Abstract
Introduction
Magnetic resonance imaging (MRI) T2* technique is used to assess iron overload in the heart, liver and pancreas of thalassaemic patients. Optimal iron chelation and expected tissue iron response rates remain under investigation. The objective of this study was to analyse serum ferritin and the iron concentration in the heart, liver and pancreas measured by MRI T2*/R2* during regular chelation therapy in a real-world cohort of patients with thalassemia.
Methods
We evaluated thalassaemic patients ≥ 7 years old undergoing chelation/transfusion therapy by MRI and assessed serum ferritin at baseline and follow-up from 2004-2011.
Results
We evaluated 136 patients, 92% major thalassaemic, with a median age of 18 years, and median baseline ferritin 2.033ng/ml (range: 59–14,123). Iron overload distribution was: liver (99%), pancreas (74%) and heart (36%). After a median of 1.2 years of follow-up, the iron overload in the myocardium reduced from 2,63 Fe mg/g to 2,05 (p 0.003). The optimal R2* pancreas cut-off was 148 Hertz, achieving 78% sensitivity and 73% specificity. However, when combining the R2* pancreas cut off ≤ 50 Hertz and a ferritin ≤ 1222 ng/ml, we could reach a negative predictive value (NPV) of 98% for cardiac siderosis. Only 28% were undergoing combined chelation at baseline assessment, which increased up to 50% on follow up evaluation.
Conclusions
Chelation therapy significantly reduced cardiac siderosis in thalassaemic patients. In patients with moderate/severe liver iron concentration undergoing chelation therapy, ferritin levels and myocardium iron improved earlier than the liver siderosis.
Keywords: Iron overload, Thalassemia, Magnetic resonance imaging, Chelation therapy
Introduction
Advances on transfusional therapy and iron chelation for thalassaemic patients improved the all-cause mortality rate from 22.8/1000 patients-year until 1960, to up to 4/1000 patients-year after 2000.1 Despite that, cardiac disease continues to be an important cause of morbidity and the leading cause of death in patients with thalassemia major.1 Thus, early detection of myocardial iron overload is a main issue among the patients. However, myocardial iron overload evaluation must not be based only on serum ferritin, because it does not have a good correlation with myocardium iron2 and could result in delayed diagnosis when patients are already suffering from heart failure and arrhythmias.1,2
The optimal iron chelation and monitoring tools to prevent and treat cardiac complications due to iron overload remain under investigation.1,2,3 Nevertheless, magnetic resonance imaging (MRI) T2* techniques have been increasingly validated as a valuable non-invasive tool to measure the iron concentration in the liver, heart and pancreas.3 The optimal MRI technique, equipment and software are not well established, and this field is advancing quickly. Signal intensity ratio (SIR) and T2 relaxometry have been the most used techniques.4 Recently, even more sophisticated MRI assessments have been developed, which add, for example, multi-echo single voxel spectroscopy (HISTO/HISTOV) to the evaluation. These techniques can differentiate liver fat from iron content more accurately.4
However, to assess organ iron load, MRI techniques had to overcome some challenging issues. For cardiac iron overload, the constant heart movement, blood flow shifts and reproducibility of the technique as a whole were problematic before MRI T2* methods were ultimately validated to measure myocardium iron.3,5,6,7 For the pancreas, studies suggested that the assessment of iron would be more accurate using the pancreas body rather than its head.8
The reported correlations between ferritin and MRI T2* assessments are heterogeneous in the literature. Some studies observed that ferritin as moderately correlated with the liver iron concentration (LIC), while lower correlation rates for heart and pancreas have been reported.9 Others, that pancreatic iron can be moderately correlated with myocardium iron concentration, and also with age, and it could increase the positive predictive value for diabetes detection.10,11,12 Some differences regarding the iron transport mechanisms/homeostasis of each organ and nonspecific ferritin oscillations related to inflammatory/hepatic injuries might affect these unexpected correlations.10,11,12,13
At the same time, many trials and a systematic review have investigated the efficacy and safety of oral iron chelators (deferasirox and deferiprone) used alone or in combination with deferoxamine in thalassaemic regularly transfused patients.14,15,16,17 In this new scenario, treatment guidelines for patients with thalassemia evolved to incorporate MRI as a diagnostic/monitoring tool of iron overload as this could guide more accurately the intensification of chelation therapy.17,18
Encouraged by these advances on iron MRI assessment, better iron chelation options and based on international guidelines; Brazilian specialized centres on thalassemia care also designed treatment protocols to guide iron chelation. However, as Brazil is a very large country, with huge heterogeneity of health resources, modest health care budgets, and a population with more cultural and social diversity, we face challenging situations that might not be observed in other countries. The genetic and structural diversity might provide some pragmatism to studies developed in this country, though, and we decided to conduct this study.
Objective
We aimed to analyse the performance of chelation protocols in Brazilian patients, conducting a prospective observational “real-world” study. With that purpose, we assessed the correlations between organ iron concentrations assessed by T2* MRI and serum ferritin levels at baseline and longitudinally during chelation therapy in thalassemia major or intermedia patients.
