measure cellular senescence. Exosomes from patients’ serum before and after neoadjuvant chemotherapy were isolated for exo-miR-181b-5p quantification.
Results
Doxorubicin-resistant BC cell lines exhibited upregulated exosomal miR-181b-5p. Addition of exo-miR-181b-5p actively fused with recipient cells and transferred a drug-resistant phenotype. Overexpression of miR-181b-5p downregulated p53/p21 levels and inhibited doxorubicin-induced G1 arrest and senescence by suppressing BCLAF1 expression in vitro. Further, in vivo experiments showed treatment of exo-miR-181b-5p inhibitors exhibited superior tumour control and reversed the doxorubicin-resistance phenotype, accompanied with increased tumoral BCLAF1.
Conclusion
Our data suggests exo-miR-181b-5p as a prognostic biomarker and a therapeutic potential for exo-miR-181b-5p inhibitors in the treatment of doxorubicin-resistant BC patients.
Subject terms: Breast cancer, Chemotherapy
Background
Despite advances in early diagnosis, breast cancer is amongst the most common cancer types, accounting for the highest morbidity and mortality in women worldwide [1]. Neoadjuvant chemotherapy (NAC) is the standard clinical approach to reduce tumour burden and is essential for successful breast-conserving surgery [2]. However, accumulating studies emphasised the fact that many cancer patients who received NAC suffer from chemotherapy resistance, which leads to increased surgical difficulty, poor postoperative long-term survival benefit and ultimately treatment failure [3]. Although a subset of patients gain considerable benefits from NAC, a large proportion suffer from the side effects caused by overtreatment [4]. Despite the relevance of chemotherapy resistance, the mechanisms behind it are unclear, and novel clinical strategies to cope with resistance in breast cancer have not been elucidated. To date, no reliable biomarkers exist to predict the efficacy of NAC to provide a guidance for clinical treatment regimen [5]. Thus, identifying subclasses of patients to increase the responsive rate and avoid unnecessary overtreatment is imperative.
Exosomes are membranous vesicles with a diameter of 30–150 nm, which can be secreted by most human cell types and carry molecular cargo that mediates inter-cellular signalling. Exosomes carry nucleic acids (such as DNA, mRNA, long and short non-coding RNAs), proteins (such as cytoskeletal proteins, heat shock proteins and tyrosine kinase receptors), enzymes (GAPDH, ATPase, pgk1) and other substances [6]. MicroRNAs (miRNAs) can be carried by exosomes, and are defined as a group of small non-coding RNAs (18–22 nucleotides) that regulate transcription and translation by binding to the 3’-UTR regions of target gene mRNAs [7–9], and have been reported to play important roles in chemotherapy resistance. For example, downregulation of miR-505, miR-145 and miR-128 have been linked to doxorubicin resistance in breast cancer, while up-regulation of these miRNAs reversed this phenotype [10]. Owing to their high stability when encapsulated by lipid bilayers, miRNAs carried by exosomes in serum exhibit high clinical potential as a diagnostic tool for prediction of response to neoadjuvant chemotherapy [11]. A particular miRNA, miR-181b-5p is reportedly increased in inflammatory breast cancer (IBC) and triple-negative breast cancer (TNBC) [12, 13], which correlates with poor prognosis [14]. consequently, miR-181b-5p encapsulated in small extracellular vesicles has been proposed as a candidate biomarker in IBC [15]. However, the relationship between miR-181b-5p and NAC, or whether transferred miR-181b-5p plays any mechanistic role in doxorubicin resistance is currently unknown.
In this study, we investigated the molecular mechanism and clinical potential of exosomal miRNA in doxorubicin resistance by using in vitro and in vivo breast models. We show that miR-181b-5p is upregulated in doxorubicin-resistant breast cancer cells and is encapsulated into exosomes. The transfer of exosomal miR-181b-5p (exo-miR-181b-5p) from resistant cells in turn, binds to 3’-UTR regions of the target gene Bcl-2-associated transcription factor 1 (BCLAF1) decreasing its expression in recipient cells. Further analyses demonstrated downregulated BCLAF1 decreased p53/p21 activity and suppressed senescence upon chemotherapy, resulting in increased chemotherapy resistance. These findings were supported by the evidence that addition of exo-miR-181b-5p inhibitors, abolished doxorubicin resistance and helped to decrease tumour volumes in mice. In breast cancer patients, serum levels of exo-miR-181b-5p were also closely associated with sensitivity to doxorubicin. This work suggests that exo-miR-181b-5p holds great potential as a predictive biomarker in doxorubicin resistance and could contribute to identify subsets of patients that would potentially respond to NAC with doxorubicin.
Methods
Clinical specimens
Twenty-four pairs of blood samples were collected from breast cancer patients before and after neoadjuvant chemotherapy in Tianjin Medical University Cancer Institute. The neoadjuvant chemotherapy regimen was formulated in strict accordance with clinical guidelines and all included doxorubicin (DOX). All patients signed the patient’s informed consent forms, and the experiments were approved by the Medical Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (Ek2022205). The clinical efficacy of chemotherapy was evaluated according to RECIST (Response Evaluation Criteria In Solid Tumours). The clinical characteristics are presented in Table S1.
Cells and drugs
Human breast cancer cell line MCF-7 and its doxorubicin-resistant derivative MCF‐7/A02, provided by Prof. Dongsheng Xiong (Institute of Hematology, PUMC, Tianjin, China), were cultured as previously reported [16]. Human breast cancer cell line Cal51 and its doxorubicin-resistant derivative CALDOX were gifts from Dr. Ernesto Yague (Imperial College London, UK) and were cultured as previously described [16]. Doxorubicin (DOX) and GW4869 were purchased from Sigma, USA. All the cell lines were tested for mycoplasma contamination using the Mycoplasma Detection Kit (Cat# LT37-701, Lonza, Hayward, CA, USA). They were tested to be free of Mycoplasma and authenticated by short tandem repeat profiling in this study.
Conditioned medium and CCK-8 cell cytotoxicity assays
Cell Counting Kit-8 (CCK-8) assay (MYBiotech, China) was used to analyse cell cytotoxicity. CALDOX and MCF-7/A02 cells were cultured in 8 ml exosome-free medium for 48 h in 10 cm dishes and supernatants (conditioned medium, CM) were collected. MCF-7 or Cal51 cells were cultured for 48 h with CM or exosomes isolated from CM as follows: CM obtained from MCF-7 cells (CM-M7) or exosomes isolated from CM-M7 (exo-MCF-7); CM from MCF-7/A02 cells (CM-MA) or exosomes from CM-MA (exo-MCF-7/A02); CM from Cal51 cells (CM-51) or exosomes from CM-51 (exo-Cal51); or CM from CALDOX cells (CM-CD) or exosomes from CM-CD (exo-CALDOX). After 48 h of exposure to CM or exosomes, cells were washed, detached and 5000 cells were reseeded into 96-well plates. After cell attachment (overnight) cells were treated with DOX at different doses for 48 h. 1:10 volume ratio of CCK-8 solution was added to each well and the plates were incubated (37 °C, 4 h) for detection. The optical density (OD) was measured at 450 nm, and the 50% maximum inhibitory concentration (IC50) of the drug was calculated from the OD value.
EdU staining
Cells were treated with CM or exosomes as described above, then were reseeded into 6-well plates and treated with DOX (250 nM for MCF-7 and 40 nM for Cal51) for 48 h, cells were seeded in 24-well plate with the density of 1 × 104–2 × 104 cells per well. The EDU assay was performed by Cell-Lighetm EDU Apollo488 In Vitro Kit (RiboBio, China) according to the manufacturer’s protocol.
Annexin V staining
Cell apoptosis was measured by flow cytometry using an annexin V-FITC apoptosis detection kit (Becton–Dickinson, USA) according to the manufacturer’s protocol.
Cell cycle analysis
Cells were centrifuged at 1200 rpm for 10 min and washed with cold PBS three times. Ethanol 70% was used to immobilise cells for 4 h, cells were then washed twice with cold PBS, and RNA was digested with RNase (10 mg/ml). Propidium iodide (15 μl) staining solution (1 mg/ml) was added to each sample and cell cycle was analysed by flow cytometry.
