The Cox-Maze biatrial lesion set, including the posterior LA box.
Central Message.
In a successful Maze, all lesions must be fully transmural, the entirety of the posterior LA must be isolated, and the LAA must be excluded.
The Cox-Maze procedure (CMP) is the most effective treatment for atrial fibrillation (AF). Despite this, many patients with AF undergoing cardiac surgery for other pathologies are not offered a concomitant CMP, whereas other patients with lone AF are not offered surgical intervention at all.1 Part of the original reticence to perform the CMP lay in the technical complexity of the “cut-and-sew” Maze (ie, the CMP III), in which multiple incisions were made in both atria to create conduction block. The introduction of radiofrequency (RF) and cryoablation devices has made the latest iteration of the CMP—the CMP-IV—not only technically simpler but also more time efficient, thereby decreasing cardiopulmonary bypass (CPB) times and procedural morbidity and mortality.2 However, the number of CMPs performed today still falls short of the number of patients who would benefit from this procedure. It has been estimated that approximately one-third of patients undergoing mitral valve surgery who have a history of AF do not receive the CMP.1 This is despite multiple societies' recommendations for the management of AF, including a class 1A recommendation from the Society of Thoracic Surgery.3
Lingering hesitancy to perform the CMP is in part due to surgeon inexperience and lack of comfort, but may also be due to a lack of understanding of the foundational concepts of the Maze and an unfamiliarity with the location and function of each individual lesion.
This article aims to provide a clear description of the biatrial lesion set and the importance of the individual lesions. We aim to do this in 3 parts: (1) a description of the goals of the CMP; (2) a description of the criteria required for successful lesions; and (3) a description of each lesion and its importance/function. We will end with a brief discussion of the various ablation modalities available with which to make Maze lesions. This is not a step-by-step description of how to perform the Maze. Our group has published multiple articles describing our operative technique in detail, both via sternotomy (Video 1) and via right minithoracotomy (Video 2).4,5 Instead, our goal is to enable a conceptual understanding of the electrical rationale for a biatrial CMP.
Goal of the Cox-Maze Procedure
The major goal of the CMP is termination of AF and restoration of normal sinus rhythm. A secondary goal is the excision or exclusion of the left atrial appendage (LAA) to prevent strokes, the most dreaded complication of AF. The intended hemodynamic outcomes are (1) restoration of the atrial kick with subsequent improvement in cardiac output and amelioration of heart failure, and (2) cessation of stagnant blood flow in the fibrillating left atrium (LA) that serves as a nidus for thromboemboli.
The core tenet of the CMP is that a pattern of lesions made in both atria block the conduction of aberrant electrical impulses, both by isolating arrhythmogenic foci and by interrupting micro- and macro-reentrant circuits, thereby preventing sustained AF. The ultimate effect is to create a constrained pathway through which electrical impulses travel from the sinoatrial node complex (SAN) to the atrioventricular node (AVN) and activate the majority of atrial tissue with the crucial exception of the posterior LA (including the region of the pulmonary veins [PVs]). The posterior LA is isolated in its entirety given this area's propensity for developing arrhythmogenic foci.
Required Criteria for Successful Lesions
There are specific criteria a lesion must meet to consistently and reproducibly block electrical conduction. Even if all lesions of the CMP are made, if each individual lesion does not meet these criteria, the CMP as a whole may be incomplete and ineffective. Fortunately, these criteria are straightforward and are as follows:
-
1.
Each lesion must be transmural throughout its entirety. Our laboratory and others have shown that even a very small gap in a lesion can allow conduction of aberrant electrical impulses.6 Furthermore, these small gaps may actually be proarrhythmic given that they often result in slow conduction of these impulses, increasing the likelihood of maintaining reentrant circuits.
-
2.
Each lesion must originate from or end in tissue that is not electrically conductive. Electrically nonconductive tissue can either be another lesion or tissue that is natively nonconductive such as a valve annulus or vena cava. This is necessary to block conduction, particularly of the rotors and micro- and macro-reentrant circuits that may be needed to sustain AF. Lesions that are not anchored on at least one side by electrically nonconductive tissue can actually serve as a nidus for reentrant circuits that can rotate around the lesions creating atypical atrial flutters.7
We will handle the latter criteria first: where to place your lesions and why. The complete biatrial CMP lesion set is described next: management of the LAA, isolation of the posterior LA, completion of the LA lesion set, and the right atrial (RA) lesion set, with numbering in parentheses corresponding to Figure 1. By ordering the lesion sets and their descriptions in this manner, we aim to focus on the conceptual importance of each lesion/set of lesions and how the final pattern of ablation lines in the biatrial CMP restricts electrical wavefronts to prevent arrhythmias. Of note, this is different from the order in which we create these lesions intraoperatively, which is dictated primarily by the practicalities of anatomy and operative approach. A brief description of our operative procedure (ie, a list of the lesions in the order in which we create them intraoperatively) is shown in Table E1.