Methods
Design, settings and ethics
This is a national multicentre cross-sectional study. We collected data prospectively from a convenience sample consisting of all consecutive patients diagnosed and treated for thalassemia major or intermedia recruited from seven Brazilian hematologic centres from 2004 until 2011. These patients were treated locally and referred to our service, a private hospital in São Paulo, SP, Brazil, for MRI and clinical evaluation only.
The study was funded by the Brazilian Programme Supporting the Institutional Development (PROADI) by the Brazilian Ministry of Health and the National Health System. All the patients studied signed informed consent forms and the procedures were approved by the Hospitals Ethics Committees. This study was conducted in accordance with the Helsinki Declaration as revised in 2008.
Participants
All consecutive thalassaemic patients admitted in the participant centres were considered eligible for this study under the following conditions:
-
‐
age ( 7 years;
-
‐
thalassemia major or intermedia patients;
-
‐
treated according to the Brazilian clinical guidelines14,15 based on regular packed red cell transfusions according to the severity of iron overload;
-
‐
chelation therapy based on the Brazilian protocol or with an indication for this treatment;14
-
‐
submitted to MRI T2* exam to evaluate pancreas, heart and liver;
-
‐
serum ferritin evaluated at admission and at least six months later (minimum follow-up of six months);
-
‐
signed informed consent forms.
Exclusion criteria were these situations:
-
‐Pacemaker carrier;
-
‐Intracranial aneurysm clipping carrier;
-
‐Claustrophobia;
-
‐Pregnancy;
-
‐History of noncompliance to chelation therapy.
-
‐
MRI technique and ferritin measurements
The MRI equipment used to assess tissue iron content consisted of 1,5T scanner (General Electric Healthcare - Milwaukee, Wisconsin - USA) with the combination of body-matrix and spine-matrix surface coils. The myocardium sequences measurements were analysed using the ReportCard V3.3 (General Electric Healthcare - Milwaukee, Wisconsin- USA). Multi-echo segmented fast gradient echo pulse (MFGRE) sequence was used with the following parameters: FOV 44 cm, PFOV = 0,7, slice thickness 10 mm, spacing 8.0 mm, 4 slices, TR = 34 ms, FLIP 20 degrees, TEmin = 2 ms, TEmax = 16 ms, 8 echoes with 2 ms inter echo, frequency matrix = 128, phase matrix = 128, NEX = 1, receiver bandwidth = 62.5 kHz; post-processing. T2* decay fitting was performed on Report Card using the following formula: S = A*e (-TE/T2*) + C. The initial scaling factor was 25 ms, and 2 parameter fit was used (C = 0). The following equation was used to calculate hepatic iron concentration (LIC): [Fe]R2* = 0.0254 x R2* + 0.202. The same parameters were used employed for cardiac iron assessment, using short-axis view focused on the myocardial interventricular septum.
The hepatic and pancreatic relaxation times at T2* MRI were assessed at the transversal plane of the upper abdomen. Regarding the liver, an experienced radiologist selected a region from the right liver lobe in segments 6 and 7 to avoid vessels and possible artefacts.19 Paraspinal musculature signal intensity was also assessed to calculate the signal intensity ratio (SIR) between these muscles and the pancreas.18,20
The severity of iron overload on each organ was classified for each organ and technique, according to Supplementary Table 1.3,10,12,20,21,22 The radiologic report was done by two experienced radiologists.
In this study, “improvements” of iron overload correspond to the difference (Δ) between initial and final measurements in a given period. It is expressed as milliseconds (ms), with the final measure being higher than the initial one (thus a positive value), or in milligrams of iron (Fe mg/g), where it is expected that the final measure be lower than the initial (negative value).
The serum ferritin measures were done by chemiluminescence.10
Potential sources of bias
All lab tests and MRI assessments were performed at our service, using the same equipment. MRI tests were analysed by two experienced radiologists who mutually agreed to the calculation of T2*. In cases where images had shown artefacts, which prevented further analysis, those patients were excluded, and results were not recorded.
All patients had been assisted by their own treating physicians at each centre, who referred them to our service for the tests (MRI and laboratory) and medical evaluation at least every six months to record all relevant clinical information (clinical symptoms, medications/chelation, transfusion requirement etc.). Therefore, our centre did not interfere with treatments. Each centre assistant physicians were allowed to assess the MRI and lab tests results, so the treatment choice was not blinded. These physicians received general guidance to follow the Brazilian chelation protocol, but each physician, according to his/her discretion, could adjust the patient's treatments according to specific individual characteristics and tolerability. They also were required to inform our Hospital about any modification on the chelation therapy or patient treatment during the follow-up. In consequence, we could not ascertain strict compliance (assistant physician-patient) to the national guidelines for treatment, which limited a reliable comparison between each type of iron chelation and explains why some patients, although eligible, were not undergoing combined chelation.
In general, the Brazilian protocol14,15 recommends chelation therapy with desferrioxamine or deferasirox to patients with hepatic iron overload and combined therapy (desferrioxamine plus deferiprone) to those with concomitant cardiac iron overload.
