Abstract
The discovery of slow-cycling cells at the corneal periphery three decades ago established the limbus as the putative corneal stem cell niche. Since then, studies have underscored the importance of the limbal stem cells in maintaining the health and function of the ocular surface. Advancements in our understanding of stem cell biology have been successfully translated into stem cell therapies for corneal diseases. However, recent developments in mouse genetics, intravital imaging, and single cell genomics have revealed the complexity of the limbal stem cells, from their molecular identity, function, and interactions with their niche environment. Continued efforts to elucidate stem cell dynamics of this extraordinary tissue are critical for not only understanding stem cell biology but advancing therapeutic innovation and development.
Keywords: cornea, limbus, epithelium, stem cells, niche, differentiation, regeneration
Introduction
A highly specialized tissue, the cornea is comprised of three distinct cellular layers, a stratified epithelium, a collagenous stroma containing specialized fibroblasts, and a single layer of endothelial cells. The cornea protects the intraocular contents from external insults and transmits and refracts light due to its transparency and curvature. Decades of research have revealed how critical the corneal epithelium is for maintaining the health and function of the ocular surface. These functions are aided by the extraordinary regenerative capacity of the corneal epithelium.
Like other stratified epithelia, the process of renewal is driven by the coordinated proliferation of cells in the basal layer and terminal differentiation and shedding of cells in the suprabasal layers. As a result, a continuous vertical flux of cells is generated as new cells are produced in the basal layer to replace those shed from the surface (1,2). However, in striking contrast to the cutaneous epidermis or esophagus, the corneal epithelium exhibits a secondary flux of cells within the basal layer, where cells are mobilized from the periphery to the center of the cornea (1,3–9). For many decades, the prevailing view was that this behavior was driven by an imbalance in the potency of the basal cells. Short-lived transient amplified cells (TACs) in the central cornea were assumed to be replenished by slow-cycling, long-lived stem cells from a niche in the corneal periphery called the limbus. The appearance of distinct radial clonal stripes in lineage tracing experiments provided critical evidence to espouse this theory (10–13). Innovative mouse genetics, intravital imaging, and single cells genomics have subsequently uncovered significant cellular heterogeneity of the corneal stem cells and have shed new light on their molecular identity, function, and regulation. New experimental evidence is challenging the current dogma and support a revised model for corneal maintenance and regeneration that accounts for greater cellular diversity and complexity at the limbus than previously assumed. Collectively, these studies reveal the complex dynamics that drive the behaviors of these captivating cells. In this review, we provide an update on recent developments on our understanding of the limbal stem cells and their therapeutic relevance.
Compartmentalization of heterogeneous stem cell populations support corneal regeneration
The corneal epithelium consists of a single layer of basal cells and 4–6 superficial layers of stratified squamous cells. It has been estimated that complete turnover of the corneal epithelium takes about 2 weeks in both humans and mice, continuously replenished and maintained by the limbal stem cells (14–16). While early studies suggested a homogenous population of stem cells, recent evidence from various organs support the concept of inherent heterogeneity within the resident stem cell populations (17–19). A large host of concurrent genomic studies, both in human and mouse corneas, previously revealed specific gene expression signatures that distinguished more quiescent, long lived limbal stem cells from their more active transiently amplified progeny. From these studies, conserved marker genes have emerged, such as GPHA2, which encodes a surface marker protein that is specifically expressed in both human and mouse limbal stem cells (20–24). Evidence from in vitro and in vivo experiments suggest that expression of GPHA2 is critical for self-renewal of limbal stem cells and is furthermore dependent on cytokine mediated crosstalk with immune cells in the niche (20,21,23,24). This, as well as other, unique markers emerging from these studies are potentially not only important for the prospect of developing more effective protocols for isolating limbal stem cells for research and clinical use but also for elucidating general mechanisms of stem cell regulation across different tissues. For example, in the skin, GPHA2 as well as IFTM3 were found to be distinctly expressed in epidermal stem cells in the G0 phase (25).
However, recent single-cell gene expression analysis data also appear to challenge some previously accepted limbal stem cell markers (20–24,26–29). Abcg2 and Abcb5, for instance, were not found to be abundantly or exclusively expressed in the targeted populations though they were implicated beforehand as limbal stem cell markers (21–23,26–29). As such, discrepancies between RNAseq data have impeded the identification of definitive stem cell marker(s). Contributing to the conflicting findings are several issues that include the inherent dynamism exhibited by the limbal stem cells (29); ill-defined topography of the limbal stem cell niche; varying approaches to isolating limbal stem cells (30); and contrasting transcriptome analysis methods. Advancements in genomic methods that include direct visualization of transcripts in intact tissue, such as spatial transcriptomics, will hopefully help to resolve some of these differing results.
