Graphical abstract
Keywords: Atrial fibrillation, Electrocardiography, e-Health, Monitoring, Catheter ablation
Abstract
Background
This multicenter prospective observational study examined the impact of additionally using a home electrocardiograph (ECG) to detect atrial fibrillation (AF) recurrence after ablation.
Methods
Between May 2019 and December 2020, 128 patients undergoing ablation were enrolled in the study. After performing ablation, they were instructed to measure their ECGs at home using Complete (ECG paired with a blood pressure monitor; Omron Healthcare, Japan) every day and to visit the hospital every 3 months until after 12 months for 24-hour Holter ECG and 12-lead ECG as usual care (UC).
Results
After ablation, 94 patients were followed up, and AF recurrence at 12 months was detected more commonly in adjudicators-interpreted Complete (31 [33 %]) than in UC (18 [9 %]) (hazard ratio 1.95, 95 % confidence interval [95 %CI] 1.35–2.81, P < 0.001). In patients with recurrent AF found via both modalities (n = 16), the time to first AF detection by Complete was 40.9 ± 73.9 days faster than that in UC (P = 0.04). Notably, when the adherence to Complete measurement was divided by 80 %, the add-on effect of Complete on the detection of recurrent AF in UC indicated the hazard ratio (HR) of 1.71 (95 %CI 0.92–3.18, P = 0.09) for the low adherence (<80 %) group, but it was significant for the high adherence (≥80 %) group, with HR of 2.19 (95 %CI 1.43–3.36, P < 0.001).
Conclusions
Despite a shorter measurement time, Complete detected recurrent AF more frequently and faster compared with UC after AF ablation. A significant adherence-dependent difference of Complete was found in detecting AF recurrence.
1. Introduction
Catheter ablation (CA) is a popular clinical treatment for symptomatic atrial fibrillation (AF). However, it is known that the percentage of patients in sinus rhythm after a single ablation procedure decreases over time: 72 % after 1 year, 65 % after 3 years, and approximately 59 % after 5 years [1]. Managing and identifying AF can be difficult for both patients and healthcare providers because many patients with AF are unable to recognize and distinguish symptoms associated with AF from other conditions, which can impede timely treatment and put them at risk for complications [2].
Electrocardiogram (ECG) monitoring is critical in post-ablation follow-up. Arrhythmia monitoring, for example, can help determine whether the patient’s “palpitations” are caused by recurrent AF. Several studies have shown that “palpitations” are frequently caused by premature atrial or ventricular beating and are not reliable predictors of AF recurrence [3], [4]. Monitoring for arrhythmias has also been shown to be beneficial in asymptomatic patients. Asymptomatic AF is common in post-CA patients, according to multiple studies [4], [5], [6], [7].
Various e-health tools have recently entered routine clinical use, and systematic evaluation has demonstrated the utility of e-health tools in identifying AF [8], [9]. Although these techniques may have a positive impact on post-ablation monitoring, few studies have demonstrated their efficacy [10]. However, it is known that adherence to the use of digital tools declines over time because it depends on the individual’s willingness to use the device [11]. Therefore, despite its clinical significance, there are no reports on the add-on effect of home ECG measurement adherence on AF detection in usual care (UC).
Thus, this prospective multicenter observational study was designed to investigate the impact of add-on effect of an at-home ECG device on the detection rate in patients with post-ablation AF. For ECG measurements, an ECG was used in conjunction with a blood pressure (BP) monitor (The Complete; Omron Healthcare Co., ltd., Kyoto, Japan). The device was approved by the Food and Drug Administration (FDA) in 2020 and has been shown to accurately distinguish between AF and normal sinus rhythm [12], [13].
2. Methods
2.1. Study population
The Institutional Review Board at each participating center approved this multicenter prospective observational study. All study participants provided written informed consent. Patients were selected from six cardiovascular centers in Japan’s Kansai region. Patients with persistent AF who were at least 20 years old, had access to a smart phone, and were scheduled for ablation were eligible for study participation. Exclusion criteria included (1) patients currently participating or planning to participate in an interventional trial, (2) patients with a confirmed diagnosis of mitral stenosis, (3) patients with an implanted pacemaker or defibrillator, and (4) patients deemed inappropriate to enroll in the study by the attending physician. Between May 2019 and December 2020, a total of 128 patients were registered. Fig. 1 depicts a patient flow chart.
