Abstract
Background
Assessing the infectious reservoir is critical in malaria control and elimination strategies. We conducted a longitudinal epidemiological study in a high-malaria-burden region in Kenya to characterize transmission in an asymptomatic population.
Methods
488 study participants encompassing all ages in 120 households within 30 clusters were followed for 1 year with monthly sampling. Malaria was diagnosed by microscopy and molecular methods. Transmission potential in gametocytemic participants was assessed using direct skin and/or membrane mosquito feeding assays, then treated with artemether-lumefantrine. Study variables were assessed using mixed-effects generalized linear models.
Results
Asexual and sexual parasite data were collected from 3792 participant visits, with 903 linked with feeding assays. Univariate analysis revealed that the 6–11-year-old age group was at higher risk of harboring asexual and sexual infections than those <6 years old (odds ratio [OR] 1.68, P < .001; and OR 1.81, P < .001), respectively. Participants with submicroscopic parasitemia were at a lower risk of gametocytemia compared with microscopic parasitemia (OR 0.04, P < .001), but they transmitted at a significantly higher rate (OR 2.00, P = .002). A large proportion of the study population who were infected at least once remained infected (despite treatment) with asexual (71.7%, 291/406) or sexual (37.4%, 152/406) parasites. 88.6% (365/412) of feeding assays conducted in individuals who failed treatment the previous month resulted in transmissions.
Conclusions
Individuals with asymptomatic infection sustain the transmission cycle, with the 6–11-year age group serving as an important reservoir. The high rates of artemether-lumefantrine treatment failures suggest surveillance programs using molecular methods need to be expanded for accurate monitoring and evaluation of treatment outcomes.
Keywords: Plasmodium falciparum, malaria surveillance and transmission dynnamics, malaria control and elimination, artemether/lumefantrine, drug resistance
Primary school children are the most important infectious reservoir for transmission of malaria. Asymptomatic participants with submicroscopic parasitemia transmit malaria at a significantly higher rate compared with microscopic parasitemia. One-third of the study population failed treatment with artemether-lumefantrine.
The goal of malaria control and, in some settings, elimination has brought focus on the need for comprehensive, high-quality, sustained surveillance to inform program planning and implementation of malaria intervention strategies [1, 2]. In endemic areas, infection with Plasmodium falciparum can lead to asymptomatic carriage, which may progress to symptomatic uncomplicated or severe malaria [3]. Both asymptomatic and symptomatic infections are important reservoirs for transmission, which is determined primarily by gametocyte density, fitness, and circulation time [4]. The presence of mature gametocytes in human peripheral blood is a prerequisite for human infectiousness to mosquitoes, with successful mosquito infections observed in those with microscopic and submicroscopic parasites [4]. With studies showing nonlinear positive relationships between gametocyte density and mosquito infection risk [5, 6], there is a need for field data in natural settings to better understand the determinants of human transmission potential.
Studies investigating the complexity and dynamics of malaria transmission have not been adequate, especially in high-transmission settings, with only a limited number of studies investigating the longitudinal infectiousness of individuals or populations in natural settings [4, 7]. We conducted a longitudinal study following natural malaria infections in a region of intense transmission to better understand the complexity and dynamics of malaria transmission in high-transmission settings. Individuals within household clusters of varying ages were followed for 12 months, and their infectiousness assessed by mosquito feeding assays before and after treatment, with testing performed using highly sensitive molecular assays.
METHODS
Study Area, Design, and Participants
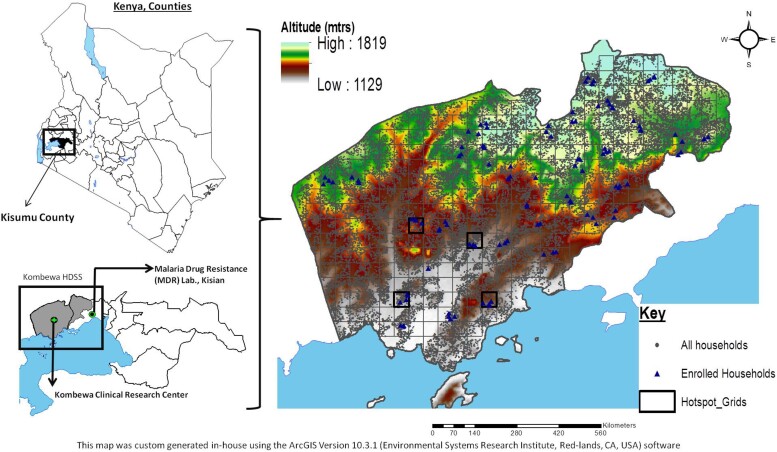
This study was conducted in Kombewa, western Kenya, a holoendemic region with year-round transmission of mostly (>95%) P. falciparum infections [8]. A grid with 1 km × 1 km cells was overlaid on a map of the study area covering 369 km [2, 9] and a total of 50 cells selected at random using ArcGIS mapping software (Esri, Redlands, CA, USA), ensuring at least a 1-km buffer zone around a selected cell and 4 or more households (Figure 1). To be considered for participation, a household had to have at least 1 adult aged older than 25 years, at least 1 child aged 5 years or younger, and at least 1 person aged between 6 and 25 years. Consents/assents were obtained from household members in a total of 30 cells referred to as clusters. The study was approved by the Walter Reed Army Institute of Research (WRAIR) Institutional Review Board and Kenya Medical Research Institute (KEMRI) Ethics Review Committee.
Figure 1.