Figure 1.
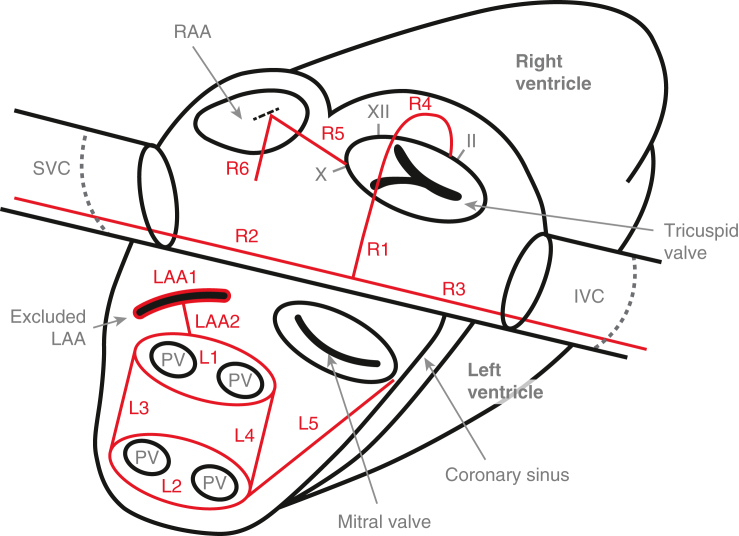
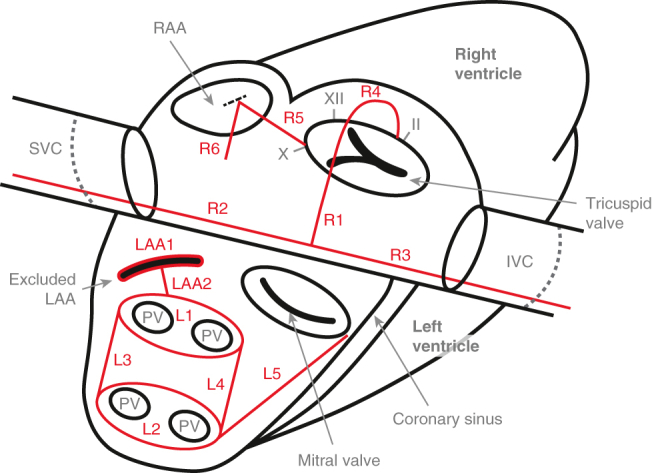
Cox-Maze lesions. LAA1: Manage the LAA. LAA2: Connect the LAA exclusion to the Box. L1-4: The Box: Isolate the entirety of the posterior LA. L5: Connect the box to the mitral annulus and the coronary sinus. R1: Make a right atriotomy. R2-3: Create a line of block between the SVC (R2) and the IVC (R3). R4: Connect the atriotomy to the tricuspid annulus at the 2 o'clock position (∗this lesion does not cross the valve itself; it arches over the valve along the anterior wall of the RA with the end of the lesion extending onto the valve annulus at the 2 o'clock position). R5: Connect the tricuspid annulus at the 10 o'clock position to the RAA. R6: Connect the RAA to the RA free wall, making sure to avoid the SAN. RAA, Right atrial appendage; SVC, superior vena cava; IVC, inferior vena cava; LAA, left atrial appendage; PV, pulmonary vein.
Discussion of Cox-Maze Lesions
LAA1-2: Manage the Left Atrial Appendage
The management of the LAA is essential to performing a CMP. Most important, because the majority of thromboembolic strokes in patients with AF originate in the LAA, removal of the LAA significantly decreases the risk of stroke or systemic embolism. This has been observed both in retrospective case series and prospective, randomized trials.8,9 In our experience, we have found either excision or application of an epicardial exclusion device (AtriClip, AtriCure Inc) to be the most effective means of eliminating the LAA. Unfortunately, stapler exclusion, oversewing, and ligation have had reasonably high failure rates.10,11 The appendage clip has had promising intraoperative and late success rates of both complete LAA occlusion and safety.12,13 In our hands, and in either minimally invasive or beating-heart applications, clipping the LAA is the most reasonable approach.
