Abstract
Background
Lung cancer is one of the leading causes of death in the world and the deadliest of all cancers. Apoptosis is a key pathway in regulating the cell growth rate, proliferation, and occurrence of lung cancer. This process is controlled by many molecules, such as microRNAs and their target genes. Therefore, finding new medical approaches such as exploring diagnostic and prognostic biomarkers involved in apoptosis is needed for this disease. In the present study, we aimed to identify key microRNAs and their target genes that could be used in the prognosis and diagnosis of lung cancer.
Methods
Signaling pathways, genes, and microRNAs involved in the apoptotic pathway were identified by bioinformatics analysis and recent clinical studies. Bioinformatics analysis was performed on databases including NCBI, TargetScan, UALCAN, UCSC, KEGG, miRPathDB, and Enrichr, and clinical studies were extracted from PubMed, web of science, and SCOPUS databases.
Results
NF-κB, PI3K/AKT, and MAPK pathways play critical roles in the regulation of apoptosis. MiR-146b, 146a, 21, 23a, 135a, 30a, 202, and 181 were identified as the involved microRNAs in the apoptosis signaling pathway, and IRAK1, TRAF6, Bcl-2, PTEN, Akt, PIK3, KRAS, and MAPK1 were classified as the target genes of the mentioned microRNAs respectively. The essential roles of these signaling pathways and miRNAs/target genes were approved through both databases and clinical studies. Moreover, surviving, living, BRUCE, and XIAP was the main inhibitor of apoptosis which act by regulating the apoptosis-involved genes and miRNAs.
Conclusion
Identifying the abnormal expression and regulation of miRNAs and signaling pathways in apoptosis of lung cancer can represent a novel class of biomarkers that can facilitate the early diagnosis, personalized treatment, and prediction of drug response for lung cancer patients. Therefore, studying the mechanisms of apoptosis including signaling pathways, miRNAs/target genes, and the inhibitors of apoptosis are advantageous for finding the most practical approach and reducing the pathological demonstrations of lung cancer.
Keywords: Lung cancer, Gene, miRNA, Signaling pathway, Apoptosis
Introduction
Lung cancer is the leading cause of cancer mortality, accounting for approximately 25% of all cancer deaths. Lung cancer is cancer that most often begins in the lungs but is sometimes the result of Cancer spreading from adjacent areas. Lung cancer is divided into two groups non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) [1, 2]. Risk factors for lung cancer include two groups of genetic and environmental factors, environmental factors such as smoking, asbestos exposure, radon gas, air pollution, and age [3–6]. It is estimated that 8 to 14% of lung cancers are caused by genetic factors [7]. This genetic damage affects normal cell function, including cell proliferation, apoptosis, and DNA repair. The greater the damage, the higher the risk of cancer [8]. Lung cancer is triggered by the activation of oncogenes or the inactivation of tumor suppressor genes. Mutations in the proto-oncogene K-ras cause 10–30% of adenocarcinomas [9, 10]. Mutations and amplification of EGFR have been investigated to be common in NSCLC [11, 12]. The p53 tumor suppressor gene is affected in 60–75% of cases [13, 14]. Other genes that are often mutated or amplified include c-MET [15], NKX2-1[16], LKB1 (STK11) [17], PIK3CA [18], Bcl-2 [19, 20] and BRAF [21].
Apoptosis is a crucial pathway in regulating the cell growth rate and proliferation and the occurrence of cancers such as lung cancer which is the result of poor function or inhibition of apoptosis [22, 23]. This process is an active and energy-dependent phenomenon in which genetic mechanisms and factors play a role in controlling and executing it with special programs [24]. In the path of apoptosis, many molecules, such as microRNAs and their target genes, are involved [25–27].
MicroRNAs are non-coding ribonucleic acids and have a length of 18–25 nucleotides [28]. MicroRNAs regulate the gene expression after transcription by mRNA degradation or inhibition of their translation [29]. These molecules can act as oncogenes or tumor inhibitors in several cancers [30, 31]. MicroRNAs play a direct role in cancer through interaction with target genes and the regulation of growth, apoptosis, cell differentiation, and proliferation [32].
In the present study, we aim to study apoptosis as one of the important signaling pathways in lung cancer. Poor function or inhibition of apoptosis plays an essential role in the occurrence of lung cancer, so identifying the genes and miRNAs that control this signaling pathway can be very effective in the diagnosis, prediction, and prevention of lung cancer.
Methods
In the present study, the genes and microRNAs involved in the apoptosis pathway of lung cancer were identified by databases and recent research studies. 100 articles were reviewed including 12 research papers and 88 review papers, which were extracted from PubMed, Web of Science, and SCOPUS databases and were published from 2000-to 2022. Additionally, the bioinformatics data were obtained using databases such as NCBI, TargetScan, UALCAN, UCSC, KEGG, miRPathDB, and Enrichr. The study is characterized in 4 sections including (1) signaling pathways of lung cancer, (2) apoptosis pathway, (3) the regulatory role of microRNAs in apoptosis, and (4) conclusion and future prospective. Following that, the IAPs and Bcl-2 pathways, which are the most important regulatory groups of apoptosis, were explained in detail. Moreover, in the section on prospects in cancer therapy, it was tried to show that the identified genes and microRNAs involved in the apoptosis pathway, can be used as Therapeutic approaches and considered appropriate biomarkers for diagnosis and prognosis of lung cancer.
Results
Signaling pathways of lung cancer
Control of important cellular processes, such as cell division or cell death, is the result of the function of molecules in cells that work in signaling pathways and interact with each other. The signaling pathways in lung cancer comprise:
RTKs pathway
RAS pathway
BRAF/MAPK pathway
PI3K pathway
LKB1/AMPK pathway,
TP53 pathway
RB1/MYC pathway
Wnt/β-catenin pathway
Epigenetic pathways
Oxidative stress
Apoptosis
The process of apoptosis, as a conserved method, is under the control of genes. This process plays important role in the development and maintenance of the body by destroying old cells, unnecessary cells, and unhealthy cells, and interferes with many immune system mechanisms or diseases. This process is crucial in regulating the growth rate, cell proliferation, development, and health of the body, and the occurrence of many autoimmune diseases, cancers and viral infections is the result of poor function or inhibition of apoptosis. Therefore, the main purpose of apoptotic studies is to focus on recognizing the molecular components and regulatory mechanisms, especially the Bcl-2 family and the IAP family as the most important regulatory groups, and this information helps to apply therapeutic agents that process this pathway. They affect the treatment of neurodegenerative diseases and reproductive diseases such as cancer.
The mechanism of apoptosis
Apoptosis is a form of programmed cell death that occurs in multicellular organisms [33]. Caspases are a family of cysteine proteases that play essential functional roles in the performance of apoptosis [34]. Mammalian caspases are functionally divided into three groups: initiator caspases (caspase 2, 8, 9, and 10), executioner caspases (caspase 3, 6, and 7), and inflammatory caspases (caspase 1, 4, 5, 11, and 12). Initiator caspases initiate the apoptosis signal while the executioner caspases conduct the mass proteolysis that leads to apoptosis [35–37]. Two main pathways of apoptosis are the Intrinsic Apoptosis Pathway and the Extrinsic Apoptosis [38].