Fig. 1.
Study flowchart. Reason for exclusions from the analysis: 8 (6.5%) had no declination reports but did not have a single measurement record.
2.2. Procedures and data collection
All patients had extensive encircling pulmonary vein isolation (PVI), isolating ipsilateral superior and inferior pulmonary veins (PVs) at the same time. The electrophysiological endpoint of PVI was a bidirectional conduction block between the LA and the PVs. The operator and/or the attending physician decided whether to perform additional ablation, such as tricuspid valve isthmus ablation, continuous fractionated atrial electrogram ablation, and LA linear ablation. Antiarrhythmic drugs (AADs) were stopped in all patients 3 months post-ablation. The decision to resume AAD use after the blanking period was usually made in the event of a recurrence of atrial tachycardia (AT)/AF. In this study, the use (including discontinuation) of antiarrhythmic drugs post-ablation was not specified.
During their hospitalization, patients were trained to take their BP and an ECG for 30 s by touching the electrodes on the top face and both sides of the monitor [12]. After discharge, patients were instructed to record at least one ECG daily in addition to further ECGs if symptoms occurred. Patients were followed up for 1 year at the outpatient clinic of the center where the ablation was performed, with 12-lead ECG and 24-hour Holter ECGs performed every 3 months until after 12 months. Notably, clinicians at the local center read 12-lead ECG and 24-hour Holter ECG records. When patients were unable to visit the outpatient clinic of the local center, follow-up data were obtained by contacting the physicians in charge or the patients. Clinicians and/or attending physicians at the local center imputed all clinical data.
Although Complete has been FDA-approved and has clinically demonstrated excellent sensitivity and specificity for detecting AF during BP measurements [12], it was not available in Japan during the study. Therefore, physicians made all clinical decisions based on their usual practice, and physicians and patients were blinded to Complete results. Only the data of Complete were used for the study.
2.3. Definitions and outcome
Recurrent AF was defined as AF documented by an ECG rhythm strip of Complete or a 12-lead ECG or 24-hour Holter ECG, and it has duration of ≥ 30 s. As adjudicators, two trained cardiologists (TN and SS) read Complete ECG rhythm strip blindly and classified the rhythm as AF, non-AF, or “uninterpretable” (due to baseline artifact, wander, or drift). Kappa (κ) coefficient of interobserver agreement was 0.73. In the event of disagreement, they reached a final decision on the diagnosis after deliberation. The primary goal of this study was to investigate the impact of at-home ECG adherence on AF detection in UC.
2.4. Statistical analyses
All demographic and clinical data are presented in the form of frequencies and percentages, or mean and standard deviation. The sensitivity and specificity were calculated by comparing the proportion of AF and sinus rhythm detected by Complete to that detected by 12-lead ECG measured simultaneously within 2 h. Over the 12-month follow-up period, Kaplan–Meier curves were created to detect AF recurrence in Complete and UC, and the difference in AF recurrence rate between them was calculated using a marginal Cox regression model. During the observation period, high adherence was defined as 80 % or more of the days with at least one ECG measurement per day in Complete, while low adherence was defined as 80 % or less. Given that the conventional threshold for “good adherence” to medication is 80 % [14], [15], in this study, the cutoff for measured adherence was also defined as 80 %.The effect of Complete on the detection of AF recurrence in UC was evaluated using Kaplan–Meier curves, which helped classify the measurement adherence to Complete into low (n = 43) or high (n = 51). Moreover, the difference in AF recurrence between them was calculated using a marginal Cox regression model. A paired sample t-test was used to compare the time to the first AF detection between each measurement modality. The distribution of possible AF within 15 days of the reference date is presented in two heatmaps, where the reference date is the date of clinically detected AF in UC and the date of at-home ECG device detected AF (interpreted by the adjudicators). The retention rate was calculated as the average of the following evaluation periods: day 24–30 for 1 month, day 84–90 for 3 months, day 174–180 for 6 months, and day 354–360 for 12 months. All analyses were carried out using R (version 3.6.1; July 5, 2019). Moreover, in all analyses, a critical P value of.05 was used to determine the significance.
