Abstract
Introduction
Exposure to blue light has seriously increased in our environment since the arrival of light emitting diodes (LEDs) and, in recent years, the proliferation of digital devices rich in blue light. This raises some questions about its potential deleterious effects on eye health. The aim of this narrative review is to provide an update on the ocular effects of blue light and to discuss the efficiency of methods of protection and prevention against potential blue light-induced ocular injury.
Methods
The search of relevant English articles was conducted in PubMed, Medline, and Google Scholar databases until December 2022.
Results
Blue light exposure provokes photochemical reactions in most eye tissues, in particular the cornea, the lens, and the retina. In vitro and in vivo studies have shown that certain exposures to blue light (depending on the wavelength or intensity) can cause temporary or permanent damage to some structures of the eye, especially the retina. However, currently, there is no evidence that screen use and LEDs in normal use are deleterious to the human retina. Regarding protection, there is currently no evidence of a beneficial effect of blue blocking lenses for the prevention of eye diseases, in particular age-related macular degeneration (AMD). In humans, macular pigments (composed of lutein and zeaxanthin) represent a natural protection by filtering blue light, and can be increased through increased intake from foods or food supplements. These nutrients are associated with lower risk for AMD and cataract. Antioxidants such as vitamins C, E, or zinc might also contribute to the prevention of photochemical ocular damage by preventing oxidative stress.
Conclusion
Currently, there is no evidence that LEDs in normal use at domestic intensity levels or in screen devices are retinotoxic to the human eye. However, the potential toxicity of long-term cumulative exposure and the dose-response effect are currently unknown.
Keywords: Blue light; Light emitting diodes; Digital devices; Ocular hazard; Prevention; Ocular health, myopia, retina, macular pigment
Key Summary Points
| Exposure to blue light raises questions about its potential deleterious effects on eye health. |
| There is no evidence that light emitting diode (LED) light sources in normal use at domestic intensity levels, or used as backlights in screen devices, are retinotoxic to the human eye. |
| However, questions remain about the long-term cumulative exposure of blue light (and particularly LEDs emitting cold white light) and the potential deleterious effects on their ocular health in late adulthood, particularly for specific light-sensitive populations (young children, older pseudophakic individuals). |
| To prevent potential photochemical damages and photoreceptor loss, it is recommended to avoid LEDs emitting cold white light with a high level of blue component (lamp or luminous objects) in areas where children could be exposed. |
| While there is no evidence of a beneficial effect of blue blocking lenses for the prevention of eye diseases, a diet rich in lutein and zeaxanthin (natural blue-light filtering retinal pigments) and antioxidants (vitamins C, E, zinc, etc.) such as the Mediterranean diet, could contribute to the prevention of ocular photochemical damage. |
Introduction
Blue light is everywhere in our environment and is emitted mainly from the sun [1]. Exposure to blue light during the day is crucial to keep our biological needs in balance and affects our body and mind, both visually and nonvisually, mainly to regulate human behavior and circadian rhythm [2]. However, inappropriate lighting exposure (especially from artificial sources of blue light in the evening or at night) may lead to harmful effects on health [2]. Due to its high energy, blue light can cause and accelerate photochemical reactions and retinal cell damage [3].
Since the nineties, in an economic and ecological approach, incandescent bulbs have been progressively replaced by light emitting diodes (LED) lights, which have gradually replaced most conventional light sources [1, 2, 4]. In addition, in recent years, we have observed a proliferation of numerous sources rich in blue light, in particular digital devices (computer monitors, smartphones, tablets) containing LED backlighting technology [5]. As we spend more than 90% of our lifetime in an indoor environment [6], these two major technologies that have recently emerged (LED lights and the last generation of screens), have increased the exposure to manufactured light sources, with potential deleterious ocular effects [5]. Therefore, we are under longer and more intense exposures to artificial light (especially blue light) and spend more and more hours in front of screens emitting blue light [2]. In an American study, about 60% of the population self-reported spending more than 5 h per day in front of digital devices [7]. This high use of high-luminance displays concerns all age groups [7, 8]. Since the COVID-19 pandemic, there is a tremendous increase in screen devices usage, particularly due to the lockdowns and remote working [9, 10]. Along with an explosion of screen use and the extensive use of LED lights, a large number of elderly people are affected by age-related eye diseases. The baby boom generation is the first historically to be bathed in intense blue light, thus there is concern that excessive exposure to blue light could possibly lead to increased rates of eye diseases. Younger generations will probably experience longer periods of life with intense exposures. Blue light exposure is thus emerging as a potential serious health issue.
Therefore, some fears and a growing number of questions have been raised about the tolerability and safety of LED technology for human health, especially the potential ocular risks, due to the specific spectral and energy characteristics of white LEDs compared with other domestic light sources [11, 12].
In addition, to prevent possible deleterious effects of blue light, especially artificial blue light, different types of external (e.g., eyeglass lenses, screen protectors, specific software) or internal [e.g., intraocular lenses (IOL)] protection that (partially) filter blue light as well as several types of supplementations have flooded the market. However, is their effectiveness confirmed?
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
The objective of this narrative review is to provide an update on the ocular consequences of blue light exposure and to discuss the efficiency of methods of protection against potential blue light-induced injury on eyes.
Methods
The search of relevant articles was conducted using the following databases: PubMed, Medline, and Google Scholar until December 2022. According to the different topics examined in the present narrative review, several keys words have been used:
(blue light) or (blue light) and effect* and health*
(blue light or blue light) and effect* and (eye* or ophthalmo* or vision* or ocular* or visual disorder or retina* or macula*)
(blue light filtering) or (blue light blocking) or (blue light-filtering) or (blue light-blocking)
(blue light or blue light or blue-blocking) and (prevent* or protect* or advice* or guideline* or blocking or filtering)
(blue light or blue light) and (filter or eye or retina or macula) and (antioxidant or carotenoid or lutein or zeaxanthin or nutrition or vitamin)
Original articles, reviews, meta-analyses, randomized controlled trials, and epidemiological studies were included. Only articles in English have been included. No selection was done on the type of population studied (human population, animal models, or in vitro trials were included). Reference lists of original research articles and reviews were also checked to identify relevant articles.
Results
Blue Light
Definition of Light
Light is an electromagnetic radiation composed of electromagnetic particles (photons) that move in waves emitting energy of varying range and strength. The shorter the wavelength, the greater the emitted energy. It can be categorized in large bands: gamma rays (less than 0.01 nm), X-rays (0.01 to 10 nm), ultraviolet (UV) rays (100–380 nm), visible light (380–760 nm), infrared light (760–10,000 nm), and radio waves (microwave, TV, radio, greater than 10,000 nm). Together, these wavelengths compose the electromagnetic spectrum [1, 4].
The human eye reacts only to the visible light from violet (380 nm) to red light (780 nm) [4, 13]. This part of the electromagnetic spectrum corresponds to the colors violet, indigo, blue, green, yellow, orange, and red. Blue light is the highest energy band of the visible spectrum (from 380 to 500 nm) and can be divided into two main categories: blue–violet and blue–turquoise. The blue–violet (380 to 450 nm) portion of the spectrum is also known as high-energy violet (HEV) [14]. It passes through the cornea and lens of the eye and can reach the retina. In laboratory settings on animal subjects and tissues, blue–violet light causes cumulative and lasting damage to retinal structures [15–17]. In particular, it causes oxidative stress that leads to the destruction of photoreceptor cells, which process light to create vision, and leads to apoptosis in primary Müller cells [18]. Most of the blue light research has studied varying blue–violet wavelengths from 405 to 455 nm. The blue–turquoise (450 to 500 nm) portion of the spectrum passes through the cornea and lens to reach the retina. Light in this range reduces melatonin levels and, therefore, has a strong influence on circadian rhythms. Moreover, light stimulates the release of dopamine and serotonin, two neurotransmitters that modulate, in part, mood [19].
Four fundamental photometric quantities characterize the light and color sensations of the human eye [4]:
The luminous intensity (candela, cd), representing the light intensity of an optical source, as perceived by the human eye.
The luminous flux (lumen) is used to express the luminous energy emitted per unit time by a light source and represents the light power of a source perceived by the human eye.
The illuminance (lux) is the amount of luminous flux per unit area and is used to characterize illumination condition.
The luminance (cd/m2) of a surface source is the luminous flux per unit solid angle and unit projected area, representing the notion of brightness [1].
Different Sources of Blue Light
Blue light is produced by both natural and artificial sources. The sun is the first producer of natural blue light. The intensity of the solar blue light spectrum fluctuates during the day, with the maximum at noon and much less at sunrise and sunset. The human body has evolved to use these differences to keep our circadian rhythm in time with our environment. Solar blue light also depends on the latitude, altitude, meteorological conditions, and season. Solar radiation of daylight consists of 24–30% blue light on average, according to the standards ASTM G173-03 and D65 [1]. The luminance of the sun at noon is 1.6 × 109 cd/m2. The luminance of a clear blue sky is around 5000 cd/m2 (compared with 300 for a TV display and 150–250 cd/m2 for a computer screen). The other sources of natural blue light are the moon and flames.
Blue light is also produced by artificial sources that vary widely in their spectral distribution, mainly from LED technology. LEDs generally produce a small wavelength spectrum compared with the phosphor emission spectrum [4]. Its conversion effectiveness depends on the emitting wavelength. Until the 1990s, LEDs existed only in red, yellow, or green, and were mainly used as indicator lights in electronic equipment such as remote controls or alarm clocks. With the creation of the first blue LED, it became possible, by covering it with a layer of yellow phosphor, to create a white light that is intense enough to be used in lighting. Since then, technological research has been constantly improving their performance, focusing on materials or combinations of different types of LEDs [20].
Besides their emission of relatively high levels of blue light, they can vary also in their brightness and color composition. The correlated color temperature (CCT) in Kelvin is also used to describe the perceived color (LED 2700–6500 K). The CCT enabled the shade of the color (cold or hot light) to be defined. Light with short wavelength is named “cold white” ranging around 5500 K.
Three methods of combination can produce white light from a LED [4]:
The first method is a diode emitting at a short wavelength coupled with a phosphor emitting at a larger wavelength producing, when arriving simultaneously on the human eye, the white light sensation. Today, this is the method the most frequently used to produce high brightness white LEDs and for domestic lighting.
The second method is a diode emitting in the near ultraviolet coupled with at least one phosphor, intrinsically avoiding direct emission of blue light. This method produces very high-quality white light with good color rendering.
The third method is at least three diodes (one of each of the fundamental color: red, green, and blue), producing white light when they are combined themselves. This method is used mainly for scenic and decorative lighting.
The flux emitted by a LED may be moderate but its luminance may be extremely high. For instance, the luminance for a LED emitting a luminous flux of 212 lm, has been estimated to be of 6.2 × 107 cd/m2 by ANSES (the French Agency for Food, Environmental, and Occupational Health & Safety), which is significantly higher than other domestic light sources [4]. In addition, future LEDs will have a higher luminance with the expected increase of LED luminance efficacy [4].
LED technology is used today in lighting systems (home and public lighting, vehicle lights, illuminated signs, domestic lighting, architectural and street lighting, car headlights). Residential lighting has changed considerably over the past two decades, from traditional incandescent bulbs to LEDs [21, 22]. In Europe, since 2016, no more incandescent lights are commercialized for domestic lighting. Nowadays, the main sources of household lighting are compact fluorescent lamps (CFL), halogen, and LED lighting [22–24]. In 2019, it was estimated that nearly half of all light sources in the world were LED. The projection for 2030 is that over 87% of all light sources will be LED [25].
Besides the use of LED technology in lighting systems, this broad-spectrum light is also used in a large range of digital screen devices (portable lighting, light-emitting screens such as televisions, computers, tablets. and smartphone screens) [26]. A considerable amount of time is spent using computers, smartphones, and tablets, with peak use during the day, and for younger individuals there is a peak during the evening or at night [27]. Thus, the use of digital display technologies during the day and at evening or at night can expose people to relatively high quantities of blue light in addition to normal daylight hours [21].
Over the past decade, non-institutionalized older American subjects aged 60 years and older have spent more than 4 h a day on their TVs, computers, tablets, or other electronic devices, and this trend has increased about 30 min per day [28]. The time spent per day on digital devices was more than 8 h for American teenagers and almost 6 h for children ages 8–12 years old [7].. Among them, almost 67% used at least three devices simultaneously. A study from the UK reported that 83% of the teenagers aged 12–15 years had their own smartphone and 50% their own tablets, versus 35% and 47%, respectively of 8–11-year-old children and 5% and 42% of 5–7-year-old children [29]. Regarding online games, 36% of children aged 3–4 years and 63% of 5–7-year-old children played online games for 6.5 h or more per week, and 74% of 8–11-year-old children and 76% of 12–15-year-old teenagers played online for 10 h or more per week. Among the teenagers with a smartphone, 71% take it to bed [29]. This rise in screen time coincides with significant growth in the adoption of digital technology by older Americans. In 2000, 14% of adults aged 65 years and older were internet users compared with 73% today. Around the turn of the twenty-first century, smartphone ownership was uncommon in all age classes. Currently, 53% of people 65 years and older own a smartphone [28].
Artificial light sources with luminance above 10,000 cd/m2 are damaging when viewed by the unprotected eye. Sunlight, arc welding, plasma cutting, and discharge lamp arcs have extremely high effective radiations for very short exposure times (0.6–40 s) [4, 30]. These data suggest that viewing these light sources is very hazardous to the retina and that over time, exposure to blue light can cause long-term damage to eyes [4, 30].
Effects of Blue Light on Eyes
Visual and Nonvisual Reception by the Eye
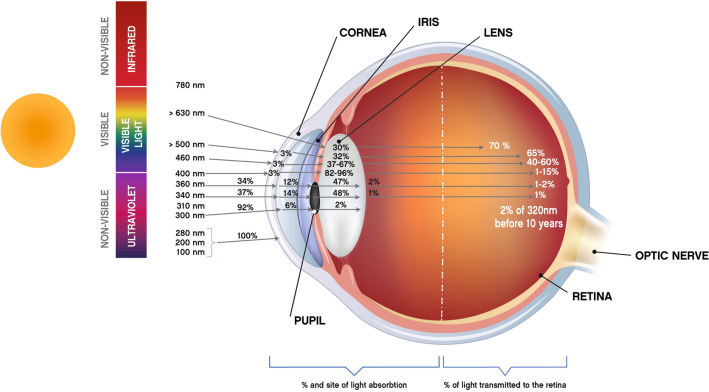
The whole sunlight spectrum (including blue light) or any artificial light source is received by the eye, sending environment information to the brain throughout visual and nonvisual processes via the retina [31, 32]. The radiations emitted by sunlight or artificial light are either absorbed or transmitted by the different eye tissues (the cornea and lens) and media before reaching the retina [4, 33, 34] (Fig. 1) (Table 1). Light entering the eye passes through these different transparent media, which focus the light on the retina where the photosensitive proteins (rhodopsin in the rods, opsin in the cones) are activated, initiating phototransduction and the triggering of a nerve impulse that will reach the brain to construct an image.