The extrinsic pathway is initiated by the activation of death receptors such as FAS, tumor necrosis factor receptors (TNFRs), and TNF-related apoptosis-inducing ligand receptors (TRAILRs). Activation of death stimuli leads to the recruitment of death domain-containing adapter proteins such as Fas-associated protein with death domain (FADD) and TNFR1-associated death domain (TRADD). Subsequently, caspase-8, which is a downstream factor and an important mediator in the external pathway, is recruited. Finally, apoptosis begins when caspase-3 is activated by caspase-8 [33, 39–41].
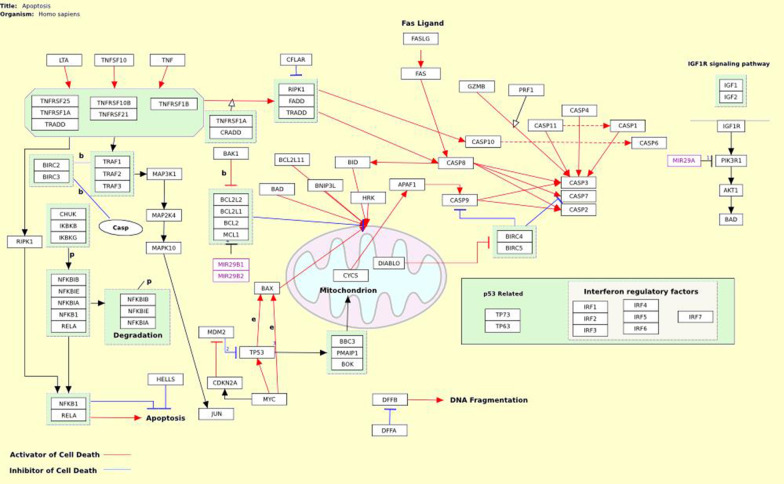
The intrinsic Apoptosis Pathway differs from extracellular signals that cause apoptosis through the extrinsic pathway. Chemotherapy, radiotherapy, and cellular stress, including DNA damage, oxidative stress, and energy starvation, activate the Intrinsic Apoptosis Pathway [42]. In the intrinsic pathway, pro-apoptotic signaling leads to the release of cytochrome C into the cytoplasm, formation of an apoptotic complex with apoptotic protease activating factor 1 (APAF1) and the inactive form of caspase-9, activation of caspase 9, activation of caspase 3, 6 and 7 and cell apoptosis [43]. The Bcl-2 family is an important regulator of apoptosis. Bcl-2 and Bcl-XL are anti-apoptotic proteins that inhibit the secretion of cytochrome C. Whereas Bax, Bak, and Bid are pro-apoptotic proteins that release it from the mitochondria [44, 45]. Research has shown that mutations in Bcl-2 cause many cancers, especially lung cancer [45] (Fig. 1).
Fig. 1.
The mechanism of apoptosis signaling pathway [46]
The inhibitors of apoptosis (IAPs)
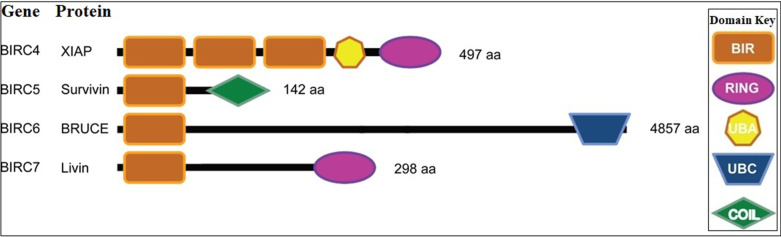
Lung cancer is the deadliest of all cancers [1, 2]. High resistance to chemotherapy and aggression has increased the need for reliable prognostic assay and effective treatments for the disease. Apoptosis is the main cellular process that plays a key role in the precise regulation of this pathway in cancer [47]. This process is regulated by several signaling pathways and three important factors affect the regulation of this process: IAP, IAP antagonists, and caspases [48, 49]. IAPs are a group of endogenous proteins that are known to control cell death and survival. These proteins play a regulatory role in apoptosis through the activation of caspases and the NF-κB signaling pathway [50, 51]. The main cause of resistance to chemotherapy and poor prognosis of lung cancer is the overexpression of IAP proteins, which are the main culprits in escaping apoptosis [52]. XIAP (BIRC4), Survivin (BIRC5), Livin (BIRC7), and BRUCE or Apollone ((BIRC6) are four members of the IAP family that play an important role in the development of chemoresistant lung cancer [51] (Fig. 2.) These data suggest that the identification of IAPs function is advantageous for finding the most practical approach and reducing the pathological demonstrations of cancers. Table 1 shows a summary of the data about IAP and IAP-based therapies which were obtained in recent clinical trials.
Fig. 2.
Schematic representation of human IAPs [53]. XIAP (BIRC4), Survivin (BIRC5), Livin (BIRC7), and BRUCE or Apollone ((BIRC6) are four members of the IAP family. The main cause of resistance to chemotherapy and poor prognosis of lung cancer is the overexpression of IAP proteins, which are the main culprits in escaping apoptosis
Table 1.