Whether the LAA is excised or clipped, it is important to leave no stump, because residual LAA tissue (in communication with the LA) has been shown to be prothrombotic.14 Even in the absence of a stump, the LAA exclusion line if not properly anchored in electrically nonconductive tissue can still serve as a nidus for a flutter circuit.7 Given this, we also create a lesion from the excluded LAA to the left PV lesion (as described next), connecting the LAA exclusion to the electrically excluded posterior LA.
Left Atrial Lesion Set
L1-4: isolation of the posterior left atrium, the box
This is the single most important part of the CMP (Figure 2). The posterior LA, including the PVs, has consistently been identified as important to both the initiation and maintenance of AF. Catheter-based electrophysiologic studies have shown that between 87% and 96% of ectopic atrial foci in patients with AF are located within this region.15,16 Moreover, our data have shown that without complete isolation of the posterior LA, the remainder of the CMP lesions only lead to a 33% freedom from recurrent atrial tachyarrhythmias at 5 years.17
Figure 2.
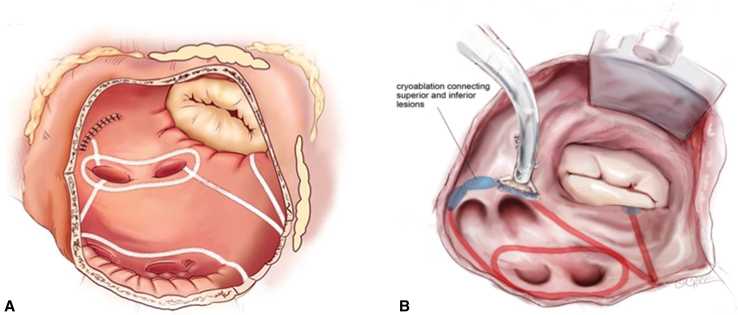
LA Cox-Maze lesion set. A, LA lesion set via a sternotomy approach.2White lines indicate lesions made by bipolar RF ablation, and the blue oval indicates a lesion made by cryoablation. B, LA lesion set via a right minithoracotomy.5Red lines indicate lesions made by bipolar RF ablation, and blue ovals indicate lesions made by cryoablation. Not pictured in B is the LAA exclusion, which we perform using an epicardial approach via the transverse sinus.
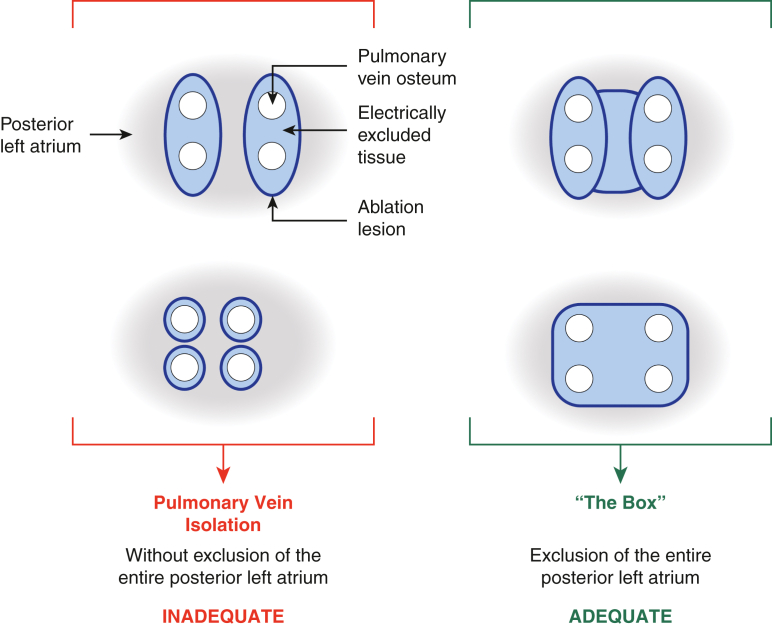
A complete box electrically isolates the whole posterior LA by encompassing the entirety of the PVs and most, if not all, of the posterior LA tissue between the PVs. The term “pulmonary vein isolation” (PVI) is inconsistently used, but in the context of surgical ablation most commonly refers to creating 2 separate ablation lesions—one around the left-sided PVs, one around the right-sided PVs—that are not connected to each other. This results in electrical isolation of the left and right PVs, but does not isolate any of the rest of the posterior LA between the PVs (Figure 3). When so defined, PVI has been shown to be an inadequate treatment for AF, with success rates no different than catheter ablation but with greater morbidity. Two relatively recent randomized controlled trials comparing catheter-based with surgical PVI support this conclusion.18,19 Our data on an incomplete box also document the inadequacy of PVI, given the 33% rate of atrial tachyarrhythmia freedom among patients who all had exit block–confirmed PVIs.17 The potential role for the box isolation as a stand-alone lesion has not been established, because no clinical trial has yet addressed this question.
