Abstract
Background
Uric acid nephropathy (UN) is a complication of hyperuricemia (HUA), which has a great impact on people's lives. Here, we evaluated the therapeutic potential of total flavonoids of Phellinus igniarius (TFPI) in vivo and studied the anti UN effect of TFPI in vitro.
Methods
Hyperuricemia was induced by intraperitoneal injection of potassium oxonate in ICR mice. After intervention with TFPI, we evaluated the levels of serum uric acid (UA) and creatinine (CR), and the contents of xanthine oxidase (XOD) and adenosine deaminase (ADA) in liver. To explore the effect and molecular mechanism of TFPI on UN, we treated HK-2 cells with monosodium urate (MSU) to study the effect of TFPI on apoptosis and inflammation. In addition, to explore the mechanism of TFPI on uric acid transport we evaluated the relationship between uric acid transporter ABCG2 and inflammatory signaling pathway TLR4-NLRP3.
Results
In the model mice, TFPI significantly decreased the levels of UA and Cr, which may be related to the inhibition of XOD enzyme activity. In HK-2 cells, the response of TFPI to MSU can effectively inhibit apoptosis and activation of TLR4-NLRP3 signaling pathway and promote the expression of ABCG2.
Conclusions
TFPI can significantly inhibit the release of inflammatory factors and promote the expression of ABCG2 by targeting TLR4 receptor and NLRP3 inflammasome. And targeted inhibition of XOD enzyme activity to reduce uric acid level and inhibit the development of UN.
Keywords: Hyperuricemia, Uric acid nephropathy, Phellinus igniarius, TLR4, NLRP3
1. Introduction
HUA is a metabolic disease caused by abnormal purine metabolism or reduced UA excretion, which is characterized by elevated serum UA [1,2]. As a product of purine nucleotide metabolism, UA is mainly synthesized by XOD, which is widely distributed in the liver, and then excreted by the kidney and intestine. Studies have shown that long-term hyperuricemia can cause secondary damage to the kidney and eventually lead to UN [3]. With the change in dietary pattern, the intake of high sugar, high purine, and alcohol increases, and the incidence rate of UN are increasing. It has become one of the most important diseases threatening human health.
The inflammasome is an inherent and natural immune response, when cells sense PAMP (pathogen associated molecular pattern) or DAMP (damage associated molecular pattern) from allergens, various pathogens, stress stimuli, and mutagens, they will form protein complexes in the cytoplasm [4]. In response to the stimulation of cells receiving antigens, such as the MSU to toll-like receptors (TLRs), when the expression of protein complexes of key inflammasome components is up-regulated, the immune response of inflammasome starts initially. TLR4 is a receptor protein on the cell membrane, which can produce a signal to foreign stimuli, activate NF-kB, and produce inflammatory response [5]. Recent studies have shown that activation of NLRP3 and TLR4 contribute to the pathogenesis of UN [6,7]. These data indicate the potential of targeting the TLR4-NLRP3 signaling pathway as a novel therapeutic strategy.
Phellinus igniarius is a kind of traditional Chinese medicine with a long history. Modern research shows that Phellinus igniarius contains a variety of compounds, including flavonoids, polysaccharides and pyranones, which have anti-tumor, immune enhancement, hypoglycemic, antioxidant and anti-inflammatory pharmacological effects [8,9]. TFPI is extracted from Phellinus igniarius, recently studies have shown that TFPI can reduce the levels of IL-18 and TNF-α, inhibit the expression of NF-κB to produce an anti-inflammatory effect [10]. Liu shuaiyang et al. proved that TFPI could inhibit the expression of XOD and HGPRT genes, which are key enzymes in the uric acid metabolism pathway. These findings support the hypouricemic and anti-inflammatory activities of TFPI, and prompt us to study the therapeutic potential of TFPI for the UN.
In this study, we established a hyperuricemic mouse model by intraperitoneal injection of potassium oxonate to study the effect of TFPI on reducing uric acid in hyperuricemic mice. We used MSU to treat HK-2 cells to simulate a hyperuricemic environment, and preliminarily elucidated the protective effect and mechanism of TFPI on the HK-2 injury model induced by MSU based on the TLR4-NLRP3 pathway. We demonstrated for the first time that TFPI could significantly inhibit the development of UN, and further explored the relationship between ABCG2 and inflammatory pathway. This study identified a novel therapeutic agent and revealed the pathogenic mechanisms of UN.
2. Materials and methods
2.1. Drug sample preparation
The fine powder of Phellinus igniarius (“Zhehuang No. 1” provided by Zhejiang Qiandao Lake Sangdu Edible Fungus Professional Cooperative) was accurately weighed, extracted with 70% ethanol under reflux, and purified by AB-8 type macroporous adsorption resin [11]. The total flavonoid content measured by the Rutin-AlCl3 method was 66.7%, and the drug was placed at −20 °C for use.