Fig. 1.
Solar radiation and filtration by the structures of the eye (adapted from F. Behar Cohen [4]).
Adapted from Behar-Cohen F, Martinsons C, Viénot F, Zissis G, Barlier-Salsi A, Cesarini JP, et al. Light-emitting diodes (LED) for domestic lighting: Any risks for the eye? Prog Retin Eye Res. 2011;30(4):239–57, Copyright (2023), with permission from Elsevier
Table 1.
Effects of blue light on eyes—Wavelengths absorption of different eye tissues and potential mechanisms of effects of blue light
| Eye tissue | Wavelengths absorption | Percentage of blue light passing through the eye structure | Potential mechanisms | References |
|---|---|---|---|---|
| Ocular surface (tear film, corneal epithelial tissue, conjunctival tissue) | Ultraviolet B (< 295 nm) | Transmits all wavelengths ≥ 295 nm |
Ocular surface inflammation Oxidative stress damage Cell apoptosis |
[4, 5, 36–39] |
| Lens |
Ultraviolet A and B (295 to –390 nm and a part of the near infrared wavelengths In young adults; peak of absorption around 365 nm At 60 or 70 years old; peak of absorption around 400 nm |
In young children, around 80–90% of blue light at 450 nm passes through the lens At about 25 years old, 20% of the light between 400 and 460 nm and 50% of wavelengths between 400 and 500 nm are transmitted to the retina; In the elderly, the transmission of blue light to the retina is notably reduced due to the yellowing of the lens absorbing most of the blue light |
Photobiological damage Oxidative stress Cell apoptosis |
[4, 41–43, 48] |
| Retina |
Visible (380–780 nm) Near infra-red (780–1400 nm) |
Photomechanical damage, caused by high irradiance and short exposure independently of the wavelength of light (e.g., therapeutic laser with YAG for iridotomy and capsulotomy); photothermal damage, caused by long exposure (e.g., therapeutic laser photocoagulation); photochemical damage, caused by incident radiation with wavelength in the high-energy portion of the visible spectrum, mainly blue light; Increase in ROS production (loss of photoreceptors, lipid peroxidation, and cell apoptosis); Activation of inflammatory reactions, DNA damage, inhibition of mitochondria, and lysosome function |
[51] [52] |
DNA deoxyribonucleic acid, LED light emitting diodes, nm nanometer, ROS reactive oxygen species
Blue light plays also a major role in the nonvisual functions and is responsible for the regulation and synchronization of our biological functions (e.g., circadian rhythm) [35]. These nonvisual functions are regulated by a subset of retinal ganglion cells that express the photopigment melanopsin, rendering them intrinsically photosensitive (ipRGCs) [35]. Melanopsin is a blue-light sensitive pigment with a maximum sensitivity between 460 and 480 nm [35]. The ipRGCs directly signal the hypothalamus, affecting the modulation of different processes (especially the circadian rhythm) [35]. The blue–turquoise light (450–500 nm) is crucial to synchronize our circadian rhythm and essential to maintain good health and well-being. Lack of blue light or inappropriate exposure to blue light results in desynchronization of the circadian rhythm and thus can lead to disturbance of sleep and alertness, seasonal affective disorder, as well as alterations of memory and cognitive performance [35].
Despite several protective mechanisms of the eye, under certain conditions, blue light can cause damage to the eye and particularly to the retina.
Effects of Blue Light on the Ocular Surface
The ocular surface is the first barrier against irradiant energy and is vulnerable to light hazard, which can potentially harm the ocular surface and intensify dry eye symptoms [4, 36]. Almost all the radiant energy below 295 nm (all UVC and most UVB) is absorbed by the cornea (Fig. 1) (Table 1).
Beyond the well-known effects of ultraviolet light, long-term exposure to blue light with short wavelengths may alter the ocular surface by three main mechanisms: oxidative stress damage, ocular surface inflammation, and cell apoptosis [4, 5, 36–40] (Table 2). These can increase the ocular phototoxicity of blue light in subjects with dry eyes or contribute to the development and pathogenesis of dry eyes [5].
Table 2.
Potential effect of blue light irradiation on ocular surface and lens
| Reference, year | Eye tissue | Species (subject/tissue/cells/animals) | Light sources of irradiation, exposure times | Effect of blue light irradiation |
|---|---|---|---|---|
|
Marek et al., 2018 [36] |
Ocular surface | In vitro; human conjunctival and corneal epithelial cells |
Xenon-based device, 380–525 nm; LED-based fiber device, 390, 420, 430, 480, and 630 nm; Exposure time: 17 h |
Alteration of the functioning of mitochondrial membrane; Inflammation; Decrease of the functioning of the cellular defense system (antioxidant protection); Alteration of cellular morphology; Overproduction of ROS; Decreases cellular viability; Oxidative stress; Conjunctival epithelial cells more prone to blue light phototoxicity than corneal epithelial cells Hyperosmolar stress |
|
Yamaguchi et al., 2018 [38] |
Ocular surface | Mice |
LED, 410 nm; Exposure time: 10 days |
Lipid oxidative stress; Inflammation and tissue damage; Inflammation of T cells; Corneal opacity and neovascularization; Dry eye disease |
|
Lee et al., 2014 [39] |
Ocular surface | In vitro; human conjunctival and corneal epithelial cells |
LED, 410, 480, 525, 580, 595, 630, and 850 nm; Exposure time: 24 h |
Decrease of corneal epithelial cell viability; Overproduction of ROS; Ocular surface damage |
|
Niwano et al., 2014 [40] |
Ocular surface | In vitro; human corneal epithelial cells |
LED, 405 nm; Exposure time: 3 min |
Decrease of corneal epithelium cell viability (in a dose- and time-dependent manner); Oxidative stress |
|
Xie et al., 2014 [47] |
Lens | Human lens epithelial cells |
LED, white light with CCTs of 2954, 5624, and 7378 K; Exposure time: First group exposed to three light–dark cycles of 16 h/8 h versus second group (control) in darkness |
Overproduction of intracellular ROS; LED light with a CCT of 7378 K; Decrease of cell viability; Severe DNA damage; Cell-cycle arrest; Apoptosis |
|
Haag et al., 2021 [49] |
Lens | Ex vivo, porcine lenses |
UV lamp, 311 nm (UVB), 370 nm (UVA), and LED, 460 nm (blue light); Exposure time: 24 h |
Cataract development for radiation with 311, 370, and 460 nm (irradiation wavelength causing the most cataract) |
|
Zeller et al., 2022 [50] |
Lens | Ex vivo, Porcine lenses |
LED, 407 nm (violet), 463 nm (blue), 635 nm (red) Exposure times: 24 h |
Irradiation with all three wavelengths induce cataract Irradiation with a wavelength of 407 nm (violet) exhibits the strongest cataract formation |
AL axial length, CCT correlated color temperatures, DNA deoxyribonucleic acid, K kelvin, LED light emitting diodes, nm nanometer, UV ultraviolet, ROS reactive oxygen species
Effects of Blue Light on the Lens
The short wavelengths in UVB (295–315 nm), all UVA wavelengths (315–390 nm), and a part of the near-infrared wavelengths are absorbed by the human lens. With age, the absorption of blue light by the lens changes, with shorter wavelengths in the blue and violet regions of the visible spectrum more prominently affected (41–43) (Fig. 1) (Table 1).
Long-term UV exposure is a recognized risk factor for cataract [44]. Blue light may induce also photodynamic damages in aging lenses. The absorption of blue light in the lens is produced by the structural proteins, protein metabolites, and enzymes absorbing blue light-producing yellow pigments, in particular through the production of reactive oxygen species (ROS) in the lens epithelial cells mitochondria [45–48]. This leads gradually to the lens darkening and yellowing, leading to cataract [45, 49, 50] (Table 2).
Effects of Blue Light on the Retina
The wavelengths reaching the retina are restricted to the visible part of the electromagnetic spectrum (380–780 nm) and a part of near infrared wavelengths (780–1400 nm). Three mechanisms are involved in light-induced damage on the retina: (1) photomechanical damage [51]; (2) photothermal damage [52]; and (3) photochemical damage [53–56] (Table 1). Because of its high energy, blue light induces and accelerates photochemical reactions and cellular damage via the production of ROS, contributing to the loss of photoreceptors, lipid peroxidation, and cell apoptosis [57, 58].
There are two types of retinal damage due to phototoxicity depending on the total dose received, including the irradiance and the exposure duration [59], and the type of affected cells.
The first types of damage are due to long periods of exposure (days to weeks) with low irradiances and affect the photoreceptors whose wavelengths are activated [60] (particularly rhodopsin that affects photoreceptors [61, 62]).
The second types of damage are due to short exposure (minutes to hours) with high irradiances of white light, and the damage is at level of the retinal pigment epithelium (RPE) [63] (leading to structural changes [64]).
A blue light wavelength in the blue–violet spectrum with long duration exposure produces ROS in the retina, which react with specific DNA components or the cell membranes, leading to cell dysfunction or cell apoptosis [57, 58]. A large number of studies on animal models or human retinal cells have reported severe photochemical injury of the retina induced by excessive exposure to blue light with wavelengths between 400 and 500 nm (blue light hazard) [60, 65–71] (Table 3). Studies that have investigated this topic, as well as proposals for pathways and signaling mechanisms triggered by blue light in the retina, are detailed in recent reviews [5, 72]. As yet, the exact mechanism is not elucidated and remains an area of ongoing research.
Table 3.
Potential effect of blue light irradiation on the retina
| Reference, year | Species (subject/tissue/cells/animals) | Light sources of irradiation, exposure times | Effect of blue light irradiation |
|---|---|---|---|
|
Arnault et al., 2013 [16] |
Porcine eye RPE cells |
LED and filter, 10 nm illumination bands centered from 380 to 520 nm in 10 nm increments; The intensity of each band was calculated according to the solar intensity received by the retina after filtering by the optics of the eye; Exposure times: 18 h |
Loss of cell viability (maximal for wavelengths from 415 to 455 nm) |
|
Noell et al., 1966 [60] |
Rat |
Fluorescent lamps Monochromatic light of various wavelengths: 1200 to 2500 lx or green filter; Variation of the temperature; Exposures times: 1–2 days |
Continuous exposure to visible light to moderate levels of damaged photoreceptor cells Light damage classified into two types: class I (damage induced by low-intensity light exposure for long durations) and class II (damage induced by relatively high-intensity light exposure for short periods) |
|
Van Norren et al., 1990 [65] |
Rat |
Xenon light, white, irradiant dose from 4 J/cm2 at 379 nm to 2000 J/cm2 at 559 nm; Narrow band spectral light; Exposure times: 10 s to 1 h |
Susceptibility for damage sharply increased towards the ultraviolet Susceptibility to photic injury in rat is comparable to that in primates |
|
Marie et al., 2020 [66] |
Porcine retina cone receptors |
LED, 10 nm wavelength bands between 390 and 510 nm, plus the 630 nm band; Exposure times: 15 h |
The near UV visible range (415 and 455 nm) is the most toxic wavelengths; Toxicity occurred in the blue–violet light (425–445 nm) for exposures at intensities of sunlight received by the retina; Macular degeneration; Retinitis pigmentosa; The toxicity originates from a porphyrin |
|
Krigel et al., 2016 [67] |
Retina of albinos and pigmented rats |
LEDs (cold white, blue, and green), fluorocompact bulbs, fluorescent; Exposure times: 24 h; Exposure at high luminance was compared with a cyclic (dark/light) exposure at domestic levels for 1 week and 1 month |
Phototoxicity; Blue light component emitted by white LEDs at usual domestic luminance induced more retinal degeneration and the development of necrosis than other light sources |
|
Chamorro et al., 2013 [68] |
Human retinal pigment epithelial cells |
LED: 468 nm (blue), 525 nm (green), 616 nm (red), and white light; Exposure times: three cycles of light–darkness (12 h/12 h) |
LED radiations: Decrease in cellular viability; Increase in cellular apoptosis; Increase in ROS production; Increase in DNA damage; Apoptosis more important in cells exposed to white and blue Three light–darkness (12 h/12 h) cycles of exposure to LED lighting affect in vitro human retinal pigment epithelial cells |
|
Kuse et al., 2014 [69] |
Murine photoreceptor-derived cells (661 W) |
LEDs: 464 nm (blue), 456–553 nm (white), 522 nm (green); Exposure times: 24 h |
Blue LED light Increase of ROS production; Alteration of the protein expression level; Blue and white LED Aggregation of short-wavelength opsins (S-opsin), leading to severe cell damage; Damage of retinal cone photoreceptor cells; N-Acetylcysteine (antioxidant) protected against the cellular damage induced by blue LED light |
|
Abdhou et al., 2022 [70] |
Human RPE cells |
Solar simulator and blue light filtering IOL Cells were exposed or not to BL, with the absence or presence of either a CIOL < 400 nm, or a YIOL Exposure time: 30 minn |
Blue light is deleterious to RPE cells due to increased oxidative stress and cell death Blue light increased cellular and mitochondrial total ROS levels These effects were attenuated by filtering this radiation. YIOL decreased cellular and mitochondrial ROS levels The increase in ROS production was coupled with an increase in cell death, which decreased when cells were protected with YIOL Pretreatment of cells with N-acetylcysteine abolished the increase in cell death |
|
Wu et al., 1999 [71] |
Rat retina |
Fluorescent lamp, Blue light 400–480 nm of 0.64 W/m3; Exposure times: 3–6 h |
Photoreceptor cell apoptosis |
|
Shang et al., 2017 [93] |
Sprague–Dawley rat |
LEDs: 460 nm (blue), 530 nm (green), 620 nm (red); Exposure times: from 3–9 to 28 days under a 12 h dark/12 h light cycle |
Blue LED group induced more: functional damage photochemical injury (apoptosis and necrosis of photoreceptors and RPE) oxidative stress than that of green or red LED groups |
|
Jaardane et al., 2015 [94] |
Wistar Rats |
LEDs (blue region), and 449 nm, 467 nm, 473 nm, 507 nm; Exposure times: 6, 12, 18, 24, 48, and 72 h |
Oxidative damage and retinal injury; Retinal degeneration; Loss of photoreceptors: Activation of caspase-independent apoptosis, necrosis, necroptosis; Wavelength dependence of the effects |
AMD age-related macular degeneration, CCTs correlated color temperatures, CIOL clear UV-filtering IOL, DNA deoxyribonucleic acid, IOL intraocular filtering, K kelvin, LED light emitting diodes, nm nanometer, RCT randomized clinical trial, RPE retinal pigment epithelium, ROS reactive oxygen species, UV ultraviolet, VEGF vascular endothelial growth factor, YIOL yellow UV- and BL-filtering IOL
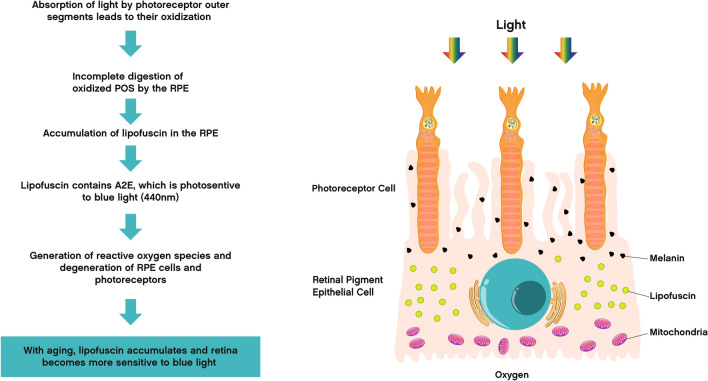
Briefly, in rod photoreceptors, absorption of a photon by rhodopsin induces isomerization and release of 11-cis-retinal to all-trans-retinal. Free all-trans-retinal is not only toxic as a reactive aldehyde, but also exhibits high sensitivity to blue light [73]. Under moderate light exposure conditions, all-trans-retinal is continuously recycled to 11-cis-retinal by RPE cells and is not hazardous for cells. When light exposure is longer or more intense, all-trans-retinal accumulates and its activation by blue light can cause oxidative stress that damages the cellular components of the outer segment of the photoreceptors. This oxidative stress is usually neutralized by the presence of various antioxidants in the retina. However, with age, and certain genetic and environmental factors such as smoking or a diet low in antioxidants, the antioxidant defenses are reduced and are no longer able to counteract the stress induced by prolonged or intense exposure to blue light [74]. RPE cells have the function of renewing the outer segment of the photoreceptors. They remove the distal portion by ingestion or “phagocytosis,” while the growth of these outer segments is continuous [75]. Photoreceptor outer segment phagocytosis by RPE cells is essential to maintain the normal function and physiological structure of the retina. When the outer segments are excessively damaged by oxidative stress, their membrane components are difficult to degrade by RPE cells. Intracellular digestion is then incomplete and generates an accumulation of lipofuscin granules, which have a high content of polyunsaturated lipids, which are targets of oxidation. Moreover, lipofuscin contains A2E, an autofluorescent pigment, with an absorption maximum in the blue range at 440 nm [76, 77]. Photoactivation of lipofuscin granules by blue light generates ROS (superoxide, hydrogen peroxide, lipid hyperoxides, and malondialdehyde) [78, 79]. Once the number of these species exceeds the capacity of the cellular defenses, RPE cells die by apoptosis. Deprived of these support cells, photoreceptors may degenerate. These processes are similar to those observed in age-related macular degeneration (AMD), in which the cones degenerate, while the RPE, located in contact with the cones, accumulates phototoxic components [80] (Fig. 2).
