Abstract
Background
Few studies have assessed participant safety in human challenge trials (HCTs). Key questions regarding HCTs include how risky such trials have been, how often adverse events (AEs) and serious adverse events (SAEs) occur, and whether risk mitigation measures have been effective.
Methods
A systematic search of PubMed and PubMed Central for articles reporting on results of HCTs published between 1980 and 2021 was performed and completed by 7 October 2021.
Results
Of 2838 articles screened, 276 were reviewed in full. A total of 15 046 challenged participants were described in 308 studies that met inclusion criteria; 286 (92.9%) of these studies reported mitigation measures used to minimize risk to the challenge population. Among 187 studies that reported on SAEs, 0.2% of participants experienced at least 1 challenge-related SAE. Among 94 studies that graded AEs by severity, challenge-related AEs graded “severe” were reported by between 5.6% and 15.8% of participants. AE data were provided as a range to account for unclear reporting. Eighty percent of studies published after 2010 were registered in a trials database.
Conclusions
HCTs are increasingly common and used for an expanding list of diseases. Although AEs occur, severe AEs and SAEs are rare. Reporting has improved over time, though not all papers provide a comprehensive report of relevant health impacts. We found very few severe symptoms or SAEs in studies that reported them, but many HCTs did not report relevant safety data. This study was preregistered on PROSPERO as CRD42021247218.
Keywords: systematic review, human challenge trial, controlled human infection model, risk mitigation, adverse events
A systematic review of health risks in human challenge trials published between 1980 and 2021 found no reported deaths or cases of permanent damage. Of challenged participants, 0.2% experienced serious adverse events and no deaths were observed.
Human challenge trials (HCTs) are a clinical research method in which volunteers are exposed to a pathogen to derive scientifically useful information about the pathogen and/or an intervention [1]. Such trials have been conducted with ethical oversight since the development of the modern institutional review system of clinical trials in the 1970s. More recently, there has been renewed discussion about the ethical and practical aspects of conducting HCTs, largely fueled by interest in conducting HCTs for severe acute respiratory syndrome coronavirus 2. Past reviews of HCTs focused on reporting methods [2] and safety for single pathogens [3–6], but these did not explicitly evaluate the safety of HCTs by assessing reported adverse events (AEs) and serious adverse events (SAEs) across a range of pathogens. Furthermore, many additional HCTs have been performed since the publication of these reviews. To better inform discussions about future uses of HCTs, including during pandemic response, this article presents a systematic review of challenge trials since 1980 and reports on their clinical outcomes, with particular focus on risk of AEs and risk mitigation strategies.
HCTs are often used to support development of therapies and vaccines more efficiently than conventional clinical trials [6, 7] and have recently been discussed as particularly valuable in the context of novel disease pandemics such as coronavirus disease 2019, Zika virus, or a future disease X [8, 9]. The benefits of such trials include defining and evaluating correlates of protection [10]; the first Food and Drug Administration (FDA)-approved cholera vaccine, Vaxchora, which proved its efficacy using a small HCT [7]; a contribution to the development of the FDA-approved therapeutic oseltamivir for influenza [11]; the Vi-tetanus toxoid conjugate vaccine for Salmonella typhi [12]; and dosing schedules and adjuvant selection for the RTS,S/AS01 malaria vaccine [13, 14].
Arguments against the use of HCTs have centered around ethics of participant compensation and the populations represented, and whether the risks and lack of personal benefit can be compatible with the principle of primum non nocere [15, 16] because of the potential risks they may inflict on a study population. Despite the debate, there is a long-standing consensus that infecting healthy volunteers is ethically justifiable as long as the risk of harm is acceptably low [15]. HCTs can therefore be ethical, based on a case-by-case assessment of risk as part of wider research ethics oversight mechanisms.
AEs related to challenge are 1 measure of health risk in HCTs. AEs refer to “any untoward medical occurrence associated with the use of a drug in humans” [17]. The FDA considers challenge agents as investigational new drugs [18], such that AEs in HCTs refer to any untoward medical occurrence associated with the challenge. AEs that result in death, hospitalization, disability, permanent damage, or other important medical events are reported as SAEs [17]. AEs graded “severe” by studies are distinct from SAEs in most cases, usually because they are not life-threatening or do not require hospitalization.
A systematic review was performed to characterize the frequency and nature of AEs and SAEs in HCTs related to the challenge and the risk mitigation measures used. The review also investigated the pathogens studied, the clinical outcomes in participants, study registration in databases, the number and uses of HCTs over time, and the quality of data reporting.
METHODS
Search Strategy
A systematic review of records from 1980 to 2021 indexed in the PubMed and PubMed Central databases was performed to identify published articles describing HCTs. Articles published before 1980 were not assessed because the modern institutional review system was not in place until after the 1979 Belmont report. The initial search was preregistered on PROSPERO as CRD42021247218 [19], but it identified few studies published before 2010. Additional searches were performed to appropriately discover studies for each decade of interest, as detailed in the amended preregistration [19] and the Supplementary Methods. The database search strategy is presented in Table 1. Further manual searches of references lists and reviews were performed to identify additional articles describing HCTs that were missed.
Table 1.