SA-β-gal staining
SA-β-gal staining solution was made fresh before each use and incubated with cells overnight at 37 °C. Cells were then washed with ice-cold PBS and fixed with 4% formaldehyde at room temperature for 10 min. The % of SA-β-gal cells was quantified using ImageJ 1.8.0.
Plasmids, oligonucleotides and cell transfection
Mimics, inhibitors and negative controls of miR-181b-5p were purchased from GenePharma, China. The overexpression and negative control plasmids of BCLAF1 were purchased from Yixiang, China. BCLAF1 siRNA and scramble siRNA were purchased from Ribobio, China. Their sequences are shown in Table S2. Transient transfection of RNA and DNA was performed using Lipofectamine 3000 (Thermo Fisher, USA) according to the manufacturer’s protocol. Briefly, cells (2 × 105) were seeded on 6-well plates one day before transfection with 25 pmol mimics/inhibitors RNAs or 2 μg plasmid DNA using 7.5 μl Lipofectamine.
Exosome purification and validation
Exosomes from serum were isolated using an exosome isolation kit (Thermo Fisher, USA). Exosomes from CM were purified by ultracentrifugation. Briefly, CALDOX or MCF-7/A02 cells were incubated in medium supplemented with 10% exosome-free fetal bovine serum (FBS, Thermo Fisher, USA) for 48 h. The supernatant was centrifuged at 2000 × g for 70 min and then 10,000 × g for 20 min to remove cell debris, apoptotic vesicles and large extracellular vesicles. Exosomes were obtained via 110,000 × g, 70 min ultracentrifugation, and suspended in PBS. Exosome characterisation was performed optically by electron microscopy and by nanoparticle tracking analysis (NTA), and biochemically by western blot against exosomal markers. For NTA, exosomes suspended in PBS at a protein concentration of 5 mg/ml were further diluted 100- to 500-fold and manually injected into the sample loading chamber at room temperature. The number and size of exosomes were directly tracked using a Nanosight NS 300 system (Malvern Technology, UK). Data were analysed using the NTA analytical software (version 2.3).
Exosome labelling with PKH26 staining and transfer to target cells
PKH26 Red Fluorescent Cell Linker Kits (Sigma, US) was used for lipid bilayer labelling. Exosomes were first resuspended in diluent C (100 ml). A dye solution was prepared by adding PKH26 (0.4 ml) ethanolic dye solution to diluent C (100 ml). The exosome suspension was then mixed with the dye solution. After incubating the cell/dye suspension for 5 min with periodic mixing, staining was stopped by adding serum (200 ml) and incubating for 1 min. Stained exosomes were washed twice with PBS and resuspended in a fresh sterile tube. Finally, moderate amounts of stained exosomes were added into cell medium to be co-incubated for 4 h before standard confocal imaging.
RNA sequencing
Illumina sequencing analysis of exosomal miRNAs was performed by Novgene using RNAs extracted from equal quantities of exosomes (quantified by NTA) collected from Cal51, CALDOX, MCF-7 and MCF-7/A02 conditional media according to manufacturer’s protocol. All small RNAs comprising 15–52 nucleotides were selected and sequenced using the HiSeq™2500/MiSeq system (Illumina). Original counts were standardised using the pruned mean mol/l value method, and the results were analysed using the Bioconductor package “edgeR” to identify the differentially expressed miRNAs between exo-Cal51 and exo-CALDOX or between exo-MCF-7 and MCF-7/A02.
RNA extraction and quantitative RT-qPCR
RNA isolation, reverse transcription, and real-time qPCR were performed as previously described [16]. miRNA was extracted using the miRNeasy Mini Kit (Qiagen, USA) for cell line-derived samples and the miRNeasy Serum/Plasma Kit for patients-derived samples (Qiagen, USA). U6 small nuclear RNA was used as an internal control. To standardise exosome-miRNAs, syngeneic cel-miR-39-3p (Qiagen, USA) was added at a final concentration of 20 fmol/μl during RNA extraction, and equal volumes of samples were extracted at each step; all following data was normalised to the level of this spiked control. The primer sequences used in RT-qPCR are as follows:
5′-TGCCTTCCAACTCTCGTCTT-3′ (CYLD, sense),
5′- AATCCGCTCTTCCCAGTAG CGG-3′ (CYLD, anti-sense),
5′-TCTGGAATAGAAGGCACTCTAGG-3′ (BCLAF1, sense),
5′-ACCCTCGTCTTTTAGAAACAGGA-3′ (BCLAF1, anti-sense),
5′-GGAGACTTGCCTGGTGAAAA-3′ (IL6, sense),
5′-GTCAGGGGTGGTTATTGCAT-3′ (IL6, anti-sense),
5′-TTGGCAGCCTTCCTGATTTC-3′ (IL8, sense),
5′-AACTTCTCCACAACCCTCTGCA-3′ (IL8, anti-sense),
5′-CAGCACATGACGGAGGTTGT-3′ (p53, sense),
5′-TCATCCAAATACTCCACACGC-3′ (p53, anti-sense),
5′-TGTCCGTCAGAACCCATGC-3′ (p21, sense),
5′-AAAGTCGAAGTTCCATCGCTC-3′ (p21, anti-sense),
5′- AGATCCCAACGCCCTGAAC-3′ (p15, sense),
5′- CCCATCATCATGACCTGGATT-3′ (p15, anti-sense),
5′-TGTTCGTCATGGGTGTGAAC-3′ (GAPDH, sense),
5′-ATGGCATGGACTGTGGTCAT-3′ (GAPDH, anti-sense),
5′-CTCGCTTCGGCAGCACA-3′ (U6, anti-sense),
5′-AACGCTTCACGAATTTGCGT-3′ (U6, anti-sense),
CCCACCGACAGCAATGAATGT(Has-miR-181b-5p),
TCACCGGGTGTAAATCAGCTTG (cel-miR-39-3p).
Western blotting
Cells and tissues were lysed in RIPA buffer (R0020; Solarbio), and proteins were separated by SDS-PAGE and transferred to polyvinyldifluoride membranes (Millipore, USA). After blocking with 5% nonfat milk for 1 h, the membranes were incubated overnight at 4 °C with cyclin D1 (1:1000, Yt1174), p21 (1:1000, YN5499), p53 (1:1000, YT3528), CD81 (1:1000, YT5394), BCLAF1 (1:1000, YT0542) (all from Immunoway, USA), TSG101 (1:1000, ab83, Abcam, UK) and β-actin (1:1000, sc47778, Santa Cruz Biotechnology, USA). The blots were then washed and incubated with peroxidase-conjugated secondary antibody (Cell Signalling Technology, USA) for 2 h at room temperature, washed 3 times and imaged using a chemiluminescence kit (Millipore, USA). All western blot images shown are representative of three independent replicates.
The miRNA target prediction and luciferase reporter assay
The miRNA target was predicted using TargetScan (http://www.targetscan.org/vert_72/), PicTar (https://pictar.mdc-berlin.de/) and miRanda (http://miranda.org.uk/). The PCR-amplified fragment containing the 3’-UTR of human BCLAF1 was digested with XbaI and NheI and inserted into pmirGLO reporter vector (Promega, USA). One-hundred nanograms of firefly plasmid, 1 ng Renilla plasmid and 100 ng mimics, inhibitors, or NTC RNAs were transfected to 5 × 104 293T cells as described in the transfection methods section. Luciferase activity was measured after 48 h using the Luciferase Reporter Assay Kit (Promega, USA).
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed using a Magna ChIP A/G Kit (Millipore, USA) in accordance with the manufacturer’s instructions. In brief, cells were first cross-linked with 1% formaldehyde. After quenched by glycine, cells were scraped from culture plate and subjected to nuclear lysis to release cross-linked protein/DNAs. Following sonication, the nuclear extract was then subjected to immunoprecipitation with anti-BCLAF1 antibody (Santa Cruz Technology, sc-101388) or control rabbit IgG (Santa Cruz Technology, sc-2027). Precipitated DNA fragments containing TP53 promotor (from −157 to +110) were quantitated by PCR using the following oligonucleotide pairs: 5′-AACTCCATTTCCTTTGCTTCCTCCGGCAGG-3′ and 5′-CAATCCAGGGAAGCGTGTCACCGTCGT-3′ under the following conditions: 1 cycle at 94 °C for 5 min, 35 cycles at 94 °C for 30 s, 56 °C for 30 s and 72 °C for 1 min and a final cycle at 72 °C for 5 min.