Variables and statistical analysis
The variables under investigation were:
-
•
Serum ferritin levels (ng/ml)
-
•
T2* MRI (results expressed in milliseconds, ms)
-
•
R2* (hertz) to assess pancreatic iron; formula R2* = 1000/T2*
-
•
Fe mg/g dry weight liver to assess LIC; [Fe]R2* = 0.0254xR2* + 0.202
-
•
Fe mg/g dry weight to assess myocardium iron, using the Carpenter equation [Fe]= 45.0•(T2*)−1,11
-
•
Severity and proportions of iron overload according to the thresholds established in the literature (Supplementary Table 1) for MRI.
-
•
Δ ferritin/year (ng/ml/year) and ΔR2*/year (hertz/year): were defined as the difference between the final and the initial ferritin or R2* organ measurement rationalized by the time (year)
-
•
Δ Fe mg/g/year: was defined as the difference between the final and the initial Fe mg/g dry weight organ measurement rationalized by the time (year)
The continuous variables (serum ferritin:, LIC, myocardium T2*, and pancreatic R2*) were described by medians, means, range and standard deviation. The Shapiro-Wilk method was applied to test for normality distribution. The correlations between those variables were analysed using the Spearman's rank test. The median variations (Δ) on iron concentrations were adjusted by the time (year) elapsed from initial to final evaluation. The Kruskal-Wallis independent test was used to compare iron variations (Δ) during follow-up between more than two chelation treatment groups.
The Wilcoxon paired test was used to compare these variables at baseline and follow-up in the same group. While, the Mann-Whitney unpaired test was used to compare those variables between different patient groups.
Compliance was assessed indirectly. Firstly, we separated patients with complete data available in two groups according to the median follow-up: short-term (1.2 years) versus long-term (5.2 years), then we compared the iron parameters as described above. Also, we analysed the estimated incidence ratios of new cases of cardiac or pancreatic iron overload.
The non-parametric receiver operating characteristic (ROC) curve was applied to test the accuracy of serum ferritin and pancreatic R2* thresholds to predict cardiac iron overload. The STATA software version 11 and SPSS version 24 were used for the statistical analysis.
Results
Patients' characteristics
In the study period, from a total of 211 patients enrolled in the study, we included 157 that we assessed at baseline and at least once more during follow up (54 were excluded due to missing final MRI results). However, from the 157 elected patients, we excluded further 21 because medical records were incomplete (lacking data from the second evaluation mostly).
The baseline clinical features of the 136 included patients were: major thalassemia (126/92,5%) and intermedia thalassemia (10/7,5%); male (47/34%) and female (89/66%); median age of 18 years old (range 7-55); median follow up time of 1.2 years (range 0.6–5.2 years); median serum ferritin of 2033 ng/ml (range 59–14.123). The proportion of patients observed on each chelation treatment was: desferrioxamine (51/37.5%), combined therapy (38/28%), deferasirox (35/26%) and deferiprone alone (12/8.5%). Only 16/57 (28%) of patients with myocardium iron overload were being treated with combined iron chelation at the baseline evaluation. During long-term follow-up, this proportion increased to 16/33 (50%).
At baseline evaluation, the organ most commonly affected by iron overload was the liver, affecting 156 (99,3%) of the patients, followed by pancreas 117 (74,3%) and heart 57 (36%). Supplementary Table 2 summarizes the distribution and severity of iron concentration in each organ.
Follow up of iron overload and ferritin during chelation therapy
Table 1 shows the MRI measures of iron concentration in each organ and serum ferritin at baseline and during follow up (median 1.2 years; range 0.6 to 5.2) on patients being treated with iron chelation. We observed a significant decrease in serum ferritin (median 2033 to 1735; p = 0.001) and myocardium Fe (median 0.86 to 0.77; p = 0.024), while we did not detect any significant improvement in LIC and pancreas R2*.
Table 1.
Iron overload and ferritin kinetics following 1.2 years (median) of chelation in thalassemic patients (n = 136).
| Baseline Median (range/SD) | Follow-up Median (range/SD) | p-valuea | |
|---|---|---|---|
|
Myocardium (Fe mg/g) |
0.86 (0.22–12.8/1.95) | 0.77 (0.34-17.2/2.0) | 0.024 |
|
Ferritin (ng/ml) |
2033 (59–14123/2347) | 1735 (180-11925/2148) | 0.001 |
|
LIC (Fe mg/g) |
8.4 (1.0–51.0/9.4) | 8.14 (1.1-25.0/6.0) | 0.118 |
|
R2* pancreas (Hertz) |
136.0 (19–526/120) | 116.9 (19 –1000/152) | 0.512 |
SD: standard deviation; LIC: liver iron concentration.
Paired Wilcoxon-Mann-Whitney test
As illustrated in Table 2, we found statistically significant moderate (0.527) correlations between LIC and ferritin parameters and also between R2* pancreas and Fe mg/g myocardium (0.476).
Table 2.
