Abstract
Background:
Lymphatic metastasis is commonly seen in patients with esophageal squamous cell carcinoma (ESCC). Both lymphatic metastasis and the number of involved nodes are prognostic for post-operative survival. To better understand lymphatic metastasis of ESCC, there is a need to develop proper animal models.
Aims:
This study is aimed to characterize the morphology and function of the lymphatic drainage system in the mouse esophagus.
Methods:
Immunostaining and fluorescence imaging were used to visualize the lymphatic drainage system in the mouse esophagus. Tracers and cancer cells were orthotopically inoculated into the submucosa of the mouse esophagus to mimic lymphatic metastasis of T1 ESCC.
Results:
Using immunostaining of a lymphatic vessel marker (LYVE1), we found that lymphatic vessels were located in the submucosa and muscularis propria of the mouse esophagus, similar to the human esophagus. In the esophagus of ProxTom mice expressing tdTomato in the lymphatic vessels, we discovered a microscopic meshwork of lymphatic vessels. Functionally, orthotopically inoculated tracers (Indian ink and FITC-dextran) were drained from the submucosa into peri-esophageal lymph nodes via lymphatic vessels. Orthotopically inoculated mouse cancer cells (LLC-eGFP, MOC2) metastasized from the submucosa to lymphatic vessels, peri-esophageal lymph nodes, and distant organs (liver and lung) in immunocompetent mice. Similarly, in immunodeficient mice, orthotopically inoculated human ESCC cells (KYSE450-eGFP-Luc) metastasized via the same route.
Conclusion:
We have characterized the morphology and function of the lymphatic drainage system of the mouse esophagus. These observations lay a foundation for mechanistic and therapeutic studies on lymphatic metastasis of T1 ESCC.
Keywords: Lymphatic drainage, Esophagus, Esophageal squamous cell carcinoma, Lymphatic metastasis
Introduction
Esophageal cancer is the seventh most prevalent cancer and the sixth leading cause of cancer-related death in the world, with more than 604,100 new cases and 544,076 deaths in 2020 [1]. Esophageal squamous cell carcinoma (ESCC) is one of the two major histological types and its 5-year survival rate is ~18%, a number that reflects late diagnosis, the aggressiveness of the disease, and a lack of effective treatment strategies[2]. At least 40% of patients have already developed lymph node metastasis when ESCC was diagnosed. The presence of metastatic lymph nodes, the number of involved nodes, and the lymph node ratio are negative prognostic factors after surgery [3,4]. ESCC cells first penetrate the epithelium’s basement membrane and gradually invade the lamina propria, muscularis mucosae, submucosa, muscularis propria, and adventitia. During this course, some of the cancer cells enter the lymphatic vessels (lymphatic permeation) and undergo metastasis into lymph nodes, bloodstream, and distant organs (most commonly lung, liver, and bone). The deeper the cancer cells can invade through the esophagus the more likely lymphatic permeation and lymph node metastasis will take place. Once cancer cells reach the muscularis mucosa, lymphatic permeation and lymph node metastasis become highly probable [5,6]. Therefore, it is critical to understand how ESCC cells spread to distant organs through the lymphatic system to develop better diagnostic and therapeutic strategies.
Human esophageal lymphatic vessels are distributed in the lamina propria, muscularis mucosa, submucosa, and muscularis propria. The presence of an abundant lymphatic network in the lamina propria supports the clinical observation that lymphatic metastasis of ESCC can take place as early as stage T1a. More importantly, esophageal lymphatic vessels run longitudinally, transversely, and perpendicularly within the esophagus, and can directly drain into the thoracic duct or through nodal relay[7–10]. Lymphatics in the proximal third of the human esophagus tend to drain into the deep cervical lymph nodes and the thoracic duct. In the middle third and the distal third, lymphatics tend to drain into the superior and posterior mediastinal nodes, and the gastric and celiac lymph nodes, respectively. These three drainage regions are highly interconnected, and thus retrograde, bidirectional, and nodal skip patterns of metastasis are commonly seen in human ESCC [10–12].
To better understand lymphatic metastasis of human ESCC, there is a need of developing proper animal models. In the literature, two previous studies reported lymphatic permeation and lymph node metastasis of human ESCC cells (i.e., TE8, KYSE150) when inoculated orthotopically into the esophagus of immunodeficient mice[13,14]. However, the lymphatic system in the mouse esophagus has not been characterized to our best knowledge. Only one previous study reported a lymphatic vessel encircling the distal esophagus[15]. In this study, we characterized the morphology and function of the lymphatic drainage system of the mouse esophagus.
Materials and Methods
Cell culture
Human ESCC cells (KYSE450) were obtained from the ATCC (Manassas, VA). Mouse LLC-eGFP and MOC2 cells were obtained from Imanis Life Sciences (Rochester, MN) and Kerafast (Boston, MA), respectively. LLC-eGFP is a GFP-labelled murine Lewis lung carcinoma cell line developed in a C57BL/6J mouse. MOC2 is a murine oral SCC cell line developed from 7,12-dimethylbenz[a]anthracene-induced oral cancer in C57BL/6J mice. ERK1/2 activation conferred MOC2 cells a highly metastatic phenotype in regional lymph nodes when the cells were inoculated subcutaneously[16]. 7,12-dimethylbenz[a]anthracene is known to induce genomic alterations in the mouse SCC cells similar to those in human ESCC[17].
