Abstract
Background: Obesity is considered a multisystem disease associated with higher mortality and morbidity in adults. This study explored the effects of two Moderate-Intensity Continuous Training (MICT) and High-Intensity Interval Training (HIIT) on body composition, maximal oxygen uptake (VO2max), and the gene expression of angiotensin-converting enzyme 2 (ACE2), fibronectin type III domain-containing protein 5 (FNDC5), and NLR family pyrin domain containing 3 (NLRP3) in adults with obesity.
Methods: In a randomized controlled trial, 36 obese, inactive subjects (age: 45.16 ± 3.13 yrs.; mean, BW: 112.38 ± 20.1 kg, Height: 1.67 ± 0.07, and BMI: 39.66 ± 6.07 kg/m2) were randomly assigned to one of three groups: HIIT: (n = 12), MICT (n = 12), and control (n = 12). Both exercise groups received 40 min of training per session (three times/week) for eight weeks. Body composition, body fat percentage (BFP), VO2max, and the gene expression of ACE2, and NLRP3, were taken pre- and post-intervention using the qRT-PCR technique. The data were analyzed using SPSS software via parametric (ANOVA and ANCOVA) and non-parametric tests (Mann Whitney U and Kruskal-Wallis).
Results: Our results showed that HIIT and MICT protocols could be effective in normalizing body composition measurements and VO2max, but HIIT could reduce body fat percentage (BFP) in obese subjects. Moreover, HIIT and MICT could significantly reduce the gene expression of NLRP3 (p < 0.0001) and ACE2 (p < 0.0001), while increasing the gene expression of FNDC5 (p < 0.0001). There were negative correlations between the gene expression of FNDC5 and NLRP3, as well as ACE2. Furthermore, increased FNDC5 was negatively correlated with BFP (r = 0.392, p < 0.001).
Conclusion: Overall, our results indicated that HIIT and MICT protocols had the greatest impact on the gene expression of NLRP3, ACE2, and FNDC5.
Keywords: Exercise training, Obesity, COVID-19, NLRP3, ACE2, FNDC5, Body composition
↑What is “already known” in this topic:
The relationship between obesity and severe COVID-19 has been reported. The beneficial effects of exercise on obesity have been proven in several studies.
→What this article adds:
In a randomized controlled trial, the effects of high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) on body composition and ACE2, NLRP3, and FNDC5 gene expression were investigated in obese adults. The result showed that protocols effectively down-regulated the gene expression of ACE2 and NLRP3 and up-regulated the FNDC5. HIIT and MICT can be used for the protection of obese subjects against COVID-19.
Introduction
Today, obesity is considered a multisystem disorder related to higher adult morbidity and mortality (1, 2). The increasing obesity prevalence rates make it a major public health problem that influences both developing and developed countries (3). Obesity can also aggravate the severity of respiratory diseases (4). Previously, it has been shown that chronic medical conditions, e.g., diabetes, obesity, hypertension, and cardiovascular diseases (CVDs), are associated with the development of adverse COVID-19 outcomes (5). This problem has been increasingly detected as a predictive factor in the current coronavirus disease 2019 (COVID-19) pandemic due to infection with SARS-CoV-2 (6, 7).
Angiotensin-converting enzyme 2 (ACE2) has been considered a critical part of the renin-angiotensin system (RAS) and is involved in the regulation of body homeostasis through the angiotensin pathways (8, 9). In addition, the development of severe illness of COVID-19 is possibly correlated with the expression of ACE2, used by SARS-CoV-2 to invade human alveolar epithelial cells (9). ACE2 is over-expressed in adipocytes, which is associated with the overactivation of RAS in obese patients, and turns adipose tissue into a viral reservoir (10). Therefore, obesity and insulin resistance are strongly linked with RAS activity. Furthermore, the function of RAS is accompanied by mitochondrial dysfunction and immune responses (11).
The NLR family pyrin domain containing 3 (NLRP3) inflammasome, a crucial part of the innate immune system, can cause inflammation following SARS-CoV-2 infection. This may worsen the release of inflammatory mediators in obese patients (12). The NLRP3 complex, consisting of NLRP3 protein, caspase-1, and apoptosis-associated speck-like protein (ASC), triggers the secretion of pro-inflammatory mediators, e.g., interleukin 1α (IL-1α) and Il-18, via gasdermin pores commonly found in pyroptotic cell death (13). Furthermore, the extracellular domain of fibronectin type III domain containing 5 (FNDC5) is cleaved into irisin, a myokine abundantly detected in the muscle (14). Irisin has been proven to participate in the control of several physiological functions in mammals (15). FNDC5 exhibits potential beneficial impacts on COVID-19 consequences via anti-obesity and anti-inflammatory functions (16-18).
Nowadays, because overweight people and the obese population are a COVID-19 vulnerable group, it is more crucial than ever to study relationships that may result in the formation of strategies with the goal of national health promotion (19, 20). Physical activity (PA, body movement that is performed by skeletal muscles that require energy) and regular exercise (a structured, purposeful, and repetitive PA) at home or in other safe environments have been considered promising strategies to boost the immune system during the COVID-19 pandemic (21, 22). PA can have benefits beyond weight loss, including improvements in quality of life, feelings, function, and sleep (23). Individuals with overweight and obesity with a high risk for COVID-19-related hospitalization are more likely to be involved in regular PA in their programs (24). PA can reduce not only the release of inflammatory markers associated with chronic kinds of low-grade inflammation but also the acute inflammatory response triggered by various adverse stimuli (25). It was shown that PA with moderate and high intensity and duration in the training period could reduce inflammatory factors (26)
In this clinical trial, our aim was to assess the impacts of two aerobic training strategies, including high-intensity interval training (HIIT) and moderate-intensity continuous exercise training (MICT), on body composition and ACE gene expression, maximal oxygen uptake (VO2max) and body composition were assessed pre-and post-intervention in the obese people. Then, the gene expression of ACE2, NLRP3, and FNDC5 was examined in the blood samples of healthy obese subjects.
