Abstract
This study examined the relationship between a validated measure of socioeconomic deprivation, such as the Area Deprivation Index (ADI), and morbid obesity. We used cross-sectional data on adult patients (≥18 years) in the Houston Methodist Cardiovascular Disease Health System Learning Registry (located in Houston, Texas, USA) between June 2016 and July 2021. Each patient was grouped by quintiles of ADI, with higher quintiles signaling greater deprivation. BMI was calculated using measured height and weight with morbid obesity defined as ≥ 40 kg/m2. Multivariable logistic regression models were used to examine the association between ADI and morbid obesity adjusting for demographic (age, sex, and race/ethnicity) factors. Out of the 751,174 adults with an ADI ranking included in the analysis, 6.9 % had morbid obesity (n = 51,609). Patients in the highest ADI quintile had a higher age-adjusted prevalence (10.9 % vs 3.3 %), and about 4-fold odds (aOR, 3.8; 95 % CI = 3.6, 3.9) of morbid obesity compared to the lowest ADI quintile. We tested for and found interaction effects between ADI and each demographic factor, with stronger ADI-morbid obesity association observed for patients that were female, Hispanic, non-Hispanic White and 40–65 years old. The highest ADI quintile also had a high prevalence (44 %) of any obesity (aOR, 2.2; 95 % CI = 2.1, 2.2). In geospatial mapping, areas with higher ADI were more likely to have higher proportion of patients with morbid obesity. Census-based measures, like the ADI, may be informative for area-level obesity reduction strategies as it can help identify neighborhoods at high odds of having patients with morbid obesity.
Abbreviations: ADI, Area Deprivation Index; BMI, Body Mass Index; CA, Catchment Area; CI, Confidence Interval; CVD, Cardiovascular Diseases; ED, Emergency Department; FIPS, Federal Information Processing Standards; HM, Houston Methodist; IRB, Internal Review Board; OR, Odds Ratio; SD, Standard Deviation; SDOH, Social Determinants of Health; SES, Socio-Economic Status; US, United States
Keywords: Data-driven, Health equity, Morbid obesity, Neighborhood deprivation
1. Introduction
The epidemic of morbid obesity (body mass index [BMI] ≥ 40 kg/m2) is a major public health concern in the United States (US), affecting nearly 10% of the adult population (Fryar et al., 1960, Hales et al., 2017). Morbid obesity is associated with increased risk of other cardiovascular risk factors such as hypertension, dyslipidemia, and type 2 diabetes, as well as cardiovascular disease (CVD) incidence and mortality (di Angelantonio et al., 2016). In addition, morbid obesity accounts for approximately 20 % of total healthcare costs in the US (Arterburn et al., 2005, Fouse and Schauer, 2016). However, existing literature often focuses on obesity in general (BMI ≥ 30 kg/m2), and fails to focus on morbid obesity, despite marked differences in disease burden and healthcare costs from less severe forms of obesity (Cecchini, 2018).
The burden of morbid obesity warrants employing innovative, accurate tools that can readily identify areas where population-level and individual-level interventions are most needed. Studies have shown that social determinants of health (SDOH), such as socioeconomic deprivation at the community or neighborhood level are strongly associated with obesity (Booth et al., 2005, Hu et al., 2021, Laraia et al., 2012, Okuyama et al., 2020, Stafford et al., 2010). Socioeconomic disadvantage has been posited to influence obesity through causal pathways attributed to greater availability of low-cost, calorie-dense foods, and lack of sporting facilities for physical exercise in poor neighborhoods, relative to their more affluent counterparts (Drewnowski, 2012, Larson et al., 2009). Yet, advances in using socioeconomic information to map social factors to health outcomes in health care systems remains largely underutilized, partly because of the inherent challenges in a standardized capture of such sensitive income-related information from patients (Cantor and Thorpe, n.d).
The area deprivation index (ADI), a proxy measure for neighborhood socioeconomic disadvantage, offers a practical and efficient opportunity for health systems to quantify the social effects on health using a single index. In fact, the ADI has been used in other studies to predict an individual’s risk for poor health outcomes such as cancer, copd, stroke, heart disease, hospital readmissions and chronic disease management (Cheruvalath et al., 2022, Durfey et al., 2019, Galiatsatos et al., 2020a, Galiatsatos et al., 2020b, Ghirimoldi et al., 2021, Kurani et al., 2021, Rosenzweig et al., 2021, Unger et al., 2021). Nevertheless, knowledge of how a composite measure such as the ADI can be leveraged in health system organizations to identify patients at risk for morbid obesity is limited, and merits greater investigation as this information can be used to inform resource targeting in this population for future practice and policy (Sheets et al., 2020).
Accordingly, the goal of our study was to examine the relationship between available measures of SDOH in health systems, such as the ADI, and morbid obesity in HM patient population. We also assessed the presence of potential moderating effects on the relationship between ADI and morbid obesity by testing for interactions between ADI and demographic variables (age, sex and race/ethnicity). Lastly, we used publicly available ADI data to map areas potentially at higher odds of having high proportions of morbid obesity.
2. Methods
2.1. Setting and study design
This study was conducted at Houston Methodist (HM) within the Center for Outcomes Research. HM is an academic medical multi-center located in Houston, Texas, US offering consultative health service and patient care to patients in the urban metropolitan area of greater Houston, while operating as a high-reliability learning health care system.
This study employed an observational cross-sectional design using data obtained from the Houston Methodist Cardiovascular Disease Health System Learning Registry. The constituents of the registry include de-identified patient information demographics, vitals, diagnoses, laboratory and imaging tests results, medications, comorbidities, and clinical outcomes from the institution’s electronic medical record system (Epic) on patients who have had at least one outpatient encounter with one of our practicing physicians. The registry repository is on a secure server that can be accessed through Microsoft SQL Server Management Studio (v 2019) by HM accredited computer scientists.
