Abstract
The gastrointestinal (GI) tract is a frequent target organ in acute graft-versus-host disease (aGVHD) which can determine the morbidity and non-relapse mortality after allogeneic hematopoietic cell transplantation (allo-HCT). Donor T cells recognize allogeneic antigens presented by host antigen presenting cells (APCs), proliferate, and differentiate into T-helper 1 (Th1) and T-helper 17 (Th17) cells that drive GVHD pathogenesis. IL-12 has been shown to play an important role in amplifying the allogeneic response in preclinical and clinical studies. The current study demonstrates that IL-12 receptor β2 (IL-12Rβ2) expression on recipient non-hematopoietic cells is required for optimal development of aGVHD in murine models of allo-HCT. aGVHD attenuation by genetic depletion of IL-12R signaling is associated with reduced major histocompatibility complex (MHC) class II expression by intestinal epithelial cells (IECs) and maintenance of intestinal integrity. We verified IL-12Rβ2 expression on activated T cells, and in the GI tract. This study reveals a novel function of IL-12Rβ2 in GVHD pathogenesis and suggests that selectively targeting IL-12Rβ2 on host non-hematopoietic cells may preserve the GI tract after allo-HCT.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a curative treatment for patients with malignant hematological diseases, solid tumors, severe aplastic anemia, immune deficiencies, and other non-malignant disorders (1). Acute graft-versus-host disease (aGVHD) is caused by the donor immune response to host tissues and is known as a major complication affecting the prognosis of primary disorders after allo-HCT (2, 3). aGVHD damage is common in the gastrointestinal (GI) tract because of early tissue injury due to the chemotherapy/irradiation pretransplant conditioning regimen, allogeneic immune response, and altered tissue repair mechanisms (4, 5). The GI tract is the second most common organ involved in aGVHD after the skin, but GI GVHD is the most difficult to treat (6). In the clinic, symptoms of GI acute GVHD occur very early after transplantation, overlap with the effects of chemoradiotherapy and predict the long-term outcomes after allo-HCT (7, 8).
aGVHD is a result of the interaction between pathogenic donor T cells and antigen presenting cells (APCs) in recipients, consisting of professional and non-professional APCs (9–11). Alloantigen presentation within MHC class II by donor-derived APCs is insignificant at inducing GVHD compared to that induced by host APCs (12). The roles of recipient hematopoietic APCs, including dendritic cells, B cells, tissue-resident macrophages, and Langerhans cells, within MHC class II-dependent GVHD are not clear (13–16). During GVHD development, host hematopoietic APCs are gradually eradicated by chemotherapy or irradiation conditioning and donor T cells, while recipient non-hematopoietic APCs persist (17). MHCII+ Lgr5+ intestinal stem cells (ISCs) and intestinal epithelial cells (IECs) have both been reported to be important for antigen presentation and stimulation of donor T helper (Th) cells (18).
Type 3 innate lymphoid cells (ILC3s) are mucosa-resident cells that act with the innate immune response at early stages of intestinal aGVHD (19). ILC3s produce the cytokine IL-22, which stimulates the secretion of antimicrobial REG3γ by paneth cells and maintains intestinal homeostasis (20). Both IL-22 and REG3γ contribute to intestinal protection against chemical toxins and irradiation damage through preventing ISC and paneth cell apoptosis, both in vivo and in vitro (21, 22). In aGVHD, donor T cells destroy paneth cells, releasing REG3α (the human homolog of REG3γ)/γ into the bloodstream, resulting in increased blood levels of REG3α/γ (23). The Mount Sinai Acute GVHD International Consortium (MAGIC) study determined that elevated REG3α is a specific biomarker of aGVHD progress and predicts long-term outcomes of patients at the onset of GVHD (24, 25).
Alloreactive donor T-cell activation is characterized by naïve CD4+ T cells differentiation into Th1 T cells in the presence of IL-12 and the production of proinflammatory cytokines that further induce T-cell expansion (26, 27). IL-12R is composed of IL-12Rβ1 and IL-12Rβ2, and is mainly expressed on activated T cells, memory T cells and NK cells (28, 29). In the current study, we validated that IL-12Rβ2 on non-hematopoietic cells of recipients contribute to aGVHD development, which provides rationale to target IL-12Rβ2 as a potential strategy in the prevention and treatment of aGVHD.
Materials and Methods:
Mice.
