Abstract
Crop rotation has widely contributed to increasing farmland biodiversity as well as to improving soil carbon pools and microbial diversity. However, there is a weak understanding of the suitability of winter crop rotation intensification in double rice fields, especially rotation with various winter crops. For this task, a long-term field experiment based on one from 2012 was conducted with five winter crop systems for double rice: winter fallow (T0), winter milk vetch (T1), winter rape (T2), winter garlic (T3), winter rotation intensification with potato, milk vetch, and rape (T4). Parameters such as crop yield, soil carbon, nitrogen, and soil microorganism were measured. It was found that compared to winter fallow, winter milk vetch, rape, garlic, and crop rotation intensification practices increased the late rice yield by 2.5%, 2.3%, 4.5%, and 3.7%, respectively; winter garlic and crop rotation intensification also increased the early rice yield by 4.6% and 3.5%, respectively. This is associated with the promotion of rice tillering. At the same time, for winter crop rotation, compared to winter fallow, the soil organic carbon increased by 21%. With the input of diversified crop residues, winter crops were effective in soil carbon sequestration, improving soil microbial structure, and increasing soil microbial diversity. The Shannon diversity index of winter crops ranged from 9.75 to 9.91, while winter fallow was 9.38. The Simpson’s diversity index of winter crops ranged from 0.997 to 0.998, while winter fallow was 0.996. In conclusion, winter crop practices, especially winter crop rotation intensification, can enhance soil health and sustainability in double rice fields through its positive feedback on crop yield, soil carbon sequestration, and microorganisms.
Keywords: Crop diversity, Green manure, Cover crop, Soil carbon sequestration, Soil microbe
1. Introduction
During the last few decades, to maximize crop yields and economic benefits, the winter fallow-double rice system has become the main cropping system in the south of China [1]. An increase in chemical fertilizer for sustained high yield has led to the loss of soil organic carbon and biodiversity, thus risking stable crop yields and reducing soil microbial diversity [[2], [3], [4], [5]]. With renewed interest in maintaining soil health and clean production on farmland, crop rotation may be an effective solution to establish a diversified planting system that can stabilize crop yields and increase soil carbon pools and biodiversity [6].
Crop rotation, as a nature-based solution, has been proven to i) increase farmland biodiversity, thus decreasing pest and disease incidence, and maintaining crop yield stability [7]; ii) reduce soil erosion because of plant cover [8], thus protecting soil nutrients in the cultivated layer of farmland; iii) maintain soil temperature and reduce water dissipation, thus improving soil hydrothermal environment [9]; and iv) affect the decomposition and transformation of soil organic carbon through the input of root residues and release of root exudates, thus enhancing soil organic carbon pools and optimizing soil microbial community structure and diversity [10,11].
Crop rotation can enhance crop yields and soil carbon sequestration, but their contribution differs depending on the combination of crops [12]. Milk vetch, a leguminous crop, can improve rice productivity, with reduced N fertilizer input and total global warming potential (GWP) per yield [13,14]. Rape, a brassica crop, may increase soil gross N immobilization rates and decrease soil gross N mineralization rates in a double rice cropping system [15]. In the upland soil, the wheat–soybean rotation can result in the greatest richness and biodiversity of the total microbial community [16]. Moreover, various crop rotations have been selected for different microbial communities, which have generated microbial products that significantly influenced soil C and N retention [17,18]. Relatively small increases in crop diversity could have large impacts on microbial community size and function, with cover crops appearing to facilitate the largest increases [19]. However, it is still not clear what winter crop rotation is suitable for this task in the double rice field and how to intensify rotation with various winter crops.
Thus, a better understanding is needed for what winter crop rotation in double rice fields, especially rotation with various winter crops, may contribute to crop yield, and how they can provide positive feedback in soil carbon and microbes. In this study, we characterized the crop yield, soil carbon, nitrogen, and soil microorganisms under various winter crop treatments in double rice fields. The main aim of this study was to investigate the effects of diverse crop rotation practices on crop yield, soil C, N, and microbes. It was hypothesized that winter crop rotation intensification in double rice fields can increase crop yield, be effective in soil carbon sequestration and nitrogen content, and improve soil microbial structure and diversity.
2. Materials and methods
2.1. Experimental site
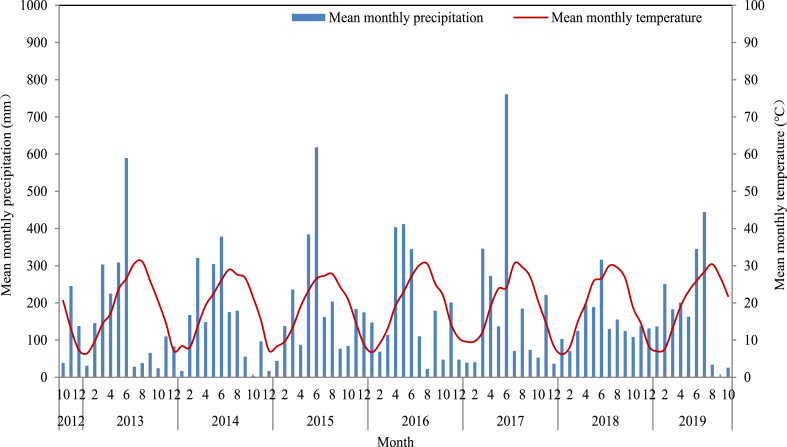
The field experiment was conducted from 2012 to 2019 at the Agricultural Science Institute of Wannian County (28°42′N, 117°04′E), Jiangxi, China. The region belongs to the subtropical monsoon climate, with abundant sunlight, heat, and precipitation resources and significant seasonal changes in temperature. The mean monthly temperature and precipitation during the experiment are shown in Fig. 1. The experimental soil is a typical double rice paddy field in the middle and lower reaches of the Yangtze River. Before the study, the soil was hydromorphic paddy soil with 6.08 pH, 41.81 g kg−1 of soil organic matter, 1.97 g kg−1 of total nitrogen, 16.38 mg kg−1 of available phosphorus, and 130 mg kg−1 of available potassium.
Fig. 1.
Mean monthly temperature and precipitation during the experiment.