Fig. 2.
Photochemical damage of the retina
The long-term effects of light exposure are difficult to assess, mainly because of the difficulty of accurately measuring such exposure. In particular, the link between sun exposure and the development of AMD has been widely debated [81–83]. Some epidemiological studies have reported that light exposure is a potential risk factor for the development of AMD [81] and that blue light has a potential contributing effect higher than any other wavelength in the electromagnetic spectrum [5, 84].
However, studies investigating associations of light exposure in human populations have reported conflicting results [85–91], which has led to controversy on this issue [81, 83]. The different studies have been detailed in two reviews in this field [81, 83]. Exposure to sunlight is not limited to blue light but also includes UV radiation, and it is difficult to disentangle the effects of the different wavelengths in epidemiological studies.
To better assess the sensitivity of the retina to precise wavelengths, research was performed on RPE cells in pig eyes [16, 66] (Table 3). They reported that the most damaging wavelengths were between 415 and 455 nm (blue–violet wavelengths) [16], for a given exposure intensity, the most toxic wavelengths for cone photoreceptors, were in the near UV visible range and, for exposures at intensities comparable to sunlight, toxicity appeared in the blue–violet (425–445 nm) [66]. Cone photoreceptors are most likely the main target of light-induced AMD. This study reinforces the importance of very precise blue–violet light filtering to prevent cone pathologies such as AMD. However, in these studies, the cells were exposed to blue–violet light for 15–18 consecutive hours at one time. Thus, exposure for 1 day to these intense lights should not induce toxicity, but the daily accumulation of this exposure could trigger these neurodegenerative processes.
Humans are currently over-exposed to artificial light rich in blue light such as LEDS, in particular at night. In vitro [68, 92] and animal studies [93] show increased photoreceptor mortality following exposure to commercially available white LEDs (Table 3). The effects of LED exposure on the retina together with those of other lighting sources, such as compact fluorescent bulbs and fluorescent tubes, have been tested in several studies. However, intense acute exposures to high luminance lighting systems have often been used in various models of light-induced retinal degeneration. Limited studies have estimated the effects of different light sources in conditions close to domestic use [67, 94] and suggested that the blue component of the white LED may cause retinal toxicity at usual domestic illuminance, and not only in extreme experimental conditions (Table 3). However, these studies were carried out in the short term (1 month maximum). In addition, extrapolations of in vitro to in vivo in animal experiments are challenging, making it difficult to extrapolate these results to real conditions of exposure. Most of these experiments do not mimic what happens in living eyes [95]. Findings from animal experiments are difficult to extrapolate to human retina, especially those on mice and rats as these animals do not have a macula and therefore do not mimic human retina characteristics.
The European Scientific Committee on Health, Environmental and Emerging Risks (SCHEER, providing opinion on risks that may negatively impact health and the environment to the European Commission) stated in a June 2018 report that there was no evidence of risk under normal conditions of use (below the international recognized ICNIRP exposure limits, LEDs form GRP 0 and GRP 1) [96]. It is the dose that would be dangerous. The brightness of the screens are 100 times lower than the doses that could be dangerous. On the other hand, the cumulative effect of this exposure could lead to potential sequelae in the long term. The consecutive time spent in front of the screens would be the main determinant. The current regulations and standards have been established on the basis of acute light exposure and do not consider the effects of repeated exposure [4]. The question is whether these very low doses during very long daily exposure times and throughout life can be harmful. Future studies are needed to better understand the mechanisms of photochemical injury and to determine the presence of potential long-term effects of blue light. However, to date there is no evidence that LEDs, in normal use at domestic intensity levels, or used as backlighting for computer screens, are retinotoxic to human eyes. Furthermore, there is not enough scientific data on the potentially deleterious effects of exposure beyond normal conditions of use. Studies investigating the dose-response relationships of LED exposure are also needed.
Effects of Blue Light on Refractive Development
Extensive research has reported that outdoor activities can prevent the occurrence and progression of myopia [97–101]. Higher time spent indoors can increase myopia, which has been reported after COVID-19 confinement [102, 103], due to lack of exposure to daylight (Table 4). Other factors mentioned were the major increase in screen use during confinement. In children of the birth cohort study (Generation R study), increased computer use was associated with myopia development [104]. In teenagers of the same cohort, continuous smartphone use was associated with greater refractive error, particularly in those with low outdoor exposure [105] (Table 4).
Table 4.
Potential effect of blue light irradiation on refractive development, human studies
| Reference, year | Species (subject) | Light sources of irradiation, exposure times | Effect of blue light irradiation |
|---|---|---|---|
|
He et al., 2015 [98] |
Asian school-aged children, RCT | Additional 40 min class of outdoor activities for intervention group | The cumulative incidence of myopia was lower in the intervention group (with the addition of outdoor school activities) |
|
Wu et al., 2020 [99] |
Asian school-aged children | Outdoors for 120 min every day | Continuous decrease of the prevalence of reduced visual acuity after intervention implementation in school children |
|
Jin et al., 2015 [100] |
Chinese school-aged children | Additional 20 min class outside classroom | Incidence of myopia, changes in refractive error towards myopia, change in axial length were lower in the intervention group |
|
French et al., 2013 [101] |
Australian cohort of school-aged children and adolescents, prospective study | Time spent outdoor/daylight/sun exposure | Less time spent outdoor and greater levels of near work significantly associated with incident myopia |
|
Wand et al., 2021 [102] |
Chinese school-aged children, prospective cross-sectional study | Effect of restriction of time spent in outdoor activities due to COVID-19 confinement and time and increased screen time | Prevalence of myopia was higher after the confinement than the years before |
|
Hu et al., 2021 [103] |
Chinese school-aged children, prospective study | Effect of restriction of time spent in outdoor activities due to COVID-19 confinement and increased digital learning | Incidence of myopia and axial length elongation increased after the COVID-19 confinement period |
|
Enthoven et al., 2020 [104] |
Dutch school-aged teenagers, prospective study | Computer use, outdoor exposure, reading distance |
Computer use was moderately associated with myopia development The effect of combined near work was decreased by outdoor exposure |
|
Enthoven et al., 2021 [105] |
Dutch school-aged children, cross-sectional study | Smartphone use, outdoor exposure | Myopic refractive errors were higher in teenagers with episodes of 20 min of continuous use of smartphone, particularly in those with low outdoor exposure |
AL axial length, CCT correlated color temperatures, DNA deoxyribonucleic acid, K kelvin, LED light emitting diodes, nm nanometer, UV ultraviolet, ROS reactive oxygen species
Sunlight is essential for the correct functioning of the retina as it allows for greater production of dopamine, a neurotransmitter that directly affects the retina. Dopamine secretion in the retina is stimulated by light, and has an inhibitory effect on eye growth [106].
Some animal models have suggested that blue light, and in particular blue–violet light (360–400 nm), had a suppressive effect against myopia progression and is the most ideal light for myopia control for efficiency and safety [107–122] (Table 5). In addition, the time (i.e., morning versus evening) of blue–violet light exposure may have a different impact on ocular growth. Exposure to monochromatic blue light in the evening may increase ocular growth relative to white light controls for all illuminance conditions (0.15, 200, 600 lx) except bright condition (1000 lx), whereas morning exposure would have no effect on eye growth [113] (Table 5).Myopia progressed less rapidly in a group of myopic children fitted with corrective contact lenses that are transparent to violet light (some lenses are transparent to violet, while others filter out about 50%) [108]. If these findings are confirmed in future epidemiological studies, the usefulness of anti-blue light optical lenses, particularly for myopic children, will be questionable.
Table 5.