Methods
Study Subjects
This study was an 8-week randomized-controlled trial (IRCT20220105053631N1, registered 01 January 2022, available on https://www.irct.ir/trial/61202). In this study, 36 obese subjects (18 males and 18 females; mean age: 45.16 ± 3.13 years; mean weight: 112.38 ± 20.1 kg; mean height: 1.67 ± 0.07; and mean body mass index [BMI]: 39.66 ± 6.07 kg/m2) were invited to the study through advertisements (posters, social media platforms, SMS, and email). This population had received two doses of the COVID-19 vaccine (including Sinopharm, Bharat, AstraZeneca, or Sputnik) and at least two months had passed since the injection of their second dose of vaccine. The subjects with the following characteristics were included: age of 40- 50 years, BMI>30 kg/m2, waist-to-height ratio (WHtR)>0.6, non-smokers, being sedentary with no chronic disorders (e.g., hypertension, CVDs, and type 2 diabetes), no treatment with mental or hormonal medication, and without drinking alcohol, not regular PA in the last six months. Individuals who took medications and dietary supplements, e.g., beta-blockers, amino acids, calcium channel blockers, beta-agonists, and corticosteroids) that influence the metabolism of adipose tissue or muscle were excluded from the study. Women were not pregnant or lactating. The physical activity readiness questionnaire (PAR-Q) was filled out by each participant (27) and their medical health status was assessed by a physician. All participants voluntarily signed an informed consent form. The study was done based on the Helsinki Declaration (Nathanson, 2013) and accepted by the Ethics Committee of the Islamic Azad University (Ethics code: IR.IAU.TNB.REC.1400.102).
Study Groups
Before collecting the baseline data, all procedures and testing were explained to the subjects. Then, three groups of subjects were randomly selected (n=12 in each group): HIIT, MICT, or control. Data collection was performed at two-time points; 1) baseline measurements 48 h before starting the investigation and 2) eight weeks measurements 48h after the last PA at the same time of the day (within ~1h) with the same conditions (~50-55% humidity and ~20°C heat). Furthermore, every subject was requested to be on his/her usual eating diet for the investigation duration. All participants were living in the same facility, as they were mostly related to middle-class households.
Sample size and randomization
The sample size was calculated to find a significant difference among study measurements with a confidence interval (CI) of 95% and a power value of ≥80%. For the sample size calculation, G*Power software (University of Trier, Trier, Germany) was used based on generic moderate effect sizes (Cohen’s ƒ=0.5), error of 0.05 of previously published studies (28). The sampling method in this study was classified as randomization. Finally, 36 people (18 men and 18 women) were studied in this investigation. Since the expression of the effect of the interventionists may have a significant difference according to the gender of the people, therefore, an equal number of each gender was studied in each group. Based on this, the sample space was divided into three blocks of 12 people. In this study, one category was related to the control group, and two categories were related to the intervention group. Therefore, each block contains two members from each of these categories, who are placed in that category based on gender and age. To randomize the sampling, online software sealed envelope was used at the website https://www.sealedenvelope.com.
Training Intervention
Before the randomization and training intervention, one week of training was done under supervision, and all subjects were familiarized with the protocols. Next, the subjects in the training groups followed HIIT or MICT protocols for 8 weeks, three sessions per week. A treadmill was used for completing the protocols. The maximal heart rate (HRmax) index was used to control and assess of the training intensity (29). An HR monitor (Speedo Int. Ltd., USA) was used for each subject during the investigation to ensure compliance with the predetermined target HR zone. At the end of the training session, velocity was enhanced by 0.1-0.5 km/h every time the HR was below the lower threshold.
1- HIIT: This protocol was performed with an intensity of 55-90% maximal heart rate (HR max). In the first week, exercise was done with a 30-second activity time and an intensity of 65% HR max, which was repeated twice. In the following, every week 5% was added to the intensity of the HR max and 30 seconds to the time, and one repetition was added every two weeks. The rest time was 1 minute, and gradually, 60 seconds to the rest times were added in each training week until the end of the 8-week training. HIIT was performed for a total of 35 min, with a 5-minute warm-up and cool-down (30).
2- MICT: This protocol was done with an intensity of 40-75% HR max. In the first session, the exercise started with an intensity of 40% HR max, and gradually with the improvement of the subjects' readiness, the intensity of the exercise was increased by 5% every week and after the subjects reached the intensity of 60% HR max, this state was maintained until the end of the protocol. MICT was carried out 35-min of continuous exercise, with a 5-minute warm-up and cool down at 40% HR max for a 40-minute exercise session (31).
Body Composition and Anthropometry
Body composition and anthropometric measurements, including height, body weight (BW), BMI, body fat percentage (BFP), and waist-to-hip ratio (WHR), were evaluated using standard methods before and after the training sessions. Participants were measured in light clothes without shoes. A calibrated weighing scale was utilized to measure BW (~0.1 kg). Height (~0.1 cm) was determined with a stadiometer. BMI was calculated by dividing BW (kg) by height in meters squared (m2) (32). BFP was measured using a bio-impedance analyzer (Medigate Company Inc.) measured body fat. To calculate WHR, waist circumference and hip circumference were measured. Waist circumference was assessed at the narrowest diameter between the iliac crest and the lower ribs. Hip circumference was measured at the widest point of the buttocks with a firm measuring tape (33, 34). BFP was measured using a bio-impedance analyzer (Medigate Company Inc.) measured body fat. One of the investigators took all measurements in duplicate and the final average values were reported.
Blood Sampling
Fasting blood samples (20 ml) were taken from 36 subjects before and after the investigation. The antecubital vein was used for collecting samples using standard procedures. Following sampling before and after training, the blood samples were centrifuged for 15 minutes at 3000 rpm at 4°C after being kept at room temperature for 30 minutes. Serum samples were stored at −80°C until molecular tests were performed.
RNA Extraction and RT-q PCR
Based on the manufacturer's protocol, TRIzol (Carlsbad, CA, USA) was used for extraction of total RNA. RNA (1µg) was reverse transcribed with a reverse transcription kit (Toyobo). The gene expression of ACE2, NLRP3, and FNDC5 was assessed by qPCR using QuantiFast SYBR® Green PCR Kit (Qiagen). Table 1 shows the designed primers. Based on the manufacturer's protocols, qPCR was conducted with a LightCycler CFX96 (BioRad, Hercules, CA, USA). Beta-2 microglobulin (B2M) was selected as a housekeeping gene. Relative mRNA expression was calculated using the 2(-Delta Delta C(T)) method.