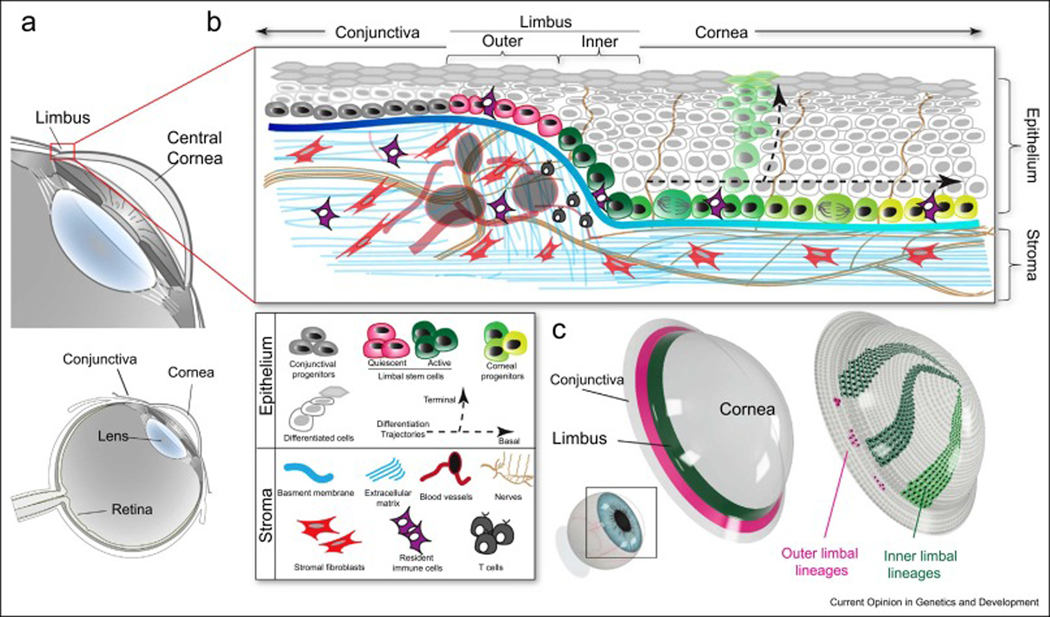
Distinctive clonal dynamics and spatial organization within the mouse limbus have disclosed a second level of heterogeneity in the limbal stem cells. Unlike the human limbus where the limbal stem cells are organized in radiating columns referred to as palisades of Vogt, the mouse limbus is an undefined, narrow area of cells encircling the cornea (Figure 1). Recent studies have identified in the mouse limbus subpopulations that primarily support homeostatic maintenance and subpopulations primarily involved in injury repair and regeneration (17–19). Using lineage tracing, quantitative clonal analyses, and intravital imaging, three independent studies dissected the activity of limbal stem cells in the mouse cornea and found at least two populations with discrete localization and growth patterns (Figure 1 a, b) (24,31,32). These subpopulations are organized circumferentially, dividing the limbus into two compartments (Figure 1 c). Stem cells in the inner limbus are active and undergo mostly symmetric cell divisions to replenish their pool and generate more differentiated progeny. These transient amplified progenitors then exit the niche and expand centripetally with increased probability to commit to terminal differentiation. When these cells differentiate, they move away from the basal layer towards the corneal surface to maintain the epithelial barrier before being shed from the corneal surface.
Figure 1. Compartmentalized organization of stem cells in the ocular surface epithelium.
(a) Schematic of the anatomy of the mammalian eye. Inset shows a magnification of the ocular anterior segment and the location of the cornea and limbus. (b) Cross section diagram of the mouse cornea illustrating the components of the limbal stem cell niche. The limbus is located at the intersection between the conjunctival and corneal epithelia, which share a common surface. Stem cells and progenitors are located in the basal layer of the stratified epithelium. At least two distinct stem cell populations exist in the limbal niche. Mostly quiescent stem cells are located in the outer limbus. These stem cells do not contribute directly to the homeostasis of either the conjunctiva or the cornea but can be activated after large scale injury to the corneal epithelium. Stem cells in the inner limbus are active and undergo mostly symmetric cell divisions to generate transient amplified progenitors. These exit the limbus and drift centripetally while continuing to proliferate. Cells in the basal layer can commit to terminal differentiation by intrinsic or extrinsic cues, to replenish the cells that are shed form the epithelial surface. The probability of basal progenitors committing to terminal differentiation increases towards the center of the cornea. The stroma consists of cellular and non-cellular components that can regulate and influence the activity and fate of stem cells. (c) 3D model of the cornea showing 11 the organization of the limbal compartments and the clonal behavior of the stem cells within.
