To the Editor—We acknowledge Dr de Silva’s letter [1] on the profile of Takeda's dengue vaccine (TAK-003). Respectfully, we disagree with the author’s assertion that the safety conclusion is inaccurate. Moreover, his assertion that TAK-003–induced immunogenicity is mainly mediated by dengue virus (DENV) serotype 2 (DENV-2) is misleading and does not reflect data from 2 large trials, demonstrating tetravalent neutralizing antibody (nAb) responses that persist for multiple years [2, 3]. Furthermore, experiments performed after anti–DENV-2 nAb depletion indicate that nAb responses to the other 3 serotypes comprise both type-specific and cross-reactive nAbs [4].
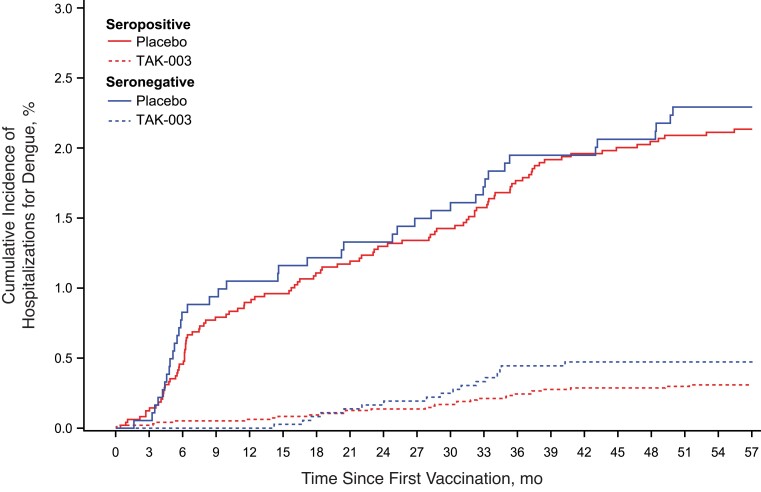
The opinion expressed by de Silva that TAK-003 shouldn’t be used in seronegative populations [1] does not accurately reflect available data, given that the vaccine had 54.3% efficacy against symptomatic and 77.1% efficacy against hospitalized dengue cases in that population over 39 months [3]. The remaining 18 months of trial data, now available, further confirm the vaccine's favorable profile, with corresponding efficacy of 53.5% and 79.3% over 57 months [5]. These latest data were presented in June 2022 at Northern European Conference on Travel Medicine and Asia Dengue Summit, and a manuscript is under peer review. The overall long-term safety profile of TAK-003 is illustrated by the cumulative incidence curves (Figure 1), which emphasize its highly promising public health impact.
Figure 1.
Cumulative incidence of hospitalization for dengue fever, by baseline serostatus in the pivotal TAK-003 vaccine efficacy trial over 57 months.
Importantly, dengue serotype distribution in the placebo seronegative group shows that DENV-1 was the predominant serotype over 57 months (50.6%), whereas the DENV-2 proportion was only 39.9% and 37.2% through 39 and 57 months, respectively. Therefore, the efficacy was not dependent on DENV-2 alone. Importantly, DENV-1 and DENV-2 are the 2 most common serotypes globally according to decades of dengue epidemiology [6, 7].
DENV-3 data from seronegative populations are complex, involving several confounding factors (eg, few case counts, different hospitalization practices, 2:1 randomization) and have been discussed in detail by Rivera et al [3]. Notably, within the placebo group (all serotypes and over 39 months), the dengue hospitalization rate at Sri Lankan sites was 68%, compared with 14.4% (range, 2.5%–37.7%) in 7 other trial countries—a considerable difference that cannot be explained by case severity alone. More plausibly, it reflects the local practice of proactive hospitalization for monitoring. Importantly, none of these DENV-3 cases in Sri Lanka was severe or dengue hemorrhagic fever grade III/IV. The totality of data did not indicate a higher risk in vaccinees. Likewise, the few DENV-4 cases in seronegative participants, while reflecting low incidence according to known epidemiology, did not indicate a higher risk of severe disease in vaccinees. Although there is no important identified safety risk, thorough monitoring will continue in the postapproval period.
In summary, the TAK-003 dengue vaccine, with a dengue backbone and components of all 4 serotypes, elicits a tetravalent immune response and has demonstrated long-term safety and efficacy regardless of serostatus in a well-designed trial conducted in 8 countries over 57 months. Given the growing burden of dengue, the lack of an efficacious vaccine that can be administered regardless of pre-exposure status, and the well-known challenges of dengue vaccine development, an all-or-none approach is not in the interest of public health. TAK-003's profile eliminates the need for a prevaccination screening, and the vaccine can meaningfully complement the current multimodal dengue control efforts.
Contributor Information
Shibadas Biswal, Takeda Vaccines Inc., Boston, Massachusetts, USA.
Sanjay S Patel, Takeda Pharmaceuticals International AG, Zurich, Switzerland.
Martina Rauscher, Takeda Pharmaceuticals International AG, Zurich, Switzerland.
Notes
Financial support. This work was supported by Takeda.
References
- 1. de Silva A. Safety of dengue vaccine? Clin Infect Dis 2023; 76:371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tricou V, Sáez-Llorens X, Yu D, et al. . Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2–17 years: a randomised, placebo-controlled, phase 2 trial. Lancet 2020; 395:1434–43. [DOI] [PubMed] [Google Scholar]
- 3. Rivera L, Biswal S, Sáez-Llorens X, et al. . Three-year efficacy and safety of Takeda's dengue vaccine candidate (TAK-003). Clin Infect Dis 2022; 75:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeMaso CR, Karwal L, Zahralban-Steele M, et al. . Specificity and breadth of the neutralizing antibody response to a live attenuated tetravalent dengue vaccine. J Infect Dis doi: 10.1093/infdis/jiac272. Published 30 June 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tricou V, Folschweiller N, Lloyd E, et al. . Efficacy and safety of Takeda’s tetravalent dengue vaccine candidate (TAK-003) after 4.5 years of follow-up. Presented at: 44th ICMM World Congress on Military Medicine 2022; 5–9 September 2022; Brussels, Belgium.
- 6. Messina JP, Brady OJ, Scott TW, et al. . Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol 2014; 22:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo C, Zhou Z, Wen Z, et al. . Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta-analysis. Front Cell Infect Microbiol 2017; 7:317. [DOI] [PMC free article] [PubMed] [Google Scholar]