2.2. Study population
Our study population comprised a sample of 865,995 individuals ≥ 18 years of age in the Houston Methodist Cardiovascular Disease Health System Learning Registry who had at least one outpatient encounter with one of the physicians in our hospital system between June 1st, 2016 and July 20th, 2021. Using the University of Wisconsin-Madison School of Medicine’s ADI dataset, we linked our patient database to the ADI dataset to assign an ADI rank score to each patient according to the census block group of their geocoded residential address. We extracted patient demographics from our clinical records management software system in compliance with our Institutional Review Board. We excluded 107,037 (12.4 %) patients because they were missing height or weight data, or because their height or weight values were abnormal, resulting in an implausible BMI value (>100 kg/m2 and < 10 kg/m2). We also excluded 9,855 (1.1 %) patients because their residential address could not be geocoded and assigned ADI rank scores. Our final study population included 751,174 patients. Please, see research study patient flow chart in Appendix A.
Appendix A.
Research Study Patient Flow Chart.
2.3. Study variables
2.3.1. Area deprivation Index
The ADI is a composite measure of 17 indicators of socioeconomic status across four domains: employment, income, education and housing quality, which provides neighborhood ranking by socioeconomic disadvantage within the state or national level (Kind et al., 2014) Appendix B. In this study, we used the 2015 ADI metric, which was constructed from a 5-year average of the 2011 to 2015 American Community Survey at the census block group level (considered the closest approximation to neighborhood). We categorized ADI percentiles into quintiles (Q): Q1 (1–20), Q2 (21–40), Q3 (41–60), Q4 (61–80) and Q5 (81–100)), and then each patient was assigned into their corresponding ADI quintile based on their mailing address as of July 20th, 2021. This method of categorizing ADI into quintiles has been previously validated in past literature (Johnson et al., 2021, Knighton et al., 2016).
2.3.2. Body Mass Index
For each patient, the BMI was calculated using the most recent clinical information about patients’ height and weight recorded in the HM database as of July 20th, 2021. Patients were classified into the following BMI categories (“Executive Summary of the Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults.,” 1998): underweight (<18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2), obesity class 1 (30–34.9 kg/m2), obesity class 2 (35–39.9 kg/m2), and obesity class 3/morbid obesity (≥40 kg/m2). (“Appropriate Body-Mass Index for Asian Populations and Its Implications for Policy and Intervention Strategies.,” 2004; “Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation.,” 2000).
2.3.3. Covariates
Covariates used in our study were age, sex and race/ethnicity. Each of these were categorized as follows: age (18–39, 40–64, 65–79 and ≥ 80 years), sex (male and female), race/ethnicity (Hispanic, non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and non-Hispanic other).
2.4. Statistical analyses
Baseline characteristics of the study population were reported as frequencies and percentages for each ADI quintile category. We also described the age-adjusted prevalence of the different BMI categories (underweight, normal weight, overweight, obesity class I, obesity class II and obesity class III) by ADI quintiles. The 2010 US Census Population Data was used to obtain age-adjusted estimates. We used unadjusted and adjusted (by age, sex and race/ethnicity) logistic regression models to examine the association between ADI and morbid obesity. We also tested for interactions between ADI and demographic variables (age, sex and race/ethnicity) to determine the presence of potential moderating effects by each demographic variable. For interaction terms with significant p-values, we stratified our logistic regression models by the demographic variable to examine differences in the association between ADI and morbid obesity among subgroups. All statistical analyses were conducted in Stata/MP 16.1 analytical software (StataCorp, College Station, TX).
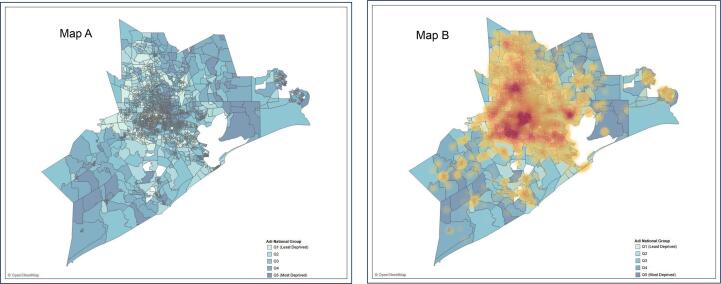
The visualization component of our analyses was conducted using Tableau; an interactive data visualization software powered by VizQL (Jones, 2014, What is VizQL, n.d.). We created the HM catchment area base layer map featured with a choropleth distribution of ADI across geospatial census block group boundaries. An overlay of the hospital’s morbid obese population was subsequently juxtaposed on this base layer map to identify geospatial boundaries with a high density of morbid obese patients at the census block group level.
3. Results
3.1. Study population
The population included in the analysis comprised 751,174 adults. The mean age was 52.4 years, and the majority of patients were between 40 and 64 years old (n = 321,659; 42.8 %), women (n = 452,414; 60.2 %), non-Hispanic White (n = 437,766; 58.3 %), located in the least socioeconomically deprived areas (n = 229,533; 30.6 %), and were overweight (n = 245,608; 32.6 %). About 6.9 % (n = 51,609) of our study population had morbid obesity.
Table 1 summarizes the baseline characteristics of the study population across ADI quintiles in the total population and across age, sex, and race/ethnicity categories. Higher ADI quintiles had lower proportions of patients aged 40–64 years when compared to ADI Q1. The proportion of females in higher ADI quintiles were higher than ADI Q1. The proportion of Black patients in higher ADI quintiles compared to ADI Q1 was also higher, while the proportion of White patients and Asians in higher ADI quintiles, compared to ADI Q1, was lower.
Table 1.