Search Strategy
| Search Number | Search Purpose | Database Accessed | Date Accessed | Query Text | Results, n |
|---|---|---|---|---|---|
| Search 1 | Articles from all decades | PMC | 20 April 2021 | (((((“human challenge”) OR (“controlled human infection”)) AND (trial OR vaccine OR model)) AND (((“adverse events”) OR (medical* AND “significant event” OR “significant events”)))) AND (“1980”[PMC Live Date] : “2021/04/20”[PMC Live Date])) |
417 |
| Search 2 | Articles before 1990 | PubMed | 6 January 2021 | ((“human challenge”) OR (“controlled human infection”) OR (“experimental” AND “infection” AND “human*”) OR (“wild-type virus” AND infection)) AND (trial OR vaccine OR model OR inoculat*) AND ((“adverse events”) OR (medical* AND “significant event” OR “significant events”) OR (illness)) AND (0:1990[pdat]) |
90 |
| Search 3 | Articles between 1990 and 2000 | PubMed | 6 January 2021 | ((experimental* AND infect*) OR (“wild-type” AND inoculat*) OR (volunteer* AND inoculat*)) AND (trial OR vaccine OR model OR inoculat* OR stud*) AND (“adverse events” OR (medical* AND “significant event*”) OR “illness”) AND (1990:2000[pdat]) |
326 |
| Search 4 | Articles between 2000 and 2010 | PubMed | 6 January 2021 | ((experimental* AND infect*) OR (“wild-type” AND inoculat*) OR (volunteer* AND inoculat*)) AND (trial OR vaccine OR model) AND (“adverse events” OR (medical* AND “significant event*”) OR “illness”) AND (2000:2010[pdat]) |
483 |
| Search 5 | Articles that were otherwise missed | PubMed | 10 July 2021 | ((human challenge AND trial) OR (human challenge AND vaccine) OR (controlled AND human AND infection AND model)) AND (severe AND events) AND (1980:2021[pdat]) |
1338 |
Abbreviation: PMC, PubMed Central.
Screening Process
Titles and abstracts of search results were manually screened by 3 authors working independently to identify articles that were eligible for full-text review. Case reports, reviews, articles not available in English, studies that did not meet the criteria for an HCT, and articles published before 1980 were excluded. Secondary reviews of 2 past reviews [2, 20] were also performed to identify more articles that were missed by the searches. Articles that described studies that performed secondary analysis of results from previously conducted HCTs were excluded, but their reference lists were reviewed to identify the original publication of these results.
Full-text Review Process
The unit of analysis is the individual study, as described within a published article detailing results. Individual studies were identified by trial registration. If trial registration was not reported, studies were counted per the article description, or as a single study if participants were challenged with a single pathogen. If multiple articles were published discussing the same study, the earliest published article was included. In some cases, multiple articles were combined (see Supplementary Methods).
There is an ongoing discussion on the precise definition of an HCT [21]. In general, studies that had been completed and involved intentional exposure of human volunteers to a pathogen were included. Challenges with candidate vaccine viruses were also included, as were studies in which previously challenged participants were challenged again with the same pathogen. Consistent with Kalil et al, studies involving live, attenuated vaccines that were not followed by intentional infection, as well as data from phases of studies involving immunization or vaccination with live, attenuated vaccines or other methods that could have potentially resulted in infection, but that are not generally referred to as HCTs, were excluded [22].
Data Collection Process
At least 2 reviewers independently examined each publication selected for full-text review and any discrepancies were either reconciled or resolved by the senior author. Data collection was performed manually and results were input into a spreadsheet.
Data Extraction
The following numerical data were extracted from each study: year of article publication, size of cohort, sex breakdowns; mean or median age, standard deviation, and age range; number of participants challenged, number of challenged participants infected with pathogen, number of participants in control group (those who did not undergo a challenge), number of control participants infected with pathogen, number of control participants with at least 1 AE, and number of challenged participants with: (1) at least 1 AE, (b) at least 1 “severe” or “very severe” (grade 3 or higher) AE, (3) at least 1 SAE.
In addition, the following nonnumerical data were extracted from each study: clinical trial registration, pathogen assessed, definition of infection, definition of AEs, treatments administered to participants, risk mitigations taken, ethics committee and review board approvals reported, and a brief description of the study design.
For articles that reported separate study arms that were all exposed to a pathogen within a single pathogen category, data were summed across all arms to be treated as a single study. Data from rechallenges were extracted separately and treated as individual studies. No treatment effect measures were extracted.
AEs among challenged participants that were not related to challenge (such as AEs related to vaccination or drug treatment) were not extracted (see Supplementary Methods). For studies that did not define and/or report AEs, reported symptom data were extracted instead. For studies that did not define and/or report SAEs, reported symptom data that met the 2016 definition of SAEs provided by the FDA [17] based on reviewer judgment were extracted as SAEs.
RESULTS
Study Selection
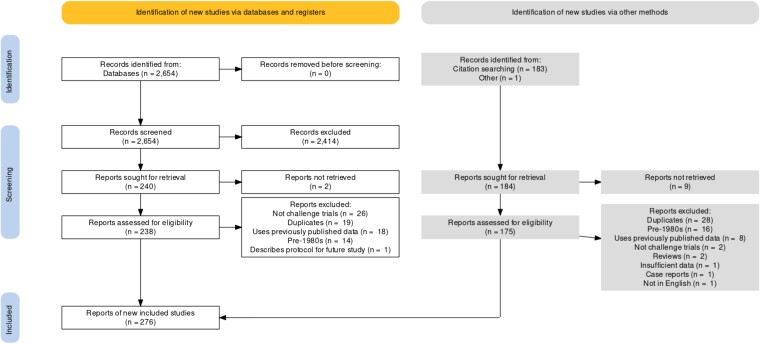
Figure 1 shows a Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of study selection. Searches yielded a total of 2654 results; 183 additional results were added by citation searching the reference lists of 2 past reviews [2, 20] and articles identified among search results that used data from prior HCTs. One article [23] provided updated data for another [24]. Eleven results were not retrieved (5 with no full text available and 6 with unpublished data) and 47 duplicates were removed. No further efforts were made to identify unpublished or unidentified work. A total of 276 articles were included, describing 308 studies from which data were extracted. Excluded results were primarily reviews and articles discussing non-HCT clinical trials. See the Supplementary references for the complete reference list of included articles.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Results of Individual Studies
Data from 284 studies, with 14 628 challenged participants, were extracted (Table 2). Additional data were extracted from 24 rechallenge studies (Supplementary Tables 3, 4, 5, and 8). Between 9917 and 10 277 challenged participants (67.8%-70.3%) were diagnosed with infection. The dataset and code used for generating all results and tables are publicly available [26].
