Abstract
The mixing of edible oils makes it possible to prevent lipid oxidation and preserve the nutritional and organoleptic quality of food. This study was conducted to determine the optimized blend ratio of groundnut (Arachis hypogea), olein, stearin, and sesame (Sesamum indicum L.) oils, to limit thermal oxidation of their lipids. The augmented simplex lattice design was used to study the effect of the edible oils (peanut, palm olein, palm stearin, and sesame oils) on the responses; peroxide value, thiobarbituric acid value, p-Anisidine value, iodine value, free fatty acid content, and total oxidation. The optimized blending oil proportions of peanut, olein, sesame, and stearin oils, were: 33.23%, 23.23%, 15.85%, and 27% respectively. Under this optimum condition, the following quality parameters were obtained: 1.75% oleic acid for the free fatty acid; 6.15 meq/kg for the peroxide value; 1.16 meq MDA/Kg for the thiobarbituric acid value; 55.39 g I2/100 g for the iodine value, 4.45 for the p-Anisidine value, and 19.85 for the total oxidation value. The resulting desirability was equal to 1. Thus, our results for the optimization indicate that the combination of oil makes it possible to best preserve the chemical quality of the lipids during heat treatments.
Keywords: Oil blending, Heat treatment, Oxidation, Lipid quality
1. Introduction
Vegetable oils and fats are an integral part of human consumption and play an important function in the global diet [1]. Having been extracted from nuts, fruits such as the olive and palm, and oilseeds such as cottonseed, sesame seed, soybeans, and peanuts; via different methods, they are rich in lipids, consisting of triglycerides and varying proportions of fatty acids [2]. However, these oils and fats can undergo multiple alterations, such as the oxidation of lipids which is likely to modify their physicochemical and organoleptic characteristics [3]. These lipid oxidations can occur during storage and heat treatments of blended oils [4,5]. The oxidation reactions that produce toxic compounds, can release radicals which later, give a rancid smell and can induce several mutations, carcinogenic and cardiovascular diseases [6,7]. Faced with this, several researchers have initiated work focused on mixing edible oils, to limit their oxidation and preserve their quality. Thus [8], demonstrated that by blending different types of oils, the consumer can be offered a better-quality product with respect to flavor and nutritive value. Also, it has been revealed that by mixing virgin olive oil with sunflower and soybean oil, an oil with a high content of palmitic and oleic acid is obtained with low lipid oxidation index and enhanced nutritional and functional qualities [9]. In addition, it has been shown that the thermal stability of virgin olive oil increased significantly when mixed with palm oil when the composition of the olive oil was around 20% or less [10]. It has been demonstrated that by mixing vegetable oils, their physicochemical properties remain stable without any modification of their chemical composition because the plants have an antioxidant activity favoring oxidative stability [11]. However, little or no scientific work has been done to determine the optimized blend oil ratio from groundnut, olein, stearin, and sesame oils. Thus, the objective of this study was to determine the optimized mixing ratio of groundnut, olein, stearin, and sesame oils, capable of preserving the lipid quality of this mixture during heating.
2. Materials and methods
2.1. Materials
The study was conducted in March 2021 where peanut oil was purchased in the local market of Ndjamena (Chad) from artisanal producers, sesame oil also produced traditionally was obtained in northern Cameroon (Garoua), olein oil and stearin (manufactured in march 2021) were obtained from oil-producing industry (SCS/RAFCA) of Cameroon. For each oil's sample, five (05) liters were collected and transported in hermetic containers to the laboratory for further analysis.
2.2. Methods
2.2.1. Optimization of edible oil blends
An augmented simplex lattice was applied in this study. It is a design that allows consideration of trials with different proportions of components [12]. Hence this plan was advantageous as the interest, was to have each trial with all the components’ proportions to study the effects that these different proportions of these oils could have in the mixtures. They were carefully mixed to form uniform mixtures. After mixing, the oils were heated in a ventilated oven (BMT Medical Technology, Czech Republic) at 180 °C for 1 h, cooled, and analyzed for their chemical characteristics using standardized methods.
2.2.2. Presentation of the components, factor levels, and design matrix
The criterion for choosing the factor levels (Table 1) was made based on literature reviews, which stipulate that when mixing oils, saturated edible oils must be in small proportions compared to unsaturated ones [13]. Also, preliminary tests were used in defining the extent of the different components. Based on this, the design matrix which presented the mixture experiments used in this study was obtained (Table 2).
Table 1.
Factors level for the optimization of an edible oil blend.
| Components | Abbreviations | Low level (−1) | High level (+1) |
|---|---|---|---|
| Groundnut oil (%) | GO | 20.00 | 60.00 |
| Olein palm (%) | OP | 10.00 | 50.00 |
| Stearin palm (%) | SP | 10.00 | 20.00 |
| Sesame oil (%) | SO | 20.00 | 40.00 |
Table 2.
Design matrix for experiments.