C57BL/6 (B6 H-2Kb, Ly5.2+), BALB/c (H-2Kd), FVB (H-2Kq, Thy1.1+) were purchased from National Cancer Institute (NCI, Frederick, MD). B6 (H2Kb, Ly5.1+), FVB (H2Kq, Thy1.2+), IL-12Rβ2KO and DBA2 (H-2Kd) were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred in the mouse breeding facility at the Medical University of South Carolina (MUSC) and Medical College of Wisconsin (MCW). Luciferase transgenic DBA2 mice were established by the C. Contag lab (30) and provided by Defu Zeng (31). All animals were maintained in pathogen-free conditions in the America Association for Laboratory Animal Care-accredited Animal Resource Center at the Medical University of South Carolina and Medical College of Wisconsin.
BMT models.
The recipient mice were lethally irradiated at 650–700 cGy for BALB/c or 1100–1200 cGy for C57BL/6 with an X-RAD 320 or Shepherd Mark I Cesium irradiator. T cells were purified from splenocytes and lymph nodes cells by negative selection using anti-biotin MicroBeads (Miltenyi Biotech, Auburn, CA). In 80 and 14-day experiments, GVHD models were established as below: BALB/c recipients were transplanted with 5×106 T cell-depleted bone marrow (TCD-BM) and 0.75 ×106 T cells from B6 mice, C57BL/6 recipients were transplanted with 5×106 TCD-BM and 0.75 ×106 T cells from FVB or DBA/2 mice. Recipient mice were monitored twice per week for weight loss, and scored for aGVHD weekly based on weight loss, posture, mobility, skin integrity, fur appearance and diarrhea. Individual clinical parameters were scored from 0 to 2 by each criterion and from 0 to 10 overall. Recipients at premorbid stage or body weight loss up to 30% were euthanized and counted toward lethality. In 4-day and 7-day experiments, GVHD models were established by transfusing 3–5×106 splenocytes from FVB mice to C57BL/6 or IL-12Rβ2KO recipients.
Bone marrow chimeric model.
B6 Ly5.1+ or Ly5.2+ recipients were lethally irradiated at 1100 cGy and transplanted with 5×106 TCD-BM from B6 (Ly5.2+) or (Ly5.1+). After 2–3 months, the chimeric mice were then used for recipients in the second BMT after successfully hematopoietic engraftment was confirmed.
IECs isolation from recipient intestines.
Isolated small and large intestines were flushed with cold medium containing 10% 10xHBSS without Ca2+ and Mg2+, 2% FBS, and 10mM HEPES. Intestines were cut into small pieces and washed until the medium was clear after shaking, followed by incubation for 45 min at 37°C in incubation solution containing 9.2% 10xHBSS, 10% FBS, 0.1 mM EDTA, and 9.2 mM HEPES. Supernatant was collected through 100 μM and 40 μM filter in turn, centrifuged by 1500g for 5 min, and pellet cells was resuspended in cold PBS.
Flow cytometry.
As previously described, mononuclear cells were isolated from the spleen, liver and intestine of recipients for surface or intracellular staining. The Live/dead yellow cell staining kit (catalog L-34968) was purchased from Invitrogen. The following antibodies were used for cell-surface staining: anti–H2Kq (clone KH114, BioLegend), anti–H2Kb (clone AF6–88.5, BD Biosciences), anti-H2Kd (clone SF1–1.1.1, BioLegend), anti-CD45 (clone 30-F11,BD Biosciences ), anti- CD45.1 (clone A20, BD Biosciences), anti-CD45.2 (clone 104, BD Biosciences), anti-CD90.2 (clone 30-H12, BD Biosciences), anti- I-Ab (AF6–120.1, BioLegend ), anti-IL-12Rβ2 (clone 305719, R&D), anti-mouse-IgG1 (clone 11711, R&D), anti–CD4 (clone RM4–5, BD Biosciences), anti–CD8 (clone 53–6.7, BD Biosciences), and anti-B220(clone RA3–6B2, BioLegend). To measure intracellular cytokines, cells were stimulated for 4–5 hours at 37°C with PMA (100 ng/ml, MilliporeSigma) and ionomycin (100 ng/ml; Calbiochem, EMD) in the presence of GolgiStop (BD Biosciences). Fix and permeabilization were performed using Cytofix/Cytoperm Plus (BD Biosciences), followed by staining with the appropriate antibodies, including anti–IFN-γ (clone XMG1.2, eBioscience), anti–IL-17A (clone TC11– 18H10.1, BioLegend), and anti–GM-CSG (clone MP1–22E9, eBioscience). Data were analyzed with FACSDiva software, LSR II (BD Biosciences), and FlowJo software (Tree Star).
Intestinal permeability assay.