2.2. Mouse model establishment and TFPI treatment
ICR mice (18–22 g) were purchased from Zhejiang Experimental Animal Center (scxk2019-0002). The animals were raised in the animal center of Zhejiang Academy of Medical Sciences and the research plan was reviewed and approved by the animal ethics committee of Hangzhou Medical College. All experiments, including animal reproduction, experimental operation and animal euthanasia, were carried out in accordance with the guidelines formulated by the Committee. Animal feeding conditions: the temperature was 18–28 °C, the humidity was 60–80%, the light and dark were alternated for 12 h, the temperature, humidity, light, pressure gradient were set with automatic control and display system, the tap water was put in the drinking water bottle for free drinking, the sterilized rats were fed by themselves, and the feed was from Zhejiang experimental animal center.
Male mice were randomly divided into normal group, model group, allopurinol group (10 mg/kg), TFPI [12] low, medium, and high dose groups (50, 150 and 450 mg/kg), with 10 mice in each group. On the 7th day, except for the normal group, the other groups were intraperitoneally injected with 350 mg/kg potassium oxonate 0.5 h before the last administration to establish the hyperuricemia model in mice. The normal group was injected with the same amount of 0.5% CMC-Na.
2.3. Serum and liver biochemical analysis
The levels of UA, Cr in the serum and XOD, ADA in the liver were detected by using commercial detection kits (Jiancheng Bioengineering Institute, China) according to the instructions of manufacturers.
2.4. Cell culture and treatment
The HK-2 cell line, a human proximal tubular epithelial cell line, was purchased from ATCC and maintained in DMEM/F12, as described in ref. To examine the effect of TFPI on the activation of inflammatory pathway, HK2 cells were treated with different concentrations of TFPI (37.5, 75 and 150 mg/L) and MSU (150 mg/L, sigma) for 24 h.
2.5. MTT assay
To examine the cell viability, HK-2 cells were seeded into 96-well plates (Corning, NY, USA) in triplicate at 5 × 105 cells/100 μL/well at 37 °C in a humidified 5% CO2 incubator. After the indicated treatments, 20 μL of MTT agent (5 mg/mL) was added into each well and incubated at 37 °C for a further 4 h. Dimethyl sulfoxide (DMSO, 150 μL/well) was added into each well to dissolve the formazan crystals. The absorbance was measured using a microplate reader at 570 nm.
2.6. Analysis of cell ROS and LDH levels
Intracellular superoxide production was detected with dichlorodihydrofluorescein diacetate (DCF-DA) (S0033-1, Biyuntian Bioengineering Institute) and observed under fluorescence microscope. The levels of LDH in the cells were detected by using commercial detection kits (E1020, Puli Gene Technology Co. Ltd, China) according to the instructions of manufacturers.
2.7. Assay for pyroptosis
After HK2 cells were treated with different concentrations of TFPI and MSU (150 mg/L, sigma) for 24 h, and then the apoptosis rate was detected by flow cytometry according to the instructions of the kit (C1062L, Biyuntian Bioengineering Institute).
2.8. Enzyme-linked immunosorbent assay (ELISA)
IL-1β and TNF-α (Sizhengbai Bioengineering Institute, China) expression an activity levels in the above cells were detected with the corresponding ELISA Kits, according to the manufacturers’ instructions.
2.9. Cell immunofluorescence (IF)
HK-2 cells were seeded on a round glass dish and grew to near confluence. After various treatments, HK-2 cells were sucked out of the upper liquid and washed with PBS for three times. 4% paraformaldehyde for 15 min, PBS for three times, 0.5% Triton X-100 for 60 min, PBS for three times. Seal with 1% BSA for 60 min, and quickly clean with PBS. The first antibody (according to the instructions of antibody: the dilution of antibody NF-κB is 1:200) was placed in a wet box at 4 °C overnight and washed with PBS for three times. The second antibody (IgG Fab2 Alexa Fluor(R) 647; 4414S; Cell Signaling) was incubated at room temperature in dark for 120 min and washed with PBS for three times. The nuclei were stained with Hoechst 33,342 for 10 min, washed with PBS for three times and observed by direct fluorescence.