Study site locations. Map showing the study area in western Kenya, which has been cartographically mapped by the HDSS using GPS, located on the northeastern shores of Lake Victoria. Each grid is 1 km2. The gray dots represent all the households on HDSS, and the blue triangle represent the households enrolled in the study. Abbreviations: GPS, Global Positioning System; HDDS, Health and Demographic Surveillance System; mtrs, meters.
Household Visits, Parasite Detection, and Treatment
Study teams collected clinical and epidemiological data from each household and cluster once per week, and blood samples were collected from each participant once a month for the duration of the study (12 months). Detection of asexual parasites by real-time polymerase chain reaction (PCR; real-time PCR [qPCR]) and sexual parasites by reverse transcriptase–real-time PCR (RT-qPCR) was performed on all blood samples within 18 hours of collection using previously published methods (Supplementary Data) [10]. Malaria blood smears were also prepared and read on a later date as previously described [11]. Participants infected with sexual parasites (based on RT-qPCR) were requested to participate in mosquito feeding assays either by direct skin feeding assays (DFAs) and/or to donate 2 mL venous blood for a membrane feeding assay (MFA) within 24 hours. Those who declined were treated immediately for uncomplicated malaria using artemether-lumefantrine following Kenya Ministry of Health–recommended case management guidelines, and those who consented/assented were treated immediately following the blood draw and/or feeding procedure. The attending clinician administered and observed the first dose of treatment. Further, to ensure compliance with the remainder of treatment dose, participants were followed up using text messaging and/or home visits.
Mosquito Feeding Assays
Mosquito feeding assays were performed either by DFA, MFA, or both for a subset of consenting adults. For DFAs, we used a previously described protocol on participants aged 7 years and older [12, 13]. For MFAs, we used the previously published procedures, with the notable exception that serum collected from participant blood samples was not replaced with nonimmune serum [13, 14]. Participants younger than 7 years old participated only in MFAs. Mosquitoes that completed a successful feed were maintained for 7–9 days post-feeding for mid-gut dissections for oocyst quantification on mercurochrome-stained slides using microscopy. A subset (30%) of the fed mosquitoes were maintained for 12–14 days for salivary gland dissection and enumeration of sporozoites.
Statistical Analysis
Detailed information on statistical analysis and modeling used in this study can be found in the Supplementary Data. Briefly, asexual and sexual parasite prevalence was based on positive samples as a percentage of the total samples examined for both microscopy and PCR-based detection, with the sensitivity and specificity of microscopy calculated relative to PCR. The spatial-temporal patterns in parasite prevalence were explored using spatial scan statistics estimated using a Bernoulli model of malaria prevalence [15] and SaTScan software (SaTScan, version 10.0 [https://www.satscan.org/]) [16]. From this, we identified significant (P < .05) clusters for which malaria prevalence was higher than expected (hotspot) or lower than expected (coldspot). We explore the risk factors associated with asexual and sexual parasite infection using univariate logistic regression before reporting the adjusted odds ratios (ORs).
Treatment failures were defined as being PCR-positive for asexual infection 1 month after being treated with artemether-lumefantrine. All data, codes, and analyses are available on Github (https://github.com/OJWatson/kenya_al_failure).
RESULTS
A total of 488 participants from 120 households within 30 clusters were enrolled in the study. A total of 4362 contacts were made with participants during the study, with each contact considered an independent event (Supplementary Table 1). We obtained complete asexual and sexual parasite data by both PCR and microscopy for 98.0% (478/488) individuals, representing 86.2% (3792/4362) of the samples collected. 84.9% (406/478) of individuals were infected with either asexual or sexual parasites at least once during the study period. For the 3792 samples with complete parasite data, the mean age was 17 years (range, 0.7–72.5 years), with 69.2% of samples collected from females (Supplementary Table 2). The overall prevalence of P. falciparum asexual parasites was 54.7% (2074/3792) by qPCR, where 27.3% (567/2074) of all infections were microscopic and 72.7% (1507/2074) of asexual infections were submicroscopic. Parasitemia positivity rate differed significantly when we compared those with microscopically patent infections with qPCR (P < .001 each), with the 6–11-year age group having the highest positivity rates at 24.7% (286/1173) by microscopy and 69.8% (819/1173) by qPCR (Supplementary Table 3).
The overall prevalence of P. falciparum sexual parasites was 29.6% (1123/3792) by RT-qPCR, where 47.6% (534/1123) of gametocyte-positive infections were from asexual microscopy infections and 52.7% (592/1123) were from asexual submicroscopic infections, with the 6–11-year age group having the highest gametocyte positivity rates at 40.7% (477/1173) (Supplementary Table 4A). The proportion of sexual infections was higher in those with asexual microscopic parasites (94.2% [534/567]) compared with submicroscopic parasites (39.3% [592/1507]) (Supplementary Table 4B). When we compared the proportion of sexual infections in different age groups, the difference in positivity rate was only apparent when we separated microscopic from submicroscopic asexual infections where the positivity rate significantly increased with age (P < .001) (Supplementary Table 4C). The temporal burden of sexual parasites mirrored that of asexual parasites (Supplementary Figure 1). Analysis of spatial-temporal clusters identified 4 significant hotspots (ie, clusters with a relative risk >1 and P < .05) and 4 significant coldspots (clusters highly significant; ie, P < .005) (Supplementary Figure 2, Supplementary Table 5).