Stem cells identified in the compartment corresponding to the outer limbus appear to be more quiescent and by lineage tracing analysis display only local clonal dynamics. Under normal physiologic conditions, the outer limbal stem cells do not contribute to the corneal epithelium nor the conjunctival epithelium that lies on the other side of the limbus. Instead, these outer limbal stem cells can be induced to mobilize after a large epithelial injury to repair and regenerate the corneal epithelium. The functional and spatial stem cell organization in the limbus mirror those found in other tissues, including the hair follicle, intestinal and hematopoietic niches, but the significance in these other organ systems is less clear (17–19,33–35). In the eye, the limbal stem cells may be critical for maintaining the distinct compartmentalization of the corneal epithelium from the conjunctival epithelium. Alternatively, or in concert, the separation of active and quiescent stem cells may ensure the presence of a reserve population in times of urgent need, like after an acute injury. The most recent studies have expanded the toolbox of available markers that distinguish the subpopulations within the limbus, such as TP63, K14, K15, Lrig1, GPHA2 and Slc1a3. However, given the discrepancies that often arise when comparing different experimental approaches used to characterize stem cells (RNA sequencing, immunohistochemistry, knock-in in vivo reporters, Cre-LoxP based lineage tracing, etc.), caution should be exercised for the absolute specificity of these markers, especially for identifying and isolating the different stem cell populations present in the limbus (5,12,24,31,32).
Clinical implications of emerging corneal single cell biology
The cornea is one of the few tissues where stem cell therapy has been successfully employed in humans. For decades, corneal epithelial stem cells have been used to for various ocular surface and corneal disorders including limbal stem cell deficiency (LSCD). LSCD is a condition characterized by the loss of limbal stem cells resulting in dysfunction of the ocular surface. Etiology may be genetic, acquired, or idiopathic, ranging from PAX6 gene mutations, aniridia, inflammatory/immunogenic disorders to trauma, chemical/thermal burns, and iatrogenic from ocular surgery (36,37). Diagnosis is primarily based on clinical findings and cytologic evidence, which include confirming the presence of goblet cells or absence of corneal epithelial markers on the corneal surface (38). While mild cases of LSCD may be managed with medical therapy, severe cases can only be treated surgically with either grafting of tissue or transplantation of cultivated cells (36,39).
Since the first transplantation performed by Jose Barraquer, limbal stem cell therapies have evolved with our growing understanding of the limbal stem cells (40). Current approaches include direct transplantation of autologous or allogenic limbal tissue, as well as transplantation of cultivated autologous or allogenic limbal stem cells. These advances have led to successful clinical outcomes that include improvement in visual acuity, corneal reepithelialization, and reduced inflammation (36,40,41). In a meta-analysis by Le et al., the authors found an overall improvement in ocular surface disease by 74.5% after limbal stem cell transplantation (39). Of the four common stem cell therapies analyzed, direct autologous limbal transplantation resulted in the greatest improvement in visual acuity by 76% (39) Nevertheless, there remains tremendous opportunity and potential to further develop limbal stem cell therapies. For example, despite its success, direct autologous limbal transplantation can only be employed in unilateral cases of LSCD because it requires tissue to be harvested from the healthy, contralateral eye. Additionally, LSCD in the healthy donor eye have been reported after this procedure since a large segment of tissue is harvested from the patient’s healthy eye (39,42,43). If direct allogeneic limbal transplantation is necessary, as in the case of bilateral disease, Le et al. found only a 52.3% improvement in visual acuity with a 27.6% rejection rate (39).
Therefore, attention has turned to alternative approaches, such as limbal epithelial grafts. These procedures require only small amounts of tissue to be harvested from a donor eye, minimizing the risk of inducing LSCD in a healthy eye (36). Cultivated limbal epithelial transplantation (CLET) and, more recently, simple limbal epithelial transplantation (SLET) involve the excision of a small limbal area from a healthy donor and transplanting either cultivated corneal epithelial sheets or fragmented donor tissue, respectively, to the ocular surface (36,42). Success rates for both procedures have been shown to be similar to direct autologous limbal transplants (36,42). Underlying this progress is the discovery of limbal stem cell markers, which have included Np63 (44), ABCB5 (45), and Keratin 15 (12). It has been shown that cases transplanted with >3% p63+ limbal stem cells yield higher success rates (46). Norrick et al. recently assessed the feasibility of using an alternative marker, ABCB5+ to isolate p63+ limbal stem cells since using p63 as a marker poses unique clinical challenges given its nuclear localization while ABCB5 is expressed on the cell surface (41). Though the researchers found that 50% of ABCB5+ cells co-express p63, the efficacy of ABCB5+ limbal stem cells in restoring the ocular surface remains to be seen from the recently completed interventional, open-label, multicenter Phase I/IIa clinical trial on allogeneic transplantation with ABCB5+ limbal stem cells (Clinicaltrials.gov NCT03549299) (41). Nevertheless, many of the established markers do not distinguish active stem cells from quiescent ones, which continues to impede the isolation of the limited stem cells from the limbus. Identification of new potential markers from recent single-cell studies (Table 1) may potentially overcome this obstacle (20–24,26–29).