3. Results
Five of the 128 patients with AF admitted for ablation who were enrolled later withdrew data usage permission, resulting in a final enrollment of 123 patients. Two patients were excluded at the time of ablation because they did not meet the eligibility criteria (n = 1) and did not undergo AF ablation (n = 1). Moreover, 121 patients received AF ablation, and 94 were included in the final analysis set, excluding patients for the following reasons: unable to continue follow-up of patients due to hospital transfer (n = 1), withdraw consent (n = 15), investigators determined that patients were unable to continue treatment (n = 3), and no measurements of Complete during the follow-up (n = 8). The mean age was 65.4 ± 9.0 years, 17.9 % of the patients were female, 55.3 % had hypertension, 47.2 % had long-term persistent AF (≥1 year), and the mean CHADS2 score was 1.1 ± 1.0. Table 1 also includes a list of remaining valuables. The results of the accuracy of Complete for AF detection were as follows: sensitivity analysis was performed on 102 patients before ablation, excluding “No ECG during pre-ablation” (n = 17) and “Sinus rhythm detected by 12-lead ECG during pre-ablation” (n = 2), and specificity analysis was performed on 94 subjects after ablation, excluding “No ECG during post-ablation” (n = 18) and “AF detected by 12-lead ECG during post-ablation” (n = 9). Out of a total of 196 simultaneous recordings, Cohen’s kappa coefficient (κ) to assess agreement between Complete and 12-lead ECG recordings was 0.85, indicating excellent agreement. The Complete had 98 % sensitivity and 89 % specificity, as well as 91 % positive and 97 % negative predictive value.
Table 1.
Patient characteristics in the study.
| Variables | n = 123 |
|---|---|
| Age, mean (SD) | 65.4 (9.0) |
| Age ≥ 75, n (%) | 24 (19.5) |
| Sex, female, n (%) | 22 (17.9) |
| Congestive heart failure, n (%) | 24 (19.5) |
| Hypertension, n (%) | 68 (55.3) |
| Diabetes mellitus, n (%) | 12 (9.8) |
| Stroke or TIA, n (%) | 5 (4.1) |
| Vascular Disease, n (%) | 1 (0.8) |
| Body Mass Index, mean (SD) | 24.9 (3.8) |
| CHADS2 score, mean (SD) | 1.1 (1.0) |
| CHA2DS2-VASc score, mean (SD) | 1.91 (1.3) |
| AF duration, n (%) | |
| <3 months | 15 (12.2) |
| 12 months | 50 (40.7) |
| >12 months | 58 (47.2) |
| Ablation (%) | |
| First procedure | 111 (90.2) |
| Repeat procedure | 12 (9.8) |
| Antiarrhythmic agents, n (%) | 48 (39.0) |
| ACE inhibitors, n (%) | 6 (4.9) |
| ARBs, n (%) | 41 (33.3) |
| CCBs, n (%) | 55 (44.7) |
| Beta blocker, n (%) | 76 (61.8) |
| Dabigatran, n (%) | 8 (6.5) |
| Rivaroxaban, n (%) | 41 (33.3) |
| Apixaban, n (%) | 11 (8.9) |
| Edoxaban, n (%) | 55 (44.7) |
| Warfarin, n (%) | 8 (6.5) |
SD: standard deviation; TIA: Transient ischemic attack; CHADS2 score: congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, stroke or TIA.
CHA2DS2-VASC score: congestive heart failure, hypertension, age ≥ 65, diabetes mellitus, stroke or TIA, female, vascular disease.
AF: atrial fibrillation; ACE inhibitor: angiotensin converting enzyme inhibitor; ARBs: Angiotensin II receptor blockers, CCBs: Calcium channel blockers.