2.2. Experimental design
The long-term field experiment consisted of five cropping systems based on double rice. T0: winter fallow, a traditional cropping system, was used as a control; T1: winter milk vetch, a green manure cropping system; T2: winter rape, an oil-bearing cropping system; T3: winter garlic, a vegetable cropping system; T4: winter potato-milk vetch-rape, a cover crop rotation intensification system. The whole experimental design consisted of five treatments (T0, T1, T2, T3, and T4) with a randomized block design and three replications in each treatment. There were 15 experimental plots in total, each with an area of 66 m2 (11 m × 6 m). The experimental design of the annual planting pattern is detailed in Table 1. Taking winter crop rotation treatment (T4) as a reference, the parameters of the third (2015) and sixth (2018) years were mainly used for analysis in this study.
Table 1.
Experimental design.
| Treatments | 2012.10–2013.10 | 2013.10–2014.10 | 2014.10–2015.10 | 2015.10–2016.10 | 2016.10–2017.10 | 2017.10–2018.10 | 2018.10–2019.10 |
|---|---|---|---|---|---|---|---|
| T0 (winter fallow) | fallow - double rice | fallow - double rice | fallow - double rice | fallow - double rice | fallow - double rice | fallow - double rice | fallow - double rice |
| T1 (winter milk vetch) | milk vetch- double rice | milk vetch - double rice | milk vetch - double rice | milk vetch - double rice | milk vetch - double rice | milk vetch - double rice | milk vetch - double rice |
| T2 (winter rape) | rape - double rice | rape - double rice | rape - double rice | rape - double rice | rape - double rice | rape - double rice | rape - double rice |
| T3 (winter garlic) | garlic - double rice | garlic - double rice | garlic - double rice | garlic - double rice | garlic - double rice | garlic - double rice | garlic - double rice |
| T4 (winter crop rotation) | potato - double rice | milk vetch- double rice | rape - double rice | potato - double rice | milk vetch- double rice | rape - double rice | potato - double rice |
The cultivar of milk vetch was Yujiang Daye, which was sown (45 kg·hm−2 seeds) in October and harvested as green manure in March. The cultivar of rape was Rongyou 05 in 2012–2014, Ganyouza 8 in 2015, and Mianfengyou 18 in 2016–2018, which was sown (23 kg·hm−2 seeds) in October and harvested in May. The cultivar of garlic was Jinxiang Dasuan, which was sown (2500 kg·hm−2 seeds) in November and harvested in March. The cultivar of potato was Kexin 18 in 2012 and 2015, and Dongnong 303 in 2018, which was sown (2000 kg·hm−2 seeds) in November and harvested in April. The cultivar of early rice was Tianyou 463 in 2013, Zhuliangyou 09 in 2014, 2016–2019, and Xinrong 08 in 2015, which was transplanted in May and harvested in July. The cultivar of early rice was Tianyou Huazhan in 2013–2019, which was transplanted in July and harvested in October. Fertilizer was applied according to local conventional fertilization, and other field management measures were the same as general field cultivation.
2.3. Sampling and analysis of crop
A sampling of rice crop yield was carried out each year (2013–2019) and sampling of rice crop yield components and dry matter weight was carried out in 2015 and 2018. The panicle length, seed setting rate, effective panicle number, thousand-grain weight, and yield of rice were measured at the harvest stage. The yield of rice was air-dried to constant weight. The shoot biomass of rice was also measured at the tillering stage, booting stage, heading stage, and harvest stage, dried at 105 °C for 30 min and then dried at 80 °C to constant weight.
2.4. Soil carbon and nitrogen
A sampling of soil was carried out at the harvest stage of rice in 2015 and 2018. Five representative soil samples were taken with the help of an auger from each replicate of the individual treatments to a depth of 15 cm and then combined to form a composite sample. All samples were sieved through a 0.25 mm sieve after air-drying. The air-drying soil samples were used to analyze soil organic carbon (SOC) and total nitrogen (TN) concentrations. SOC concentrations were analyzed by combustion analysis (TOC-L SSM-5000A, SHIMADZU). TN concentration was analyzed by Kjeldahl determination (Kjeltec™ 2300, FOSS).
2.5. Soil microorganisms
Sampling for soil microbes was collected from each plot in April 2019 before the winter crop harvest. Fifteen soil samples were taken with the help of an auger from each plot to a depth of 15 cm. All samples were individually removed from roots and stones, and then a portion of each fresh mixed sample was bagged with a sterile spoon, placed in ice buckets to bring them back to the laboratory, and stored at −80 °C immediately for measuring microorganisms.
Total genomic DNA in the soil samples was extracted using the CTAB method. DNA concentration and purity were detected in 1% agarose gels, and DNA was diluted to 1 ng μL−1 using sterile water. Bacterial 16S rRNA gene fragments from the V4 region were amplified by a PCR method using the universal primers 515F (GTGCCAGCMGCCGCGGTAA) and 806R (GGACTACHVGGGTWTCTAAT) [20]. All PCRs were conducted with Phusion® High-Fidelity PCR Master Mix (New England Biolabs). The same quantity of 1 × loading buffer (containing SYB green) was mixed with the PCR products according to their concentrations, and the samples were subjected to electrophoresis on 2% agarose gels for detection. Samples with a bright main band around 300 bp for bacteria, were selected for further examination. The target strips were purified using a Gel Extraction Kit (Qiagen). Sequencing libraries were built using a TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, USA). The qualified libraries were assessed using a Qubit@ 2.0 Fluorometer (Thermo Scientific) as well as PCR quantitative detection and were sequenced using a HiSeq 2500 PE250 platform by the Novogene Company (Beijing, China). The sequencing data has been uploaded to the National Center for Biotechnology Information (NCBI) database, and the BioProject accession number is PRJNA911586.