Characteristics of study participants overall and by ADI-based level of social disadvantage.
| Characteristic | Overall |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
|---|---|---|---|---|---|---|
| (n = 751 174) | (n = 229533) | (n = 216629) | (n = 145328) | (n = 97911) | (n = 61773) | |
| Baseline | ||||||
| Age, mean (SD), years | 52.4 (18.0) | 51.8 (17.7) | 52.2 (18.1) | 52.4 (18.2) | 53.8 (18.1) | 53.3 (18.5) |
| Age groups, n (%), years | ||||||
| 18–39 | 208,453 (27.7) | 63,363 (27.6) | 61,401 (28.3) | 41,365 (28.4) | 25,266 (25.8) | 17,058 (27.6) |
| 40–64 | 321,659 (42.8) | 103,744 (45.1) | 92,244 (42.5) | 60,497 (41.6) | 40,299 (41.1) | 24,875 (40.2) |
| 65–79 | 175,412 (23.3) | 49,741 (21.6) | 49,637 (22.9) | 34,735 (23.9) | 25,917 (26.4) | 15,382 (24.9) |
| 80 up | 45,650 (6.07) | 12,685 (5.52) | 13,347 (6.16) | 8731 (6.00) | 6429 (6.56) | 4458 (7.21) |
| Female sex, n (%) | 452,414 (60.2) | 134,627 (58.6) | 130,655 (60.3) | 89,540 (61.6) | 59,679 (60.9) | 37,913 (61.3) |
| Race/Ethnicity, n (%) | ||||||
| Hispanic | 106,886 (14.2) | 19,802 (8.62) | 26,794 (12.3) | 25,512 (17.5) | 20,821 (21.2) | 13,957 (22.5) |
| Non-Hispanic White | 437,766 (58.2) | 152,133 (66.2) | 135,305 (62.4) | 77,035 (53.0) | 49,715 (50.7) | 23,578 (38.1) |
| Non-Hispanic Black | 104,251 (13.8) | 15,979 (6.96) | 24,252 (11.1) | 27,361 (18.8) | 18,278 (18.6) | 18,381 (29.7) |
| Non-Hispanic Asian | 48,989 (6.52) | 23,440 (10.2) | 14,765 (6.81) | 5924 (4.07) | 2970 (3.03) | 1890 (3.05) |
| Non-Hispanic Other | 53,282 (7.09) | 18,179 (7.91) | 15,513 (7.16) | 9496 (6.53) | 6127 (6.25) | 3967 (6.42) |
| BMI Groups, n (%), | ||||||
| Under Weight | 12,679 (1.68) | 4354 (1.89) | 3588 (1.65) | 2199 (1.51) | 1508 (1.54) | 1030 (1.66) |
| Normal Weight | 211,742 (28.1) | 82,783 (36.0) | 59,845 (27.6) | 34,122 (23.4) | 21,435 (21.8) | 13,557 (21.9) |
| Over Weight | 245,608 (32.6) | 79,397 (34.5) | 72,015 (33.2) | 45,637 (31.4) | 30,241 (30.8) | 18,318 (29.6) |
| Obese Class I | 155,554 (20.7) | 39,426 (17.1) | 46,067 (21.2) | 33,166 (22.8) | 22,827 (23.3) | 14,068 (22.7) |
| Obese Class II | 73,982 (9.84) | 15,161 (6.60) | 21,295 (9.83) | 17,340 (11.9) | 12,273 (12.5) | 7913 (12.8) |
| Obese Class III | 51,609 (6.87) | 8412 (3.66) | 13,819 (6.37) | 12,864 (8.85) | 9627 (9.83) | 6887 (11.1) |
Abbreviations: N = number; ADI = Area Deprivation Index; BMI = Body Mass Index; Q = Quintile groups of Area Deprivation Index.
3.2. Age-adjusted prevalence of BMI by ADI
There was an inverse relationship between the prevalence of underweight, normal weight and overweight BMI categories and ADI. In other words, as ADI quintiles increased, the prevalence of patients falling within the underweight, normal weight and overweight BMI categories decreased.
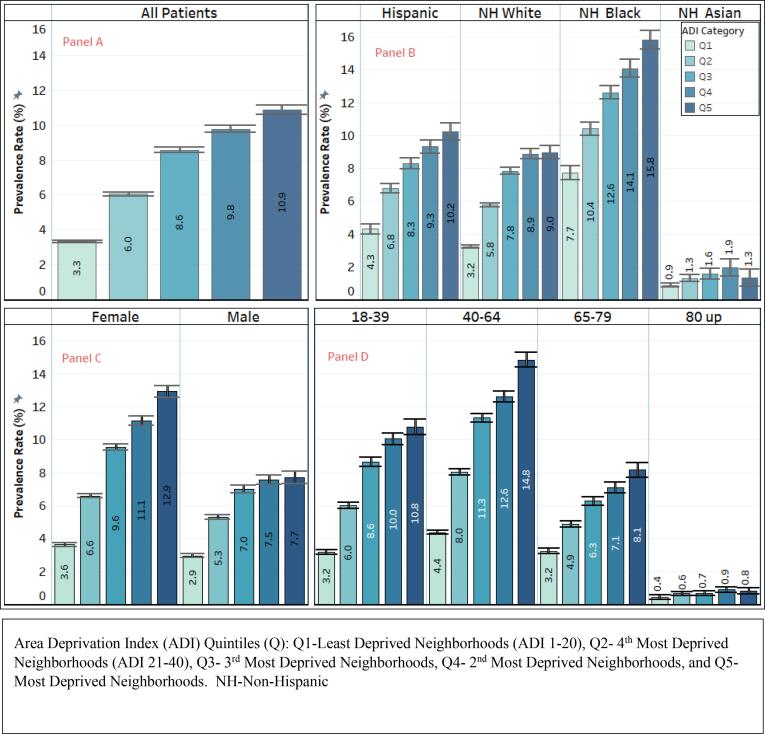
We observed a positive relationship between ADI and all classes of obesity (class I, II and III). In other words, the prevalence of obesity, irrespective of the class, increased with increasing ADI. (Fig. 1). As ADI increased, the age-adjusted prevalence of morbid obesity increased from 3.3 % (in ADI quintile 1) to 10.9 % in ADI quintile 5. These trends were consistent across age, sex, and racial/ethnic subgroups (Fig. 2).
Fig. 1.
Age-adjusted Prevalence of BMI Categories by ADI Quintiles.
Fig. 2.