Table 1.
| Gene | Function | Species | Reference | |
|---|---|---|---|---|
| Limbal Stem Cells | SCRG1 | Secreted ligand | Human | Ligocki et al. [16] |
| FRZB | Wnt-binding protein | Human | Ligocki et al. [16] | |
| GPHA2 | Subunit of the dimeric glycoprotein hormone family | Human, Mouse | Collin et al. [19], Altshuler et al. [24], Ligocki et al. [16] | |
| CAV1 | Scaffolding protein within caveolar membranes | Human | Catala et al. [17] | |
| TSPAN7 | Cell-surface, signal transduction protein | Human | Collin et al. [19], Li et al. [18] | |
| SOX17 | Transcription factor | Human | Li et al. [18] | |
| IFITM3 | Antiviral membrane protein | Mouse, Human | Altshuler et al. [24], Dou et al. [20] | |
| TXNIP | Metabolic protein involved in redox regulation | Mouse, Human | Kaplan et al. [34], Collin et al. [19] | |
| Corneal Transient Amplified Progenitors | CKS2 | Protein subunit of cyclindependent kinases | Human | Ligocki et al. [16], Catala et al. [17] |
| UBE2C | Ubiquitin-conjugating enzyme | Human | Ligocki et al. [16], Catala et al. [17], Li et al. [18] | |
| CDC20 | Cell cycle regulatory protein | Human | Li et al. [18] | |
| STMN1 | Microtubule regulatory protein | Human | Catala et al. [17] |
Another area of great interest in limbal stem cell therapy is the reconstitution of the stem cell niche, driven by our greater understanding of limbal stem cell dynamics and niche interactions. LSCD due to injuries such as severe chemical/thermal burns or ocular surgeries is not only characterized by limbal stem cell loss but destruction of the limbal niche. Recent studies have investigated the contributions of the limbal niche extracellular matrix (ECM) to limbal stem cell survival and cellular programming. Unlike the central corneal stroma, the limbal ECM is enriched with fibronectin, α2 and β2-laminin, Tenascin C, and Wnt ligands, as well as mesenchymal stem cells (MSCs) (36,47,48). MSCs, in particular, are of clinical interest since they have been shown to affect the innate and acquired immune response via anti-inflammatory and growth factors, which could mitigate the loss of limbal stem cells and stem cell markers from prolonged inflammation (49,50). While epithelial cells from the host have been detected on the corneal surface after LSCD transplantation, suggesting the limbal niche may be revitalized (51), other evidence indicate limbal stem cell transplantation and a healthy stroma are not sufficient in restoring the stem cell niche (52,53). In vivo confocal microscopy has shown that CLET alone, for example, fails to recreate the limbal niche (52,53). As such, early phase clinical trials are underway to examine the efficacy of transplanting MSCs with limbal stem cells to reconstruct the limbal niche (54–57). Additional effort in understanding the underlying cellular programming of LSCs is also ongoing to develop therapies using transformed human induced pluripotent stem cells and various biocompatible scaffolding to replenish the stem cell population and replicate the niche (58,59).
Concluding remarks
Easy accessibility to the limbal stem cells and niche has facilitated not only our understanding of limbal stem cell biology but also the development of limbal stem cell-based therapies. Current data paint a vibrant picture of the complex and often intersecting molecular processes that underlie the identity, functions, and fates of the limbal stem cells. However, the intrinsic and extrinsic regulatory mechanisms that determine the state of differentiation of stem cells in the cornea remain unresolved and are unlikely to be determined by a single factor. Thus far, intrinsic mechanisms have included epigenetic regulatory elements, transcription factors, autocrine and paracrine molecular signals, and circadian clock oscillations (60–71). However, it is becoming increasingly clear that stem cells rely on cues from the tissue microenvironment to control their fate. These extrinsic factors not only comprise static factors, such as the ECM, but modifiable niche factors as well, such as immune cells, that are capable of modulating corneal stem cell behavior in response to various stimuli (24,72–78). While present-day concepts suggest each of these regulatory mechanisms are appointed specific roles, it remains to be seen how they are all integrated at the cellular level to maintain tissue homeostasis. These recent studies highlight the dynamism of stem cell regulation and heterogeneity, which also reflect their complexity. Advancements in understanding limbal stem cell programming will not only expand our understanding of stem cells in general but could have immediate translational therapeutic impact. Therefore, the study of limbal stem cells can help preserve vision in individuals and shed light on the broader field of stem cell research.
Acknowledgements
We apologize to our esteemed colleagues whose work could not be cited due to space limitations. This work was supported by the National Institutes of Health and National Eye Institute (R01EY030599) to P.R. and (R01EY033291) to V.L., and the Yanoff Endowment Fund (V.L.)
Footnotes
Conflict of interest statement
The authors declare no direct competing financial or non-financial interests.