A median follow-up was 12.0 months (range 7.1–12.3 months), and the mean number and duration of ECG measurements during the follow-up were 326.0 ± 300.6 times and 163 ± 150 min in Complete vs 2.2 ± 1.6 times and 3217 ± 2239 min in UC (both P < 0.001). At 12 months, 18 (19 %) AF recurrences were detected in UC and 31 (33 %) in the adjudicators-interpreted Complete (hazard ratio [HR] 1.95, 95 % confidence interval [CI] 1.35–2.81, P < 0.001, Fig. 2). Of the 31 detected with Complete, 15 were detected solely using Complete, whereas the remaining 16 were also detected using UC. In patients with recurrent AF found via both modalities (n = 16), the time to first AF detection by Complete was 40.9 ± 73.9 days faster than UC (Mean 112.2 ± 45.9 days in Complete vs 153.1 ± 76.7 days in UC, P = 0.04). Of the 18 detected in UC, 2 were unable to be detected in Complete.
Fig. 2.
Kaplan–Meier analysis of time from ablation to first detection of recurrent AF. Observation period: After the blanking period until the last observation point or until discontinuation or endpoint.
We further explored the combined effects of Complete on AF detection rates in UC, assuming that both Complete and UC were in combination in daily practice. When the adherence to Complete measurement was divided by 80 %, the combined effect of Complete on detection of recurrent AF in UC indicated HR of 1.71 (95 % CI 0.92–3.18, P = 0.09) for the low adherence (<80 %) group, but this was significant for the high adherence (≥80 %) group, with HR of 2.19 (95 % CI 1.43–3.36, P < 0.001) (Fig. 3). Notably, a significant adherence-dependent difference of Complete was found in the detection of AF recurrence.
Fig. 3.
The add-on effect of Complete on detection rates in UC according to measurement adherence of Complete. Adherence: Percentage of days with at least one measurement per day during the observation period.
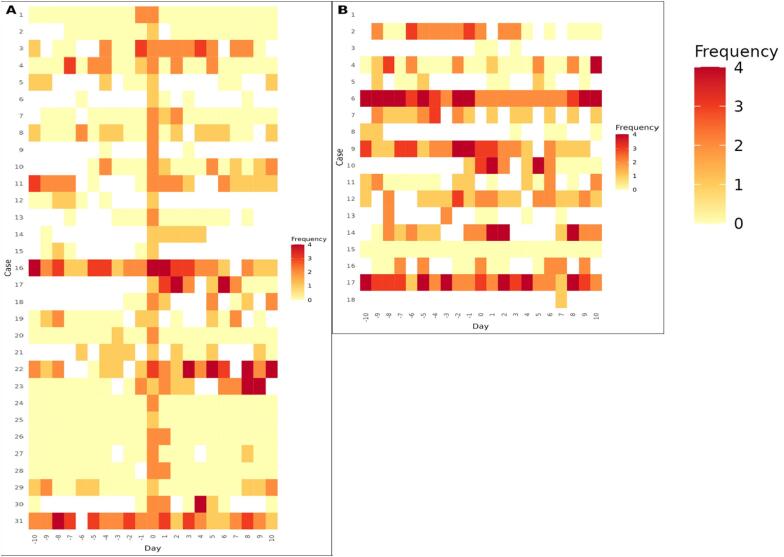
We used the date when AF was detected (by the adjudicators’ interpretation) in Complete ECG (Fig. 4A) and the date when AF was clinically confirmed in UC (Fig. 4B) as reference date, and the distribution of normal and possible AF cases within ± 15 days of the reference date were shown in two heat maps. Overall, in 20 of 31 Complete ECGs that the adjudicators classified as AF, Complete was used on both the day before and the day after the reference date. Moreover, in 16 cases, sinus rhythm was detected both on the day before and on the day after the reference date, i.e., 80 % (n = 16/20) of the recurrence patterns were seen in < 48 h (i.e., short-lasting). In contrast, of the 18 patients diagnosed with AF in UC, 11 were also measuring Complete on both the day before and the day after the reference date. <40 % (n = 4/11) had < 48 h (a short-lasting) recurrence pattern.
Fig. 4.