Figure 3.
The Box: Adequate exclusion of the entire posterior LA. A satisfactory result with long-term efficacy relies on isolation of the entire posterior LA. Specifically, a box-shaped area of tissue, incorporating the ostia of all PVs and the intervening posterior LA wall, must be electrically excluded via a continuous and fully transmural circumferential line of ablation. Note that this box does not need to be made with a single application of a device, but can be constructed from a number of applications.
L5: connecting line to the mitral annulus, coronary sinus lesion
A linear lesion connecting the box to the mitral annulus, which is electrically nonconductive, should be created. This has been termed the “LA isthmus line” and is critical to prevent both typical and atypical LA flutter.20 The coronary sinus should be ablated at this level. If the latter is not performed, the coronary sinus can conduct aberrant electrical signals leading to LA flutter around the mitral annulus.21
Right Atrial Lesion Set
The goal of the RA lesion set is to create a complete line of block from the superior vena cava (SVC) to the inferior vena cava (IVC) and connect this line to the natively nonconductive tissue of the tricuspid annulus, and further connect the annulus to the right atrial appendage (RAA), while not damaging the SAN or AVN (Figure 4). There is good evidence to support the importance of the RA (particularly the cavotricuspid isthmus) in the initiation and maintenance of typical atrial flutter as well as AF.22,23 We have shown that up to one-third of AF drivers originate in the RA.23 Moreover, the best late results of surgical ablation have been shown after biatrial lesion sets.17
Figure 4.
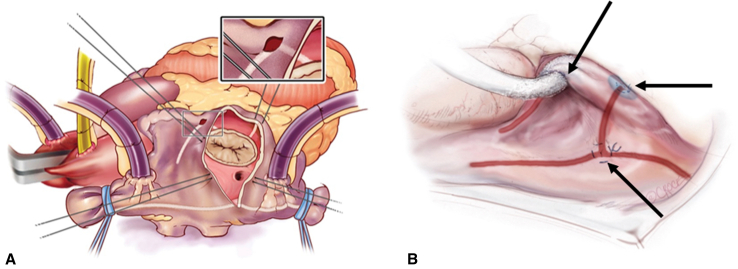
RA Cox-Maze lesion set. A, RA lesion set via a sternotomy approach.2White lines indicate lesions made by bipolar RF ablation, and blue ovals indicate lesions made by cryoablation. B, RA lesion set via a right minithoracotomy.5Red lines indicate lesions made by bipolar RF ablation, blue ovals indicate lesions made by cryoablation, and black arrows indicate the 3 purse-string stab-incisions.
An in-depth discussion of the need for a postoperative pacemaker (PPM) is beyond the scope of this article, particularly in regard to performing a biatrial CMP in combination with other cardiac surgery, and ultimately is dependent on surgeon experience and patient selection. It is important to note that the 2 main indications for PPM placement after a biatrial CMP are sick sinus syndrome (SSS) and complete heart block (CHB). CHB after a CMP is most likely secondary to intraoperative AVN injury that occurred during a concomitant procedure, that is, not due to the CMP itself. The CMP does not include any ablation in the immediate area of the AVN. SSS is most likely present at baseline and simply uncovered (rather than caused) by the CMP. The incidence of SSS increases with patient age and duration of preoperative AF. Over the past decade, we have found that approximately 12% of our patients require PPM placement post-CMP, with approximately half of these patients requiring PPM for SSS and the other half for CHB.24 We do not test SAN or AVN function intraoperatively; all patients have temporary pacing wires placed before closure, which are removed or replaced postoperatively once patients have had time to recover from potentially transient postoperative rhythm abnormalities. There is a high incidence of early junctional rhythms due to SAN dysfunction, and we recommend waiting at least 5 days before making a decision regarding permanent PPM.
R1: right atriotomy
The right atriotomy should have a relatively perpendicular orientation in relation to the axis of the SVC and IVC. An atriotomy is transmural and therefore is electrically nonconductive. We only perform a right atriotomy via a sternotomy. When performing the CMP via a right minithoracotomy, we create all right-sided lesions through purse-string stab-incisions.
R2-3: vena cavae lesions
The vena cavae lesions form a continuous straight line that is perpendicular to the atriotomy and that connects the SVC (R2) and IVC (R3). These lesions are made as posterior and lateral as possible to avoid injury to the SAN near the SVC-RA junction and the pericardial reflection by the IVC. These lesions must extend several centimeters onto the vena cavae to ensure the combined vena cavae line in its entirety starts and ends in electrically nonconductive tissue.