2.6. Data statistics
Statistical analysis of all experimental data was conducted using SPSS 17.0 and Microsoft EXCEL 2010. Crop yield, soil carbon, nitrogen, and microbe data were averaged for each treatment and were evaluated with one-way ANOVA. The mean values of all data in different treatments were compared using Duncan's multiple comparisons for each sampling point. The significant difference was assessed at P < 0.05. Species abundance information was normalized using a standard of sequence number corresponding to the sample with the least sequences. Subsequent analysis of alpha diversity and beta diversity were all performed based on this output normalized data. Alpha diversity is applied in analyzing the complexity of species diversity for a sample through 2 indices, including Shannon and Simpson's. All these indices in our samples were calculated with QIIME (Version 1.7.0) and displayed with R software (Version 2.15.3). Cluster analysis was preceded by principal component analysis (PCA), which was applied to reduce the dimension of the original variables using the FactoMineR package and ggplot2 package in R software (Version 2.15.3). The unweighted pair-group method with arithmetic means (UPGMA) clustering was performed as a type of hierarchical clustering method to interpret the distance matrix using average linkage and was conducted by QIIME software (Version 1.7.0).
3. Results
3.1. Crop yield
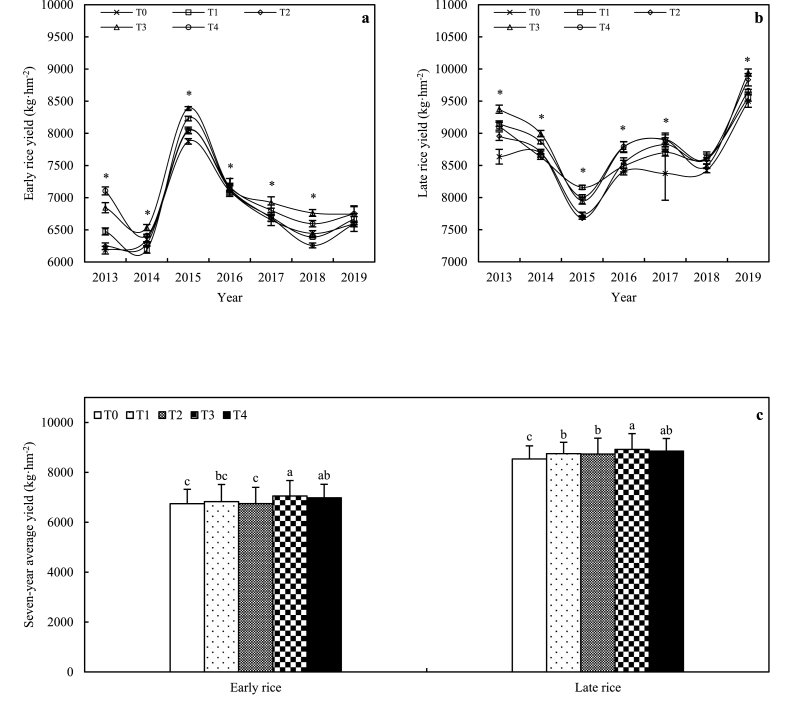
Rice yields under the winter garlic and crop rotation treatments were consistently at high levels, while rice yields under the winter fallow and rape treatments were relatively low (Fig. 2a and b). The results of the 7-year average rice yield showed that early rice yield was 4.6% and 3.5% higher in winter garlic and crop rotation treatments than in winter fallow, respectively; and late rice yield was 2.5%, 2.3%, 4.5%, and 3.7% higher in winter milk vetch, rape, garlic, and crop rotation treatments than in winter fallow, respectively (Fig. 2c). Winter cover crops can significantly increase the rice yield, among which the most significant effects of winter garlic and crop rotation treatments on rice yield were observed.
Fig. 2.
Effects of winter cover crops on rice crop yield. Note:Fig. 2a and b represent the rice yield in each year under different cover crops (Means of three replicates and standard deviations). Fig. 2c represents the seven-year average rice yield under different cover crops (Means of seven years and standard deviations). The asterisks and letters above means indicate significant differences at P < 0.05.
3.2. Crop yield components
The effects of different winter crops on panicle length, seed setting rate, and thousand-grain weight of double rice were not significant, but the effects on effective panicle number were significant (Table 2). In 2015, the effective panicle number of early rice in winter milk vetch, garlic, and crop rotation significantly increased by 4.6%, 6.7%, and 4.0%, respectively, compared with winter fallow, and the effective panicle number of late rice in winter milk vetch, garlic, and crop rotation significantly increased by 3.1%, 3.1%, and 2.8% compared with winter fallow. In 2018, the effective panicle number of early rice in winter milk vetch and crop rotation also significantly increased by 26.0% and 32.0%, respectively, compared with winter fallow. It can be observed that winter crops increase rice yield mainly by enhancing the effective panicle number.
Table 2.
Effects of winter cover crops on rice crop yield components.