Table 2.
Number of Studies, Number of Participants, and Number of Infections in Published HCTs by Decade
| Decade | Studies, n | Participants Challenged, n | Control Participants, n | Challenged Participants Diagnosed With Infectiona, n |
|---|---|---|---|---|
| 1980s | 31 | 1761 | 18 | 1272-1385 |
| 1990s | 68 | 4181 | 47 | 2956-3040 |
| 2000s | 57 | 2907 | 37 | 2172-2193 |
| 2010s | 106 | 4789 | 256 | 2860-2980 |
| 2020s | 22 | 990 | 75 | 657-679b |
| Total | 284 | 14 628 | 433 | 9917-10 277b |
Abbreviation: HCT, human challenge trial.
A range of values is given to account for unclear data reporting by some studies.
One additional control (nonchallenged) participant was diagnosed with infection with influenza in a human challenge-transmission model [25].
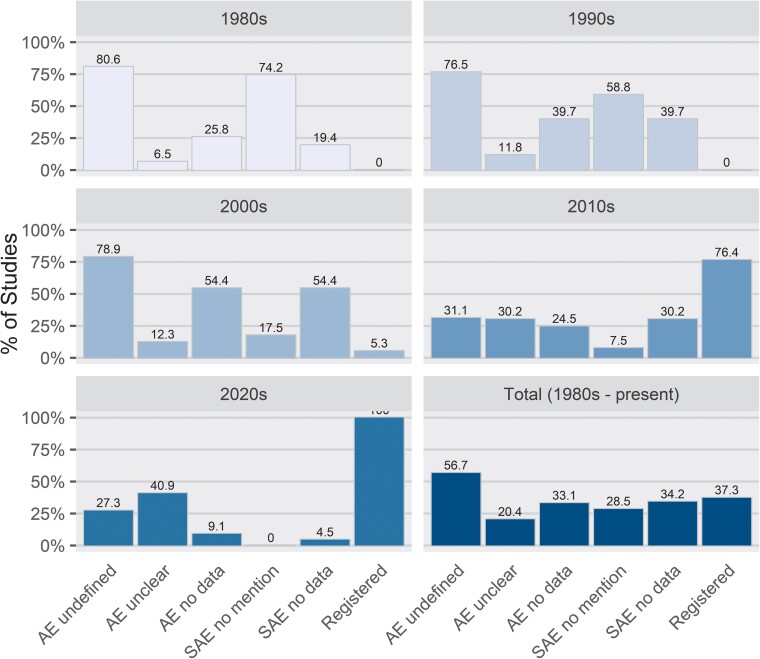
Reported AEs and Unreported Data
Among 284 studies, 94 and 97 did not report any AE or SAE data, respectively (Table 3, Figure 2). The precise number of participants experiencing at least 1 SAE could not be extracted from 2 studies: 1 lost challenged subjects' records in a flooded storage facility [27] and the other did not provide any detail on the AEs observed [28].
Table 3.
Data Reporting and Database Registration in Published HCTs by Decade
| Decade | Studies, n | Studies That Do Not Define AEs, n (%) | Studies With Unclear AE Data, n (%) | Studies With No AE Data, n (%) | Studies That Do Not Mention SAEs, n (%) | Studies With No SAE Data, n (%) |
|---|---|---|---|---|---|---|
| 1980s | 31 | 25 (80.6) | 2 (6.5) | 8 (25.8) | 23 (74.2) | 6 (19.4) |
| 1990s | 68 | 52 (76.5) | 8 (11.8) | 27 (39.7) | 40 (58.8) | 27 (39.7) |
| 2000s | 57 | 45 (78.9) | 7 (12.3) | 31 (54.4) | 10 (17.5) | 31 (54.4) |
| 2010s | 106 | 33 (31.1) | 32 (30.2) | 26 (24.5) | 8 (7.5) | 32 (30.2) |
| 2020s | 22 | 6 (27.3) | 9 (40.9) | 2 (9.1) | 0 (0.0) | 1 (4.5) |
| Total | 284 | 161 (56.7) | 58 (20.4) | 94 (33.1) | 81 (28.5) | 97 (34.2) |
Abbreviations: AE, adverse event; HCT, human challenge trial; SAE, serious adverse effect.
Figure 2.
Reporting and database registration in published human challenge trials.
Among 10 325 challenged participants in studies that reported AEs, between 4317 (41.8%) and 5730 (55.5%) experienced at least 1 AE (Table 4). Among 5083 challenged participants in studies that graded severity of AEs, between 285 (5.6%) and 801 (15.8%) experienced at least 1 severe or very severe (grade 3 or higher) AE (Table 5). The range in possible AE values is greater in more recent decades as a result of more studies reporting AEs by individual or symptom, rather than reporting the total number of participants with at least 1 AE. Nineteen studies included control (nonchallenged) participants (n = 433); only 2 of these studies reported AE data for control participants (n = 69). Between 7 (10.1%) and 12 (17.4%) control participants experienced at least 1 AE.
Table 4.
AEs in Published HCTs by Decade
| Decade | Studiesa, n | Participants Challenged, n | Challenged Participants With AEs (minimumb), n (%) | Challenged Participants With AEs (maximumb), n (%) |
|---|---|---|---|---|
| 1980s | 23 | 1448 | 389 (26.9) | 428 (29.6) |
| 1990s | 41 | 2875 | 1192 (41.5) | 1384 (48.1) |
| 2000s | 26 | 1984 | 743 (37.4) | 1001 (50.5) |
| 2010s | 80 | 3139 | 1576 (50.2) | 2210 (70.4) |
| 2020s | 20 | 879 | 417 (47.4) | 707 (80.4) |
| Total | 190 | 10 325 | 4317 (41.8) | 5730 (55.5) |
Abbreviations: AE, adverse event; HCT, human challenge trial.