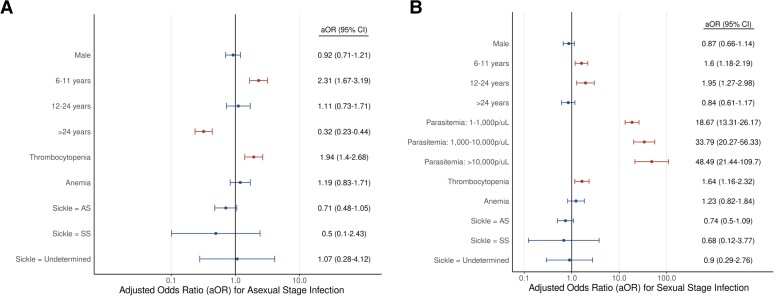
Univariate analyses were initially performed to explore risk factors associated with asexual and sexual infections (Table 1) before reporting the adjusted ORs (aORs) after adjusting for other individual covariates (Figure 2). Age was a significant predictor of both asexual and sexual stage infection. In comparison to the younger-than-6-year age group, the 6–11-year age group was at a higher risk for both asexual (OR = 1.68; 95% confidence interval [CI]: 1.27–2.22; P < .001) and sexual infections (OR = 1.81; 95% CI: 1.38–2.39; P < .001) compared with the younger-than-6-year age group. The 12–24-year age group was at higher risk of sexual infection (OR = 1.83; 95% CI: 1.23–2.72; P = .003) and the older-than-24-year age group was at lower risk for asexual infections (OR = .26; 95% CI: .2–.34; P < .001), while also at a lower risk for sexual infections in the univariate analysis (OR = .49; 95% CI: .36–.65; P < .001); however, this was not significant in the adjusted analysis (aOR = .84; 95% CI: .61–1.17; P = .302). Overall, study participants with submicroscopic parasites were at a lower risk of having gametocytes compared with microscopically patent infections (OR = .04; 95% CI: .02–.05; P < .001). While anemia (OR = 1.78; 95% CI: 1.25–2.53; P < .001) and the AS sickle cell (heterozygous) genotype (.63; .4–.98; P = .041) were significant risk factors for asexual infections in the univariate analysis, these effects were nonsignificant in the adjusted analysis (Figure 2). On the contrary, thrombocytopenia was a significant predictor of asexual (OR = 1.78; 95% CI: 1.25–2.53; P = .001) and sexual (OR = 2.63; 95% CI: 1.89–3.66; P < .001) parasites and remained significant in the adjusted analysis (Figure 2).
Table 1.
Univariate Analyses of Risk Factors for Asexual and Sexual Parasite Infections
| Asexual Parasite | Sexual Parasites | |||
|---|---|---|---|---|
| Variables | OR [95% CI] | P a | OR [95% CI] | P |
| Gender | ||||
| ȃFemale, n = 2281 | 1 | 1 | ||
| ȃMale, n = 1511 | 1.28 [.96–1.71] | .099 | 1.21 [.93–1.58] | .153 |
| Age category | ||||
| ȃ<6 years, n = 1100 | 1 | 1 | ||
| ȃ6–11 years, n = 1173 | 1.68 [1.27–2.22] | <.001 | 1.81 [1.38–2.39] | <.001 |
| ȃ12–24 years, n = 389 | 1.02 [.69–1.51] | .911 | 1.83 [1.23–2.72] | .003 |
| ȃ>24 years, n = 1130 | .26 [.2–.34] | <.001 | .49 [.36–.65] | <.001 |
| Parasitemia (p/µL)b | ||||
| ȃ0, n = 3024 | … | … | 1 | |
| ȃ1–1000, n = 354 | … | … | 22.17 [16.01–30.69] | <.001 |
| ȃ1000–10 000, n = 205 | … | … | 42.41 [25.87–69.52] | <.001 |
| ȃ10 000–100 000, n = 88 | … | … | 64.9 [29.48–142.89] | <.001 |
| Thrombocytopenia | ||||
| ȃNo, n = 3195 | 1 | 1 | ||
| ȃYes, n = 311 | 1.41 [1.03–1.94] | .03 | 2.24 [1.66–3.02] | <.001 |
| Anemia | ||||
| ȃNo, n = 3,184 | 1 | 1 | ||
| ȃYes, n = 322 | 1.78 [1.25–2.53] | .001 | 2.63 [1.89–3.66] | <.001 |
| Sickle cell trait | ||||
| ȃAA, n = 3062 | 1 | 1 | ||
| ȃAS, n = 556 | .63 [.4–.98] | .041 | .68 [.45–1.03] | .068 |
| ȃSS, n = 18 | .47 [.07–2.99] | .423 | 1.23 [.23–6.59] | .811 |
| ȃUndetermined, n = 39 | 1.01 [.23–4.47] | .995 | 1.26 [.36–4.42] | .72 |
Abbreviations: CI, confidence interval; OR, odds ratio; p/uL, parasites per microliter.
P values and OR from univariate logistic regression model with random intercepts for each individual, household, and cluster.
Parasitemia based on microscopy.
Figure 2.
Risk factors associated with asexual and sexual stage infections. The aOR for (A) asexual and (B) sexual stage infection for each predictor assessed is shown, with 95% CIs as whiskers surrounding each point. Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
We then investigated transmission rates in our study population by performing DFA and MFA. DFAs accounted for 69.4% (831/1198) of the mosquito feeding events and MFAs accounted for 30.6% (367/1198), with 2.5% (30/1198) having DFA and MFA feeding events performed from the same study participant. DFAs and MFAs had similar infection success (which led to the development of oocysts in at least 1 mosquito during the feeding assay in the mid-gut; 83.4% [693/831] vs 86.5% [315/367]; P = .17) and in the salivary gland (73.4% [608/831] vs 70.3% [258/367]; P = .26) dissections. There was no significant difference in the prevalence of oocysts in participants with dual feeds (DFA and MFA) (Supplementary Figure 3).