Tumour xenograft
In order to study whether exosomal miR-181b-5p is associated with resistance to doxorubicin in vivo, tumour xenografts were established in nude mice by injecting 2 × 106 Cal51 or CALDOX cells into the mammary fat pad (#4) of 5 mice per condition. After 14 days, serum was collected, and exosomes were isolated to measure expression levels of exosomal miR-181b-5p.
To investigate the combined effects of DOX and exosomal miR-181b-5p inhibitors, inhibitors of miR-181b-5p and negative controls (NC) were transfected into 293T cells. 293T cells were then incubated for 48 h in medium without exosomes. Exosomes from CM were purified and suspended in PBS. Tumour xenografts were established in nude mice by injecting 2 × 106 CALDOX cells as mentioned above, using 15 mice in total. Tumour sizes were measured with a caliper every 3 days, and tumour volumes were calculated using the formula: tumour volume (mm3) = 0.5 × ab2 (a and b are the longest and shortest tumour diameters, respectively). When tumour sizes reached 50 mm3 (Day 14), mice were randomly divided into three groups for treatment. The groups PBS, exo-inhibitors NC and exo-inhibitors miR-181b-5p received s.c. injections around the tumours every 2 days for a total of 12 days. From the same starting time, DOX was injected intraperitoneally every 4 days for a total of 12 days at the same time. Tumours were collected 2 days after the last treatment. The process of drug administration was performed in a double-blinded manner. All animal experiments were approved by the Tianjin Medical University Cancer Institute and Hospital Laboratory Animal Care and Use Committee (NSFC-AE-2022259). Five-week-old female BALB/c nude mice (~19–20 g) were purchased from SPF (Beijing) Biotechnology Co., Ltd. and kept under specific pathogen-free conditions. Mice experiments were performed after 1 week of settling.
Immunohistochemistry (IHC)
Tumour tissues were fixed with 4% paraformaldehyde, embedded in paraffin and stained with anti-BCLAF1 antibody (1:100, YT0542), anti-p53 antibody (1:100, YT3528) and anti-p21 antibody (1:100, YN5499), all purchased from Immunoway, USA.
Statistical analysis
The Cancer Genome Atlas (TCGA) (https://www.genome.gov/Funded-Programs-Projects/Cancer-Genome-Atlas) was used to analyse gene expression and clinical data.
All quantitative data are presented as mean ± standard deviation (SD) and were analysed using GraphPad Prism 7.0. The means of the two datasets were compared using paired t-tests. One-way ANOVA was used to evaluate multiple independent groups. The nonparametric Wilcoxon test was used to compare breast cancer samples before and after neoadjuvant chemotherapy. P-value < 0.05 was considered as statistically significant.
Results
Conditioned medium from doxorubicin-resistant cells causes senescence-mediated doxorubicin resistance in drug-sensitive cells
DOX-resistant breast cancer cells were established by treatment with escalating DOX concentrations in vitro (Fig. S1A). Compared to parental cells, MCF-7/A02 and CALDOX cells exhibited resistance to DOX, with significantly higher IC50 (Fig. S1B). After culture with conditioned medium from DOX-resistant cell lines (CMCD or CMMA), the IC50 of DOX-sensitive cells increased significantly, indicating acquired DOX-resistant capabilities (Fig. 1a). To evaluate further the capabilities of CMCD or CMMA to transfer drug resistance, we performed EdU assays after 48 h of incubation of DOX-sensitive cells with CM (drug concentrations and time points were determined empirically in a series of preliminary experiments). These experiments allowed us to assess proliferation in the presence of toxic drug concentrations. CMCD or CMMA increased the percentage of proliferative cells in the presence of DOX by 2–3-fold compared to conditional medium from normal DOX-sensitive cells (CM51 or CMM7) (Fig. 1b, c). Cell cycle analysis showed that CMCD or CMMA significantly decreased the population of cells in G0/G1 (Fig. 1d, e). Cyclin D1 is a key regulator of the G1 to S transition via allosteric modulation of the cyclin-dependent kinases (CDK) CDK4 and CDK6 [17], therefore we assessed Cyclin D1 expression levels by western blotting. In agreement with the previous data, exposure of sensitive cells to CMCD or CMMA prior to DOX treatment led to decreased Cyclin D1 expression (Fig. 1f), indicating that CM from resistant cells, transfers a phenotype that decreases cell cycle progression. Next, we assessed whether drug resistance caused by CMCD or CMMA on drug-sensitive cells was additionally mediated by apoptosis blockade by means of Annexin V staining. Treatment of sensitive cells with CMCD or CMMA led to modestly decreased apoptosis after DOX compared to treatment with normal media CM51 and CMM7 (Fig. 1g), suggesting that inhibition of apoptosis was not a mediator of transferred drug resistance.
Fig. 1. Conditioned medium from DOX-resistant cells transfers a senescence-mediated DOX-resistant phenotype in breast cancer cells.
Conditioned medium (CM) collected from DOX-sensitive or DOX-resistant cells was added to DOX-sensitive cells for 48 h, before treatment with DOX. a Effects on cell viability were detected by CCK-8 assay as described in materials and methods. EdU incorporation assays were performed to quantify (b) and visualise (c) cell proliferation. d Cell cycle analysis of sensitive cells was conducted by flow cytometry. Representative plots per condition are shown (average in red, black lines show auto-scaled individual phases). e Quantitative analysis of cell cycle data from panel d. f Cyclin D1 analysis by western blot. g Apoptotic analysis by flow cytometry conducted using Annexin V/PI staining as indicated in methods. Representative dot-plots (left) and quantification summary (right) are shown. h DOX-sensitive cells were stained for SA-β-gal (representative images). i The percentages of cells positive for SA-β-gal from panel h were quantified. j The level of senescence relative proteins was assessed by western blot. *p < 0.05; **p < 0.01; ***p < 0.001. Numerical data represent mean ± S.D. based on three independent experiments, and the immunoblots are representative of three replicates.
Accumulating evidence supports that cellular senescence is a physiologically important tumour suppressor process, which can be triggered by multiple chemotherapeutic agents to mediate tumour control [18]. In agreement with previous reports, we observed a strikingly altered cellular morphology following DOX treatment. In response to DOX, cells became enlarged and with decreased nucleus/cytoplasm ratio, suggesting cellular senescence. Thus, to confirm this observation, we stained cells with SA-β-gal, a well-established senescence marker [19]. CM from DOX-resistant cells decreased SA-β-gal staining in the presence of DOX by 40–70% (Fig. 1h, i). A key feature of cellular senescence is a stable cell cycle arrest, normally mediated by the p53 and p21 tumour suppressor pathways [20, 21]. Senescent cells also exhibit other features such as increased secretion of multiple cytokines including interleukin-6 (IL-6) and interleukin-8 (IL-8), a process known as senescence-associated secretory phenotype (SASP) [22]. Consistent with the decreased senescence phenotype observed in CMCD- or CMMA-incubated cells, we found that exposure of sensitive cells to these DOX-resistant CMs caused a significant decrease in the protein levels of p53 and p21, as well as the mRNA levels of SASP genes IL6, IL8 and also in p53, p21 and p15 in Cal51 and MCF-7 cells (Figs. 1j and S1C). Together, these results indicate that the DOX-resistant phenotype transferred by CMCD or CMMA is caused by a combination of inhibition of cellular senescence.