Correlations between iron parameters at baseline (n = 136).
| Rho Spearman correlations | R2⁎ pancreas | LIC | Ferritin | Myocardium | |
|---|---|---|---|---|---|
| R2⁎ pancreas (Hertz) |
Coefficient | ——- | 0.296a | 0.338a | 0.476a |
| P-value | ——- | 0.000 | 0.000 | 0.000 | |
| LIC (Fe mg/g) |
Coefficient | 0.296a | ——— | 0.527a | 0.323a |
| P-value | 0.000 | ——— | 0.000 | 0.000 | |
| Ferritin (ng/ml) |
Coefficient | 0.338a | 0.527a | ——– | 0.329a |
| P-value | 0.000 | 0.000 | ——– | 0.000 | |
| Myocardium (Fe mg/g) |
Coefficient | 0.476a | 0.323a | 0.329a | ——— |
| P-value | 0.000 | 0.000 | 0.000 | ——— | |
LIC: liver iron concentration.
The correlation was considered significant if ≤ 0.01 (bilateral).
As shown in Table 3, the subgroup of patients (n = 50) with cardiac iron overload had a significant improvement in the myocardium Fe mg/g, reaching a median reduction of 0.27 Fe mg/g per year (p = 0.003). The majority of patients improved the myocardium iron concentration, corresponding to 38/50 (75%).
Table 3.
Iron response (Δ) following chelation in the myocardium overloaded subgroup
| Number of patients(n = 50) |
Baseline (Fe mg/g) |
Follow-up (Fe mg/g) |
Δ(Fe mg/g) per year | p-value |
|---|---|---|---|---|
| Mean (SD) | 3.4 (2.3) | 3.0 (2.7) | - 0.3 (0.9) | 0.003 |
| Median | 2.63 | 2.05 | - 0.27 | |
| Range | 1.1–12.8 | 0.5–17.1 | -2.5–2.0 |
We also analysed the heart using T2* MRI (ms), which resulted in a significant median improvement of 1 ms/year (range 0.4–2.1ms/year). For the R2* pancreas and LIC, we did not observe a significant iron load improvement during follow up.
Analysing each iron-chelating therapy in the short-term follow up (median 1.2 years), we did not find any differences between combined, desferrioxamine, deferasirox and deferiprone therapies in neither iron parameter, as shown in Table 4.
Table 4.
Evolution (Δ) of iron concentration in the myocardium, liver (LIC) and pancreas (R2*) according to chelating therapy (n = 136).
|
Myocardium (Δ Fe mg.g/year) |
Combined deferiprone + deferrioxamine (n = 37) | Deferasirox (n = 32) | Deferiprone (n = 14) | Deferrioxamine (n = 53) |
|---|---|---|---|---|
| Mean (SD) | -0.125 (0.68) | -0.206 (0.54) | -0.077 (0.60) | -0.028 (0.63) |
| Median | -0.034 | -0.087 | 0.017 | -0.053 |
| Interquartile (25/75) | -0.358/0.121 | -0.315/0.092 | -0.315/0.092 | -0.239/0.303 |
| p-valuea | 0.685 | |||
| LIC (Δ Fe mg.g/year) | Combined deferiprone + deferrioxamine | Deferasirox | Deferiprone | Deferrioxamine |
| Mean (SD) | 0.311 (3.01) | -5.224 (12.02) | 0.568 (6.77) | 1.211 (5.65) |
| Median | 0.000 | -0.835 | -0.691 | -0.226 |
| Interquartile (25/75) | -1.252/1.261 | -2.615/1.297 | -1.591/-0.078 | -1.484/0.885 |
| p-valuea | 0.107 | |||
| Pancreas (R2* ΔHertz/year) | Combined deferiprone + deferrioxamine | Deferasirox | Deferiprone | Deferrioxamine |
| Mean (SD) | 19.364 (110.6) | -8.652 (63.3) | -4.496 (40.2) | -10.820 (80.3) |
| Median | -2.704 | -2.004 | 0.00000 | 0.246 |
| Interquartile (25/75) | -25.278/24.960 | -39.594/28.413 | -27.226/10.665 | -19.111/25.444 |
| p-valuea | 0.998 | |||
Kruskal-Wallis test.