2.10. Western blotting
Whole cell lysate was prepared from cultured HK-2 cells in RIPA buffer (Biyuntian Bioengineering Institute, China). Soluble proteins from the culture medium were prepared as described previously. After measuring the protein concentration using BCA kit (Biyuntian Bioengineering Institute), equal amounts of total proteins were separated on SDS-PAGE gel and transferred onto a polyvinylidene difluoride (PVDF) membrane. The target protein was detected with one of the following primary antibodies (all from Cell Signaling, MA, USA, unless otherwise indicated) at 4 °C overnight: NLRP3 (15101S; Cell Signaling, MA, USA), TLR4 (bs-20594R; Bioss Bioengineering Institute, China), cleaved caspase 3 (9664S; Cell Signaling), caspase-3 (9665S; Cell Signaling), BAX (5023S; Cell Signaling), NF-κB (bs-0465R; Bioss Bioengineering Institute), p–NF–κB (3033T; Cell Signaling), TXNIP (14715S; Cell Signaling), and ABCG2 (bs-0662R; Bioss Bioengineering Institute). After the incubation with horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 h. The signal was developed using the ECL system (Biyuntian Bioengineering Institute) according to the manufacturer's instructions. The signal density was analyzed using Quantity One Software (Bio-Rad, CA, USA) and the relative protein level was calculated as the density ratio of the target protein to β-actin (4970T; Cell Signaling).
2.11. Statistical analysis
Statistical analysis of the experimental data was performed using SPSS 22.0 software, and the results are presented as the mean ± SE. Measurement data were analyzed by one-way analysis of variance (one-way ANOVA). Multiple comparisons of the means of two samples were performed using independent-samples T-tests, and the correlations between two variables were assessed using Pearson correlation analysis. P < 0.05 indicated that the difference was statistically significant.
3. Results
3.1. TFPI ameliorated UN development in a dose-dependent manner in vivo
In the experiment, there was no significant difference in body weight between TFPI groups and normal group (Table 1), which indicated that TFPI was safe. It is not known whether TFPI presents any therapeutic efficacy on UN. In this study, hyperuricemic mice were induced by intraperitoneal injection of potassium oxonate. The results showed that the serum UA and Cr levels were significantly increased, and the liver XOD enzyme activities were significantly increased in the model group, when compared with control group (Table 2, Table 3). Following the treatment with increasing concentrations of TFPI, we found that the levels of serum UA and liver XOD were significantly decreased in a dose-dependent manner (Tables 2 and 3), when compared with model group. In mice treated with 450 mg/kg TFPI, the content of serum creatinine in mice decreased significantly, which indicated that TFPI had protective effect on kidney.
Table 1.
Effect of TFPI on body weight of hyperuricemia mice ( ± s, n = 10).
| Group | Dosage (mg/kg) | Start (g) | End (g) |
|---|---|---|---|
| Control | – | 22.13 ± 1.73 | 29.25 ± 1.49 |
| Model | – | 21.88 ± 1.96 | 30.25 ± 1.58 |
| Allopurinol | 10 | 22.56 ± 1.51 | 31.22 ± 2.28 |
| TFPI | 50 | 21.75 ± 1.75 | 30.63 ± 1.77 |
| TFPI | 150 | 22.38 ± 1.41 | 30.25 ± 2.31 |
| TFPI | 450 | 21.11 ± 1.54 | 30.00 ± 1.58 |
Note: TFPI - total flavonoids of Phellinus igniarius.
*P < 0.01.
**P < 0.05, as compared to control.
#P < 0.01.
##P < 0.05, as compared to model.
Table 2.
Effects of TFPI on serum UA and Cr levels in hyperuricemic mice ( ± s, n = 10).
| Group | Dosage (mg/kg) | UA (μmol/L) | Cr (μmol/L) |
|---|---|---|---|
| Control | – | 39.2 ± 13.9 | 31.33 ± 8.60 |
| Model | – | 105.0 ± 9.9* | 44.30 ± 10.00** |
| Allopurinol | 10 | 49.8 ± 9.1# | 33.06 ± 7.29## |
| TFPI | 50 | 86.5 ± 21.6 | 45.85 ± 10.77 |
| TFPI | 150 | 83.7 ± 25.3## | 34.51 ± 10.46 |
| TFPI | 450 | 82.3 ± 11.3## | 33.37 ± 6.22## |
Note: TFPI – total flavonoids of Phellinus igniarius; UA – the levels of serum uric acid; CR – the levels of serum creatinine.
*P < 0.01.
**P < 0.05, as compared to control.
#P < 0.01.
##P < 0.05, as compared to model.
Table 3.
Effects of TFPI on XOD and ADA activities in liver of hyperuricemic mice ( ± s, n = 10).
| Group | Dosage (mg/kg) | XOD (U/g prot) | ADA (U/g prot) |
|---|---|---|---|
| Control | – | 43.09 ± 4.34 | 30.47 ± 2.39 |
| Model | – | 48.91 ± 4.43* | 29.34 ± 3.02 |
| Allopurinol | 10 | 38.69 + 2.56# | 25.43 ± 2.84## |
| TFPI | 50 | 42.48 + 2.69# | 27.52 ± 4.48 |
| TFPI | 150 | 44.11 ± 4.07## | 27.77 ± 2.12 |
| TFPI | 450 | 44.09 ± 2.93## | 27.76 ± 4.14 |
Note: TFPI – total flavonoids of Phellinus igniarius; XOD – the levels of xanthine oxidase; ADA – the levels of adenosine deaminase.