94 studies that did not report AE data are excluded, see Supplementary Data, Tables 1 and 2.
Minimum and maximum values are given to account for unclear data reporting by some studies.
Table 5.
Severe AEs in Published HCTs by Decade
| Decade | Studiesa, n | Participants Challenged, n | Challenged Participants With Severe or Very Severe (≥Grade 3) AEs (Minimumb), n (%) | Challenged Participants With Severe or Very Severe (≥Grade 3) AEs (Maximumb), n (%) |
|---|---|---|---|---|
| 1980s | 3 | 77 | 9 (11.7) | 25 (32.5) |
| 1990s | 8 | 429 | 23 (5.4) | 23 (5.4) |
| 2000s | 12 | 1984 | 31 (1.6) | 102 (5.1) |
| 2010s | 57 | 1954 | 179 (9.2) | 473 (24.2) |
| 2020s | 14 | 639 | 43 (6.7) | 178 (27.9) |
| Total | 94 | 5083 | 285 (5.6) | 801 (15.8) |
Abbreviations: AE, adverse event; HCT, human challenge trial.
190 studies that did not report severe AE data are excluded, see Supplementary Data, Tables 1 and 2.
Minimum and maximum values are given to account for unclear data reporting by some studies.
Among 10 016 challenged participants in studies that reported SAEs, 23 (0.2%) experienced at least 1 SAE (Table 6). Among 146 rechallenged participants in studies that reported SAEs, 1 additional participant (0.7%) experienced at least 1 SAE (Supplementary Table 6). No fatalities were reported. SAEs are described in more detail in Table 7, and some SAEs deemed not related to challenge are discussed further in Supplementary Table 7.
Table 6.
Serious AEs in Published HCTs by Decade
| Decade | Studiesa, n | Participants Challenged, n | Challenged Participants With SAEs, n (%) |
|---|---|---|---|
| 1980s | 25 | 1469 | 6 (0.4) |
| 1990s | 41 | 2799 | 1 (0.0) |
| 2000s | 26 | 1623 | 1 (0.1) |
| 2010s | 74 | 3194 | 13 (0.4) |
| 2020s | 21 | 931 | 2 (0.2) |
| Total | 187 | 10 016 | 23b (0.2) |
Abbreviations: AE, adverse event; HCT, human challenge trial; SAE, serious adverse event.
97 studies that did not report SAE data are excluded, see Supplementary Data, Tables 1 and 2.
One additional SAE from a rechallenge is described in Table 7 but not included in this total.
Table 7.
Descriptions of SAEs in Published HCTs by Pathogen Category
| Pathogen Category | Participants With ≥1 SAE, n | Description | Outcomes | Long-term Follow-up | Dataset File Name | Supplementary Reference Numbersa |
|---|---|---|---|---|---|---|
| Escherichia coli | ||||||
| … | 2 | Clinical relapse of diarrhea and vomiting with trimethoprim-resistant strain isolated in stools, after initial improvement following trimethoprim treatment. | ND | ND | Black 1982 | 27 |
| … | 4 | “…four subjects became sufficiently ill that they received adjunctive therapy,” including intravenous fluids, antiemetics, or oral antibiotics. | ND | ND | Graham 1983 | 100 |
| Influenza viruses | ||||||
| … | 1 | A 21-y-old male developed dilated cardiomyopathy, possibly related to experimental influenza B infection. | Resolved with ACE-I treatment. | Clinically stable with low-normal cardiac output on echocardiography after ∼5 y. | Barroso 2005 | 18 |
| Norovirus | ||||||
| … | 4 | Severe vomiting and/or diarrhea. | ND | No further SAE reported over 12 mo. | Bernstein 2015 | 22 |
| Plasmodium spp | … | |||||
| … | 1 | Probable case of acute myocarditis 12 d after challenge and 1 d after diagnosis and treatment with atovaquone/proguanil for malaria. Definite etiology and mechanism have not been established. | Clinical and biochemical recovery within ∼2 wk. | Normal cardiac MRI after ∼5 mo, edema resolved, with decreased but persistently delayed enhancement of subepicardial and mid-wall regions. | Bastiaens 2016 | 19 |
| … | 1 | Asymptomatic molecular relapse with unexpected positive qPCR on day 28 (smear negative) after treatment with atovaquone/proguanil. | Remained asymptomatic. Single further borderline positive qPCR. Repeated negative smears. Retreated with chloroquine. Smear results and qPCR subsequently negative. | Plasmodium falciparum culture of blood from day 28 was negative after 4 wk incubation. | Lyke 2015 | 165 |
| … | 3 | Hepatitis temporally related and considered as likely attributable to ferroquine treatment. | ND | ND | McCarthy 2016 | 175 |
| … | 1 | Overnight hospital admission for treatment with acetaminophen and chloroquine. Mild transient thrombocytopenia, leukopenia, pyuria, hematuria. | ND | ND | Rickman 1990 | 209 |
| … | 1 | Chest pain 1 d after treatment initiated with atovaquone/proguanil, initially considered as possibly consistent with angina pectoris. | Spontaneous resolution of pain within 1 h. Brief admission for cardiac monitoring. Single abnormal ECG (negative T-wave in V2) reverting to baseline. Normal serial troponin levels. | ND | Roestenberg 2013 | 210 |
| Respiratory syncytial virus | ||||||
| … | 1 | Acute myocarditis. | ND | ND | DeVincenzo 2020 | 68 |
| Salmonella spp. | ||||||
| … | 1 | Persistent nausea, vomiting, tachycardia, not improved by oral antiemetic treatment. | Overnight admission for intravenous fluid and ceftriaxone. Discharged to complete oral ciprofloxacin course. | ND | Gibani 2020 | 95 |
| … | 1 | Elevated alanine aminotransferase (898 IU/L) 5 d after diagnosis, ascribed to paratyphoid fever plus possible adverse drug reaction. | Complete biochemical recovery. Further acetaminophen withheld and azithromycin switched to ciprofloxacin. | ND | Gibani 2020 | 95 |
| … | 1 | Reactive arthritis possibly related to challenge or antibiotic treatment. | ND | ND | Jin 2017 | 143 |
| Shigella spp | ||||||
| … | 2 | Two subjects with asymptomatic hyperbilirubinemia at day 14 visit. | Total bilirubin levels returned to normal by day 28, without treatment. | No concerns at day 42 telephone assessment. | Bodhidatta 2012 | 30 |
Abbreviations: ACE-I, angiotensin converting enzyme inhibitor; ECG, electrocardiogram; HCT, human challenge trials; MRI, magnetic resonance imaging; ND, no data; qPCR, quantitative polymerase chain reaction SAE, serious adverse event.