Exosomes in conditioned medium mediate senescence-associated drug resistance
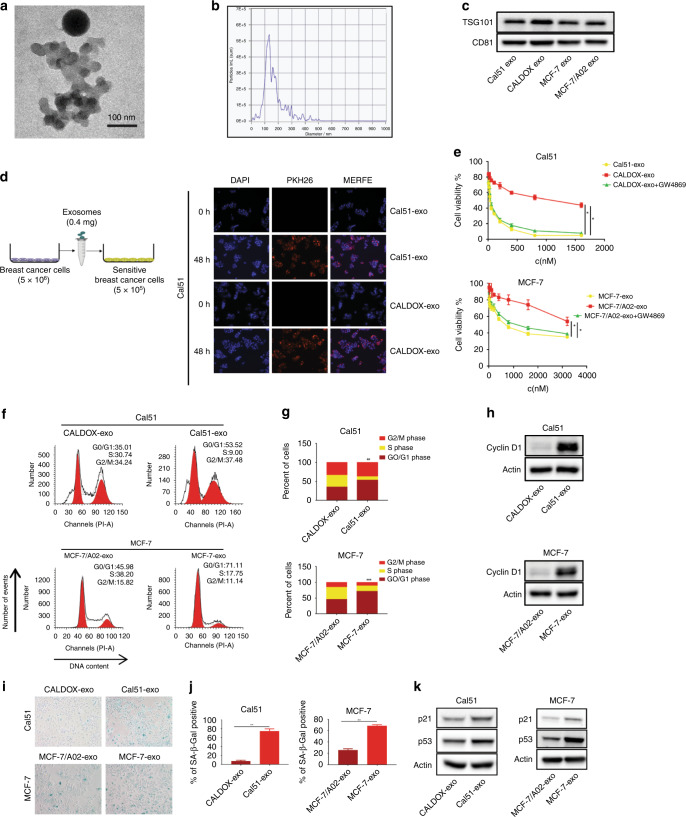
Reportedly, exosome-mediated cell communication is a novel mechanism underlying drug resistance [23–27]. We hypothesised that exosomes were involved in the CM-induced drug resistance transfer that we observed in BC cell lines. To verify this, we firstly isolated exosomes from the CM by ultracentrifuge method. The vesicles exhibited the classic cup-shaped form of exosomes, with a diameter ranging from 30 to 150 nm observed by transmission electron microscope (Fig. 2a). We also conducted state-of-the-art nanoparticle particle tracking analysis (NTA) using a Nanosight system, and observed that the modal size of the vesicles was 100 nm with minimal contamination of larger vesicles (Fig. 2b). We further characterised our extracellular vesicles biochemically using western blot, and detected the hallmark exosomal markers CD81 and TSG101 (Fig. 2c). These results indicate that the vesicles isolated from CM display typical characteristics of exosomes.
Fig. 2. Exosomes derived from DOX-resistant cells transfer a DOX-resistant phenotype consistent with inhibition of DOX-induced cellular senescence.
Characterisation of exosomes. a Transmission electron microscope representative image of exosomes isolated from CM of DOX-resistant cells (scale bar, 100 nm). b Nanoparticle tracking analysis (NTA) size profile of exosomes isolated from CM of DOX-resistant cells, showing the predominant size of exosomes between 50–150 nm. c Biochemical characterisation of exosomes via western blot of CD81 and TSG101. d Left panel: schematic description of the experimental design of exosomal transfer. Exosomes from breast cancer cells were isolated and 0.4 mg exosomes were used to culture with 5 × 105 sensitive cells. Right panel: confocal microscopy images showing internalisation of fluorescently labelled exosomes (PKH26) in breast cancer cells after 48 h of incubation. e A CCK-8 assay was conducted to assess cell viability in sensitive breast cancer cells treated with exosomes derived from CM of DOX-resistant cells (CALDOX-exo and MCF-7/A02-exo), or from cells pre-treated with exosome-release inhibitor GW4869 (10 µM). Doxorubicin-sensitive breast cancer cells were incubated with exosomes for 48 h and the cell viability curves are shown as indicated. f Cell cycle analysis of sensitive cells was conducted by flow cytometry. Representative plots per condition are shown (average in red, black lines show auto-scaled individual phases). g Quantitative analysis of cell cycle data from panel f. h Cyclin D1 analysis by western blot with treatments indicated. i Sensitive cells were stained for SA-β-gal. The percentages of cells positive for SA-β-gal were quantified as shown in j. k The level of senescence related proteins p53/p21 was detected by western blot. *p < 0.05; **p < 0.01; ***p < 0.001. Numerical data represent mean ± S.D. based on three independent experiments, and the immunoblots are representative of three replicates.
To visualise exosome transfer, we labelled exosomes from CM with the fluorescent tracer PKH26. After incubation with labelled exosomes, we observed strong PKH26 fluorescence in the cytoplasm of recipient cells via confocal microscopy, suggesting that secreted exosomes were successfully incorporated by recipient cells (Figs. 2d and S2A). To determine whether DOX-resistance transmission is mediated by exosomes, we used GW4869 to block exosome secretion by drug-resistant cells. When co-cultured with exosomes isolated from CMCD or CMMA (CALDOX-exo or MCF/A02-exo), wild type cell lines presented an increased viability in the presence of varies doses of DOX compared to co-culture with exosome from wild type cell lines (Cal51-exo or MCF-7-exo), but remarkably this effect was reversed after blocking exosome secretion with GW4869 from DOX-resistant cells (Fig. 2e). Additionally, cell cycle arrest and Cyclin D1 expression showed similar trends compared to CMCD or CMMA, namely CALDOX-exo or MCF/A02-exo significantly decreased cell cycle arrest and Cyclin D1 expression (Fig. 2f–h), indicating that exosomes mediate DOX-resistance transfer. In line with these findings, mechanistically, cells showed lower SA-β-gal activity, lower activity of the p53 pathway and lower activity of SASP genes in the presence of DOX after 48 h incubation with CALDOX-exo or MCF/A02-exo (exosomes from CMCD or CMMA, Fig. 2i–k and S2B). Taken together, these results indicate that exosomes from CM are responsible for mediating drug resistance via transferring a cellular senescence suppressive phenotype.
MiR-181b-5p is enriched in drug-resistant cell-derived exosomes and enhances DOX resistance of recipient cells via exosome transfer
As miRNAs have been reported to mediate drug resistance, we sought to identify the key exosomal miRNAs related to DOX resistance in our setting, thus we isolated exosomes from our 4 types of CM (CMCD, CM51, CMMA and CMM7), then sequenced the miRNAs within these exosomes. The results of differential analyses showed that 5 miRNAs, including miR-181b-5p, miR-196a-5p, miR-3074-5p, miR-378a-3p and miR-22-3p, were substantially increased in exosomes from drug-resistant cells (CALDOX-exo or MCF/A02-exo) compared with exosomes from drug-sensitive cells (Cal51-exo or MCF-7-exo) (p < 0.05) (Fig. 3a and Tables S3 and S4). To validate the sequencing results, we examined the levels of miR-181b-5p, miR-196a-5p, miR-3074-5p, and miR-22-3p in exosomes, except for miR-378a-3p, which has already been confirmed to play a role in chemoresistance [28]. As the RT-qPCR cycle numbers for miR-196a-5p, miR-3074-5p and miR-22-3p were considerably higher than 40, indicating their low expression, we excluded them for further analyses and focused on miR-181b-5p, which was actually the highest miRNA ranked from the sequencing data in Fig. 3a. As shown in Fig. 3b, miR-181b-5p expression in CALDOX-exo and MCF-7/A02-exo was significantly higher than that in CM or Cal51-exo or MCF-7-exo. Additionally, we transfected sensitive cells with miR-181b-5p mimics and observed an increase in IC50 to DOX treatment, reconfirming that miR-181b-5p can mediate DOX resistance (Fig. S3A–D). Thus, we reasoned that miR-181b-5p from drug-resistant cells may confer DOX resistance to sensitive cells via exosome transfer.