Age-adjusted Prevalence of Morbid Obesity by ADI Quintiles, Overall (Panel A) and by Race/Ethnicity (Panel B), Age (Panel C), and Gender (Panel D) Groups.
3.3. Association between ADI and morbid obesity
In logistic regression models evaluating the association between ADI and morbid obesity, we observed stepwise increasing odds of morbid obesity with increasing ADI quintile levels. In unadjusted models, patients in ADI quintiles 2, 3, 4 and 5 had 2.2-fold (OR = 2.3; 95 % CI = 2.2, 2.3), 3.7-fold (OR = 3.6; 95 % CI = 3.6, 3.8), 4.4-fold (OR = 4.4; 95 % CI = 4.3, 4.6) and 5-fold (OR = 5.0; 95 % CI = 4.8, 5.2) odds of morbid obesity compared to those in quintile 1 (least deprived group). After adjustment for age, sex and race/ethnicity, this relationship was attenuated, but remained statistically significant, with patients in the highest ADI quintile roughly 4 times more likely to be morbidly obese compared to patients in the lowest ADI quintile (OR = 3.8; 95 % CI = 3.6, 3.9) (Table 2, Fig. 3).
Table 2.
Association between ADI and morbid obesity overall, and by race/ethnicity.
| All Patients |
Hispanic |
Non-Hispanic White |
Non-Hispanic Black |
Non-Hispanic Asian |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | ||||||
| Characteristic | n (%) | OR (95 % CI) | OR (95 % CI) | n (%) | OR (95 % CI) | OR (95 % CI) | n (%) | OR (95 % CI) | OR (95 % CI) | n (%) | OR (95 % CI) | OR (95 % CI) | n (%) | OR (95 % CI) | OR (95 % CI) |
| ADI | |||||||||||||||
| Q1 | 8412 (3.3) | Ref | Ref | 903 (4.3) | Ref | Ref | 5398 (3.2) | Ref | Ref | 1417 (7.7) | Ref | Ref | 204 (0.9) | Ref | Ref |
| Q2 | 13,819 (6.0) | 2.3 (2.2, 2.3) | 2.2 (2.1, 2.2) | 1987 (6.8) | 2.3 (2.1, 2.5) | 2.3 (2.1, 2.5) | 8127 (5.8) | 2.2 (2.1, 2.2) | 2.3 (2.2, 2.4) | 2716 (10.4) | 1.5 (1.3, 1.6) | 1.5 (1.4,1.6) | 193 (1.3) | 1.7 (1.35, 2.01) | 1.7 (1.38, 2.06) |
| Q3 | 12,864 (8.6) | 3.7 (3.6, 3.8) | 3.1 (3.0, 3.2) | 2272 (8.3) | 3.2 (2.9,3.5) | 3.4 (3.1, 3.7) | 5987 (7.8) | 3.1 (3.0, 3.3) | 3.4 (3.2, 3.5) | 3790 (12.6) | 1.9 (1.8, 2.0) | 1.9 (1.8, 2.1) | 95 (1.6) | 2.0 (1.54, 2.52) | 2.1 (1.61, 2.64) |
| Q4 | 9627 (9.8) | 4.4 (4.3, 4.6) | 3.7 (3.6, 3.8) | 2025 (9.3) | 3.7 (3.4, 4.0) | 4.0 (3.6, 4.3) | 4340 (8.9) | 3.7 (3.6, 3.9) | 4.0 (3.8, 4.2) | 2695 (14.1) | 2.0 (1.9, 2.2) | 2.2 (2.0, 2.4) | 57 (1.9) | 2.3 (1.74, 3.16) | 2.6 (1.90, 3.46) |
| Q5 | 6887 (10.9) | 5.0 (4.8, 5.2) | 3.8 (3.6, 3.9) | 1470 (10.2) | 3.9 (3.5, 4.3) | 4.3 (3.9, 4.7) | 2090 (9.0) | 3.6 (3.4, 3.8) | 3.9 (3.7, 4.2) | 2952 (15.8) | 2.1 (2.0, 2.3) | 2.4 (2.2, 2.6) | 27 (1.3) | 1.8 (1.17, 2.63) | 1.7 (1.13, 2.58) |
Abbreviations: ADI = Area Deprivation Index; CI = Confidence Interval; OR = Odds Ratio; Q = Quintile groups of Area Deprivation Index. Model was adjusted by age, sex and race/ethnicity+ n (%) represents frequency and age-adjusted prevalence.
Fig. 3.
Association between ADI Quintiles and Morbid Obesity, Overall (Panel A) and by Race/Ethnicity (Panel B), Age (Panel C), and Gender (Panel D) Groups.
3.4. Association between ADI and Morbid Obesity by Demographic Subgroup
We observed statistically significant p-values for the interaction terms between neighborhood deprivation and each demographic variable (age, sex, race/ethnicity). Age (p-interaction, p < 0.001), sex (p-interaction, p < 0.001), and race/ethnicity (p-interaction, p < 0.001) appeared to be strong effect modifiers in the relationship between neighborhood deprivation and morbid obesity (Appendix C).
In stratified analyses, by race/ethnicity, we found similar patterns of higher odds of morbid obesity as ADI increased, across all racial/ethnic subgroups for both unadjusted and adjusted models (Table 2, Fig. 3). Age and sex subgroups also had higher odds of morbid obesity with increasing ADI quintile levels (Fig. 3).
Specifically, among racial/ethnic subgroups, the strength of the association between ADI and morbid obesity was greater among Hispanic patients, then White patients and Black patients. Also, for sex subgroups, females had a stronger association between ADI and morbid obesity. For age groups, age groups 18–39 and 40–64 also had a strong association between ADI and morbid obesity.
3.5. Plotting of choropleth ADI maps overlaid with patient density map
The results of the juxtaposition of the morbid obesity patient density map on the ADI choropleth map containing the distribution of neighborhood deprivation across geospatial boundaries showed that areas with higher ADI quintile category were more likely to have higher proportion of patients with morbid obesity. Inner-city neighborhoods in the Houston area had more deprivation and more morbid obese patients (Fig. 4).