Potential effect of blue light irradiation on refractive development, animal studies
| Reference, year | Species (subject/tissue/cells/animals) | Light sources of irradiation, exposure times | Effect of blue light irradiation |
|---|---|---|---|
|
Ashby et al., 2010 [106] |
Chickens |
Experiment 1: 500 lx (normal illuminance) or 15,000 lx (high ambient illuminance) Monocular lenses: −7 or +7 D Exposure times: 5 days; Experiment 2: 500 lx or 15,000 lx; Diffusers; Exposure time: 4 days |
Exposure to high illuminances (15,000 lx) for 5 h per day significantly slowed compensation for negative lenses, compared with that seen under 500 lx; Compensation for positive lenses was accelerated by exposure to high illuminances; High illuminance reduced deprivation myopia of about 60%, compared with normal illuminance |
|
Karouta et al., 2014 [107] |
Chicks |
Experiment 1: One of five light intensities 500, 10,000, 20,000, 30,000, and 40,000 lx; Translucent diffusers monocularly; Exposure times: 7 days; Experiment 2: three groups: 500 lx, 40,000 lx, 500 lx for the first 4 days and 40,000 lx for the remaining 7 days; Translucent diffusers monocularly; Exposure times: 11 days |
The level of protection from the development of form-deprivation myopia increases with increasing light intensity Daily exposure to 40,000 lx prevents the onset of form-deprivation myopia and halts further progression, once myopia is established |
|
Torii et al., 2017 [108] |
Chick Children |
Chicks with goggles: Fluorescent light, 365 nm (violet), 470 nm (blue), and UVB light; Exposure times: were illuminated for 7 days in 12-h on and off cycles Children: non-violet light-transmitting eyeglasses, partially violet light-blocking contact lenses, and violet light-transmitting contact lenses Exposure times: retrospective, 1 year |
In chicks: violet light (360–400 nm) suppresses myopia progression and suppressed the axial length elongation: this suppression increases the expression of the EGR1 gene known to prevent myopia In children: myopia progressed less rapidly in a group of myopic children fitted with corrective contact lenses that are transparent to violet light (some lenses are transparent to violet, while others, filter out about 50%) Violet light, suppressed myopia progression for individuals under 20 years of age |
|
Smith et al., 2012 [110] |
Monkeys |
Normal lighting levels (15–630 lx) to high light (25,000 lx) Exposure times: 6 h, during 23 ± 2 days to 132 ± 8 days. Monocular form deprivation |
High ambient lighting retards the development of form-deprivation myopia; The treated eyes to high light were more hyperopic than the form-deprived eyes to the normal light Eyes to the high light were more hyperopic than those of normal light |
|
Nickla et al., 2022 [113] |
Chicks |
Narrow band blue light (460 nm) or white light (588 lx) Illuminances, for evening: 0.15 lx, 200 lx, 600 lx, or 1000 lx For morning: 200 lx, 600 lx, and 1000 lx Exposure times: evening or morning, 4 h for 9 days |
Exposure to 4 h of blue light at lower illuminances (less than 1000 lx) at transition times of lights on and lights off stimulates ocular growth rates and affects ocular rhythms in chicks; Exposure to monochromatic blue light in the evening increases ocular growth except for bright illuminance, whereas morning exposure would have no effect on eye growth; Evening exposures caused circadian disruptions in the choroidal thickness rhythms, and morning exposures disrupted both axial and choroidal rhythms |
|
Najar et al., 2021 [114] |
Chicken model of form-deprivation myopia |
LED: ambient standard white (233.1 lx, 3900 K), blue-enriched white (223.8 lx, 9700 K) Exposure times: 29 days on a 12 h/12 h light–dark cycle |
At moderate light levels, blue-enriched white light (223.8 lx, 9700 K) decreased aberrant axial elongation and accelerated recovery from form deprivation, compared with ambient standard white light (233.1 lx, 3900 K) |
|
Foulds et al., 2013 [115] |
Chicks |
LED, 33.37 cd/m2 red light or blue light with a 12 h on/off cycle for 14–42 days |
Blue light induced progressive hyperopia; Red light induced progressive myopia; Light-induced myopia or hyperopia was reversed to hyperopia or myopia by changing the chromaticity of the ambient light |
| Turnbull et al., 2015 [116] | Squid |
Blue filter: 447 nm Orange filter: 557 nm Exposure times: 30 days A switch in other light environments (blue, orange, or white, period variable) |
Blue light induced shorter eyes than orange light and less myopic refractions; Switch between wavelengths, conducted changes in eye size and refractive status changed appropriately within a few days |
|
Wand et al., 2018 [117] |
Chicks |
Experiment 1: white room light, 430–630 nm or in the dark; Or unilateral exposure to 470 nm (blue), 620 nm (red), or 375 nm (UV) lighting, fellow eyes covered with black occluders Exposure times: 30 min Experiment 2: deprivation myopia in one eye; Exposure times: 5 days |
Blue, red, and UV lighting increased the release of retinal dopamine but there were wavelength-dependent differences in retinal dopamine release and metabolism; Less deprivation myopia and shorter eyes developed in blue and UV lighting, compared with white and red light |
|
Seideman et al., 2002 [118] |
Experiment 1: Chickens were refracted in quasi-monochromatic ambient illumination Experiment 2: monochromatic light for 2 days and subsequently refracted both in complete darkness, in monochromatic light, and in white light, both without and with cycloplegia |
Blue light inhibited the growth of the eye axis had a tendency toward myopia; Red light was associated with a longer focal length, made eyes grow longer |
|
|
Lieu et al., 2011 [119] |
Guinea pigs |
LED: Different monochromatic light; 430 nm, 530 nm, or broad-band light, equal luminance Exposure times: 12 weeks |
Exposure to blue light (430 nm) exerted more hyperopic effects with suppressed axial elongation; Exposure to green light (530 nm) exerted more myopia |
|
Zou et al., 2018 [120] |
Guinea Pigs |
Short-wavelength light, middle-wavelength light, or white light; Exposure times: 10 weeks |
In the short-wavelength light, the guinea pigs developed relative hyperopia; In the middle-wavelength light, the guinea pigs developed relative myopia |
|
Rucker et al., 2015 [121] |
Chicks |
LEDs, sinusoidal luminance modulation of white light (with blue) or yellow light (without blue), at 80% contrast, at one of six temporal frequencies: 0, 0.2, 1, 2, 5, 10 Hz. Mean illumination was 680 lx Time exposure: 3 days |
With blue light: refraction did not change across frequencies and little difference in eye growth across frequencies; Without blue light: a hyperopic shift at high frequencies, and a myopic shift at low frequencies and eyes grew more at low temporal frequencies and less at high temporal frequencies; |
|
Rucker et al., 2018 [122] |
Chicks |
LEDs, sinusoidal color modulation of blue/yellow or red/green at 80% contrast, at one of six temporal frequencies: 0, 0.2, 1, 2, 5, 10 Hz Time exposure: 3 days |
Eyes grew less when exposed to high temporal frequencies and more at low temporal frequencies Blue/yellow modulation, small temporal variation, 16.4% growth reduction Red/green modulation, 35% growth reduction Red/green modulation produced maximal growth, at low temporal frequencies |
AL axial length, CCT correlated color temperatures, DNA deoxyribonucleic acid, Hz Hertz, K kelvin, LED light emitting diodes, nm nanometer, UV ultraviolet, ROS reactive oxygen species
Prevention of Blue Light-Induced Ocular Damage
Exposure Limit, Advice, and Recommendations
International Exposure Limit Values and European Standard
To prevent light-induced retinal photochemical damage (blue light hazard), exposure limit values (ELV) have been proposed by the ICNIRP [123]. These ELV are the internationally recommended values for the evaluation of the toxicity of optical radiation [124–127].
There are four risk groups for optical radiation sources related to the maximum acceptable exposure time of the eye to light [127]:
GR0, risk group 0 (zero): no risk below an observation time (at 20 cm from the source) of 10,000 s;
GR1, risk group 1 (low risk): maximum exposure time at 20 cm of 100 s;
GR2, risk group 2 (moderate risk): maximum exposure time at 20 cm of 0.25 s (duration of onset of the palpebral reflex);
GR3, risk group 3 (high risk): potential lesions appearing during observation (at 20 cm from the source) of less than 0.25 s duration.
Commercialized artificial light sources must respond to certain requirements, including the assessment of the potential risk to the retina for sources that contain a significant proportion of blue light wavelengths. Thus, when introducing lamps or luminaires to the European market, manufacturers are required to assess their products according to the standards in force, and to classify them in one of the existing risk groups. The European standard EN 62560 [128], which sets out the design requirements for LED lamps, only allows the marketing of lamps in risk groups 0 or 1 (no risk or low risk). For luminaires for professional use (floodlights, emergency lighting, etc.), the regulations also authorize the moderate risk group (for which the protection is constituted by the palpebral reflex) but require specific marking and installation beyond a safety distance reducing the risk to a low level.
It is important to note that the current regulations and standards have been established based on acute light exposure and do not consider the effects of repeated exposure [67]. The ICNIRP specifies in its recommendations that the exposure limit values defined for the general population, and included in standard NF EN 62471, do not apply to chronic and repeated exposure to blue light [123]. Remarkably, these values do not consider the repeated exposures known as “subcritical,” below the ELVs, accumulated over very long periods of time. Nor does the standard NF EN 62471 take into account the increased sensitivity to optical radiation for specific sensitive populations (children, pseudophakic elderly individuals with eye disease, etc.).
Advice and Recommendations from Health Authorities
In October 2010, an initial report by ANSES alerted to the risks associated with LED exposure [129]. They stated that “the risks identified as the most worrying, both in terms of the seriousness of the associated hazards and the probability of occurrence in the context of widespread use of LEDs are linked to the photochemical effects of blue light on the eye and to glare.” These risks would be due to the photochemical effects of blue light and to glare. They mentioned that particular populations would be more at risk than others, such as children, people with specific eye diseases, or certain professional populations exposed to high-intensity lighting, as they would be more sensitive to photochemical risk or more exposed to blue light.
In June 2018, the SCHEER related that “there is no evidence of direct adverse health effects from LEDs emission in normal use (lamps and displays) by the general healthy population” [96]. However, they did not exclude a potential risk for vulnerable and susceptible population such as children, adolescents, and older individuals. Although the scientific literature has not yet provided reliable evidence on potential long-term adverse effects of LED emissions on the health of the general healthy population, the SCHEER stressed the need of monitoring these potential health risks linked to long-term use.
The French National Research and Safety Institute for the Prevention of Occupational Accidents and Diseases (INRS) considers that “for lighting devices for indoor use belonging to groups GR0 and GR1, there is no a priori risk to the eyes under normal conditions of use. Visual hazards seem to be present when using LEDs with a risk group higher than 1, under particular conditions of use, especially in direct vision” [130]. As underlined by the SCHEER, specific populations may show increased sensitivity to blue light, such as aphakic or pseudophakic individuals or subjects with AMD. Regarding screen devices, they related that “LEDs used in backlighting, in computer, tablet or telephone screens, according to current scientific data, do not represent a risk to the retina.” The retinal risk in blue light is in fact considered to be zero when the light source has a luminance of less than 10,000 candelas per m2, which is 10–100 times higher than the luminance typical of a LED LCD screen. However, the Institute points out that “the blue light emitted by LEDs can have a significant effect on the biological clock, which regulates many of the body’s functions such as appetite, alertness and body temperature.”
In May 2019, ANSES published a new report on the effects of LEDs on human health and the environment. To limit exposure to blue-rich light, ANSES issued several recommendations [20]. The agency recalls the importance of favoring “warm white” domestic lighting (color temperature below 3000 kJ) and to avoid the use of light sources emitting cold white light (with a strong blue component) in places frequented by children (maternity wards, nurseries, schools, leisure centers, etc.) or in the items they use (toys, electronic display panels, game consoles and joysticks, night lights, etc.). In addition, ANSES recommends to limit the exposure of the population, and in particular children, to blue light from LED screens (mobile phones, laptops, computers, etc.), before bedtime and during the night to prevent disturbing biological rhythms.
Regarding manufacturers and professionals designing lighting systems using LEDs, ANSES requests that they apply all standards concerning the quality of lighting (control of quality of LEDs and qualification of these products according to the different risk groups). In addition, ANSES recommends that manufacturers set up a clear, easy-to-understand labeling system for consumers, with a mandatory indication of the photobiological safety risk group on the packaging for all types of lighting. They ask that these standards should be adapted to specific light-sensitive populations (children and aphakic or pseudophakic individuals).
Also, ANSES recommends to change the regulatory framework for all LED systems and to limit the sale of LEDs for domestic use or for the general public, which are in risk groups equal to or higher than 1 (when assessed at an observation distance of 200 mm); to limit the light intensity of vehicle headlights, while ensuring road safety; and to minimize the level of temporal modulation of light emitted by all light sources (lighting, displays, LED devices).
Methods of Protection and Prevention
Various methods have been developed to reduce eye fatigue and discomfort, to improve sleep quality, and potentially to prevent or reduce the damage caused by blue light on the various eye tissues, particularly retinal phototoxicity. Concerning protection against blue light, traditional blue light protection measures are external, such as eyeglass lenses blocking blue light, contact lenses, screen protections, and blue light emission control software. They can filter high-energy short-wave blue light to improve vision and may protect against blue light damage. Also, for elderly people with cataract surgery, IOL’s are used to replace the natural lens and some have been designed to filter blue and violet light in addition to UV light. Regarding prevention of ocular damage, specific diets and some nutrients with antioxidant effects seem to play a key role in the prevention of the development of age-related eye diseases such as AMD. Is there evidence that these different protective or preventive methods are effective in reducing light toxicity and ocular damage?
Protection against Blue Light
Blue Light Filtering Eyeglasses
At the present time, the potential digital eye strain (eye fatigue, strain, blurred vision, irritated or burning eyes, dry eyes, headache, discomfort, etc.) induced by prolonged exposure to blue light emitted by digital screens and their potential damaging effect on eyes [84, 131, 132] are the target of particular interest from eyewear manufacturers. Most of the available standard spectacle lenses on the market protect against UV (up to wavelengths of 380 nm). The addition of a yellow chromophore reduces or eliminates blue light transmission [133]. A coating on both the anterior and posterior spectacle lens surfaces can selectively attenuate parts of the blue–violet light spectrum (415–455 nm corresponding to the hazard parts of the blue–violet), whereas the spectacle lens transmits all other wavelengths of visible light. Thus, an increasing number of blue-blocking filters are proposed by optical lens manufacturers that claim to reduce the symptoms of digital eye strain and potentially reduce phototoxic retinal damage, with variable degrees of short-wavelength light protection (from 10% to 100%) [12, 134]. However, as highlighted in two recent reviews, few studies have been conducted to assess their clinical efficacy in reducing eye fatigue and symptoms of eye strain, or on sleep quality, while no study evaluated their preventive effect in blue light-related ocular disorders (such as AMD progression) [12, 132, 133]. These studies were generally small (often less than 50 people) and short term. Moreover, one bias raised by Lawrenson et al. [133] is that all of these studies have used yellow-tinted blue-blocking lenses, limiting the adequate masking of the participants [133]. Most of these studies did not find significant differences between blue-blocking lenses and non-blue-blocking lenses.
Thus, to date, there is no consistent evidence to support the use of blue-blocking filters in spectacle lenses, or their introduction in the clinical practice as a treatment for eyestrain or eye fatigue [12, 132, 135]. Rosenfield et al. suggested that patients presenting with these symptoms have a complete ocular examination (including refractive error, binocular vision, oculomotor and ocular surface assessments) to determine their eye health [132]. In addition, currently no studies have reported a protective effect of blue light filtering lenses on myopia progression (refractive power or axial length progression) in schoolchildren compared with those with single vision lenses with conventional coating [136].
Finally, there are no published studies evaluating the benefit of such blue-blocking protections for the prevention of eye diseases, in particular AMD. Large medium- to long-term (at least 2–3 years) randomized trials are required to demonstrate this potential preventive effect of blue-blocking spectacle lenses.
In addition, although there is no evidence of retinal toxicity from exposure to screens (computers, tablets, smartphones), regarding myopia, the World Health Organization (WHO) [137] and the Erasmus Myopia Research Group [138], recommend no close-up screen use at all for children up to 2 years of age. Moreover, the Erasmus Myopia Research Group recommends no more than 1 h per day for children up to 5 years of age, and 2 h maximum per day for children aged 5–12 years [138].
Blue Light-Filtering Intraocular Lens (IOL)
Cataract surgery with IOL implantation is the most common ocular surgery [139, 140]. While all IOLs include UV filters, blue light filtering (BLF) IOLs have been commercialized since the early 2000s, putatively to protect against retinal phototoxicity and prevent AMD.
Since their introduction, more than a hundred articles have compared UV filtering IOLs and BLF IOLs [141]. The main concerns of BLF IOL are the potential alterations of color perception, color vision, scotopic visual function, and contrast sensitivity in mesopic and scotopic conditions. There are also concerns regarding disruption of circadian rhythm [141, 142], since BFL IOL were produced almost a decade before the discovery of photosensitive retinal ganglion cells and their essential role in maintaining good health and quality of life [143, 144]. Sensitivity spectra show that for vision in dim environments and circadian photoreception, the best IOL should transmit all possible blue light [143, 144].