Pre-clinical data about IAP and IAP-based therapies in clinical trials
| IAP | Mechanism of inhibition | Increased sensitivity to | Refs. | Drug | Mode of action | Refs. | The regulatory miRNAs | Refs. |
|---|---|---|---|---|---|---|---|---|
| XIAP | Antisense |
Doxorubicin Taxol Vinorelbine Etoposide |
[69] | AEG35156 | Antisense | [70] | miR-142 | [71] |
| Embelin | Small molecule targeting BIR3 domain | [72] |
miR-192-5p miR-215 |
[73] | ||||
| Polyphenylureas/Xantags | Small molecule targeting BIR2 domain | [74] | ||||||
| Arylsulfonamides (TWX006, TWX024) | [75] | |||||||
| siRNA | Cisplatin | [64] | ||||||
| Survivin | siRNA |
Adriamycin Cisplatin Paclitaxol |
[76] [77] |
LY2181308 | Antisense | [78] | miR-195 | [79] |
| YM155 | Small molecule antagonist | [80] | miR-320 | [81] | ||||
| Shepherdin | Small molecule targeting Hsp90 | [82] |
miR-205 miR-218 |
[83] | ||||
| AICAR | [84] | |||||||
| Anti-Survivin Ab | Antibody | [85] | miR-203 | [86] |
Survivin
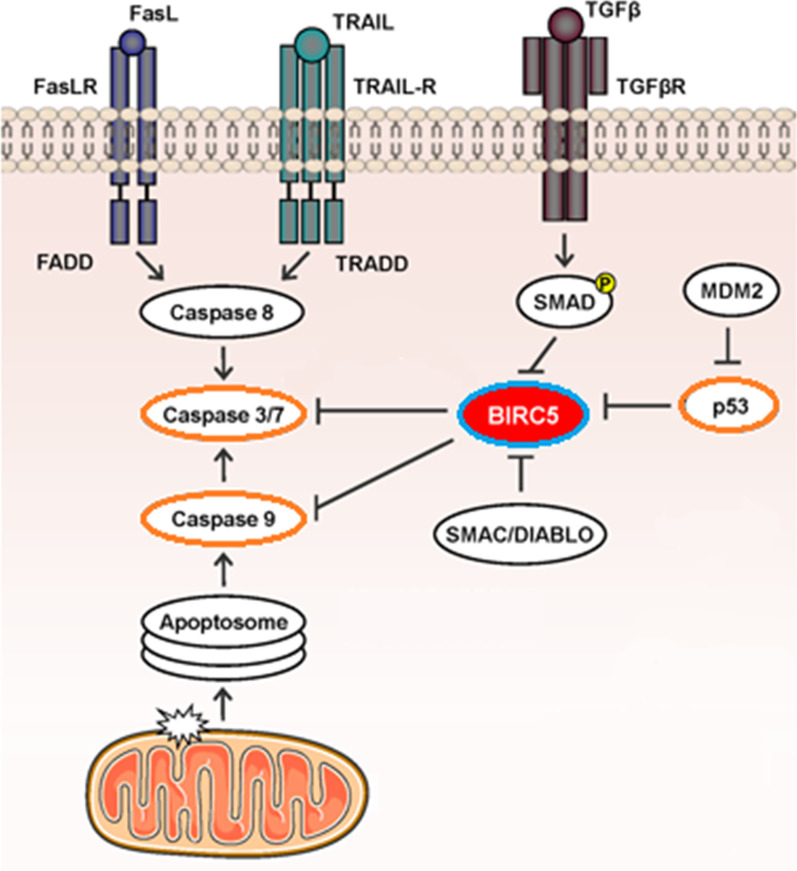
Survivin is a protein that is encoded by the BIRC5 gene in humans. Survivin is a member of the IAP family. This protein negatively regulates apoptosis through the inhibition of caspase3 and caspase7, which are the effector caspases in the signaling pathway of apoptosis [54, 55]. Survivin is only expressed in the G2-M phase and is highly regulated by the cell cycle [56, 57]. Moreover, the regulatory role of this protein in mitosis was shown in research [58, 59]. WNT/β-catenin Signaling and p53 protein were known to have essential roles in Survivin regulation [60–63]. Figure 3 shows the function of survivin (BIRC5) in the apoptosis pathway (http://atlasgeneticsoncology.org/).
Fig. 3.
BIRC5 (survivin) acts on cytoplasm and nucleus and is involved in different cellular functions: cell survival, cell cycle progression, and apoptosis. Survivin decreases apoptosis by suppressing caspase-3, 7, and 9. Also, survivin expression is regulated by WNT/β-catenin Signaling and p53 protein; p53 represses survivin expression and the Wnt signaling pathway upregulates survivin
XIAP
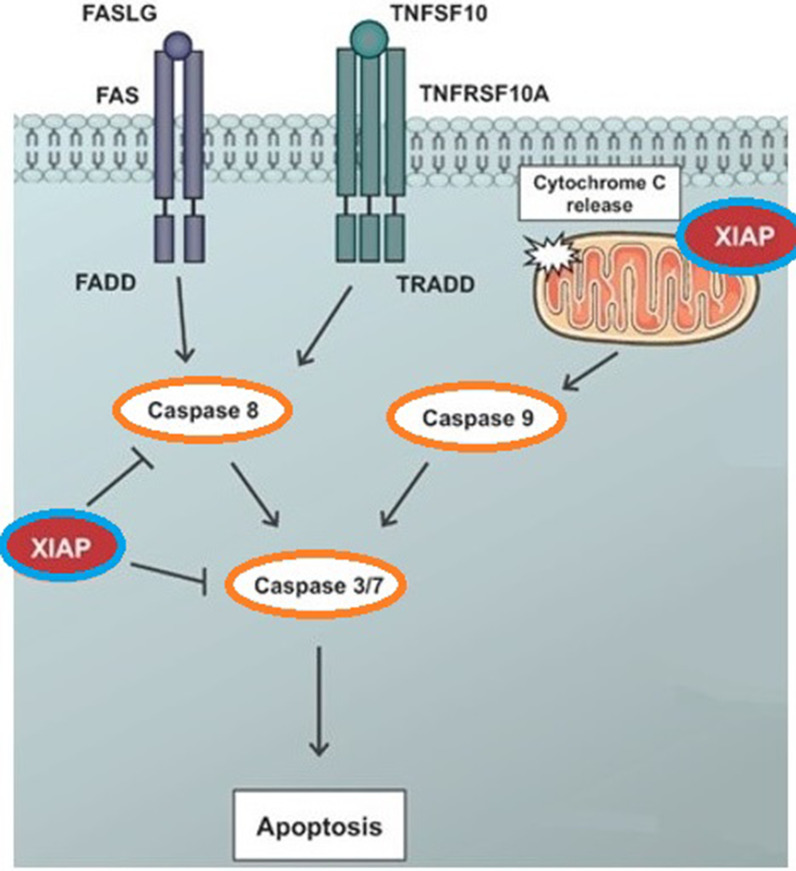
X-linked inhibitor of apoptosis protein (XIAP) is another member of the IAP family. XIAP also known as BIRC4, is a protein that is produced by the XIAP gene. This protein is a well-known apoptosis inhibitor in cancers such as colorectal, breast, pancreatic, and lung cancer [53]. XIAP inhibits apoptosis in humans, by inhibiting the activities of caspase 9 and its effectors caspase 3 and caspase 7 [64, 65]. In addition, Smac/DIABLO and Omi/HtrA2 are the two main XIAP inhibitors. Smac/DIABLO, can enhance apoptosis by binding to XIAP and preventing it from binding to caspases. This allows normal caspase activity to proceed [66–68]. Figure 4 shows the function of XIAP (BIRC4) in the apoptosis pathway (http://atlasgeneticsoncology.org/).
Fig. 4.
XIAP (BIRC4) is a multi-functional protein that is involved in cell death, cell cycle, cell migration, and apoptosis. The main function of XIAP is its antiapoptotic activity, which is performed by inhibiting caspase 9 and its effector's caspase 3 and caspase 7
The regulatory role of microRNAs in the apoptosis
The known regulatory role of microRNAs in the apoptosis pathway confirms their direct function in lung cancer. Figure 5 which was extracted from the KEGG database [87], showed the interaction of microRNAs with target genes in NSCLC. Apoptosis is involved in different stages of a living organism's biological evolution and, if left unchecked, can lead to cancer. The apoptosis signaling pathways include NF-κB, PI3K/AKT, MAPK, and P53. Many molecules, such as microRNAs are involved in the apoptosis pathway. The research and bioinformatics studies led to the identification of several microRNAs that have essential roles in the regulation of apoptosis. The involved miRNAs/Target Genes in the apoptosis signaling pathway were obtained from the miRPathDB database [88] and were shown in Table 2. In addition, the characteristics of miRNAs involved in apoptosis were extracted from miRDB [89] and the TargetScan database and were demonstrated in Table 3. MicroRNAs involved in apoptosis are classified into two categories based on their function: Proapoptotic and Antiapoptotic.
Fig. 5.