KYSE450 cells were cultured in RPMI 1640 with 10% FBS and 1% penicillin-streptomycin. LLC-eGFP cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. MOC2 cells were cultured in IMDM with Ham’s nutrient mixture (Gibco), supplemented with 5% FBS, 1% penicillin-streptomycin, 5 mg/L insulin, 40 μg/L hydrocortisone, and 5 μg/L EGF. KYSE450 cells were labeled with GFP and luciferase via stable transfection with firefly luciferase + eGFP Lentifect™ purified lentiviral particles (GeneCopoeia, Rockville, MD) according to the manufacturer’s instructions. In brief, 5 × 104 of KYSE450 cells per well were plated in a 24-well plate 24 hours before viral infection. After removing the old culture medium from each well, polybrene (5 μg/ml) and diluted lentivirus were added. The eGFP fluorescence can be visualized under a fluorescent microscope after 4 days. The culture medium was replaced with a fresh complete medium containing the selection chemical once every 3–4 days to select drug-resistant colonies. This subline was named KYSE450-eGFP-Luc.
Animals
Wild-type C57BL/6J mice and B6;129S-Tg(Prox1-tdTomato)12Nrud/J mice (ProxTom in short) were obtained from the Jackson Laboratory (Bar Harbor, ME). ProxTom mice have tdTomato expressed under the direction of the promoter of Prox1, which encodes a transcription factor (prospero-related homeobox 1) specific for lymphatic vessels[15]. Immunocompromised R2G2 mice were obtained from Envigo (Dublin, VA) and used for orthotopic inoculation of KYSE450-eGFP-Luc cells. The R2G2 mice are double knockout mice (Il2rg and Rag2) with an ultra immunodeficient phenotype. All animal experiments were approved by the IACUC at the North Carolina Central University (protocol number XC06142019).
To test the drainage of tracers or cells through the lymphatic system in the mouse esophagus, we developed a surgical procedure of orthotopic inoculation into the submucosa of the abdominal and cervical esophagus. Eight-week-old mice were anesthetized with a mixture of ketamine (100mg/kg) and xylazine (10mg/kg, i.p.). To expose the abdominal esophagus, a skin incision was made in the middle of the upper abdomen. The stomach was pulled out and a cotton swab was placed under the esophagus to enhance the exposure of the abdominal esophagus. To expose the cervical esophagus, a skin incision was made on the neck. The salivary glands were separated and the trachea was pulled aside to expose the cervical esophagus for orthotopic injection.
Indian ink or FITC-dextran (MW: 200,000; Cat. #FD2000S, Sigma, St. Louis, MO) were injected with a SMARTouch microinjection syringe pump (World Precision Instruments, Sarasota, FL; 4μl per site). LLC-eGFP, MOC2, and KYSE450-eGFP-Luc cells were suspended in Matrigel at a concentration of 1×106 cells/10 μl. The cells were injected into the esophageal submucosa slowly with an insulin syringe. Mouse skin was closed with surgical clips.
When sacrificed, mice were anesthetized and perfused with 4% PFA. The esophagus, peri-esophageal lymph nodes, and organs (lung and liver) were harvested to obtain fresh tissues, to be fixed with PBS-buffered formalin, or to be frozen in OCT. Indian ink-positive lymph nodes were first identified by visual examination and confirmed by tissue sectioning and H&E staining. FITC dextran-positive lymph nodes were first identified under a dissecting fluorescence microscope (MVX10, Olympus corporation; Tokyo, Japan) and confirmed by tissue sectioning and fluorescence microscopy.
IVIS imaging
The growth of KYSE450-eGFP-Luc cells was monitored by IVIS imaging. Luciferin was injected intraperitoneally into mice at a dose of 150 mg/kg body weight. The mice were then anesthetized and placed in the supine position on the imaging stage inside the IVIS apparatus (Lumina Series III, Perkin Elmer, Waltham, MA). Images were collected once every few minutes after luciferin injection. The GFP signal is directly visualized with the fluorescence channel.
H&E staining and immunohistochemical staining (IHC)
We used a routine protocol for hematoxylin and eosin (H&E) staining. For IHC, we used a streptavidin–peroxidase reaction kit with DAB as a chromogen (ABC kit, Vector Laboratories, Burlingame, CA). In brief, deparaffinized sections were pretreated with an antigen unmasking solution to retrieve antigens before blocking with 10% normal serum. Tissue sections were then incubated with a rabbit polyclonal anti-LYVE1 (lymphatic vessel endothelial hyaluronan receptor 1; 1:6,400; Cat. #Ab33682; Abcam, Waltham, MA), a rabbit polyclonal anti-CD31 (1:50; Cat. #Ab28364; Abcam), a mouse monoclonal anti-PROX1 (1:25; Cat. #Ab199359; Abcam), a mouse monoclonal anti-pan-cytokeratin (1:50; Cat. #Ab86734; Abcam), a mouse monoclonal anti-GFP (1:500; Cat. #SC-9996, Santa Cruz, Dallas, TX), a rabbit monoclonal anti-E-cadherin (1:500; Cat. #Ab40772; Abcam), or a rabbit polyclonal anti-SNAIL/SLUG (1:500; Cat. #Ab85936; Abcam) at 4C overnight. For double IHC staining, we used an ImmPRESS Duet Double Staining HRP/AP Polymer Kit (MP-7714, anti-rabbit IgG-brown, anti-mouse IgG-red) (Vector Laboratories) according to the manufacturer’s instructions.
Confocal microscopy and light-sheet microscopy
PFA-fixed tissues were cut into 100μm–thick frozen sections. Nuclei were counterstained with DAPI (Cat. # H-1200, Vector Laboratories). Digital images were captured using a confocal microscope (LSM 800, Carl Zeiss GmbH, Jena, Germany). Images were analyzed and the 3D image was reconstructed using Imaris software (Bitplane, Oxford Industries; Concord, MA).