Table 1. Primer sequences.
| Genes | Sequence of Primers | bp | Temperature (˚C) |
|---|---|---|---|
| ACE2 | F:TCCATTGGTCTTCTGTCACCCG | 245 | 60 |
| R:GACCATCCACCTCCACTTCTCT | |||
| NLRP3 | F:GGACTCTTGCACCCCGACT | 115 | 61.9 |
| R:GGTCGCCCAGGTCATTGTTG | |||
| FNDC5 | F:CGGATTTGCCATCTCCCAGC | 104 | 59 |
| R:TTGAAGAGCACAGGCTCGCT | |||
| B2MG (reference) | F:TGAGTCCAAGCTAGGCCCTTT | 180 | 59.1 |
| F:ACCAGCCACCACTTTCTGAT |
Air Force Fitness Test
The VO2max of the subjects was measured using the one-mile walking test. In this test, after the training, a one-mile route was specified, and the individual walked the one-mile route at maximum speed, and upon completion, the HR was counted for one minute. In this formula, the BW (lb), age (year), gender factor (gender = Males: 1 & Females: 0), time taken to walk one mile in minutes, and HR were used to calculate VO2max. VO2max calculated from the 1-mile walk was calculated using the Kline et al. regression equation (35):
VO2max = 132.853 − (BW × 0. 0769) − (age × 0.3877) + (gender × 6.315) − (time ×3.2649) − (HR × 0.1565)
Dietary Analysis
Food records were done in three days (two weekdays and one weekend day) before and after the study were obtained to assess the alterations of the usual diet over time (36). We used a duplicate diet approach to analyze dietary intake. Food consumption was recorded by a trained research staff to make sure that the facts were inserted into the Nutritionist software. Furthermore, every food item was individually entered into Nutritionist software (Nutritionist IV, Version. 3.5.2) and the amount of energy from fats, carbohydrates, and proteins, as well as total energy consumption, were calculated (Table 2).
Table 2. Pre- and post-mean of nutritional intake parameters in the three groups of study.
| Nutritional intake parameters | HIIT | MICT | Control | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Energy (kcal/d) | 2198±85 | 2224±72 | 2358±104 | 2414±98 | 2248±112 | 2263±93 |
| CHO (g/d) | 285±18 | 291±16 | 293±14 | 298±16 | 281±30 | 282±24 |
| Fat (g/d) | 86.3±11 | 83.5±9 | 85.4±8 | 83.2±10 | 80.7±9 | 81.2±10 |
| Protein (g/d) | 108±37 | 110±35 | 101±28 | 102±30 | 106±32 | 108±28 |
Statistical Analysis
Blind data analysis was done. Data analyses were done using SPSS software (Version 23.0, IBM® SPSS® software for Windows). Data were summarized as the mean±standard deviation (mean ± SD).The normality of data was examined using the Kolmogorov-Smirnov test. To compare the variables among the three groups, ANOVA and ANCOVA tests were used. In addition, nonparametric tests of Mann Whitney U and Kruskal-Wallis were run on non-normally distributed data. P ˂0.05 were conventionally regarded as statistically significant.
Results
The CONSORT flowchart was used to show the participant flow (Figure 1). Of a total of 55 subjects screened in the study, 15 were not eligible based on the inclusion criteria, 40 enrolled randomly to receive one of the interventions, and 36 participated in the eight-week training, leading to a drop-out rate of 34.54%. No significant differences were seen in baseline features (including age, weight, height, and BMI) of the three groups (P > 0.05, Table 3).
Figure 1.
Study flow chart. HIIT, MICT, and control groups. (m: males & F: females)
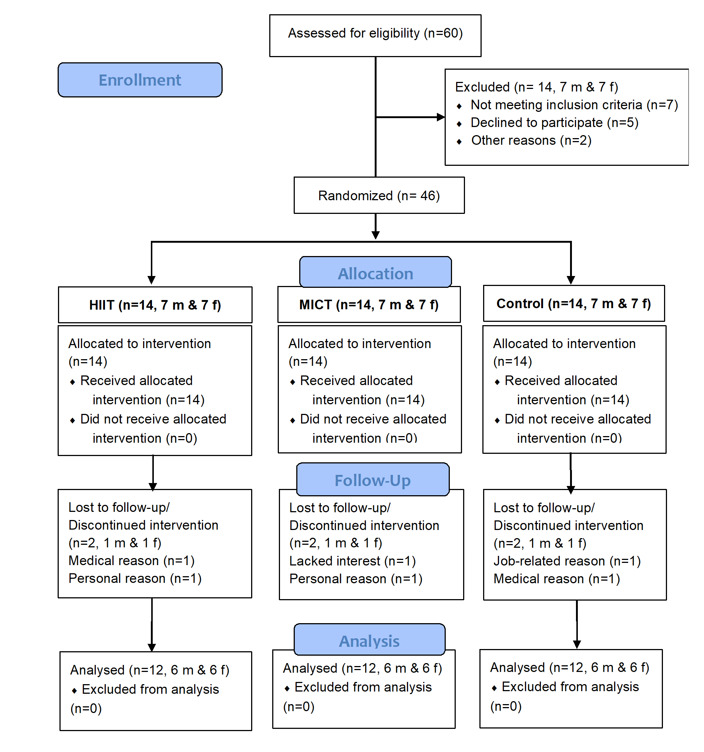
Table 3. Values of baseline characteristics of HIIT, MICT, and Control groups.
| HIIT | MICT | Control | P-value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age | 45.33 ± 3.55 | 45.17 ± 3.24 | 45.00 ± 2.86 | 0.969 |
| Weight (Kg) | 108.92 ± 18.14 | 110.65 ± 17.30 | 111.23 ±24.84 | 0.978 |
| Height (m) | 1.69± 0.08 | 1.70 ±0.07 | 1.66 ± 0.07 | 0.546 |
| BMI | 39.55 ± 5.39 | 39.33 ± 6.03 | 40.11 ± 7.17 | 0.951 |
BW: Body weight, HIIT: High-intensity interval training, MICT: Moderate-intensity continuous exercise training, SD: Standard Deviation, BMI: Body mass index
Anthropometry and Body Composition
According to Table 4, there were no significant differences in weight (P = 0.201), BMI (P = 0.146), BFP (P = 0.729), WHR (P = 0.267), and VO2max (P= 0.854) in both training group relative to the control group before investigation. After investigation, no significant differences were recorded in the mean weight (P = 0.111), BMI (P = 0.37), WHR (P = 0.105), and VO2max (P = 0.058). According to Table 4, reduced BFP was recorded in the MICT (P = 0.019) and HIIT (P = 0.035). groups relative to the control group.