Fig. 3. Identification of miR-181b-5p secreted in exosomes by DOX-resistant cells as a key mediator of DOX-resistance transfer in breast cancer cell lines; and correlates with DOX-resistance in patient samples.
a Sequencing analysis of exosomal microRNAs from exosomes isolated from DOX-resistant and DOX-sensitive breast cancer cells, data shown as volcano plots and the arrow indicates the highest score for miR-181b-5p, also shown in the left panel. qRT-PCR assay was performed to measure miR-181b-5p expression in exosomes from resistant and sensitive cells (b), miR-181b-5p expression in resistant and sensitive cells (c) and miR-181b-5p expression in sensitive cells after incubation with exosomes (d). e Inhibitors of miR-181b-5p (Inhibitors.miR-181b-5p) and negative controls (inhibitors.NC) were transiently transfected into resistant cells, and the CM was used for exosome isolation. DOX was added to sensitive and resistant cells after incubation with CM exosomes for 48 h. CCK-8 assays were conducted to quantify cell viability of cells under the conditions indicated. f Using the conditions described in panel e, cell cycle was tested through flow cytometry. g Quantitative analysis of the data shown in panel f. h Cyclin D1 levels were analysed by western blot. i Sensitive cells were stained for SA-β-gal after treatment as indicated. The percentages of cells positive for SA-β-gal were quantified and are shown in j. k The levels of senescence relative proteins were detected by western blot. l Kaplan–Meier analysis of the overall survival in patients with different miR-181b-5p expression levels (data obtained from the TCGA database), indicate correlation of poor prognosis with miR-181b-5p. m Exosomes were isolated from the serum of breast cancer patients before and after neoadjuvant chemotherapy (n = 24). Variation in serum exosomal miR-181b-5p in groups with different responses to neoadjuvant chemotherapy assessed by RECIST criteria. *p < 0.05; **p < 0.01; ***p < 0.001. Numerical data represent mean ± S.D. based on three independent experiments, and the immunoblots are representative of three replicates.
To validate this further, first we tested cellular miR-181b-5p expression and found it was more abundant in CALDOX and MCF-7/A02 cells compared to Cal51 and MCF-7 cells (Fig. 3c). Furthermore, after incubation with exosomes from CMCD or CMMA, wild type cells showed dramatically upregulated miR-181b-5p levels, suggesting high rate of uptake of miR-181b-5p by recipient cells (Fig. 3d). These data demonstrated that miR-181b-5p might be directly transferred from drug-resistant donor cells to drug-sensitive recipient cells via exosomes. To further determine whether exosomal miR-181b-5p is functionally responsible for transferring drug resistance, we transfected CALDOX and MCF-7/A02 cells with a miR-181b-5p inhibitor or a negative control (NC). After 48 h, exosomes were collected from different CMs and then used to incubate Cal51 or MCF-7 cells. As previously, CALDOX-exo or MCF-7/A02-exo incubation significantly increased cell viability of cells treated with DOX compared with Cal51-exo or MCF-7-exo. However, this phenotype was remarkably reversed by the inhibitor of miR-181b-5p (Fig. 3e). To expand this data using our whole experimental readout, we analysed cell cycle, Cyclin D1 expression levels, SA-β-gal activity and p53/p21 levels using the inhibitor of miR-181b-5p. In line with the previous results, the miR-181b-5p inhibitor reversed the decreased cell cycle arrest, cell senescence, changes in Cyclin D1, p53 and p21, as well as SASP genes caused by CALDOX-exo or MCF-7/A02-exo (Figs. 3f–k and S3E, F). Further, we transfected miR-181b-5p inhibitors into resistant cell models and observed a decrease in IC50 to DOX (Fig. S3g–j). Collectively, these results confirmed that DOX resistance is mainly mediated via exosomal transfer of miR-181b-5p.
To further extrapolate these preclinical findings with a translational angle, we analysed patient data from the Cancer Genome Atlas (TCGA) database. Importantly, the overall survival (OS) for 2102 breast cancer patients with poor prognosis correlated with higher miR-181b-5p levels (p < 0.05) (Fig. 3l). Based on this result, we collected serum samples (pre- and post-neoadjuvant chemotherapy, pre-NAC and post-NAC) from 24 patients with available clinical outcome data for further validation analysis. Remarkably, we observed that miR-181b-5p levels were increased in exosomes isolated from the serum of patients post-NAC compared to pre-NAC exosomes, and this difference was greater in the RECIST-assessed chemo-insensitive group (Progressive Disease (PD)/ Stable Disease (SD)) than in the treatment-sensitive group (Complete Response (CR)/ Partial Response (PR)) (Fig. 3m), indicating that the increased serum levels of exosomal miR-181b-5p were indeed associated with drug resistance in patients. Taken together, these results confirm a role for exosomal miR-181b-5p in the inter-cellular transfer of DOX resistance, and importantly suggest that exosomal miR-181b-5p could be used as a potential biomarker of chemoresistance in breast cancer patients during treatment.
Exosomal miR-181b-5p derived from drug-resistant cells confers doxorubicin resistance by targeting BCLAF1 in recipient cells
miR-181b has recently been linked to the pathogenesis of breast cancer via targeting CYLD [14]. However, we did not observe a regulation of CYLD upon incubation with either MCF-7/A02-exo or CALDOX-exo (Fig. S4A), suggesting that the miR-181b/CYLD axis may be more relevant in oncogenesis than treatment resistance. To elucidate further which could be the miR-181b-5p targets upon exosomal uptake, we used bioinformatic analyses, which predicted 10 targets for miR-181b-5p (Fig. 4a). Among these targets, BCLAF1 presented the highest score. Furthermore, BCLAF1 was reported to promote TP53 gene transcription through interaction with PKCδ in response to DNA damage and be involved in therapeutic drug-induced senescence in multiple cancer cells [29, 30]. To address the contribution of BCLAF1 in DOX-induced p53 activation, we treated scramble and siBCLAF1 breast cancer cells with DOX and observed that DOX-induced p53 was decreased when knocking-down BCLAF1 (Figure S4B–D). In concert with previous study [29], ChIP-PCR analysis was also performed and showed that BCLAF1 was more enriched on the p53 promoter post DOX treatment (Fig. S4E), demonstrating that BCLAF1 was a transcriptional factor induced by DOX to regulate p53 expression. To validate binding between miR-181b-5p and BCLAF1 3′-UTR mRNA, we using a dual-luciferase reporter assay, which showed that the relative luciferase activity was inhibited when miR-181b-5p mimics were co-transfected with the luciferase reporters, while the relative luciferase activity was noticeably increased when miR-181b-5p inhibitors were used. Moreover, the interaction was lost when a plasmid carrying a mutated sequence was used instead (Fig. 4b).
Fig. 4. Exosomal miR-181b-5p induces DOX-resistance in recipient cells via downregulation of BCLAF1 and subsequent evasion of senescence mediated by p53/p21.
a Bioinformatic analysys was used to predict target genes affected by miR-181b-5p, ranking BCLAF1 with the highest score. For the following panels, inhibitors of miR-181b-5p (inhibitors.miR-181b-5p) and negative controls (inhibitors.NC) or mimics of miR-181b-5p (mimic.miR-181b-5p) and negative controls (mimic.NC) were transiently transfected into resistant cells. b miR-181b-5p was found to be directly bound to the BCLAF1 3′-UTR using a dual-luciferase reporter assay. BCLAF1 mRNA (c) and protein level (d) analyses from breast cancer cells treated with exosomes. e CM from resistant cells transfected with inhibitors.NC and inhibitors.miR-181b-5p was collected and exosomes were isolated. BCLAF1 overexpression plasmid was transiently transfected into sensitive cells, which had been incubated with exosomes from detailed previously for 48 h, and CCK-8 assays were conducted to measure the cell viability of sensitive cells as indicated. f Using the conditions described above, cell cycle was tested through flow cytometry. g Quantitative analysis of the data shown in panel f. h Cyclin D1 analysis by western blot. i Sensitive cells were stained for SA-β-gal. The percentages of cells positive for SA-β-gal were quantified as shown in panel j. k The levels of BCLAF1 and senescence relative proteins were detected by western blot. *p < 0.05; **p < 0.01; ***p < 0.001. Numerical data represent mean ± S.D. based on three independent experiments, and the immunoblots are representative of three replicates.