| Coded values |
Actual values |
|||||||
|---|---|---|---|---|---|---|---|---|
| Tests | GO | OP | SP | SO | GO (%) | OP (%) | SP (%) | SO (%) |
| 1 | 1.00 | −1.00 | −1.00 | −1.00 | 60.00 | 10.00 | 10.00 | 20.00 |
| 2 | −1.00 | −0.50 | 0.00 | −1.00 | 20.00 | 40.00 | 20.00 | 20.00 |
| 3 | −0.43 | −0.68 | −0.25 | 0.50 | 31.25 | 16.25 | 17.50 | 35.00 |
| 4 | 0.50 | −1.00 | 0.00 | −1.00 | 50.00 | 10.00 | 20.00 | 20.00 |
| 5 | −1.00 | 0.00 | −1.00 | 1.00 | 20.00 | 30.00 | 10.00 | 40.00 |
| 6 | −1.00 | −0.50 | 0.00 | 1.00 | 20.00 | 20.00 | 20.00 | 40.00 |
| 7 | −0.73 | −0.18 | −0.75 | 0.50 | 25.25 | 26.25 | 12.50 | 35.00 |
| 8 | −0.68 | 0.06 | −0.25 | −0.50 | 26.25 | 31.25 | 17.50 | 25.00 |
| 9 | −0.37 | −0.37 | −0.50 | 0.00 | 32.50 | 22.50 | 15.00 | 30.00 |
| 10 | 0.06 | −0.68 | −0.25 | −0.50 | 41.25 | 16.25 | 17, 50 | 25.00 |
| 11 | −0.68 | 0.31 | −0.75 | −0.50 | 26.25 | 36.25 | 12.50 | 25.00 |
| 12 | 0.00 | −1.00 | −1.00 | 1.00 | 40.00 | 10.00 | 10.00 | 40.00 |
| 13 | 0.31 | −0.68 | −0,75 | −0.50 | 46.25 | 16.25 | 12.50 | 25.00 |
| 14 | −0.50 | −1.00 | 0.00 | 1.00 | 30.00 | 10.00 | 20.00 | 40.00 |
| 15 | −0.18 | −0.68 | −0.75 | 0.50 | 36.25 | 16.25 | 12.50 | 35.00 |
| 16 | −0.68 | −0.43 | −0.25 | 0.50 | 26.25 | 21.25 | 17.50 | 35.00 |
| 17 | −1.00 | 1.00 | −1.00 | −1.00 | 20.00 | 50.00 | 10.00 | 20.00 |
GO = groundnut oil; OP = olein palm; SP = stearin palm; SO = sesame oil.
2.2.3. Evaluation of responses
The responses evaluated were the parameters used to determine the lipid quality of oils.
-
a
Peroxide value (PV)
The peroxide value was determined according to the standard spectrophotometric method of IDF 74 A:1991 [14]. Indeed, in a glass test tube of 10 ml volume containing 50–100 mg of oil sample, 9.8 ml of a chloroform-methanol mixture (7:3) was added, and the mixture was vortexed for 2–4 s. Subsequently, 50 μl of an aqueous solution of 30% ammonium thiocyanate was also added, and the mixture was again vortexed for 2–4 s. This was followed by the addition of 50 μl of an aqueous solution of iron II chloride and vortexed for 2–4 s. After 5 min of incubation at room temperature, the absorbance of the reaction mixture was read at 500 nm against a blank containing all the reagents except the oil, using a PerkinElmer UV–Visible Spectrophotometer (Norwalk CT, USA). The manipulation took place in a dimly lit enclosure and was accomplished within a maximum of 8 min per test of a sample. The samples were tested in triplicate. The peroxide index expressed in ppm was calculated from the Fe III calibration curve according to the formula (Eq. (1)):
| (1) |
where, PV = peroxide index; k = Slope, obtained from the calibration curve (m was 38.46); AS = Sample absorbance; m = mass of the sample in g; Ab = Absorbance of blank; 55.84 = Molar mass of iron.
-
b
Thiobarbituric acid value (TBA)
The thiobarbituric acid value was evaluated according to the method described by Ref. [15]. Here, 0.25 g of oil was weighed into a 10 ml test tube, then an aqueous solution of 0.1% trichloroacetic acid was added and the mixture vigorously vortexed. Subsequently, 1 ml of a 0.375% thiobarbituric acid solution, 1 ml of a 15% trichloroacetic acid solution, and 1 ml of a 0.25 N hydrochloric acid solution were successively added to this tube and the contents of the tube were shaken again before being incubated in a water bath at 95 °C for 30 min. After removing the tubes from the bath and cooling them to ambient temperature, the aqueous phase was sampled and its optical density was measured at 532 nm against a blank consisting of all the reagents except the oil. The thiobarbituric acid value was calculated using the following formula (Eq. (2)):
| (2) |
where, TBA = thiobarbituric acid value; Abs = Corrected absorbance of the sample; VTCA= Volume of TCA solutions; m = Mass of the sample; M = molecular mass of malonaldehyde (72 g/mol).
-
c
p-Anisidine value (p-AnV)
The p-Anisidine value was determined according to the official AOCS Cd 18–90 “p anisidine value” method [16] using a PerkinElmer UV–Visible Spectrophotometer (Norwalk CT, USA). A mass of oil between 0.5 and 1.0 g was weighed in a 25 ml volumetric flask, then dissolved and diluted with isooctane to the mark. The solution obtained was stirred and its absorbance (Abs1) was measured at 350 nm using a “PerkinElmer” brand spectrophotometer using isooctane as a blank. Five milliliters of this solution were taken using a pipette and introduced into a test tube. In a second tube, the same volume of isooctane was added. Subsequently, in each of these tubes, 1 ml of a p-anisidine solution prepared in glacial acetic acid was added and the mixture was vortexed for a few seconds. After 10 min of incubation at room temperature, the absorbance (Abs2) of the solution from the first tube was measured at 350 nm using the solution from the second tube as a blank. The p-Anisidine value was calculated according to the formula (Eq. (3)):
| (3) |
where, p-AnV = p-Anisidine value; Abs1 = Absorbance of the lipid solution after reaction with isooctane solution; Abs2 = Absorbance of the lipid solution after reaction with p-anisidine solution; m = mass of the sample (g).
-
d
Iodine value (IV)
This parameter was determined according to the official AOCS Cd 1–25 method [16]. A mass of 0.2 g of oil was weighed into a flask into which 15 ml of carbon tetrachloride solution (CCl4) and 25 ml of Wijs reagent were introduced. The tightly closed bottle was shaken gently and placed in a dark place for 1 h, then 20 ml of the aqueous solution of potassium iodide (KI) (10 g/100 ml), 15 ml of distilled water, and 5 drops of 1% starch solution paste were added. The solution in the flask was titrated with a 0.1 N sodium thiosulfate solution (Na2S2O3) and the volume V1 of sodium thiosulfate used to turn the solution (disappearance of the blue color) from the flask was noted. This titration was also made with the blank test and the volume V0 of sodium thiosulfate used was noted. The iodine value (IV) expressed in Iodine was calculated according to the formula (Eq. (4)):
| (4) |
where, Vo (ml): Volume of the thiosulphate solution for the blank; V1 (ml): Volume of thiosulfate solution for the sample; T: Titer of the sodium thiosulfate solution used; M (g): Mass of the test portion.