A meta-analysis of 15 randomized clinical trials (RCT) reported that the postoperative visual performance (including best corrected visual acuity (BCVA), contrast sensitivity, and overall color vision) of patients with implantation of BLF IOLs was comparable to those with implantation of non-BLF IOL, except for color vision in blue light spectrum under mesopic conditions, in favor of the non-BLF IOL group [142]. Similarly, in a Cochrane review based on 51 randomized clinical trials (more than 5000 eyes), no difference was found in short-term BCVA and contrast sensitivity between the two types of IOLs [145]. Regarding longer-term effects on AMD, only two small-sized short-term RCT’s were available for review, preventing any reliable conclusion. Regarding potential effects on macular pigment density, contrast sensitivity, color discrimination, daytime alertness, reaction time, or patient satisfaction, the authors were unable to draw reliable conclusions, mainly because of heterogeneity in the measurements and low design quality. Recently, in a large prospective cohort study of patients who underwent cataract surgery and were followed for 4 years, incidence of neovascular AMD was similar in the 5425 eyes that received BLF IOLs and the 5972 that received non-BLF IOLs (HR 1.07, 95% confidence interval 0.79–1.47) [146]. Another large prospective cohort study, with a 10-year follow-up included 186,591 patients with cataract surgery. The authors reported no statistical difference between the two groups of patients (21,126 with BLF IOLs and 165,465 with non-BFL IOLs) in the incidence rate of AMD (non-exudative AMD HR 0.95, 95% CI 0.88–1.03; exudative AMD HR 0.96, 95% CI 0.77–1.18) [147].
Thus, although the rationale for BLF IOL is scientifically grounded, the academic debate on the merit of these devices to protect macular health and function continues [145, 148–152]. Currently, it is unclear whether BLF IOLs confer any potential clinical benefits over non-BLF IOLs, with respect to aspects such as distance BCVA, contrast sensitivity, and the development or progression of AMD. Regarding the choice of the optimal lenses for the surgeon, Downes et al. highlighted the importance to implant an IOL of which photoreception and photoprotection are as close as possible to the young adult healthy crystalline lens [141].
External Protection Mode—Blue Light Filtering for Screen Devices
Regarding computers, recent digital screens have “Night Shift” software to reduce high-energy visible light transmission. Various apps are also available online to adjust the color temperature of the screen according to the time of day (F.lux, EyeDefender, etc.). The function of this software is to reduce the blue content [153], orientating the light towards the orange or red end of the spectrum. Use of the Night Shift function at any or all times of the day and turning down the overall brightness may reduce potential eye strain and eye fatigue due to blue light [134].
Also, advice should be given about adequate ergonomics and environmental issues when subjects spend a prolonged period of time viewing electronic devices. Regarding “eye ergonomic tips,” the American Academy of Ophthalmologists (AAO), gives several simple recommendations to reduce symptoms of computer vision syndrome, digital eye strain, and occupational fatigue [154, 155]. There is the “20–20–20” rule. Once every 20 min, people have to take a break for at least 20 s and to focus their eyes on an object further than 20 feet away (about 6 m). In addition, people have to sit about 20–26 inches away (about 63 cm) from their computer screen (which should be tilted slightly down). They have to reduce screen glare as much as possible, and to keep the digital screen not much brighter than the surrounding light [155]. To our knowledge, no scientific study has examined the effect of a blue light computer filter on eye damage as well as the effect of a combination of blue filters in glasses and on the computer.
Nutritional Prevention of Blue Light Ocular Damage
The vision system has an effective antioxidant network that protects it from the action of light. The different structures of the eye (the lens, the pupil, the aqueous and vitreous humors, and the retina) contain natural antioxidants: carotenoids lutein and zeaxanthin, which represent a natural blue light filter in the retina (and, to a lesser extent, in the lens), and work in synergy with vitamins C and E, and minerals such as zinc, to fight against photochemical damage, by preventing oxidative stress.
Lutein and Zeaxanthin
The central part of the human retina is named “macula lutea” (yellow spot), because of the characteristic presence of a yellow pigment by two carotenoids, lutein and zeaxanthin, present at high concentrations (30–10,000 times higher than in other tissues) [156, 157] in the macular pigment. Lutein and zeaxanthin are also the only carotenoids present in the lens, albeit at lower concentrations [158]. These two yellow carotenoids are not synthesized de novo and must be obtained from the diet (mainly from vegetables and fruits, especially green leafy vegetables). They are abundant in the macula of children but decrease with aging [159].
These carotenoids are known for their ability to absorb energetic blue light radiation (with absorption between 400 and 500 nm and a peak absorption at 460 nm), thus reducing photochemical damage [160]. They also prevent the development and promote the scavenging of ROS [156, 157]. These compounds also act to slow the inflammatory reactions that aggravate light-induced damage, and they may prevent the development of blood vessel proliferation typical of neovascular AMD [156, 157].
Numerous publications on the preventive and therapeutic effects of lutein and zeaxanthin have shown encouraging results on AMD and cataract. Regarding AMD, observational studies have shown that higher dietary intake [161–164], as well as serum measurements [165–167] of lutein and zeaxanthin are associated with lower risk for both incident and prevalent AMD. In cataract, high dietary intakes of lutein and zeaxanthin are also associated with a lower risk for incident cataract in numerous epidemiological cohorts, with a 19% reduction in risk [168]. Meta-analyses of RCTs found improvements in visual acuity [169–171] and significant increases in macular pigment ocular density (MPOD) [172] in response to lutein and zeaxanthin supplementation in people with AMD in comparison with the placebo group.
To date, studies show significant and consistent benefits of lutein, zeaxanthin, and meso-zeaxanthin on retinal health and the prevention of AMD development [171, 173], as well as possible effects on cataract [174] in restoring the eye’s natural blue light shield.
Other Nutritional Compounds and Diets
Several experimental studies have reported that blue light damages the retinal pigment epithelium and choriocapillaris through generation of ROS, and may be a factor in the pathogenesis of AMD and cataract [175]. The retinal antioxidant defense system provides protection against oxidative stress (an imbalance of ROS and antioxidants) due to the potential harmful effects of blue light. This includes vitamins C [176–179] and E, carotenoids (including beta-carotene, lutein, and zeaxanthin) as well as zinc and other minerals, which act as cofactors for antioxidant enzymes [180–183].
Vitamins E and C have important roles in preventing oxidative stress and protecting cell membranes [184, 185]. The retina cell membranes (RPE and outer segments of the photoreceptor cells) are particularly rich in vitamin E, and vitamin C can be found in different eye tissues (the aqueous and vitreous humor, the lens). Zinc is well known for its antioxidative properties [186], and the eye, especially the retina–choroid complex, has an unusually high concentration of zinc compared with other tissues [187]. With lutein and zeaxanthin, these three components are key nutrients in protection against blue light damage.
The AREDS study [180], evaluated the effect of high-dose vitamins C and E, beta-carotene, and zinc supplements on AMD progression and visual acuity. The AREDS formulation contained 500 mg vitamin C, 400 UI vitamin E, 15 mg beta-carotene, 80 mg zinc (as zinc oxide), and 2 mg copper (as cupric oxide). This study reported a 25% decreased risk of progression to advanced AMD among participant taking the AREDS formulation. They also reported a reduction in rates of at least moderate visual acuity loss in participants taking the AREDS formulation.
The Mediterranean diet (MeDi) is characterized by the high consumption of plant foods and fish, low consumption of meat and dairy products, olive oil as the primary fat source, and a moderate consumption of wine, traditionally consumed by people living in countries bordering the Mediterranean Sea [188]. This diet is associated with a high intake of lutein; zeaxanthin; and vitamins including A, C, and E. Dietary patterns take into account the synergistic effect of many nutrients and their potential interactions.
Recently a high adherence to the MeDi has been associated with a lower risk of age-related eye diseases such as AMD [189–194], diabetic retinopathy [195], and visual impairment [196].
In summary, a diet rich in lutein and zeaxanthin (to increase natural filters of the eyes; the macular pigment) and antioxidants (as vitamins C, E, or zinc, to fight the oxidative stress responsible for the loss of photoreceptors), such as the Mediterranean diet, may be a preventive measure to reduce risk associated with cumulative blue-light exposure [183].
Conclusions
Because they can be powerful and potentially blue-rich, LEDs need to be systematically assessed for photobiological safety. Standards exist (in France, only for lamps and not for luminous objects) and should be applied, but there are currently no standards for limiting values for chronic exposure. There is no evidence to date that LEDs, in normal use at domestic intensity levels, or used as backlights in screen devices, are retinotoxic to humans or can led to cataract, with respect to short-term exposure for the use of LEDs with warm white (yellow) light [45]. Regarding young children, there is some concern about the effect of blue light, since they have an immature optical system allowing blue light to reach the retina [4]. Conversely, exposure to blue–violet light has been shown to reduce the risk for myopia in children, making it difficult to draw conclusions regarding the safety of blue light exposure in children [108, 197–199].
Despite the wide diversity of blue light filtering ophthalmic devices (BFL lenses or BFL IOL), there is no evidence-based research demonstrating the beneficial effect of blue light filter eyeglasses, blue blocking lenses, or BFL-IOL to decrease digital eye strain, improve visual performance or contrast sensitivity, or to prevent the progression of eye-related diseases such as AMD [12, 133, 149]. In humans, macular pigment (composed of lutein and zeaxanthin) represents a natural protection against blue light, which can be improved through increased dietary intake and/or supplementation of lutein, zeaxanthin, and meso-zeaxanthin. High dietary intake and plasma concentrations of lutein and zeaxanthin have been consistently associated with lower risk for AMD and cataract in numerous epidemiological studies. Other antioxidants (in particular vitamins C, E, and zinc) and their combination in the Mediterranean diet might also contribute to the prevention of photochemical ocular damage.
Regarding future perspectives, the ocular effect of exposure over decades needs to be evaluated in future epidemiological studies as we have insufficient data on the dose-response effect of blue light and on the spectral imbalance exposure especially in children, adolescents, and sensitive populations. Further studies are needed to better understand the mechanisms of photochemical injury related to blue light exposure, and to determine whether long-term, low-level exposure to artificial blue light is a risk factor for AMD or other eye conditions [4, 21, 57]. In the meantime, to prevent potential ocular hazards of blue light, it may be advised to limit time of exposure to blue light (in particular by LED lights) and have adequate nutritional intake of carotenoids present in the macular pigment and other antioxidants, in particular in sensitive populations such as children and older adults.
Acknowledgements
Funding
Laboratoires Théa, Clermont-Fd, France covered the publication costs, including the journal’s Rapid Service fees, but had no role in the collection of articles, analysis of their contents, or interpretation for the present review.
Author Contributions
All authors contributed to the study conception and design. Material preparation, selection of articles and analysis were performed by Audrey Cougnard-Gregoire, Cécile Delcourt and Bénédicte Merle. The first draft of the manuscript was written by Audrey Cougnard-Gregoire, Cécile Delcourt and Bénédicte Merle and all authors commented on versions of the manuscript. All authors read, edited and approved the final manuscript.
Disclosures
Audrey Cougnard-Gregoire: Consultant for Laboratoires Théa. Bénédicte MJ Merle: Travel fees from Laboratoires Théa. Tariq Aslam: has no conflicts of interest to disclose. Johanna M Seddon: Gemini Therapeutics, Inc and Apellis Pharmaceuticals- stock options, Consultant for Laboratoires Théa. Isabelle Aknin: Consultant for Laboratoires Bayer, Roche, Horus, Baush Et Lomb, Laboratoires Théa. Caroline CW Klaver has no conflicts of interest to disclose. Gerhard Garhöfer has no conflicts of interest to disclose. Angelo Maria Minnella: Consultant for Laboratoires Théa, and received meeting participation and travel expenses from Bayer, Novartis and AbbVie. Alfredo Garcia Layana: Consultant for AbbVie, Apellis, Bayer, Novartis, Roche, Laboratoires Théa. Rufino Silva: Consultant for Alimera, Abbvie, Bayer, Novartis, Laboratoires Théa, Roche, NovoNordisk. Cécile Delcourt: Consultant for Allergan-Abbvie, Apellis, Chauvin-Bausch + Lomb, Laboratoires Théa and Novartis. Grant from Laboratoires Théa. All authors have nothing to declare specifically related to the topic of the manuscript.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Leid J. Blue light: what are the risks to our eyes? Points de Vue. Int Rev Opht Opt 2016;1–7. October 2016. https://www.pointsdevue.com.
- 2.Gomes CC, Preto S. Blue light: a blessing or a curse? Proc Manuf. 2015;3:4472–4479. [Google Scholar]
- 3.Bi W, Sun K. Light-induced retinal damage and potential benefits and side effects of blue light-filtering intraocular lens. Recent Adv Ophthalmol. 2014;34(3):289–293. [Google Scholar]
- 4.Behar-Cohen F, Martinsons C, Viénot F, Zissis G, Barlier-Salsi A, Cesarini JP, et al. Light-emitting diodes (LED) for domestic lighting: any risks for the eye? Prog Retin Eye Res. 2011;30(4):239–257. doi: 10.1016/j.preteyeres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Ouyang X, Yang J, Hong Z, Wu Y, Xie Y, Wang G. Mechanisms of blue light-induced eye hazard and protective measures: a review. Biomed Pharmacother. 2020;130:110577. doi: 10.1016/j.biopha.2020.110577. [DOI] [PubMed] [Google Scholar]
- 6.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 7.The Vision Council. Eyes overexposed: The digital device dilemma: digital eye strain report 2016.
- 8.Kabali HK, Irigoyen MM, Nunez-Davis R, Budacki JG, Mohanty SH, Leister KP, et al. Exposure and use of mobile media devices by young children. Pediatrics. 2015;136(6):1044–1050. doi: 10.1542/peds.2015-2151. [DOI] [PubMed] [Google Scholar]
- 9.Jakhar D, Kaul S, Kaur I. Increased usage of smartphones during COVID-19: is that blue light causing skin damage? J Cosmet Dermatol. 2020;19(10):2466–2467. doi: 10.1111/jocd.13662. [DOI] [PubMed] [Google Scholar]
- 10.Ratan ZA, Zaman SB, Islam SMS, Hosseinzadeh H. Smartphone overuse: a hidden crisis in COVID-19. Health Policy Technol. 2021;10(1):21–22. doi: 10.1016/j.hlpt.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golebiowski B, Long J, Harrison K, Lee A, Chidi-Egboka N, Asper L. Smartphone use and effects on tear film, blinking and binocular vision. Curr Eye Res. 2020;45(4):428–434. doi: 10.1080/02713683.2019.1663542. [DOI] [PubMed] [Google Scholar]
- 12.Vagge A, Ferro Desideri L, Del Noce C, Di Mola I, Sindaco D, Traverso CE. Blue light filtering ophthalmic lenses: a systematic review. Semin Ophthalmol. 2021;36(7):541–548. doi: 10.1080/08820538.2021.1900283. [DOI] [PubMed] [Google Scholar]
- 13.Norton B, Balick M, Hobday R, Fournier C, Scartezzini JL, Solt J, et al. Daylight: contexts and concepts, in Changing perspectives on daylight: science, technology and culture. 2017. In: AAAS Membership Make the connection [Internet]. Washington DC: Science/AAAS [4–8]. https://www.researchgate.net/profile/Arthur-Gessler/publication/321485288_Light_as_a_source_of_information_in_ecosystems/links/5a25108caca2727dd87e7574/Light-as-a-source-of-information-in-ecosystems.pdf.