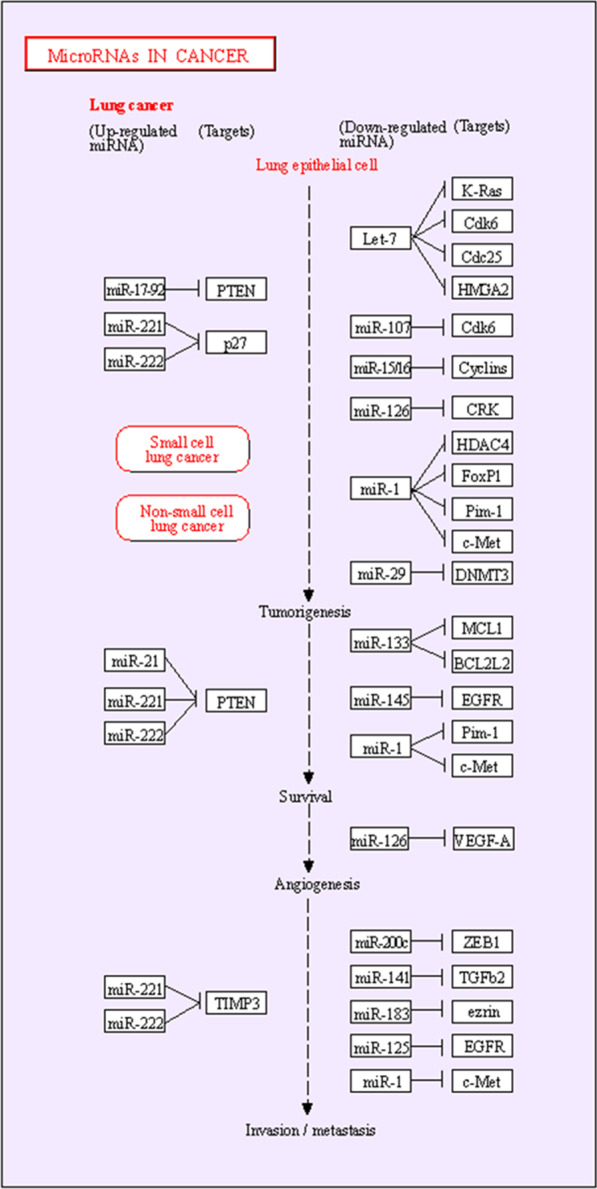
Schematic representation of the interaction between miRNAs and genes in NSCLC
Table 2.
The involved miRNAs/Target Genes in apoptosis signaling pathway
| miRNA | P-value | Targets |
|---|---|---|
| hsa-miR-221-3p | 0.005 | APAF1,BBC3,BCL2L11,BMF,BNIP3,FOS,NAIP,TNFSF10 |
| hsa-miR-125b-5p | 0.016 | BAK1,BBC3,BCL2,BCL2L2,BMF,CDKN2A,MCL1,TP53 |
| hsa-miR-21-5p | 0.004 | APAF1,BCL2,CASP8, FAS,FASLG,IRAK1,MYD88,NFKB |
| hsa-miR-181a-5p | 0.009 | BAX,BCL2,BCL2L11,FOS,MCL1,XIAP |
| hsa-miR-146a-5p | 0.011 | CASP7,FADD,FAS,IRAK1,NFKB1, TRAF6 |
| hsa-miR-139-5p | 0.001 | BCL2,FOS,JUN,MCL1,NFKB1 |
| hsa-miR-181b-5p | 0.014 | BCL2,BCL2L11,FOS,MCL1,XIAP |
| hsa-let-7g-5p | 0.003 | BCL2L1,CASP3,CDKN2A,TNFRSF10B |
| hsa-miR-106b-5p | 0.026 | BCL2L11,CASP7,CASP8,TNFRSF10A |
| hsa-miR-7-5p | 0.031 | BAX,BCL2,FOS,XIAP |
| hsa-miR-133b | 0.037 | BCL2L1,BCL2L2,FAS,MCL1 |
| hsa-miR-148a-3p | 0.041 | BAX,BCL2,BCL2L11,IKBKB |
| hsa-miR-582-5p | 7.70e-4 | CASP3,CASP9,MCL1 |
| hsa-miR-491-5p | 0.009 | BCL2L1,CAPNS1,TP53 |
| hsa-miR-146b-5p | 0.012 | IRAK1,NFKB1,TRAF6 |
| hsa-miR-708-5p | 0.020 | BCL2,BIRC5,CASP2 |
| hsa-miR-149-5p | 0.026 | BBC3,FASLG,MYD88 |
| hsa-miR-497-5p | 0.038 | BCL2,BIRC5,IKBKB |
| hsa-let-7c-5p | 0.039 | BCL2L1,CASP3,TNFRSF10B |
| hsa-miR-224-5p | 0.041 | BCL2,CASP3,CASP7 |
| hsa-miR-339-3p | 0.002 | MCL1,NFKB1 |
| hsa-miR-365a-3p | 0.019 | BAX,BCL2 |
| hsa-miR-630 | 0.027 | BCL2,BCL2L2 |
| hsa-miR-197-3p | 0.034 | BMF,PMAIP1 |
| hsa-miR-33b-5p | 0.037 | BCL2,XIAP |
| hsa-miR-363-3p | 0.038 | BCL2L11,CASP3 |
| hsa-miR-301a-3p | 0.040 | BCL2L11,MAP3K5 |
| hsa-miR-125b-1-3p | 0.044 | BIK,TP53 |
| hsa-miR-30e-5p | 0.049 | CASP3,TP53 |
Table 3.
Characteristics of miRNAs involved in apoptosis
| Signaling pathway | miRNA name | Gene symbol | Gene description | Target Score | Number of 3P-seq tags supporting UTR + 5 | Total context + + score |
|---|---|---|---|---|---|---|
| NF-κB | MiR-146b-5p | IRAK1 | Interleukin 1 Receptor Associated Kinase 1 | 99 | 54 | − 0.56 |
| MiR-146a-5p | TRAF6 | TNF receptor associated factor 6 | 100 | 91 | − 1.00 | |
| MiR-21 | Bcl-2 | B-cell lymphoma 2 | 85 | 1380 | − 0.12 | |
| PI3K/Akt | MiR-21 | PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha | 87 | 112 | − 0.21 |
| MiR-1269 | PTEN | Phosphatase and tensin homolog | 54 | 118 | − 0.44 | |
| MiR-23a | 99 | 118 | − 0.52 | |||
| MiR-181 | 67 | 118 | − 0.18 | |||
| MiR-135a | Akt | AKT Serine/Threonine Kinase | 70 | 525 | − 0.21 | |
| PIK3R2 | Phosphoinositide-3-Kinase Regulatory Subunit 2 | 89 | 1484 | − 0.35 | ||
| MiR-30a-5p | PIK3CD | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Delta | 93 | 5 | − 0.09 | |
| PIK3R2 | Phosphoinositide-3-Kinase Regulatory Subunit 2 | 53 | 1484 | − 0.07 | ||
| MAPK | MiR-202 | KRAS | KRAS Proto-Oncogene, GTPase | 94 | 40 | − 0.58 |
| MiR-181a | MAPK1 | Mitogen-Activated Protein Kinase 1 | 79 | 630 | − 0.16 |
NF-κB
MiR-21 is an Antiapoptotic miR and plays an inhibitory role in the apoptotic pathway by regulating the PI3K / Akt / NF-κB signaling pathway. Studies have shown that the miR-21 inhibitor in NSCLC cells can induce apoptosis by inhibiting the PI3K / Akt / NF-κB pathway. Induction of apoptosis by miR-21 inhibitor occurs as a result of caspase-dependent pathway regulation and increased caspase-3, 8, and 9 activities. Additionally, inactivation of Bcl-2 was observed following treatment with the miR-21 inhibitor [90].
miR-146b is another molecule that has been identified to play an inhibiting role in the apoptosis pathway. So that miR-146b inhibits IRAK4 which is an important gene in the NF-κB pathway. On the other hand, miR-146b-5p has an inhibitory effect on IL-6 and IL-8, which are NF-κB regulated chemokines, in lung cancer cells [91] .