| Year | Rice season | Treatment | Panicle Length (cm) | Seed setting rate (%) | Effective panicle number (104 hm−2) | Thousand grain weight (g) |
|---|---|---|---|---|---|---|
| 2015 | Early rice | T0 | 20.54 ± 0.24a | 86.23 ± 0.65b | 298.45 ± 0.82c | 25.82 ± 0.06a |
| T1 | 21.41 ± 0.38a | 85.77 ± 0.41b | 312.23 ± 1.86b | 26.12 ± 0.33a | ||
| T2 | 21.21 ± 0.28a | 88.48 ± 1.31 ab | 300.91 ± 2.02c | 26.14 ± 0.38a | ||
| T3 | 21.39 ± 0.32a | 89.10 ± 0.05a | 318.58 ± 2.14a | 26.00 ± 0.21a | ||
| T4 | 20.70 ± 0.47a | 86.78 ± 1.13 ab | 310.48 ± 1.21b | 26.10 ± 0.23a | ||
| Late rice | T0 | 20.25 ± 0.19a | 83.03 ± 0.40a | 304.18 ± 3.24b | 25.20 ± 0.35a | |
| T1 | 21.15 ± 0.29a | 85.71 ± 0.90a | 313.74 ± 1.25a | 26.06 ± 0.39a | ||
| T2 | 20.77 ± 0.44a | 83.13 ± 0.63a | 309.34 ± 0.24 ab | 25.91 ± 0.12a | ||
| T3 | 20.70 ± 0.36a | 84.86 ± 1.21a | 313.65 ± 2.34a | 25.01 ± 0.47a | ||
| T4 | 20.50 ± 0.08a | 84.52 ± 0.60a | 312.82 ± 2.09a | 25.65 ± 0.03a | ||
| 2018 | Early rice | T0 | 17.43 ± 0.72a | 81.86 ± 1.19a | 296.67 ± 0.67c | 23.61 ± 0.25a |
| T1 | 16.54 ± 1.33a | 82.33 ± 0.65a | 373.80 ± 1.15b | 23.65 ± 0.35a | ||
| T2 | 18.06 ± 0.23a | 81.94 ± 1.91a | 332.27 ± 0.88bc | 23.45 ± 0.61a | ||
| T3 | 17.66 ± 0.45a | 84.39 ± 2.03a | 320.40 ± 1.15bc | 24.37 ± 0.45a | ||
| T4 | 17.88 ± 0.28a | 84.99 ± 1.84a | 391.60 ± 0.58a | 24.44 ± 0.12a | ||
| Late rice | T0 | 22.79 ± 0.44a | 89.68 ± 0.82a | 315.00 ± 12.60a | 23.9838 ± 0.44a | |
| T1 | 23.58 ± 0.34a | 89.93 ± 2.43a | 346.50 ± 44.10a | 24.0645 ± 0.30a | ||
| T2 | 22.75 ± 0.02a | 90.29 ± 0.42a | 327.60 ± 6.30a | 24.1157 ± 0.26a | ||
| T3 | 23.34 ± 0.54a | 90.80 ± 1.91a | 327.6 ± 35.08a | 24.1306 ± 0.56a | ||
| T4 | 23.16 ± 0.47a | 90.58 ± 0.64a | 384.30 ± 12.60a | 24.2719 ± 0.09a |
Note: Means of three replicates and standard deviations. Letters indicate significant differences between the crops at P < 0.05.
3.3. Crop dry matter weight
The effect of different winter crops on the dry matter weight of early and late rice varied (Table 3). In 2015, winter crops mainly affected the dry matter weight of early rice, whereas winter garlic significantly improved the dry matter weight of early rice by 24.6%, 17.0%, and 12.2% at the booting, heading and harvest stages, respectively, compared with winter fallow, while it had some effect on the dry matter weight of late rice at the tillering stage. In 2018, the effect of winter crops on the dry matter weight of early and late rice was weak, whereas winter garlic and crop rotation only significantly improved the dry matter weight of early rice by 16.5% and 20.0%, respectively, at the heading stage compared with winter fallow. Therefore, the effect of winter crops on the dry matter weight of rice was found to be limited.
Table 3.
Effects of winter cover crops on rice crop dry matter weight.
| Year | Rice season | Treatment | Tillering stage (103 kg hm−2) | Booting stage (103 kg hm−2) | Heading stage (103 kg hm−2) | Harvest stage (103 kg hm−2) |
|---|---|---|---|---|---|---|
| 2015 | Early rice | T0 | 1.3 ± 0.10a | 3.05 ± 0.16b | 5.30 ± 0.07c | 11.72 ± 0.38b |
| T1 | 1.65 ± 0.07a | 3.58 ± 0.18ab | 5.90 ± 0.13ab | 12.43 ± 0.24ab | ||
| T2 | 1.62 ± 0.14a | 3.29 ± 0.10ab | 5.78 ± 0.06b | 12.98 ± 0.23ab | ||
| T3 | 1.76 ± 0.28a | 3.80 ± 0.19a | 6.20 ± 0.15a | 13.15 ± 0.40a | ||
| T4 | 1.56 ± 0.08a | 3.37 ± 0.21ab | 5.71 ± 0.06b | 11.96 ± 0.57ab | ||
| Late rice | T0 | 3.33 ± 0.05b | 6.02 ± 0.68a | 8.73 ± 0.12a | 13.91 ± 0.03a | |
| T1 | 3.52 ± 0.20 ab | 5.87 ± 0.67a | 9.08 ± 0.31a | 15.66 ± 0.78a | ||
| T2 | 3.47 ± 0.02 ab | 6.82 ± 0.10a | 8.94 ± 0.19a | 14.33 ± 0.39a | ||
| T3 | 3.86 ± 0.19a | 6.97 ± 0.26a | 9.07 ± 0.22a | 15.24 ± 0.92a | ||
| T4 | 3.40 ± 0.03b | 6.70 ± 0.06a | 8.98 ± 0.08a | 14.73 ± 0.62a | ||
| 2018 | Early rice | T0 | 1.26 ± 0.12a | 1.69 ± 0.14a | 4.49 ± 0.23b | 7.35 ± 0.32a |
| T1 | 1.33 ± 0.16a | 1.82 ± 0.24a | 5.01 ± 0.20ab | 8.25 ± 0.81a | ||
| T2 | 1.37 ± 0.11a | 1.82 ± 0.12a | 4.52 ± 0.29b | 7.57 ± 0.52a | ||
| T3 | 1.40 ± 0.09a | 2.11 ± 0.09a | 5.23 ± 0.08a | 8.25 ± 0.14a | ||
| T4 | 1.59 ± 0.10a | 2.08 ± 0.16a | 5.39 ± 0.16a | 8.73 ± 0.21a | ||
| Late rice | T0 | 4.20 ± 0.18a | 6.51 ± 0.35a | 9.18 ± 0.31a | 12.19 ± 0.22a | |
| T1 | 4.35 ± 0.21a | 6.69 ± 0.36a | 9.39 ± 0.21a | 12.48 ± 0.57a | ||
| T2 | 4.31 ± 0.58a | 6.78 ± 0.34a | 9.40 ± 0.22a | 12.50 ± 0.16a | ||
| T3 | 4.55 ± 0.29a | 7.19 ± 0.63a | 9.42 ± 0.28a | 12.45 ± 0.34a | ||
| T4 | 4.54 ± 0.37a | 6.89 ± 0.49a | 9.58 ± 0.43a | 13.20 ± 0.84a |
Note: Means of three replicates and standard deviations. Letters indicate significant differences between the crops at P < 0.05.