Diagnostic accuracy on cardiac iron overload
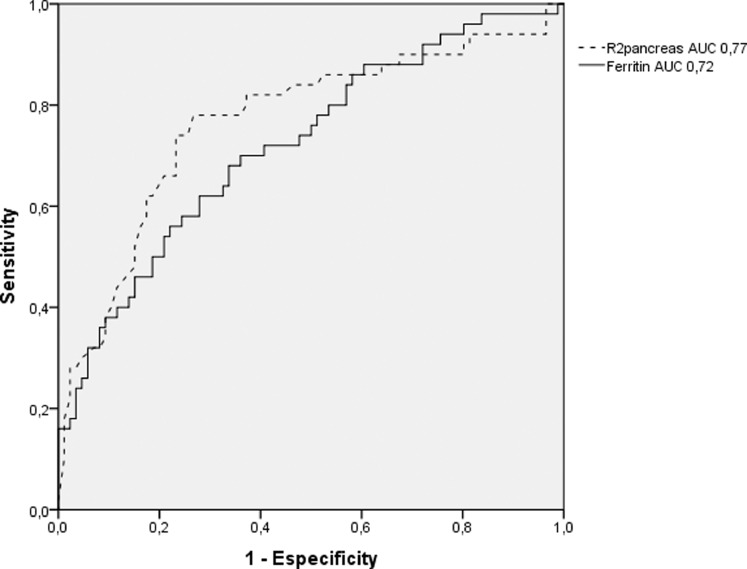
Because we observed a moderate correlation between R2* pancreas and myocardium Fe mg/g and considering that ferritin could be a very sensitive parameter of iron overload, we performed a non-parametric ROC analysis using these variables. We observed that R2* pancreas or ferritin alone would not be enough to guide us for myocardium iron. The optimal R2* pancreas cut-off was 148 Hertz, achieving 78% sensitivity and 73% specificity (Figure 1). However, when combining the R2* pancreas cut off ≤ 50 Hertz and a ferritin ≤ 1222 ng/ml, we could reach a negative predictive value (NPV) of 98% for cardiac siderosis.
Figure 1.
Diagnostic accuracy of R2* pancreas (dashed line) and serum ferritin (solid line) assessments to predict myocardium iron overload.
Impact of chelation compliance
As compliance is a critical issue affecting the effectiveness of iron chelation, we indirectly assessed the adherence to treatment verifying if patients with a longer follow-up could achieve better iron parameters after chelation than those with a short-term. Our follow-up compliance seems to be much lower than the optimal target (> 80%). We had complete data from 136 patients at a median of 1.2 years (short term), but from only 33 patients at 5.2 years of follow-up (long term).
We compared the Δ Fe mg/g in the myocardium in the subgroup of 33 patients that had been compliant to MRI assessments and persisted with the same chelation therapy (i.e., a “long-term” subgroup) with the patients in the “short-term” subgroup. We observed that the median improvement rates were not significant different: -0.035 Δ Fe mg/g/year (equivalent T2* rate of +0.9 ms/year) in the long-term versus -0.023 Δ Fe mg/g/year in the short-term (p = 0.611). Half of those patients followed on long-term were using combined chelation, 40% desferrioxamine and 10% deferiprone.
Furthermore, to indirectly assess compliance, we also analysed the estimated annual incidence rates of new cases diagnosed with pancreatic or myocardium siderosis at follow-up, as one would not expect new cases of iron overload following adequate chelation. We found incidence rates of new cases of 6,1% (95% IC: 3,0-12,3%) in the myocardium and 16,4% (95%IC: 6,8-39%) in the pancreas.
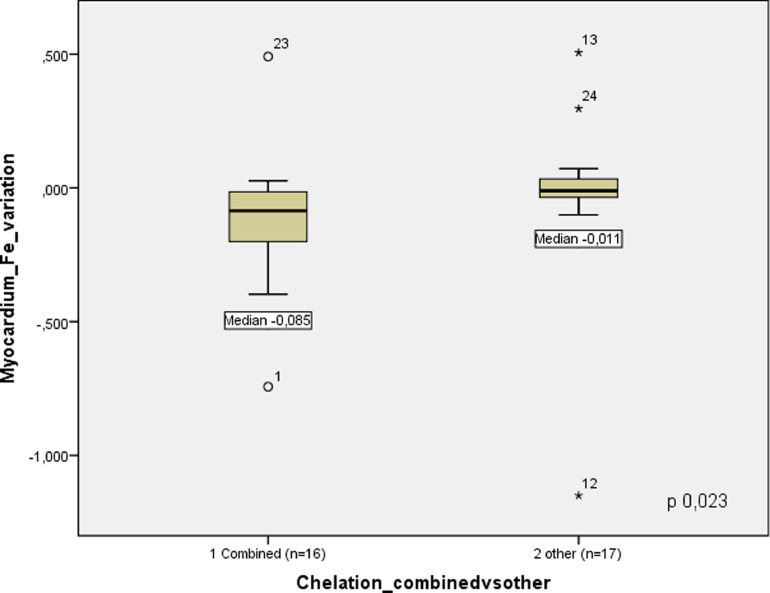
We also compared if patients doing combined iron chelation were performing better than those with other chelators, and we found a significant difference in the median rate of myocardium iron overload improvement (Δ Fe mg/g/year): -0.085 in combined versus -0.011 in other chelating therapies (p 0.023), as illustrated in Figure 2.
Figure 2.
Long-term (median 5.2 years) follow-up of myocardium iron concentration according to chelation therapy: combined versus other types.
Comparing each organ iron improvement between the subgroups with longer versus shorter follow-up, as shown in Supplementary Table 3, we could not observe a significant difference in iron overload measures.
Discussion
The results from this study and others show that almost all patients with major thalassemia on regular transfusion regimens will have some degree of siderosis at the median age of 18 years.20 Almost all patients in our cohort (99%) suffered from hepatic iron overload at baseline assessment, followed by pancreatic (74,3%) and myocardium (36%) iron overload. Moreover, our observed annual incidence rate of pancreatic siderosis was superior to that in the myocardium (16,4% versus 6,1%). When taken together, these findings suggest that organ iron deposits overtime are usually occurring, as previously described in the literature,22 first in the liver, then in the pancreas and finally in the heart.