Fig. 4.
Visualization of HM Catchment area ADI map layered with Morbid Obese Patient Density Map.
4. Discussion
Utilizing a data-driven approach with hospital system population data containing patient health information linked to location-based socioeconomic data, our study confirms that there was a higher prevalence of patients with a higher body mass index (obesity class I, II and III/morbid obesity) as ADI (neighborhood socioeconomic disadvantage) increased in the total population and across age, sex, and race/ethnicity subgroups. Furthermore, our study confirms that there is a significant and positive association between morbid obesity (the highest category of BMI) and ADI (neighborhood socioeconomic disadvantage) in the total population, and across age, sex and race/ethnic subgroups. Lastly, our study showed that a visualization software can be successfully used in identifying areas at high odds of having patients with morbid obesity, an at-risk BMI category.
Despite the growing popularity of utilizing ADI in health outcomes research, the understanding of how neighborhood deprivation contexts relate with body weight compositions is still quite elusive in literature. One recent study has aimed to elucidate this relationship by establishing that there was a positive association between neighborhood disadvantage and obesity in a Midwestern US Medicare population (Sheets et al., 2020), but that study was limited to only obese elderly patients. Other prior cross-sectional studies have aimed to provide evidence of a relationship between neighborhood SES and prevalent obesity, however, not only are these studies quite outdated, they are also limited by self-selection bias, or likelihood of healthier and richer individuals living in areas of higher SES (Lovasi et al., 2009, Mujahid et al., 2005, Powell-Wiley et al., 2014).
As an improvement to these studies, our study describes the characteristics and prevalence of all BMI categories in relation to ADI and furthers this cause by focusing on an important but understudied subpopulation, patients with morbid obesity, at most risk for cardiovascular morbidity and mortality. Evidenced from our study, there is a relationship between ADI and BMI. For non-obese BMI categories, the prevalence of underweight, normal and overweight patients decreases as neighborhood disadvantage worsens. However, and unsurprisingly, this relationship reverses at BMI cut-off >=29.9 kg/m2 (obesity class I, II, III/morbid obesity) where the prevalence of these patients increases as neighborhood socioeconomic disadvantage worsens.
Our study’s finding of a high prevalence of obese patients in more socioeconomically disadvantage neighborhoods are indeed consistent with a recent meta-analyses that reported higher odds of obesity among individuals living in high SES neighborhoods (Mohammed et al., 2019). In corroboration with this finding, some studies have posited that neighborhood deprivation affects weight by influencing the energy balance between caloric intake and loss (Cohen, 2008, Hill, 2006). More deprived neighborhoods are usually characterized by high availability of unhealthy food supply chains, poor/non-existent street walkability, sports amenities and parks, high crime rate, more psychosocial stressors and high degree of depression which may be mechanistic influences of chronic conditions like obesity (Mohammed et al., 2019). In addition, the relationship between obesity and socioeconomic disadvantage may be bidirectional (Booth et al., 2017, Finkelstein et al., 2005). Just as more deprived neighborhoods are obesogenic hotspots because of unhealthy food options and unavailable physical activity amenities, reverse causation may lead to discrimination against persons with obesity in the workplace, thereby affecting their socioeconomic position (Villar & Quintana-Domeque, 2009).
In our study, after stratifying by age, sex and race/ethnicity, we observed that females, black patients and those between age 40- and 64 had the highest prevalence of morbid obesity; this finding was consistent with the most recent CDC report (Hales et al., 2017) on severe obesity trends in the United States. Prior evidence suggests that neighborhood deprivation affects women more strongly than men (Assari et al., 2016, Flegal et al., n.d., Flegal et al., 1988, Sobal and Stunkard, 1989, Zhang and Wang, 2004), partly through unhealthy food consumption as a chronic stress coping mechanism (Phelan et al., 2010, Sobal and Stunkard, 1989). Furthermore, the higher prevalence of morbid obesity among black individuals in deprived neighborhoods can be explained by empirical findings which reveal that black individuals from disadvantaged backgrounds also engage in unhealthy eating behaviors as a way to buffer the effects of stress on mental health. (Keith et al., 2006).
Our study’s finding of the socioeconomic inequity experienced by patients with morbid obesity maybe ameliorated by timely resource targeting to patients directly or to deprived neighborhoods where majority of these patients reside. Resource (development of parks, walking spaces, and healthy food stores) targeting and clinical planning requires knowing the hotspot communities’ endemic to these patients (Dodson et al., 2018). Our study responds to this need by modeling the feasibility of employing digital tools like geographical visualization software to visually locate hot spots endemic to high prevalence of patients with chronic diseases. Given that population health management entails understanding the full picture of patient health including their SDOH, a tool that provides actionable information on where patients are coming from will result in targeted prevention, screening, diagnosis, intervention, post-care coordination, and an overall improvement in health for these patients. With increasingly available preventive and therapeutic interventions for patients at-risk of morbid obesity, the clinical team can readily find patients who will benefit from these interventions. Moreover, the availability of such a resource deployment tool provides opportunities for collaboration with local community partners in prioritizing and distributing resources to deprived areas.
As we strive for value-based and equitable healthcare, it has become nationally recognized that SDOH needs to be part of the healthcare discourse as the healthcare community works on devising a robust population health management strategy (Houlihan and Leffler, n.d). Unfortunately, the lack of systematic and standardized capture of social determinants of health at the patient-level pose inherent difficulties in using this information for actionable insights in delivery of patient care. Our study circumvents this challenge of capturing SDOH metrics in a standardized way, by leveraging publicly available neighborhood-level data on social determinants of health, and integrating this data into our electronic health records system to reveal health inequities and treatment gaps (Cantor and Thorpe, n.d).