- 14.Giannos SA, Kraft ER, Lyons LJ, Gupta PK. Spectral evaluation of eyeglass blocking efficiency of ultraviolet/high-energy visible blue light for ocular protection. Optom Vis Sci. 2019;96(7):513–522. doi: 10.1097/OPX.0000000000001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wasowicz M, Morice C, Ferrari P, Callebert J, Versaux-Botteri C. Long-term effects of light damage on the retina of albino and pigmented rats. Invest Ophthalmol Vis Sci. 2002;43(3):813–820. [PubMed] [Google Scholar]
- 16.Arnault E, Barrau C, Nanteau C, Gondouin P, Bigot K, Viénot F, et al. Phototoxic action spectrum on a retinal pigment epithelium model of age-related macular degeneration exposed to sunlight normalized conditions. PLoS ONE. 2013;8(8):e71398. doi: 10.1371/journal.pone.0071398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marie M, Gondouin P, Pagan D, Barrau C, Villette T, Sahel J, et al. Blue-violet light decreases VEGFa production in an in vitro model of AMD. PLoS ONE. 2019;14(10):e0223839. doi: 10.1371/journal.pone.0223839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fietz A, Hurst J, Schnichels S. Out of the shadow: blue light exposure induces apoptosis in Müller cells. Int J Mol Sci. 2022;23(23):14540. doi: 10.3390/ijms232314540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gronfier C. Circadian clock and non-visual functions: the role of light in humans. Biol Aujourdhui. 2014;208(4):261–267. doi: 10.1051/jbio/2015008. [DOI] [PubMed] [Google Scholar]
- 20.ANSES. (French National Agency for Food, Environmental and Occupational Health Safety). « LED : les recommandations de l’Anses pour limiter l’exposition à la lumière bleue». 2019.
- 21.Hatori M, Gronfier C, Van Gelder RN, Bernstein PS, Carreras J, Panda S, et al. Global rise of potential health hazards caused by blue light-induced circadian disruption in modern aging societies. NPJ Aging Mech Dis. 2017;3:9. doi: 10.1038/s41514-017-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royal Society NZ. Blue Light Aotearoa: Impacts of Artificial Blue Light on Health and the Environment : Evidence Summary. 2018. https://www.royalsociety.org.nz/assets/Uploads/Blue-light-Aotearoa-evidence-summary.pdf..
- 23.Wooliscroft B. National Household Survey of Energy and Transportation: Energy Cultures Two: Centre for Sustainability, University of Otago; 2015.
- 24.Baumgartner T, Wunderlich F, Jaunich A, Sato T, Bundy G, Grießmann N, et al. Lighting the way: Perspectives on the global lighting market. 2012.
- 25.LED lighting in the United States—statistics & facts. Published by Statista Research Department, Feb 23, 2021.
- 26.Chen HW, Lee JH, Lin BY, Chen S, Wu ST. Liquid crystal display and organic light-emitting diode display: present status and future perspectives. Light Sci Appl. 2018;7:17168. doi: 10.1038/lsa.2017.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen MA, Bettencourt L, Kaye L, Moturu ST, Nguyen KT, Olgin JE, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS ONE. 2016;11(11):e0165331. doi: 10.1371/journal.pone.0165331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pew Research Center. Americans 60 and older are spending more time in front of their screens than a decade ago 2019. https://www.pewresearch.org/fact-tank/2019/06/18/americans-60-and-older-are-spending-more-time-in-front-of-their-screens-than-a-decade-ago/.
- 29.Ofcom. Children and Parents: Media Use and Attitudes Report 2018. 2018. https://ofcom.org.uk/__data/assets/pdf_file/0024/134907/Children-and-Parents-Media-Use-and-Attitudes-2018.pdf.
- 30.Okuno T, Saito H, Ojima J. Evaluation of blue-light hazards from various light sources. Dev Ophthalmol. 2002;35:104–112. doi: 10.1159/000060814. [DOI] [PubMed] [Google Scholar]
- 31.Marshall J. The Blue Light Paradox: Problemor Panacea? Points de Vue. Int Rev Ophthalmic Opt 2017;1–8. February 2017. https://www.pointsdevue.com.
- 32.Arshavsky VY, Burns ME. Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem. 2012;287(3):1620–1626. doi: 10.1074/jbc.R111.305243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sliney DH. How light reaches the eye and its components. Int J Toxicol. 2002;21(6):501–509. doi: 10.1080/10915810290169927. [DOI] [PubMed] [Google Scholar]
- 34.Sliney DH. Exposure geometry and spectral environment determine photobiological effects on the human eye¶. Photochem Photobiol. 2005;81(3):483–489. doi: 10.1562/2005-02-14-RA-439.1. [DOI] [PubMed] [Google Scholar]
- 35.Mure LS. Intrinsically photosensitive retinal ganglion cells of the human retina. Front Neurol. 2021;12:636330. doi: 10.3389/fneur.2021.636330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marek V, Mélik-Parsadaniantz S, Villette T, Montoya F, Baudouin C, Brignole-Baudouin F, et al. Blue light phototoxicity toward human corneal and conjunctival epithelial cells in basal and hyperosmolar conditions. Free Radic Biol Med. 2018;126:27–40. doi: 10.1016/j.freeradbiomed.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Niwano Y, Iwasawa A, Tsubota K, Ayaki M, Negishi K. Protective effects of blue light-blocking shades on phototoxicity in human ocular surface cells. BMJ Open Ophthalmol. 2019;4(1):e000217. doi: 10.1136/bmjophth-2018-000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi T. Inflammatory response in dry eye. Invest Ophthalmol Vis Sci. 2018;59(14):DES192–DES199. doi: 10.1167/iovs.17-23651. [DOI] [PubMed] [Google Scholar]
- 39.Lee J-B, Kim S-H, Lee S-C, Kim H-G, Ahn H-G, Li Z, et al. Blue light–induced oxidative stress in human corneal epithelial cells: protective effects of ethanol extracts of various medicinal plant mixtures. Invest Ophthalmol Vis Sci. 2014;55(7):4119–4127. doi: 10.1167/iovs.13-13441. [DOI] [PubMed] [Google Scholar]
- 40.Niwano Y, Kanno T, Iwasawa A, Ayaki M, Tsubota K. Blue light injures corneal epithelial cells in the mitotic phase in vitro. Br J Ophthalmol. 2014;98(7):990–992. doi: 10.1136/bjophthalmol-2014-305205. [DOI] [PubMed] [Google Scholar]
- 41.Bron A, Vrensen G, Koretz J, Maraini G, Harding J. The ageing lens. Ophthalmologica. 2000;214(1):86–104. doi: 10.1159/000027475. [DOI] [PubMed] [Google Scholar]
- 42.Van De Kraats J, Van Norren D. Optical density of the aging human ocular media in the visible and the UV. J Opt Soc Am A. 2007;24(7):1842–1857. doi: 10.1364/JOSAA.24.001842. [DOI] [PubMed] [Google Scholar]
- 43.Kessel L, Lundeman JH, Herbst K, Andersen TV, Larsen M. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg. 2010;36(2):308–312. doi: 10.1016/j.jcrs.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 44.Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. Lancet. 2017;390(10094):600–612. doi: 10.1016/S0140-6736(17)30544-5. [DOI] [PubMed] [Google Scholar]
- 45.Zhao ZC, Zhou Y, Tan G, Li J. Research progress about the effect and prevention of blue light on eyes. Int J Ophthalmol. 2018;11(12):1999–2003. doi: 10.18240/ijo.2018.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babizhayev MA. Mitochondria induce oxidative stress, generation of reactive oxygen species and redox state unbalance of the eye lens leading to human cataract formation: disruption of redox lens organization by phospholipid hydroperoxides as a common basis for cataract disease. Cell Biochem Funct. 2011;29(3):183–206. doi: 10.1002/cbf.1737. [DOI] [PubMed] [Google Scholar]
- 47.Xie C, Li X, Tong J, Gu Y, Shen Y. Effects of white light-emitting diode (LED) light exposure with different correlated color temperatures (CCTs) on human lens epithelial cells in culture. Photochem Photobiol. 2014;90(4):853–859. doi: 10.1111/php.12250. [DOI] [PubMed] [Google Scholar]
- 48.Gaillard ER, Merriam J, Zheng L, Dillon J. Transmission of light to the young primate retina: possible implications for the formation of lipofuscin. Photochem Photobiol. 2011;87(1):18–21. doi: 10.1111/j.1751-1097.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 49.Haag R, Sieber N, Heßling M. Cataract development by exposure to ultraviolet and blue visible light in porcine lenses. Medicina (Kaunas) 2021;57(6):535. doi: 10.3390/medicina57060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeller K, Mühleisen S, Shanmugarajah P, Fehler N, Haag R, Hessling M. Influence of visible violet, blue and red light on the development of cataract in porcine lenses. Medicina (Kaunas) 2022;58(6):721. doi: 10.3390/medicina58060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marshall J. Thermal and mechanical mechanisms in laser damage to the retina. Invest Ophthalmol Vis Sci. 1970;9(2):97–115. [PubMed] [Google Scholar]
- 52.Singh AD. Ocular phototherapy. Eye (Lond) 2013;27(2):190–198. doi: 10.1038/eye.2012.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010;29(2):113–134. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wenzel A, Grimm C, Samardzija M, Remé CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24(2):275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Youssef PN, Sheibani N, Albert DM. Retinal light toxicity. Eye (Lond) 2011;25(1):1–14. doi: 10.1038/eye.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Norren D, Vos J. Light damage to the retina: an historical approach. Eye. 2016;30(2):169–172. doi: 10.1038/eye.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tosini G, Ferguson I, Tsubota K. Effects of blue light on the circadian system and eye physiology. Mol Vis. 2016;22:61–72. [PMC free article] [PubMed] [Google Scholar]
- 58.Marie M, Bigot K, Angebault C, Barrau C, Gondouin P, Pagan D, et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018;9(3):287. doi: 10.1038/s41419-018-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lawwill T, Crockett S, Currier G. Retinal damage secondary to chronic light exposure, thresholds and mechanisms. Doc Ophthalmol. 1977;44(2):379–402. doi: 10.1007/BF00230089. [DOI] [PubMed] [Google Scholar]
- 60.Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol Vis Sci. 1966;5(5):450–473. [PubMed] [Google Scholar]
- 61.West SK, Rosenthal FS, Bressler NM, Bressler SB, Munoz B, Fine SL, et al. Exposure to sunlight and other risk factors for age-related macular degeneration. Arch Ophthalmol. 1989;107(6):875–879. doi: 10.1001/archopht.1989.01070010897038. [DOI] [PubMed] [Google Scholar]
- 62.Young RW. Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci. 1976;15(9):700–725. [PubMed] [Google Scholar]
- 63.Ham WT., Jr Ocular hazards of light sources: review of current knowledge. J Occup Med. 1983;25(2):101–103. [PubMed] [Google Scholar]
- 64.Pang J, Seko Y, Tokoro T, Ichinose S, Yamamoto H. Observation of ultrastructural changes in cultured retinal pigment epithelium following exposure to blue light. Graefe's Arch Clin Exp Ophthalmol. 1998;236(9):696–701. doi: 10.1007/s004170050143. [DOI] [PubMed] [Google Scholar]
- 65.Van Norren D, Schellekens P. Blue light hazard in rat. Vision Res. 1990;30(10):1517–1520. doi: 10.1016/0042-6989(90)90032-G. [DOI] [PubMed] [Google Scholar]
- 66.Marie M, Forster V, Fouquet S, Berto P, Barrau C, Ehrismann C, et al. Phototoxic damage to cone photoreceptors can be independent of the visual pigment: the porphyrin hypothesis. Cell Death Dis. 2020;11(8):711. doi: 10.1038/s41419-020-02918-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krigel A, Berdugo M, Picard E, Levy-Boukris R, Jaadane I, Jonet L, et al. Light-induced retinal damage using different light sources, protocols and rat strains reveals LED phototoxicity. Neuroscience. 2016;339:296–307. doi: 10.1016/j.neuroscience.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 68.Chamorro E, Bonnin-Arias C, Pérez-Carrasco MJ, Muñoz de Luna J, Vázquez D, Sánchez-Ramos C. Effects of light-emitting diode radiations on human retinal pigment epithelial cells in vitro. Photochem Photobiol. 2013;89(2):468–473. doi: 10.1111/j.1751-1097.2012.01237.x. [DOI] [PubMed] [Google Scholar]
- 69.Kuse Y, Ogawa K, Tsuruma K, Shimazawa M, Hara H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci Rep. 2014;4:5223. doi: 10.1038/srep05223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdouh M, Lu M, Chen Y, Goyeneche A, Burnier JV, Burnier MN., Jr Filtering blue light mitigates the deleterious effects induced by the oxidative stress in human retinal pigment epithelial cells. Exp Eye Res. 2022;217:108978. doi: 10.1016/j.exer.2022.108978. [DOI] [PubMed] [Google Scholar]
- 71.Wu J, Seregard S, Spångberg B, Oskarsson M, Chen E. Blue light induced apoptosis in rat retina. Eye (Lond) 1999;13(Pt 4):577–583. doi: 10.1038/eye.1999.142. [DOI] [PubMed] [Google Scholar]
- 72.Fan B, Zhang C, Chi J, Liang Y, Bao X, Cong Y, et al. The molecular mechanism of retina light injury focusing on damage from short wavelength light. Oxid Med Cell Longev. 2022;2022:8482149. doi: 10.1155/2022/8482149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maeda T, Golczak M, Maeda A. Retinal photodamage mediated by all-trans-retinal. Photochem Photobiol. 2012;88(6):1309–1319. doi: 10.1111/j.1751-1097.2012.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaya S, Weigert G, Pemp B, Sacu S, Werkmeister RM, Dragostinoff N, et al. Comparison of macular pigment in patients with age-related macular degeneration and healthy control subjects—a study using spectral fundus reflectance. Acta Ophthalmol. 2012;90(5):e399–403. doi: 10.1111/j.1755-3768.2012.02423.x. [DOI] [PubMed] [Google Scholar]
