Abstract
Objective
This study aimed to evaluate the impact of cardiopulmonary bypass for thoraco-abdominal normothermic regional perfusion on the metabolic milieu of donation after cardiac death organ donors before transplantation.
Methods
Local donation after cardiac death donor offers are assessed for suitability and willingness to participate. Withdrawal of life-sustaining therapy is performed in the operating room. After declaration of circulatory death and a 5-minute observation period, the cardiac team performs a median sternotomy, ligation of the aortic arch vessels, and initiation of thoraco-abdominal normothermic regional perfusion via central cardiopulmonary bypass at 37 °C. Three sodium chloride zero balance ultrafiltration bags containing 50 mEq sodium bicarbonate and 0.5 g calcium carbonate are infused. Arterial blood gas measurements are obtained every 15 minutes after every zero balance ultrafiltration bag is infused, and blood is transfused as needed to maintain hemoglobin greater than 8 mg/dL. Cardiopulmonary bypass is weaned with concurrent hemodynamic and transesophageal echocardiogram evaluation of the donor heart. The remainder of the procurement, including the abdominal organs, proceeds in a similar controlled fashion as is performed for a standard donation after brain death donor.
Results
Between January 2020 and May 2022, 18 donation after cardiac death transplants using the thoraco-abdominal normothermic regional perfusion protocol were performed at our institution. The median donor age was 42.5 years (range, 20-51 years), and 88.9% (16/18) were male. The mean total donor cardiopulmonary bypass time was 88.8 ± 51.8 minutes. At the beginning of cardiopulmonary bypass, the average donor lactate was 9.4 ± 1.5 mmol/L compared with an average final lactate of 5.3 ± 2.7 mmol/L (P<.0001). The average beginning potassium was 6.5 ± 1.8 mmol/L compared with an average end potassium of 4.2 ± 0.4 mmol/L (P<.0001) . The average beginning hemoglobin was 6.8 ± 0.7 g/dL, and the average end hemoglobin was 8.2 ± 1.3 g/dL (P<.001) . On average, donation after cardiac death donors received transfusions of 2.3 ± 1.5 units of packed red blood cells. Of the 18 donors who underwent normothermic regional perfusion, all hearts were deemed suitable for recovery and successfully transplanted, a yield of 100%. Other organs successfully recovered and transplanted include kidneys (80.6% yield), livers (66.7% yield), and bilateral lungs (27.8% yield).
Conclusions
The use of cardiopulmonary bypass for thoraco-abdominal normothermic regional perfusion is a burgeoning option for improving the quality of organs from donation after cardiac death donors. Meticulous intraoperative management of donation after cardiac death donors with a specific focus on improving their metabolic milieu may lead to improved graft function in transplant recipients.
Key Words: donation after circulatory death, heart transplantation, normothermic regional perfusion
Abbreviations and Acronyms: CIT, cold ischemic time; CPB, cardiopulmonary bypass; DBD, donation after brain death; DCD, donation after circulatory death; DWIT, donor warm ischemic time; ICU, intensive care unit; NRP, normothermic regional perfusion; OPO, Organ Procurement Organization; TEE, transesophageal echocardiography; UF, ultrafiltration; WLST, withdrawal of life-sustaining therapy; Z-BUF, zero-balance ultrafiltration
Co-location enables uniform DCD-NRP procurement, focusing the expertise of multiple teams.
Central Message.
A standardized protocol for DCD cardiac transplantation using NRP is reproducible, allows for metabolic optimization of the donor, and ensures minimal ischemic time for the heart.
Perspective.
A standardized protocol for the use of CPB for thoracoabdominal NRP is a burgeoning option for improving the quality of cardiac allografts from DCD donors.
In 2021, despite the ongoing worldwide COVID-19 pandemic and severely constrained medical resources, a total of 3817 heart transplants were performed in the United States—the highest number ever recorded.1 However, the number of heart transplants performed annually continues to be outpaced by the increasing number of waitlisted patients. The 2019 annual data report from the Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients demonstrated a 42.5% increase in the number of newly listed heart transplant candidates from 2008 to 2019.2 The persistent gap between the number of hearts available and the number of patients in need of heart transplantation has led to novel efforts to expand the donor pool, as well as a renewed interest in obtaining hearts from donation after circulatory death (DCD) donors.
The majority of deceased donor hearts in the United States are obtained from patients with irreversible cessation of neurological function, otherwise known as organ donation after brain death (DBD). In contrast, DCD involves the intentional withdrawal of life-sustaining therapy (WLST) to permit controlled circulatory death to occur. If the patient fails to progress to circulatory death within an allotted time frame, DCD donation is aborted and the patient is placed on palliative measures until the patient dies.
There have been 3 primary methods described for DCD heart transplantation. The original method, as described by Barnard3,4 and used in the first heart transplants, was direct procurement and transplantation. The donor and recipient were co-located in the same facility to minimize time between the withdrawal of donor life support, heart implantation, and subsequent reperfusion in the recipient. An alternative strategy involves direct procurement after circulatory death followed by ex situ perfusion using a specialized perfusion device (eg, TransMedics, Andover, Mass, Organ Care System). This was first described by the Sydney group and subsequently by the UK group.5, 6, 7 Most recently, a randomized trial has been completed in the United States with favorable clinical results, and the device has been approved by the Food and Drug Administration for ex vivo reanimation, functional monitoring, and beating-heart preservation of DCD hearts.8,9 The third method, normothermic regional perfusion (NRP), involves the use of extracorporeal membrane oxygenation or cardiopulmonary bypass (CPB) to establish in situ reperfusion of the heart and other organs with oxygenated blood after isolation and ligation of the aortic arch vessels.10 After NRP procurement, the heart is either transported using cold storage or in the Organ Care System.11, 12, 13 NRP has the following significant advantages:
-
1.