4.1. Policy and practical implications
Findings from this study have implications for policy makers. Policies should be aimed at assessing and addressing the environmental and socioeconomic components of neighborhood that influence proximal behavioral risk factors of morbid obesity. (Gillman, 2015; Gillman & Ludwig, 2013) For this purpose, mapping ADI with tools such as those used in our analysis may help identify areas where those interventions are likely most needed. In addition, health organizations can use ADI for various purposes, including identifying high-risk morbid obese patients for intensive bariatric intervention and informing administrative planning decisions related to targeted outreach, delivery system design and redesign, disparities analysis, and resource allocation (in terms of community investment). Furthermore, ADI-enhanced predictive risk models are useful for multidisciplinary entities, including healthcare providers, disparity researchers, insurance companies, employers and governmental agencies seeking to leverage such data to better serve morbid obese patients with health-related social needs. Greater understanding of the interplay of socio-environmental context and morbid obesity is essential to moving closer to holistic healthcare delivery that fully encompasses the effects of genetics, environment and lifestyle factors.
4.2. Strengths, limitations and future perspective
Our study has several strengths. For one, given the large representative sample of HM patients, we are not short on statistical power. Secondly, the use of anthropometric measurements of height and weight measured using standardized approaches as part of clinical care at HM, as opposed to self-reported measures of height and weight in previous studies, increases internal validity. Thirdly, our study included a heterogeneous population, and we conducted a wide range of subgroup analyses by age, sex, and race/ethnicity, providing more insight into the associations between ADI quintiles and BMI categories, morbid obesity in particular, in each of those groups.
Despite the strengths of our study, it is not without limitations. The generalizability of our study to larger geographical boundaries like regions or the entire US nation is limited because our study population consisted of only one healthcare institution whose main catchment area is the Houston gulf region and surrounding counties. Nonetheless, we expect similar contributions of ADI to morbid obesity in other settings; in fact, the prevalence of morbid obesity at 7 % in our study was consistent with that in US national samples (Sturm & Hattori, 2013). Additionally, we excluded a substantial number of patients due to missing data; nonetheless, our final sample size was sufficiently large and adequately powered to provide reliable estimates of morbid obesity across ADI quintiles. We also compared demographic characteristics for participants with missing vs complete data, and found similar distribution of age, sex and race/ethnicity across the main subgroup types. Combined, these findings mitigate any concerns related to loss of power. Still, our study may have suffered from selection bias given the non-probability sampling of patients from our hospital system alone. Nonetheless, this risk is expected to be minimal since our hospital provides care to a diverse and substantial representation of patients from all racial/ethnic, sex, age and socioeconomic group, regardless of their financial status. Also, although ADI is a robust and validated measure of neighborhood-level social determinants of health, it still lacks other neighborhood-level predictors of disease outcomes including area crime rates, green space, and racial segregation (Johnson et al., 2021). Nevertheless, the independent associations between those features and obesity after accounting for the SDOH components included in the ADI are expected to be modest, if any at all. Lastly, the cross-sectional design employed in our study contributes to our inability to infer causality from this analysis. A more compelling picture of a potential causal and bidirectional relationship may be deduced from longitudinal or experimental studies. Future studies could focus on the mediating effect of neighborhood characteristics, like access to fitness amenities and healthy food options, neighborhood safety, walkability, and structural racism/discrimination (redlining), on morbid obesity in this region. An understanding of the causal pathway will inform policymakers on the needed resources to be prioritized in this region to reduce the burden of morbid obesity in socioeconomically deprived neighborhoods.
5. Conclusion
As evidenced from our study, publicly available location-based data, like the ADI, can be integrated into the electronic health record system and leveraged to further our understanding of the relationship between social determinants of health and BMI, particularly morbid obesity. This study revealed that increasing ADI was associated with prevalence of obesity. Patients with morbid obesity were also more likely to be found in areas with higher socioeconomic deprivation. With this association being established, measures of area deprivation can be used in identifying neighborhoods at high odds of having patients with morbid obesity for comprehensive intervention strategies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the anonymous reviewers for their constructive feedback.
Appendices
Appendix B.
The 17 components of the Area Deprivation Index.
| Domain | Variable |
|---|---|
| Education | % Population aged 25 years or older with<9 years of education |
| % Population aged 25 years or older with at least a high school diploma | |
| Income/employment | % Employed population aged 16 years or older in white-collar occupations |
| % Civilian labor force population aged 16 years and older who are unemployed | |
| Housing | % Owner-occupied housing units |
| % Families below federal poverty level | |
| % Population below 150 % of federal poverty level | |
| % Households with more than 1 person per room | |
| Median monthly mortgage in US dollars | |
| Median gross rent in US dollars | |
| Median home value in US dollars | |
| Poverty | Median family income in US dollars |
| Income disparity | |
| % Families below federal poverty level | |
| % Population below 150 % of federal poverty level | |
| % Households without a motor vehicle | |
| % Households without a telephone | |
| % Occupied housing units without complete plumbing |
Appendix C.
Interaction Effects between ADI and each stratifying group of age, gender and race/ethnicity.
| Characteristic | OR (95 % CI) |
|---|---|
| ADI X Age Group | 0.946 (0.942, 0.950) |
| ADI X Sex | 1.037 (1.030, 1.045) |
| ADI X Race/Ethnicity | 1.009 (1.007, 1.011) |
Data availability
Data will be made available on request.