References:
Papers of particular interest, published within the period of review, have been highlighted as: • of special interest
- 1.Di Girolamo N. Moving epithelia: Tracking the fate of mammalian limbal epithelial stem cells. Prog Retin Eye Res. 2015. Sep;48:203–25. [DOI] [PubMed] [Google Scholar]
- 2.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983. Oct;24(10):1442–3. [PubMed] [Google Scholar]
- 3.Collinson JM, Morris L, Reid AI, Ramaesh T, Keighren MA, Flockhart JH, et al. Clonal analysis of patterns of growth, stem cell activity, and cell movement during the development and maintenance of the murine corneal epithelium. Dev Dyn Off Publ Am Assoc Anat. 2002. Aug;224(4):432–40. [DOI] [PubMed] [Google Scholar]
- 4.Mort RL, Ramaesh T, Kleinjan DA, Morley SD, West JD. Mosaic analysis of stem cell function and wound healing in the mouse corneal epithelium. BMC Dev Biol. 2009. Dec;9(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Girolamo N, Bobba S, Raviraj V, Delic NC, Slapetova I, Nicovich PR, et al. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells Dayt Ohio. 2015. Jan;33(1):157–69. [DOI] [PubMed] [Google Scholar]
- 6.Amitai-Lange A, Altshuler A, Bubley J, Dbayat N, Tiosano B, Shalom-Feuerstein R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells Dayt Ohio. 2015. Jan;33(1):230–9. [DOI] [PubMed] [Google Scholar]
- 7.Dorà NJ, Hill RE, Collinson JM, West JD. Lineage tracing in the adult mouse corneal epithelium supports the limbal epithelial stem cell hypothesis with intermittent periods of stem cell quiescence. Stem Cell Res. 2015. Nov;15(3):665–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasetti RB, Gaddipati S, Tian S, Xue L, Kao WWY, Lu Q, et al. Study of corneal epithelial progenitor origin and the Yap1 requirement using keratin 12 lineage tracing transgenic mice. Sci Rep. 2016. Dec;6(1):35202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West JD, Mort RL, Hill RE, Morley SD, Collinson JM. Computer simulation of neutral drift among limbal epithelial stem cells of mosaic mice. Stem Cell Res. 2018. Jul;30:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amitai-Lange A, Berkowitz E, Altshuler A, Dbayat N, Nasser W, Suss-Toby E, et al. A Method for Lineage Tracing of Corneal Cells Using Multi-color Fluorescent Reporter Mice. J Vis Exp. 2015. Dec 18;(106):53370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson A, Lobo EP, Delic NC, Myerscough MR, Lyons JG, Wakefield D, et al. Keratin-14-Positive Precursor Cells Spawn a Population of Migratory Corneal Epithelia that Maintain Tissue Mass throughout Life. Stem Cell Rep. 2017. Oct;9(4):1081–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasser W, Amitai-Lange A, Soteriou D, Hanna R, Tiosano B, Fuchs Y, et al. Corneal-Committed Cells Restore the Stem Cell Pool and Tissue Boundary following Injury. Cell Rep. 2018. Jan;22(2):323–31. [DOI] [PubMed] [Google Scholar]
- 13.Park M, Richardson A, Pandzic E, Lobo EP, Whan R, Watson SL, et al. Visualizing the Contribution of Keratin-14+ Limbal Epithelial Precursors in Corneal Wound Healing. Stem Cell Rep. 2019. Jan;12(1):14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cenedella RJ, Fleschner CR. Kinetics of corneal epithelium turnover in vivo. Studies of lovastatin. Invest Ophthalmol Vis Sci. 1990. Oct;31(10):1957–62. [PubMed] [Google Scholar]
- 15.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell. 1989. Apr;57(2):201–9. [DOI] [PubMed] [Google Scholar]
- 16.Buck RC. Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Invest Ophthalmol Vis Sci. 1985. Sep;26(9):1296–9. [PubMed] [Google Scholar]
- 17.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990. Jun 29;61(7):1329–37. [DOI] [PubMed] [Google Scholar]
- 18.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008. Dec 12;135(6):1118–29. [DOI] [PubMed] [Google Scholar]
- 19.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011. Dec 9;334(6061):1420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ligocki AJ, Fury W, Gutierrez C, Adler C, Yang T, Ni M, et al. Molecular characteristics and spatial distribution of adult human corneal cell subtypes. Sci Rep. 2021. Aug 11;11(1):16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collin J, Queen R, Zerti D, Bojic S, Dorgau B, Moyse N, et al. A single cell atlas of human cornea that defines its development, limbal progenitor cells and their interactions with the immune cells. Ocul Surf. 2021. Jul;21:279–98. • Comprehensive scRNA-and ATAC-Seq analysis of human corneal cell populations at three developmental stages (embryonic, fetal, adult). This study identified GPHA2 as a putative limbal stem cell marker that is downregulated when limbal cells are expanded ex vivo.