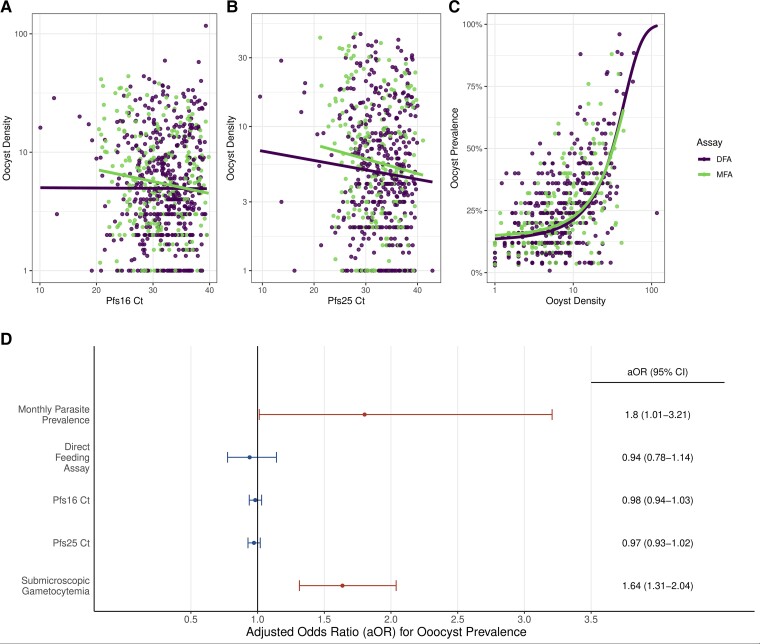
For the 903 feeding experiments that could be linked to individual-level covariates, the sexual stage–specific RT-qPCR analyses revealed that oocyst density was significantly correlated with gametocyte density for the MFA but not DFA (Figure 3A and 3B, Supplementary Table 6). Mean oocyst density was observed to be sigmoidally related to oocyst prevalence, with no significant difference observed in this relationship between the MFA and DFA (Figure 3C and 3D, Supplementary Figure 4). When assessing the risk factors for infectiousness, univariate analysis revealed that age and asexual parasite density were not significant risk factors for infection success or oocyst prevalence (Table 2). Participants with submicroscopic sexual parasites had a significantly higher infection success when compared with those with microscopic sexual parasites (Table 2). A similar finding was observed when comparing oocyst prevalence (the proportion of mosquitoes infected during the feeding assay) between submicroscopic and microscopically detectable sexual infection (Table 2). In the adjusted analysis, submicroscopic sexual infections continued to be a significant risk factor for oocyst prevalence along with average monthly cluster malaria prevalence (Figure 3D).
Figure 3.
Relationship and predictors of oocyst density and prevalence. The relationship between oocyst density (the average number of oocysts per feeding assay) and (A) Pfs16 and (B) Pfs25 RT-qPCR Ct is shown for both DFA and MFA. A linear relationship was found to best explain the relationship in both panels A and B, although this was within 2 AIC differences compared with a power law relationship. A significant correlation is observed between oocyst density and Ct in MFA but not DFA (Pfs16 Ct [MFA: P = .002; DFA: P = .509] and Pfs25 Ct [MFA: P = .040; DFA: P = .262]). In panel C, a significant logistic relationship observed between oocyst prevalence (proportion of mosquitoes with at least 1 oocyst per feeding assay) is shown to be positively correlated against oocyst density (P < .001). In panel D, factors significantly associated (P < .05) with oocyst prevalence are shown in red, indicating oocyst prevalence is significantly higher during months with higher parasite prevalence and from submicroscopic sexual infections. The aORs and 95% CIs are shown on the right. Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; Ct, cycle threshold; DFA, direct skin feeding assay; MFA, membrane feeding assay; RT-qPCR, reverse transcriptasereal-time quantitative polymerase chain reaction.
Table 2.
ȃUnivariate Analysis of Infection Success and Oocyst Prevalence
| Infection Successa | Oocyst Prevalenceb | |||||
|---|---|---|---|---|---|---|
| Variables | Mean % [95% Binomial CI] | OR [95% CI] | P | Mean % [95% Bootstrapped CI] | OR [95% CI] | P |
| Asexual microscopy status | ||||||
| ȃMicroscopic asexual parasitemia, n = 343 | 84.3 [80–87.7] | 1 | 17.0 [14.9–18.7] | 1 | ||
| ȃSubmicroscopic asexual parasitemia, n = 429 | 82.9 [79–86.2] | .91 [.62–1.34] | .635 | 17.4 [15.8–19.1] | .99 [.85–1.15] | .851 |
| Sexual microscopy status | ||||||
| ȃMicroscopic gametocytemia, n = 144 | 74.8 [67.1–81.2] | 1 | 13.1 [10.3–15.5] | 1 | ||
| ȃSubmicroscopic gametocytemia, n = 594 | 85.5 [82.4–88.1] | 2.00 [1.28–3.14] | .002 | 18.1 [16.8–19.4] | 1.50 [1.23–1.83] | <.001 |
| Age category | ||||||
| ȃ<6 years, n = 246 | 85.2 [80.2–89.1] | 1 | 18.8 [16.5–20.9] | 1 | ||
| ȃ6–11 years, n = 364 | 86.8 [82.9–89.9] | 1.15 [.72–1.83] | .573 | 18.6 [16.8–20.2] | 1.02 [.86–1.22] | .814 |
| ȃ12–24 years, n = 119 | 79.0 [70.8–85.3] | .67 [.37–1.19] | .171 | 15.1 [12.2–18] | .81 [.64–1.04] | .097 |
| ȃ>24 years, n = 174 | 85.1 [79–89.6] | 1.00 [.58–1.74] | .999 | 18.3 [15.3–21] | .99 [.8–1.22] | .922 |
Abbreviations: CI, confidence interval; OR, odds ratio.