*P < 0.01.
**P < 0.05, as compared to control.
#P < 0.01.
##P < 0.05, as compared to model.
3.2. TFPI protected HK-2 cells from pyroptosis induced by MSU
The precipitation of MSU crystals in renal tubules is the main reason for the development of UN. Thus, we used the proximal tubular epithelial cells HK-2 as the model system. Firstly, we profiled the safe concentration of TFPI on HK-2 cells by MTT assay. As shown in Fig. 1A, TFPI at different concentrations from 18.75 mg/L to 300 mg/L after either 24 h or 48 h presented non-significant alterations on cell viability when compared to cells not treated with TFPI, suggesting the relative safety of TFPI on HK-2 cells. Therefore, we chose 37.5, 75, and 150 mg/L for subsequent experiments. Secondly, we treated HK-2 cells with TFPI + MSU, as shown in Fig. 1B, compared with the model group, the cell viability of HK-2 cells treated with TFPI increased significantly, the release rate of LDH decreased significantly (Fig. 1C), and the total apoptosis rate and late apoptosis rate decreased significantly (Fig. 1D and E), suggesting that TFPI potently protected HK-2 cells from pyroptosis induced by MSU stimulation.
Fig. 1.
The survival rate of HK-2 cells at different times under different concentrations of TFPI (A); cell viability in each group was detected by MTT assay (B); the LDH release rate of each group of cells was detected by LDH kit (C); the total apoptosis rate of cells in each group (D); the late apoptosis rate of cells in each group (E); *P < 0.01, **P < 0.05, compared with the control; #P < 0.01, ##P < 0.05, compared with the model.
3.3. TFPI protects HK-2 cells from MSU induced apoptosis via mitochondrial pathway
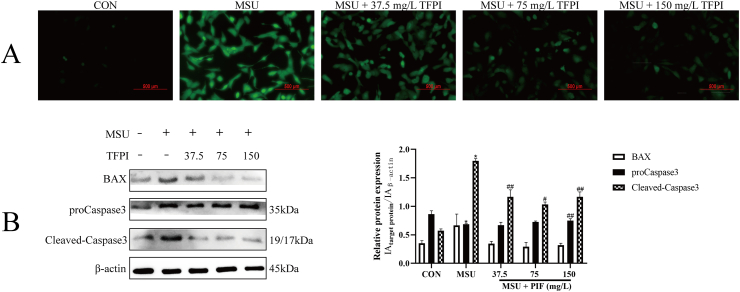
As shown in Fig. 2A, MSU could aggravate the oxidative stress response of HK-2 cells, while the ROS level of HK-2 cells treated with TFPI gradually decreased in a concentration dependent manner. Oxidative stress often mediates apoptosis through mitochondrial pathway. As shown in Fig. 2B, the expression of Caspase-3, cleaved-caspase-3 and Bax in HK-2 cells stimulated by MSU increased significantly, while the expression of Caspase-3, cleaved-caspase-3 and Bax in HK-2 cells treated by TFPI was inhibited. This suggests that TFPI protects HK-2 cells from MSU induced apoptosis through mitochondrial pathway.
Fig. 2.
Effect of TFPI on ROS level of HK-2 cells injured by MSU (A); The protein levels of indicated targets were examined by Western blot (B); *P < 0.01, **P < 0.05, compared with the control; #P < 0.01, ##P < 0.05, compared with the model.
3.4. TFPI inhibits the activation of TLR4-NLRP3 inflammatory pathway in vitro
Immunofluorescence showed that NF-κB nuclear shift occurred after MSU stimulation, and nuclear shift was inhibited after TFPI treatment, which indicated that TFPI could inhibit the inflammation induced by MSU stimulation (Fig. 3). To evaluate the status of TLR4-NLRP3 inflammatory pathway in response to TFPI treatment, we detected the protein expression of TLR4, NF-κB, p–NF–κB, TXNIP and NLRP3 in cell lysate and IL-1β, TNF-α in cell supernatant. The results showed that the expression of these proteins and the content of inflammatory factors increased significantly after MSU stimulation (Fig. 4). However, after TFPI treatment, the levels of IL-1β and TNF-α in cell supernatant were significantly decreased in a concentration dependent manner (Fig. 4B and C). Compared with the model group, the protein expressions of TLR4, NF-κB, p–NF–κB, TXNIP and NLRP3 were decreased in all treatment groups, suggesting that TFPI may protect HK-2 cells by inhibiting the activity of TLR4-NLRP3 inflammatory pathway induced by MSU stimulation (Fig. 4A).
Fig. 3.
Effect of TFPI on nuclear factor shift in HK-2 cells injured by MSU.