Expedient restoration of blood flow mitigates the impact of donor warm ischemic time (DWIT).
-
2.
Metabolic abnormalities that accompany circulatory death, primarily acidosis and hyperkalemia, can be corrected.14
-
3.
The heart can be evaluated for suitability in situ after it has been volume loaded to function under physiologic conditions.
-
4.
A controlled procurement process for all teams involved facilitates a safe dissection and allows for accurate assessment of all thoracic and abdominal organs.
In DCD cardiac transplantation, there is considerable concern regarding the heart's exposure to injury in 3 distinct phases: (1) warm hypoxic/ischemic injury during the period from WLST to decompensation and death; (2) warm ischemic injury during the standoff period after donor death; and (3) ischemia and reperfusion injury in the period of organ storage or ex vivo heart perfusion, transportation, and implantation.15 DWIT is defined as the period from WLST to the end of the standoff period.6 Minimizing DWIT and mitigating the effects of reperfusion injury are of the utmost importance, because they are the major contributors to irreversible myocardial damage. In the context of DCD heart transplantation, DCD donors decompensate in a highly variable and unpredictable fashion after WLST, and neither donor vital signs nor DWIT can reliably predict graft performance.16 The rapid initiation of NRP after donor death aims to minimize DWIT and optimize the donor heart before acceptance and implantation.
As the use of NRP for DCD heart transplantation continues to expand both nationally and internationally, a standardized protocol with proven results is needed.11,17, 18, 19 We have previously reported17 on our early experience with DCD heart transplantation using CPB for NRP and hereby publish our institutional protocol.
Donor Selection
Potential DCD donors are stratified according to the modified Maastricht classification system (Table 1). Categories 1 and 2 would be considered “uncontrolled” DCD, whereas categories 3 and 4 are considered “controlled” DCD, where the duration and conditions of warm ischemia are known and the precise course of circulatory arrest can be followed.20,21
Table 1.
Modified Maastricht classification of donation after circulatory death
| Category | Circumstances | Definition |
|---|---|---|
| I. Uncontrolled | Found dead IA. Out of hospital IB. In hospital |
Sudden unexpected circulatory arrest without any attempt of resuscitation by a medical team |
| II. Uncontrolled | Witnessed cardiac arrest IIA. Out of hospital IIB. In hospital |
Sudden unexpected irreversible circulatory arrest with unsuccessful resuscitation by a medical team |
| III. Controlled | Withdrawal of life-sustaining therapy | Planned WLST; expected circulatory arrest |
| IV. Uncontrolled/controlled | Circulatory arrest after neurological determination of death. | Sudden circulatory arrest after neurological determination of death diagnosis during donor management but before planed organ recovery |
WLST, Withdrawal of life-sustaining therapy.
We accept donors between the ages of 18 and 49 years. Recipients are selected on the basis of standard criteria according to United Network for Organ Sharing transplant listing order, blood group, cross-match, size match, and clinical stability. All organ functions of the donor are assessed for suitability on notification of the intent to withdraw support on an active referral. If we have a suitable recipient, transthoracic echocardiography is performed to assess the potential donor's baseline cardiac function. If the echocardiographic assessment is satisfactory, in select cases, such as a significant smoking history, uncontrolled hypertension, diabetes, age more than 40 years, or any clinical concern for coronary artery disease, cardiac catheterization may be requested before acceptance. If the organ function is determined to be acceptable, upon authorization by the patient's health care proxy, the patient is transferred from the initial hospital to our surgical intensive care unit (ICU). The patient is transported using the same standard policies and procedures that govern all interfacility transfers at our institution. All other assessments and determinations are made by our ICU team (Table 2).
Table 2.