3.4. Soil carbon and nitrogen
Winter crops can significantly affect soil carbon and nitrogen (Table 4). In 2015, winter crops significantly increased the total soil organic carbon content by 7.3%–11.6% and winter garlic increased the total soil nitrogen content by 20.5% in the late rice season compared with winter fallow. In 2018, winter crops significantly increased the total soil organic carbon content by 6.1%–7.2% and winter milk vetch, garlic and winter crop rotation significantly increased the total soil nitrogen content by 12.8%, 7.3%, and 10.4%, respectively, in the early rice season compared with winter fallow. At the same time, winter garlic and winter crop rotation also significantly increased the total soil organic carbon content by 19.3% and 20.8%, respectively, in the late rice season compared with winter fallow. This indicates that winter crops promote the accumulation of soil organic carbon, which is especially stable and obvious with winter crop rotation.
Table 4.
Effects of winter cover crops on soil carbon and nitrogen.
| Rice season | Treatment | 2015 |
2018 |
||
|---|---|---|---|---|---|
| SOC/(g·kg−1) | TN (g·kg−1) | SOC/(g·kg−1) | TN (g·kg−1) | ||
| Early rice | T0 | 20.85 ± 0.07a | 1.20 ± 0.07a | 20.91 ± 0.13b | 1.64 ± 0.01c |
| T1 | 21.00 ± 0.88a | 1.23 ± 0.04a | 22.23 ± 0.25a | 1.85 ± 0.05a | |
| T2 | 21.07 ± 0.73a | 1.22 ± 0.06a | 22.19 ± 0.27a | 1.71 ± 0.01bc | |
| T3 | 21.64 ± 1.30a | 1.24 ± 0.11a | 22.22 ± 0.21a | 1.76 ± 0.04ab | |
| T4 | 21.99 ± 1.43a | 1.23 ± 0.00a | 22.41 ± 0.12a | 1.81 ± 0.01ab | |
| Late rice | T0 | 20.45 ± 0.22b | 1.32 ± 0.06b | 18.14 ± 1.43b | 1.40 ± 0.07a |
| T1 | 22.82 ± 1.13a | 1.34 ± 0.03b | 20.08 ± 0.88ab | 1.49 ± 0.05a | |
| T2 | 22.11 ± 1.06a | 1.44 ± 0.12b | 20.35 ± 0.23ab | 1.44 ± 0.07a | |
| T3 | 22.35 ± 0.71a | 1.59 ± 0.06a | 21.65 ± 0.22a | 1.45 ± 0.05a | |
| T4 | 21.94 ± 0.57a | 1.39 ± 0.03b | 21.91 ± 0.43a | 1.50 ± 0.07a | |
Note: Means of three replicates and standard deviations. Letters indicate significant differences between the crops at P < 0.05.
3.5. Soil microbial community abundance
Among the studied phylum classifications, Proteobacteria accounted for 34.83%–50.97%, Acidobacteria for 10.26%–15.50%, Bacteroidetes for 9.03%–12.93%, Nitrospirae for 4.73%–12.45%, and Gemmatimonadetes for 4.33%–7.40% (Fig. 3a). The various winter crops significantly increased the abundance of Proteobacteria and Bacteroidetes, and significantly decreased the abundance of Acidobacteria and Nitrospirae compared to the winter fallow. Winter crops significantly increased the abundance of bacterial phyla in the order of T2 > T4 > T3 > T1 > T0, by 3.39%, 2.87%, 2.64%, and 1.75%, respectively, compared to the winter fallow.
Fig. 3.
Effects of winter cover crops on the relative abundance of soil bacteria at the phylum and genus level. Note:Fig. 3a shows the histogram of the relative abundance of soil bacteria at the phylum level. The horizontal coordinate indicates the treatments of different winter cover crops; the vertical coordinate indicates the relative abundance of soil bacteria. Proteobacteria, Acidobacteria, Bacteroidetes, Nitrospirae, Gemmatimonadetes, Thaumarchaeota, Actinobacteria, Chloroflexi, Rokubacteria, and Crenarchaeota were the top 10 dominant bacteria at the phylum level. Others were the sum of the relative abundances of all other phyla. Fig. 3b shows the clustering map of the relative abundance of soil bacteria at the genus level. The top 35 dominant genera were selected based on bacteria annotation and abundance information of all samples at the genus level. Vertical is the sample information, horizontal is the bacteria annotation information, and the clustering tree on the left side of Fig. 3b is the bacteria clustering tree.
The highest relative abundance of unidentified_Rokubacteria, Geobacter, and unidentified_Deltaproteobacteria was found in the winter fallow treatment; Methylotenera, Pseudomonas, and Mycobacterium in the winter garlic treatment; and Cellvibrio and Bryobacter in the winter crop rotation treatment (Fig. 3b). Observed at the genus level, species abundance varied greatly between winter crops and winter fallow treatments, with winter garlic and crop rotation being the most pronounced.
3.6. Soil microbial community composition
The results of PCA analysis based on OTU levels showed that the soil microbial community composition differed significantly between the treatments (Fig. 4a). The soil microbial community composition under winter crops showed a large divergence in PC1 and PC2 compared to the winter fallow, which demonstrated the strong influence of winter crops on the soil microbial community. Large differences were also shown between winter milk vetch and garlic, while the differences between winter rape and crop rotation were smaller.
Fig. 4.
PCA and cluster analysis for soil microbial community composition under different winter cover crops. Note:Fig. 4a shows PCA scores for soil microbial community composition. PCA was performed on soil microbial community composition based on OTU levels. Percentage values in brackets indicate the variation explained by the principal components. Fig. 4b shows cluster analysis for soil microbial community composition. The clustering tree on the left side of Fig. 4b is based on the weighted unifrac distance UPGMA (Unweighted Pair-group Method with Arithmetic Mean). The right side of Fig. 4b shows the distribution of the relative abundance of soil bacteria at the phylum level for each treatment.
Cluster analysis at the phylum level revealed that the different treatments eventually clustered into two categories, one for the winter fallow treatment and one for the winter crop treatment, where winter milk vetch and crop rotation clustered into one category and winter rape and garlic clustered into another category among the winter crop treatments (Fig. 4b). It further showed that there was a great difference between the soil microbial community composition of winter fallow and crops.