Fig. 4.
The protein levels of indicated targets were examined by Western blot (A); the levels of TNF-α from indicated groups were examined by ELISA (B); the levels of IL-1β from indicated groups were examined by ELISA (C); *P < 0.01, **P < 0.05, compared with the control; #P < 0.01, ##P < 0.05, compared with the model.
3.5. TFPI promotes ABCG2 expression by blocking TLR4-NLRP3 pathway activation
As shown in Fig. 5, the expression of ABCG2 increased significantly after TFPI treatment. The relationship between the inhibition of TLR4-NLRP3 pathway by TFPI treatment and the expression of ABCG2 protein is unknown. According to Fig. 4C, the release rate of IL-1β in cells decreased significantly under the action of TFPI. Recent studies have shown that IL-1β is related to ABCG2 protein expression through PDZK1 [13]. As a result, the change of ABCG2 protein expression is closely related to the decrease of IL-1β. Therefore, our research group will further explore the relationship between IL-1β and ABCG2 in the later research and explore the mechanism between ABCG2 expression and TLR4-NLRP3 signal axis under the action of TFPI.
Fig. 5.
Effects of TFPI on ABCG2 proteins in HK-2 cells injured by MSU; *P < 0.01, **P < 0.05, compared with the control; #P < 0.01, ##P < 0.05, compared with the model.
4. Discussion
In recent years, the number of patients with hyperuricemia continues to rise [14]. Chronic hyperuricemia can cause secondary damage to the cardiovascular endothelium, liver and kidney, and the kidney is the main organ excreting uric acid. Therefore, the renal injury will further aggravate hyperuricemia. Modern drugs can well control the level of uric acid in patients with hyperuricemia, but the strong side effects limit their clinical application [[15], [16], [17]]. Hence, there is no doubt that more effective anti-hyperuricemic medicines with fewer side effects are needed. According to the literature review and the previous research of the research group, the 70% alcohol extract of Phellinus igniarius has good anti-hyperuricemia characteristics. The existing research shows that the main active components in TFPI obtained by macroporous resin adsorption and purification are protocatechuic aldehyde, inoscavin A, hispidin, hypholomine B, phelligridimer A and davallialactone. The above substances isolated from TFPI have good antioxidant, anti-inflammatory and XOD inhibitory effects in previous studies [[18], [19], [20], [21]]. In this study, we confirmed that TFPI can inhibit the development of HUA and protect the kidney.
A high level of UA is the main feature of hyperuricemia [22]. Some studies have shown that HUA can damage the kidney, lead to the obstruction of renal excretion of UA, and aggravate hyperuricemia [23,24]. The level of Cr is an important index to evaluate renal function [25]. The present study found that TFPI can reduce the level of Cr in hyperuricemia model mice, suggesting that TFPI has the function of protecting the kidney. XOD is an important enzyme of uric acid metabolism in vivo, and the enhancement of XOD activity will increase the production of uric acid [26]. This study showed that TFPI could significantly reduce UA content and inhibit XOD enzyme activity, suggesting that the effect of TFPI on decreasing UA might be due to the inhibitory effect on XOD level.
Apoptosis is a process of active cell death controlled by genes to maintain the stability of the internal environment and regulate the development of the body under certain stimulation [27]. At present, there are three pathways involved in the regulation of apoptosis, namely death receptor pathway, mitochondrial pathway, and endoplasmic reticulum pathway. Studies have confirmed that apoptosis of renal tubular epithelial cells is regulated by Bax, Bcl-2 and Caspase3 [28]. In the process of regulating apoptosis, Bax can form homologous or heterodimer with Bcl-2, antagonize the inhibitory effect of Bcl-2 on apoptosis, release inhibitory cytochrome c, activate Caspase3, and cause apoptosis [29]. In this study, treatment with TFPI (37.5, 75, and 150 mg/L) could reduce the expression of Bax, caspase3, and cleaved-caspase3 in a dose-dependent manner. Therefore, we speculate that the mechanism of TFPI protecting kidney may be through the mitochondrial pathway, antagonizing the expression of Pro apoptotic gene Bax, inhibiting the release of cytochrome c [30], thus reducing the activation of apoptosis executive protein caspase, inhibiting apoptosis, and reducing MSU induced HK-2 cell damage.