- 22.Dou S, Wang Q, Qi X, Zhang B, Jiang H, Chen S, et al. Molecular identity of human limbal heterogeneity involved in corneal homeostasis and privilege. Ocul Surf. 2021. Jul;21:206–20. [DOI] [PubMed] [Google Scholar]
- 23.Li DQ, Kim S, Li JM, Gao Q, Choi J, Bian F, et al. Single-cell transcriptomics identifies limbal stem cell population and cell types mapping its differentiation trajectory in limbal basal epithelium of human cornea. Ocul Surf. 2021. Apr;20:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Altshuler A, Amitai-Lange A, Tarazi N, Dey S, Strinkovsky L, Hadad-Porat S, et al. Discrete limbal epithelial stem cell populations mediate corneal homeostasis and wound healing. Cell Stem Cell. 2021. Jul;28(7):1248–1261.e8. • This study demonstrated the compartmentalized organization of the mouse limbus by single cell gene expression analysis. It identified GPHA2 as a marker of limbal stem cells whose expression is inhibited following immune suppression, suggesting T-cells as a niche factor that may be critical for regulating limbal stem cell activity.
- 25.Dekoninck S, Hannezo E, Sifrim A, Miroshnikova YA, Aragona M, Malfait M, et al. Defining the Design Principles of Skin Epidermis Postnatal Growth. Cell. 2020. Apr;181(3):604–620.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Català P, Groen N, Dehnen JA, Soares E, van Velthoven AJH, Nuijts RMMA, et al. Single cell transcriptomics reveals the heterogeneity of the human cornea to identify novel markers of the limbus and stroma. Sci Rep. 2021. Dec;11(1):21727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JM, Kim S, Zhang Y, Bian F, Hu J, Lu R, et al. Single-Cell Transcriptomics Identifies a Unique Entity and Signature Markers of Transit-Amplifying Cells in Human Corneal Limbus. Investig Opthalmology Vis Sci. 2021. Jul 23;62(9):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaplan N, Wang J, Wray B, Patel P, Yang W, Peng H, et al. Single-Cell RNA Transcriptome Helps Define the Limbal/Corneal Epithelial Stem/Early Transit Amplifying Cells and How Autophagy Affects This Population. Investig Opthalmology Vis Sci. 2019. Aug 16;60(10):3570. • This study pioneered scRNA-seq analysis of corneal epithelial and stromal heterogeneity, identifying novel putative limbal stem cell markers and providing insight on the role of autophagy in corneal epithelial cell proliferation.
- 29.Guo ZH, Jia YYS, Zeng YM, Li ZF, Lin JS. Transcriptome analysis identifies the differentially expressed genes related to the stemness of limbal stem cells in mice. Gene. 2021. Apr;775:145447. [DOI] [PubMed] [Google Scholar]
- 30. Song Z, Tsai CH, Mei H. Comparison of different methods to isolate mouse limbal epithelial cells. Exp Eye Res. 2021. Nov;212:108767. • Four methods to isolate mouse limbal stem cells were compared for cell yield and viability. Enzymatic digestion of whole globes with an intact limbus yielded higher cell numbers and viability than digestion of excised limbal tissue.
- 31.Ishii R, Yanagisawa H, Sada A. Defining compartmentalized stem cell populations with distinct cell division dynamics in the ocular surface epithelium. Development. 2020. Dec 15;147(24):dev197590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farrelly O, Suzuki-Horiuchi Y, Brewster M, Kuri P, Huang S, Rice G, et al. Two-photon live imaging of single corneal stem cells reveals compartmentalized organization of the limbal niche. Cell Stem Cell. 2021. Jul;28(7):1233–1247.e4. • Using longitudinal intravital imaging of the mouse cornea combined with single-cell lineage tracing this study identified two subpopulations of stem cells in the limbal niche with distinct dynamics and roles in homeostasis and wound healing. This study showcased how the spatiotemporal coordination of stem cell activities across the entire tissue support corneal maintenance.
- 33.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004. Sep 3;118(5):635–48. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009. May 29;137(5):811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010. Jan 29;327(5965):542–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elhusseiny AM, Soleimani M, Eleiwa TK, ElSheikh RH, Frank CR, Naderan M, et al. Current and Emerging Therapies for Limbal Stem Cell Deficiency. Stem Cells Transl Med. 2022. Mar 31;11(3):259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y, Yung M, Huang L, Tseng C, Deng SX. Limbal Stem Cell Deficiency After Glaucoma Surgery. Cornea. 2020. May;39(5):566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng SX, Borderie V, Chan CC, Dana R, Figueiredo FC, Gomes JAP, et al. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea. 2019. Mar;38(3):364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Le Q, Chauhan T, Yung M, Tseng CH, Deng SX. Outcomes of Limbal Stem Cell Transplant: A Meta-analysis. JAMA Ophthalmol. 2020. Jun 1;138(6):660. • A meta-analysis to evaluate the outcomes of four limbal stem cell transplantation procedures was performed, which included: direct autologous limbal transplant, direct allogenic limbal transplant, cultivated autologous limbal stem cells transplant, and cultivated allogenic limbal stem cells transplant. Autologous limbal transplantation resulted in the greatest visual acuity improvement, while the most common adverse events were recurrent/persistent epithelial erosion and elevated intraocular pressure with direct allogenic limbal transplant having the most adverse events.