At least 1 mid-gut positive for oocytes in a pool of at least 25 mosquitoes that fed on a single study participant. P values and ORs from univariate logistic regression model with random intercepts for household.
Number of mid-guts positive for oocysts in a pool of at least 25 mosquitoes that fed on a single study participant. Due to overdispersion in oocyst prevalence, CIs are given as basic bootstrapped CIs (1000 repetitions). P values and ORs from univariate beta-binomial regression model with random intercepts for each household.
After each mosquito feeding event, participants were treated with artemether-lumefantrine to clear the parasites. We investigated how quickly participants cleared parasites after each treatment and the subsequent ability to transmit. Participants were considered to have had an inadequate treatment response (treatment failure) if the following month they remained positive for infection by PCR. Of individuals, 67.1% (321/478) were at 1 point during the study positive for sexual infections and had at least 2 continuous months of sampling. Of these, 30.0% (95/321), 14.6% (47/321), and 3.1% (10/321) had 1, 2, or 3 distinct treatment failure episodes, respectively (Supplementary Figure 5). Of individuals, 78.0% (373/478) were at 1 point during the study positive for asexual infections and had at least 2 continuous months of sampling. Of these, 39.4% (147/373), 33.5% (125/373), and 5.1% (19/373) had 1, 2, or 3 treatment failure episodes, respectively (Supplementary Figure 5). There were also 412 feeding events that were conducted in individuals who had failed treatment the previous month. Of these 412 feeding events, 88.6% (365/412) resulted in onward infections. The duration of consecutive treatment failures was also assessed for both sexual and asexual infections. For both types of infection, the majority were cleared within 3 months, with over 90% of infections clearing after 6 months. While sexual infections displayed a clear exponential distribution in clearance times, the distribution of parasite clearance times for asexual infections was overdispersed, with an excess of long-lasting infections lasting longer than 6 months (Supplementary Figure 6).
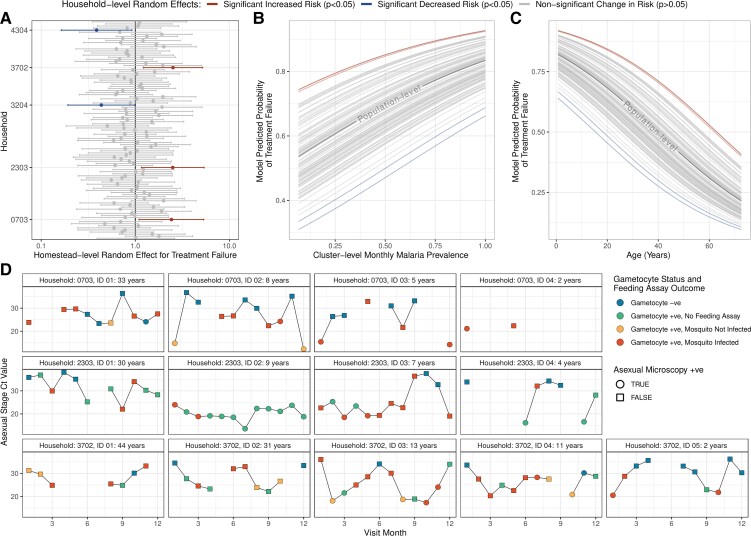
Failure to clear sexual stage parasites after treatment was expected, with artemether-lumefantrine designed to target asexual parasites rather than mature gametocytes. However, the higher proportion of inadequate treatment responses for asexual infections compared with sexual infections was surprising. To further explore these findings, focusing on the 1537 individuals with asexual infections and known treatment outcomes the following month, we explored the risk of treatment failure. We observed that both age and average monthly, cluster-level malaria prevalence were significant predictors of treatment failures (Figure 4B and 4C). Younger individuals were significantly more likely to experience treatment failures (age [years], aOR = .96; 95% CI: .95–.97; P < .001), and more instances of treatment failures were observed during period of higher transmission (monthly cluster-level malaria prevalence [range, 0–1]; aOR = 4.86; 95% CI: 2.29–10.3; P < .001). When we controlled for variation at the household level using random effects, 3 households were significantly more likely to experience treatment failures after adjusting for age and malaria prevalence (Figure 4A). We observed individuals in these households who were frequently positive for asexual infection despite treatment with artemether-lumefantrine (Figure 4D). While some individuals were likely exhibiting very slow parasite clearance, as typified by slowly increasing asexual stage RT-qPCR cycle threshold (Ct) values (eg, Household 3702, ID 03), some individuals maintained high parasite-density infections every month and were observed to infect mosquitoes during conducted feeding assays (eg, Household 2303).
Figure 4.