Serum ferritin is a sensitive exam for iron overload. However, its variations may not reflect the iron concentrations increases in each organ directly, as it is influenced by other factors such as inflammation and liver injuries. In spite of that, we observed a significant decrease in ferritin levels with treatment. Moreover, this serum decrease occurred earlier than the iron overload in the liver (LIC), as shown in Table 1, possibly because as it has the capacity to estimate the total body iron, serum ferritin levels also reflect the improvements in other organs altogether.
Many studies have demonstrated that the correlation between myocardium and pancreatic iron assessments range from moderate to good, which is also a better correlation than with the ferritin.8,10,11,12,20 As one could observe in Table 2, we also found a moderate correlation between ferritin and LIC (r = 0.527), reproducing the correlation rates found by the ASIMILA study in non-thalassaemic patients with sickle cell or myelodysplastic diseases (r = 0.47/r = 0.61).9 Furthermore, to our knowledge, no study could objectively establish optimal cut-offs to predict cardiac iron. We were especially interested in this issue because we envisaged that we could design a more cost-effective algorithm based on this data, that could perform better in low incoming countries. In this context, centres would first perform an abdominal MRI iron assessment (liver and pancreas) and combine those findings with the ferritin, guiding them to optimally select patients to proceed with a cardiac T2* MRI. We observed that using R2* pancreas alone would offer an optimal cut-off of 148 hertz, reaching a 78% sensitivity and 73% specificity (Figure 1). However, when combining the R2* pancreas cut-offs of ≤ 50 hertz and ferritin ≤1222 ng/ml, we could reach a negative predictive value (NPV) of 98% for cardiac siderosis. In this subset of patients, clinicians might not need to request cardiac MRI T2*.
Our finding of a median T2* myocardium improvement rate of + 0.9 (range 2.1 to 0.4) ms/year following iron chelation was inferior to rates reported in the literature.23,24,25,26 One randomized trial described median improvement rates of +1.5 ms/year versus 1.24 ms/year for combined therapy and desferrioxamine alone, respectively.14,24,25,26,27 There is some heterogeneity in the literature regarding the patient cohorts, the baseline myocardium iron levels and how to report the iron improvement rates. Nevertheless, the rates range from +0.5 ms/year to +2.6 ms/year only.14,24,25,26,27,28
Notably, Casale et al. reported real-world results of MRI T2* myocardium improvement of 99 thalassaemic patients after 6.9 (mean) years on deferasirox chelation, therefore observing an improvement of +2.6 ms (±11.9 ms; P = 0.035), even better in myocardium overload subgroup +11.6 ms (±15.5 ms, P = 0.019). However, in that study, it was not reported in a time-adjusted unit.27,28 By adjusting for time, one could estimate improvement rates ranging from +0.4 to +1.6 ms/year. Furthermore, interpreting the study by Casale et al. deeply, we could calculate these rates in a subgroup of 13 patients with abnormal cardiac function in Casale et al. study,28 which would result in a median rate of + 0.67 ms/year (range +10.6 ms-1.73 ms). These rates are both closer to our findings, which however are still inferior from clinical trials outcomes, probably due to compliance issues22 that become more evident in the real-world setting. Reinforcing the observed compliance problem, we observed new cases of pancreatic and myocardium siderosis despite the ongoing iron chelation therapy: an unfavourable outcome that reflects treatment failure, rarely reported by chelation trials.
The median LIC improvement was -0.4 mg Fe/g/year in our study, while the literature has demonstrated improvement rates as high as -1.5 mg Fe/g/year.24 It seems that hepatic improvement occurs slower and latter than in the myocardium. We hypothesized that it could be due to differences on iron kinetics of each organ,29,30 considering that heart has proportionally lower macrophagic (reticuloendothelial system) iron stores, is less influenced by the enterobiliary tract recycling and favourably transports the circulating non-transferrin-bound iron (NTBI).29,30 Some experts argued that when there is cardiac iron overload, clinicians and patients become aware to this dangerous and potentially fatal condition, which leads to greater compliance to treatment and better chelation than in the subgroup with only hepatic involvement.17 This hypothesis might explain why we observed iron improvement only in the subgroup with cardiac siderosis.
Compliance to chelation treatment might be a source of bias in this study. The short follow-up period in this multicentre study might have resulted in underestimation of compliance; i.e., we would need more time to see iron overload reduction. However, this does not diminish the value of MRI T2* as a tool for monitoring iron overload across time, regardless of chelation therapy type or time.
Transfusion load and its relationship with iron overload is another potential source of confusion which we cannot discard. The correlation between transfusion load and iron overload over time should be evaluated in future studies.
Overall, MRI T2* is a useful tool to guide chelation therapy, but it still has some limitations mainly in tissues with high-fat content, such as in liver (due to steatosis), and the pancreas. In the near future, we might be incorporating new MRI techniques such as HISTO/HISTOV4 to solve the interference of fat tissue during iron load assessments.