- 40.Saghizadeh M, Kramerov AA, Svendsen CN, Ljubimov AV. Concise Review: Stem Cells for Corneal Wound Healing. Stem Cells Dayt Ohio. 2017. Oct;35(10):2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Norrick A, Esterlechner J, Niebergall-Roth E, Dehio U, Sadeghi S, Schröder HM, et al. Process development and safety evaluation of ABCB5+ limbal stem cells as advanced-therapy medicinal product to treat limbal stem cell deficiency. Stem Cell Res Ther. 2021. Dec;12(1):194. • With the subsequent identification of ABCB5+ as a potential marker for LSCs, this study developed and evaluated the safety and preclinical efficacy of validated Good Manufacturing Practice and European Pharmacopeia expansion of ABCB5+ limbal stem cells (LSCs). Preclinical studies using two methods of LSCs delivery in mice revealed no toxicity and tumorgenicity.
- 42.Jackson CJ, Myklebust Ernø IT, Ringstad H, Tønseth KA, Dartt DA, Utheim TP. Simple limbal epithelial transplantation: Current status and future perspectives. Stem Cells Transl Med. 2020. Mar 1;9(3):316–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Figueiredo FC, Glanville JM, Arber M, Carr E, Rydevik G, Hogg J, et al. A systematic review of cellular therapies for the treatment of limbal stem cell deficiency affecting one or both eyes. Ocul Surf. 2021. Apr;20:48–61. [DOI] [PubMed] [Google Scholar]
- 44.Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci. 2001. Mar 13;98(6):3156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014. Jul;511(7509):353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N Engl J Med. 2010. Jul 8;363(2):147–55. [DOI] [PubMed] [Google Scholar]
- 47.Yazdanpanah G, Haq Z, Kang K, Jabbehdari S, Rosenblatt M, Djalilian AR. Strategies for reconstructing the limbal stem cell niche. Ocul Surf. 2019. Apr;17(2):230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polisetti N, Zenkel M, Menzel-Severing J, Kruse FE, Schlötzer-Schrehardt U. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem Cells. 2016. Jan 1;34(1):203–19. [DOI] [PubMed] [Google Scholar]
- 49.Wang M, Yuan Q, Xie L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018. Jun 14;2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res Ther. 2020. Dec;11(1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Djalilian AR, Mahesh SP, Koch CA, Nussenblatt RB, Shen D, Zhuang Z, et al. Survival of Donor Epithelial Cells after Limbal Stem Cell Transplantation. Investig Opthalmology Vis Sci. 2005. Mar 1;46(3):803. [DOI] [PubMed] [Google Scholar]
- 52.Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000. Jun;48(2):83–92. [PubMed] [Google Scholar]
- 53.Pedrotti E, Passilongo M, Fasolo A, Nubile M, Parisi G, Mastropasqua R, et al. In Vivo Confocal Microscopy 1 Year after Autologous Cultured Limbal Stem Cell Grafts. Ophthalmology. 2015. Aug;122(8):1660–8. [DOI] [PubMed] [Google Scholar]
- 54.Calonge M, Pérez I, Galindo S, Nieto-Miguel T, López-Paniagua M, Fernández I, et al. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl Res. 2019. Apr;206:18–40. [DOI] [PubMed] [Google Scholar]
- 55.Liang L, Luo X, Zhang J, Su W, Zhu W, Xie Y, et al. Safety and feasibility of subconjunctival injection of mesenchymal stem cells for acute severe ocular burns: A single-arm study. Ocul Surf. 2021. Oct;22:103–9. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe S, Hayashi R, Sasamoto Y, Tsujikawa M, Ksander BR, Frank MH, et al. Human iPS cells engender corneal epithelial stem cells with holoclone-forming capabilities. iScience. 2021. Jun;24(6):102688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galindo S, de la Mata A, López-Paniagua M, Herreras JM, Pérez I, Calonge M, et al. Subconjunctival injection of mesenchymal stem cells for corneal failure due to limbal stem cell deficiency: state of the art. Stem Cell Res Ther. 2021. Dec;12(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shalom-Feuerstein R, Serror L, De La Forest Divonne S, Petit I, Aberdam E, Camargo L, et al. Pluripotent Stem Cell Model Reveals Essential Roles for miR-450b-5p and miR-184 in Embryonic Corneal Lineage Specification. STEM CELLS. 2012. May;30(5):898–909. [DOI] [PubMed] [Google Scholar]