Donor demographics
| Characteristics | Donor 1 | Donor 2 | Donor 3 | Donor 4 | Donor 5 | Donor 6 | Donor 7 | Donor 8 | Donor 9 | Donor 10 | Donor 11 | Donor 12 | Donor 13 | Donor 14 | Donor 15 | Donor 16 | Donor 17 | Donor 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 43 | 44 | 29 | 26 | 42 | 47 | 43 | 44 | 44 | 51 | 35 | 20 | 39 | 34 | 29 | 43 | 44 | 40 |
| Sex | M | M | M | F | M | M | M | M | M | M | M | M | M | M | M | M | M | F |
| Height, m | 2.03 | 1.68 | 1.75 | 1.70 | 1.78 | 1.80 | 1.72 | 1.80 | 1.75 | 1.73 | 1.83 | 1.96 | 1.80 | 1.88 | 1.83 | 1.83 | 1.83 | 1.70 |
| Weight, kg | 96.1 | 70.0 | 117.4 | 99.1 | 78.9 | 82.1 | 85.0 | 77.1 | 106.5 | 90.7 | 102.8 | 81.0 | 93.0 | 89.4 | 76.5 | 91.0 | 77.0 | 103.9 |
| BMI, kg/m2 | 23.3 | 24.8 | 38.3 | 34.2 | 25.0 | 25.3 | 28.6 | 23.7 | 34.8 | 30.3 | 30.7 | 21.1 | 28.7 | 25.3 | 22.8 | 27.2 | 23.0 | 36.0 |
| Blood type | A | O | B | A | O | A | O | O | O | B | O | O | B | O | O | O | O | O |
| Terminal condition | ESLD | HBI | HBI | HBI | SAH | TBI | SAH | ICH | HBI | HBI | TBI | HBI | HBI | HBI | CVA | HBI | HBI | TBI |
| Toxicology | BZD, THC | BZD, cocaine, PCP, THC | BZD, cocaine | BZD, cocaine, opioid | Negative | Negative | Negative | Negative | BZD, cocaine | Negative | BZD, opioid, THC | Negative | Opioid | THC | THC | THC | PSA | Vaping, prior tobacco |
| LVEF, % | 68 | 70 | 75 | 65 | 65 | 60 | 65 | 60 | 65 | 65 | 60 | 60 | 75 | 65 | 60 | 55 | 60 | 70 |
| Preoperative coronary angiogram | Yes | No | No | No | No | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes |
| Organs procured and successfully transplanted | Heart | Heart Liver Kidneys | Heart Liver Kidneys | Heart Liver Kidneys | Heart Lungs Liver∗ Kidneys† |
Heart Liver Kidneys‡ |
Heart Liver Kidneys | Heart Kidneys |
Heart Liver Kidneys | Heart Lungs Liver Kidneys |
Heart Lungs∗ Liver∗ Kidneys |
Heart Kidneys† |
Heart Liver Kidneys |
Heart Lungs Liver Kidneys |
Heart Liver† Kidneys |
Heart Lungs Liver Kidneys |
Heart Lungs Liver Kidneys |
Heart Lungs∗ Liver Kidneys |
BMI, Body mass index; ESLD, end-stage liver disease; HBI, hypoxic brain injury; SAH, subarachnoid hemorrhage; TBI, traumatic brain injury; ICH, intracerebral hemorrhage; CVA, cerebrovascular accident; BZD, benzodiazepine; THC, tetrahydrocannabinol; PCP, phencyclidine; PSA, polysubstance abuse; LVEF, left ventricular ejection fraction.
Recovered for research.
Recovered but not transplanted.
Both kidneys recovered, only 1 transplanted.
Informed Consent
In 2012, the American Thoracic Society, International Society for Heart and Lung Transplantation, Association of Organ and Procurement Organizations, and United Network for Organ Sharing established a framework to guide ethics and health policy considerations in adult DCD organ donation.22 With respect to informed consent, discussions about DCD should be coordinated jointly by clinicians and Organ Procurement Organization (OPO) representatives, and should be obtained by individuals with appropriate experience and training. These individuals' organizational affiliations should always be clearly disclosed. For patients who are potentially suitable candidates for DCD, donation should be presented to the surrogate after a decision has been made to withdraw life-sustaining treatments. Before initiating the DCD program, we met with the OPO and their coordinators to describe thoroughly our NRP protocol. The goal of these training sessions was to provide OPO representatives with a complete understanding of the NRP process so they could obtain informed consent from the surrogates of potential donors with full transparency. The local OPO discusses organ donation with the family only after the following 3 criteria are met: The decision has been made to withdraw support, organ function is determined to be acceptable, and the donor is likely to progress to cardiac arrest within the time allotted after WLST.23 The coordinator reviews the DCD process, including all relevant antemortem interventions and medications. This includes the administration of heparin before WLST and the possibility that the patient will not proceed to cardiac arrest and may be ultimately ineligible for organ donation.
Co-location of Donor and Recipient
Our requirement for co-location of the donor and recipient is to maintain uniformity of the DCD-NRP procurement process, minimize cold ischemic time (CIT), and allow for efficient and improved team member communication. There are several benefits for co-location, the most important of which are as follows: (1) minimizing total warm ischemic time and CIT for the heart; (2) standardizing the methodology of organ reperfusion to ensure safety and reproducibility; (3) reducing subjective variability in the assessment of whether or not organs are suitable for transplantation; (4) standardizing the WLST by dedicated ICU physicians and staff who understand the process; and (5) focusing expertise in a single location with multiple experienced teams in cardiothoracic surgery, cardiology, critical care, anesthesiology, and abdominal transplant surgery.
There are limitations that arise in the concept of co-location of the donor and recipient. Institutions based in densely populated metropolitan areas have the ability to perform interfacility transfers easily, which may not be feasible or practical for centers located in smaller cities or in remote rural areas. In these instances, a protocol using a portable extracorporeal circuit, blood pump, and blood reservoir has been successful for NRP procurement of DCD hearts.18 However, this method does not address potential inexperience in ICU management and lack of standard practices for the withdrawal of support and antemortem interventions.
We have previously proposed that DCD heart transplantation using NRP should be concentrated in specialized centers accustomed to cardiac surgery.24 In this model, 1 or 2 cardiac transplant centers within the donor service area of each OPO could be selected to serve as designated DCD heart centers. Once a recipient who is willing to accept the heart is identified, the potential donor would be transferred to the designated DCD heart center in that donor service area. At this center, a dedicated cardiothoracic surgical team would conduct the reperfusion, assessment for heart suitability, and eventual heart procurement. The heart would then be transported to the distant recipient center using standard cold storage. Each heart would undergo a standardized resuscitation and evaluation, thereby ensuring a greater yield of transplantable hearts.