To test whether exosomal miR-181b-5p was functional and able to suppress BCLAF1 expression in recipient cells, we incubated Cal51 cells for 48 h with Cal51-exo, CALDOX-exo+inhibitor.NC and CALDOX-exo+inhibitor.miR-181b-5p, respectively. Incubation with exosomes from CALDOX-exo+inhibitor.NC led to a sharp reduction in BCLAF1 expression, whereas the inhibition of miR-181b-5p enhanced the expression of BCLAF1 (Fig. 4c, d). An equivalent phenotype was observed in MCF-7 treated with MCF-7-exo, MCF-7/A02-exo+inhibitor.NC or MCF-7/A02-exo+inhibitor.miR-181b-5p (Fig. 4c, d). Then we evaluated whether exosomal miR-181b-5p induces senescence-associated DOX resistance in Cal51 or MCF-7 cells by targeting BCLAF1. Overexpressing BCLAF1 phenotypically mimicked the effects induced by miR-181b-5p inhibitor. As expected, overexpressing BCLAF1, similar to inhibitors.miR-181b-5p, decreased the cell viability of Cal51 or MCF-7 cells when treated with CALDOX-exo or MCF-7/A02-exo in the presence of DOX (Fig. 4e). Thus, miR-181b-5p knockdown and BCLAF1 overexpression showed equivalent capacity to reverse the effects of drug resistance induced by exosomes from drug-resistant cell lines. Additionally, decreased cell cycle arrest and cell senescence caused by CALDOX-exo or MCF-7/A02-exo were also reversed by the overexpression of BCLAF1. (Figs. 4f–k and S4F). Taken together, these results suggested that the acquisition of doxorubicin-resistance achieved by exosomal uptake and processing of miR-181b-5p is mediated by the inhibition of BCLAF1 translation in breast cancer cells.
Systemically injected inhibitors of miR-181b-5p sensitise the response to DOX in vivo
To investigate the relationship between circulating exosomal miR-181b-5p and doxorubicin-resistance in vivo, Cal51 and CALDOX xenografts were established (n = 5 per group) by injection in nude mice as detailed in materials and methods. After 14 days, serum was collected, and exosomes were isolated to measure the expression levels of exosomal miR-181b-5p. As shown in Fig. S5A, miR-181b-5p was observed to be significantly upregulated in serum exosomes from CALDOX group. Several studies have shown the advantages of exosomes as naturally derived delivery vehicles for therapeutic agents for tumour-therapy [31–33]. To explore in vivo the DOX-sensitising effect of exosomal inhibitors of miR-181b-5p, we established CALDOX xenograft tumours (n = 15) and after 14 days we isolated exosomes from 293T cells with the aim to use them to treat separate mice groups that had tumours established and were undergoing DOX treatment. These mice had CALDOX tumours established (14 days), and then received i.p. systemic injections of DOX in combination with s.c. injections around the tumour of exosomes isolated from 293T cells that had been pre-treated with either inhibitors of miR-181b-5p or negative control inhibitors (diagram in Fig. 5a). After 14 days, with 6 cycles of exosome treatment and 4 cycles of DOX treatment, tumours and serum were collected for subsequent analyses. Injection of exosomes pre-treated with inhibitors of miR-181b-5p (exo-inhibitors.miR-181b-5p) led to a clear and significant reduction in tumour volumes (Fig. 5b), indicating that they were able to reverse the DOX-resistant phenotype of CALDOX xenografts in vivo. We also analysed the serum exosomes from these mice and conducted qRT-PCR analysis, which showed significantly decreased miR-181b-5p expression in the group treated with exo-inhibitors.miR-181b-5p compared to the group treated with negative control inhibitors (exo-inhibitors.NC, Fig. 5c). The tumours were additionally analysed by western blot, showing that tumours treated with exo-inhibitors.miR-181b-5p were able to rescue the expression levels of p53/p21 and also rescued the protein levels of the miRNA target BCLAF1 (Fig. 5d). IHC staining from tumours validated the increased levels of cellular senescence markers p51 and p21 in the exo-inhibitors.miR-181b-5p group (Fig. 5e). Altogether, these results demonstrate that application of exosomes treated with inhibitors of miR-181b-5p alleviated tumour burden, increased the efficacy of DOX by reversing the DOX-resistant phenotype, via a mechanism involving cellular senescence mediated by p53/p21 and BCLAF1 in vivo.
Fig. 5. Systemically injected exosomes pre-loaded with inhibitors of miR-181b-5p sensitises the response to DOX in vivo.
a Diagram depicting the in vivo experimental design and administration of DOX injections and exosome injections (details in methods and results sections). b Left panel: images of tumours in mice (n = 15); right panel: quantification of tumour volumes in the treatment and two control groups. c Expression levels of miR-181b-5p in serum exosomes from mice were detected by qRT-PCR. d Senescence-associated target proteins were analysed by western blot of tumours. e IHC analysis of senescence-associated target proteins in tumours. f Schematic diagram of the role of exosomal miR-181b-5p in transferring a doxorubicin-resistant phenotype in breast cancer. **p < 0.01. Numerical data represent mean ± S.D. based on three independent experiments, and the immunoblots are representative of three replicates.
Discussion
Exosomes are increasingly regarded as novel inter-cellular communication vehicles, with important roles in mediating cancer progression and resistance to chemotherapy. Although many other components in exosomes exert effects on recipient cells, miRNAs are considered key functional signalling messengers transferred by exosomes that can regulate gene expression via translational control [34–36]. Drug-resistant cancer cells have been reported to release exosomal miRNAs into the microenvironment and confer drug resistance to recipient cells [37–42]. Here, we found that miR-181b-5p is directly transferred from DOX-resistant cells to sensitive cells via exosomes, transferring DOX resistance by targeting BCLAF1, which decreases the ability of recipient cells to undergo cellular senescence upon exposure to DOX (Fig. 5f). These results not only provide a new mechanism of DOX-resistance in breast cancer cells mediated by exosomes and miRNAs, but also suggest that exosomal miR-181b-5p holds biomarker potential in assessing response to chemotherapy using liquid biopsies from patients.
Several studies have identified that horizontal transfer of exosomal miRNAs induces chemoresistance and malignant phenotypic traits. Exosomal miR-522 has been reported to be involved in chemotherapy resistance of gastric cancer [43]. Chen et al. demonstrated that hypoxic tumour cell-derived exosomal miR-340-5p promotes radiotherapy resistance in oesophageal squamous cell carcinoma via KLF10 [44]. MiR-24-3p is delivered from cancer-associated fibroblast to colon cancer cells via exosomes and suppresses the CDX2/HEPH pathway to induce methotrexate-resistance [45]. Exosome-mediated miR-7-5p delivery enhances the anticancer effect of everolimus via blocking MNK/eIF4E axis in non-small cell lung cancer [46]. Our group previously demonstrated that chemotherapy increases exosomal miR-378a-3p and miR-378d in breast cancer cells, transferring stemness and chemoresistance via the activation of EZH2/STAT3 signalling [28].
In this study, we show that secreted exosomal miR-181b-5p by doxorubicin-resistant cells can be taken up by recipient cells and downregulate BCLAF1. The effects of miR-181b-5p/ BCLAF1 axis has also been detected on drug-resistant phenotype in vitro and in vivo, showing that either overexpressing BCLAF1 in recipient cells or the application of miR-181b-5p inhibitors blocked exosomes (derived from drug-resistant cells)-mediated doxorubicin resistance and senescence. BCLAF1 was firstly identified to bind E1B19K (an adenoviral Bcl2 homologue) after screened in a yeast two-hybrid system [47], indicating its important role in regulating cell survival. Moreover, the silence of BCLAF1 leads to embryonic lethal due to the impairment in lung development [48]. In multiple cell types, dysregulated expression of BCLAF1 has also been shown to induce apoptosis or autophagy-related cell death [47, 49]. The structure analysis identifies that BCLAF1 protein has Myb DNA-binding domain [47], suggesting its potential DNA-binding effects. Indeed, this is supported by several studies pointing out the involvement of BCLAF1 in DNA damage response. Specifically, in response to DNA damage, BCLAF1 can interact with PKCδ to co-occupy the promoter of TP53, resulting in the activation of P53 and cell death [29, 30]. Consistently, the BCLAF1/ P53 regulatory axis was reconfirmed in our results. Additionally, by co-operation with C/EBPβ, BCLAF1 is observed to regulate transcription of SASP components, which belong to senescence-associated signature [50]. Here, we confirmed that exosomal miR-181b-5p derived from DOX-resistant cells suppresses senescence to mediate DOX resistance in recipient cells through modulating BCLAF1/p53/p21 signalling.