References
- Arterburn D.E., Maciejewski M.L., Tsevat J. Impact of morbid obesity on medical expenditures in adults. Int. J. Obesity. 2005;29(3):334–339. doi: 10.1038/sj.ijo.0802896. [DOI] [PubMed] [Google Scholar]
- Assari S., Nikahd A., Malekahmadi M.R., Lankarani M.M., Zamanian H. Race by Gender Group Differences in the Protective Effects of Socioeconomic Factors Against Sustained Health Problems Across Five Domains. J. Racial Ethnic Health Disparities. 2016 doi: 10.1007/s40615-016-0291-3. [DOI] [PubMed] [Google Scholar]
- Booth H.P., Charlton J., Gulliford M.C. Socioeconomic inequality in morbid obesity with body mass index more than 40 kg/m(2) in the United States and England. SSM – Popul. Health. 2017;3:172–178. doi: 10.1016/j.ssmph.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth K.M., Pinkston M.M., Poston W.S.C. Obesity and the built environment. J. Am. Dietetic Assoc. 2005;105(5 Suppl 1):S110–S117. doi: 10.1016/j.jada.2005.02.045. [DOI] [PubMed] [Google Scholar]
- Cantor, M. N., & Thorpe, L. (n.d.). Integrating Data On Social Determinants Of Health Into Electronic Health Records. doi:10.1377/hlthaff.2017.1252. [DOI] [PMC free article] [PubMed]
- Cecchini M. Use of healthcare services and expenditure in the US in 2025: The effect of obesity and morbid obesity. PLOS ONE. 2018;13(11):e0206703. doi: 10.1371/journal.pone.0206703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheruvalath H., Homa J., Singh M., Vilar P., Kassam A., Rovin R.A. Associations Between Residential Greenspace, Socioeconomic Status, and Stroke: A Matched Case-Control Study. J. Patient-Centered Res. Rev. 2022;9(2):89–97. doi: 10.17294/2330-0698.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D.A. Obesity and the built environment: changes in environmental cues cause energy imbalances. Int. J. Obesity. 2008;32(7):S137–S142. doi: 10.1038/ijo.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Angelantonio E., Bhupathiraju S.N., Wormser D., Gao P., Kaptoge S., de Gonzalez A.B., Cairns B.J., Huxley R., Jackson C.L., Joshy G., Lewington S., Manson J.A.E., Murphy N., Patel A.V., Samet J.M., Woodward M., Zheng W., Zhou M., Bansal N., Hu F.B. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–786. doi: 10.1016/S0140-6736(16)30175-1/ATTACHMENT/E2A32D35-AEAE-445A-8FEB-FE6D9C03DA38/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson Z.M., Yoo E.H.E., Martin-Gill C., Roth R. Spatial methods to enhance public health surveillance and resource deployment in the opioid epidemic. Am. J. Publ. Health. 2018;108(9):1191–1196. doi: 10.2105/AJPH.2018.304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A. Obesity treatment and prevention: new directions. Karger Publishers; 2012. The economics of food choice behavior: why poverty and obesity are linked; pp. 95–112. [DOI] [PubMed] [Google Scholar]
- Durfey S.N.M., Kind A.J.H., Buckingham W.R., DuGoff E.H., Trivedi A.N. Neighborhood disadvantage and chronic disease management. Health Serv. Res. 2019;54:206–216. doi: 10.1111/1475-6773.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E.A., Ruhm C.J., Kosa K.M. Economic causes and consequences of obesity. Annu. Rev. Publ. Health. 2005;26(1):239–257. doi: 10.1146/annurev.publhealth.26.021304.144628. [DOI] [PubMed] [Google Scholar]
- Flegal, K. M., Carroll, M. D., Kit, B. K., & Ogden, C. L. (n.d.). Prevalence of Obesity and Trends in the Distribution of Body Mass Index Among US Adults, 1999-2010. doi:10.1001/jama.2012.39. [DOI] [PubMed]
- Flegal K.M., Harlan W.R., Landis J.R. Secular trends in body mass index and skinfold thickness with socioeconomic factors in young adult women. Am. J. Clin. Nutr. 1988;48(3):535–543. doi: 10.1093/ajcn/48.3.535. [DOI] [PubMed] [Google Scholar]
- Fouse T., Schauer P. The Socioeconomic Impact of Morbid Obesity and Factors Affecting Access to Obesity Surgery KEYWORDS Prevalence Obesity Economic impact Bariatric and metabolic surgery Access to care KEY POINTS. Surg. Clin. NA. 2016;96:669–679. doi: 10.1016/j.suc.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Fryar C.D., Carroll M.D., Afful J. Health E-Stats December 2020. Natl. Health Nutr. Examination Survey. 1960 doi: 10.1001/jama.2020.14590. [DOI] [Google Scholar]
- Galiatsatos, P., Woo, H., Paulin, L. M., Kind, A., Putcha, N., Gassett, A. J., Cooper, C. B., Dransfield, M. T., Parekh, T. M., Oates, G. R., Graham Barr, R., Comellas, A. P., Han, M. K., Peters, S. P., Krishnan, J. A., Labaki, W. W., Mccormack, M. C., Kaufman, J. D., & Hansel, N. N., 2020. The Association Between Neighborhood Socioeconomic Disadvantage and Chronic Obstructive Pulmonary Disease. 10.2147/COPD.S238933. [DOI] [PMC free article] [PubMed]
- Galiatsatos P., Woo H., Paulin L.M., Kind A., Putcha N., Gassett A.J., Cooper C.B., Dransfield M.T., Parekh T.M., Oates G.R., Barr R.G., Comellas A.P., Han M.K., Peters S.P., Krishnan J.A., Labaki W.W., McCormack M.C., Kaufman J.D., Hansel N.N. The Association Between Neighborhood Socioeconomic Disadvantage and Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstructive Pulmonary Dis. 2020;15:981. doi: 10.2147/COPD.S238933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirimoldi F.M., Schmidt S., Simon R.C., Wang C.P., Wang Z., Brimhall B.B., Damien P., Moffett E.E., Manuel L.S., Sarwar Z.U., Shireman P.K. Association of Socioeconomic Area Deprivation Index with Hospital Readmissions After Colon and Rectal Surgery. J. Gastrointestinal Surg. 2021;25(3):795–808. doi: 10.1007/S11605-020-04754-9/TABLES/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales, C. M., Carroll, M. D., Fryar, C. D., & Ogden, C. L., 2017. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018 Key findings Data from the National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/products/index.htm.