Prerecovery Sequence of Events
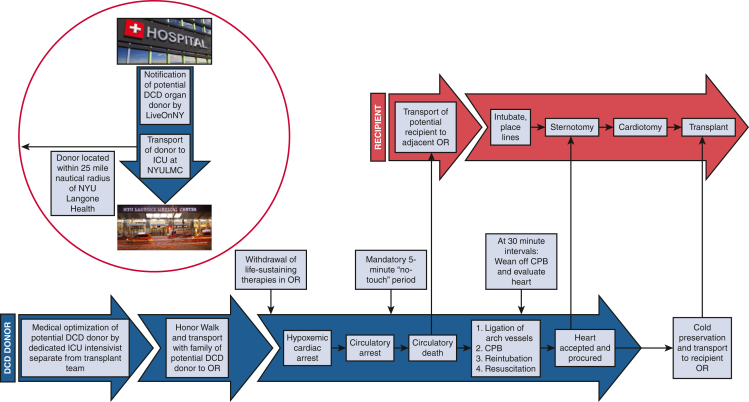
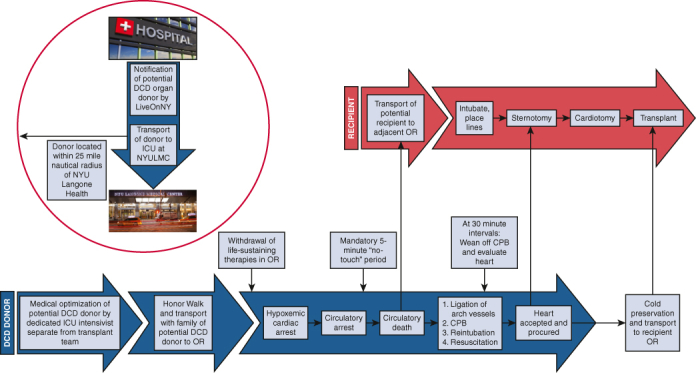
Efficiency is of paramount importance in the DCD-NRP procurement process to limit DWIT and initiate CPB as soon as possible. Accordingly, in our protocol WLST is performed in the operating room rather than in the ICU to eliminate the DWIT during the transfer process to the operating room after cardiac arrest. While the patient is in the ICU before transfer to the operating room, a femoral arterial line and pulmonary artery (Swan-Ganz) catheter are placed. Operating room staff are briefed on the procedure and how this type of donation differs from that of DBD donors. When the time comes for the patient to be transferred to the operating room, physicians, nurses, and staff line the pathway from the ICU to the patient transport elevators in a ritual known as the Honor Walk. The Honor Walk provides the families and patients of intended organ donors, as well as the medical care team, an opportunity to acknowledge the donor for sharing the gift of life.25 The Honor Walk is a symbol of compassion and unity among families, friends, and care teams of the donor and is a powerful way to say goodbye.
Family members or friends who have accompanied the donor from the ICU are escorted to an adjacent conference room on the same floor as the operating room. The patient is transferred to the operating room and prepped and draped before WLST, with the CPB circuit prepared on the surgical field and ready for cannulation. Another sterile sheet is used to cover the draped patient, which allows the intensivist to confirm death after the 5-minute “no-touch” period without contaminating the surgical field. After confirmation of circulatory death, the cover drape is removed and discarded so that the operation may commence (Figure 1).
Figure 1.
Sequence of events for DCD-NRP. DCD, Donation after circulatory death; ICU, intensive care unit; OR, operating room; CPB, cardiopulmonary bypass.
Withdrawl of Life-Sustaining Treatment
Before WLST, an arterial blood gas is drawn to establish a metabolic baseline. The presence of 4 units of cross-matched packed red blood cells in the operating room is confirmed and verified. The intensivist administers 50,000 units of heparin 3 minutes before WLST. At this point, the organ recovery teams and perfusion team exit the operating room. Family members or friends may choose to be present with the patient during the WLST and are escorted to the operating room after the patient has been draped. WLST is performed by the critical care team, who are not involved in the organ procurement process. During this period, comfort care measures are taken to ensure a dignified and appropriate end of life for the patient. When the patient is draped, their arm remains exposed so that family members may hold their hand after WLST. Family members typically remain in the operating room until the declaration of death. No members of the organ recovery teams reenter until the family has been escorted out the operating room. We strictly adhere to this protocol to maintain complete separation of WLST and declaration of death from the initiation of NRP and organ procurement.
Pronouncement of Death
Our policy for pronouncement of death adheres to the recommendations of the Institute of Medicine (1997 Non-Heart-Beating Organ Transplantation: Medical and Ethical Issues in Procurement).26 These recommendations include separating decisions about management of care with respect to WLST and those of organ donation, as well as the determination of death and the act of organ procurement. Furthermore, the responsibilities of the critical care attending physicians and other personnel charged with patient care must be kept separate from those of the transplant or procurement physicians and personnel. An interval of 5 minutes must elapse between the cardiopulmonary arrest and the declaration of death. This time is described in the Electronic Death Records as the “Start of the Observation Period” and is documented accordingly. Cardiopulmonary arrest must be verified by clinical observation, electrocardiographic monitor, or pulseless electrical activity with direct blood pressure monitoring. Death is pronounced by the critical care attending physician who is wholly separate from the transplant and procurement teams. The organ recovery teams only reenter the operating room if the patient meets criteria for cardiopulmonary arrest and the obligatory 5-minute standoff period has elapsed.