NLRP3 inflammasome is an immunosensor, which can participate in the immune, inflammatory, and metabolic processes of a variety of diseases [31]. It can respond to a variety of damage related molecular patterns and induce inflammatory response [32]. Modern studies have shown that three mechanisms can activate NLRP3 inflammasome [33]. The mechanism of activating inflammasome through ROS accumulation and release of TXNIP is an important way for stimulants to participate in inflammatory response through oxidative stress [34]. After the NLRP3 inflammasome is activated and assembled, procaspase1 is enzymolysis into the active state. Activated caspase1 can cleave and induce the precursor of IL-1β to mature and secrete out of the cell. Among them, the precursor of IL-1β is generally produced by activation of the NF-κB pathway [35]. Under normal conditions, NF-κB and IκBα exist in the cytoplasm as a complex. When the body is stimulated, IκBα is phosphorylated, which eventually leads to NF-κB phosphorylation activation and translocation to the nucleus, thus inducing immune and inflammatory reactions [36]. TLR4 is a recognition receptor on the cell membrane [37,38]. It has been reported that MSU can be recognized by TLR4 and activate its downstream signaling pathway. Through cascade reaction, it can finally induce NF-κB activation into the nucleus and release pro-inflammatory cytokines such as TNF-α and IL-1β [39]. In the present study, the protein expressions of TLR4, NF-κB, NLRP3, TXNIP, and p–NF–κB were significantly decreased after the treatment with TFPI, indicating that TFPI may inhibit MSU-induced inflammatory response through TLR4-NLRP3 pathway.
The excretion of UA needs the help of UA transporters. The current research shows that UA transporters mainly promote the reabsorption of UA and promote the excretion of UA [40]. ABCG2 is expressed in many organs of the body and in the brush border of proximal tubular epithelial cells in the kidney. Recently, more and more studies have proved that ABCG2 is a key urate transporter responsible for UA excretion, which is closely related to the occurrence of hyperuricemia [41]. In this study, the expression of ABCG2 protein increased after TFPI treatment, indicating that TFPI may be able to promote the expression of ABCG2 to increase renal uric acid excretion. At present, it has been suggested that IL-1β can inhibit the expression of ABCG2 mainly by inhibiting the expression of PDZK1 protein [13]. PDZK1 has no ability to transport urate but can regulate the expression of UA transporter. Therefore, the mechanism of TFPI regulating ABCG2 protein expression may be through reducing the release of IL-1β cytokines, thus promoting ABCG2 protein activation.
To sum up, TFPI helps to reduce UA and has a certain protective effect on renal injury caused by UA. However, this study also has some limitations. First, animal models can reflect pharmacological effects, but models are still limited because of the differences in UA metabolism between humans and animals [42]. Secondly, in this study, the bioactive components were not completely separated from the raw material extracts, and only total flavonoids were used for the study. Therefore, it is necessary to conduct a comprehensive and in-depth study of TFPI against HUA to provide support for the clinic.
5. Conclusions
In conclusion, our findings demonstrated for the first time that TFPI not only inhibited the development of UN but also inhibited the apoptosis and inflammatory response of HK-2 cells stimulated by MSU. Furthermore, TFPI can regulate the expression of ABCG2 by inhibiting the activation of the TLR4-NLRP3 inflammatory pathway. This study demonstrates the potential of TFPI in the treatment of UN and the main therapeutic mechanisms regulating the TLR4-NLRP3 pathway.
Author contribution statement
-
1)
Dongming Chen and Hong Lu conceived and designed the experiments.
-
2)
Dongming Chen and Chenlei Jiang performed the experiments.
-
3)
Dongming Chen analyzed and interpreted the data; wrote the paper.
-
4)
Hong Lu contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e12979.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Kanda E., Muneyuki T., Kanno Y., Suwa K., Nakajima K. Uric acid level has a U-shaped association with loss of kidney function in healthy people: a prospective cohort study. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0118031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su X.L., Xu B.Y., Yan B.J., Qiao X., Wang L.H. Effects of uric acid-lowering therapy in patients with chronic kidney disease: a meta-analysis. PLoS One. 2017;12(11):17. doi: 10.1371/journal.pone.0187550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roughley M.J., Belcher J., Mallen C.D., Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res. Ther. 2015;17 doi: 10.1186/s13075-015-0610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel S. Inflammasomes, the cardinal pathology mediators are activated by pathogens, allergens and mutagens: a critical review with focus on NLRP3. Biomed. Pharmacother. 2017;92:819–825. doi: 10.1016/j.biopha.2017.05.126. [DOI] [PubMed] [Google Scholar]