- 75.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85(3):845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 76.Rózanowska M, Wessels J, Boulton M, Burke JM, Rodgers MA, Truscott TG, et al. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic Biol Med. 1998;24(7–8):1107–1112. doi: 10.1016/S0891-5849(97)00395-X. [DOI] [PubMed] [Google Scholar]
- 77.Gaillard ER, Atherton SJ, Eldred G, Dillon J. Photophysical studies on human retinal lipofuscin. Photochem Photobiol. 1995;61(5):448–453. doi: 10.1111/j.1751-1097.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 78.Boulton M, Dontsov A, Jarvis-Evans J, Ostrovsky M, Svistunenko D. Lipofuscin is a photoinducible free radical generator. J Photochem Photobiol B. 1993;19(3):201–204. doi: 10.1016/1011-1344(93)87085-2. [DOI] [PubMed] [Google Scholar]
- 79.Rózanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, Sarna T. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995;270(32):18825–18830. doi: 10.1074/jbc.270.32.18825. [DOI] [PubMed] [Google Scholar]
- 80.Fletcher EL, Chung ST, Downie LE, Guymer RH, Vingrys AJ. Age-related macular degeneration: what's new and on the horizon. Optom Vis Sci. 2014;91(8):816–818. doi: 10.1097/OPX.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 81.Sui G-Y, Liu G-C, Liu G-Y, Gao Y-Y, Deng Y, Wang W-Y, et al. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br J Ophthalmol. 2013;97(4):389–394. doi: 10.1136/bjophthalmol-2012-302281. [DOI] [PubMed] [Google Scholar]
- 82.Yam JC, Kwok AK. Ultraviolet light and ocular diseases. Int Ophthalmol. 2014;34(2):383–400. doi: 10.1007/s10792-013-9791-x. [DOI] [PubMed] [Google Scholar]
- 83.Zhou H, Zhang H, Yu A, Xie J. Association between sunlight exposure and risk of age-related macular degeneration: a meta-analysis. BMC Ophthalmol. 2018;18(1):1–8. doi: 10.1186/s12886-018-1004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006;84(1):4–15. doi: 10.1111/j.1600-0420.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 85.Klein R, Meuer SM, Knudtson MD, Klein BE. The epidemiology of progression of pure geographic atrophy: the Beaver Dam Eye Study. Am J Ophthalmol. 2008;146(5):692.e1–9.e1. doi: 10.1016/j.ajo.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knudtson MD, Klein BE, Klein R. Biomarkers of aging and falling: the Beaver Dam eye study. Arch Gerontol Geriatr. 2009;49(1):22–26. doi: 10.1016/j.archger.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tomany SC, Cruickshanks KJ, Klein R, Klein BE, Knudtson MD. Sunlight and the 10-year incidence of age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol. 2004;122(5):750–757. doi: 10.1001/archopht.122.5.750. [DOI] [PubMed] [Google Scholar]
- 88.Taylor HR, West S, Muñoz B, Rosenthal FS, Bressler SB, Bressler NM. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992;110(1):99–104. doi: 10.1001/archopht.1992.01080130101035. [DOI] [PubMed] [Google Scholar]
- 89.Fletcher AE, Bentham GC, Agnew M, Young IS, Augood C, Chakravarthy U, et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch Ophthalmol. 2008;126(10):1396–1403. doi: 10.1001/archopht.126.10.1396. [DOI] [PubMed] [Google Scholar]
- 90.Delcourt C, Cougnard-Grégoire A, Boniol M, Carrière I, Doré JF, Delyfer MN, et al. Lifetime exposure to ambient ultraviolet radiation and the risk for cataract extraction and age-related macular degeneration: the Alienor Study. Invest Ophthalmol Vis Sci. 2014;55(11):7619–7627. doi: 10.1167/iovs.14-14471. [DOI] [PubMed] [Google Scholar]
- 91.Schick T, Ersoy L, Lechanteur YT, Saksens NT, Hoyng CB, den Hollander AI, et al. History of sunlight exposure is a risk factor for age-related macular degeneration. Retina. 2016;36(4):787–790. doi: 10.1097/IAE.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 92.Kuse Y, Ogawa K, Tsuruma K, Shimazawa M, Hara H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci Rep. 2014;4(1):1–12. doi: 10.1038/srep05223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shang YM, Wang GS, Sliney DH, Yang CH, Lee LL. Light-emitting-diode induced retinal damage and its wavelength dependency in vivo. Int J Ophthalmol. 2017;10(2):191–202. doi: 10.18240/ijo.2017.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jaadane I, Boulenguez P, Chahory S, Carré S, Savoldelli M, Jonet L, et al. Retinal damage induced by commercial light emitting diodes (LEDs) Free Radic Biol Med. 2015;84:373–384. doi: 10.1016/j.freeradbiomed.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 95.Ratnayake K, Payton JL, Lakmal OH, Karunarathne A. Blue light excited retinal intercepts cellular signaling. Sci Rep. 2018;8(1):10207. doi: 10.1038/s41598-018-28254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scientific Committee on Health, Environmental and Emerging Risks (SCHEER). Opinion on Potential risks to human health of Light Emitting Diodes (LEDs). 5–6 June 2018: SCHEER; 2018. https://ec.europa.eu/health/sites/health/files/scientific_committees/scheer/docs/scheer_o_011.pdf.
- 97.Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, et al. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018;62:134–149. doi: 10.1016/j.preteyeres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 98.He M, Xiang F, Zeng Y, Mai J, Chen Q, Zhang J, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015;314(11):1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 99.Wu PC, Chen CT, Chang LC, Niu YZ, Chen ML, Liao LL, et al. Increased time outdoors is followed by reversal of the long-term trend to reduced visual acuity in Taiwan primary school students. Ophthalmology. 2020;127(11):1462–1469. doi: 10.1016/j.ophtha.2020.01.054. [DOI] [PubMed] [Google Scholar]
- 100.Jin JX, Hua WJ, Jiang X, Wu XY, Yang JW, Gao GP, et al. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast China: the Sujiatun Eye Care Study. BMC Ophthalmol. 2015;15:73. doi: 10.1186/s12886-015-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.French AN, Morgan IG, Mitchell P, Rose KA. Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology. 2013;120(10):2100–2108. doi: 10.1016/j.ophtha.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 102.Wang J, Li Y, Musch DC, Wei N, Qi X, Ding G, et al. Progression of myopia in school-aged children after COVID-19 home confinement. JAMA Ophthalmol. 2021;139(3):293–300. doi: 10.1001/jamaophthalmol.2020.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hu Y, Zhao F, Ding X, Zhang S, Li Z, Guo Y, et al. Rates of myopia development in young Chinese schoolchildren during the outbreak of COVID-19. JAMA Ophthalmol. 2021;139(10):1115–1121. doi: 10.1001/jamaophthalmol.2021.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Enthoven CA, Tideman JWL, Polling JR, Yang-Huang J, Raat H, Klaver CCW. The impact of computer use on myopia development in childhood: the Generation R study. Prev Med. 2020;132:105988. doi: 10.1016/j.ypmed.2020.105988. [DOI] [PubMed] [Google Scholar]
- 105.Enthoven CA, Polling JR, Verzijden T, Tideman JWL, Al-Jaffar N, Jansen PW, et al. Smartphone use associated with refractive error in teenagers: the myopia app study. Ophthalmology. 2021;128(12):1681–1688. doi: 10.1016/j.ophtha.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 106.Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51(10):5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- 107.Karouta C, Ashby RS. Correlation between light levels and the development of deprivation myopia. Invest Ophthalmol Vis Sci. 2014;56(1):299–309. doi: 10.1167/iovs.14-15499. [DOI] [PubMed] [Google Scholar]
- 108.Torii H, Kurihara T, Seko Y, Negishi K, Ohnuma K, Inaba T, et al. Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine. 2017;15:210–219. doi: 10.1016/j.ebiom.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Norton TT, Siegwart JT., Jr Light levels, refractive development, and myopia–a speculative review. Exp Eye Res. 2013;114:48–57. doi: 10.1016/j.exer.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith EL, 3rd, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53(1):421–428. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47. doi: 10.1016/j.exer.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tkatchenko TV, Shen Y, Braun RD, Bawa G, Kumar P, Avrutsky I, et al. Photopic visual input is necessary for emmetropization in mice. Exp Eye Res. 2013;115:87–95. doi: 10.1016/j.exer.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nickla DL, Rucker F, Taylor CP, Sarfare S, Chen W, Elin-Calcador J, et al. Effects of morning and evening exposures to blue light of varying illuminance on ocular growth rates and ocular rhythms in chicks. Exp Eye Res. 2022;217:108963. doi: 10.1016/j.exer.2022.108963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Najjar RP, Chao De La Barca JM, Barathi VA, Ho CEH, Lock JZ, Muralidharan AR, et al. Ocular growth and metabolomics are dependent upon the spectral content of ambient white light. Sci Rep. 2021;11(1):7586. doi: 10.1038/s41598-021-87201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Foulds WS, Barathi VA, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Invest Ophthalmol Vis Sci. 2013;54(13):8004–8012. doi: 10.1167/iovs.13-12476. [DOI] [PubMed] [Google Scholar]
- 116.Turnbull PR, Backhouse S, Phillips JR. Visually guided eye growth in the squid. Curr Biol. 2015;25(18):R791–R792. doi: 10.1016/j.cub.2015.07.073. [DOI] [PubMed] [Google Scholar]
- 117.Wang M, Schaeffel F, Jiang B, Feldkaemper M. Effects of light of different spectral composition on refractive development and retinal dopamine in chicks. Invest Ophthalmol Vis Sci. 2018;59(11):4413–4424. doi: 10.1167/iovs.18-23880. [DOI] [PubMed] [Google Scholar]
- 118.Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002;42(21):2409–2417. doi: 10.1016/S0042-6989(02)00262-6. [DOI] [PubMed] [Google Scholar]
- 119.Liu R, Qian YF, He JC, Hu M, Zhou XT, Dai JH, et al. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res. 2011;92(6):447–453. doi: 10.1016/j.exer.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 120.Zou L, Zhu X, Liu R, Ma F, Yu M, Liu H, et al. Effect of altered retinal cones/opsins on refractive development under monochromatic lights in guinea pigs. J Ophthalmol. 2018;2018:9197631. doi: 10.1155/2018/9197631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rucker F, Britton S, Spatcher M, Hanowsky S. Blue light protects against temporal frequency sensitive refractive changes. Invest Ophthalmol Vis Sci. 2015;56(10):6121–6131. doi: 10.1167/iovs.15-17238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rucker F, Britton S, Taylor C. Color and temporal frequency sensitive eye growth in chick. Invest Ophthalmol Vis Sci. 2018;59(15):6003–6013. doi: 10.1167/iovs.18-25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.ICNIRP Statement on light-emitting diodes (LEDS) and laser diodes: implications for hazard assessment. International Commission on Non-Ionizing Radiation Protection. Health Phys. 2000;78(6):744–752. doi: 10.1097/00004032-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 124.Jaadane I, Villalpando Rodriguez GE, Boulenguez P, Chahory S, Carré S, Savoldelli M, et al. Effects of white light-emitting diode (LED) exposure on retinal pigment epithelium in vivo. J Cell Mol Med. 2017;21(12):3453–3466. doi: 10.1111/jcmm.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ham WT, Mueller HA. The photopathology and nature of the blue light and near-UV retinal lesions produced by lasers and other optical sources. Laser applications in medicine and biology. New York: Springer; 1989. pp. 191–246. [Google Scholar]
- 126.Sliney D, Aron-Rosa D, DeLori F, Fankhauser F, Landry R, Mainster M, et al. Adjustment of guidelines for exposure of the eye to optical radiation from ocular instruments: statement from a task group of the International Commission on Non-Ionizing Radiation Protection (ICNIRP) Appl Opt. 2005;44(11):2162–2176. doi: 10.1364/AO.44.002162. [DOI] [PubMed] [Google Scholar]
- 127.International Commission on Non-Ionizing Radiation Protection (ICNIRP) Guidelines on Limits of Exposure to Laser Radiation of Wavelengths between 180 nm and 1,000 μm. International Commission on Non-Ionizing Radiation Protection. Health Phys. 2013;105(3):271–295. doi: 10.1097/HP.0b013e3182983fd4. [DOI] [PubMed] [Google Scholar]
- 128.Rusnati F. Standardization of led for lighting. Light Eng. 2010;18(4).
- 129.ANSES. (French National Agency for Food, Environmental and Occupational Health Safety). « Effets sanitaires des systèmes d’éclairage utilisant des diodes électroluminescentes (LED) » 2010.
- 130.Institut National de Recherche et de Sécurité (INRS). Rayonnements optiques. Eclairage à LED. 2017. https://www.inrs.fr/risques/rayonnements-optiques/eclairage-led.html#c373d418-fd2a-4cd0-8dbd-afb4e2bc59ea. Accessed 06 Jul 2017.