-
e
Free fatty acid (FFA)
The free fatty acid content of the various oil samples was determined according to standard NFT60-204 of the French Association for Standardization [17]. Approximately, 0.5 g of oil (M) was weighed into a 250 ml beaker into which 12.5 ml of 95 °C of ethanol was introduced. Two drops of 1% phenolphthalein solution were then added to the contents of the beaker and titrated with 0.1 N potassium hydroxide solution. The volume V1 of KOH solution was used to reach the indicator end-point (pink phenolphthalein staining, persisting for 10 s was noted). The blank test was also made and its volume V0 of KOH used was noted. The free fatty acid (FFA) content was calculated by the following formula (Eq. (5)):
| (5) |
where, Vo (ml): Volume of the KOH solution for the blank; V (ml): Volume of KOH solution for the sample; T: Titer of the ethanolic KOH solution used; M (g): Mass of the test portion.
-
f
Total oxidation value
The total oxidation value (Totox) of oil samples was determined based on the obtained peroxide and p-Anisidine values using the following formula (Eq. (6)), according to Ref. [18].
| (6) |
Where, PV = Peroxide value; p-AnV = Anisidine value.
2.2.4. Mathematical model, validation of postulated models, and optimal conditions for individual responses
The postulated model can either be linear, quadratic, or cubic. This model has the property of representing well the experimental responses studied in the experimental domain of interest and thus, making it possible to obtain an estimate of the value of the response studied for the acceptable quality. The mathematical models used were:
| (7) |
| (8) |
| (9) |
where Y is the expected response; b₁, b₂, b₃, and b₄ are the coefficients of the equations; X₁, X2, X₃, and X₄ are the proportions of the different components.
To put the observed phenomenon in the form of an equation and make it possible to predict the responses in the field defined for the study, it was important to validate the empirical models obtained. So, the coefficient of determination (R2), the Absolute Mean Deviation Analysis (AMDA) which provides information on the average handling error, and the Bias factor (Bf) were determined [19]. For a valid model, the value of R2 must be greater than 75%; the AMDA must be equal to 0 and the Bf must be between 0.75 and 1.25. Concerning the validation of the optimum conditions, the desirability results were determined, and also the level of significance (p < 0.05) of the experimental and the predicted values of the responses were evaluated [20].
2.2.5. Statistical analysis
Minitab software version 18.0 was used for the experimental design and the statistical analysis of the data. All the responses were determined in triplicate (n = 3) and the validity of the model was determined by evaluating the coefficient of determination R2 obtained from the analysis of variance (ANOVA). The statistical significance of the model variables was determined at the 5% probability level. Contour plots were also made using the same software.
3. Results and discussion
3.1. Results
Table 3 shows the experimental values of the free fatty acid content, the peroxide value, thiobarbituric acid value, iodine value, anisidine value, and total oxidation value of the blend oils. From this table, it has been observed that the free fatty acid content is in the range of 0.55%−1.65% Oleic acid, the peroxide value varies from 2.46 to 8.2 meq/kg, the thiobarbituric acid value from 0.44 to 1.64 meq MDA/kg, iodine value from 37.43 to 45.04 g I2/100 g, the p-anisidine value from 2.63 to 26.66 and the total oxidation values vary from 11.96 to 32.55.
Table 3.
Experimental design and experimental values of the responses.
| Trail number | GO (%) | OP (%) | SP (%) | SO (%) | PV (meq/kg) | TBA (meq MDA/kg) | p-AnV | FFA (% Oleic acid) | IV (I2/100 g) | Totox |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60.00 | 10.00 | 10.0 | 20 | 2.46 | 1.32 | 26.66 | 0.82 | 43.78 | 32.55 |
| 2 | 20.00 | 40.00 | 20.0 | 20 | 4.95 | 0.81 | 5.18 | 0.55 | 37.43 | 11.96 |
| 3 | 31.25 | 16.25 | 17.5 | 35 | 3.05 | 0.94 | 4.35 | 0.82 | 41.24 | 14.31 |
| 4 | 50.00 | 10.00 | 20.0 | 20 | 8.20 | 0.86 | 4.04 | 0.55 | 43.78 | 18.89 |
| 5 | 20.00 | 30.00 | 10.0 | 40 | 3.92 | 1.23 | 5.51 | 1.10 | 42.51 | 12.80 |
| 6 | 20.00 | 20.00 | 20.0 | 40 | 4.42 | 1.54 | 6.82 | 0.55 | 41.24 | 15.54 |
| 7 | 26.25 | 26.25 | 12.5 | 35 | 5.04 | 0.78 | 6.38 | 0.82 | 42.51 | 21.89 |
| 8 | 26.25 | 31.25 | 17.5 | 25 | 6.24 | 1.27 | 2.63 | 0.55 | 39.94 | 15.85 |
| 9 | 32.50 | 22.50 | 15.0 | 30 | 5.26 | 1.12 | 3.43 | 0.82 | 39.97 | 12.05 |
| 10 | 41.25 | 16.25 | 17.5 | 25 | 6.90 | 1.25 | 4.24 | 0.55 | 41.24 | 18.39 |
| 11 | 26.25 | 36.25 | 12.5 | 25 | 5.53 | 1.42 | 11.81 | 0.55 | 41.24 | 21.75 |
| 12 | 40.00 | 10.00 | 10.0 | 40 | 5.55 | 1.64 | 11.60 | 0.82 | 43.78 | 23.45 |
| 13 | 46.25 | 16.25 | 12.5 | 25 | 5.20 | 1.10 | 11.44 | 0.82 | 42.51 | 24.35 |
| 14 | 30.00 | 10.00 | 20.0 | 40 | 4.98 | 0.91 | 9.15 | 1.65 | 42.51 | 21.36 |
| 15 | 36.25 | 16.25 | 12.5 | 35 | 5.25 | 0.44 | 10.76 | 0.55 | 45.04 | 23.37 |
| 16 | 26.25 | 21.25 | 17.5 | 35 | 4.93 | 1.23 | 18.19 | 1.10 | 42.51 | 26.71 |
| 17 | 20.00 | 50.00 | 10.0 | 20 | 4.42 | 0.93 | 21.32 | 0.55 | 41.24 | 28.79 |
FFA: free fatty acid (in % Oleic acid); PV: peroxide value (in meq/Kg of oil), TBA: thiobarbituric acid value (in meq MDA/Kg), IV: iodine value (in g I2/100 g), p-AnV: p-anisidine value, Totox: total oxidation value, GO: groundnut oil; OP: olein palm; SP: stearin palm; SO: sesame oil.