- 5.Yuan F., Kolb R., Pandey G., Li W., Sun L., Liu F., Sutterwala F.S., Liu Y., Zhang W. Involvement of the NLRC4-inflammasome in diabetic nephropathy. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0164135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An M.-F., Wang M.-Y., Shen C., Sun Z.-R., Zhao Y.-L., Wang X.-J., Sheng J. Isoorientin exerts a urate-lowering effect through inhibition of xanthine oxidase and regulation of the TLR4-NLRP3 inflammasome signaling pathway. J. Nat. Med. 2021;75(1):129–141. doi: 10.1007/s11418-020-01464-z. [DOI] [PubMed] [Google Scholar]
- 7.Ma C., Yang X., Lv Q., Yan Z., Chen Z., Xu D., Liu X., Yang W., Xing S. Soluble uric acid induces inflammation via TLR4/NLRP3 pathway in intestinal epithelial cells. Iran. J. Basic Med. Sci. 2020;23(6):744–750. doi: 10.22038/ijbms.2020.44948.10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H., Tian T., Miao H., Zhao Y.-Y. Traditional uses, fermentation, phytochemistry and pharmacology of Phellinus linteus: a review. Fitoterapia. 2016;113:6–26. doi: 10.1016/j.fitote.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen Tan T., Nguyen Ngoc T., Kuo P.-C., Doan Manh D., Doan Lan P., Dinh Thi Truong G., Wu T.-S., Tran Dinh T. Chemical constituents from the fruiting bodies of Phellinus igniarius. Nat. Prod. Res. 2018;32(20):2392–2397. doi: 10.1080/14786419.2017.1413572. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Z., Jin M., Zhou W., Li R., Zhao Y., Jin X., Li G. Anti-inflammatory activity of chemical constituents isolated from the willow bracket medicinal mushroom Phellinus igniarius (Agaricomycetes) Int. J. Med. Mushrooms. 2018;20(2):119–128. doi: 10.1615/IntJMedMushrooms.2018025536. [DOI] [PubMed] [Google Scholar]
- 11.Zheng Y.Y., Li C., Feng S.L., Huang X.Y., Liu Y.F., Chen X.F., Di D.L. Study on the content determination of total flavonoids in Olea europaea L. Leaves. Spectrosc. Spectr. Anal. 2011;31(2):547–550. [PubMed] [Google Scholar]
- 12.Li H.X., Zhang X.Y., Gu L.L., Li Q., Ju Y., Zhou X.B., Hu M. Anti-Gout effects of the medicinal fungus Phellinus igniarius in hyperuricaemia and acute gouty Arthritis rat models. Front. Pharmacol. 2022;12 doi: 10.3389/fphar.2021.801910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen M., Lu X., Lu C., Shen N., Jiang Y., Chen M., Wu H. Soluble uric acid increases PDZK1 and ABCG2 expression in human intestinal cell lines via the TLR4-NLRP3 inflammasome and PI3K/Akt signaling pathway. Arthritis Res. Ther. 2018;20 doi: 10.1186/s13075-018-1512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y., Li C., Duan S., Yuan X., Liang J., Hou S. Curcumin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Biomed. Pharmacother. 2019;118 doi: 10.1016/j.biopha.2019.109195. [DOI] [PubMed] [Google Scholar]
- 15.Burns C.M., Wortmann R.L. Gout therapeutics: new drugs for an old disease. Lancet. 2011;377(9760):165–177. doi: 10.1016/S0140-6736(10)60665-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu D., Liang X.C., Zhang H. Effects of high glucose on cell viability and differentiation in primary cultured schwann cells: potential role of ERK signaling pathway. Neurochem. Res. 2016;41(6):1281–1290. doi: 10.1007/s11064-015-1824-6. [DOI] [PubMed] [Google Scholar]
- 17.Rees F., Hui M., Doherty M. Optimizing current treatment of gout. Nat. Rev. Rheumatol. 2014;10(5):271–283. doi: 10.1038/nrrheum.2014.32. [DOI] [PubMed] [Google Scholar]
- 18.Lee I.-K., Yun B.-S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011;64(5):349–359. doi: 10.1038/ja.2011.2. [DOI] [PubMed] [Google Scholar]
- 19.Lung M.Y., Tsai J.C., Huang P.C. Antioxidant properties of edible basidiomycete Phellinus igniarius in submerged cultures. J. Food Sci. 2010;75(1):E18–E24. doi: 10.1111/j.1750-3841.2009.01384.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Mo S.Y., Wang S.J., Li S., Yang Y.C., Shi J.G. A unique highly oxygenated pyrano 4,3-c 2 benzopyran-1 6-dione derivative with antioxidant and cytotoxic activities from the fungus Phellinus igniarius. Org. Lett. 2005;7(9):1675–1678. doi: 10.1021/ol0475764. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., Wang S.J., Mo S.Y., Li S., Yang Y.C., Shi J.G., Phelligridimer A. A highly oxygenated and unsaturated 26-membered macrocyclic metabolite with antioxidant activity from the fungus Phellinus igniarius. Org. Lett. 2005;7(21):4733–4736. doi: 10.1021/ol0520875. [DOI] [PubMed] [Google Scholar]