Table 4. Values of body composition and maximal oxygen consumption at pre-and post-intervention.
| Variables | HIIT | MICT | Control | P-value | |||
|---|---|---|---|---|---|---|---|
| Pre N=12 | Post N=12 | Pre N=12 | Post N=12 | Pre N=12 | Post N=12 | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| BW (kg) | 112.75 ± 18.46 | 105.08 ± 17.76 | 113.04 ± 18.47 | 108.25 ± 16.49 | 111.38 ± 24.56 | 111.08 ± 24.96 | 0.918 |
| BMI | 39.55 ± 5.39 | 36.86 ± 5.19 | 39.33 ± 6.03 | 37.67 ± 5.45 | 40.11 ± 7.17 | 39.98 ± 7.21 | 0.533 |
| BFP | 47.02 ± 6.76 | 44.06 ± 6.47* | 44.35 ± 9.21 | 41.69 ± 8.61* | 48.23 ± 5.02 | 48.39 ± 4.86 | 0.037 |
| WHR | 1.03 ± 0.09 | 0.99 ± 0.09* | 1.00 ± 0.09 | 0.96 ± 0.09 | 1.01 ± 0.08 | 1.02± .09 | 0.346 |
| VO2max | 28.42 ± 8.23 | 30.45 ± 8.19 | 28.65 ± 9.14 | 29.90 ± 8.78 | 28.13 ± 8.17 | 28.23 ± 8.05 | 0.849 |
Pre: pre-investigation, Post: post-investigation, BW: Body weight, HIIT: High-intensity interval training, MICT: Moderate-intensity continuous exercise training, SD: Standard Deviation, BMI: Body mass index, WHR: Waist-to-hip ratio, VO2max: Maximal oxygen consumption, BFP: Body fat percentage. * P < 0.05 significant differences compared to the control group
Gene expression of ACE2, NLRP3, and FNDC5
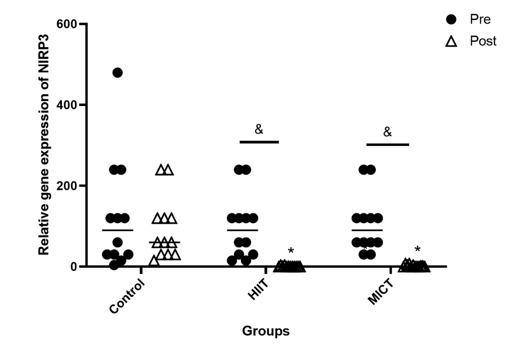
Gene expression levels of ACE2 (P < 0001, Figure 2) and NLRP3 (P < 0001, Figure 3) were significantly downregulated in both training groups, compared to the control group. Furthermore, the gene expression of ACE2 (P < 0001, Figure 2) and NLRP3 (P < 0001, Figure 3) was significantly reduced in the pre-investigation values relative to that of the post-investigation values of both training groups. ACE2 was highly expressed in the HIIT group relative to those of the MICT group (P < 0.001, Figure 3).
Figure 2.
Pre- and post-investigation values (Median) of the gene expression of ACE2 in HIIT, MICT, and control groups. *P < 0.001 relative to the control group, &P < 0.001 between pre- and post-investigation values. Pre: pre-investigation, Post: post-investigation. HIIT: Moderate-Intensity Continuous Training, MICT: High-Intensity Exercise Training
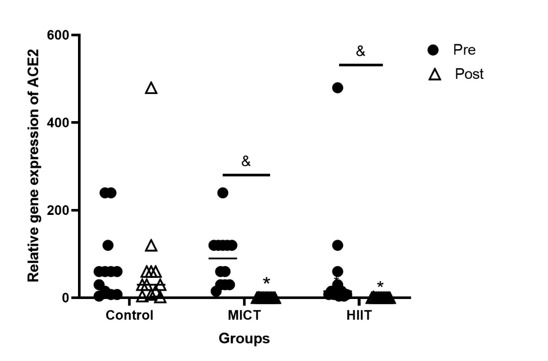
Figure 3.
Pre- and post-investigation values (Median) the gene expression of NLRP3 in HIIT, MICT, and control groups < 0.001 compared to the control group, &P < 0.001 between pre- and post-investigation values. Pre: pre-investigation, Post: post-investigation. HIIT: Moderate-Intensity Continuous Training, MICT: High-Intensity Exercise Training
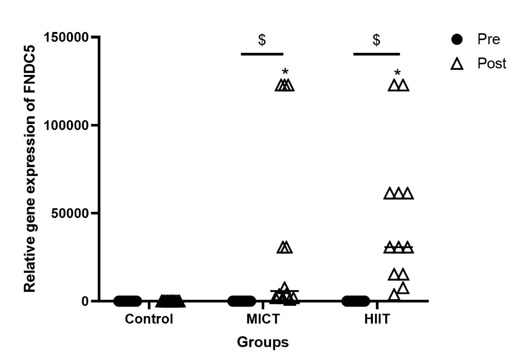
However, the gene expression of FNDC5 (P < 0001, Figure 4) was significantly greater in the HIIT and MICT groups compared to the control group. Besides, a significant increase was observed in the gene expression of FNDC5 (P < 0001, Figure 4) in the pre-investigation values relative to the post-investigation values of both training groups.
Figure 4.
Pre- and post-investigation values (Median) of the gene expression of FNDC5 in HIIT, MICT, and control groups. *P < 0.0001 relative to the control group, &P < 0.0001 between pre- and post-investigation values. Pre: pre-investigation, Post: post-investigation. HIIT: Moderate-Intensity Continuous Training, MICT: High-Intensity Exercise Training
Correlation between quantitative data
According to Table 5, there were significant correlations between BW and BMI (r = 0.873, P < 0.001), BFP (r = 0.244, P=0.039), WHR (r = 0.639, P < 0.001), and VO2max (r = − 0.532, P < 0.001). Significant correlations were also recorded between BFP and WHR (r = 0.434, P < 0.001), as well as VO2max (r = − 0.326, P =0.005). There was a significant correlation between WHR and VO2max (r = − 0.551, P < 0.001). Additionally, significant correlations were detected between gene expression of ACE2 and NLRP3 (r = 0.379, P < 0.001), and FNDC5 (r = − 0.252, P = 0.032). A significant correlation was recorded between FNDC5 and NLRP3 (r = − 0.328, P = 0.005). Interestingly, there was a significant correlation between BFP and the gene expression of FNDC5 (r = 0.392, P < 0.001).