Number of “possible AF” cases detected in Complete within ± 15 days from the reference date. Reference date in A: the date of the adjudicators’ interpretation of AF in Complete ECG. Reference date in B: the date of clinically confirmation of AF in UC. Frequency: Number of “possible AF” in Complete per day. White: Days with no “normal” or “possible AF”.
4. Discussion
Complete detected AF recurrence more frequently and faster because of its ability to detect short-lasting AF compared to UC. It also exerted a significant add-on effect on diagnosis rates in UC by maintaining high measurement adherence. To the best of our knowledge, this is the first report demonstrating the importance of constant monitoring of home ECGs in post-ablation patients.
The main reason for arrhythmia monitoring after ablation is to help determine whether the patient’s palpitations are caused by recurrent AF or other AT. Palpitations are often caused by premature heartbeats and do not accurately predict recurrent AF [4]. As several studies, including the present study, have demonstrated the ability to distinguish recurrent AF from more benign symptomatic arrhythmias, at-home ECG has the potential to enable more effective and timely clinical decision-making in post-ablation AF patients [12], [13]. In addition, once telemedicine is widely used, after checking the ECG transmission, the physician can remotely and appropriately guide the patient’s postoperative treatment by reassuring the patient, changing the treatment, or asking the patient to visit the hospital [16]. Intermittent arrhythmia monitoring may also influence anticoagulation decision-making after ablation. Several studies have shown that asymptomatic AF is common in post-ablation patients. For example, in some previous studies, compared to symptomatic AF, the rate of asymptomatic AF increased from 11 % to 35 % before ablation to 53 %–65 % after ablation [17], [18], [19]. Another study also found that 53.8 % of AF episodes after ablation were asymptomatic and that the number of asymptomatic episodes increased from the acute to the chronic phase of the procedure, indicating that AF success is not solely based on the absence of symptoms [3]. Indeed, Complete could detect higher numbers of AF lasting for < 48 h (i.e., short-lasting AF). Given the highly irregular patterns of AF occurrence, more frequent but short-duration monitoring (i.e., at-home ECG) may be more effective at detecting arrhythmias than less frequent but longer monitoring (Holter monitors).
Based on the results of the present study, there is an add-on effect on the detection rate of AF during UC with increased frequency of intermittent at-home ECG monitoring. Notably, as the usage of mobile application tools decreases over time [11], [20], maintaining a high frequency of use should be important to maximize the effectiveness of home ECG monitoring.
As a method for routine monitoring of patients undergoing AF ablation, implantable loop recorders (ILR) currently shows promise as an accurate method for assessing postoperative AF, although this is not recommended in the guideline [21], [22]. However, ILRs are invasive and expensive and have diagnostic limitations, such as beat under-sensing, myoelectric potential oversensing, reduced specificity due to irregular premature beats, and limitations in the number of ECGs read out and memory to validate correct rhythm diagnosis [23], [24], [25]. Importantly, few countries have approved its use for postoperative AF management. Therefore, arrhythmia monitoring using at-home ECG can be an important component in evaluating the results of ablation procedures.
Some studies have attempted to estimate changes in AF burden (or sinus duration) using various methods in the absence of an implantable device [26], [27]. Similarly, the present study attempted to demonstrate “normal” and “possible AF” before and after the day when AF was detected clinically or using Complete. The percentage of “normal” (sinus rhythm time) was significantly higher on the days before and after the day when AF was detected using Complete than that detected using UC. In other words, Complete might detect many cases of < 48 h AF (short-lasting AF) that would not have been detected clinically. However, the accuracy of the estimated sinus duration is limited by the duration and frequency of monitoring with intermittent monitoring devices; thus, it will be necessary in the future to calculate estimated sinus duration by combining symptom reports and ECG status at each time point.
5. Limitations
First, the attached application instructions were in English, and the ECG display was customized to be blind to the user due to the nature of the study, which may have contributed to Japanese users' difficulty in operating the device. Although enrolled patients were instructed on how to use the device during hospitalization, and that call center support and face-to-face instruction were also provided during follow-up, the trial had a high dropout rate (22 %). However, most patients (78 % of total patients) who could be followed up were able to continue to measure ECG during the observation period. (Supplementary Figure) Second, because symptom records were not obtained during the ECG recording of Complete, the percentage of asymptomatic AF recurrences is unknown. Third, all patients in this study were Japanese. Generalizing our findings to all patients with AF should be done with caution, as results may differ in other patient populations.