R4: connecting line from the right atriotomy to the tricuspid annulus at the 2 o'clock position relative to the valve
This connecting line anchors the previous RA lesions (R1-3) to the electrically inactive tissue of the tricuspid annulus (the first pair of anchors being the SVC and IVC). This forms a cavotricuspid isthmus ablation and is therefore important for the prevention of atrial flutter.25
R5: connecting line from the tricuspid annulus (10 o'clock position) to the right atrial appendage
This lesion completes the line of block across the RA, connecting the tricuspid annulus anchor to the RAA. This theoretically prevents rotation of macro-reentrant circuits around the RAA and may be particularly important in patients with RA enlargement. RA access to allow for creation of this lesion is gained via a small purse-string stab-incision at the base of the RAA.
R6: connecting line from the right atrial appendage to the right atrium free wall
We routinely create a final additional lesion from the base of the RAA onto the RA free wall down the aortic side of the RAA to avoid the SAN. Access for this lesion is gained via the same small incision used for lesion R5.
We will now discuss the second key criteria for a successful CMP lesion: transmurality throughout the entirety of the lesion. We will briefly discuss how this can be achieved with our recommended ablation devices, either a bipolar RF clamp or cryoablation.
Ensuring Transmurality: The Use of Various Ablation Devices
Every Cox-Maze lesion must be transmural throughout its entirety. This can be achieved by using any of the following techniques in isolation or in combination: surgical incisions, bipolar RF ablation, and cryoablation. It is our practice to use a combination of these techniques, with each of the 3 methods matched to specific lesions to facilitate performance of the procedure. All 3 modalities are effective if used properly.
Transmurality is always ensured with an incision. However, this is the most technically difficult and time-consuming option. Therefore, we minimize its use to only what is required to gain access to the interior of the atria. Our right atriotomy performs double-duty as an atriotomy and an ablation lesion. We make our left atriotomy to connect to the posterior box.
Extensive experience in our laboratory has shown that the only devices that reliably create transmural lesions are bipolar RF clamps (as opposed to unipolar RF probes) and cryoablation. Both achieve a more than 99% rate of transmurality as judged histologically in animal models.26,27 Bipolar RF clamps require access to the interior of the atria and therefore CPB, except for the right and left PV lesions that can be created by encircling within the clamp as large a cuff of LA tissue as possible around the base of the PV ostia from the exterior of the atrium, therefore ablating 2 layers of atrial wall simultaneously. Creating an oval around each set of PVs (left and right) is not strictly necessary as the superior and inferior connecting lesions (L3 and L4) will effectively exclude all tissue between the PVs (compare the 2 adequate box lesions in Figure 3). However, we recommend treating the PVs in this fashion when using bipolar RF clamps, because it is possible to do so on the beating heart on CPB but before crossclamping. This in turn allows for exit-block testing to confirm PV isolation and appropriate function of the RF clamp. A novel device has recently been introduced to the market making it possible to perform the entirety of the LA box exclusion with a single application and without opening the LA. Our laboratory has found that the AtriCure EnCompass RF clamp (AtriCure Inc) reliably creates transmural lesions in a porcine model.28 However, there are no currently available data on the clinical effectiveness of this clamp.
Cryoablation energy can be applied via a flexible linear probe and therefore can be used to draw a lesion onto the epicardial or endocardial surface of the atrium. Cryoablation has been shown to be most effective while on CPB from the endocardial surface.29 This is likely due to the heat sink of warm intracavitary blood counteracting the cooling needed to facilitate transmural lesions, in addition to the insulating nature of epicardial fat. In a porcine beating-heart model in which the posterior LA box was created using epicardial cryoablation in 13 animals, none of the box lesions were fully transmural.30
We make the majority of our CMP lesions using bipolar RF clamps; however, we do use a cryoprobe to extend lesions to both valve annuli. This is done primarily to eliminate the risk of RF-associated thermal injury to the valve apparatus. Cryothermy (as applied via cryoprobe) does not denature protein or degrade the extracellular matrix, rendering it safe around valvular tissue.31 We additionally favor the cryoprobe in this region given the difficulty in clamping up to the valve annuli due to the bulk of the atrioventricular groove and the presence of coronary arteries in this area.31 It is important to note that neither bipolar RF nor cryothermy is safe to use on the coronary arteries.31 We use cryothermy in this region on the beating heart only. Warm coronary blood flow, in addition to the epicardial fat around the RCA, protects the RCA from injury.