3.1.1. Analysis of variance and regression coefficient for the optimization of edible oil blends
The analysis of variance, p-values, and coefficients of determination (R2) of the factors are summarized in Table 4. It has been observed that the oil's quality parameters assessed in this study are significantly (p < 0.05) influenced by the different components. Specifically, the free fatty acid (FFA) content was influenced by the interactions of the components GO, OP, and SP; GO and OP (GO-OP), GO and OP (GO-OP); GO and SP (GO-SP). The peroxide value (PV) on the other hand was influenced by GO and SP interactions of OP and SP. However, the thiobarbituric acid value (TBA), iodine value (IV), p-anisidine value (p-AnV), and total oxidation value (Totox) were significantly influenced by the individual components.
Table 4.
Analysis of variance of the responses.
| Parameters | FFA | PV | TBA | IV | p-AnV | Totox |
|---|---|---|---|---|---|---|
| GO | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| OP | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| SP | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| SO | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| GO*SP | / | 0.018 | / | / | / | |
| OP*SP | / | 0.042 | / | / | / | / |
| GO*OP*SP | 0.039 | / | / | / | / | / |
| GO*OP (GO-OP) | 0.047 | / | / | / | / | / |
| GO*SP (GO-SP) | 0.043 | / | / | / | / | / |
FFA: free fatty acid (in % Oleic acid); PV: peroxide value (in meq/Kg of oil), TAV: thiobarbituric acid value (in meq MDA/Kg), IV: iodine value (in g I2/100g), p-AnV: p-anisidine value, Totox: total oxidation value, GO: groundnut oil; OP: olein; SP: stearin; SO: sesame oil;/: not significant; *: interaction.
3.1.2. Effects of the different components on the responses
The following figures represent the iso-response curves of the different responses as a function of the different proportions of groundnut oil (GO), olein (OP), stearin (SP), and sesame oil (SO). These different iso-response curves make it possible to study the effect of the different components on the responses. Each curve is materialized by 3 factors and the last factor was maintained at optimum.
-
a
Peroxide value (PV)
Fig. 1 shows the effects of the components on the peroxide value (PV). It can be observed that the increase in groundnut oil and olein leads to an increase in the peroxide value. On the other hand, the increase in stearin leads to a decrease in this parameter.
Fig. 1.
-
bThiobarbituric acid value
PV: peroxide value (in meq/Kg of oil), GO: groundnut oil; OP: olein; SP: stearin; SO: sesame oil.
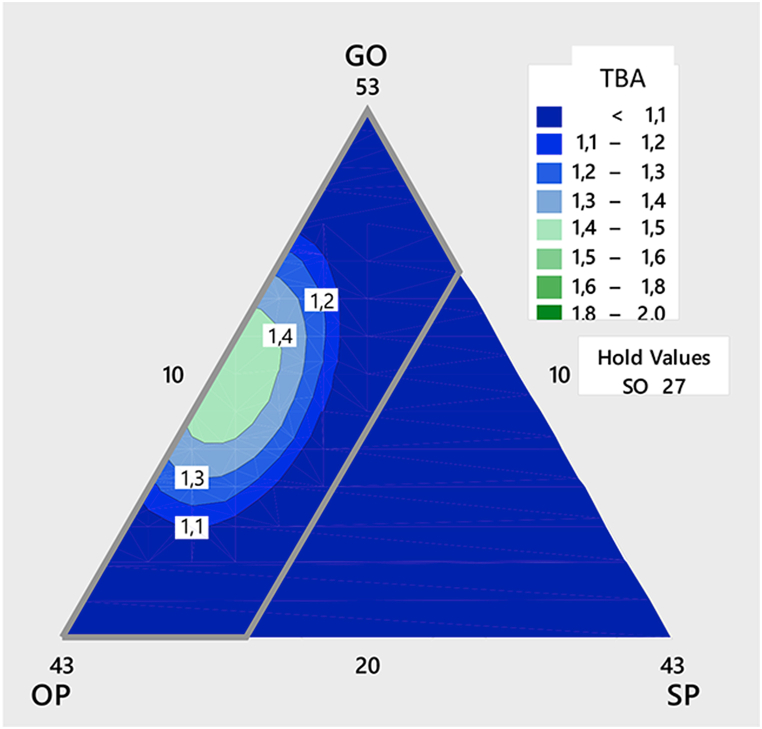
The effects of the components on the thiobarbituric acid value (TBA) are shown in Fig. 2. It appears that increases in GO, and olein, lead to an increase in the thiobarbituric acid value. However, an increase in stearin leads to a decrease in the thiobarbituric acid value.
Fig. 2.
-
cp-Anisidine value
TBA: thiobarbituric acid value (in meq MDA/Kg), GO: groundnut oil; OP: olein; SP: stearin; SO: sesame oil.
The effects of the components on the anisidine value are illustrated in Fig. 3. Here, the increase in the proportion of stearin and groundnut oil leads to the increase of this parameter; while the increases in olein lead to a decrease in the anisidine value.
Fig. 3.
-
dIodine value
p-AnV: anisidine value, GO: groundnut oil; OP: olein; SP: stearin; SO: sesame oil.