Characterization of asexual infection treatment failures. Individuals who were treated with artemether-lumefantrine but remained infected the following month were regarded as treatment failures. A mixed-effects logistic regression model with random intercepts at the household level (effect size and 95% CI) shown in panel A was used to identify that (B) cluster-level monthly malaria prevalence and (C) age were significant predictors of treatment failure. The parasite profiles of all individuals in the 3 households with a significantly increased risk of treatment failure identified in panel A are shown in panel D. For each individual, the RT-qPCR Ct for asexual parasite stages is plotted over time. Gametocyte status and feeding assay outcome are indicated by the color of the point, and the shape of the point indicates whether the individual was microscopy positive for asexual parasites. Continual observation (ie, observations the following month) are indicated by points connected by lines. Abbreviations: CI, confidence interval; Ct, cycle threshold; RT-qPCR, reverse transcriptase–real-time quantitative polymerase chain reaction; +ve, positive; –ve, negative.
DISCUSSION
We conducted a longitudinal epidemiological study to assess the infectiousness of asymptomatic individuals in a region of high malaria burden in western Kenya. Malaria-control programs often focus on children under 5 years due to their vulnerability and role as an infectious reservoir for transmission [3, 17]. Our study had 2 key findings with important programmatic implications. First, children in the 6–11-year age group, and not those under 6 years of age, carried the highest parasite burden, suggesting that school-age children likely function as an important infectious reservoir for transmission in this population. Therefore, targeting this age group for intensive malaria-control efforts such as chemoprevention may be an important new strategic approach—in particular, given the elevated rates of treatment failures observed in younger ages. Higher rates of infection in this age group may be attributable to activities that put them at risk for exposure to mosquito bites [9]. Second, our monthly follow-up diagnosis and treatment of participants revealed that artemether-lumefantrine did not adequately clear asexual parasites or disrupt transmission in a large proportion of our study population. Treatment failures of this nature occurred more frequently during periods of higher malaria prevalence and in younger individuals. These findings were only possible due to the application of molecular diagnosis, whose adoption will become increasingly invaluable as the primary endpoint for surveillance studies and therapeutic efficacy studies (TESs).
The observed high rates of parasite recurrence despite monthly treatment are consistent with an earlier study in western Kenya [18] and recent findings in Africa of persistent submicroscopic P. falciparum parasitemia 72 hours after treatment with artemether-lumefantrine, which predicted 42-day treatment failure [19–21]. This finding has previously been observed to vary depending on transmission intensity [20], which we also observed in our study. Additional considerations may be necessary when formulating artemether-lumefantrine dosing for transmission disruption based on transmission intensity, which may explain 1 of the reasons along with poor coverage as to why mass drug administration studies in high-transmission settings have not been successful [22, 23].
Our feeding assay experiments revealed that individuals with submicroscopically detectable gametocytes infected more mosquitoes than those with microscopically detectable gametocytes. One possible explanation may be due to the difference in a participant’s immune presentation at the time of mosquito bite, which has been shown to modulate the probability of onward infection [17, 24]. This effect may be driven by density-dependent transmission-blocking immunity, where high densities may inhibit successful transmission by eliciting a strong, specific immune response [25].
Studies show that individuals with submicroscopic and microscopic parasites are infectious, whether they are asymptomatic or symptomatic [26, 27]. Notably, most of this information has been obtained from clinical or cross-sectional studies, which have, in general, relied on microscopy and/or malaria rapid diagnostic tests for diagnosis [28, 29]. Using molecular diagnosis, we identified a large proportion of study participants who were continuously infected with asexual and/or sexual parasites for up to 1 year. Chronic infections can produce significantly more secondary infections than short-lived clinical infections, with modelling previously showing that chronic infections contribute heavily towards sustaining transmission in regions where malaria would not be likely to invade, a phenomenon known as bistability [30]. Persistence of such infections in the asymptomatic phase presents a resource allocation equilibrium relative to within-host and between-parasite interactions that temper growth/survival versus reproduction, and current versus future reproduction [24]. Indeed, we discovered hotspot clusters, with sustained recurrent parasitemia, geographically close to regions with very low malaria prevalence, a likely bistability phenomenon in this region of intense transmission.
Therapeutic efficacy for artemisinin-based combination therapy is determined by the rate of parasite clearance in the first 3 days, and the presence of parasites on day 28 and/or 42 [31]. Artemether-lumefantrine clears asexual parasites rapidly in the first 3 days [31] and has also been shown to be effective in clearing gametocytes [32, 33]. Mature gametocytes usually appear 7–15 days after the initial wave of asexual parasites from which they are derived [34, 35]. Although our study was not a TES, after every blood collection and/or mosquito feeding event in gametocytemic individuals, participants were treated with artemether-lumefantrine. Surprisingly, more than one-third of the study population was continuously and continually infected with asexual and sexual parasites, with some individuals having episodes lasting several months, up to 1 year, regardless of the monthly treatment to clear the parasites during the monthly surveillance. Therapeutic efficacy studies are conducted in a highly controlled environment, with most studies not enrolling hyperparasitemic individuals. Our study represents a “real life” effectiveness study, where many variables may influence treatment outcome, as often observed in naturally infected individuals receiving self-dosing scheduled prescriptions for uncomplicated malaria. In a longitudinal study conducted in children under 6 years of age in the same patient population in 2005, none of the participants had microscopically detected asexual or sexual parasites on day 28 after treatment with artemether-lumefantrine [36]. Our data raise the concern that the efficacy of artemether-lumefantrine may have declined in this study population over time, corroborating recent reports across the continent [19–21, 37–39].