Further, we found that compared to doxorubicin-sensitive cells, DOX-resistant cells showed more abundant endogenous miR-181b-5p. To be consistent, the similar expression pattern can be observed in corresponding exosome as well, with more miR-181b-5p enriched in DOX-resistant cells derived exosomes than doxorubicin-sensitive cells derived counterparts. The observation that the uptake of exosomes from resistant cells by recipient cells endowed recipient cells resistant capacity suggested exosomal miR-181b-5p might keep stable and mediate drug-resistant effects and even senescence phenotype. It is well-established exosomes can be secreted into a variety of bodily fluids [51–53], which is further supported by the recent evidence of the detection of cancerous exosomal miRNAs in blood, urine and saliva [54, 55]. Therefore, it is reasonable to propose that circulating exosomal miR-181b-5p is a potential prognostic marker for predicting response of chemotherapy in patients with breast cancer. Here, correspondingly, we found that increased serum levels of exosomal miR-181b-5p were associated with a poor response to chemotherapy by analysing 24 pair patient-derived serum samples.
Recent studies have pointed out that combining exosomes with RNA interference can be a promising gene therapy approach. For instance, plasma exosomes have been identified to achieve MAPK silencing in monocytes and lymphocytes by delivering exogenous siRNA [31]. Moreover, exogenous siRNA imported into exosomes are also shown to decrease the expression of other target genes in cancer cells [32]. More importantly, the silencing effects mediated by exosome-siRNA gene can be verified in vivo (preclinical mouse models) [33]. Nanotechnology has revolutionised drug delivery design in recent years, and nanoparticles designed to reverse multidrug resistance by bypassing drug efflux mediated resistance are also being actively developed. Therefore, our results showed that exosomal inhibitors of miR-181b-5p combined with doxorubicin can synergise to limit tumour growth in chemotherapy resistant mice, which is an interesting idea that needs to be further studied for future patient application as therapeutic purposes.
In conclusion, our current study demonstrates on one hand that DOX resistance and reduced senescence in response to DOX can be induced by the horizontal transfer of exosomal miR-181b-5p via the suppression of BCLAF1 in breast cancer. On the other hand, we show that exosomal miR-181b-5p holds great biomarker potential and might serve as a diagnostic tool and possibly be the target to novel therapies for breast cancer.
Supplementary information
Table S1. Breast cancer patient characteristics
Table S2. Sequences used in cell transfection
Figure S1. Conditioned medium (CM) from doxorubicin-resistant cells suppressed senescence in breast cancer cells.
Figure S2: Uptake of exosomes derived from DOX-resistant cells decreased DOX-induced senescence in breast cancer cells.
Figure S3. Exosomal miR-181b-5p from DOX-resistant cells is responsible for drug resistance and suppresses cellular senescence.
Figure S4. Exosomal miR-181b-5p induced senescence reduction in recipient cells via downregulation of BCLAF1.
Figure S5. Exosomal miR-181b-5p is related to DOX-resistance in vivo.
Table S3-Cal51vsCALDOX.Differential_analysis_results
Table S4-MCF-7vsMCF-7A02.Differential_analysis_results
Acknowledgements
This work was supported by The Tianjin Science Foundation (No. 19YFZCSY00030 to JZ); The China Scholarship Council Award (No. 202006940028 to TP, No. 201806010012 to JD); Tianjin Municipal Education Research Project (2020KJ136 to SZ); Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A); and CRUK Early Detection and Diagnosis Committee Project (No. C1519/A27375 to JMV).
Author contributions
SZ, TP, JD and LC have contributed equally to this work. JZ is the corresponding author to this work. Conceptualisation, resources, supervision and project administration were done by JZ; Methodology, software, formal analysis, investigation, data curation and visualisation were done by TP, SZ, JD, LC and GZ. Writing—original draft and writing—review & editing were done by JZ, TP, SZ, JD and LC. JMV, JL and TN reviewed and edited the manuscript. The authors reviewed and approved the final manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conformed to the principles of the Declaration of Helsinki, and authorised by the Medical Ethics Committee of Tianjin Medical University Cancer Institute and Hospital and Tianjin Medical University Cancer Institute and Hospital Laboratory Animal Care and Use Committee.
Consent for publication
We have obtained consent to publish from the participant to report individual patient data.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shaorong Zhao, Teng Pan, Jinhai Deng, Lixia Cao.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-02077-x.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015[J] CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Kutanzi KR, Yurchenko OV, Beland FA, Checkhun VF, Pogribny IP. MicroRNA-mediated drug resistance in breast cancer[J] Clin Epigenetics. 2011;2:171–85. doi: 10.1007/s13148-011-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression[J] J Pathol. 2011;223:307–17. doi: 10.1002/path.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnefoi H, Litiere S, Piccart M, MacGrogan G, Fumoleau P, Brain E, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial[J] Ann Oncol. 2014;25:1128–36. doi: 10.1093/annonc/mdu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnefoi H, Jacot W, Saghatchian M, Moldovan C, Venat-Bouvet L, Zaman K, et al. Neoadjuvant treatment with docetaxel plus lapatinib, trastuzumab, or both followed by an anthracycline-based chemotherapy in HER2-positive breast cancer: results of the randomised phase II EORTC 10054 study[J] Ann Oncol. 2015;26:325–32. doi: 10.1093/annonc/mdu551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vader P, Breakefield XO, Wood MJ. Extracellular vesicles: emerging targets for cancer therapy[J] Trends Mol Med. 2014;20:385–93. doi: 10.1016/j.molmed.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function[J] Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs[J] Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 9.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels[J] Nature. 2010;466:835–40. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu W, Tan C, He Y, Zhang G, Xu Y, Tang J. Functional miRNAs in breast cancer drug resistance[J] Onco Targets Ther. 2018;11:1529–41. doi: 10.2147/OTT.S152462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Martinez A, de Miguel-Perez D, Ortega FG, Garcia-Puche JL, Robles-Fernandez I, Exposito J, et al. Exosomal miRNA profile as complementary tool in the diagnostic and prediction of treatment response in localized breast cancer under neoadjuvant chemotherapy[J] Breast Cancer Res. 2019;21:21. doi: 10.1186/s13058-019-1109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed SH, Shakir G, Badawy MA, Zakhary NI, Greve B, El-Shinawi M, et al. Inflammatory breast carcinoma: elevated microRNA miR-181b-5p and reduced miR-200b-3p, miR-200c-3p, and miR-203a-3p expression as potential biomarkers with diagnostic value. Biomolecules. 2020;10:1059. [DOI] [PMC free article] [PubMed]
- 13.Ouyang M, Li Y, Ye S, Ma J, Lu L, Lv W, et al. MicroRNA profiling implies new markers of chemoresistance of triple-negative breast cancer. PLoS ONE. 2014;9:e96228. doi: 10.1371/journal.pone.0096228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andalib A, Rashed S, Dehbashi M, Hajati J, Noorbakhsh F, Ganjalikhani-Hakemi M. The upregulation of hsa-mir-181b-1 and downregulation of its target CYLD in the late-stage of tumor progression of breast cancer. Indian J Clin Biochem. 2020;35:312–21. doi: 10.1007/s12291-019-00826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed SH, Espinoza-Sanchez NA, El-Damen A, Fahim SA, Badawy MA, Greve B. Small extracellular vesicle-encapsulated miR-181b-5p, miR-222-3p and let-7a-5p: Next generation plasma biopsy-based diagnostic biomarkers for inflammatory breast cancer. PLoS ONE. 2021;16:e250642. doi: 10.1371/journal.pone.0250642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Yague E, Zhao J, Wang L, Bai J, Yang Q, et al. Sabutoclax, pan-active BCL-2 protein family antagonist, overcomes drug resistance and eliminates cancer stem cells in breast cancer. Cancer Lett. 2018;423:47–59. doi: 10.1016/j.canlet.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 17.Montalto FI, De Amicis F. Cyclin D1 in cancer: a molecular connection for cell cycle control, adhesion and invasion in tumor and stroma. Cells. 2020,9:2648. [DOI] [PMC free article] [PubMed]