For light-sheet microscopy, tissue clearing was performed on two ProxTom esophagi according to the iDISCO+ protocol [18]. In brief, mice were perfused via transcardial perfusion with 4% PFA. The esophagus was fixed in 4% PFA for an additional day. Samples were then washed in PBS, dehydrated in a graded series of methanol, and pretreated with 66% dichloromethane methanol and 5% H2O2/methanol, followed by rehydration, permeabilization (20% DMSO, 1.6% Triton X-100, 23mg/mL glycine), and blocking with 6% goat serum. Samples were then incubated with rabbit anti-RFP antibody (1:400, Rockland, Limerick, PA) for 1 day at 37°C in PTwH buffer (PBS, 0.5% Tween-20, 10mg/L Heparin). After 2 days of washing with PTwH, samples were incubated with goat anti-rabbit Alexa Fluor 555 (ab150078, 1:400, Abcam) for 1 day at RT. Samples were then washed for 12 hours with PTwH, dehydrated again using a graded methanol series, and incubated in 66% dichloromethane/methanol for 3 hours, followed by a 30-minute incubation in 100% dichloromethane before storing in a dibenzyl ether solution (RI = 1.56, Sigma-Aldrich) at RT. The light-sheet imaging was performed using the Ultramicroscope II (LaVision Biotec, Germany) equipped with MVPLAPO 2X/0.5 NA objective (Olympus), sCMOS camera, and ImSpector control software. The zoom body was set to 2.5x magnification (yielding 1.21 μm/pixel). The cleared esophagus was positioned horizontally or parallelly to the single illuminating light-sheet and imaged in dibenzyl ether. Images were acquired using a 561nm laser line. The 3D images of the entire esophagus were constructed using Imaris software.
Results
Lymphatic vessels in the mouse esophagus
In the mouse esophagus, IHC of two lymphatic vessel markers (LYVE1 and PROX1) showed lymphatic vessels mostly in the esophageal muscle. Both LYVE1 and PROX1 showed the same staining pattern (data not shown). On the cross-section of the mouse esophagus, LYVE1+ lymphatic vessels were observed in the submucosa and muscularis propria (Figure 1A). Lymphatic vessels in the muscularis propria were larger than those in the submucosa in size (Figure 1B, C). It should be noted that the mouse esophagus does not contain muscularis submucosa as in the human esophagus, and thus lamina propria and submucosa are merged.
Figure 1. Lymphatic vessels in the esophagus of wild-type mice.

(A) LYVE1+ lymphatic vessels (arrows) on a cross-section of mouse esophagus. Scale bar=100μm. (B) Lymphatic vessels in the submucosa. Scale bar=50μm. (C) Lymphatic vessels in the muscularis propria. Scale bar=50μm.
In the thoracic esophagus of ProxTom mice, dtTomato fluorescence was observed in the muscularis propria under the confocal microscope (Figure 2A, 2B). Observing from the lumen side of the esophagus with a confocal microscope, we found a network of lymphatic vessels (Figure 2C).
Figure 2. Lymphatic vessels in the esophagus of a ProxTom mouse under a confocal microscope.

(A) tdTomato+ (red fluorescence) lymphatic vessel (white arrows) on a cross-section of ProxTom mouse esophagus. White solid line: adventitia; White dotted line: inner boundary of muscularis propria; White dashed line: base membrane; Green solid line: surface of the epithelium; Blue fluorescence: DAPI staining of the nuclei. Scale bar=200μm. (B) Enlarged part of Panel A. Scale bar=20μm. (C) Two-dimensional view of tdTomato+ lymphatic vessel network. Scale bar=200μm.
Under the light-sheet microscope, a meshwork of the lymphatic vessels was observed in the whole esophagus (Figure 3A, 3B). On the anterior-posterior view, esophageal lymphatic vessels and peri-esophageal lymphatic vessels were connected. The forestomach was particularly rich in lymphatic vessels (Figure 3C, 3E). Interestingly, on the cross-sectional view, lymphatic vessels were observed only in the musclaris propria of the cervical and thoracic esophagus (Figure 3D), but in both the submucosa and the musclaris propria of the abdominal esophagus near gastroesophageal junction (Figure 3F). Consistent with the 2D observation, 3D reconstruction of the light-sheet image of the thoracic esophagus showed details of the lymphatic vessels (Suppl. Video 1). These data demonstrated that the mouse esophagus contains a meshwork of lymphatic vessels in the submucosa and muscularis propria, which is similar to that in the human esophagus.
Figure 3. Lymphatic vessels in the whole esophagus of a ProxTom mouse under a light-sheet microscope.