MiR-135b is an oncogene miRNA in lung cancer that is overexpressing and enhances the invasiveness, angiogenesis, proliferation, and antiapoptosis of cancer cells. miR-135b significantly activates NF-κB. The luciferase reporter assay showed that NF-κB reporter luciferase activity in miR-135b transfected cells is higher than in control A549 cells. In addition, the upregulation of NF-κB downstream genes, such as Bcl-2, Bcl-xL, A20VEGFC, IL-1β, and IL-6 was observed as a result of miR-135b overexpression [92].
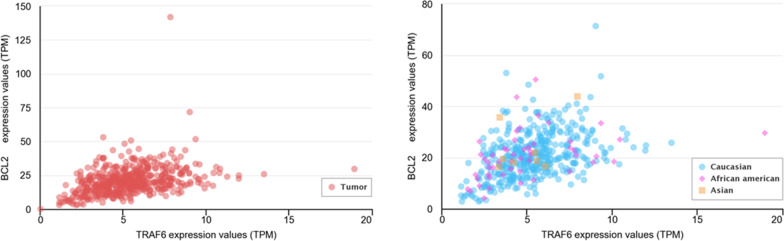
MiR-146a-5p Inhibits apoptosis and Plays an oncogenic role in lung cancer. TRAF6 is an important gene in the NF-κB signal pathway and has a critical role in lung cancer tumorigenesis. TRAF6 is a direct target of miR-146a-5p in lung cancer cells. Therefore, miR-146a-5p binds to TRAF6 directly and suppresses this gene. MiR-146a regulates the main gene TRAF6 and continually Bcl2 via the NF-KB signaling pathway that induces apoptosis in lung cancer [93] (Fig. 6) [94]. In addition, the results of statistical analyzes in the miRDB and Target Scan databases show that miR-146a targets part 3'UTR of the TRAF6 gene in the NF-KB pathway and Bcl2 is one of the important genes involved in apoptosis.
Fig. 6.
Gene expression correlation between TRAF6 and BCL-2 in lung cancer
PI3K/AKT
The PI3K/AKT signaling pathway is a particularly important pathway with a key role in apoptosis [95]. The PTEN gene is one of the most important genes in the apoptotic pathway. This gene acts as a suppressor tumor and increases the death of cancer cells and prevents their proliferation. Mutations in this gene are an important factor in the progression of lung cancer. Inactivation of PTEN increases the proliferation and invasion of cancer cells by activating the PI3K-AKT-NFkB signaling pathway. This gene is regulated by several microRNAs.
MiR-1269 targets PTEN directly in lung cancer cells. Using Luciferase Assay, it was shown that increasing the expression of miR-1269 in A549 cells significantly reduces the expression of PTEN compared to normal cells. In contrast, the miR-1269 inhibitor increases PTEN expression, thereby increasing the apoptosis of cancer cells [96]. In addition, Luciferase Assay in cancer stem cells (CSCs) isolated from NSCLC cells showed that PTEN is the target of miR-23a. As a result, suppression of proliferation and activation of apoptosis occurs through the activation of the PI3K-AKT pathway [97]. miR-181 regulates the PTEN / PI3K / AKT signaling pathway in A549 lung cells. Decreased expression of miR-181 suppresses the PTEN / PI3K / AKT pathway and thus activates lung cancer apoptotic cells [98]. MiR-135a promotes cell apoptosis through the IGF-1/PI3K/Akt signaling pathway in NSCLC [99]. Moreover, miR-30a-5p induces apoptosis by regulating the PI3K/AKT pathway. MiR-30a-5p inhibits the expression of PIK3R2 and PIK3CD which are two subunits of PI3K [100].
MAPK
The MAPK pathway (also known as the Ras-Raf-MEK-ERK pathway), plays an essential role in the apoptosis pathway. MAPKs can both activate or inhibits apoptosis depending on the cell type and the stimulus. Three subfamilies of MAPKs have been identified: extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38-MAPKs. The regulation of apoptosis is by JNKs and p38-MAPKs whereas, ERKs are important for cell survival [101–103]. This signaling pathway is regulated by several microRNAs.
MiR-202 inhibits the Ras / MAPK pathway by targeting the KRas gene and consequently, promotes apoptotic signaling in NSCLC A549 cells [104]. Furthermore, the upregulation of miR-181a leads to inhibition of the MAPK pathway by suppressing MAPK1 and MAP2K1 expression. This results in increased induction of apoptosis.
Conclusion and future prospective
Apoptosis is a crucial pathway in regulating cell proliferation. So, the occurrence of many cancers such as lung cancer is the result of poor function or inhibition of apoptosis. This process is under the control of genes. In addition, the interaction of microRNAs with target genes determines their role in apoptosis and confirms the direct function of microRNAs in cancer. Some microRNAs create the oncogenic phenotype by reducing the expression of genes that suppress tumors, while others target proto-oncogenic mRNAs and turn them off, to reduce the process of becoming cancerous.
Both apoptosis and microRNAs are important in cancer. Identification of microRNAs and their target molecules has provided a clear horizon for understanding the pathways that lead to cancer. Therefore, microRNAs can be used as potential biomarkers in the diagnosis, prognosis, and treatment of cancer. With the knowledge gained from microRNAs, new therapies have been developed. Recently, the treatment of cancer cells by inserting microRNAs that are involved in apoptosis has been proposed in research. Therefore, studying oncogenes, tumor suppressor genes, miRNAs/target genes, and their associated pathways/genetic networks is advantageous for finding the most practical approach and reducing the pathological demonstrations of cancers. However, more clinical studies are needed to find the role of biomarkers in important cellular pathways such as apoptosis. In addition, understanding the diagnostic and therapeutic potential of microRNAs and target genes in cancer requires the integration of these biomarker data. According to preclinical data existing to date, using IAPs in combination with standard anti-cancer therapy yields in favorable outcome. Further extensive research validates these data on clinical grounds, and identify whether apoptosis pathway targeting miRNAs have utility as a novel class of biomarkers which can facilitate the early diagnosis, personalized treatment, and prediction of drug response for lung cancer patients.
Acknowledgements
We thank our colleagues from the Department of Mycobacteriology and Pulmonary Research, Microbiology Research Center, Pasteur Institute of Iran.