- 18.Te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–83. [PubMed] [Google Scholar]
- 19.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 21.Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–16. doi: 10.1016/S0092-8674(03)00401-X. [DOI] [PubMed] [Google Scholar]
- 22.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lappano R, Rigiracciolo DC, Belfiore A, Maggiolini M, De, Francesco EM. Cancer associated fibroblasts: role in breast cancer and potential as therapeutic targets. Expert Opin Ther Targets. 2020;24:559–72. doi: 10.1080/14728222.2020.1751819. [DOI] [PubMed] [Google Scholar]
- 25.Matei I, Kim HS, Lyden D. Unshielding exosomal RNA unleashes tumor growth and metastasis. Cell. 2017;170:223–5. doi: 10.1016/j.cell.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–16. [PubMed] [Google Scholar]
- 27.Taube JM, Galon J, Sholl LM, Rodig SJ, Cottrell TR, Giraldo NA, et al. Implications of the tumor immune microenvironment for staging and therapeutics. Mod Pathol. 2018;31:214–34. doi: 10.1038/modpathol.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Q, Zhao S, Shi Z, Cao L, Liu J, Pan T, et al. Chemotherapy-elicited exosomal miR-378a-3p and miR-378d promote breast cancer stemness and chemoresistance via the activation of EZH2/STAT3 signaling. J Exp Clin Cancer Res. 2021;40:120. doi: 10.1186/s13046-021-01901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Lu ZG, Miki Y, Yoshida K. Protein kinase C delta induces transcription of the TP53 tumor suppressor gene by controlling death-promoting factor Btf in the apoptotic response to DNA damage. Mol Cell Biol. 2007;27:8480–91. doi: 10.1128/MCB.01126-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarras H, Alizadeh Azami S, McPherson JP. In search of a function for BCLAF1. Sci World J. 2010;10:1450–61. doi: 10.1100/tsw.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahlgren J, De L, Karlson T, Brisslert M, Vaziri Sani F, Telemo E, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV, et al. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan Q, Ramakrishnaiah V, Henry S, Fouraschen S, de Ruiter PE, Kwekkeboom J, et al. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi) Gut. 2012;61:1330–9. doi: 10.1136/gutjnl-2011-300449. [DOI] [PubMed] [Google Scholar]
- 34.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237–48. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiba M, Kimura M, Asari S. Exosomes secreted from human colorectal cancer cell lines contain mRNAs, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep. 2012;28:1551–8. doi: 10.3892/or.2012.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–66. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao L, Li J, Chen WX, Cai YQ, Yu DD, Zhong SL, et al. Exosomes decrease sensitivity of breast cancer cells to adriamycin by delivering microRNAs. Tumour Biol. 2016;37:5247–56. doi: 10.1007/s13277-015-4402-2. [DOI] [PubMed] [Google Scholar]
- 38.Yu DD, Wu Y, Zhang XH, Lv MM, Chen WX, Chen X, et al. Exosomes from adriamycin-resistant breast cancer cells transmit drug resistance partly by delivering miR-222. Tumour Biol. 2016;37:3227–35. doi: 10.1007/s13277-015-4161-0. [DOI] [PubMed] [Google Scholar]
- 39.Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng L, et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer. 2017;16:132. doi: 10.1186/s12943-017-0694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alharbi M, Zuniga F, Elfeky O, Guanzon D, Lai A, Rice GE, et al. The potential role of miRNAs and exosomes in chemotherapy in ovarian cancer. Endocr Relat Cancer. 2018;25:R663–85. doi: 10.1530/ERC-18-0019. [DOI] [PubMed] [Google Scholar]
- 41.Fu X, Liu M, Qu S, Ma J, Zhang Y, Shi T, et al. Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37:52. doi: 10.1186/s13046-018-0677-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Wang M, Qiu R, Yu S, Xu X, Li G, Gu R, et al. Paclitaxelresistant gastric cancer MGC803 cells promote epithelialtomesenchymal transition and chemoresistance in paclitaxelsensitive cells via exosomal delivery of miR1555p. Int J Oncol. 2019;54:326–38. doi: 10.3892/ijo.2018.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. 2020;19:43. doi: 10.1186/s12943-020-01168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen F, Xu B, Li J, Yang X, Gu J, Yao X, et al. Hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10. J Exp Clin Cancer Res. 2021;40:38. doi: 10.1186/s13046-021-01834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang HW, Shi Y, Liu JB, Wang HM, Wang PY, Wu ZJ, et al. Cancer-associated fibroblast-derived exosomal microRNA-24-3p enhances colon cancer cell resistance to MTX by down-regulating CDX2/HEPH axis. J Cell Mol Med. 2021;25:3699–713. doi: 10.1111/jcmm.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S, Wang W, Ning Y, Zheng H, Zhan Y, Wang H, et al. Exosome-mediated miR-7-5p delivery enhances the anticancer effect of Everolimus via blocking MNK/eIF4E axis in non-small cell lung cancer. Cell Death Dis. 2022;13:129. doi: 10.1038/s41419-022-04565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasof GM, Goyal L, White E. Btf, a novel death-promoting transcriptional repressor that interacts with Bcl-2-related proteins. Mol Cell Biol. 1999;19:4390–404. doi: 10.1128/MCB.19.6.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McPherson JP, Sarras H, Lemmers B, Tamblyn L, Migon E, Matysiak-Zablocki E, et al. Essential role for Bclaf1 in lung development and immune system function. Cell Death Differ. 2009;16:331–9. doi: 10.1038/cdd.2008.167. [DOI] [PubMed] [Google Scholar]
- 49.Lamy L, Ngo VN, Emre NC, Shaffer ALRD, Yang Y, Tian E, et al. Control of autophagic cell death by caspase-10 in multiple myeloma. Cancer Cell. 2013;23:435–49. doi: 10.1016/j.ccr.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shao AW, Sun H, Geng Y, Peng Q, Wang P, Chen J, et al. Bclaf1 is an important NF-kappaB signaling transducer and C/EBPbeta regulator in DNA damage-induced senescence. Cell Death Differ. 2016;23:865–75. doi: 10.1038/cdd.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteom Clin Appl. 2015;9:358–67. doi: 10.1002/prca.201400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salido-Guadarrama I, Romero-Cordoba S, Peralta-Zaragoza O, Hidalgo-Miranda A, Rodriguez-Dorantes M. MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. Onco Targets Ther. 2014;7:1327–38. doi: 10.2147/OTT.S61562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fanale D, Taverna S, Russo A, Bazan V. Circular RNA in exosomes. Adv Exp Med Biol. 2018;1087:109–17. doi: 10.1007/978-981-13-1426-1_9. [DOI] [PubMed] [Google Scholar]
- 54.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 55.Hu G, Drescher KM, Chen XM. Exosomal miRNAs: biological properties and therapeutic potential. Front Genet. 2012;3:56. doi: 10.3389/fgene.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Breast cancer patient characteristics
Table S2. Sequences used in cell transfection
Figure S1. Conditioned medium (CM) from doxorubicin-resistant cells suppressed senescence in breast cancer cells.
Figure S2: Uptake of exosomes derived from DOX-resistant cells decreased DOX-induced senescence in breast cancer cells.
Figure S3. Exosomal miR-181b-5p from DOX-resistant cells is responsible for drug resistance and suppresses cellular senescence.
Figure S4. Exosomal miR-181b-5p induced senescence reduction in recipient cells via downregulation of BCLAF1.
Figure S5. Exosomal miR-181b-5p is related to DOX-resistance in vivo.
Table S3-Cal51vsCALDOX.Differential_analysis_results
Table S4-MCF-7vsMCF-7A02.Differential_analysis_results
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.