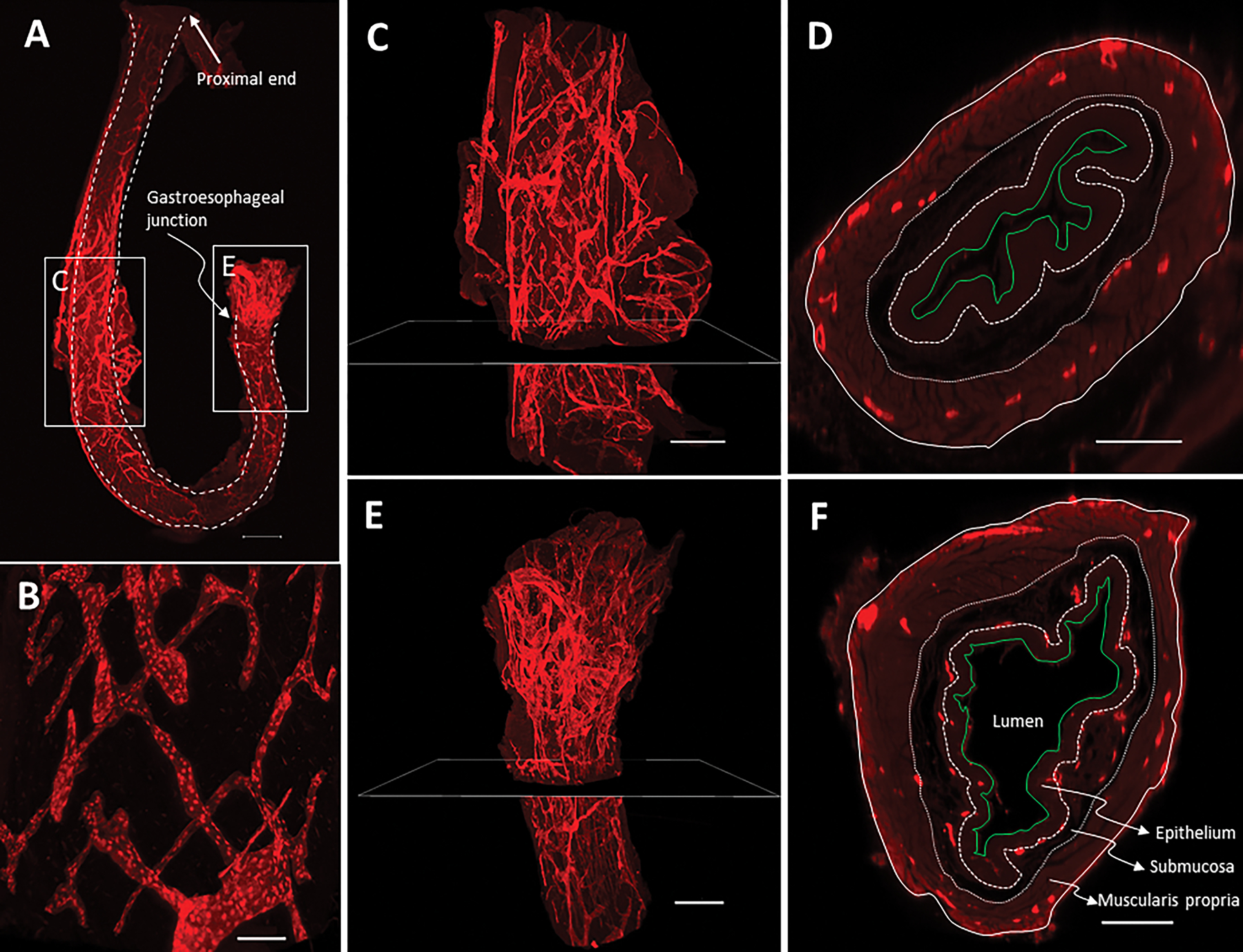
(A) Lymphatic vessels labeled with anti-RFP (red fluorescence). Due to its length, the mouse esophagus had to be curved to fit in the chamber for observation. As a result, the cervical esophagus and the curved area were not well exposed to laser light and thus showed less strong staining. White line: esophagus boundary. Scale bar=1,000μm. (B) Enlarged part of Panel A. Scale bar=100μm. (C) Lymphatic vessels in the thoracic esophagus. Scale bar=500μm. (D) Cross-section of the thoracic esophagus at the position shown in Panel C. White solid line: adventitia; White dotted line: inner boundary of muscularis propria; White broken line: base membrane; Green solid line: surface of the epithelium. Scale bar=150μm. (E) Lymphatic vessels in the abdominal esophagus. Scale bar=500μm. (F) Cross-section of the abdominal esophagus at the position shown in Panel E. Scale bar=200μm. Note: Panel C and D are still shots of Supplementary Video 1.
Lymphatic drainage of Indian ink and FITC-dextran
We surgically exposed the esophagus and injected tracers into the submucosa to monitor lymphatic drainage of the cervical and abdominal esophagus (Suppl. Figure 1A, B). Indian ink drained from the submucosa of the abdominal esophagus and cervical esophagus longitudinally upward and downward into local lymph nodes (Figure 4A, B, C; Suppl. Figure 1C, D). Inside the lymph node, Indian ink particles were found mainly in the subcapsular sinus (Figure 4D, E). Similarly, FITC was drained through the lymphatic vessels in the submucosa to peri-esophageal lymph nodes (Suppl. Figure 1E, F). These data indicate that the mouse esophageal lymphatic system functions by draining tracer molecules from the submucosa into lymph nodes longitudinally in a bidirectional manner.
Figure 4. Lymphatic drainage of Indian ink in the wild-type mouse esophagus.

(A) Distribution of Indian ink-positive lymph nodes after Indian ink is injected into the submucosa of the cervical or abdominal esophagus. (B) Peri-esophageal lymph nodes identified by orthotopic injection of Indian ink. Green dot, lymph node; Black dot, injection site of Indian ink; Red arrow, direction of lymphatic drainage. (C) Indian ink-positive drainage lymph nodes (green arrowhead) on Day 9 after Indian ink (black arrowhead) was injected into the abdominal esophagus. (D) Indian ink particles inside an Indian ink-positive lymph node. Scale bar=200μm. (E) Enlarged inset of Panel D. Scale bar=50μm.
Lymphatic drainage of mouse and human cancer cells and distant metastasis
As a preliminary experiment, we first orthotopically inoculated the abdominal esophagus (n=3) with mouse LLC-eGFP cells which are known to be highly metastatic. Double staining of LYVE1 and GFP clearly showed lymphatic permeation in the primary tumor (Suppl. Figure 2A, C), and double staining of CD31 and GFP showed vascular permeation (Suppl. Figure 2B, D). Metastatic cancer cells were found in the lung and liver by GFP IHC (Suppl. Figure 2E, F, G, H). 3D image reconstruction of the ProxTom esophagus demonstrated the presence of LLC-eGFP cells inside the esophageal lymphatic vessel (lymphatic permeation; Suppl. Figure 2I; Suppl. Video 2).