These numbers refer to the reference number of each study in the Supplementary reference list in the Supplementary Materials.
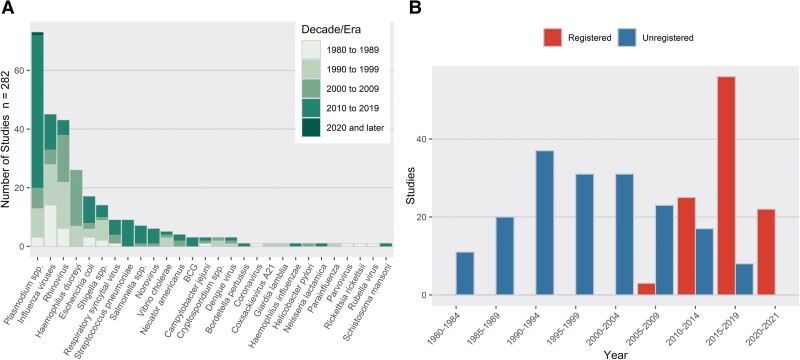
Studies by Pathogen
The numbers of studies and participants challenged within each category of pathogen are presented in Table 8, and Figure 3A illustrates studies of different pathogens have occurred over time. There were 28 pathogen categories, with the most commonly studied being Plasmodium spp (73 studies, 1689 participants), influenza viruses (45 studies, 3536 participants), and rhinovirus (43 studies, 4332 participants). Studies investigating Plasmodium spp had the greatest number of challenged participants with SAEs, with 7 SAEs (of 23 in all nonrechallenge studies) occurring among 1129 participants in 52 studies. Studies investigating norovirus had the greatest proportion of SAEs to number challenged, with 4 SAEs occurring among 163 participants in 3 studies.
Table 8.
Number of Published HCTs, Number of Participants, Number Infected, and Number With SAEs by Pathogen Category
| Pathogen Category | Studies, n | Participants Challenged Across All Studies, n | Challenged Participants Diagnosed With Infection Across All Studies, n (%) | Studies That Reported SAEs, n | Participants Challenged Across Studies That Reported SAEs, n | Challenged Participants With SAEs Across Studies That Reported SAEs, n (%) |
|---|---|---|---|---|---|---|
| BCG | 3 | 128 | 88 (68.8) | 3 | 128 | 0 (0.0) |
| Bordetella pertussis | 1 | 34 | 19 (55.9) | 1 | 34 | 0 (0.0) |
| Campylobacter jejuni | 3 | 197 | 178 (90.4) | 3 | 197 | 0 (0.0) |
| Coronavirus | 1 | 55 | 50 (90.9) | 1 | 55 | 0 (0.0) |
| Coxsackievirus A21 | 1 | 31 | 29 (93.5) | 1 | 31 | 0 (0.0) |
| Cryptosporidium spp | 3 | 79 | 45 (57.0) | 3 | 79 | 0 (0.0) |
| Dengue virus | 3 | 104 | 70 (67.3) | 2 | 63 | 0 (0.0) |
| Escherichia coli | 17 | 559 | 395 (70.7) | 9 | 300 | 6 (2.0) |
| Giardia lamblia | 1 | 19 | 5 (26.3) | 0 | 0 | 0 (0.0) |
| Haemophilus ducreyi | 26 | 218 | 180 (82.6) | 0 | 0 | 0 (0.0) |
| Haemophilus influenzae | 1 | 15 | 9 (60.0) | 0 | 0 | 0 (0.0) |
| Helicobacter pylori | 1 | 20 | 18 (90.0) | 1 | 20 | 0 (0.0) |
| Influenza viruses | 45 | 3536 | 2224 (62.9) | 38 | 3011 | 1 (0.0) |
| Necator americanus | 4 | 69 | 44 (63.8) | 2 | 45 | 0 (0.0) |
| Neisseria lactamica | 1 | 292 | 97 (33.2) | 1 | 292 | 0 (0.0) |
| Norovirus | 6 | 293 | 150 (51.2) | 3 | 163 | 4 (2.5) |
| Parainfluenza | 1 | 83 | 34 (41.0) | 1 | 83 | 0 (0.0) |
| Parvovirus | 1 | 9 | 5 (55.6) | 1 | 9 | 0 (0.0) |
| Plasmodium spp | 73 | 1689 | 1313 (77.7) | 52 | 1129 | 7 (0.6) |
| Respiratory syncytial virus | 9 | 502 | 332 (66.1) | 7 | 420 | 1 (0.2) |
| Rhinovirus | 43 | 4332 | 3285 (75.8) | 31 | 2560 | 0 (0.0) |
| Rickettsia rickettsii | 1 | 22 | 18 (81.8) | 1 | 22 | 0 (0.0) |
| Rubella virus | 2 | 40 | 28 (70.0) | 2 | 40 | 0 (0.0) |
| Salmonella spp | 7 | 374 | 197 (52.7) | 6 | 282 | 2 (0.7) |
| Schistosoma mansoni | 1 | 17 | 17 (100.0) | 1 | 17 | 0 (0.0) |
| Shigella spp | 14 | 708 | 386 (54.5) | 10 | 445 | 2 (0.4) |
| Streptococcus pneumoniae | 10 | 936 | 330 (35.3) | 5 | 530 | 0 (0.0) |
| Vibrio cholerae | 5 | 267 | 199 (74.5) | 2 | 61 | 0 (0.0) |
| Total | 284 | 14 628 | 9745 (66.6) | 187 | 10 016 | 23 (0.2) |
Abbreviations: HCT, human challenge trial; SAE, serious adverse event.