The effects of the components on the iodine value (IV) are shown in Fig. 4. It is apparent that the increases in olein, groundnut oil, and stearin, lead to an increase in the iodine value.
Fig. 4.
-
eFree fatty acid
IV: iodine value (in g I2/100 g), GO: peanut oil; OP: olein; SP: stearin; SO: sesame oil.
Fig. 5 shows the effects of components on free fatty acid (FFA) content. It appears that an increase in stearin leads to a decrease in the free fatty acid. However, increases in groundnut oil and olein lead to an increase in this parameter.
Fig. 5.
-
fTotal oxidation value
FFA: free fatty acid (in % Oleic acid), GO: groundnut oil; OP: olein; PS: palm stearin; SO: sesame oil.
The effects of the components on the total oxidation value (Totox) are shown in Fig. 6. It can be observed that an increase in olein and groundnut oil increases the total oxidation value; while an increase in stearin leads to a decrease in this parameter.
Fig. 6.
Iso-response curve of total oxidation value.
Totox: total oxidation value, GO: groundnut oil; OP: olein; SP: stearin; SO: sesame oil.
3.1.3. Mathematic models of the responses
The regression equations obtained for the independent and response variables for the peroxide value (Y1); thiobarbituric acid value (Y2); p-anisidine value (Y3); iodine value (Y4); free fatty acid content (Y5) and total oxidation (Y6) are as shown in equations (10),(11),(12),(13),(14),(15).
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
3.1.4. Validation of the model for the responses
Table 5 presents the values of the coefficient of determination (R2), the Absolute Mean Deviation Analysis (AMDA), and the Bias Factor (Bf) obtained for the various responses. It can be highlighted that the R2 of the different responses is greater than 75%, the AMDA is between 0 and 0.07 and the Bf is between 0.75 and 1.25; thus, the models of the responses are valid.
Table 5.
Coefficient of determination (R2), AMDA, and Bf of the different responses.
| Responses | R2 | AMDA | Bf |
|---|---|---|---|
| FFA | 98.50 | 0.07 | 1.21 |
| PV | 79.96 | 0.1 | 1.05 |
| TBA | 90.29 | 0.02 | 1.24 |
| IV | 93.21 | 0.00 | 0.99 |
| p-AnV | 80.43 | 0.03 | 1.24 |
| Totox | 89.58 | 0.01 | 1.03 |
| Standard values | >75% | 0 | 0.75 < Bf < 1.25 |
FFA: free fatty acid (in % Oleic acid); PV: peroxide value (in meq/Kg of oil), TBA: thiobarbituric acid value (in meq MDA/Kg), IV: iodine value (in g I2/100 g), p-AnV: p-anisidine value, Totox: total oxidation value, R2: coefficient of determination, AMDA: absolute mean deviation analysis, Bf: Bias factor.
3.1.5. Multiple optimizations and validation of compromised optimum condition
After analyzing the different responses, a compromise optimum condition was made for all the different responses shown by the arrow in Fig. 7. This condition is as thus 33.23%, 23.23%, 15.85%, and 27.67% respectively for the groundnut oil, olein, stearin, and sesame oil. This resulted in experimental values of the quality parameters that were not significantly different from the model predicted value with high desirability (Table 6).
Fig. 7.
Overlaid iso-respond curve for the responses.
FFA: free fatty acid (in % Oleic acid); PV: peroxide value (in meq/Kg of oil), TBA: thiobarbituric acid value (in meq MDA/Kg), IV: iodine value (in g I2/100 g), p-AnV: p-anisidine value, Totox: total oxidation value, GO: groundnut oil; OP: olein; SP: stearin; SO: sesame oil.
Table 6.
Compromised optimum condition and validation.
| Compromised condition |
GO | OP | SP | SO |
| 33.23% |
23.23% |
15.85% |
27% |
|
| Verification of the conditions of the optima | ||||
| Responses |
model predicted value |
experimental values |
||
| FFA | 1.75a | 1.69 ± 0.01a | ||
| PV | 6.15a | 6.14 ± 0.03a | ||
| TBA | 1.16a | 1.05 ± 0.06a | ||
| IV | 55.39a | 54.18 ± 0.26a | ||
| p-AnV | 4.45a | 4.47 ± 0 .02a | ||
| Totox Desirability |
19.85a 0.99 |
19.81 ± 0.15a | ||
The values of the column with the same letter do not differ significantly (p > 0.05). FFA: free fatty acid (in % Oleic acid); PV: peroxide value (in meq/Kg of oil), TBA: thiobarbituric acid value (in meq MDA/Kg), IV: iodine value (in g I2/100 g), p-AnV: p-anisidine value, Totox: total oxidation value, GO: groundnut oil; OP: olein; PS: palm stearin; SO: sesame oil.
3.2. Discussion
The peroxide value is a good indicator to assess the quality of the main primary oxidation products such as hydroperoxides. The R2 of the peroxide index (79.96%) indicates that these mixtures contribute to 79% of the variation in this response [21]. Regarding the absolute mean deviation analysis (AMDA) and the Bias factor (Bf), these values were within the norm, which is 0 and 0.75 ≤ Bf ≤ 1.25 respectively [22]. The decrease in peroxide value with the increase in stearin is due to the low content of this oil in unsaturated fatty acids which is more sensitive to oxidation than saturated fatty acids, hence the low rate of primary oxidation products [13]. However, the increase in the peroxide value with the increase in groundnut and olein oil is attributed to their high content in unsaturated fatty acids which would have been transformed into oxidation products such as hydroperoxides during heating. In fact, it has been shown that oil extracted from Manga groundnut (Arachis hypogea), contains high levels of oleic acid (53.18%), followed by linoleic acid (29.74%) and palmitic acid (12.43%) [23]. These observations agree with the statement of [24] who suggested that an increase in the peroxide value of oils would be due to the formation of hydroperoxides by degradation of the double bonds of fatty acids, while a drop would be attributed to low production or the volatilization of compounds resulting from the decomposition of hydroperoxides formed during the primary oxidation phase. In addition [25], demonstrated that mixtures of Boerhavia and groundnut seed oils as well as mixtures of moringa, sunflower and soybean oils, treated at a high temperature (180 °C/2 h) gave low peroxide values (6.97–6.02 meq O2/kg of oil). Also, it has been observed a decrease of peroxide value in a mixture made up of sesame and corn oils during frying, compared to that of sesame oil alone, and this was associated to the proportion of saturated fatty acid present in corn oil [5]. The value of peroxide value obtained from the optimal combination in this study is lower than that recommended by Codex [26] which is 10 meqO2/kg, indicating a good quality of the oil.