Postexpiration Sequence of Events
After declaration of death, the cardiothoracic organ procurement team begins first to minimize the number of people competing for space around the operating table. A rapid median sternotomy is performed. and the pericardium is partially opened to expose the aorta and right atrium. Consistent with standard practice, the innominate vein is ligated and divided, and the innominate artery, carotid artery, and subclavian artery are clamped to achieve complete cerebral circulatory isolation.13 After placement of a purse-string suture in the distal aorta, the aortic cannula is placed and connected to the bypass circuit. The right atrium is entered through a stab incision, and the patient is placed on circulatory support. When the bypass cannulas have been secured, the donor is reintubated. We generally place a left ventricle vent through the right superior pulmonary vein or left atrial appendage depending on consideration for lung procurement. Once normothermic CPB (37 °C) has been established, the perfusion team works diligently and efficiently to correct fluid and electrolyte abnormalities and optimize the metabolic milieu of the donor. This process begins with immediate administration of 3 sodium chloride zero-balance ultrafiltration (Z-BUF) bags containing 50 mEq sodium bicarbonate and 0.5 g calcium carbonate. The use of ultrafiltration (UF) for patients undergoing cardiac surgery with CPB is a well-accepted technique for hemoconcentration and correcting electrolyte imbalances.27 The most common UF modality is conventional UF, which typically denotes the use of UF during CPB as a hemoconcentrating process without the addition of asanguineous solutions, whereas Z-BUF is performed in a euvolemic manner with equal volumes of fluid added and removed. Z-BUF provides a means to reduce circulating elements generated most often through inflammatory processes or electrolyte imbalances created by cardioplegic solutions, and in this case the hyperkalemia that accompanies circulatory death.27 Arterial blood gas measurements are obtained at 15-minute intervals after each Z-BUF bag is infused. Blood is transfused as needed to maintain hemoglobin greater than 8 mg/dL. We have found that the use of CPB for NRP improves the metabolic milieu of the donors, which may lead to improved graft function in transplant recipients (Table 3).
Table 3.
Intraoperative donor ischemic times and laboratory values
| Parameter | Donor 1 | Donor 2 | Donor 3 | Donor 4 | Donor 5 | Donor 6 | Donor 7 | Donor 8 | Donor 9 | Donor 10 | Donor 11 | Donor 12 | Donor 13 | Donor 14 | Donor 15 | Donor 16 | Donor 17 | Donor 18 | Mean ± SD for all donors | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WLST to agonal phase, min | 5 | 0 | 1 | 2 | 1 | 1 | 12 | 3 | 0 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2.17 ± 2.71 | |
| Agonal phase to asystole, min | 14 | 14 | 15 | 23 | 16 | 9 | 20 | 3 | 14 | 14 | 12 | 18 | 13 | 7 | 3 | 16 | 16 | 11 | 13.22 ± 5.23 | |
| WLST to asystole, min | 19 | 14 | 16 | 25 | 17 | 10 | 32 | 6 | 14 | 15 | 13 | 20 | 15 | 8 | 5 | 18 | 18 | 12 | 15.39 ± 6.51 | |
| WLST to skin incision, min | 29 | 19 | 21 | 25 | 23 | 16 | 38 | 11 | 20 | 20 | 18 | 25 | 20 | 14 | 11 | 23 | 24 | 24 | 21.17 ± 6.42 | |
| Skin incision to CPB, min | 12 | 9 | 12 | 12 | 9 | 11 | 11 | 12 | 7 | 9 | 9 | 12 | 9 | 7 | 8 | 10 | 6 | 0 | 9.17 ± 3.00 | |
| Return of cardiac activity, min | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1.0 ± 0.0 | |
| Total donor CPB time, min | 40 | 32 | 35 | 39 | 68 | 33 | 115 | 119 | 84 | 64 | 38 | 118 | 102 | 100 | 141 | 130 | 109 | 231 | 88.78 ± 51.76 | |
| Functional WIT, min | 36 | 28 | 32 | 35 | 31 | 26 | 37 | 20 | 27 | 28 | 26 | 35 | 27 | 20 | 17 | 31 | 28 | 23 | 28.17 ± 5.74 | |
| Total WIT, min | 41 | 28 | 33 | 37 | 32 | 27 | 49 | 23 | 27 | 29 | 27 | 37 | 29 | 21 | 19 | 33 | 30 | 24 | 30.33 ± 7.36 | |
| Total CIT, min | 78 | 70 | 85 | 80 | 84 | 80 | 65 | 68 | 70 | 86 | 67 | 69 | 95 | 71 | 76 | 117 | 85 | 68 | 78.56 ± 12.75 | |
| Lactate at beginning of CPB, mmol/L | 7.7 | 8.3 | 11.3 | 10.1 | 10.2 | 7.3 | 10.7 | 9.4 | 11.0 | 8.1 | 9.9 | 8.6 | 9.6 | 8.7 | 6.9 | 9.8 | 8.6 | 12.6 | 9.4 ± 1.5 | P < .0001 |
| Lactate at end of CPB, mmol/L | 10.2 | 7.7 | 8.2 | 5.9 | 4.8 | 7.6 | 2.4 | 8.1 | 2.5 | 3.1 | 10.3 | 4.1 | 3.9 | 3.9 | 3.4 | 3.5 | 2.3 | 3.4 | 5.3 ± 2.7 | |
| K+ at beginning of CPB, mmol/L | 7.2 | 9.7 | 6.4 | 6.6 | 4.6 | 7.7 | 9.0 | 5.8 | 4.5 | 6.8 | 4.4 | 7.9 | 6.2 | 6.2 | 3.7 | 7.4 | 4.2 | 9.5 | 6.5 ± 1.8 | P < .0001 |
| K+ at end of CPB, mmol/L | 3.5 | 4.8 | 4.0 | 4.3 | 5.2 | 4.3 | 4.4 | 3.8 | 4.2 | 4.0 | 4.7 | 3.9 | 4.4 | 4.2 | 3.9 | 4.5 | 3.6 | 4.2 | 4.2 ± 0.4 | |