3.7. Soil microbial community alpha diversity
The soil microbial community alpha diversity analysis between different treatments revealed that the Shannon diversity index of winter crops ranged from 9.75 to 9.91, while that of winter fallow was 9.38, and that of winter crops was in all cases extremely significantly higher than that of winter fallow (Fig. 5a). Furthermore, the Simpson's diversity index of winter crops ranged from 0.997 to 0.998, while that of winter fallow was 0.996, and that of winter crops was in all cases also extremely significantly higher than that of winter fallow (Fig. 5b). The results showed that winter crops significantly increased soil microbial community diversity in a double rice field.
Fig. 5.
Effects of winter cover crops on soil microbial community diversity. Note:Fig. 5a represents the Shannon diversity index under different treatments. Fig. 5b represents the Simpson diversity index under different treatments. Means of three replicates and standard deviations. The double asterisks above means indicate extremely significant differences at P < 0.01.
4. Discussion
Compared to winter fallow, winter milk vetch, rape, garlic, and crop rotation practice increased the late rice yield by 2.5%, 2.3%, 4.5% and 3.7%, respectively, and winter garlic and crop rotation also increased the early rice yield by 4.6% and 3.5%, respectively (Fig. 2). This illustrated that winter crop rotation can improve late rice yield, and winter garlic and crop rotation also have advantages in enhancing early crop yield. The increase in rice yield was directly attributed to the fact that the winter crop promoted rice tillering, thus increasing the effective panicle number of rice (Table 2). Furthermore, the indirect reason for the increase in crop yield may be that winter crops increase farmland biodiversity, soil carbon, and nitrogen content [16,21], thus improving soil microbial structure and increasing soil microbial diversity [19,22,23].
Legume winter crop (milk vetch) can apply more nitrogen by N-fixing for the subsequent crop [24]; non-legume winter crops (rape, garlic, potato) are most useful for suppressing weeds [25], controlling the loss of soil nutrients [26] and inputting crop residue [27] that can add soil organic carbon content; legume and non-legume cover crop rotation over many years further increases the biodiversity of farmland, and the input of crop residue will be more diversified, which can regulate the decomposition of high and low-quality residues [28] and provide a more balanced soil carbon and nitrogen supply [29], thus being more conducive to the regulation of soil C/N ratio [27]. This is why, compared to winter fallow, winter crop rotation intensification practice increased the soil organic carbon and total nitrogen content by 21% and 7%, respectively (Table 4).
Winter crops, compared to winter fallow, increased the abundance of bacteria (Fig. 3), changed soil microbial community composition (Fig. 4), and increased soil microbial community diversity (Fig. 5). Generally, soil microbial abundance and diversity were closely associated with soil environment changes: pH, temperature, moisture, cation exchange capacity, C/N ratio, crop residues, and soil organic carbon [30]. In this study, the rotation of various winter crops input diversified crop residues and released more kinds of root exudates, thus changing soil microbial structure and diversity [31]. Soil microbial diversity was found to be positively correlated with crop yields [32], potentially leading to more-efficient processing of soil nutrients and thus more nutrient uptake and subsequently higher yields. Therefore, our results show that winter cover crop rotation, as a biodiversity solution in double rice fields, can increase soil carbon sequestration, regulate soil C/N ratio, and improve soil microbial structure and diversity, which can keep soil healthy and sustainable, thus promoting rice tillering and enhancing crop yield stability.
Finally, we have only set up one winter crop rotation system in this study, which is still not enough to fully prove its advantages. In the future, it is necessary to design more diversified combinations of winter crop rotations, which will provide a better understanding of how to rotate with diversified cover crops and provide greater insight into their effectiveness.
5. Conclusions
-
i)
Winter crops can increase double rice yield, which is associated with the promotion of rice tillering.
-
ii)
Winter crops with the input of diversified residues, compared to winter fallow, contribute to soil carbon sequestration, improve soil microbial structure, and increase soil microbial diversity.
-
iii)
Winter crop rotation was not only 3.5% (early rice) and 3.7% (late rice) higher in terms of crop yield than winter fallow, but the soil organic carbon content also increased by 21%.
In conclusion, winter crop practices, especially winter crop rotation intensification, can largely keep soil healthy and sustainable for double rice fields through its positive feedback in terms of crop yield, soil carbon and microorganism.
Author contribution statement
QUAN ZHOU: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Peng Zhang: Performed the experiments; Analyzed and interpreted the data.
Zhiqiang Wang; Lixian Wang: Performed the experiments.
Shubin Wang; Guoqin Huang: Conceived and designed the experiments.
Wenting Yang; Binjuan Yang: Contributed reagents, materials, analysis tools or data.
Funding statement
Dr. QUAN ZHOU was supported by National Natural Science Foundation of China [31901476], Natural Science Foundation of Jiangxi Province [20202ACBL215002].
Guoqin Huang was supported by National Key Research & Development Program [2016YFD0300208].
Data availability statement
The authors do not have permission to share data.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Quan Zhou, Email: zhouquanyilang@163.com.
Peng Zhang, Email: zhangpeng20207@163.com.
Zhiqiang Wang, Email: wangzhiqiang06278143@sina.com.
Lixian Wang, Email: wanglixian0214@126.com.
Shubin Wang, Email: shubinwjxau@126.com.
Wenting Yang, Email: ywt111@163.com.
Binjuan Yang, Email: yangbinjuan@jxau.edu.cn.
Guoqin Huang, Email: hgqjxes@sina.com.