Table 5. Correlation between quantitative data.
| Variable | BW (kg) | BMI (kg/m2) | BFP (%) | WHR | VO2max | ACE2 | NLRP3 | FNDC5 | |
|---|---|---|---|---|---|---|---|---|---|
| BW (kg) | r | 1 | 0.873 | 0.244 | 0.639 | -0.532 | 0.094 | -0.061 | 0.033 |
| P-value | ˂0.001 | 0.039 | ˂0.001 | ˂0.001 | 0.433 | 0.610 | 0.785 | ||
| BMI (kg/m2) | r | - | 1 | -0.077 | -0.031 | -0.091 | -0.069 | -0.083 | 0.392 |
| P-value | - | 0.521 | 0.798 | 0.446 | 0.564 | 0.486 | ˂ 0.001 | ||
| BFP (%) | r | - | - | 1 | 0.434 | -0.326 | 0.070 | 0.182 | -0.155 |
| P-value | - | - | ˂0.001 | 0.005 | 0.561 | 0.125 | 0.193 | ||
| WHR | r | - | - | - | 1 | -0.551 | -0.021 | 0.064 | -0.047 |
| P-value | - | - | - | ˂0.001 | 0.861 | 0.593 | 0.695 | ||
| VO2max | r | - | - | - | - | 1 | 0.132 | 0.116 | 0.051 |
| P-value | - | - | - | - | 0.268 | 0.330 | 0.668 | ||
| ACE2 | r | - | - | - | - | - | 1 | 0.379 | -0.252 |
| P-value | - | - | - | - | - | ˂ 0.001 | 0.032 | ||
| NLRP3 | r | - | - | - | - | - | - | 1 | -.328 |
| P-value | - | - | - | - | - | - | 0.005 | ||
| FNDC5 | r | - | - | - | - | - | - | - | 1 |
| P-value | - | - | - | - | - | - | - | ||
BW: Body weight, BMI: Body mass index, WHR: Waist-to-hip ratio, VO2max: Maximal oxygen consumption, BFP: Body fat percentage
Discussion
In this clinical study, we evaluated the effects of aerobic training with high and moderate intensity on the body composition measures, VO2max, and the gene expression of FNDC5, ACE2, and NLRP3 in obese subjects. The subjects of the study were in the age range of 40 to 50 years. The results of our study indicated that HIIT and MICT could equally affect the variables of the study. While HIIT and MICT reduced weight, BMI, and WHR in the intervention groups, the values were not significant. Our findings indicated that HIIT was effective in the reduction of BFP. Therefore, HIIT and MICT were not effective for the regulation of composition measures and BFP in adult subjects 40–50 years of age and a BMI of 30–50 kg/m2. Exercise has been shown in several studies to improve body composition. For instance, using a 12-week HIIT protocol could reduce body weight, BFP, waist circumference (WC), WHR, and WHtR in sedentary obese college students with a BMI ≥ 27 kg/m2 and an age range of 18–26 years (37). Our finding can be due to the duration of the training, age range, and BMI range of the studied population. In addition, HIIT and MICT were not effective in the regulation of VO2max. VO2max has been defined as the maximum amount of oxygen that an individual can uptake during maximal or intense PA, which is an indicator of cardiovascular fitness and aerobic endurance. In agreement with our findings, Borgomaster et al. (2005) pointed out that HIIT did not show a significant change in VO2max despite increasing the oxidative potential of the muscle (38). Similarly, Bickham et al. (2004) could not show a significant change in VO2max despite the implementation of a HIIT program for 6 weeks (39).
The gene expression of NLRP3, ACE2, and FNDC5 was evaluated pre- and post-investigation in obese cases. Our findings indicated that HIIT and MICT could equally reduce the gene expression of ACE2. Obesity increases the susceptibility to different infections and chronic diseases (40, 41). It was shown that the risk of progressing to severe pneumonia following COVID-19 is fivefold higher compared with people of normal weight, emphasizing the particular involvement of SARS-CoV-2 for both the prevalence and severity of illness in obese individuals (42). Obesity is a condition characterized by the accumulation of excess body fat (43). Adipocytes were reported to highly express ACE2, the main receptor for SARS-CoV-2 entry into host cells (44, 45). In line with our findings, Klöting et al. (2020) revealed that a 4-week intensive exercise intervention reduced circulating ACE2 in healthy young men, indicating the potential impact of PA on SARS-CoV-2 pathogenesis (46). In another study, Klöting et al. (2022) reported that high intrinsic exercise capacity and low exercise capacity could effectively reduce circulating ACE2, as well as ACE2 expression in the lung, heart, muscle, and kidney in a rat model. They also showed that BW was negatively associated with serum ACE2 (47). In addition, Boschetti et al. reported that aerobic physical training could inhibit obesity-associated insulin resistance via alterations in the skeletal muscle phenotype (oxidative independent one) via the regulation of the ACE2 axis in a mouse model (48). Therefore, exercise could change the muscular cells’ function via the regulation of ACE2 and prevent SARS-CoV-2 infection via downregulation of ACE2.
Furthermore, our results showed a higher expression of NLRP3 in individuals with obesity who completed 8 weeks of exercise training, including HIIT and MICT. These protocols could equally downregulate the gene expression of NLRP3, indicating the suppressive effects of PA on the activation of the inflammasome. In addition, increased expression of ACE2 was correlated with the upregulation of NLRP3. Previously, studies have shown that the NLRP3 inflammasome contributes to the molecular mechanisms of several chronic inflammatory disorders, e.g., obesity, type 2 diabetes, insulin resistance, etc. (49, 50). A growing body of evidence has shown the enhanced levels of pro-inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), IL-1β, and IL-6, in severe obesity (51, 52). The activation of the NLRP3 inflammasome in adipose tissue stimulates the secretion of pro-inflammatory IL-1β and IL-18, a key step in innate immunity (53). Ratajczak et al. (2021) discovered for the first time that interactions between SARS-CoV-2 spike protein and ACE2 activated the NLRP3 inflammasome in endothelial progenitor cells and human hematopoietic stem cells, leading to pyroptosis after hyperactivation (25). COVID-19 can promote the pre-existing systemic inflammatory state of obese subjects through the activation of the NLRP3 inflammasome and the overproduction of inflammatory mediators (12).
Regular PA has been reported to enhance immune responses and promote resistance to several microbial infections (54). Different molecular mechanisms contribute to the regulation of inflammatory responses via PA, including reducing fat mass, which is active in the production of inflammatory mediators (e.g., TNF-α) and stimulation of muscular cells to produce IL6, which is responsible for the reduction of pro-inflammatory cytokines and regulation of other anti-inflammatory factors (55). Recently, the inhibitory effects of exercise on inflammasome activation have been demonstrated. For instance, Stensvold et al. (2012) showed that a 12-week protocol of aerobic interval training (three times a week) could downregulate the pro-inflammatory cytokine IL-18, triggered by the Nlrp3 inflammasome, by 43% in people with metabolic syndrome (56). Similarly, reduced mRNA content of IL-18 by 20% was observed in obese cases in response to a 8-week HIIT (57). Furthermore, a 12-week MICT protocol regulated the expression of inflammasome constituents, including NLRP3 and Toll-like receptor 4 (TLR4, activator of NLRP3 through the nuclear factor kappa B [NF-κB] signaling pathway) and serum levels of pro-inflammatory factors, e.g., TNFα, IL-18, IL-1β, and IL-6, in a population of elderly women (58). In line with our findings, Hosseini et al. (2022) also reported that both moderate and high intensity were beneficial in the regulation of inflammatory factors (26). Therefore, exercise training can protect obese patients against SARS-CoV-2 infection via the downregulation of ACE2 and NLRP3.