The thiobarbituric acid value provides information on the presence of secondary lipid oxidation products such as aldehydes, alcohols, and ketones, specifically malondialdehydes. The coefficient of determination (R2) of the thiobarbituric acid value is greater than 75% (90.29%). The absolute mean deviation analysis (AMDA) is close to 0 and the Bias factor (Bf) is between 0.75 and 1.25; thus, the model response is valid [22]. Concerning the effect of the components on the thiobarbituric acid value, the increase of this parameter with the increase in groundnut and olein oils can be attributed to the rapid decomposition of hydroperoxides which are unstable substances to secondary oxidation products such as malondialdehyde which are responsible for the change in odour and color [27]. This result corroborates with those obtained by Ref. [10]; who found a production of a high amount of thiobarbituric acid value by mixing palm oil with extra virgin olive oil during heating. However, the decrease in the thiobarbituric acid value with the increase in stearin would be due to the low content of this oil in unsaturated fatty acids likely to undergo primary and secondary oxidation [13].
The p-Anisidine value is the measure of the secondary product produced when hydroperoxide decomposed to non-volatile carbonyls during lipid oxidation leading to the rancid flavor of the oil [28]. The value of the coefficient of determination (R2) of 80.43% indicates there is a match between the experimental values and the values predicted by the model. Also, the AMDA and Bf values are within the required range. The decrease in the anisidine value with groundnut and olein oil would be due to the less polyunsaturated fatty acids (PUFA) content in white sesame oil as described by Ref. [29]. Yacoub et al. (2008) noted that the anisidine value increased much slower in nuts that contain less PUFA during roasting than in other nuts which contain high PUFA. Also, the fact that oxidative degradation of oleic acid forms fewer α and β-unsaturated aldehydes than do linoleic and linolenic acid, explains the lower value of the response observed [30]. These results are consistent with those of [3] who demonstrated that the mixture of groundnut, palm olein, and sunflower oils significantly reduces the anisidine value when frying potato chips. Furthermore, the increase in the anisidine value with the increase in palm stearin would be the consequence of a rapid formation of hydroperoxides and alkoxyl radicals due to the absence of antioxidants, and their decomposition in favor of secondary oxidation products, mainly carbonyls, 2-alkenals and 2,4-decadienals [31].
The iodine value is a parameter that measures the degree of fatty acid unsaturation in an oil. The coefficient of determination (R2) of the iodine value (93.21%) is greater than 75%, which indicates that there is a match between the experimental values and the values predicted by the model. Regarding the absolute mean deviation analysis (AMDA) and the Bias factor (Bf), these values were within the norm, which is 0 and 0.75 ≤ Bf ≤ 1.25 respectively [22]. The high values of the iodine number with the increase in the proportion's components would be due to the high proportion of unsaturated fatty acids in the mixture of oils; and also, the low destruction of these unsaturated fatty acids during heating [32]. This result corroborates that obtained by Roiaini et al. (2015) who found a high content of iodine value when mixing canola, olive, and palm olein oils. When oil with high content of linoleic acid is blended with palm olein, the linoleic acid tends to migrate into the oil blends [33]. Then, the increase of iodine value could be due to the fact that after the oils are blended together, their degree of unsaturation changed leading to change iodine value [34].
The free fatty acid content is a parameter used to assess the presence of free fatty acids in the oil. The coefficient of determination (R2) is an indicator that makes it possible to judge the quality of simple linear regression. Thus, the R2 of the free fatty acid content (98.50%) is greater than 75%, which indicates that there is a match between the experimental values and the values predicted by the model [21]. Regarding the absolute mean deviation analysis (AMDA) and the Bias factor (Bf), these values were within the norm, which is 0 and 0.75≤ Bf ≤ 1.25 respectively [22]. This means that the model is valid. Indeed, the decrease in the free fatty acid content with the increase in stearin could be explained by the rapid transformation of the fatty acids released into primary and secondary oxidation products. These results obtained do not differ from those obtained by Ref. [35] who showed that the decrease in the free acidity of oil would be the consequence of a faster formation of hydroperoxides. However, the increase in the acid number with the increase in groundnut oil and palm olein would be explained by the high content of these oils in free fatty acids. This can also be due to a high hydrolysis rate of their triglycerides during heating [36].
The Totox value reflects the initial and later stages of the oil oxidation. It measures both hydroperoxides and their break-down products and provides a better estimation of the progressive deterioration of the oil. The lower Totox value indicates a good quality of oil [37]. The coefficient of determination (R2) of the total oxidation index is greater than 75% (89.58%). This justifies that the response meets the quality of the optimal combination and that there is a match between the experimental values and the values predicted by the model. The absolute mean deviation analysis (AMDA) is close to 0 and the Bias factor (Bf) is between 0.75 and 1.25 showing that the model is valid [22]. The increase in the total oxidation index with the increase in groundnut oil and palm olein is explained by the alteration of the unsaturated fatty acids of these oils during heating. Furthermore, the decrease in the Totox value with the increase in stearin is due to the stability of these oils at a high temperature by their saturated fatty acids. Indeed, it has been shown that oil mixtures can limit the total oxidation of oils during storage [38]. However, the products of oxidation reactions have been concerned with negative health effects, and as they destroy vitamins, prohibit enzymes and could cause mutations or gastrointestinal irritations [39].