6. Conclusion
After AF ablation, the use of Complete—at-home ECG self-recording device—allows for better and faster detection of AF recurrence. The recurrent AF patterns detected by Complete include many short-lasting cases that would go undetected clinically. A significant adherence-dependent difference from Complete was found in the detection of AF recurrences. This indicates the importance of constant monitoring of home ECGs during follow-up.
Disclosures
Dr. K Senoo had university research contracts with the Omron Healthcare company. The other authors have declared no conflicts of interest.
Ethics approval statement
The study was approved by the Medical Ethics Review Committee of the Kyoto Prefectural University of Medicine (approval number: ERB-C-1347).
Data availability statement
The data supporting the findings of this study are available upon request from the corresponding author. Due to privacy and ethical concerns, the data are not publicly available.
Author contributions
KS and SM contributed to the conception and design of the work. AY, TO, HI, KI, TS, KK, TH, HK, KN, and SN all helped with data collection and interpretation for this study. TN and SS independently assessed Complete ECGs. MN and ST helped with data analysis. Moreover, the manuscript was drafted by KS. The manuscript was also revised critically by the other authors. All agreed to be accountable for all aspects of the work, ensuring integrity and accuracy, and gave final approval.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement of grant support
The Omron Healthcare company provided Complete device and an iPhone (Apple Inc., CA, USA) for this study but was not involved in the study design, analysis, or drafting of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2023.101177.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Takigawa M., Takahashi A., Kuwahara T., et al. Long-term follow-up after catheter ablation of paroxysmal atrial fibrillation: the incidence of recurrence and progression of atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2014;7:267–273. doi: 10.1161/CIRCEP.113.000471. [DOI] [PubMed] [Google Scholar]
- 2.McCabe P.J., Schad S., Hampton A., Holland D.E. Knowledge and self-management behaviors of patients with recently detected atrial fibrillation. Heart Lung: J. Critical Care. 2008;37:79–90. doi: 10.1016/j.hrtlng.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Klemm H.U., Ventura R., Rostock T., et al. Correlation of symptoms to ECG diagnosis following atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2006;17:146–150. doi: 10.1111/j.1540-8167.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 4.Vasamreddy C.R., Dalal D., Dong J., et al. Symptomatic and asymptomatic atrial fibrillation in patients undergoing radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 2006;17:134–139. doi: 10.1111/j.1540-8167.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 5.Hindricks G., Piorkowski C., Tanner H., et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation. 2005;112:307–313. doi: 10.1161/CIRCULATIONAHA.104.518837. [DOI] [PubMed] [Google Scholar]
- 6.Oral H., Veerareddy S., Good E., et al. Prevalence of asymptomatic recurrences of atrial fibrillation after successful radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 2004;15:920–924. doi: 10.1046/j.1540-8167.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 7.Pokushalov E., Romanov A., Corbucci G., et al. Ablation of paroxysmal and persistent atrial fibrillation: 1-year follow-up through continuous subcutaneous monitoring. J. Cardiovasc. Electrophysiol. 2011;22:369–375. doi: 10.1111/j.1540-8167.2010.01923.x. [DOI] [PubMed] [Google Scholar]
- 8.Perez M.V., Mahaffey K.W., Hedlin H., et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Y., Wang H., Zhang H., et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J. Am. Coll. Cardiol. 2019;74:2365–2375. doi: 10.1016/j.jacc.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Huang S., Zhao T., Liu C., et al. Portable device improves the detection of atrial fibrillation after ablation. Int. Heart J. 2021;62:786–791. doi: 10.1536/ihj.21-067. [DOI] [PubMed] [Google Scholar]