Besides, increased expression of FNDC5 was recorded in the obese cases exposed to the 8 weeks of HIIT and MICT. The upregulation of FNDC5 was correlated with the downregulation of NLRP3 and ACE2. As well, higher gene expression of FNDC5 was negatively associated with BFP, confirming the anti-obesity effects of FNDC5.
FNDC5/irisin produced by adipose tissue has been proven to be an adipokine that can regulate adipocyte action (59, 60). Rabiee et al. (2020) reported that FNDC5/irisin could suppress adipogenesis and promote fat browning via the extracellular-signal-regulated kinase (ERK)/mitogen activated protein kinase (MAPK) cascade, which is consistent with our findings (61). To show the anti-inflammatory effects, overexpression of FNDC5 was stated to lower adipose tissue inflammation by suppressing the production of pro-inflammatory cytokines and M1 macrophage polarization (62, 63). To confirm the potential antiviral function, FNDC5 was shown to influence several genes associated with SARS-CoV-2 infection in human adipocytes (64). Interestingly, treatment with FNDC5 could reduce the gene expression of SARS-CoV-2 host cell receptors, e.g., ACE2, in adipocytes. Notably, gene silencing of FNDC5 resulted in increased ACE2 mRNA levels in human visceral adipocytes (65). Irisin exhibits a beneficial effect on inflammation; besides, subcutaneous adipose tissue and plasma levels of irisin are reduced in obese patients (66, 67). On the other hand, FNDC5 is known to mediate some beneficial effects of exercise via the browning of white adipose tissue (68). As reported in previous studies, acute and chronic exercise protocols enhance plasma levels of irisin in different conditions and age groups (69, 70).
Conclusion
In general, HIIT and MICT protocols were not effective in the modulation of body composition measurements and VO2max in obese patients due to the older population and higher BMI. Nevertheless, HIIT was beneficial in reducing BFP. On the other hand, HIIT and MICT protocols could regulate the gene expression of ACE2, NLRP3, and FNDC5 in patients with obesity, highlighting the potential effects of physical exercise in the protection of obese individuals against SARS-CoV-2 infection. The novelty of this study was that the HIIT and MICT effects on the regulation of genes actively involved in the pathophysiology of obesity, including ACE2, NLRP3, and FNDC5, were examined and a strategy for the prevention of viral infection, particularly COVID-19, in the obese population was suggested.
Limitations
In this study, six men and six women were randomized to each training group with the same training protocol. Although the sex, body composition, and anthropometric indices were matched in each group, doing exercise was hard for women during menstruation. To solve this problem, women were asked to continue the program after finishing the third day of menstruation. Nevertheless, most of the women were in perimenopause or menopause.
Conflict of Interests
The authors declare that they have no competing interests.
Acknowledgment
This research is conducted as a part of Ph.D. thesis of the first author. (No.: 162497476).
Authors' contributions
FA and FG contributed substantially to the conception and design of the study. FA, FG, MS, HK, and MG contributed to performing the investigation, FA and HK contributed to analyzing the data, FA, MS, and MG drafted or provided critical revision of the article. FA and FG provided the final approval of the version to publish. All authors discussed the results and contributed to the final manuscript.
Data availability statements
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics approval
The research reported in this publication was approved by the ethics committee of Islamic Azad University, Tehran, Iran (IR.IAU.TNB.REC.1400.102).
Consent to participate
An informed consent form was signed by all subjects.
Consent for publication
An informed consent form was signed by all subjects.
Cite this article as : Armannia F, Ghazalian F, Shadnoush M, Keyvani H, Gholami M. Effects of High-Intensity Interval Vs. Moderate-Intensity Continuous Training on Body Composition and Gene Expression of ACE2, NLRP3, and FNDC5 in Obese Adults: A Randomized Controlled Trial. Med J Islam Repub Iran. 2022 (22 Dec);36:161. https://doi.org/10.47176/mjiri.36.161
References
- 1.Abdelaal M, le Roux, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5(7):161. doi: 10.21037/atm.2017.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari S, Haboubi H, Haboubi N. Adult obesity complications: challenges and clinical impact. Ther Adv Endocrinol Metab. 2020;11:2042018820934955.. doi: 10.1177/2042018820934955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarma S, Sockalingam S, Dash SJD, Obesity as. Diabetes Obes Metab. 2021;23:3–16. doi: 10.1111/dom.14290. [DOI] [PubMed] [Google Scholar]
- 4.Zammit C, Liddicoat H, Moonsie I, Makker H. Obesity and respiratory diseases. Int J Gen Med. 2010;3:335. doi: 10.2147/IJGM.S11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vas P, Hopkins D, Feher M, Rubino F, B Whyte. Diabetes, obesity and COVID-19: A complex interplay. Diabetes Obes Metab. 2020;22(10):1892. doi: 10.1111/dom.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21(6):e13034. doi: 10.1111/obr.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callender LA, Curran M, Bates SM, Mairesse M, Weigandt J, Betts CJJFii. The impact of pre-existing comorbidities and therapeutic interventions on COVID-19. Front Immunol. 2020;11:1991. doi: 10.3389/fimmu.2020.01991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157:104833.. doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng H, Wang Y, Wang GQJJomv. Organ‐protective effect of angiotensin‐converting enzyme 2 and its effect on the prognosis of COVID‐19. J Med Virol. 2020;92(7):726. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruglikov IL, Scherer PE. The Role of Adipocytes and Adipocyte-Like Cells in the Severity of COVID-19 Infections. Obesity (Silver Spring) 2020;28(7):1187. doi: 10.1002/oby.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med. 2020;19:100283.. doi: 10.1016/j.obmed.2020.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Reyes A, Martinez-Armenta C, Espinosa-Velazquez R, Vazquez-Cardenas P, Cruz-Ramos M, Palacios-Gonzalez B. et al. NLRP3 inflammasome: the stormy link between obesity and COVID-19. Front Immunol. 2020;11:570251.. doi: 10.3389/fimmu.2020.570251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammed I, Ijaz S, Mokhtari T, Gholaminejhad M, Mahdavipour M, Jameie B. et al. Subventricular zone-derived extracellular vesicles promote functional recovery in rat model of spinal cord injury by inhibition of NLRP3 inflammasome complex formation. Metab Brain Dis. 2020;35(5):809. doi: 10.1007/s11011-020-00563-w. [DOI] [PubMed] [Google Scholar]