This study had several limitations. First, participants were sampled only once a month; more frequent sampling would have provided more comprehensive information. Immunological analyses were not performed and may have provided important information, especially in elucidating the difference in the infectiousness between submicroscopic compared with microscopic infections. Parasite infections were not genetically characterized and did not quantify gametocyte densities. However, regardless of limitations, this comprehensive study was designed to minimize selection bias and provides relevant data.
In conclusion, we have identified possible new approaches that, if implemented, may reduce transmission in high-malaria-burden regions. Our data show that, in high-burden settings, all age groups are important reservoirs for transmission, especially older children, and individuals are continuously and continually transmitting, making R0 from a single infection high, creating a bistability phenomenon, in which household dynamics and the environment are risk factors.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Dr. Veronica Manduku, KEMRI Center for Clinical Research, and Dr. Steve Munga, KEMRI Center for Global Health Research, for supporting this study and giving their permission to publish these data. They also thank all clinical staff at Kombewa District Hospitals, the Clinical Research Centre in Kombewa, the amazing field staff, and all those who unwaveringly supported this study. It literally took the whole village. Last, they thank Dr. Andre Lin Ouédraogo (Institute for Disease Modeling, Bill and Melinda Gates Foundation; aouedraogo@idmod.org) for reviewing the initial draft and providing thoughtful comments.
Disclaimer. The material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. This paper has been approved for public release with unlimited distribution. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25. J. C. and E. K. are government employees and this work was prepared as part of their official duties. Title 17 USC §105 provides that copyright protection under this title is not available for any work of the US government. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Army, Department of Defense, or the US government. The study sponsor had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The corresponding author confirms that he had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support. This work was supported by the Malaria Vaccine Branch of the Walter Reed Army Institute of Research. O. J. W. is supported by a Schmidt Science Fellowship in partnership with the Rhodes Trust.
Supplementary Material
Contributor Information
Ben Andagalu, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Oliver J Watson, Medical Research Council, Centre for Global Infectious Disease Analysis, Department of Infectious Disease Epidemiology, Imperial College London, London, United Kingdom.
Irene Onyango, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Benjamin Opot, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Raphael Okoth, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Gladys Chemwor, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Peter Sifuna, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Dennis Juma, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Agnes Cheruiyot, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Redemptah Yeda, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Charles Okudo, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Jackline Wafubwa, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Santos Yalwala, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
David Abuom, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Bernhards Ogutu, Kenya Medical Research Institute (KEMRI), Nairobi, Kenya.
Jessica Cowden, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Hoseah M Akala, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya.
Edwin Kamau, Department of Emerging and Infectious Diseases (DEID), US Army Medical Research Directorate-Africa (USAMRD-A), Kenya Medical Research Institute (KEMRI)/Walter Reed Project, Kisumu, Kenya; US Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Department of Pathology and Laboratory Medicine, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA.
References
- 1. Mueller I, Slutsker L, Tanner M. Estimating the burden of malaria: the need for improved surveillance. PLoS Med 2011; 8:e1001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nahlen BL, Low-Beer D. Building to collective impact: the global fund support for measuring reduction in the burden of malaria. Am J Trop Med Hyg 2007; 77:321–7. [PubMed] [Google Scholar]
- 3. Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev 2009; 22:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone W, Goncalves BP, Bousema T, Drakeley C. Assessing the infectious reservoir of falciparum malaria: past and future. Trends Parasitol 2015; 31:287–96. [DOI] [PubMed] [Google Scholar]
- 5. Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 2011; 24:377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Churcher TS, Bousema T, Walker M, et al. . Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. Elife 2013; 2:e00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ouedraogo AL, Goncalves BP, Gneme A, et al. . Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis 2016; 213:90–9. [DOI] [PubMed] [Google Scholar]
- 8. Zhou G, Afrane YA, Vardo-Zalik AM, et al. . Changing patterns of malaria epidemiology between 2002 and 2010 in Western Kenya: the fall and rise of malaria. PLoS One 2011; 6:e20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sifuna PM, Ouma C, Atieli H, et al. . Spatial epidemiology of tuberculosis in the high-burden counties of Kisumu and Siaya. Western Kenya 2012–2015. Int J Tuberc Lung Dis 2019; 23:363–70. [DOI] [PubMed] [Google Scholar]
- 10. Kamau E, Tolbert LS, Kortepeter L, et al. . Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J Clin Microbiol 2011; 49:2946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Odhiambo G, Bergmann-Leitner E, Maraka M, et al. . Correlation between malaria-specific antibody profiles and responses to artemisinin combination therapy for treatment of uncomplicated malaria in Western Kenya. J Infect Dis 2019; 219:1969–79. [DOI] [PubMed] [Google Scholar]
- 12. Bonnet S, Gouagna LC, Paul RE, Safeukui I, Meunier JY, Boudin C. Estimation of malaria transmission from humans to mosquitoes in two neighbouring villages in south Cameroon: evaluation and comparison of several indices. Trans R Soc Trop Med Hyg 2003; 97:53–9. [DOI] [PubMed] [Google Scholar]
- 13. Bousema T, Dinglasan RR, Morlais I, et al. . Mosquito feeding assays to determine the infectiousness of naturally infected plasmodium falciparum gametocyte carriers. PLoS One 2012; 7:e42821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Da DF, Dixit S, Sattabonkot J, et al. . Anti-Pfs25 human plasma reduces transmission of Plasmodium falciparum isolates that have diverse genetic backgrounds. Infect Immun 2013; 81:1984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kulldorff M. SaTScan—software for the spatial, temporal, and space-time scan statistics. Harvard Medical School and Harvard Pilgrim Health Care Boston, 2010. Available at: https://www.satscan.org/. Accessed 1 April 2022.