| Hgb at beginning of CPB, g/dL | 6.0 | 6.0 | 6.0 | 6.4 | 6.1 | 6.0 | 6.9 | 7.1 | 8.3 | 6.5 | 6.6 | 6.6 | 8.1 | 6.0 | 6.9 | 7.5 | 7.8 | 6.8 | 6.8 ± 0.7 | P < .001 |
| Hgb at end of CPB, g/dL | 8.0 | 9.8 | 9.3 | 8.1 | 7.4 | 6.4 | 10.1 | 6.0 | 8.9 | 7.6 | 8.8 | 7.0 | 10.2 | 7.9 | 9.2 | 8.2 | 8.4 | 6.4 | 8.2 ± 1.3 | |
| Transfused pRBC, units | 2 | 2 | 2 | 2 | 7 | 2 | 2 | 4 | 0 | 2 | 2 | 1 | 2 | 2 | 4 | 2 | 2 | 1 | 2.0 ± 1.5 |
SD, Standard deviation; WLST, withdrawal of life-sustaining therapy; CPB, cardiopulmonary bypass; WIT, warm ischemic time; CIT, cold ischemic time; HgB, hemoglobin; pRBC, packed red blood cells.
At 30 minutes of reperfusion, CPB is weaned completely as the heart is refilled with blood for cardiac assessment by transesophageal echocardiography (TEE) and hemodynamic monitoring under the normal physiologic state. If cardiac function is determined to be adequate and the heart is accepted for transplant, CPB is resumed to rest the heart as the recipient operation is commenced in the proximate operating room. If the heart has not yet recovered, full CPB is resumed and the heart is permitted to beat empty for further resuscitation. This evaluation is repeated every 30 minutes for a maximum of 180 minutes. Inotropes are not used during the weaning process, although patients may require vasopressors for blood pressure support. We aim to maintain the mean arterial pressure between 70 and 90 mm Hg and a heart rate less than 120 beats per minute. If patients are hypertensive or tachycardic to greater than 120 beats per minute, we administer low-dose esmolol.
Transesophageal Echocardiographic Assessment Protocol
-
1.
Standard midesophageal 2-dimensional TEE views (at 0°, 60°, 90°, and 120°) are used to assess left and right ventricular wall motion, valve anatomy and function, presence or absence of a patent foramen ovale or ventricular septal defect, left atrial appendage anatomy, and pulmonary vein anatomy. Objectively, left ventricular systolic function can be calculated by obtaining the left ventricular end-diastolic and systolic volumes (left ventricular end-diastolic volume and left ventricular end-systolic volume, respectively) using the modified Simpson's rule. Left ventricular systolic function 50% or greater is the suggested threshold for adequate cardiac function.
-
2.
Standard transgastric TEE views are used to assess global and regional left and right wall motions, whereas a deep, transgastric 5-chamber view allows visualization of left ventricular outflow tract velocity time integral; velocity time integral 15 cm or greater is the suggested threshold.
-
3.
Tissue Doppler is used to measure the S wave at the lateral aspects of both mitral and tricuspid valves in the mid-esophageal 4-chamber view, at 0° to 15°; S wave 10 cm/sec or greater is the suggested threshold.
-
4.
Full-volume 3-dimensional TEE acquisition and mid-esophageal views from 0° to 120° encompassing the full left ventricle and right ventricle are obtained to visually assess ventricular function.
Recovery of the Heart and Other Organs
Although the listed criteria serve as the framework for our approach to the assessment of DCD-NRP hearts, the ultimate decision to accept or reject the donor heart rests with the decision of the transplanting heart team based on their clinical judgment. Once the heart is accepted, the implanting surgeon can begin the recipient operation. The decision of whether to return to CPB or not is primarily determined by the adequacy of lung function, the amount of bleeding encountered, and the patient's hemodynamic stability. If there are any concerns, the patient can be maintained on CPB until crossclamp. When all teams are prepared for crossclamp, the patient's blood volume is emptied into the CPB circuit and the organs are flushed with preservation solution. This terminal venting strategy results in a very clean field and excellent cardiac decompression. Between January 2020 and May 2022, we performed 18 heart transplants using the DCD-NRP protocol. Of the 18 donors who underwent NRP, all hearts were deemed suitable for recovery and successfully transplanted, a yield of 100% (Table 4). Other organs successfully recovered and transplanted include kidneys (80.6% yield), livers (66.7% yield), and bilateral lungs (27.8% yield).
Table 4.
Organs recovered from donation after circulatory death donors and successfully transplanted
| Organ | DCD-NRP donors (n = 18) | Yield |
|---|---|---|
| Heart | 18 | 100.0% |
| Bilateral lungs | 5 | 27.8% |
| Liver | 12 | 66.7% |
| Kidneys | 29 | 80.6% |
DCD-NRP, Donation after circulatory death normothermic regional perfusion.