- 14.Bubak MP, Heesch MWS, Shute RJ, Dinan NE, Laursen TL, DT LAS. et al. Irisin and Fibronectin Type III Domain-Containing 5 Responses to Exercise in Different Environmental Conditions. International journal of exercise science. Int J Exerc Sci. 2017;10(5):666. doi: 10.70252/XKMW8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkoff HJM, endocrinology c. Fish as models for understanding the vertebrate endocrine regulation of feeding and weight. Mol Cell Endocrinol. 2019;497:110437.. doi: 10.1016/j.mce.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Saleh BO, Majeed MJ, Oreaby GMJAJRC. Irisin peptide is myokine, anti-obesity and anti-lipidemic factor. Medicine. 2015;3(1):20–30. [Google Scholar]
- 17.Bosma M, Gerling M, Pasto J, Georgiadi A, Graham E, Shilkova O. et al. FNDC4 acts as an anti-inflammatory factor on macrophages and improves colitis in mice. Nat Commun. 2016;7:11314.. doi: 10.1038/ncomms11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazur-Bialy AI, Pocheć E, Zarawski MJIjoms. Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int J Mol Sci. 2017;18(4):701. doi: 10.3390/ijms18040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castañeda-Babarro A, Arbillaga-Etxarri A, Gutiérrez-Santamaría B, Coca A. Physical Activity Change during COVID-19 Confinement. Int J Environ Res Public Health. 2020;17(18):6878. doi: 10.3390/ijerph17186878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Fang W, Hu Y, Wang Y, Li J. Characterization of fibronectin type III domain-containing protein 5 (FNDC5) gene in chickens: Cloning, tissue expression, and regulation of its expression in the muscle by fasting and cold exposure. Gene. 2015;570(2):221. doi: 10.1016/j.gene.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Chen P, Mao L, Nassis GP, Harmer P, Ainsworth BE, Li F. Coronavirus disease (COVID-19): The need to maintain regular physical activity while taking precautions. J Sport Health Sci. 2020;9(2):103. doi: 10.1016/j.jshs.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dasso NA. Dasso NA. How is exercise different from physical activity. A concept analysis. 2019;54(1):45–52. doi: 10.1111/nuf.12296. [DOI] [PubMed] [Google Scholar]
- 23.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA. et al. The physical activity guidelines for Americans. Jama. 2018;320(19):2020. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozturk B, Duruturk N. Effect of telerehabilitation applied during COVID-19 isolation period on physical fitness and quality of life in overweight and obese individuals. Int J Obes (Lond) 2022;46(1):95. doi: 10.1038/s41366-021-00965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratajczak MZ, Bujko K, Ciechanowicz A, Sielatycka K, Cymer M, Marlicz W, Kucia M. SARS-CoV-2 Entry Receptor ACE2 Is Expressed on Very Small CD45- Precursors of Hematopoietic and Endothelial Cells and in Response to Virus Spike Protein Activates the Nlrp3 Inflammasome. Stem Cell Rev Rep. 2021;17(1):266. doi: 10.1007/s12015-020-10010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoseini SM, Khajei RJRJoMS. The effect of physical activity on motor function and serum levels of anti-inflammatory factors in athletes. Razi J Med Sci
- 27.Bredin SS, Gledhill N, Jamnik VK, Warburton DE. PAR-Q+ and ePARmed-X+: new risk stratification and physical activity clearance strategy for physicians and patients alike. Can Fam Physician. 2013;59(3):273. [PMC free article] [PubMed] [Google Scholar]
- 28.Ataeinosrat A, Haghighi MM, Abednatanzi H, Soltani M, Ghanbari-Niaki A, Nouri-Habashi A. et al. Effects of Three Different Modes of Resistance Training on Appetite Hormones in Males With Obesity. Front Physiol. 2022:21;13:827335.. doi: 10.3389/fphys.2022.827335. [DOI] [PMC free article] [PubMed]
- 29.Inbar O, Oren A, Scheinowitz M, Rotstein A, Dlin R, Casaburi R. Normal cardiopulmonary responses during incremental exercise in 20- to 70-yr-old men. Med Sci Sports Exerc. 1994;26(5):538. [PubMed] [Google Scholar]
- 30.May BK, Treadwell RE. Increasing Exercise Intensity: Teaching High-Intensity Interval Training to Individuals with Developmental Disabilities Using a Lottery Reinforcement System. Behav Anal Pract. 2020;13(4):826–837. doi: 10.1007/s40617-020-00428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vella CA, Taylor K, Drummer D. High-intensity interval and moderate-intensity continuous training elicit similar enjoyment and adherence levels in overweight and obese adults. Eur J Sport Sci. 2017;17(9):1203–1211. doi: 10.1080/17461391.2017.1359679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Na W, Kim J, Kim H, Lee Y, Jeong B, Lee SP. et al. Evaluation of Oral Nutritional Supplementation in the Management of Frailty among the Elderly at Facilities of Community Care for the Elderly. Clin Nutr Res. 2021;10(1):24–35. doi: 10.7762/cnr.2021.10.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha PM, Barata JT, Teixeira PJ, Ross R, Sardinha LB. Independent and opposite associations of hip and waist circumference with metabolic syndrome components and with inflammatory and atherothrombotic risk factors in overweight and obese women. Metabolism. 2008;57(10):1315. doi: 10.1016/j.metabol.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Snijder MB, Dekker JM, Visser M, Yudkin JS, Stehouwer CD, Bouter LM. et al. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obes Res. 2003;11(1):104. doi: 10.1038/oby.2003.18. [DOI] [PubMed] [Google Scholar]
- 35.Kline GM, Porcari JP, Hintermeister R, Freedson PS, Ward A, McCarron RF. et al. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Med Sci Sports Exerc. 1987;19(3):253. [PubMed] [Google Scholar]
- 36.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Can J Sport Sci. 1992;17(4):338. [PubMed] [Google Scholar]
- 37.Chiu C-H, Ko M-C, Wu L-S, Yeh D-P, Kan N-W, Lee P-F. et al. Benefits of different intensity of aerobic exercise in modulating body composition among obese young adults: a pilot randomized controlled trial. Health Qual Life Outcomes. 2017;15(1):168. doi: 10.1186/s12955-017-0743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol (1985) 2005;98(6):1985. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- 39.Bickham D, Le Rossignol. Effects of high-intensity interval training on the accumulated oxygen deficit of endurance-trained runners. Professional Exerc Physiol. 2004;7(1) [Google Scholar]
- 40.Wang Y, Chen Y. Increased risk of bacterial infections among the obese with chronic diseases. J Nutr Health Aging. 2015;19(5):595–600. doi: 10.1007/s12603-015-0472-5. [DOI] [PubMed] [Google Scholar]