Conclusion
Young adults with major thalassemia treated with regular transfusion regimens have a high prevalence of hepatic (99%) and cardiac siderosis (36%). Chelation therapy significantly reduced cardiac siderosis in thalassaemic patients. In the subset of patients with moderate/severe liver iron concentration undergoing chelation therapy, the ferritin levels and myocardium iron overload appear to improve earlier than liver iron. Patients and assistant physicians may be more compliant to iron chelation when there is heart iron overload, as it is a more severe scenario, which could justify why liver iron was not improving as expected.
Supplementary Table 1. Iron overload severity criteria 6,20,22,34-36
Supplementary Table 2. Baseline iron overload distribution in thalassemic patients (n = 157)
Supplementary Table 3. Impact of short-term versus long-term follow-up on iron overload improvement
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
The study was funded by the Brazilian Programme Supporting the Institutional Development (PROADI) by the Brazilian Ministry of Health and the National Health System.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.htct.2021.01.014.
Appendix. Supplementary materials
References
- 1.Betts M, Flight PA\, Paramore LC, Tian L, Milenković D, Sheth S. Systematic literature review of the burden of disease and treatment for transfusion-dependent β-thalassemia. Clin Ther. 2019;42(2) doi: 10.1016/j.clinthera.2019.12.003. 322–337.e2. [DOI] [PubMed] [Google Scholar]
- 2.Davis BA, Porter JB. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood. 2000;95(4):1229–1236. [PubMed] [Google Scholar]
- 3.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22(23):2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 4.Fu C, Kannengiesser S, Cheng S, Shen J, Dong H, Yan F. Quantitative analysis of hepatic iron in patients suspected of coexisting iron overload and steatosis using multi-echo single-voxel magnetic resonance spectroscopy: Comparison with fat-saturated multi-echo gradient echo sequence. J Magn Reson Imaging. 2018 Jul;48(1):205–213. doi: 10.1002/jmri.25967. [DOI] [PubMed] [Google Scholar]
- 5.Tanner MA, He T, Westwood MA, Firmin DN, Pennell DJ, Thalassemia International Federation Heart T2* Investigators Multi-center validation of the transferability of the magnetic resonance T2* technique for the quantification of tissue iron. Haematologica. 2006;91(10):1388–1391. [PubMed] [Google Scholar]
- 6.Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, et al. Calibration of myocardial T2 and T1 against iron concentration. J Cardiovasc Magn Reson. 2014;16:62. doi: 10.1186/s12968-014-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, et al. On T2* magnetic resonance and cardiac iron. Circulation. 2011;123(14):1519–1528. doi: 10.1161/CIRCULATIONAHA.110.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meloni A, Restaino G, Missere M, De Marchi D, Positano V, Valeri G, et al. Pancreatic iron overload by T2* MRI in a large cohort of well treated thalassemia major patients: can it tell us heart iron distribution and function? Am J Hematol. 2015;90(9):E189–E190. doi: 10.1002/ajh.24081. [DOI] [PubMed] [Google Scholar]
- 9.Watman NP, Lobo C, Chona Z, Manzur F, Traina F, Park M, Drelichman G, Zarate JP, Marfil L. Assessment of liver and cardiac iron overload using MRI in patients with chronic anemias in Latin American countries: results from ASIMILA study. Hematology. 2018 Oct;23(9):676–682. doi: 10.1080/10245332.2018.1461292. [DOI] [PubMed] [Google Scholar]
- 10.de Assis RA, Ribeiro AA, Kay FU, Rosemberg LA, Nomura CH, Loggetto SR, et al. Pancreatic iron stores assessed by magnetic resonance imaging (MRI) in beta thalassemic patients. Eur J Radiol. 2012;81(7):1465–1470. doi: 10.1016/j.ejrad.2011.03.077. [DOI] [PubMed] [Google Scholar]
- 11.Noetzli LJ, Papudesi J, Coates TD, Wood JC. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood. 2009;114(19):4021–4026. doi: 10.1182/blood-2009-06-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Au WY, Lam WW, Chu W, Tam S, Wong WK, Liang R, et al. A T2* magnetic resonance imaging study of pancreatic iron overload in thalassemia major. Haematologica. 2008;93(1):116–119. doi: 10.3324/haematol.11768. [DOI] [PubMed] [Google Scholar]