Abbreviations
- NSCLC
Non-small cell lung cancer
- SCLC
Small cell lung cancer
- EGFR
Epidermal growth factor receptor precursor
- NKX2-1
NK2 Homeobox 1
- STK11
Serine/Threonine Kinase 11
- PIK3CA
Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha
- Bcl-2
B-cell lymphoma 2
- BRAF
B-Raf Proto-Oncogene, Serine/Threonine Kinase
- MAPK
Mitogen-Activated Protein Kinase
- FAS
Fas cell surface death receptor
- TNFR
Tumor necrosis factor receptor
- TRAILR
TNF-related apoptosis-inducing ligand receptors
- FADD
Fas-associated protein with death domain
- TRADD
TNFR1-associated death domain
- APAF1
Apoptotic protease activating factor 1
- BAX
BCL2 Associated X, Apoptosis Regulator
- BAK
BCL2 Antagonist/Killer
- Bid
BH3 Interacting Domain Death Agonist
- IAP
Inhibitors of apoptosis
- BIRC
Baculoviral IAP repeat-containing protein
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- TRAF6
TNF receptor associated factor 6
- PTEN
Phosphatase and tensin homolog
- PI3K
Phosphatidylinositol-4,5-Bisphosphate 3-Kinase
- ERK
Extracellular signal-regulated kinas
- JNK
C-Jun N-terminal kinas
- FASLG
Fas Ligand (TNF Superfamily, Member 6)
- TNFSF10
TNF Superfamily Member 10
Author contributions
HA, analyzed data, interpreted data; performed the experiments, MA, contributed data or analysis tools, read and edited the manuscript, MSH: designed and supervised, contributed data or analysis tools, read and approved the manuscript. All authors read and approved the final manuscript.
Funding
No funding.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abolfathi H, et al. The comparison and evaluation of the miR-16, miR-155 and miR-146a expression pattern in the blood of TB and NSCLC patients: a research paper. Gene Reports. 2021;22:100967. doi: 10.1016/j.genrep.2020.100967. [DOI] [Google Scholar]
- 2.Abolfathi H, et al. Studies in lung cancer cytokine proteomics: a review. Expert Rev Proteomics. 2021;18(1):49–64. doi: 10.1080/14789450.2021.1892491. [DOI] [PubMed] [Google Scholar]
- 3.Yu F, et al. Combined effects of lung disease history, environmental exposures, and family history of lung cancer to susceptibility of lung cancer in Chinese non-smokers. Respir Res. 2021;22(1):210. doi: 10.1186/s12931-021-01802-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baratella E, et al. Accuracy of CT-guided core-needle biopsy in diagnosis of thoracic lesions suspicious for primitive malignancy of the lung: a five-year retrospective analysis. Tomography. 2022;8(6):2828–2838. doi: 10.3390/tomography8060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baratella E, et al. Aging-related findings of the respiratory system in chest imaging: pearls and pitfalls. Curr Radiol Rep. 2023;11(1):1–11. doi: 10.1007/s40134-022-00405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang HJ, et al. Relationship of the lung microbiome with PD-L1 expression and immunotherapy response in lung cancer. Respir Res. 2021;22(1):322. doi: 10.1186/s12931-021-01919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanwal M, Ding X-J, Cao Y. Familial risk for lung cancer. Oncol Lett. 2017;13(2):535–542. doi: 10.3892/ol.2016.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borges HL, Linden R, Wang JYJ. DNA damage-induced cell death: lessons from the central nervous system. Cell Res. 2008;18(1):17–26. doi: 10.1038/cr.2007.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jancík S, et al. Clinical relevance of KRAS in human cancers. J Biomed Biotechnol. 2010;2010:150960–150960. doi: 10.1155/2010/150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 2017;9(5):52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metro G, Crinò L. Advances on EGFR mutation for lung cancer. Translational lung cancer research. 2012;1(1):5–13. doi: 10.3978/j.issn.2218-6751.2011.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noronha V, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124–136. doi: 10.1200/JCO.19.01154. [DOI] [PubMed] [Google Scholar]
- 13.Rivlin N, et al. Mutations in the p53 tumor suppressor gene: important milestones at the various steps of tumorigenesis. Genes Cancer. 2011;2(4):466–474. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, et al. Upregulation and activation of p53 by erastin-induced reactive oxygen species contribute to cytotoxic and cytostatic effects in A549 lung cancer cells. Oncol Rep. 2018;40(4):2363–2370. doi: 10.3892/or.2018.6585. [DOI] [PubMed] [Google Scholar]
- 15.Awad MM, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-met overexpression. J Clin Oncol. 2016;34(7):721–730. doi: 10.1200/JCO.2015.63.4600. [DOI] [PubMed] [Google Scholar]
- 16.Mollaoglu G, et al. The lineage-defining transcription factors SOX2 and NKX2-1 determine lung cancer cell fate and shape the tumor immune microenvironment. Immunity. 2018;49(4):764–779.e9. doi: 10.1016/j.immuni.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, et al. CPS1 maintains pyrimidine pools and DNA synthesis in KRAS/LKB1-mutant lung cancer cells. Nature. 2017;546(7656):168–172. doi: 10.1038/nature22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savli H, et al. TP53, EGFR and PIK3CA gene variations observed as prominent biomarkers in breast and lung cancer by plasma cell-free DNA genomic testing. J Biotechnol. 2019;300:87–93. doi: 10.1016/j.jbiotec.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Gu RH, et al. Diagnostic value of the combined detection of CEA, NSE and IL-18 for lung cancer and their relationship with apoptosis gene Bcl-2. J Biol Regul Homeost Agents. 2020;34(5):1637–1646. doi: 10.23812/20-34-A. [DOI] [PubMed] [Google Scholar]
- 20.Li S, et al. Oridonin enhances the radiosensitivity of lung cancer cells by upregulating Bax and downregulating Bcl-2. Exp Ther Med. 2018;16(6):4859–4864. doi: 10.3892/etm.2018.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Planchard D, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18(10):1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 22.Ji K, et al. Regulation of apoptosis and radiation sensitization in lung cancer cells via the Sirt1/NF-κB/Smac pathway. Cell Physiol Biochem. 2018;48(1):304–316. doi: 10.1159/000491730. [DOI] [PubMed] [Google Scholar]
- 23.Tang X, et al. Sirtuin 3 induces apoptosis and necroptosis by regulating mutant p53 expression in small-cell lung cancer. Oncol Rep. 2020;43(2):591–600. doi: 10.3892/or.2019.7439. [DOI] [PubMed] [Google Scholar]
- 24.Sevrioukova IF. Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid Redox Signal. 2011;14(12):2545–2579. doi: 10.1089/ars.2010.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amri J, et al. Combination of two miRNAs has a stronger effect on stimulating apoptosis, inhibiting cell growth and increasing erlotinib sensitivity relative to single miRNA in A549 lung cancer cells. Biotechnol Appl Biochem. 2021 doi: 10.1002/bab.2211. [DOI] [PubMed] [Google Scholar]