- 41.Pugliese G, Liccardi A, Graziadio C, Barrea L, Muscogiuri G, Colao A. Obesity and infectious diseases: pathophysiology and epidemiology of a double pandemic condition. Int J Obes. 2022;46(3):449. doi: 10.1038/s41366-021-01035-6. [DOI] [PubMed] [Google Scholar]
- 42.Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q. et al. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care. 2020;43(7):1392. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 43.Arner P, Spalding KL. Fat cell turnover in humans. Biochem Biophys Res Commun. 2010;396(1):101. doi: 10.1016/j.bbrc.2010.02.165. [DOI] [PubMed] [Google Scholar]
- 44.Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP. et al. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26(7):1270. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC. et al. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R781. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klöting N, Ristow M, Blüher MJO. Effects of exercise on ACE2. Obesity (Silver Spring) 2020;28(12):2266. doi: 10.1002/oby.23041. [DOI] [PubMed] [Google Scholar]
- 47.Klöting N, Schwarzer M, Heyne E, Ceglarek U, Hoffmann A, Krohn K. et al. Intrinsic Exercise Capacity Affects Glycine and Angiotensin-Converting Enzyme 2 (ACE2) Levels in Sedentary and Exercise Trained Rats. Metabolites. 2022;12(6) doi: 10.3390/metabo12060548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boschetti D, Muller CR, Américo ALV, Vecchiatto B, Martucci LF, Pereira RO. et al. Aerobic Physical Exercise Improves Exercise Tolerance and Fasting Glycemia Independent of Body Weight Change in Obese Females. Front Endocrinol (Lausanne) 2021:12. doi: 10.3389/fendo.2021.772914. [DOI] [PMC free article] [PubMed]
- 49.Lee H-M, Kim J-J, Kim HJ, Shong M, Ku BJ, Jo E-KJD. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62(1):194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aygun AD, Gungor S, Ustundag B, Gurgoze MK, Sen YJMoi. Proinflammatory cytokines and leptin are increased in serum of prepubertal obese children. Mediators Inflamm. 2005;2005(3):180. doi: 10.1155/MI.2005.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kern L, Mittenbühler MJ, Vesting AJ, Ostermann AL, Wunderlich CM, Wunderlich FT. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers. 2018;11(1) doi: 10.3390/cancers11010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wani K, AlHarthi H, Alghamdi A, Sabico S, Al-Daghri NM. Role of NLRP3 Inflammasome Activation in Obesity-Mediated Metabolic Disorders Int J Environ Res Public Health. 2021;18(2) doi: 10.3390/ijerph18020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng Q, Cui G, Chen J, Gao H, Wei Y, Uede T. et al. Regular exercise enhances the immune response against microbial antigens through up-regulation of toll-like receptor signaling pathways. Cell Physiol Biochem. 2015;37(2):735. doi: 10.1159/000430391. [DOI] [PubMed] [Google Scholar]
- 55.Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis. 2012;3(1):130. [PMC free article] [PubMed] [Google Scholar]
- 56.Stensvold D, Slørdahl SA, Wisløff U. Effect of exercise training on inflammation status among people with metabolic syndrome. Metab Syndr Relat Disord. 2012;10(4):267. doi: 10.1089/met.2011.0140. [DOI] [PubMed] [Google Scholar]
- 57.Leick L, Lindegaard B, Stensvold D, Plomgaard P, Saltin B, Pilegaard HJO. Adipose tissue interleukin‐18 mRNA and plasma interleukin‐18: effect of obesity and exercise. Obesity (Silver Spring) 2007;15(2):356. doi: 10.1038/oby.2007.528. [DOI] [PubMed] [Google Scholar]
- 58.Gomarasca M, Micielska K, Faraldi M, Flis M, Perego S, Banfi G. et al. Impact of 12-Week Moderate-Intensity Aerobic Training on Inflammasome Complex Activation in Elderly Women. Front Physiol. 2022;13:792859-. doi: 10.3389/fphys.2022.792859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F. et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):E769. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 60.Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Crujeiras AB. et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8(4):e60563. doi: 10.1371/journal.pone.0060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rabiee F, Lachinani L, Ghaedi S, Nasr-Esfahani MH, Megraw TL, Ghaedi K. New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci. 2020;10:51. doi: 10.1186/s13578-020-00413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiong XQ, Geng Z, Zhou B, Zhang F, Han Y, Zhou YB. et al. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism. 2018;83:31–41. doi: 10.1016/j.metabol.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Hou N, Liu Y, Han F, Wang D, Hou X, Hou S. et al. Irisin improves perivascular adipose tissue dysfunction via regulation of the heme oxygenase-1/adiponectin axis in diet-induced obese mice. J Mol Cell Cardiol. 2016;99:188. doi: 10.1016/j.yjmcc.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 64.de Oliveira, De Sibio, Mathias LS, Rodrigues BM, Sakalem ME, Nogueira CR. Irisin modulates genes associated with severe coronavirus disease (COVID-19) outcome in human subcutaneous adipocytes cell culture. Mol Cell Endocrinol. 2020;515:110917.. doi: 10.1016/j.mce.2020.110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frühbeck G, Catalán V, Valentí V, Moncada R, Gómez-Ambrosi J, Becerril S. et al. FNDC4 and FNDC5 reduce SARS-CoV-2 entry points and spike glycoprotein S1-induced pyroptosis, apoptosis, and necroptosis in human adipocytes. Cell Mol Immunol. 2021;18(10):2457. doi: 10.1038/s41423-021-00762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frühbeck G, Fernández-Quintana B, Paniagua M, Hernández-Pardos AW, Valentí V, Moncada R. et al. FNDC4, a novel adipokine that reduces lipogenesis and promotes fat browning in human visceral adipocytes. Metabolism. 2020;108:154261.. doi: 10.1016/j.metabol.2020.154261. [DOI] [PubMed] [Google Scholar]
- 67. Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):E769-78. [DOI] [PubMed]
- 68.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu S, Cai X, Yin H, Zügel M, Sun Z, Steinacker JM. et al. Association between circulating irisin and insulin resistance in non-diabetic adults: a meta-analysis. Metabolism. 2016;65(6):825. doi: 10.1016/j.metabol.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 70.Dinas PC, Lahart IM, Timmons JA, Svensson PA, Koutedakis Y, Flouris AD. et al. Effects of physical activity on the link between PGC-1a and FNDC5 in muscle, circulating Ιrisin and UCP1 of white adipocytes in humans A systematic review. F1000Res. 2017;17:6:286.. doi: 10.12688/f1000research.11107.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