- 13.Noetzli LJ, Carson SM, Nord AS, Coates TD, Wood JC. Longitudinal analysis of heart and liver iron in thalassemia major. Blood. 2008;112(7):2973–2978. doi: 10.1182/blood-2008-04-148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pennell DJ, Porter JB, Piga A, Lai Y, El-Beshlawy A, Belhoul KM, et al. A 1-year randomized controlled trial of deferasirox vs deferoxamine for myocardial iron removal in β-thalassemia major (CORDELIA) Blood. 2014;123(10):1447–1454. doi: 10.1182/blood-2013-04-497842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassinerio E, Orofino N, Roghi A, Duca L, Poggiali E, Fraquelli M, et al. Combination of deferasirox and deferoxamine in clinical practice: an alternative scheme of chelation in thalassemia major patients. Blood Cells Mol Dis. 2014;53(3):164–167. doi: 10.1016/j.bcmd.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Veríssimo MP, Loggetto SR, Fabron Junior A, Baldanzi GR, Hamerschlak N, Fernandes JL, Araujo Ada S, Lobo CL, Fertrin KY, Berdoukas VA, Galanello R. Brazilian Thalassemia Association protocol for iron chelation therapy in patients under regular transfusion. Rev Bras Hematol Hemoter. 2013;35(6):428–434. doi: 10.5581/1516-8484.20130106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappellini MD, Cohen A, Eleftheriou A, Piga A, Porter J, Taher A. Thalassemia International Federation (TIF); Nicosia: 2008. Guidelines for the Clinical Management of Thalassaemia. 2nd Revised edition. [PubMed] [Google Scholar]
- 18.Wood JC. Guidelines for quantifying iron overload. Hematology Am Soc Hematol Educ Program. 2014;2014(1):210–215. doi: 10.1182/asheducation-2014.1.210. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani LF, Santos FPS, Perini GF, Nascimento CMB, Silva LP, Wroclawski CK, Esposito BP, Ribeiro MSS, Velloso EDRP, Nomura CH, Kay FU, Baroni RH, Hamerschlak N, Schuster S. Hepatic and cardiac and iron overload detected by T2* magnetic resonance (MRI) in patients with myelodisplastic syndrome: A cross-sectional study. Leuk Res. 2019 Jan;76:53–57. doi: 10.1016/j.leukres.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Papakonstantinou O, Ladis V, Kostaridou S, Maris T, Berdousi H, Kattamis C, et al. The pancreas in beta-thalassemia major: MR imaging features and correlation with iron stores and glucose disturbances. Eur Radiol. 2007;17(6):1535–1543. doi: 10.1007/s00330-006-0507-8. [DOI] [PubMed] [Google Scholar]
- 21.Assis RA, Kay FU, Conti FM, Campregher PV, Szarf G, Diniz MS, Rodrigues M, Helman R, Funari MB, Wood J, Hamerschlak N. The role of magnetic resonance imaging-T2* in the evaluation of iron overload early in hereditary hemochromatosis. A cross-sectional study with 159 patients. Am J Hematol. 2015 Dec;90(12):E220–E221. doi: 10.1002/ajh.24189. [DOI] [PubMed] [Google Scholar]
- 22.Gandon Y, Olivié D, Guyader D, Aubé C, Oberti F, Sebille V, et al. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363(9406):357–362. doi: 10.1016/S0140-6736(04)15436-6. [DOI] [PubMed] [Google Scholar]
- 23.Aydinok Y, Porter JB, Piga A, Elalfy M, El-Beshlawy A, Kilinç Y, et al. Prevalence and distribution of iron overload in patients with transfusion-dependent anemias differs across geographic regions: results from the CORDELIA study. Eur J Haematol. 2015;95(3):244–253. doi: 10.1111/ejh.12487. [DOI] [PubMed] [Google Scholar]
- 24.Fisher SA, Brunskill SJ, Doree C, Gooding S, Chowdhury O, Roberts DJ. Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion-dependent thalassaemia. Cochrane Database Syst Rev. 2013;8 doi: 10.1002/14651858.CD004450.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang HH, Lu MY, Peng SS, Yang YL, Lin DT, Jou ST, et al. The long-term efficacy and tolerability of oral deferasirox for patients with transfusion-dependent β-thalassemiain Taiwan. Ann Hematol. 2015;94(12):1945–1952. doi: 10.1007/s00277-015-2476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennell DJ, Porter JB, Piga A, Lai YR, El-Beshlawy A, Elalfy M, et al. Sustained improvements in myocardial T2* over 2 years in severely iron-overloaded patients with beta thalassemia major treated with deferasirox or deferoxamine. Am J Hematol. 2015;90(2):91–96. doi: 10.1002/ajh.23876. [DOI] [PubMed] [Google Scholar]
- 27.Orofino N, Roghi A, Duca L, Poggiali E, Fraquelli M, et al. Combination of deferasirox and deferoxamine in clinical practice: an alternative scheme of chelation in thalassemia major patients. Blood Cells Mol Dis. 2014;53(3):164–167. doi: 10.1016/j.bcmd.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Casale M, Filosa A, Ragozzino A, Amendola G, Roberti D, Tartaglione I, De Michele E, Cozzolino D, Rispoli G, Palmieri F, Pugliese U, Scianguetta S, Signoriello G, Musallam KM, Perrotta S. Long-term improvement in cardiac magnetic resonance in β-thalassemia major patients treated with deferasirox extends to patients with abnormal baseline cardiac function. Am J Hematol. 2019 Mar;94(3):312–318. doi: 10.1002/ajh.25370. [DOI] [PubMed] [Google Scholar]
- 29.Oudit GY, Trivieri MG, Khaper N, Liu PP, Backx PH. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J Mol Med (Berl) 2006;84(5):349–364. doi: 10.1007/s00109-005-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127(3):348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.