- 26.Li J, et al. miRNA-1284 inhibits cell growth and induces apoptosis of lung cancer cells. Mol Med Rep. 2017;16(3):3049–3054. doi: 10.3892/mmr.2017.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amri J, Molaee N, Karami H. Up-regulation of MiRNA-125a-5p inhibits cell proliferation and increases EGFR-TKI induced apoptosis in lung cancer cells. Asian Pac J Cancer Prev. 2019;20(11):3361–3367. doi: 10.31557/APJCP.2019.20.11.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratti M, et al. MicroRNAs (miRNAs) and long non-coding RNAs (lncRNAs) as new tools for cancer therapy: first steps from bench to bedside. Target Oncol. 2020;15(3):261–278. doi: 10.1007/s11523-020-00717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomankova T, Petrek M, Kriegova E. Involvement of microRNAs in physiological and pathological processes in the lung. Respir Res. 2010;11(1):159. doi: 10.1186/1465-9921-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan W, et al. MicroRNAs and cancer: key paradigms in molecular therapy (Review) Oncol Lett. 2018;15(3):2735–2742. doi: 10.3892/ol.2017.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoballa MH, et al. Identification of a novel intergenic miRNA located between the human DDC and COBL genes with a potential function in cell cycle arrest. Mol Cell Biochem. 2018;444(1–2):179–186. doi: 10.1007/s11010-017-3242-3. [DOI] [PubMed] [Google Scholar]
- 32.Si W, et al. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin Epigenetics. 2019;11(1):25. doi: 10.1186/s13148-018-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaczanowski S, Sajid M, Reece SE. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasit Vectors. 2011;4(1):44. doi: 10.1186/1756-3305-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22(53):8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 35.Chang HY, Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64(4):821–846. doi: 10.1128/MMBR.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan TJ, et al. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37(11):719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 37.Sakamaki K, Satou Y. Caspases: evolutionary aspects of their functions in vertebrates. J Fish Biol. 2009;74(4):727–753. doi: 10.1111/j.1095-8649.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, et al. Virus infection and death receptor-mediated apoptosis. Viruses. 2017;9(11):316. doi: 10.3390/v9110316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G, et al. Role of autophagy and apoptosis in non-small-cell lung cancer. Int J Mol Sci. 2017;18(2):367. doi: 10.3390/ijms18020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashkenazi A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008;19(3–4):325–331. doi: 10.1016/j.cytogfr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Fulda S, et al. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrido C, et al. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13(9):1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 44.Hata AN, Engelman JA, Faber AC. The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015;5(5):475–487. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26(9):1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelder T, et al. WikiPathways: building research communities on biological pathways. Nucleic Acids Res. 2011;40(D1):D1301–D1307. doi: 10.1093/nar/gkr1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pistritto G, et al. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8(4):603–619. doi: 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong RSY. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30(1):87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasudevan D, Ryoo HD. Regulation of cell death by IAPs and their antagonists. Curr Top Dev Biol. 2015;114:185–208. doi: 10.1016/bs.ctdb.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fulda S. Molecular pathways: targeting inhibitor of apoptosis proteins in cancer–from molecular mechanism to therapeutic application. Clin Cancer Res. 2014;20(2):289–295. doi: 10.1158/1078-0432.CCR-13-0227. [DOI] [PubMed] [Google Scholar]
- 51.Mohamed MS, et al. Inhibitors of apoptosis: clinical implications in cancer. Apoptosis. 2017;22(12):1487–1509. doi: 10.1007/s10495-017-1429-4. [DOI] [PubMed] [Google Scholar]
- 52.Obexer P, Ausserlechner MJ. X-linked inhibitor of apoptosis protein - a critical death resistance regulator and therapeutic target for personalized cancer therapy. Front Oncol. 2014;4:197–197. doi: 10.3389/fonc.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Owens TW, et al. Inhibitor of apoptosis proteins: promising targets for cancer therapy. J Carcinog Mutagen. 2013 doi: 10.4172/2157-2518.S14-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, et al. Survivin and tumorigenesis: molecular mechanisms and therapeutic strategies. J Cancer. 2016;7(3):314–323. doi: 10.7150/jca.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Altieri DC. Survivin and IAP proteins in cell-death mechanisms. Biochem J. 2010;430(2):199–205. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chandele A, et al. Upregulation of survivin in G2/M cells and inhibition of caspase 9 activity enhances resistance in staurosporine-induced apoptosis. Neoplasia. 2004;6(1):29–40. doi: 10.1016/S1476-5586(04)80051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jaiswal PK, Goel A, Mittal RD. Survivin: a molecular biomarker in cancer. Indian J Med Res. 2015;141(4):389–397. doi: 10.4103/0971-5916.159250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Temme A, et al. Localization, dynamics, and function of survivin revealed by expression of functional survivinDsRed fusion proteins in the living cell. Mol Biol Cell. 2003;14(1):78–92. doi: 10.1091/mbc.e02-04-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mita AC, et al. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14(16):5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 60.Lai Y-J, et al. Expression of survivin and p53 modulates honokiol-induced apoptosis in colorectal cancer cells. J Cell Biochem. 2014;115(11):1888–1899. doi: 10.1002/jcb.24858. [DOI] [PubMed] [Google Scholar]
- 61.Or CR, et al. Obatoclax, a Pan-BCL-2 inhibitor, downregulates survivin to induce apoptosis in human colorectal carcinoma cells via suppressing WNT/β-catenin signaling. Int J Mol Sci. 2020 doi: 10.3390/ijms21051773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu G, et al. SPAG5 contributes to the progression of gastric cancer by upregulation of Survivin depend on activating the wnt/β-catenin pathway. Exp Cell Res. 2019;379(1):83–91. doi: 10.1016/j.yexcr.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 63.Chawla D, et al. Role of survivin and p53 expression in response of primary culture of ovarian cancer cells to treatment with chemotherapeutic agents. Int J Gynecol Cancer. 2018;28(6):1239–1246. doi: 10.1097/IGC.0000000000001281. [DOI] [PubMed] [Google Scholar]
- 64.Cheng YJ, et al. XIAP-mediated protection of H460 lung cancer cells against cisplatin. Eur J Pharmacol. 2010;627(1–3):75–84. doi: 10.1016/j.ejphar.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3(6):401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 66.Yang Q-H, et al. Omi/HtrA2 catalytic cleavage of inhibitor of apoptosis (IAP) irreversibly inactivates IAPs and facilitates caspase activity in apoptosis. Genes Dev. 2003;17(12):1487–1496. doi: 10.1101/gad.1097903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzuki Y, et al. Mitochondrial protease Omi/HtrA2 enhances caspase activation through multiple pathways. Cell Death Differ. 2004;11(2):208–216. doi: 10.1038/sj.cdd.4401343. [DOI] [PubMed] [Google Scholar]
- 68.Maas C, et al. Smac/DIABLO release from mitochondria and XIAP inhibition are essential to limit clonogenicity of Type I tumor cells after TRAIL receptor stimulation. Cell Death Differ. 2010;17(10):1613–1623. doi: 10.1038/cdd.2010.39. [DOI] [PubMed] [Google Scholar]
- 69.Hu Y, et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res. 2003;9(7):2826–2836. [PubMed] [Google Scholar]