Whatever energy method is used, it is imperative that the surgeon understand the capabilities of their chosen device. Specific parameters must be met when using each of these devices to consistently produce transmural lesions. Our laboratory showed that when using bipolar RF clamps, each lesion should be ablated twice, without unclamping the targeted tissue in between ablations to ensure greater than 99% transmurality of lesions.26 To ensure 100% transmurality, our practice is to perform each lesion 2 to 3 times, with each lesion ablated twice without unclamping, and slightly rotating the clamp between each double-ablation. It is also important to ensure all char is cleaned off the clamp jaws between each set of ablations when using a nonirrigated RF clamp. When using any type of clamp, it is important to ensure excellent tissue contact and avoid tissue bunching. Anything that impairs conductance between the 2 electrodes, such as air, fat, char, or intravenous or cardioplegia catheters, will prevent transmural lesion creation.
Generally speaking, cryoablation probes require a single application duration of 3 minutes for nitrous oxide devices and 2 minutes for argon probes.30,31 The probe needs to be in direct contact with atrial tissue along its entire surface. Ice is an excellent insulator, so if there are any gaps between the probe and the tissue, the ice-ball that forms will insulate that area and lead to focal nontransmurality.
Conclusions
The CMP is the most effective treatment for AF, but is underused in current practice. We recommend performing a complete biatrial Maze in the majority of patients undergoing cardiac surgery who have a history of AF and are otherwise good surgical candidates. To ensure a good electrophysiologic result, surgeons must ensure that all lesions are fully transmural, and that all lesions both start or end in electrically inactive tissue. The LAA and the entirety of the posterior LA (including the PV ostia and the intervening tissue) must always be excluded. Surgeons must know the specific characteristics of whatever device they plan to use to create transmural lesions and modify their technique accordingly. If surgeons are able to meet these criteria and increase the appropriate use of this effective surgical treatment, this would make a significant positive impact for patients with AF, not only by restoring sinus rhythm and reducing the risk of stroke but also by improving long-term survival.24
Conflict of Interest Statement
R.J.D.: AtriCure, Inc: Speaker and receives research funding; Medtronic: Consultant and receives research funding; Edwards Lifesciences: Consultant. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
This work was supported by the National Institutes of HealthR01-HL032257 to R.J.D. and the Barnes-Jewish Foundation.
Associated presentation given by Dr Damiano at the American Association for Thoracic Surgery 2021 Annual Meeting.
Appendix E1
Table E1.
Steps of the Cox-Maze procedure as we perform it
| Sternotomy approach | Right minithoracotomy approach |
|---|---|
|
|
| Left atrial lesion set—part 1 | Left atrial lesion set—part 1 |
Box Lesion 1: Right PV lesion (L1)
|
Box Lesion 1: Right PV lesion (L1)
|
Box Lesion 2: Left PV lesion (L2)
| |
Right atrial lesion set
|
Right atrial lesion set
|
Connecting line from the RAA to the RA free wall (R6)
|
Vena cavae lesions (R2, R3)
|
| Right atriotomy (R1) | Connecting line from the vena cavae ablation to the tricuspid annulus (2 o'clock position; R1, R4)
|
Vena cavae lesions (R2, R3)
|
Connecting line from the RAA to the RA free wall (R6)
|
Connecting line from the right atriotomy to the tricuspid annulus (2 o'clock position; R4)
|
Connecting line from the RAA to the tricuspid annulus (10 o'clock position; R5)
|
Connecting line from the RAA to the tricuspid annulus (10 o'clock position; R5)
| |
|
|
| Left atrial lesion set—part 2 | Left atrial lesion set—part 2 |
Connecting line from the LAA to the previously made left PV lesion (LAA2)
|
Box Lesions 3 and 4: Connecting lines superiorly and inferiorly connecting the previously made right PV lesion to where the left PV lesion will be (L3, 4)
|
| LAA exclusion (clip; LAA1) | Box Lesion 2: Left PV lesion (L2)
|
Box Lesions 3 and 4: Connecting lines superiorly and inferiorly connecting the previously made PV lesions (L3, 4)
|
Connecting line from the base of the LAA to the previously-made left PV lesion (LAA2)
|
Connecting line to the mitral annulus, with coronary sinus lesion (L5)
|
Connecting line to the mitral annulus, with coronary sinus lesion (L5)
|
| LAA exclusion (clip; LAA1) |
Lesions are listed in the order in which we typically perform them, with adjustments made for the operative approach, that is, sternotomy or right minithoracotomy. Our standard practice is to perform the latter. Numbering listed in parentheses corresponds to numbering used in Figure 1. CPB, Cardiopulmonary bypass; PV, pulmonary vein; RAA, right atrial appendage; RA, right atrium; RF, radiofrequency; SVC, superior vena cava; IVC, inferior vena cava; LA, left atrium; LAA, left atrial appendage.