- Hill J.O. Understanding and Addressing the Epidemic of Obesity: An Energy Balance Perspective. Endocrine Rev. 2006;27(7):750–761. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- Houlihan, J., & Leffler, S. (n.d.). Assessing and Addressing Social Determinants of Health A Key Competency for Succeeding in Value-Based Care. doi:10.1016/j.pop.2019.07.013. [DOI] [PubMed]
- Hu M.D., Lawrence K.G., Bodkin M.R., Kwok R.K., Engel L.S., Sandler D.P. Neighborhood Deprivation, Obesity, and Diabetes in Residents of the US Gulf Coast. Am. J. Epidemiol. 2021;190(2):295–304. doi: 10.1093/aje/kwaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.E., Zhu J., Garrard W., Thoma F.W., Mulukutla S., Kershaw K.N., Magnani J.W. Area Deprivation Index and Cardiac Readmissions: Evaluating Risk-Prediction in an Electronic Health Record. J. Am. Heart Assoc. 2021;10(13):e020466. doi: 10.1161/JAHA.120.020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. O’Reilly Media, Inc; 2014. Communicating data with Tableau: Designing, developing, and delivering data visualizations. [Google Scholar]
- Knighton A.J., Savitz L., Belnap T., Stephenson B., VanDerslice J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. EGEMs. 2016;4(3) doi: 10.13063/2327-9214.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurani S.S., Lampman M.A., Funni S.A., Giblon R.E., Inselman J.W., Shah N.D., Allen S., Rushlow D., McCoy R.G. Association Between Area-Level Socioeconomic Deprivation and Diabetes Care Quality in US Primary Care Practices. JAMA Network Open. 2021;4(12):e2138438–e. doi: 10.1001/JAMANETWORKOPEN.2021.38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraia B.A., Karter A.J., Warton E.M., Schillinger D., Moffet H.H., Adler N. Place matters: neighborhood deprivation and cardiometabolic risk factors in the Diabetes Study of Northern California (DISTANCE) Social Sci. Med. (1982) 2012;74(7):1082–1090. doi: 10.1016/j.socscimed.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson N.I., Story M.T., Nelson M.C. Neighborhood environments: disparities in access to healthy foods in the US. Am. J. Prevent. Med. 2009;36(1):74–81. doi: 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Lovasi G.S., Neckerman K.M., Quinn J.W., Weiss C.C., Rundle A. Effect of individual or neighborhood disadvantage on the association between neighborhood walkability and body mass index. Am. J. Publ. Health. 2009;99(2):279–284. doi: 10.2105/AJPH.2008.138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed S.H., Habtewold T.D., Birhanu M.M., Sissay T.A., Tegegne B.S., Abuzerr S., Esmaillzadeh A. Neighbourhood socioeconomic status and overweight/obesity: a systematic review and meta-analysis of epidemiological studies. BMJ Open. 2019;9(11):e028238. doi: 10.1136/bmjopen-2018-028238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujahid M.S., Diez Roux A.V., Borrell L.N., Nieto F.J. Cross-Sectional and Longitudinal Associations of BMI with Socioeconomic Characteristics. Obesity Res. 2005;13(8):1412–1421. doi: 10.1038/OBY.2005.171. [DOI] [PubMed] [Google Scholar]
- Okuyama K., Li X., Abe T., Hamano T., Franks P.W., Nabika T., Sundquist K. Fast food outlets, physical activity facilities, and obesity among adults: a nationwide longitudinal study from Sweden. Int. J. Obesity. 2020;44(8):1703–1711. doi: 10.1038/s41366-020-0588-5. [DOI] [PubMed] [Google Scholar]
- Phelan J.C., Link B.G., Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J. Health Soc. Behav. 2010;51(1_suppl):S28–S40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- Powell-Wiley T.M., Ayers C., Agyemang P., Leonard T., Berrigan D., Ballard-Barbash R., Lian M., Das S.R., Hoehner C.M. Neighborhood-level socioeconomic deprivation predicts weight gain in a multi-ethnic population: Longitudinal data from the Dallas Heart Study. Prevent. Med. 2014;66:22–27. doi: 10.1016/j.ypmed.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig M.Q., Althouse A.D., Sabik L., Arnold R., Chu E., Smith T.J., Smith K., White D., Schenker Y. The Association between Area Deprivation Index and Patient-Reported Outcomes in Patients with Advanced Cancer. Health Equity. 2021;5(1):8–16. doi: 10.1089/HEQ.2020.0037/ASSET/IMAGES/LARGE/HEQ.2020.0037_FIGURE4.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets L.R., Henderson Kelley L.E., Scheitler-Ring K., Petroski G.F., Barnett Y., Barnett C., Kind A.J.H., Parker J.C. An index of geospatial disadvantage predicts both obesity and unmeasured body weight. Prevent. Med. Rep. 2020;18 doi: 10.1016/J.PMEDR.2020.101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobal J., Stunkard A.J. Socioeconomic status and obesity: a review of the literature. Psychol. Bull. 1989;105(2):260–275. doi: 10.1037/0033-2909.105.2.260. [DOI] [PubMed] [Google Scholar]
- Stafford M., Brunner E.J., Head J., Ross N.A. Deprivation and the development of obesity a multilevel, longitudinal study in England. Am. J. Prevent. Med. 2010;39(2):130–139. doi: 10.1016/j.amepre.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Sturm R., Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int. J. Obesity. 2013;37:889–891. doi: 10.1038/ijo.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger J.M., Moseley A.B., Cheung C.K., Osarogiagbon R.U., Symington B., Ramsey S.D., Hershman D.L. Persistent Disparity: Socioeconomic Deprivation and Cancer Outcomes in Patients Treated in Clinical Trials. J. Clin. Oncol. 2021;39(12):1339–1348. doi: 10.1200/JCO.20.02602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J.G., Quintana-Domeque C. Income and body mass index in Europe. Econ. Hum. Biol. 2009;7(1):73–83. doi: 10.1016/j.ehb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- What is VizQL? (n.d.). Retrieved February 13, 2022, from https://www.tableau.com/drive/what-is-vizql.
- Zhang Q., Wang Y. Socioeconomic inequality of obesity in the United States: do gender, age, and ethnicity matter? Social Sci. Med. (1982) 2004;58(6):1171–1180. doi: 10.1016/s0277-9536(03)00288-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.