Multiple optimizations make it possible to construct an appropriate iso-response curve model that aggregates all the responses and then tries to find defined operating conditions for all the responses by keeping them within the desired range [40]. Here, the desirability of the optimum responses is close to 1, so the condition is satisfied. Moreover, the fact that there is no significant difference between the experimental result and the predicted result of these responses shows that the condition is valid [20].
4. Conclusion
This study showed that the combination of groundnut, olein, stearin, and sesame oil in the proportion of 33.23%, 23.23%, 15.85%, and 27% respectively, make it possible to best preserve the chemical quality of their lipids during heat treatment. Thus, it may be possible to combine these edible oils in households to limit their oxidation during cooking.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Additional information
No additional information is available for this paper.
Authors' contribution
Serge Cyrille Ndomou, Hervé Togyam, Annick Pougoue, and Bilkissou Njapndounke Conceived and designed the experiments; Performed the experiments; Analysed and interpreted the data, analysis tools, or data; Wrote the paper. Cristelle Tiwo contributed materials; Hilaire Womeni conceived and designed the experiments; Contributed reagents, analysis tools, or data; Wrote the paper.
Declaration of competing interest
This paper has no conflict of interest and all authors have read, approved, and are aware of the submission to the journal.
Acknowledgments
We acknowledge Dr. Gires Teboukeu, Dr. Cerile Woumbo, and Ms. Alix MBOUKAP, for their support during the manipulations and editing of the manuscript.
References
- 1.Rosillo-calle F., Pelkmans L., Walter A. 2009. A Global Overview of Vegetable Oils, with Reference to Biodiesel A Report for the IEA Bioenergy Task 40. [Google Scholar]
- 2.Britannica Oil extraction. T. Editors of Encyclopaedia. Encyclopedia Britannica. 2022. https://www.britannica.com/science/oil-extraction
- 3.Nor-Aini I., Hasmadi M., Mamot S., Radzuan J. Palm oil and sunflower oil: effect of blend composition and stirrer types during fractionation on the yield and physicochemical properties of the oleins. J. Food Lipids. 2005;12(1):48–61. doi: 10.1111/J.1745-4522.2005.00005.X. [DOI] [Google Scholar]
- 4.Giuffrè A.M., Caracciolo M., Zappia C., Capocasale M., Poiana M. Effect of heating on chemical parameters of extra virgin olive oil, pomace olive oil, soybean oil and palm oil. Ital. J. Food Sci. 2018;30(4):715–739. doi: 10.14674/IJFS-1269. [DOI] [Google Scholar]
- 5.Ramroudi F., Ardakani S.A.Y., Dehghani-tafti A., Sadrabad E.K. Investigation of the physicochemical properties of vegetable oils blended with sesame oil and their oxidative stability during frying. Int. J. Food Sci. 2022;2022 doi: 10.1155/2022/3165512. Article ID 3165512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiralan M., Ramadan M.F. Volatile oxidation compounds and stability of safflower, sesame and canola cold-pressed oils as affected by thermal and microwave treatments. J. Oleo Sci. 2016;65(10):825–833. doi: 10.5650/jos.ess16075. [DOI] [PubMed] [Google Scholar]
- 7.Giuffrè A., Capocasale M., Macrì R., Caracciolo M., Zappia C., Poiana M. Volatile profiles of extra virgin olive oil, olive pomace oil, soybean oil and palm oil in different heating conditions. LWT--Food Sci. Technol. 2020;117 doi: 10.1016/j.lwt.2019.108631. [DOI] [Google Scholar]
- 8.Chopra R., Kumari K.K., Nagraj G. Fatty acid profile and shelf life of linseed-groundnut, linseed-sunflower and linseed-palm oil blends. J. Oil Technol. Assoc. India. 2004;36:21–24. doi: 10.1007/s40011-014-0324-9. [DOI] [Google Scholar]
- 9.Roiaini M., Ardiannie T., Norhayati H. Physicochemical properties of canola oil, olive oil and palm olein blends. Int. Food Res. J. 2015;22(3):1228–1234. [Google Scholar]
- 10.Leonardis A.D., Macciola V. Heat-oxidation stability of palm oil blended with extra virgin olive oil. J. Food Chem. 2012;135:1769–1776. doi: 10.1016/j.foodchem.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Chu Y.H., Kung Y.L. A study on vegetable oil blends. Food Chem. 1998;62(2):191–195. doi: 10.1016/S0308-8146(97)00200-8. [DOI] [Google Scholar]
- 12.Myers M., Raymond H., Douglas C., Christine M., Anderson Cook . ume 705. John Wiley & Sons Edition; 2009. p. 680. (Response Surface Methodology: Process and Product Optimization Using Designed Experiments). of Wiley Series in Probability and Statistics, ISSN 1940-6517. [Google Scholar]
- 13.Abdel-Razek A.G., El-Shami S.M., El-Mallah M.H., Hassanien M.M.M. Blending of Virgin olive oil with less stable edible oils to strengthen their antioxidative potencies. Aust. J. Basic Appl. Sci. 2011;5(10):312–318. [Google Scholar]
- 14.IDF (International Dairy Federation Standards) International dairy federation. IDF-Square Vergote. 1991;41:74A. Brussels, Belgium, sec. [Google Scholar]
- 15.Draper H.H., Hadley M. Malondialdéhyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 16.AOCS (American Oil Chemists’ Society) Firestone D, American Oil Chemists' Society. fifth ed. AOCS Press; Champaign, Illinois, USA: 2003. Official methods and recommended practices of the American oil chemists' society. [Google Scholar]
- 17.AFNOR (Association Française de Normalisation) AFNOR; Paris, France: 1981. Recueil des normes françaises. Corps gras, graines oléagineuses, produits dérivés. 2ème édition; p. 438p. [Google Scholar]