- 70.Dean E, et al. Phase I trial of AEG35156 administered as a 7-day and 3-day continuous intravenous infusion in patients with advanced refractory cancer. J Clin Oncol. 2009;27(10):1660–1666. doi: 10.1200/JCO.2008.19.5677. [DOI] [PubMed] [Google Scholar]
- 71.Wu XB, et al. MiR-142 inhibits lung cancer cell proliferation and promotes apoptosis by targeting XIAP. Eur Rev Med Pharmacol Sci. 2019;23(17):7430–7437. doi: 10.26355/eurrev_201909_18852. [DOI] [PubMed] [Google Scholar]
- 72.Dai Y, et al. Natural IAP inhibitor Embelin enhances therapeutic efficacy of ionizing radiation in prostate cancer. Am J Cancer Res. 2011;1(2):128–143. [PMC free article] [PubMed] [Google Scholar]
- 73.Ye M, et al. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015;357(1):196–205. doi: 10.1016/j.canlet.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 74.Schimmer AD, et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5(1):25–35. doi: 10.1016/S1535-6108(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 75.Wu TY, et al. Development and characterization of nonpeptidic small molecule inhibitors of the XIAP/caspase-3 interaction. Chem Biol. 2003;10(8):759–767. doi: 10.1016/S1074-5521(03)00157-1. [DOI] [PubMed] [Google Scholar]
- 76.Yonesaka K, et al. Small interfering RNA targeting survivin sensitizes lung cancer cell with mutant p53 to adriamycin. Int J Cancer. 2006;118(4):812–820. doi: 10.1002/ijc.21350. [DOI] [PubMed] [Google Scholar]
- 77.Yang H, et al. Influence of SiRNA targeting survivin on chemosensitivity of H460/cDDP lung cancer cells. J Int Med Res. 2008;36(4):734–747. doi: 10.1177/147323000803600416. [DOI] [PubMed] [Google Scholar]
- 78.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcinogenesis. 2007;28(6):1133–1139. doi: 10.1093/carcin/bgm047. [DOI] [PubMed] [Google Scholar]
- 79.Yu X, et al. miR-195 targets cyclin D3 and survivin to modulate the tumorigenesis of non-small cell lung cancer. Cell Death Dis. 2018;9(2):193. doi: 10.1038/s41419-017-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iwasa T, et al. Marked anti-tumour activity of the combination of YM155, a novel survivin suppressant, and platinum-based drugs. Br J Cancer. 2010;103(1):36–42. doi: 10.1038/sj.bjc.6605713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li C, et al. LncRNA HCP5 stimulates the proliferation of non-small cell lung cancer cells by up-regulating survivin through the down-regulation of miR-320. Cancer Manag Res. 2020;12:1129–1134. doi: 10.2147/CMAR.S222221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Plescia J, et al. Rational design of shepherdin, a novel anticancer agent. Cancer Cell. 2005;7(5):457–468. doi: 10.1016/j.ccr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 83.Zarogoulidis P, et al. MiR-205 and miR-218 expression is associated with carboplatin chemoresistance and regulation of apoptosis via Mcl-1 and Survivin in lung cancer cells. Cell Signal. 2015;27(8):1576–1588. doi: 10.1016/j.cellsig.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 84.Meli M, et al. Small-molecule targeting of heat shock protein 90 chaperone function: rational identification of a new anticancer lead. J Med Chem. 2006;49(26):7721–7730. doi: 10.1021/jm060836y. [DOI] [PubMed] [Google Scholar]
- 85.Yagihashi A, et al. Detection of autoantibodies to survivin and livin in sera from patients with breast cancer. Clin Chim Acta. 2005;362(1–2):125–130. doi: 10.1016/j.cccn.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 86.Wang S, et al. MicroRNA-mediated epigenetic targeting of Survivin significantly enhances the antitumor activity of paclitaxel against non-small cell lung cancer. Oncotarget. 2016;7(25):37693–37713. doi: 10.18632/oncotarget.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kanehisa M, et al. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49(D1):D545–d551. doi: 10.1093/nar/gkaa970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kehl T, et al. miRPathDB 2.0: a novel release of the miRNA Pathway Dictionary Database. Nucleic Acids Res. 2019;48(D1):D142–D147. doi: 10.1093/nar/gkz1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y, Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48(D1):D127–D131. doi: 10.1093/nar/gkz757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou B, et al. Effect of miR-21 on apoptosis in lung cancer cell through inhibiting the PI3K/ Akt/NF-κB signaling pathway in vitro and in vivo. Cell Physiol Biochem. 2018;46(3):999–1008. doi: 10.1159/000488831. [DOI] [PubMed] [Google Scholar]
- 91.Liu YN, et al. miR-146b-5p enhances the sensitivity of NSCLC to EGFR Tyrosine Kinase Inhibitors by Regulating the IRAK1/NF-κB Pathway. Mol Ther Nucleic Acids. 2020;22:471–483. doi: 10.1016/j.omtn.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao J, et al. STAT3/miR-135b/NF-κB axis confers aggressiveness and unfavorable prognosis in non-small-cell lung cancer. Cell Death Dis. 2021;12(5):493. doi: 10.1038/s41419-021-03773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X, et al. miR-146a-5p plays an oncogenic role in NSCLC via suppression of TRAF6. Front Cell Dev Biol. 2020;8:847. doi: 10.3389/fcell.2020.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chandrashekar DS, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meng Y, et al. Role of the PI3K/AKT signalling pathway in apoptotic cell death in the cerebral cortex of streptozotocin-induced diabetic rats. Exp Ther Med. 2017;13(5):2417–2422. doi: 10.3892/etm.2017.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang W, et al. miR-1269b drives cisplatin resistance of human non-small cell lung cancer via modulating the PTEN/PI3K/AKT signaling pathway. Onco Targets Ther. 2020;13:109–118. doi: 10.2147/OTT.S225010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han Z, et al. Inhibition of miR-23a increases the sensitivity of lung cancer stem cells to erlotinib through PTEN/PI3K/Akt pathway. Oncol Rep. 2017;38(5):3064–3070. doi: 10.3892/or.2017.5938. [DOI] [PubMed] [Google Scholar]
- 98.Liu J, Xing Y, Rong L. miR-181 regulates cisplatin-resistant non-small cell lung cancer via downregulation of autophagy through the PTEN/PI3K/AKT pathway. Oncol Rep. 2018;39(4):1631–1639. doi: 10.3892/or.2018.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou Y, et al. Effect of microRNA-135a on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell Physiol Biochem. 2017;42(4):1431–1446. doi: 10.1159/000479207. [DOI] [PubMed] [Google Scholar]
- 100.Wang F, et al. Combination therapy of gefitinib and miR-30a-5p may overcome acquired drug resistance through regulating the PI3K/AKT pathway in non-small cell lung cancer. Ther Adv Respir Dis. 2020;14:1753466620915156. doi: 10.1177/1753466620915156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kralova J, et al. p38 MAPK plays an essential role in apoptosis induced by photoactivation of a novel ethylene glycol porphyrin derivative. Oncogene. 2008;27(21):3010–3020. doi: 10.1038/sj.onc.1210960. [DOI] [PubMed] [Google Scholar]
- 102.Guo Y-J, et al. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li L, et al. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol Lett. 2016;12(5):3045–3050. doi: 10.3892/ol.2016.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun W, et al. miR-202 enhances the anti-tumor effect of cisplatin on non-small cell lung cancer by targeting the Ras/MAPK pathway. Cell Physiol Biochem. 2018;51(5):2160–2171. doi: 10.1159/000495835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.