Ethical Concerns
Although a complete review of the ethical framework for DCD-NRP cardiac transplantation is far beyond the scope of this review, it is nevertheless necessary to touch on some of the ethical concerns raised in the face of our innovative transplant protocol. In April 2021, the American College of Physicians asserted that DCD-NRP raises serious ethical considerations and recommended a pause in its use, pending further professional and public discussion.28 The American College of Physicians claim that the act of restoring the circulatory capacity of the heart within the deceased donor's body for in situ preservation of organs before procurement violates the dead donor rule, invalidates the declaration of circulatory death, and poses disproportionate risks to stigmatized and marginalized groups. We have previously published comprehensive responses addressing these concerns.14,29,30 These assertions rely on an incomplete understanding of DCD-NRP as well as a limited interpretation of the dead donor rule. Both standard DCD and DCD-NRP begin when a decision is made by the patient or their surrogate(s) to withdraw further life-sustaining therapies and to allow death to occur. Support is subsequently withdrawn and the patient is declared dead; the 5-minute hands-off period is observed to ensure there is no spontaneous auto-resuscitation. At this point in time, the patient is no longer alive and has transitioned to a deceased organ donor. As with standard DCD, in DCD-NRP death is declared using acceptable medical criteria by a physician who is wholly separate from the transplant team. The argument put forth by the ACP hinges on the idea that restoring cardiac function with CPB invalidates the declaration of circulatory death. We believe that during DCD-NRP, the inability of the heart to function within the donor is definitively established by the declaration of death and is not reversed by the institution of CPB. As DCD-NRP becomes more widely adopted in the United States, it will be necessary to maintain an ongoing dialogue about the ethics of DCD-NRP organ donation with physicians, surgeons, community stakeholders, ethicists, professional organizations, and, most important, patients and their friends.
Conclusions
We have previously published the results for our early experience on 8 DCD donor heart transplantations.17 Our protocol, which combines donor and recipient co-localization with TA-NRP using CPB, results in remarkable improvement and in some cases complete correction of electrolyte imbalances and metabolic derangements in donors before organ recovery. The combination of donor resuscitation and the decrease in warm ischemic time and CIT afforded by our co-localization strategy has resulted in procurement of excellent quality of all recovered organs and leads to results comparable to those for DBD transplantation.
Conflict of Interest Statement
A.G. has intellectual property and receives royalties from Medtronic for valve repair devices, and has intellectual property and receives royalties from Edwards Lifesciences. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
This study was funded by the Department of Cardiothoracic Surgery at NYU Grossman School of Medicine.
References
- 1.UNOS All-time records again set in 2021 for organ transplants, organ donation from deceased donors. 2022. https://unos.org/news/2021-all-time-records-organ-transplants-deceased-donor-donation/ Accessed January 2, 2023.
- 2.Colvin M., Smith J.M., Ahn Y., Skeans M.A., Messick E., Goff R., et al. OPTN/SRTR 2019 annual data report: heart. Am J Transplant. 2021;21(Suppl 2):356–440. doi: 10.1111/ajt.16492. [DOI] [PubMed] [Google Scholar]
- 3.Barnard C.N. Human heart transplantation. Can Med Assoc J. 1969;100:91–104. [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard C.N. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967;41:1271–1274. [PubMed] [Google Scholar]
- 5.Dhital K.K., Chew H.C., Macdonald P.S. Donation after circulatory death heart transplantation. Curr Opin Organ Transplant. 2017;22:189–197. doi: 10.1097/MOT.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 6.Dhital K.K., Iyer A., Connellan M., Chew H.C., Gao L., Doyle A., et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385:2585–2591. doi: 10.1016/S0140-6736(15)60038-1. [DOI] [PubMed] [Google Scholar]
- 7.Smail H., Garcia-Saez D., Stock U., Ahmed-Hassan H., Bowles C., Zych B., et al. Direct heart procurement after donation after circulatory death with ex situ reperfusion. Ann Thorac Surg. 2018;106:e211–e214. doi: 10.1016/j.athoracsur.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Schroder J.N., Shah A., Pretorius V., Smith J., Daneshmand M., Geirsson A., et al. Expanding heart transplants from donors after circulatory death (DCD)–results of the first randomized controlled trial using the organ care system (OCSTM) heart–(OCS DCD Heart Trial) J Heart Lung Transplant. 2022;41:S72. [Google Scholar]
- 9.TransMedics Receives FDA PMA Approval of OCS DCD Heart Indication. TransMedics [Internet], 2022. Accessed January 3, 2023. https://investors.transmedics.com/news-releases/news-release-details/transmedics-receives-fda-pma-approval-ocstm-dcd-heart-indication
- 10.Messer S.J., Axell R.G., Colah S., White P.A., Ryan M., Page A.A., et al. Functional assessment and transplantation of the donor heart after circulatory death. J Heart Lung Transplant. 2016;35:1443–1452. doi: 10.1016/j.healun.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Minambres E., Royo-Villanova M., Perez-Redondo M., Coll E., Villar-Garcia S., Canovas S.J., et al. Spanish experience with heart transplants from controlled donation after the circulatory determination of death using thoraco-abdominal normothermic regional perfusion and cold storage. Am J Transplant. 2021;21:1597–1602. doi: 10.1111/ajt.16446. [DOI] [PubMed] [Google Scholar]