We then orthotopically inoculated MOC2 cells into the abdominal esophagus of immunocompetent wild-type mice (n=3). On Day 10, MOC2 cells in three mice developed primary tumors in the submucosa and invaded the muscularis propria, as shown by pan-cytokeratin IHC (Figure 5A). Lymphatic permeation was observed at the margin of the primary tumor (Figure 5B). In all three mice, MOC2 cells also metastasized into peri-esophageal lymph nodes, mainly in the subcapsular sinus, as shown by H&E staining (Figure 5C) and pan-cytokeratin IHC (Figure 5D). Metastasis was detected in the liver of one mouse and in the lungs of another mouse by pan-cytokeratin IHC. Consistent with the mechanism of metastasis, MOC2 cells in the metastatic lymph node (Figure 5F) expressed a higher level of SNAIL/SLUG than those in the primary tumor (Figure 5E).
Figure 5. MOC2 cells form primary esophageal tumors and undergo lymphatic metastasis after being orthotopically inoculated into the abdominal esophagus of wild-type mice.

(A) Double staining of pan-cytokeratin (red) and LYVE1 (brown) of a primary tumor in the mouse esophagus. Scale bar=200μm. (B) Enlarged inset of Panel A showed MOC2 cells (red) inside esophageal lymphatic vessels (brown) (lymphatic permeation; red arrows). Scale bar=100μm. (C) H&E staining showed metastatic MOC2 cells in the subcapsular sinus of a lymph node. Scale bar=200μm. (D) Most metastatic MOC2 cells were localized in the subcapsular sinus as shown by pan-cytokeratin staining (brown). Scale bar=200μm. (E) SNAIL/SLUG expression in the primary MOC2 tumor. (F) SNAIL/SLUG expression in the MOC2 cells inside the metastatic lymph node. E-F: scale bar=50μm.
We also orthotopically inoculated luciferase/GFP-labeled human ESCC cells (KYSE450-eGFP-Luc) into the abdominal esophagus of immunodeficient R2G2 mice (n=5). At Week 4, a large primary tumor developed in the esophagus of all five mice and became visible under IVIS (Figure 6A). Metastasis to the lungs and the liver was visible under IVIS after the organs were removed from two mice (Figure 6B). Double staining of pan-cytokeratin and LYVE1 showed the presence of KYSE450 cells inside the lymphatic vessels of the primary tumor (lymphatic permeation; Figure 6C, D). There was a dramatic increase in the density of lymphatic vessels in the primary tumor, suggesting lymphangiogenesis induced by KYSE450 cells (Figure 6E). Furthermore, metastatic lesions were observed in the lungs of all five mice (Figure 6F to K) and the liver of three mice (Figure 6L to M) by H&E staining (Figure 6F, G, L), pan-cytokeratin IHC (Figure 6H, I, J, K, M), and GFP IHC (Figure 6J, K, N). Metastatic ESCC cells in the lung expressed a lower level of E-cadherin and a higher level of SLUG/SNAIL in the metastatic tumor (Figure 6P, R) than those in the primary tumor (Figure 6O, Q), respectively.
Figure 6. Lymphatic metastasis of human ESCC cells (KYSE450-eGFP-Luc) after being orthotopically inoculated into the abdominal esophagus of immunocompromised R2G2 mice.

(A) IVIS image of the primary esophageal tumor at Week 4 after inoculation. (B) Ex vivo IVIS image of the primary esophageal tumor and metastatic lesions in the left lung, right lung, and liver. (C) Pan-cytokeratin/LYVE1 double staining of the primary esophageal tumor. Scale bar=100μm. (D) Enlarged inset of Panel C showing pan-cytokeratin+ ESCC cells (red) in the LYVE1+ lymphatic vessel (brown). Scale bar=100μm. (E) LYVE1+ lymphatic vessels on the margin of the primary esophageal tumor. Scale bar=100μm. (F, G) Metastatic lesions in the lung on H&E-stained sections. (H, I) Pan-cytokeratin+ metastatic lesions in the lung on neighboring sections. (J, K) GFP+ metastatic lesions in the lung on neighboring sections. (L) Metastatic lesions (arrows) in the liver on H&E-stained sections. (M) Pan-cytokeratin+ metastatic lesions in the liver on neighboring sections. (N) GFP+ metastatic lesions in the liver on neighboring sections. Panel G, I, and K were enlarged insets of Panel F, H, and J, respectively. F, H, J: scale bar=500μm; G, I, K: scale bar=100μm; L, M, N: scale bar=200μm. (O) E-cadherin expression in the primary esophageal tumor. (P) E-cadherin expression in the lung metastatic lesion. (Q) SNAIL/SLUG expression in the primary esophageal tumor. (R) SNAIL/SLUG expression in the lung metastatic lesion. O-R: scale bar=50μm.
Discussion
Given the clinical significance of lymphatic metastasis of ESCC, there is a great need of developing animal models to better understand the molecular mechanisms and develop therapeutic strategies. In this study, we characterized the morphology of the lymphatic system in the mouse esophagus. The drainage function of the esophageal lymphatic system was further tested with tracer molecules and cancer cells. Our data demonstrated a lymphatic system in the mouse esophagus similar to that in the human esophagus in its morphology and functions.