Supplementary Data
Operative video of the Cox-Maze IV procedure performed via sternotomy. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00589-2/fulltext.
Operative video of the Cox-Maze IV procedure performed via right minithoracotomy. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00589-2/fulltext.
References
- 1.Mehaffey J.H., Krebs E., Hawkins R.B., Charles E.J., Tsutsui S., Kron I.L., et al. Variability and utilization of concomitant atrial fibrillation ablation during mitral valve surgery. Ann Thorac Surg. 2021;111:29–34. doi: 10.1016/j.athoracsur.2020.05.125. [DOI] [PubMed] [Google Scholar]
- 2.Weimar T., Schena S., Bailey M.S., Maniar H.S., Schuessler R.B., Cox J.L., et al. The Cox-Maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol. 2012;5:8–14. doi: 10.1161/CIRCEP.111.963819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badhwar V., Rankin J.S., Damiano R.J., Jr., Gillinov A.M., Bakaeen F.G., Edgerton J.R., et al. The Society of Thoracic Surgeons 2017 clinical practice guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2017;103:329–341. doi: 10.1016/j.athoracsur.2016.10.076. [DOI] [PubMed] [Google Scholar]
- 4.MacGregor R.M., Khiabani A.J., Damiano R.J. The surgical treatment of atrial fibrillation via median sternotomy. Oper Tech Thorac Cardiovasc Surg. 2019;24:19–37. [Google Scholar]
- 5.Robertson J.O., Saint L.L., Leidenfrost J.E., Damiano R.J., Jr. Illustrated techniques for performing the Cox-Maze IV procedure through a right mini-thoracotomy. Ann Cardiothorac Surg. 2014;3:105–116. doi: 10.3978/j.issn.2225-319X.2013.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melby S.J., Lee A.M., Zierer A., Kaiser S.P., Livhits M.J., Boineau J.P., et al. Atrial fibrillation propagates through gaps in ablation lines: implications for ablative treatment of atrial fibrillation. Heart Rhythm. 2008;5:1296–1301. doi: 10.1016/j.hrthm.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillette P.C., Kugler J.D., Garson A., Jr., Gutgesell H.P., Duff D.F., McNamara D.G., et al. Mechanisms of cardiac arrhythmias after the Mustard operation for transposition of the great arteries. Am J Cardiol. 1980;45:1225–1230. doi: 10.1016/0002-9149(80)90482-8. [DOI] [PubMed] [Google Scholar]
- 8.Whitlock R.P., Belley-Cote E.P., Paparella D., Healey J.S., Brady K., Sharma M., et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021;384:2081–2091. doi: 10.1056/NEJMoa2101897. [DOI] [PubMed] [Google Scholar]
- 9.Pet M., Robertson J.O., Bailey M., Guthrie T.J., Moon M.R., Lawton J.S., et al. The impact of CHADS2 score on late stroke after the Cox maze procedure. J Thorac Cardiovasc Surg. 2013;146:85–89. doi: 10.1016/j.jtcvs.2012.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Squiers J.J., Edgerton J.R. Surgical closure of the left atrial appendage: the past, the present, the future. J Atr Fibrillation. 2018;10:1642. doi: 10.4022/jafib.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee R., Jivan A., Kruse J., McGee E.C., Jr., Malaisrie S.C., Bernstein R., et al. Late neurologic events after surgery for atrial fibrillation: rare but relevant. Ann Thorac Surg. 2013;95:126–132. doi: 10.1016/j.athoracsur.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 12.Ailawadi G., Gerdisch M.W., Harvey R.L., Hooker R.L., Damiano R.J., Jr., Salamon T., et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142:1002–1009. doi: 10.1016/j.jtcvs.2011.07.052. 1009.e1. [DOI] [PubMed] [Google Scholar]
- 13.Emmert M.Y., Puippe G., Baumuller S., Alkadhi H., Landmesser U., Plass A., et al. Safe, effective and durable epicardial left atrial appendage clip occlusion in patients with atrial fibrillation undergoing cardiac surgery: first long-term results from a prospective device trial. Eur J Cardio Thorac Surg. 2014;45:126–131. doi: 10.1093/ejcts/ezt204. [DOI] [PubMed] [Google Scholar]
- 14.Al-Saady N.M., Obel O.A., Camm A.J. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547–554. doi: 10.1136/hrt.82.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haissaguerre M., Jais P., Shah D.C., Takahashi A., Hocini M., Quiniou G., et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 16.Lin W.S., Tai C.T., Hsieh M.H., Tsai C.F., Lin Y.K., Tsao H.M., et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–3183. doi: 10.1161/01.CIR.0000074206.52056.2D. [DOI] [PubMed] [Google Scholar]