Abbreviations
- T0
Winter fallow
- T1
Winter milk vetch
- T2
Winter rape
- T3
Winter garlic
- T4
Winter rotation intensification with potato, milk vetch and rape
- C
Carbon
- SOC
Soil organic carbon
- N
Nitrogen
- TN
Total nitrogen
- GWP
Global warming potential
- PCA
Principal component analysis
References
- 1.Zhu B., Yi L., Xu H., Guo L., Hu Y., Zeng Z., Chen F., Liu Z. Non-leguminous winter cover crop and nitrogen rate in relation to double rice grain yield and nitrogen uptake in Dongting Lake Plain, Hunan Province, China. J. Integr. Agric. 2016;15:2507–2514. doi: 10.1016/S2095-3119(16)61331-X. [DOI] [Google Scholar]
- 2.Mandal B., Majumder B., Adhya T.K., Bandyopadhyay P.K., Gangopadhyay A., Sarkar D., Kundu M.C., Choudhury S.G., Hazra G.C., Kundu S., Samantaray R.N., Misra A.K. Potential of double-cropped rice ecology to conserve organic carbon under subtropical climate. Global Change Biol. 2008;14:2139–2151. doi: 10.1111/j.1365-2486.2008.01627.x. [DOI] [Google Scholar]
- 3.Xuan D.T., Guong V.T., Rosling A., Alström S., Chai B., Högberg N. Different crop rotation systems as drivers of change in soil bacterial community structure and yield of rice, Oryza sativa. Biol. Fertil. Soils. 2012;48:217–225. doi: 10.1007/s00374-011-0618-5. [DOI] [Google Scholar]
- 4.Sun M., Zhan M., Zhao M., Tang L.L., Qin M.G., Cao C.G., Cai M.L., Jiang Y., Liu Z.H. Maize and rice double cropping benefits carbon footprint and soil carbon budget in paddy field. Field Crop. Res. 2019;243 doi: 10.1016/j.fcr.2019.107620. [DOI] [Google Scholar]
- 5.Shi X., Hu K., Batchelor W.D., Liang H., Wu Y., Wang Q., Fu J., Cui X., Zhou F. Exploring optimal nitrogen management strategies to mitigate nitrogen losses from paddy soil in the middle reaches of the Yangtze River. Agric. Water Manag. 2020;228 doi: 10.1016/j.agwat.2019.105877. [DOI] [Google Scholar]
- 6.Zhang K., Maltais-Landry G., Liao H.-L. How soil biota regulate C cycling and soil C pools in diversified crop rotations. Soil Biol. Biochem. 2021;156 doi: 10.1016/j.soilbio.2021.108219. [DOI] [Google Scholar]
- 7.Dainese M., Martin E.A., Aizen M.A., Albrecht M., Bartomeus I., Bommarco R., Carvalheiro L.G., Chaplin-Kramer R., Gagic V., Garibaldi L.A., Ghazoul J., Grab H., Jonsson M., Karp D.S., Kennedy C.M., Kleijn D., Kremen C., Landis D.A., Letourneau D.K., Marini L., Poveda K., Rader R., Smith H.G., Tscharntke T., Andersson G.K.S., Badenhausser I., Baensch S., Bezerra A.D.M., Bianchi F.J.J.A., Boreux V., Bretagnolle V., Caballero-Lopez B., Cavigliasso P., Ćetković A., Chacoff N.P., Classen A., Cusser S., e Silva F.D. da S., de Groot G.A., Dudenhöffer J.H., Ekroos J., Fijen T., Franck P., Freitas B.M., Garratt M.P.D., Gratton C., Hipólito J., Holzschuh A., Hunt L., Iverson A.L., Jha S., Keasar T., Kim T.N., Kishinevsky M., Klatt B.K., Klein A.-M., Krewenka K.M., Krishnan S., Larsen A.E., Lavigne C., Liere H., Maas B., Mallinger R.E., Pachon E.M., Martínez-Salinas A., Meehan T.D., Mitchell M.G.E., Molina G.A.R., Nesper M., Nilsson L., O’Rourke M.E., Peters M.K., Plećaš M., Potts S.G., Ramos D. de L., Rosenheim J.A., Rundlöf M., Rusch A., Sáez A., Scheper J., Schleuning M., Schmack J.M., Sciligo A.R., Seymour C., Stanley D.A., Stewart R., Stout J.C., Sutter L., Takada M.B., Taki H., Tamburini G., Tschumi M., Viana B.F., Westphal C., Willcox B.K., Wratten S.D., Yoshioka A., Zaragoza-Trello C., Zhang W., Zou Y., Steffan-Dewenter I. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019 doi: 10.1126/sciadv.aax0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammed S., Hassan E., Abdo H.G., Szabo S., Mokhtar A., Alsafadi K., Al-Khouri I., Rodrigo-Comino J. Impacts of rainstorms on soil erosion and organic matter for different cover crop systems in the western coast agricultural region of Syria. Soil Use Manag. 2021;37:196–213. doi: 10.1111/sum.12683. [DOI] [Google Scholar]
- 9.Stipešević B., Kladivko E.J. Effects of winter wheat cover crop desiccation times on soil moisture, temperature and early maize growth. Plant Soil Environ. 2011;51:255–261. doi: 10.17221/3583-PSE. [DOI] [Google Scholar]
- 10.Frasier I., Noellemeyer E., Figuerola E., Erijman L., Permingeat H., Quiroga A. High quality residues from cover crops favor changes in microbial community and enhance C and N sequestration. Glob. Ecol. Conserv. 2016;6:242–256. doi: 10.1016/j.gecco.2016.03.009. [DOI] [Google Scholar]
- 11.Vukicevich E., Lowery T., Bowen P., Úrbez-Torres J.R., Hart M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016;36:48. doi: 10.1007/s13593-016-0385-7. [DOI] [Google Scholar]
- 12.Higashi T., Yunghui M., Komatsuzaki M., Miura S., Hirata T., Araki H., Kaneko N., Ohta H. Tillage and cover crop species affect soil organic carbon in Andosol, Kanto, Japan. Soil Tillage Res. 2014;138:64–72. doi: 10.1016/j.still.2013.12.010. [DOI] [Google Scholar]
- 13.Kim S.Y., Lee C.H., Gutierrez J., Kim P.J. Contribution of winter cover crop amendments on global warming potential in rice paddy soil during cultivation. Plant Soil. 2013;366:273–286. doi: 10.1007/s11104-012-1403-4. [DOI] [Google Scholar]