Mice have been widely used to model human ESCC in vivo [19]. The mouse esophagus is histologically similar to the human esophagus albeit with some differences, i.e., keratinization of the epithelium, lack of muscularis mucosa, and lack of submucosal gland. In this study, we first examined the distribution of lymphatic vessels in the mouse esophagus. Similar to the human esophagus, lymphatic vessels are localized in the submucosa and muscularis propria and form a meshwork (Figure 1, 2). It is known that the depth of ESCC tumor invasion is highly correlated with the probability of lymphatic metastasis and indicative of proper treatment. Stage Tis (i.e., ESCC cells within the epithelium) is not associated with lymphatic permeation or lymph node metastasis, whereas Stage T1 (i.e., cancer cells invading the lamina propria or submucosa) and Stage T2 (i.e., cancer cells invading muscularis propria) are associated with increased incidences of lymphatic metastasis [20–22]. Therefore, the presence of lymphatic vessels in the submucosa of the mouse esophagus suggests that lymphatic metastasis of ESCC cells orthotopically inoculated in this area recapitulates the metastatic process of T1 tumors.
This is the first study to demonstrate a meshwork of lymphatic vessels in the mouse esophagus (Figure 2C and Figure 3). Orthotopically injected tracers (Indian ink and FITC-dextran) were found to drain via the esophageal lymphatic vessels longitudinally in two directions (Figure 4B; Suppl. Figure 1). Bidirectional drainage suggested that lymphatic metastasis of ESCC cells in the mouse esophagus can mimic retrograde, bidirectional, and nodal skip metastasis which is commonly seen in human ESCC[10–12]. Moreover, orthotopically inoculated mouse and human cancer cells formed T1 tumors and developed into T2 tumors in the esophagus, and underwent lymphatic permeation, lymph node metastasis, and distant organ metastasis (Suppl. Figure 2; Figure 5 and 6). The presence of lymphatic vessels in both the submucosa and the musclaris propria of the mouse abdominal esophagus (Figure 3F) suggests that cancer cells in the abdominal esophagus may more likely undergo lymphatic metastasis than those in the cervical and thoracic esophagus. Our functional study with Indian ink supported this notion. Indian ink in the abdominal esophagus was more likely to drain into lymph nodes than that in the cervical esophagus (Figure 4A) Several studies on human T1 ESCC have shown that the risk of lymphatic metastasis was associated with the location of the primary tumor. ESCC in the lower esophagus was more likely to metastasize to the lymph nodes than ESCC in other locations [23–26]. However, it remains to be investigated whether the differential distribution of lymphatic vessels in various regions of the human esophagus provides an anatomic basis for the differential risk of lymphatic metastasis of primary T1 tumors.
This study is significant in establishing mouse models of lymphatic metastasis of ESCC for developing diagnostic and therapeutic strategies. It is known that mouse ESCC induced by carcinogens do not undergo lymphatic metastasis. Only one genetically modified mouse line with cyclin D1 overexpression in combination with p53+/− deficiency in the esophagus developed lymphatic metastasis at the age of 12 months. Approximately 25% of cancers showed enlarged lymph nodes and pan-cytokeratin+ cancer cells inside the lymph nodes in the para-tracheo-pharyngeal-esophageal regions [19]. Orthotopic xenograft models using human ESCC cells (i.e., TE8, KYSE150) in immunodeficient mice generated lymphatic permeation and lymph node metastasis in two previous reports [13,14]. In this study, mouse MOC2 cells and LLC-eGFP cells were drained from the submucosa to esophageal lymphatic vessels (lymphatic permeation), peri-esophageal lymph nodes, and distant organs (lung and liver) in immunocompetent mice (Suppl. Figure 2; Suppl. Video 2; Figure 5). Orthotopically inoculated human ESCC cells (KYSE450-eGFP-Luc) in immunodeficient mice also underwent lymphatic permeation and distant organ metastasis (Figure 6). Moreover, the metastatic cancer cells expressed a higher level of SLUG/SNAIL and a lower level of E-cadherin than cancer cells in the primary tumor (Figure 5E–F; Figure 6O–R). These data suggest the orthotopic inoculation of mouse cancer cells in immunocompetent mice or human ESCC cells in immunodeficient mice can be used as mouse models to mimic lymphatic metastasis of human T1 ESCC. It should be noted that orthotopic inoculation of human ESCC cells in immunodeficient mice lacks immune-cancer cell interactions which are known to be critical for lymphatic metastasis [29].
This study on mouse models is relevant to the clinical management of human ESCC in several aspects: (1) Mouse models can help us develop novel diagnostic measures of lymphatic metastasis. For example, it is technically challenging to diagnose micrometastasis in surgically resected lymph nodes with immunostaining of serial tissue sections. Mouse models would allow us to develop light-sheet microscopy for this purpose. In fact, light-sheet microscopy has been used in analyzing clinical samples with great success in revealing fine details of human pathology [27,28]. (2) Recent whole-genome analyses of metastatic solid tumors have discovered millions of somatic variants [30]. Mutations or altered expression of multiple genes (e.g., SYNE1, TBL1XR1, AKIP1) were associated with lymphatic metastasis of human ESCC[31–33]. Once cancer cells enter the lymphatic system, they are believed to adapt to the new environment by adopting new molecular and metabolic features[34,35]. However, the detailed molecular mechanisms remain to be further elucidated. We believe our mouse models will greatly enhance our mechanistic understanding of lymphatic metastasis of human ESCC and help us identify therapeutic targets. (3) Currently there is no clinical therapy specifically targeting lymphatic metastasis. Mouse models would allow us to perform preclinical tests of therapeutic agents for lymphatic metastasis.
This study has its limitations. Likely, some of the peri-esophageal lymph nodes were not identified since they are localized deep in the thoracic and abdominal cavities. The small size of lymph nodes in wild-type mice also prevented precise mapping of peri-esophageal lymph nodes. This study is a pilot and descriptive one in nature. With small sample sizes, our primary goal was to establish the procedures and test the feasibility of the mouse models. More animals and time points need to be studied in the future to show a full profile of draining lymph nodes and metastatic capabilities of various cancer cells with statistical considerations. In addition, serial sectioning and IHC were used for the detection of metastasis or tracer in lymph nodes and distant organs. This method is prone to false-negative outcomes. More importantly, our findings in the mouse models must be taken with caution when translated into understandings of human ESCC biology.