- 17.Henn M.C., Lancaster T.S., Miller J.R., Sinn L.A., Schuessler R.B., Moon M.R., et al. Late outcomes after the Cox maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2015;150:1168–1176. doi: 10.1016/j.jtcvs.2015.07.102. 1178.e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adiyaman A., Buist T.J., Beukema R.J., Smit J.J., Delnoy P.P., Hemels M.E., et al. Randomized controlled trial of surgical versus catheter ablation for paroxysmal and early persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.118.006182. [DOI] [PubMed] [Google Scholar]
- 19.Castellá M., Kotecha D., van Laar C., Wintgens L., Castillo Y., Kelder J., et al. Thoracoscopic vs. catheter ablation for atrial fibrillation: long-term follow-up of the FAST randomized trial. Europace. 2019;21:746–753. doi: 10.1093/europace/euy325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knecht S., Hocini M., Wright M., Lellouche N., O’Neill M.D., Matsuo S., et al. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J. 2008;29:2359–2366. doi: 10.1093/eurheartj/ehn302. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez A., Shorofsky S.R., Dickfeld T.M., Anand R., Saliaris A.P., Saba M. Left-sided atrial flutter originating in the coronary sinus after radiofrequency ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2010;33:e96–e99. doi: 10.1111/j.1540-8159.2010.02718.x. [DOI] [PubMed] [Google Scholar]
- 22.Shah D.C., Jaïs P., Haïssaguerre M., Chouairi S., Takahashi A., Hocini M., et al. Three-dimensional mapping of the common atrial flutter circuit in the right atrium. Circulation. 1997;96:3904–3912. doi: 10.1161/01.cir.96.11.3904. [DOI] [PubMed] [Google Scholar]
- 23.Cuculich P.S., Wang Y., Lindsay B.D., Faddis M.N., Schuessler R.B., Damiano R.J., Jr., et al. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khiabani A.J., MacGregor R.M., Bakir N.H., Manghelli J.L., Sinn L.A., Maniar H.S., et al. The long-term outcomes and durability of the Cox-Maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2022;163:629–641.e7. doi: 10.1016/j.jtcvs.2020.04.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cosio F.G., López-Gil M., Goicolea A., Arribas F., Barroso J.L. Radiofrequency ablation of the inferior vena cava-tricuspid valve isthmus in common atrial flutter. Am J Cardiol. 1993;71:705–709. doi: 10.1016/0002-9149(93)91014-9. [DOI] [PubMed] [Google Scholar]
- 26.Khiabani A.J., MacGregor R.M., Manghelli J.L., Ruaengsri C., Carter D.I., Melby S.J., et al. Bipolar radiofrequency ablation on explanted human hearts: how to ensure transmural lesions. Ann Thorac Surg. 2020;110:1933–1939. doi: 10.1016/j.athoracsur.2020.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schill M.R., Melby S.J., Speltz M., Breitbach M., Schuessler R.B., Damiano R.J., Jr. Evaluation of a novel cryoprobe for atrial ablation in a chronic ovine model. Ann Thorac Surg. 2017;104:1069–1073. doi: 10.1016/j.athoracsur.2017.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yates T.A., McGilvray M., Razo N., McElligott S., Melby S.J., Zemlin C., et al. Efficacy of a novel bipolar radiofrequency clamp: an acute porcine model. Innovations. 2022 doi: 10.1177/15569845221126524. 15569845221126524. [DOI] [PubMed] [Google Scholar]
- 29.Lustgarten D.L., Keane D., Ruskin J. Cryothermal ablation: mechanism of tissue injury and current experience in the treatment of tachyarrhythmias. Prog Cardiovasc Dis. 1999;41:481–498. doi: 10.1016/s0033-0620(99)70024-1. [DOI] [PubMed] [Google Scholar]
- 30.Masroor S., Jahnke M.E., Carlisle A., Cartier C., LaLonde J.P., MacNeil T., et al. Endocardial hypothermia and pulmonary vein isolation with epicardial cryoablation in a porcine beating-heart model. J Thorac Cardiovasc Surg. 2008;135:1327–1333. doi: 10.1016/j.jtcvs.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 31.MacGregor R.M., Melby S.J., Schuessler R.B., Damiano R.J. Energy sources for the surgical treatment of atrial fibrillation. Innovations. 2019;14:503–508. doi: 10.1177/1556984519878166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Operative video of the Cox-Maze IV procedure performed via sternotomy. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00589-2/fulltext.
Operative video of the Cox-Maze IV procedure performed via right minithoracotomy. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00589-2/fulltext.