- 16. Mosha JF, Sturrock HJ, Greenwood B, et al. . Hot spot or not: a comparison of spatial statistical methods to predict prospective malaria infections. Malar J 2014; 13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McQueen PG, Williamson KC, McKenzie FE. Host immune constraints on malaria transmission: insights from population biology of within-host parasites. Malar J 2013; 12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beshir KB, Sutherland CJ, Sawa P, et al. . Residual plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis 2013; 208:2017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silva-Pinto A, Domingos J, Cardoso M, et al. . Artemether-lumefantrine treatment failure of uncomplicated Plasmodium falciparum malaria in travellers coming from Angola and Mozambique. Int J Infect Dis 2021; 110:151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beshir KB, Diallo N, Some FA, et al. . Persistent submicroscopic plasmodium falciparum parasitemia 72 hours after treatment with artemether-lumefantrine predicts 42-day treatment failure in Mali and Burkina Faso. Antimicrob Agents Chemother 2021; 65:e0087321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sutherland CJ, Lansdell P, Sanders M, et al. . pfk13-Independent treatment failure in four imported cases of plasmodium falciparum malaria treated with artemether-lumefantrine in the United Kingdom. Antimicrob Agents Chemother 2017; 61:e02382–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris U, Msellem MI, Mkali H, et al. . A cluster randomised controlled trial of two rounds of mass drug administration in Zanzibar, a malaria pre-elimination setting-high coverage and safety, but no significant impact on transmission. BMC Med 2018; 16:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shekalaghe SA, Drakeley C, van den Bosch S, et al. . A cluster-randomized trial of mass drug administration with a gametocytocidal drug combination to interrupt malaria transmission in a low endemic area in Tanzania. Malar J 2011; 10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Jong RM, Tebeje SK, Meerstein-Kessel L, et al. . Immunity against sexual stage plasmodium falciparum and plasmodium vivax parasites. Immunol Rev 2020; 293:190–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mideo N, Day T. On the evolution of reproductive restraint in malaria. Proc Biol Sci 2008; 275:1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaumeau V, Kajeechiwa L, Fustec B, et al. . Contribution of asymptomatic plasmodium infections to the transmission of malaria in Kayin State, Myanmar. J Infect Dis 2019; 219:1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Slater HC, Ross A, Felger I, et al. . Author correction: the temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat Commun 2019; 10:2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alonso PL, Brown G, Arevalo-Herrera M, et al. . A research agenda to underpin malaria eradication. PLoS Med 2011; 8:e1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. malERA Consultative Group on Monitoring, Evaluation, and Surveillance . A research agenda for malaria eradication: monitoring, evaluation, and surveillance. PLoS Med 2011; 8:e1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aguas R, Maude RJ, Gomes MGM, White LJ, White NJ, Dondorp AM. Infectivity of chronic malaria infections and its consequences for control and elimination. Clin Infect Dis 2018; 67:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gansane A, Moriarty LF, Menard D, et al. . Anti-malarial efficacy and resistance monitoring of artemether-lumefantrine and dihydroartemisinin-piperaquine shows inadequate efficacy in children in Burkina Faso 2017–2018. Malar J 2021; 20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lefevre G, Looareesuwan S, Treeprasertsuk S, et al. . A clinical and pharmacokinetic trial of six doses of artemether-lumefantrine for multidrug-resistant Plasmodium falciparum malaria in Thailand. Am J Trop Med Hyg 2001; 64:247–56. [DOI] [PubMed] [Google Scholar]
- 33. Omondi P, Burugu M, Matoke-Muhia D, et al. . Gametocyte clearance in children, from western Kenya, with uncomplicated plasmodium falciparum malaria after artemether-lumefantrine or dihydroartemisinin-piperaquine treatment. Malar J 2019; 18:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Day KP, Hayward RE, Dyer M. The biology of plasmodium falciparum transmission stages. Parasitology 1998; 116:S95–109. [DOI] [PubMed] [Google Scholar]
- 35. Eichner M, Diebner HH, Molineaux L, Collins WE, Jeffery GM, Dietz K. Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malariatherapy data. Trans R Soc Trop Med Hyg 2001; 95:497–501. [DOI] [PubMed] [Google Scholar]
- 36. Andagalu B, Mativo J, Kamau E, Ogutu B. Longitudinal study on Plasmodium falciparum gametocyte carriage following artemether-lumefantrine administration in a cohort of children aged 12–47 months living in Western Kenya, a high transmission area. Malar J 2014; 13:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balikagala B, Fukuda N, Ikeda M, et al. . Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 2021; 385:1163–71. [DOI] [PubMed] [Google Scholar]
- 38. Uwimana A, Legrand E, Stokes BH, et al. . Emergence and clonal expansion of in vitro artemisinin-resistant plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med 2020; 26:1602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. World Health Organization . Global database on antimalarial drug efficacy and resistance. Global Malaria Programme, World Health Organization. Geneva, Switzerland, 2021. Available at: https://www.who.int/activities/monitoring-malaria-drug-efficacy-and-resistance. Accessed 1 April 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.