Supplementary Material
Supplementary Video 1. 3D image reconstruction of lymphatic vessels in the thoracic esophagus of a ProxTom mouse.
Supplementary Figure 1. Orthotopic injection of tracers into the cervical or abdominal esophagus of mice and lymphatic drainage of tracers (Indian ink or FITC) in the mouse esophagus. (A) Injection of Indian ink into the cervical esophagus. Tracers or cells were injected into the submucosa between the muscle and the epithelium (inset). (B) Injection of Indian ink into the abdominal esophagus. (C) Day 9 after orthotopic injection of Indian ink into the cervical esophagus; (D) Enlarged inset of Panel C to show Indian ink-positive lymph nodes (green arrowheads) after injection of Indian ink (black arrowheads). (E) Injection of FITC (green) in the abdominal esophagus. (F) FITC signal (green) in the subcapsular sinus of a peri-esophageal lymph node. The nuclei were stained positive for DAPI which signal was converted to red. Scale bar=100μm.
Supplementary Video 2. 3D image reconstruction of LLC-eGFP cells inside the esophageal lymphatic vessel of a ProxTom mouse.
Supplementary Figure 2. Metastasis of LLC-eGFP cells after being orthotopically inoculated into the esophagus of wild-type mice. (A) Double staining of GFP (red) and LYVE1 (brown) of an esophageal primary tumor. Scale bar=200μm. (B) Double staining of GFP (red) and CD31 (brown) of an esophageal primary tumor. Scale bar=200μm. (C) Enlarged inset of Panel A. Scale bar=50μm. (D) Enlarged inset of Panel B. Scale bar=50μm. (E) GFP+ metastatic lesion in the lung. Scale bar=200μm. (F) Enlarged inset of Panel E. Scale bar=50μm. (G) GFP+ metastatic lesion in the liver. Scale bar=200μm. (H) Enlarged inset of Panel G. Scale bar=50μm. (I) A still shot of Supplementary Video 2 showing LLC-eGFP cells (green) inside lymphatic vessels (brown) after they migrate from the primary tumor (lower left corner).
Acknowledgment:
This work was supported by research grants from the National Institutes of Health (R01 CA244236, U54 CA156735, U54 MD012392) and the Beijing Natural Science Foundation (7222153). We thank Dr. Pablo Ariel, Microscopy Services Laboratory, Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill for providing excellent light-sheet microscopy service. The Microscopy Services Laboratory, Department of Pathology and Laboratory Medicine, is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center, and in part by the North Carolina Biotech Center Institutional Support Grant 2016-IDG-1016.
Abbreviations:
- ESCC
esophageal squamous cell carcinoma
- H&E
hematoxylin and eosin
- IHC
immunohistochemical staining
- LYVE1
lymphatic vessel endothelial hyaluronan receptor 1
- PROX1
prospero-related homeobox 1
Footnotes
Disclosure Statement: The authors report no conflicts of interest.
REFERENCE
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Kang X, Chen K, Li Y, et al. Personalized targeted therapy for esophageal squamous cell carcinoma. World journal of gastroenterology. 2015;21:7648–7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kayani B, Zacharakis E, Ahmed K, Hanna GB. Lymph node metastases and prognosis in oesophageal carcinoma--a systematic review. Eur J Surg Oncol. 2011;37:747–753. [DOI] [PubMed] [Google Scholar]
- 4.Wu N, Chen Z, Pang L, Ma Q, Chen G. Prognostic significance of lymph node characteristics on survival in esophageal squamous cell carcinomas. Wien Klin Wochenschr. 2013;125:26–33. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsumi S, Saeki H, Nakashima Y, et al. Distant lymph node metastases caused by esophageal cancer invasion to the lamina propria: a case report. Surg Case Rep. 2016;2:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. 2019;16:25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuge K, Murakami G, Mizobuchi S, et al. Submucosal territory of the direct lymphatic drainage system to the thoracic duct in the human esophagus. J Thorac Cardiovasc Surg. 2003;125:1343–1349. [DOI] [PubMed] [Google Scholar]
- 8.Ji X, Cai J, Chen Y, Chen LQ. Lymphatic spreading and lymphadenectomy for esophageal carcinoma. World J Gastrointest Surg. 2016;8:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachimori Y Pattern of lymph node metastases of squamous cell esophageal cancer based on the anatomical lymphatic drainage system: efficacy of lymph node dissection according to tumor location. J Thorac Dis. 2017;9:S724–S730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Zhu L, Xia W, Wang F. Anatomy of lymphatic drainage of the esophagus and lymph node metastasis of thoracic esophageal cancer. Cancer Manag Res. 2018;10:6295–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang QX, Yang YS, Xu LY, et al. Prognostic Role of Nodal Skip Metastasis in Thoracic Esophageal Squamous Cell Carcinoma: A Large-Scale Multicenter Study. Ann Surg Oncol. 2021. [DOI] [PubMed] [Google Scholar]
- 12.Oshiro H, Osaka Y, Tachibana S, et al. Retrograde Lymphatic Spread of Esophageal Cancer: A Case Report. Medicine (Baltimore). 2015;94:e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ip JC, Ko JM, Yu VZ, et al. A versatile orthotopic nude mouse model for study of esophageal squamous cell carcinoma. Biomed Res Int. 2015;2015:910715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuroda S, Kubota T, Aoyama K, et al. Establishment of a Non-Invasive Semi-Quantitative Bioluminescent Imaging Method for Monitoring of an Orthotopic Esophageal Cancer Mouse Model. PLoS One. 2014;9:e114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truman LA, Bentley KL, Smith EC, et al. ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. Am J Pathol. 2012;180:1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Judd NP, Winkler AE, Murillo-Sauca O, et al. ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer Res. 2012;72:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nassar D, Latil M, Boeckx B, Lambrechts D, Blanpain C. Genomic landscape of carcinogen-induced and genetically induced mouse skin squamous cell carcinoma. Nat Med. 2015;21:946–954. [DOI] [PubMed] [Google Scholar]