- 22.Ichida K., Matsuo H., Takada T., Nakayama A., Murakami K., Shimizu T., Yamanashi Y., Kasuga H., Nakashima H., Nakamura T., Takada Y., Kawamura Y., Inoue H., Okada C., Utsumi Y., Ikebuchi Y., Ito K., Nakamura M., Shinohara Y., Hosoyamada M., Sakurai Y., Shinomiya N., Hosoya T., Suzuki H. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012;3 doi: 10.1038/ncomms1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaffo A.L., Jacobs D.R., Jr., Lewis C.E., Mikuls T.R., Saag K.G. Association between being African-American, serum urate levels and the risk of developing hyperuricemia: findings from the Coronary Artery Risk Development in Young Adults cohort. Arthritis Res. Ther. 2012;14(1) doi: 10.1186/ar3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S.Y., Guevara J.P., Kim K.M., Choi H.K., Heitjan D.F., Albert D.A. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res. 2010;62(2):170–180. doi: 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N., Li P., Pan J., Wang M., Long M., Zang J., Yang S. Bacillus velezensis A2 fermentation exerts a protective effect on renal injury induced by Zearalenone in mice. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-32006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Si Y., Park J.W., Jung S., Hwang G.-S., Goh E., Lee H.J. Layer-by-layer electrochemical biosensors configuring xanthine oxidase and carbon nanotubes/graphene complexes for hypoxanthine and uric acid in human serum solutions. Biosens. Bioelectron. 2018;121:265–271. doi: 10.1016/j.bios.2018.08.074. [DOI] [PubMed] [Google Scholar]
- 27.Xu X., Lai Y., Hua Z.-C. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci. Rep. 2019;39 doi: 10.1042/BSR20180992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao X.-C., Livingston M.J., Liang X.-L., Dong Z. In: Renal Fibrosis: Mechanisms and Therapies. Liu B.C., Lan H.Y., Lv L.L., editors. 2019. Cell apoptosis and autophagy in renal fibrosis; pp. 557–584. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Yang X., Ge X., Zhang F. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed. Pharmacother. 2019;109:726–733. doi: 10.1016/j.biopha.2018.10.161. [DOI] [PubMed] [Google Scholar]
- 30.Brentnall M., Rodriguez-Menocal L., De Guevara R.L., Cepero E., Boise L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14 doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui D., Liu S., Tang M., Lu Y., Zhao M., Mao R., Wang C., Yuan Y., Li L., Chen Y., Cheng J., Lu Y., Liu J. Phloretin ameliorates hyperuricemia-induced chronic renal dysfunction through inhibiting NLRP3 inflammasome and uric acid reabsorption. Phytomedicine. 2020;66 doi: 10.1016/j.phymed.2019.153111. [DOI] [PubMed] [Google Scholar]
- 32.Mangan M.S.J., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discovery. 2018;17(8):588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 33.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20(13) doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L.J., Jiang S.J., Liu X.Q., Tang Q., Chen Y., Qu J.J., Wang L., Wang Q., Wang Y.L., Wang J.M., Zhang Y., Kang W.Y. Inflammatory response and oxidative stress as mechanism of reducing hyperuricemia of gardenia jasminoides-poria cocos with network pharmacology. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/8031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 36.DiDonato J.A., Mercurio F., Karin M. NF-kappa B and the link between inflammation and cancer. Immunol. Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 37.Deng G., He H., Chen Z., OuYang L., Xiao X., Ge J., Xiang B., Jiang S., Cheng S. Lianqinjiedu decoction attenuates LPS-induced inflammation and acute lung injury in rats via TLR4/NF-kappa B pathway. Biomed. Pharmacother. 2017;96:148–152. doi: 10.1016/j.biopha.2017.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milanesi S., Verzola D., Cappadona F., Bonino B., Murugavel A., Pontremoli R., Garibotto G., Viazzi F. Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. J. Cell. Physiol. 2019;234(7):10868–10876. doi: 10.1002/jcp.27929. [DOI] [PubMed] [Google Scholar]
- 39.Huang Q., Gao W., Mu H., Qin T., Long F., Ren L., Tang H., Liu J., Zeng M. HSP60 regulates monosodium urate crystal-induced inflammation by activating the TLR4-NF-kappa B-MyD88 signaling pathway and disrupting mitochondrial function. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/8706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okuda C., Koyama H., Tsutsumi Z., Yamamoto A., Kurajoh M., Moriwaki Y., Yamamoto T. Serum CRP in patients with gout and effects of benzbromarone. Int. J. Clin. Pharm. Ther. 2011;49(3):191–197. doi: 10.5414/cp201425. [DOI] [PubMed] [Google Scholar]
- 41.Nigam S.K., Bhatnagar V. The systems biology of uric acid transporters: the role of remote sensing and signaling. Curr. Opin. Nephrol. Hypertens. 2018;27(4):305–313. doi: 10.1097/MNH.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu H.R., Song D.N., Zhao X. Potential applications and preliminary mechanism of action of dietary polyphenols against hyperuricemia: a review. Food Biosci. 2021;43 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.