- 14.Zhu B., Yi L., Hu Y., Zeng Z., Lin C., Tang H., Yang G., Xiao X. Nitrogen release from incorporated 15N-labelled Chinese milk vetch (Astragalus sinicus L.) residue and its dynamics in a double rice cropping system. Plant Soil. 2014;374:331–344. doi: 10.1007/s11104-013-1808-8. [DOI] [Google Scholar]
- 15.Ren T., Bu R., Liao S., Zhang M., Li X., Cong R., Lu J. Differences in soil nitrogen transformation and the related seed yield of winter oilseed rape (Brassica napus L.) under paddy-upland and continuous upland rotations. Soil Tillage Res. 2019;192:206–214. doi: 10.1016/j.still.2019.05.008. [DOI] [Google Scholar]
- 16.González-Chávez Ma. del C.A., Aitkenhead-Peterson J.A., Gentry T.J., Zuberer D., Hons F., Loeppert R. Soil microbial community, C, N, and P responses to long-term tillage and crop rotation. Soil Tillage Res. 2010;106:285–293. doi: 10.1016/j.still.2009.11.008. [DOI] [Google Scholar]
- 17.Loranger-Merciris G., Barthes L., Gastine A., Leadley P. Rapid effects of plant species diversity and identity on soil microbial communities in experimental grassland ecosystems. Soil Biol. Biochem. 2006;38:2336–2343. doi: 10.1016/j.soilbio.2006.02.009. [DOI] [Google Scholar]
- 18.Cheng L., Leavitt S.W., Kimball B.A., Pinter P.J., Ottman M.J., Matthias A., Wall G.W., Brooks T., Williams D.G., Thompson T.L. Dynamics of labile and recalcitrant soil carbon pools in a sorghum free-air CO2 enrichment (FACE) agroecosystem. Soil Biol. Biochem. 2007;39:2250–2263. doi: 10.1016/j.soilbio.2007.03.031. [DOI] [Google Scholar]
- 19.McDaniel M.D., Grandy A.S. Soil microbial biomass and function are altered by 12 years of crop rotation. SOIL. 2016;2:583–599. doi: 10.5194/soil-2-583-2016. [DOI] [Google Scholar]
- 20.Wagner M.R., Lundberg D.S., Coleman-Derr D., Tringe S.G., Dangl J.L., Mitchell-Olds T. Natural soil microbes alter flowering phenology and the intensity of selection on flowering time in a wild Arabidopsis relative. Ecol. Lett. 2014;17:717–726. doi: 10.1111/ele.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poeplau C., Don A. Carbon sequestration in agricultural soils via cultivation of cover crops – a meta-analysis. Agric. Ecosyst. Environ. 2015;200:33–41. doi: 10.1016/j.agee.2014.10.024. [DOI] [Google Scholar]
- 22.D’Acunto L., Andrade J.F., Poggio S.L., Semmartin M. Diversifying crop rotation increased metabolic soil diversity and activity of the microbial community. Agric. Ecosyst. Environ. 2018;257:159–164. doi: 10.1016/j.agee.2018.02.011. [DOI] [Google Scholar]
- 23.Guo Z., Wan S., Hua K., Yin Y., Chu H., Wang D., Guo X. Fertilization regime has a greater effect on soil microbial community structure than crop rotation and growth stage in an agroecosystem. Appl. Soil Ecol. 2020;149 doi: 10.1016/j.apsoil.2020.103510. [DOI] [Google Scholar]
- 24.Etemadi F., Hashemi M., Zandvakili O., Dolatabadian A., Sadeghpour A. Nitrogen contribution from winter-killed Faba bean cover crop to spring-sown sweet corn in conventional and No-till systems. Agron. J. 2018;110:455–462. doi: 10.2134/agronj2017.08.0501. [DOI] [Google Scholar]
- 25.Campiglia E., Paolini R., Colla G., Mancinelli R. The effects of cover cropping on yield and weed control of potato in a transitional system. Field Crop. Res. 2009;112:16–23. [Google Scholar]
- 26.Askegaard M., Eriksen J. Residual effect and leaching of N and K in cropping systems with clover and ryegrass catch crops on a coarse sand. Agric. Ecosyst. Environ. 2008;123:99–108. doi: 10.1016/j.agee.2007.05.008. [DOI] [Google Scholar]
- 27.Li F., Sørensen P., Li X., Olesen J.E. Carbon and nitrogen mineralization differ between incorporated shoots and roots of legume versus non-legume based cover crops. Plant Soil. 2020;446:243–257. doi: 10.1007/s11104-019-04358-6. [DOI] [Google Scholar]
- 28.McDaniel M.D., Grandy A.S., Tiemann L.K., Weintraub M.N. Crop rotation complexity regulates the decomposition of high and low quality residues. Soil Biol. Biochem. 2014;78:243–254. doi: 10.1016/j.soilbio.2014.07.027. [DOI] [Google Scholar]
- 29.Singh G., Thilakarathne A.D.G.M., Williard K.W.J., Schoonover J.E., Cook R.L., Gage K.L., McElroy R. Tillage and legume non-legume cover cropping effects on corn–soybean production. Agron. J. 2020;112:2636–2648. doi: 10.1002/agj2.20221. [DOI] [Google Scholar]
- 30.Lienhard P., Tivet F., Chabanne A., Dequiedt S., Lelièvre M., Sayphoummie S., Leudphanane B., Prévost-Bouré N.C., Séguy L., Maron P.-A., Ranjard L. No-till and cover crops shift soil microbial abundance and diversity in Laos tropical grasslands. Agron. Sustain. Dev. 2013;33:375–384. doi: 10.1007/s13593-012-0099-4. [DOI] [Google Scholar]
- 31.Kim N., Zabaloy M.C., Guan K., Villamil M.B. Do cover crops benefit soil microbiome? A meta-analysis of current research. Soil Biol. Biochem. 2020;142 doi: 10.1016/j.soilbio.2019.107701. [DOI] [Google Scholar]
- 32.Garland G., Edlinger A., Banerjee S., Degrune F., García-Palacios P., Pescador D.S., Herzog C., Romdhane S., Saghai A., Spor A., Wagg C., Hallin S., Maestre F.T., Philippot L., Rillig M.C., van der Heijden M.G.A. Crop cover is more important than rotational diversity for soil multifunctionality and cereal yields in European cropping systems. Nat. Food. 2021;2:28–37. doi: 10.1038/s43016-020-00210-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.