Figure 3.
(A) Studies by pathogen. (B) Data reporting and database registration in published human challenge trials.
Reporting AEs and Use of Trial Registries Over Time
Overall, the number of challenge studies has been increasing each decade (Figure 3B). Before the 2000s, many studies did not report AEs, but instead reported comparable symptom data. These were extracted as AEs. Of the 283 included studies, 123 explicitly mentioned or defined AEs, but not all reported them for the challenge phase specifically. The proportion of studies with definitions has increased over time, from only 19.4%, 23.9%, and 21.1% in the 1980s, 1990s, and 2000s, respectively, to 68.9% and 72.7% in the 2010s and 2020s (thus far), respectively. Results that exclude studies that did not explicitly mention AEs and SAEs are presented in Supplementary Tables 9 and 10.
The National Institutes of Health launched ClinicalTrials.gov on 29 February 2000. For National Institutes of Health-funded research after 2007, “applicable clinical trials” are required to be registered [29]. However, publication year lags year of registration, so it is unclear how much of the lack of registration is noncompliance and how much is delayed publication. Still, only 5.3% of included studies published in the 2000s were registered in at least 1 registry; 76.4% of included studies published in the 2010s were registered in at least 1 registry (Figure 2). Every included study published so far this decade was registered (Figure 2).
Risk Mitigation
Text describing specific risk mitigation measures was found in 286 of the 308 studies, which is included in the dataset [26], and a descriptive summary follows. The qualitative nature of these mitigation descriptions precluded meaningful quantitative analysis.
Risk mitigation measures typically include evaluating participants' risk of disease if exposed to a challenge agent by using medical screening and assessing participants' medical histories. In some cases, checking for previous exposure to the pathogen was a risk mitigation strategy, but it could also be done for other reasons. Demographic criteria, pregnancy screening, assessment of cardiac risk, and assessment of weight and/or body mass index were often used to evaluate risk.
Some studies reported mitigation strategies for risks to nonparticipants, such as isolation throughout the duration of the study, requiring birth control, or excluding participants with employment posing risk of spread (for example, excluding food handlers in HCTs investigating Escherichia coli, norovirus, and Salmonella spp). Validity of informed consent was sometimes assessed by testing participants' understanding of the study protocol.
DISCUSSION
The present review found a total of 24 (23 reported in traditional challenges, 1 in a rechallenge) SAEs and 0 reported deaths or cases of permanent damage among 15 046 participants in 308 studies spanning 1980 to 2021. It is unlikely that any SAEs captured in this review (Table 7) were life-threatening because the events were primarily brief hospitalizations for observation or supportive care requiring noninvasive interventions or falling under the broad category of “other serious (important medical events)” in the FDA definition of SAEs. The proportions of studies that define AEs and mention SAEs have increased over time, although inconsistent definitions make it challenging to compare reported data, particularly across studies investigating different pathogens. Unfortunately, the proportions of studies that do not report AE and SAE data related to challenges remained unacceptably high in the 2010s at 24.5% and 30.2%, respectively (Table 3). Although a high rate of failing to report SAEs may be indicative of their rarity in the HCT setting, clearer reporting would allow for better understanding of the risks and benefits of HCTs.
Issues surrounding AE reporting in clinical trials are not exclusive to HCTs [30]. However, confusion related to reporting challenge-related AEs is an issue specific to HCTs. For example, some studies identified “expected symptoms” as being distinct from AEs, only reported AEs related to interventions, or omitted discussion of AEs entirely. Additionally, clinical endpoints (such as moderate to severe diarrhea in E coli HCTs) were not always reported as AEs by the study. There is a greater degree of consistency for SAE reporting generally in agreement with the FDA definition [17], but many studies, especially those published before 2000, did not define or report SAEs. Guidelines for HCT reporting have been suggested [22] but have not yet been adopted. Accordingly, a major conclusion of this review is that in addition to a greater effort to standardize AE reporting in general, which others have postulated [30], these standardization efforts are particularly valuable to HCTs.
The number of new HCTs has been increasing; however, it is unclear whether this increase is proportional to the general growth trend in the number of new (non-HCT) clinical trials. Since 2010, pathogens such as Bordetella pertussis, Schistosoma mansoni, and Streptococcus pneumoniae have been studied in HCTs for the first time. Figure 3A shows that the number of influenza and rhinovirus HCTs has declined somewhat over time, following the discontinuation of several research programs focused on common cold, whereas the number of Plasmodium spp HCTs sharply increased in the 2010s. These trends demonstrate that HCTs are an increasingly ubiquitous tool and that their relative speed allows researchers to investigate new pathogens of interest more rapidly than in traditional clinical trials.
Limitations of this review are primarily related to uncertainties around the accuracy of AE reporting. This includes potential bias in AE reporting, inconsistent reporting, and difficulty in precisely estimating the rates of events based on provided data. Many studies reported either no or unclear AE and/or SAE data, and issues of censoring and misclassification are common with respect to AE reporting in general [31]. To partially address issues with different standards for reporting over time, we extracted symptom data as AE and/or SAE data from studies that did not mention or define AEs/SAEs, but this means that AEs for decades in which these studies occurred are not fully comparable. The review is further limited by our inability to locate some results, including published HCTs that were not on PubMed [32] and HCTs whose results have only been published as case reports [33]. These limitations further highlight the need for improvements in the field of HCTs with respect to AE reporting and availability of results. Future work building off of this review includes policy recommendations around the issues of standardization and AE reporting, investigating the registration of HCTs in databases, and further qualitative analysis of risk mitigation measures in published articles.