- 59.Sacchetti M, Rama P, Bruscolini A, Lambiase A. Limbal Stem Cell Transplantation: Clinical Results, Limits, and Perspectives. Stem Cells Int. 2018;2018:8086269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li M, Huang H, Wang B, Jiang S, Guo H, Zhu L, et al. Comprehensive 3D epigenomic maps define limbal stem/progenitor cell function and identity. Nat Commun. 2022. Dec;13(1):1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M, Huang H, Li L, He C, Zhu L, Guo H, et al. Core transcription regulatory circuitry orchestrates corneal epithelial homeostasis. Nat Commun. 2021. Dec;12(1):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo ZH, Zeng YM, Lin JS. Dynamic spatiotemporal expression pattern of limbal stem cell putative biomarkers during mouse development. Exp Eye Res. 2020. Mar;192:107915. [DOI] [PubMed] [Google Scholar]
- 63.Portal C, Wang Z, Scott DK, Wolosin JM, Iomini C. The c-Myc Oncogene Maintains Corneal Epithelial Architecture at Homeostasis, Modulates p63 Expression, and Enhances Proliferation During Tissue Repair. Investig Opthalmology Vis Sci. 2022. Feb 1;63(2):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kesper C, Viestenz A, Wiese-Rischke C, Scheller M, Hammer T. Impact of the transcription factor IRF8 on limbal epithelial progenitor cells in a mouse model. Exp Eye Res. 2022. May;218:108985. [DOI] [PubMed] [Google Scholar]
- 65.Kamarudin TA, Bojic S, Collin J, Yu M, Alharthi S, Buck H, et al. Differences in the Activity of Endogenous Bone Morphogenetic Protein Signaling Impact on the Ability of Induced Pluripotent Stem Cells to Differentiate to Corneal Epithelial-Like Cells. Stem Cells. 2018. Mar 1;36(3):337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ikkala K, Stratoulias V, Michon F. Unilateral zebrafish corneal injury induces bilateral cell plasticity supporting wound closure. Sci Rep. 2022. Dec;12(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaplan N, Ventrella R, Peng H, Pal-Ghosh S, Arvanitis C, Rappoport JZ, et al. EphA2/Ephrin-A1 Mediate Corneal Epithelial Cell Compartmentalization via ADAM10 Regulation of EGFR Signaling. Investig Opthalmology Vis Sci. 2018. Jan 19;59(1):393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun CC, Lee SY, Kao CH, Chen LH, Shen ZQ, Lai CH, et al. Cisd2 plays an essential role in corneal epithelial regeneration. EBioMedicine. 2021. Nov;73:103654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao S, Wan X, Dai Y, Gong L, Le Q. WNT16B enhances the proliferation and self-renewal of limbal epithelial cells via CXCR4/MEK/ERK signaling. Stem Cell Rep. 2022. Apr;17(4):864–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He J, Jiao X, Sun X, Huang Y, Xu P, Xue Y, et al. Short-Term High Fructose Intake Impairs Diurnal Oscillations in the Murine Cornea . Investig Opthalmology Vis Sci. 2021. Aug 20;62(10):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pal-Ghosh S, Tadvalkar G, Karpinski BA, Stepp MA. Diurnal Control of Sensory Axon Growth and Shedding in the Mouse Cornea. Investig Opthalmology Vis Sci. 2020. Sep 1;61(11):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y, Di G, Wang Y, Chong D, Cao X, Chen P. Aquaporin 5 Facilitates Corneal Epithelial Wound Healing and Nerve Regeneration by Reactivating Akt Signaling Pathway. Am J Pathol. 2021. Nov;191(11):1974–85. [DOI] [PubMed] [Google Scholar]
- 73.Tuck H, Park M, Carnell M, Machet J, Richardson A, Jukic M, et al. Neuronal-epithelial cell alignment: A determinant of health and disease status of the cornea . Ocul Surf. 2021. Jul;21:257–70. [DOI] [PubMed] [Google Scholar]
- 74.Wang LY, Zhang YT, Du LQ, Wu XY, Zhu J. The Effect of SPARC on the Proliferation and Migration of Limbal Epithelial Stem Cells During the Corneal Epithelial Wound Healing. Stem Cells Dev. 2021. Mar 15;30(6):301–8. [DOI] [PubMed] [Google Scholar]
- 75.Zhu J, Wang L yi, Li C yun, Wu J yin, Zhang Y ting, Pang K peng, et al. SPARC promotes self-renewal of limbal epithelial stem cells and ocular surface restoration through JNK and p38-MAPK signaling pathways. Stem Cells. 2020. Jan 1;38(1):134–45. [DOI] [PubMed] [Google Scholar]
- 76.Polisetti N, Roschinski B, Schlötzer-Schrehardt U, Maier P, Schlunck G, Reinhard T. A Decellularized Human Limbal Scaffold for Limbal Stem Cell Niche Reconstruction. Int J Mol Sci. 2021. Sep 17;22(18):10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ashworth S, Harrington J, Hammond GM, Bains KK, Koudouna E, Hayes AJ, et al. Chondroitin Sulfate as a Potential Modulator of the Stem Cell Niche in Cornea. Front Cell Dev Biol. 2021. Jan 12;8:567358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Polisetti N, Gießl A, Zenkel M, Heger L, Dudziak D, Naschberger E, et al. Melanocytes as emerging key players in niche regulation of limbal epithelial stem cells. Ocul Surf. 2021. Oct;22:172–89. [DOI] [PubMed] [Google Scholar]