- 11.Senoo K., Miki T., Ohkura T., et al. A smartphone app to improve oral anticoagulation adherence in patients with atrial fibrillation: prospective observational study. JMIR Mhealth Uhealth. 2022;10:e30807. doi: 10.2196/30807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senoo K., Miki T., Okura T., et al. Diagnostic value of atrial fibrillation by built-in electrocardiogram technology in a blood pressure monitor. Circulat. Rep. 2020;2:345–350. doi: 10.1253/circrep.CR-20-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senoo K., Yukawa A., Ohkura T., et al. Screening for untreated atrial fibrillation in the elderly population: A community-based study. PLoS One. 2022;17:e0269506. doi: 10.1371/journal.pone.0269506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrade S.E., Kahler K.H., Frech F., Chan K.A. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol. Drug Saf. 2006;15:565–574. doi: 10.1002/pds.1230. discussion 575-567. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner P.C., Haynes R.B., Hersberger K.E., Arnet I. A systematic review of medication adherence thresholds dependent of clinical outcomes. Front. Pharmacol. 2018;9:1290. doi: 10.3389/fphar.2018.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aljuaid M., Marashly Q., AlDanaf J., et al. Smartphone ECG monitoring system helps lower emergency room and clinic visits in post-atrial fibrillation ablation patients. Clin. Med. Insights. Cardiol. 2020;14 doi: 10.1177/1179546820901508. 1179546820901508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wokhlu A., Monahan K.H., Hodge D.O., et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J. Am. Coll. Cardiol. 2010;55:2308–2316. doi: 10.1016/j.jacc.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Piorkowski C., Kottkamp H., Tanner H., et al. Value of different follow-up strategies to assess the efficacy of circumferential pulmonary vein ablation for the curative treatment of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2005;16:1286–1292. doi: 10.1111/j.1540-8167.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 19.Janse P.A., van Belle Y.L., Theuns D.A., Rivero-Ayerza M., Scholten M.F., Jordaens L.J. Symptoms versus objective rhythm monitoring in patients with paroxysmal atrial fibrillation undergoing pulmonary vein isolation. Eur. J. Cardiovasc. Nurs. 2008;7:147–151. doi: 10.1016/j.ejcnurse.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi S., Waki K., Nannya Y., Nangaku M., Kadowaki T., Ohe K. Usage patterns of GlucoNote, a self-management smartphone app, based on ResearchKit for patients with Type 2 diabetes and prediabetes. JMIR Mhealth Uhealth. 2019;7:e13204. doi: 10.2196/13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Task Force members, M. Brignole, P. Vardas et al., Indications for the use of diagnostic implantable and external ECG loop recorders. Europace: Eur. Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology 11 (2009) 671–687. [DOI] [PubMed]
- 22.Pokushalov E., Romanov A., Corbucci G., et al. Use of an implantable monitor to detect arrhythmia recurrences and select patients for early repeat catheter ablation for atrial fibrillation: a pilot study. Circ. Arrhythm. Electrophysiol. 2011;4:823–831. doi: 10.1161/CIRCEP.111.964809. [DOI] [PubMed] [Google Scholar]
- 23.Kapa S., Epstein A.E., Callans D.J., et al. Assessing arrhythmia burden after catheter ablation of atrial fibrillation using an implantable loop recorder: the ABACUS study. J. Cardiovasc. Electrophysiol. 2013;24:875–881. doi: 10.1111/jce.12141. [DOI] [PubMed] [Google Scholar]
- 24.Damiano R.J., Jr., Lawrance C.P., Saint L.L., et al. Detection of atrial fibrillation after surgical ablation: conventional versus continuous monitoring. Ann. Thoracic Surgery. 2016;101:42–47. doi: 10.1016/j.athoracsur.2015.07.039. discussion 47-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.C. Eitel, D. Husser, G. Hindricks, et al., Performance of an implantable automatic atrial fibrillation detection device: impact of software adjustments and relevance of manual episode analysis. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology 13 (2011) 480–485. [DOI] [PMC free article] [PubMed]
- 26.Cosedis Nielsen J., Johannessen A., Raatikainen P., et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N. Engl. J. Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 27.Verma A., Jiang C.Y., Betts T.R., et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available upon request from the corresponding author. Due to privacy and ethical concerns, the data are not publicly available.