CONCLUSIONS
The recent literature contains hundreds of HCTs involving more than 10 000 participants and only 24 SAEs. With the qualification that systematic AE reporting in many studies has been incomplete, reports of severe symptoms and SAEs related to infectious challenge in HCTs are notably infrequent. Specifically, participation in an HCT has not been associated with permanent impairment or death. HCTs are now routinely used to understand infectious dose, disease progression, clinical efficacy of novel interventions, and immune response for a wide variety of pathogens. As evidenced by recent HCTs for coronavirus disease 2019, they may be conducted for novel as well as familiar diseases. This review can help support public discussion and expert deliberation regarding the safety of HCTs. It may also inform future discussions among HCT researchers and members of ethics review committees regarding the planning, conduct, and reporting of future HCTs.
Preregistration, Protocol, and Conflict of Interest Disclosures
The review was preregistered on PROSPERO as CRD42021247218, risk outcomes and risk mitigation measures in human challenge trials: a systematic review. As mentioned previously, the preregistration was amended to include additional searches and data. The review protocol is available online as Supplementary Material—Protocol.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. D. M., W. W., and V. S. conceived of the idea. E. J., J. O., M. R., and W. W. provided initial feedback and refined the idea. D. T. and D. M. designed and preregistered the systematic review, with feedback and expert guidance from E. J., J. O., and M. R. D. T. led the initial review for inclusion, with J. A. P. and K. S. The full-text reviews were done by J. A. P., D. T., and 2 nonauthor reviewers thanked in the Acknowledgments: S. K. and D. K. Disputes were resolved by D. M. Guidance on inclusion criteria and interpretation was provided by E. J., K. S., and J. O. J. A. P. and D. T. led writing of the manuscript, with supervision and assistance by D. M., V. S., W. W., K. S., E. J., and J. O. J. W. managed the dataset, performed analysis, led creation of visualizations, and produced tables and data summaries. D. M. and W. W. contributed to the visualizations.
Acknowledgments . Thank you to 1DaySooner for supporting this work, and to the 1DaySooner Scientific Advisory board, which includes coauthors D. M., V. S., and W. W., for reviewing the proposed study. The authors thank Steffen Kamenicek and Daniel Kaufman, who assisted with full-text review of papers and data extraction. They also thank Steffen Kamenicek and River Bellamy for additional assistance with reviewing the text, tables, and figures before submission.
Financial support . This work was supported by 1Day Sooner (D. T., J. A. P., K. S., W. W.). D. M. was supported by grants from the Center for Effective Altruism’s Long Term Future Fund and Guarding Against Pandemics (materials, Article Processing Charges). E. J.'s work was supported by the Wellcome Trust, including current grants 221 719 and 216 355. J. O. reports support from Melbourne Children's campus (Royal Children's Hospital, University of Melbourne, Murdoch Children's Research Institute) and the Australian National Health and Medical Research Council (NHMRC). During manuscript preparation/submission, V. S. reports a position as the director of research for 1Day Sooner, which funded the time spent working on the manuscript.
Availability of data, code, and other materials. The complete dataset of included studies is publicly available online [26].
Supplementary Material
Contributor Information
Jupiter Adams-Phipps, 1Day Sooner Research Team, Lewes, Delaware, USA.
Danny Toomey, 1Day Sooner Research Team, Lewes, Delaware, USA; Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Witold Więcek, 1Day Sooner Research Team, Lewes, Delaware, USA.
Virginia Schmit, 1Day Sooner Research Team, Lewes, Delaware, USA.
James Wilkinson, 1Day Sooner Research Team, Lewes, Delaware, USA.
Keller Scholl, RAND Corporation, Pardee RAND Graduate School, Santa Monica, California, USA.
Euzebiusz Jamrozik, The Ethox Centre & Wellcome Centre for Ethics and the Humanities, Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom; Monash Bioethics Centre, Monash University, Clayton, VIC, Australia; Royal Melbourne Hospital Department of Medicine, University of Melbourne, Parkville, VIC, Australia.
Joshua Osowicki, Murdoch Children's Research Institute, Parkville, VIC, Australia; Infectious Diseases Unit, Department of General Medicine, Royal Children's Hospital Melbourne, Parkville, VIC, Australia.
Meta Roestenberg, Department of Parasitology, Leiden University Medical Centre, Leiden, The Netherlands; Department of Infectious Diseases, Leiden University Medical Center, Leiden, ZA, The Netherlands.
David Manheim, 1Day Sooner Research Team, Lewes, Delaware, USA; Technion, Israel Institute of Technology, Haifa, Israel; ALTER, Association for Long Term Existence and Resilience, Rehovot, Israel.