- 131.Mainster MA, Sparrow JR. How much blue light should an IOL transmit? Br J Ophthalmol. 2003;87(12):1523–1529. doi: 10.1136/bjo.87.12.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rosenfield M, Li RT, Kirsch NT. A double-blind test of blue-blocking filters on symptoms of digital eye strain. Work. 2020;65(2):343–348. doi: 10.3233/WOR-203086. [DOI] [PubMed] [Google Scholar]
- 133.Lawrenson JG, Hull CC, Downie LE. The effect of blue-light blocking spectacle lenses on visual performance, macular health and the sleep-wake cycle: a systematic review of the literature. Ophthalmic Physiol Opt. 2017;37(6):644–654. doi: 10.1111/opo.12406. [DOI] [PubMed] [Google Scholar]
- 134.Palavets T, Rosenfield M. Blue-blocking filters and digital eyestrain. Optom Vis Sci. 2019;96(1):48–54. doi: 10.1097/OPX.0000000000001318. [DOI] [PubMed] [Google Scholar]
- 135.Mainster MA, Findl O, Dick HB, Desmettre T, Ledesma-Gil G, Curcio CA, et al. The blue light hazard versus blue light hype. Am J Ophthalmol. 2022;240:51–57. doi: 10.1016/j.ajo.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao HL, Jiang J, Yu J, Xu HM. Role of short-wavelength filtering lenses in delaying myopia progression and amelioration of asthenopia in juveniles. Int J Ophthalmol. 2017;10(8):1261–1267. doi: 10.18240/ijo.2017.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.World Health Organization (WHO) Guidelines on physical activity, sedentary behaviour and sleep for children under 5 years of age. Geneva: World Health Organization; 2019. [PubMed] [Google Scholar]
- 138.Klaver C, Polling JR. Myopia management in the Netherlands. Ophthalmic Physiol Opt. 2020;40(2):230–240. doi: 10.1111/opo.12676. [DOI] [PubMed] [Google Scholar]
- 139.Daien V, Le Pape A, Heve D, Carriere I, Villain M. Incidence and characteristics of cataract surgery in France from 2009 to 2012: a national population study. Ophthalmology. 2015;122(8):1633–1638. doi: 10.1016/j.ophtha.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 140.Schweitzer C, Brezin A, Cochener B, Monnet D, Germain C, Roseng S, et al. Femtosecond laser-assisted versus phacoemulsification cataract surgery (FEMCAT): a multicentre participant-masked randomised superiority and cost-effectiveness trial. Lancet. 2020;395(10219):212–224. doi: 10.1016/S0140-6736(19)32481-X. [DOI] [PubMed] [Google Scholar]
- 141.Downes SM. Ultraviolet or blue-filtering intraocular lenses: what is the evidence? Eye (Lond) 2016;30(2):215–221. doi: 10.1038/eye.2015.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhu XF, Zou HD, Yu YF, Sun Q, Zhao NQ. Comparison of blue light-filtering IOLs and UV light-filtering IOLs for cataract surgery: a meta-analysis. PLoS ONE. 2012;7(3):e33013. doi: 10.1371/journal.pone.0033013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mainster MA, Turner PL. Blue-blocking intraocular lenses: myth or reality? Am J Ophthalmol. 2009;147(1):8–10. doi: 10.1016/j.ajo.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 144.Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006;90(6):784–792. doi: 10.1136/bjo.2005.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Downie LE, Busija L, Keller PR. Blue-light filtering intraocular lenses (IOLs) for protecting macular health. Cochrane Database Syst Rev. 2018;5(5):CD011977. doi: 10.1002/14651858.CD011977.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Achiron A, Elbaz U, Hecht I, Spierer O, Einan-Lifshitz A, Karesvuo P, et al. The Effect of Blue-Light Filtering Intraocular Lenses on the Development and Progression of Neovascular Age-Related Macular Degeneration. Ophthalmology. 2021. [DOI] [PubMed]
- 147.Lee JS, Li PR, Hou CH, Lin KK, Kuo CF, See LC. Effect of blue light-filtering intraocular lenses on age-related macular degeneration: a nationwide cohort study with 10-year follow-up. Am J Ophthalmol. 2022;234:138–146. doi: 10.1016/j.ajo.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 148.Falkner-Radler CI, Benesch T, Binder S. Blue light-filter intraocular lenses in vitrectomy combined with cataract surgery: results of a randomized controlled clinical trial. Am J Ophthalmol. 2008;145(3):499–503. doi: 10.1016/j.ajo.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 149.Downie LE, Wormald R, Evans J, Virgili G, Keller PR, Lawrenson JG, et al. Analysis of a systematic review about blue light-filtering intraocular lenses for retinal protection: understanding the limitations of the evidence. JAMA Ophthalmol. 2019;137(6):694–697. doi: 10.1001/jamaophthalmol.2019.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee RM, Lam FC, Liu CS. Blue-blocking intraocular implants should be used routinely during phacoemulsification surgery–no. Eye (Lond) 2012;26(11):1400–1401. doi: 10.1038/eye.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mainster MA, Turner PL. Blue-blocking IOLs vs. short-wavelength visible light: hypothesis-based vs. evidence-based medical practice. Ophthalmology. 2011;118(1):1–2. doi: 10.1016/j.ophtha.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 152.Symes RJ, Cuthbertson FM. Blue-blocking intraocular implants should be used routinely during phacoemulsification surgery–yes. Eye (Lond) 2012;26(11):1397–1399. doi: 10.1038/eye.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smith AK, Conger JR, Hedayati B, Kim JJ, Amoozadeh S, Mehta M. The effect of a screen protector on blue light intensity emitted from different hand-held devices. Middle East Afr J Ophthalmol. 2020;27(3):177–181. doi: 10.4103/meajo.MEAJO_2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.American Optometric Association (AA. Computer vision syndrome. 2020. https://www.aoa.org/patients-and-public/caring-for-your-vision/protecting-yourvision/computer-vision-syndrome.
- 155.American Academy of Ophthalmology (AAO). Computers, digital devices and eye strain. 2016.
- 156.Kijlstra A, Tian Y, Kelly ER, Berendschot TT. Lutein: more than just a filter for blue light. Prog Retin Eye Res. 2012;31(4):303–315. doi: 10.1016/j.preteyeres.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 157.Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72(3):215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- 158.Li X, Holt RR, Keen CL, Morse LS, Zivkovic AM, Yiu G, et al. Potential roles of dietary zeaxanthin and lutein in macular health and function. Nutr Rev. 2022 doi: 10.1093/nutrit/nuac076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Davies NP, Morland AB. Macular pigments: their characteristics and putative role. Prog Retin Eye Res. 2004;23(5):533–559. doi: 10.1016/j.preteyeres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 160.Bian Q, Gao S, Zhou J, Qin J, Taylor A, Johnson EJ, et al. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic Biol Med. 2012;53(6):1298–1307. doi: 10.1016/j.freeradbiomed.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye disease case-control study group. JAMA. 1994;272(18):1413–1420. doi: 10.1001/jama.1994.03520180037032. [DOI] [PubMed] [Google Scholar]
- 162.Ma L, Dou HL, Wu YQ, Huang YM, Huang YB, Xu XR, et al. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr. 2012;107(3):350–359. doi: 10.1017/S0007114511004260. [DOI] [PubMed] [Google Scholar]
- 163.Agrón E, Mares J, Clemons TE, Swaroop A, Chew EY, Keenan TDL. Dietary nutrient intake and progression to late age-related macular degeneration in the age-related eye disease studies 1 and 2. Ophthalmology. 2021;128(3):425–442. doi: 10.1016/j.ophtha.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL, 3rd, Elman MJ, et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report no. 3. JAMA Ophthalmol. 2014;132(2):142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Delcourt C, Carrière I, Delage M, Barberger-Gateau P, Schalch W. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci. 2006;47(6):2329–2335. doi: 10.1167/iovs.05-1235. [DOI] [PubMed] [Google Scholar]
- 166.Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G, Millen AE, et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol. 2001;153(5):424–432. doi: 10.1093/aje/153.5.424. [DOI] [PubMed] [Google Scholar]
- 167.Merle BMJ, Cougnard-Grégoire A, Korobelnik JF, Schalch W, Etheve S, Rougier MB, et al. Plasma lutein, a nutritional biomarker for development of advanced age-related macular degeneration: the alienor study. Nutrients. 2021;13(6):2047. doi: 10.3390/nu13062047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jiang H, Yin Y, Wu CR, Liu Y, Guo F, Li M, et al. Dietary vitamin and carotenoid intake and risk of age-related cataract. Am J Clin Nutr. 2019;109(1):43–54. doi: 10.1093/ajcn/nqy270. [DOI] [PubMed] [Google Scholar]
- 169.Liu R, Wang T, Zhang B, Qin L, Wu C, Li Q, et al. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;56(1):252–258. doi: 10.1167/iovs.14-15553. [DOI] [PubMed] [Google Scholar]
- 170.Li SS, Wang HH, Zhang D. Efficacy of different nutrients in age-related macular degeneration: a systematic review and network meta-analysis. Semin Ophthalmol. 2022;37(4):515–523. doi: 10.1080/08820538.2021.2022165. [DOI] [PubMed] [Google Scholar]
- 171.Csader S, Korhonen S, Kaarniranta K, Schwab U. The effect of dietary supplementations on delaying the progression of age-related macular degeneration: a systematic review and meta-analysis. Nutrients. 2022;14(20):4273. doi: 10.3390/nu14204273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ma L, Liu R, Du JH, Liu T, Wu SS, Liu XH. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients. 2016;8(7):426. doi: 10.3390/nu8070426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mrowicka M, Mrowicki J, Kucharska E, Majsterek I. Lutein and zeaxanthin and their roles in age-related macular degeneration-neurodegenerative disease. Nutrients. 2022;14(4):827. doi: 10.3390/nu14040827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hankinson SE, Stampfer MJ, Seddon JM, Colditz GA, Rosner B, Speizer FE, et al. Nutrient intake and cataract extraction in women: a prospective study. BMJ. 1992;305(6849):335–339. doi: 10.1136/bmj.305.6849.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fletcher AE. Free radicals, antioxidants and eye diseases: evidence from epidemiological studies on cataract and age-related macular degeneration. Ophthalmic Res. 2010;44(3):191–198. doi: 10.1159/000316476. [DOI] [PubMed] [Google Scholar]
- 176.Garland DL. Ascorbic acid and the eye. Am J Clin Nutr. 1991;54(6 Suppl):1198s–s1202. doi: 10.1093/ajcn/54.6.1198s. [DOI] [PubMed] [Google Scholar]
- 177.Jacques PF, Chylack LT., Jr Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. Am J Clin Nutr. 1991;53(1 Suppl):352s–s355. doi: 10.1093/ajcn/53.1.352S. [DOI] [PubMed] [Google Scholar]
- 178.Jacques PF, Taylor A, Hankinson SE, Willett WC, Mahnken B, Lee Y, et al. Long-term vitamin C supplement use and prevalence of early age-related lens opacities. Am J Clin Nutr. 1997;66(4):911–916. doi: 10.1093/ajcn/66.4.911. [DOI] [PubMed] [Google Scholar]
- 179.Rautiainen S, Lindblad BE, Morgenstern R, Wolk A. Vitamin C supplements and the risk of age-related cataract: a population-based prospective cohort study in women. Am J Clin Nutr. 2010;91(2):487–493. doi: 10.3945/ajcn.2009.28528. [DOI] [PubMed] [Google Scholar]
- 180.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119(10):1439–52. [DOI] [PMC free article] [PubMed]
- 181.Newsome DA, Swartz M, Leone NC, Elston RC, Miller E. Oral zinc in macular degeneration. Arch Ophthalmol. 1988;106(2):192–198. doi: 10.1001/archopht.1988.01060130202026. [DOI] [PubMed] [Google Scholar]
- 182.Vishwanathan R, Chung M, Johnson EJ. A systematic review on zinc for the prevention and treatment of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(6):3985–3998. doi: 10.1167/iovs.12-11552. [DOI] [PubMed] [Google Scholar]
- 183.Pameijer EM, Heus P, Damen JAA, Spijker R, Hooft L, Ringens PJ, et al. What did we learn in 35 years of research on nutrition and supplements for age-related macular degeneration: a systematic review. Acta Ophthalmol. 2022;100(8):e1541–e1552. doi: 10.1111/aos.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mustacich DJ, Bruno RS, Traber MG. Vitamin E. Vitam Horm. 2007;76:1–21. doi: 10.1016/S0083-6729(07)76001-6. [DOI] [PubMed] [Google Scholar]
- 185.Brubaker RF, Bourne WM, Bachman LA, McLaren JW. Ascorbic acid content of human corneal epithelium. Invest Ophthalmol Vis Sci. 2000;41(7):1681–1683. [PubMed] [Google Scholar]
- 186.Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008;43(5):370–377. doi: 10.1016/j.exger.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 187.Gilbert R, Peto T, Lengyel I, Emri E. Zinc nutrition and inflammation in the aging retina. Mol Nutr Food Res. 2019;63(15):e1801049. doi: 10.1002/mnfr.201801049. [DOI] [PubMed] [Google Scholar]
- 188.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6 Suppl):1402s–s1406. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 189.Merle BMJ, Colijn JM, Cougnard-Grégoire A, de Koning-Backus APM, Delyfer MN, Kiefte-de Jong JC, et al. Mediterranean diet and incidence of advanced age-related macular degeneration: the EYE-RISK consortium. Ophthalmology. 2019;126(3):381–390. doi: 10.1016/j.ophtha.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 190.Mares JA, Voland RP, Sondel SA, Millen AE, Larowe T, Moeller SM, et al. Healthy lifestyles related to subsequent prevalence of age-related macular degeneration. Arch Ophthalmol. 2011;129(4):470–480. doi: 10.1001/archophthalmol.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hogg RE, Woodside JV, McGrath A, Young IS, Vioque JL, Chakravarthy U, et al. Mediterranean diet score and its association with age-related macular degeneration: the European eye study. Ophthalmology. 2017;124(1):82–89. doi: 10.1016/j.ophtha.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 192.Nunes S, Alves D, Barreto P, Raimundo M, da Luz CM, Farinha C, et al. Adherence to a Mediterranean diet and its association with age-related macular degeneration. The Coimbra Eye Study-Report 4. Nutrition. 2018;51–52:6–12. doi: 10.1016/j.nut.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 193.Raimundo M, Mira F, Cachulo MDL, Barreto P, Ribeiro L, Farinha C, et al. Adherence to a Mediterranean diet, lifestyle and age-related macular degeneration: the Coimbra Eye Study—report 3. Acta Ophthalmol. 2018;96(8):e926–e932. doi: 10.1111/aos.13775. [DOI] [PubMed] [Google Scholar]
- 194.Merle BM, Silver RE, Rosner B, Seddon JM. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am J Clin Nutr. 2015;102(5):1196–1206. doi: 10.3945/ajcn.115.111047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Díaz-López A, Babio N, Martínez-González MA, Corella D, Amor AJ, Fitó M, et al. Mediterranean diet, retinopathy, nephropathy, and microvascular diabetes complications: a post hoc analysis of a randomized trial. Diabetes Care. 2015;38(11):2134–2141. doi: 10.2337/dc15-1117. [DOI] [PubMed] [Google Scholar]
- 196.Merle BMJ, Moreau G, Ozguler A, Srour B, Cougnard-Grégoire A, Goldberg M, et al. Unhealthy behaviours and risk of visual impairment: the CONSTANCES population-based cohort. Sci Rep. 2018;8(1):6569. doi: 10.1038/s41598-018-24822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang P, Zhu H. Light signaling and myopia development: a review. Ophthalmol Ther. 2022;11(3):939–957. doi: 10.1007/s40123-022-00490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Torii H, Ohnuma K, Kurihara T, Tsubota K, Negishi K. Violet light transmission is related to myopia progression in adult high myopia. Sci Rep. 2017;7(1):14523. doi: 10.1038/s41598-017-09388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mori K, Torii H, Hara Y, Hara M, Yotsukura E, Hanyuda A, et al. Effect of violet light-transmitting eyeglasses on axial elongation in myopic children: a randomized controlled trial. J Clin Med. 2021;10(22):5462. doi: 10.3390/jcm10225462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.