- 18.Renier N, Adams EL, Kirst C, et al. Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes. Cell. 2016;165:1789–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tetreault MP. Esophageal Cancer: Insights From Mouse Models. Cancer Growth Metastasis. 2015;8:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriya H, Ohbu M, Kobayashi N, et al. Lymphatic tumor emboli detected by D2–40 immunostaining can more accurately predict lymph-node metastasis. World J Surg. 2011;35:2031–2037. [DOI] [PubMed] [Google Scholar]
- 21.Wang A, Lu L, Fan J, Wang S, Chen X. Lymph node metastatic patterns and its clinical significance for thoracic superficial esophageal squamous cell carcinoma. J Cardiothorac Surg. 2020;15:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gockel I, Sgourakis G, Lyros O, et al. Risk of lymph node metastasis in submucosal esophageal cancer: a review of surgically resected patients. Expert Rev Gastroenterol Hepatol. 2011;5:371–384. [DOI] [PubMed] [Google Scholar]
- 23.Duan X, Shang X, Yue J, et al. A nomogram to predict lymph node metastasis risk for early esophageal squamous cell carcinoma. BMC Cancer. 2021;21:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Chen H, Xiang J, et al. Prevalence of lymph node metastases in superficial esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2013;146:1198–1203. [DOI] [PubMed] [Google Scholar]
- 25.Shen W, Shen Y, Tan L, Jin C, Xi Y. A nomogram for predicting lymph node metastasis in surgically resected T1 esophageal squamous cell carcinoma. J Thorac Dis. 2018;10:4178–4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Chen QX, Shen DJ, Zhao Q. A prediction model for lymph node metastasis in T1 esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2018;155:1902–1908. [DOI] [PubMed] [Google Scholar]
- 27.Glaser AK, Reder NP, Chen Y, et al. Light-sheet microscopy for slide-free non-destructive pathology of large clinical specimens. Nat Biomed Eng. 2017;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka N, Kanatani S, Tomer R, et al. Whole-tissue biopsy phenotyping of three-dimensional tumours reveals patterns of cancer heterogeneity. Nat Biomed Eng. 2017;1:796–806. [DOI] [PubMed] [Google Scholar]
- 29.Janssen LME, Ramsay EE, Logsdon CD, Overwijk WW. The immune system in cancer metastasis: friend or foe? J Immunother Cancer. 2017;5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Priestley P, Baber J, Lolkema MP, et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature. 2019;575:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai W, Ko JMY, Choi SSA, et al. Whole-exome sequencing reveals critical genes underlying metastasis in oesophageal squamous cell carcinoma. J Pathol. 2017;242:500–510. [DOI] [PubMed] [Google Scholar]
- 32.Lin C, Song L, Liu A, et al. Overexpression of AKIP1 promotes angiogenesis and lymphangiogenesis in human esophageal squamous cell carcinoma. Oncogene. 2015;34:384–393. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Lin C, Liang W, et al. TBL1XR1 promotes lymphangiogenesis and lymphatic metastasis in esophageal squamous cell carcinoma. Gut. 2015;64:26–36. [DOI] [PubMed] [Google Scholar]
- 34.Ubellacker JM, Tasdogan A, Ramesh V, et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 2020;585:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CK, Jeong SH, Jang C, et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363:644–649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1. 3D image reconstruction of lymphatic vessels in the thoracic esophagus of a ProxTom mouse.
Supplementary Figure 1. Orthotopic injection of tracers into the cervical or abdominal esophagus of mice and lymphatic drainage of tracers (Indian ink or FITC) in the mouse esophagus. (A) Injection of Indian ink into the cervical esophagus. Tracers or cells were injected into the submucosa between the muscle and the epithelium (inset). (B) Injection of Indian ink into the abdominal esophagus. (C) Day 9 after orthotopic injection of Indian ink into the cervical esophagus; (D) Enlarged inset of Panel C to show Indian ink-positive lymph nodes (green arrowheads) after injection of Indian ink (black arrowheads). (E) Injection of FITC (green) in the abdominal esophagus. (F) FITC signal (green) in the subcapsular sinus of a peri-esophageal lymph node. The nuclei were stained positive for DAPI which signal was converted to red. Scale bar=100μm.
Supplementary Video 2. 3D image reconstruction of LLC-eGFP cells inside the esophageal lymphatic vessel of a ProxTom mouse.
Supplementary Figure 2. Metastasis of LLC-eGFP cells after being orthotopically inoculated into the esophagus of wild-type mice. (A) Double staining of GFP (red) and LYVE1 (brown) of an esophageal primary tumor. Scale bar=200μm. (B) Double staining of GFP (red) and CD31 (brown) of an esophageal primary tumor. Scale bar=200μm. (C) Enlarged inset of Panel A. Scale bar=50μm. (D) Enlarged inset of Panel B. Scale bar=50μm. (E) GFP+ metastatic lesion in the lung. Scale bar=200μm. (F) Enlarged inset of Panel E. Scale bar=50μm. (G) GFP+ metastatic lesion in the liver. Scale bar=200μm. (H) Enlarged inset of Panel G. Scale bar=50μm. (I) A still shot of Supplementary Video 2 showing LLC-eGFP cells (green) inside lymphatic vessels (brown) after they migrate from the primary tumor (lower left corner).