References
- 1. Gordon SB, Rylance J, Luck A, et al. A framework for Controlled Human Infection Model (CHIM) studies in Malawi: report of a Wellcome Trust workshop on CHIM in low income countries held in Blantyre, Malawi. Wellcome Open Res 2017; 2:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roestenberg M, Hoogerwerf M-A, Ferreira DM, Mordmüller B, Yazdanbakhsh M. Experimental infection of human volunteers. Lancet Infect Dis 2018; 18:e312–22. [DOI] [PubMed] [Google Scholar]
- 3. Church LWP, Le TP, Bryan JP, et al. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. J Infect Dis 1997; 175:915–20. [DOI] [PubMed] [Google Scholar]
- 4. Epstein JE, Rao S, Williams F, et al. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: an update. J Infect Dis 2007; 196:145–54. [DOI] [PubMed] [Google Scholar]
- 5. Balasingam S, Wilder-Smith A. Randomized controlled trials for influenza drugs and vaccines: a review of controlled human infection studies. Int J Infect Dis 2016; 49:18–29. [DOI] [PubMed] [Google Scholar]
- 6. Sherman AC, Mehta A, Dickert NW, Anderson EJ, Rouphael N. The future of flu: a review of the human challenge model and systems biology for advancement of influenza vaccinology. Front Cell Infect Microbiol 2019; 9:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mosley JF, Smith LL, Brantley P, Locke D, Como M. Vaxchora: the first FDA-approved cholera vaccination in the United States. Pharm Ther 2017; 42:638–40. [PMC free article] [PubMed] [Google Scholar]
- 8. Palacios R, Shah SK. When could human challenge trials be deployed to combat emerging infectious diseases? Lessons from the case of a Zika virus human challenge trial. Trials 2019; 20:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen LC, Bakerlee CW, McKelvey TG, et al. Evaluating use cases for human challenge trials in accelerating SARS-CoV-2 vaccine development. Clin Infect Dis 2020; 72:710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Memoli MJ, Shaw PA, Han A, et al. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. mBio 2016; 7:e00417–00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayden FG, Treanor JJ, Fritz RS, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA 1999; 282:1240–6. [DOI] [PubMed] [Google Scholar]
- 12. Jin C, Gibani MM, Moore M, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella typhi: a randomised controlled, phase 2b trial. Lancet 2017; 390:2472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Regules JA, Cicatelli SB, Bennett JW, et al. Fractional third and fourth dose of RTS, S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis 2016; 214:762–71. [DOI] [PubMed] [Google Scholar]
- 14. Leroux-Roels G, Leroux-Roels I, Clement F, et al. Evaluation of the immune response to RTS, S/AS01 and RTS, S/AS02 adjuvanted vaccines: randomized, double-blind study in malaria-naïve adults. Hum Vaccin Immunother 2014; 10:2211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hope T, McMillan J. Challenge studies of human volunteers: ethical issues. J Med Ethics 2004; 30:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grimwade O, Savulescu J, Giubilini A, et al. Payment in challenge studies: ethics, attitudes and a new payment for risk model. J Med Ethics 2020; 46:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Investigational New Drug Application. Code of Federal Regulations. Sect. 312.32 IND safety reporting.
- 18. Guidance for Clinical Investigators, Sponsors, and IRBs; Investigational New Drug Applications (INDs)—Determining Whether Human Research Studies Can Be Conducted Without an IND [Internet]. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), and Center for Food Safety and Applied Nutrition (CFSAN); 2013 September. Available at: https://www.fda.gov/files/drugs/published/Investigational-New-Drug-Applications-%28INDs%29-Determining-Whether-Human-Research-Studies-Can-Be-Conducted-Without-an-IND.pdf. Accessed 15 June 2022.
- 19. Manheim D, Toomey D, Wiecek W, Schmit V, Adams-Phipps J, Scholl K. Risk outcomes and risk mitigation measures in human challenge trials: a systematic review. PROSPERO: International prospective register of systematic reviews; 2021. Available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021247218. Accessed 15 June 2022.
- 20. Jamrozik E, Selgelid MJ. Human challenge studies in endemic settings: ethical and regulatory issues. Switzerland: Springer Nature; 2021: 134.(SpringerBriefs in Ethics). [Google Scholar]
- 21. Pollard AJ, Sauerwein R, Baay M, et al. Third human challenge trial conference, Oxford, United Kingdom, 6–7 February 2020, a meeting report. Biologicals 2020; 66:41–52. [DOI] [PubMed] [Google Scholar]
- 22. Kalil JA, Halperin SA, Langley JM. Human challenge studies: a review of adequacy of reporting methods and results. Future Microbiol 2012; 7:481–95. [DOI] [PubMed] [Google Scholar]
- 23. Atmar RL, Opekun AR, Gilger MA, et al. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis 2014; 209:1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Atmar RL, Opekun AR, Gilger MA, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis 2008; 14:1553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen-Van-Tam JS, Killingley B, Enstone J, et al. Minimal transmission in an influenza A (H3N2) human challenge-transmission model within a controlled exposure environment. PLoS Pathog 2020; 16:e1008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [dataset] Wilkinson J, Manheim D. 1DaySooner/HCTSystematicReview: human challenge trial systematic review dataset, graphs, and explanation. Zenodo, 2022. doi: 10.5281/zenodo.6711717. [DOI]
- 27. Hickey BW, Lumsden JM, Reyes S, et al. Mosquito bite immunization with radiation-attenuated Plasmodium falciparum sporozoites: safety, tolerability, protective efficacy and humoral immunogenicity. Malar J 2016; 15:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen WH, Cohen MB, Kirkpatrick BD, et al. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with Vibrio cholerae O1 El Tor. Clin Infect Dis 2016; 62:1329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clinical trials registration and results information submission. US code of federal regulations, title 42(I)(A) Part 11 2016. Available at:https://www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-11. Accessed 15 June 2022.
- 30. Neuer A. A fresh take on the adverse event landscape. Clin Res. 12 February 2019 [cited 2021 Nov 16]; 33(2). Available at:https://acrpnet.org/2019/02/12/a-fresh-take-on-the-adverse-event-landscape/. Accessed 15 June 2022. [Google Scholar]
- 31. Schroll JB, Maund E, Gøtzsche PC. Challenges in coding adverse events in clinical trials: a systematic review. PLoS One 2012; 7:e41174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Osowicki J, Azzopardi KI, Fabri L, et al. A controlled human infection model of Streptococcus pyogenes pharyngitis (CHIVAS-M75): an observational, dose-finding study. Lancet Microbe 2021; 2:e291–9. [DOI] [PubMed] [Google Scholar]
- 33. Nieman A-E, de Mast Q, Roestenberg M, et al. Cardiac complication after experimental human malaria infection: a case report. Malar J 2009; 8:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.