- 12.Vandendriessche K., Tchana-Sato V., Ledoux D., Degezelle K., Rex S., Neyrinck A., et al. Transplantation of donor hearts after circulatory death using normothermic regional perfusion and cold storage preservation. Eur J Cardiothorac Surg. 2021;60:813–819. doi: 10.1093/ejcts/ezab139. [DOI] [PubMed] [Google Scholar]
- 13.Messer S., Cernic S., Page A., Berman M., Kaul P., Colah S., et al. A 5-year single-center early experience of heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2020;39:1463–1475. doi: 10.1016/j.healun.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 14.James L, Hill F, Smith DE, Carillo J, Ngai J, Galloway AC, eds, et al. Use of Cardiopulmonary Bypass for Thoracoabdominal Normothermic Regional Perfusion Organ Donation Allows for Metabolic Optimization of the Donor. The American Association for Thoracic Surgery 102nd Annual Meeting; 2022
- 15.Kearns M.J., Miller S.D., Cheung A., Bashir J., Wong S., Seidman M.A., et al. A rodent model of cardiac donation after circulatory death and novel biomarkers of cardiac viability during ex vivo heart perfusion. Transplantation. 2017;101:e231–e239. doi: 10.1097/TP.0000000000001815. [DOI] [PubMed] [Google Scholar]
- 16.Allen M.B., Billig E., Reese P.P., Shults J., Hasz R., West S., et al. Donor hemodynamics as a predictor of outcomes after kidney transplantation from donors after cardiac death. Am J Transplant. 2016;16:181–193. doi: 10.1111/ajt.13432. [DOI] [PubMed] [Google Scholar]
- 17.Smith D.E., Kon Z.N., Carillo J.A., Chen S., Gidea C.G., Piper G.L., et al. Early experience with donation after circulatory death heart transplantation using normothermic regional perfusion in the United States. J Thorac Cardiovasc Surg. 2022;164:557–568.e1. doi: 10.1016/j.jtcvs.2021.07.059. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman J.R.H., McMaster W.G., Rali A.S., Rahaman Z., Balsara K., Absi T., et al. Early US experience with cardiac donation after circulatory death (DCD) using normothermic regional perfusion. J Heart Lung Transplant. 2021;40:1408–1418. doi: 10.1016/j.healun.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Messer S., Page A., Axell R., Berman M., Hernandez-Sanchez J., Colah S., et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2017;36:1311–1318. doi: 10.1016/j.healun.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Detry O., Le Dinh H., Noterdaeme T., De Roover A., Honoré P., Squifflet J.P., et al. Categories of donation after cardiocirculatory death. Transplant Proc. 2012;44:1189–1195. doi: 10.1016/j.transproceed.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Thuong M., Ruiz A., Evrard P., Kuiper M., Boffa C., Akhtar M.Z., et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int. 2016;29:749–759. doi: 10.1111/tri.12776. [DOI] [PubMed] [Google Scholar]
- 22.Gries C.J., White D.B., Truog R.D., Dubois J., Cosio C.C., Dhanani S., et al. An official American Thoracic Society/International Society for Heart and Lung Transplantation/Society of Critical Care Medicine/Association of Organ and Procurement Organizations/United Network of Organ Sharing Statement: ethical and policy considerations in organ donation after circulatory determination of death. Am J Respir Crit Care Med. 2013;188:103–109. doi: 10.1164/rccm.201304-0714ST. [DOI] [PubMed] [Google Scholar]
- 23.Ngai J., Masuno K., Moazami N. Anesthetic considerations during heart transplantation using donation after circulatory death. J Cardiothorac Vasc Anesth. 2020;34:3073–3077. doi: 10.1053/j.jvca.2020.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moazami N., Smith D., Galloway A. Logistics for expanding heart transplantation from donation after circulatory death using normothermic regional perfusion. JTCVS Tech. 2022;12:110–112. doi: 10.1016/j.xjtc.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honor Walk Resources [Internet]. LifeSource. Accessed January 3, 2023. https://www.life-source.org/partners/hospitals/honor-walk-resources/
- 26.Potts J.T., Jr., Herdman R. National Academies Press; 1997. Non-Heart-Beating Organ Transplantation: Medical and Ethical Issues in Procurement. [PubMed] [Google Scholar]
- 27.Stammers A.H., Tesdahl E.A., Mongero L.B., Patel K.P., Petersen C.C., Vucovich J.A., et al. Zero-balance ultrafiltration during cardiopulmonary bypass is associated with decreased urine output. J Extra Corpor Technol. 2021;53:27–37. doi: 10.1182/ject-2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American College of Physicians. Ethics, determination of death, and organ transplantation in normothermic regional perfusion (NRP) with controlled donation after circulatory determination of death (cDCD): American College of Physicians Statement of concern. 2022. 2021. https://www.acponline.org/acp_policy/policies/ethics_determination_of_death_and_organ_transplantation_in_nrp_2021.pdf 2021. Accessed October 1, 2022.
- 29.Parent B., Caplan A., Moazami N., Montgomery R.A. Response to American College of Physician's statement on the ethics of transplant after normothermic regional perfusion. Am J Transplant. 2022;22:1307–1310. doi: 10.1111/ajt.16947. [DOI] [PubMed] [Google Scholar]
- 30.Parent B., Moazami N., Wall S., et al. Ethical and logistical concerns for establishing NRP-cDCD heart transplantation in the United States. Am J Transplant. 2020;20:1508–1512. doi: 10.1111/ajt.15772. [DOI] [PubMed] [Google Scholar]