- 18.Shahidi F., Wanasundara U.N. In: Food Lipids: Chemistry, Nutrition and Biotechnology. Akoh C.C., Min D.B., editors. CRC Press; New York: 2008. Methods for measuring oxidative stability in edible oils; pp. 387–388. [Google Scholar]
- 19.Baranyi J., Pin C., Ross T. Validating and comparing predictive models. Int. J. Food Microbiol. 1999;(48):159–166. doi: 10.1016/S0168-1605(99)00035-5. [DOI] [PubMed] [Google Scholar]
- 20.Madamda P.S. The response surface methodology: an application to optimize dehydration operations of selected agricultural crops. LWT--Food Sci. Technol. 2002;(35):584–592. doi: 10.1006/fstl.2002.0914. [DOI] [Google Scholar]
- 21.Joglekar A.M., May A.I. Product excellence through design of experiments. CFW (Cereal Foods World) 1987;32:857–868. [Google Scholar]
- 22.Rose T. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 1996;81:501–508. doi: 10.1111/j.1365-2672.1996.tb03539.x. [DOI] [PubMed] [Google Scholar]
- 23.Dzondo-Gadet M., Nsikabaka S., Nkama Niamayoua R., Ossoko J.P.L., Enzonga J., Djimi L.S., Pambou-Tobi N.P.G., Silou T., Desobry S. Nutritional value of Manga groundnut (Arachis hypogea) and characterization of oil extracted by solvent. Adv. J. Food Sci. Technol. 2014;7:914–920. doi: 10.19026/ajfst.7.2533. [DOI] [Google Scholar]
- 24.Eymard S. 2003. Mise en évidence et suivi de l'oxydation des lipides au cours de la conservation et de la transformation du chinchard (Trachurus trachurus): choix des procédés, Archimer, archive institutionnelle de l'Ifremer, ID. 10670/1.rhlvur. [Google Scholar]
- 25.Al-Fargaa A., Baeshenb M., Aqlanc F.M., Siddeegd A., Afifia M., Alia H.A., Alayafib A., Al-Dalalie S., Alkaladib A. Chemical composition, oxidative stability, and sensory properties of Boerhavia elegana Choisy (alhydwan) seed oil/peanut oil blends. Grasas Aceites. 2020;71(3):367. doi: 10.3989/gya.0463191. [DOI] [Google Scholar]
- 26.Alimentarius Codex, International Food Standards . FAO-WHO; Rome, Italia: 2015. Standard for Name Animal Fats. Adopted in 1999 and Amendment in 2015; p. 6.www.codexalimentarius.org Codex Stan 211-1999. [Google Scholar]
- 27.Shermer W. United State of America; Maryland: 1990. Effects of Oxidation on the Quality of Ingredients and Feed of Poultry. 37° Maryland Nutrition Conference. [Google Scholar]
- 28.Laguerre M., Lecomte J., Villeneuve P. Evaluation of the ability of antioxidants to counteract lipid oxidation: existing methods, new trends and challenges. J. Lipid Res. 2007;46:244–282. doi: 10.1016/j.plipres.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Tenyang N., Ponka R., Tiencheu B., Djikeng F.T., Azmeera T., Karuna M.S.L., Prasad R.B.N., Womeni H.M. Effects of boiling and roasting on proximate composition, lipid oxidation, fatty acid profile and mineral content of two sesame varieties commercialized and consumed in Far-North Region of Cameroon. Food Chem. 2017;(221):1308–1316. doi: 10.1016/j.foodchem.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Muik B., Lendl B., Molina-Diaz A., Ayora-Cañada M.J. Direct monitoring of lipidoxidation in edible oils by Fourier transform Raman spectroscopy. Chem. Phys. Lipids. 2005;134:173–182. doi: 10.1016/J.CHEMPHYSLIP.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Roman O. Thèse de Doctorat AgroParisTech, Institut des Sciences et Industries du Vivant et de l'Environnement; Paris, France: 2012. Mesure et prédiction de la réactivité des lipides au cours du chauffage d’huiles végétales à haute température; p. 168. [Google Scholar]
- 32.Tynek M., Hazuka Z., Pawlowicz R., Dudek M. Changes in the frying medium during deep frying of food rich in proteins and carbohydrates. J. Food Lipids. 2001;8:251–261. doi: 10.1111/J.1745-4522.2001.TB00200.X. [DOI] [Google Scholar]
- 33.Abdulkarim S.M., Myat M.W., Ghazali H.M. Sensory and physicochemical qualities of palm olein and sesame seed oil blends during frying of banana chips. J. Agric. Sci. 2010;2(4):18–29. doi: 10.5539/JAS.V2N4P18. [DOI] [Google Scholar]
- 34.Siddique B.M., Ahmad A., Ibrahim M.H., Hena S., Rafatullah M., Mohd Omar A.K. Physicochemical properties of blends of palm olein with other vegetable oils. Grasas Aceites. 2010;61(4):423–429. doi: 10.3989/gya.010710. [DOI] [Google Scholar]
- 35.Nassif D. vol. 40p. Mémoire de DEA, Institut national agronomique Paris-Grignon; France: 2004. (Valorisation des polyphénols extraits des margines en tant qu'antioxydants naturels dans les huiles végétales). [Google Scholar]
- 36.Frega N., Mozzon M., Lercker G. Effect of free fatty acids on the oxidative stability of vegetable oils. J. Am. Oil Chem. Soc. 1999;76:325–329. doi: 10.1007/s11746-999-0239-4. [DOI] [Google Scholar]
- 37.O’keefe S.F., Pike O.A. In: Food Analysis. fourth ed. Nielsen S.S., editor. Springer Science and Business Media; New York, NY, USA: 2010. Fat characterization; pp. 239–260. [Google Scholar]
- 38.Ramli N., Nafar M., Jaswir I. Oxidative stability of blend oil during deep-fat frying of potato chips. Pakistan J. Nutr. 2012;11(9):828–832. doi: 10.3923/pjn.2012.828.832. [DOI] [Google Scholar]
- 39.Clark W.L., Serbia G.W. Safety aspects of frying fats and oils. Food Technol. 1991;45:84–89. [Google Scholar]
- 40.Derringer G., Suich R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980;(12):214–219. doi: 10.1080/00224065.1980.11980968. [DOI] [Google Scholar]