After 4 hours of fasting, recipient mice were gavaged with 200 μl of 80 mg/ml FITC-dextran (MilliporeSigma, FD4) in sterile 1x PBS. Four hours later, blood was collected and centrifuged at 5,000 rpm for 10 minutes to separate serum. Serum was diluted 1:1 with 1x PBS, and analyzed on a plate reader (BioTek Instruments, catalog FLx800) at emission wavelength of 535 nm with excitation at 485 nm. Serum concentrations of FITC-dextran in experimental samples were determined on the basis of standard serum from naive B6 mice. Standard curve (0, 125, 250, 500, 1000, 2000, 4000, and 8000 ng/ml) was used to calculate the serum concentrations of FITC-dextran.
Quantitative PCR.
As described in our previously published work, snap-frozen ileum and colon were cut into small chunks and crashed in a mortar on dry ice. Total RNA was extracted using TRIzol reagent (Invitrogen, Thermo Fisher Scientific) according to the manufacturer’s recommendations. cDNA synthesis was performed using the Superscript first-strand cDNA synthesis system (Bio-Rad). Real-time quantitative PCR was performed using the SYBR Green method. The specific primer pairs were used as follows: Reg3γ, 5′-TTCCTGTCCTC CATGA TCAAAA-3′ and 5′-CATCCACCTCTGTTGGGTTCA-3′; IL-22, 5′-GTG GAG A GATCAAGGCGATT-3′ and 5′-CAGACGCA AGC ATT T CT CAG-3′. 2^-dct was used to determine the relative gene expression.
Statistics.
Log-rank (Mantel-Cox) test was used for comparison of survival between groups. Nonparametric Mann-Whitney U test was used to compare body weight loss and clinical scores between groups. A 2-tailed Student’s t test was performed to determine the difference between two groups. One way analysis of variance (ANOVA) with the Tukey’s multiple comparison test was used for multiple group comparisons unless otherwise stated. P < 0.05 was considered statistically significant, and data are shown as mean ± SD.
Study approval.
All animal experiments were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the MUSC in Charleston, South Carolina, and the MCW in Milwaukee, Wisconsin.
Results
IL-12Rβ2 on recipients is required for development of aGVHD.
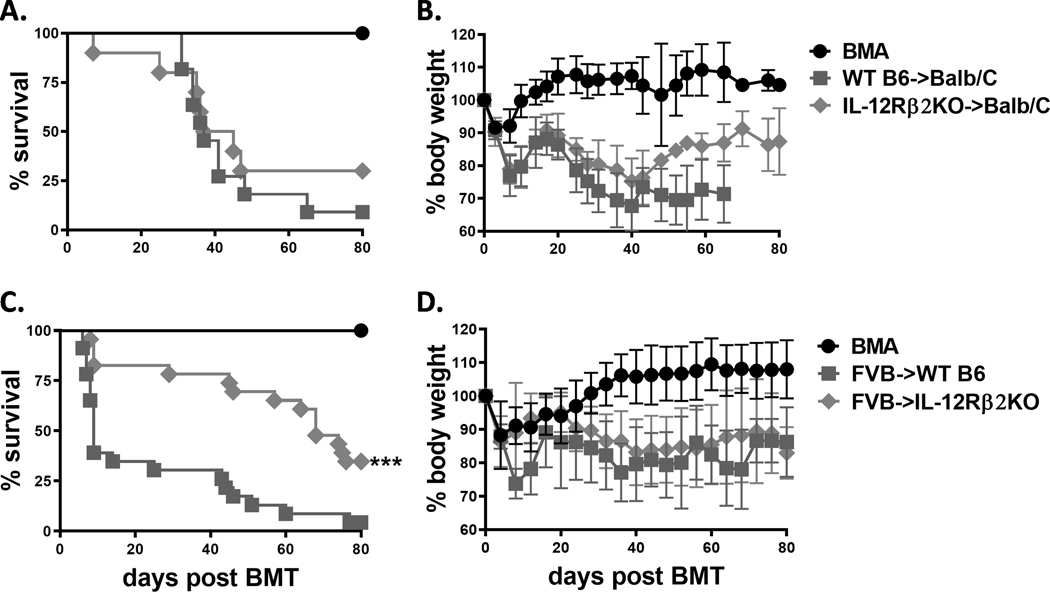
IL-12R signaling is an important factor in accelerating acute GVHD development by promoting Th1 differentiation and proinflammatory cytokine production (27). IL-12R, formed by two heterodimeric subunits: IL-12Rβ1 and IL-12Rβ2, is mainly expressed on activated T cells and NK cells. We therefore hypothesized that IL-12R signaling contributes to development of GVHD after allogeneic HCT. In a previous study, we demonstrated that IL-12Rβ1 on donor T cells was dispensable for aGVHD pathogenesis (28). We also found that lack of IL-12Rβ1 in recipients did not affect aGVHD development (Supplemental Figure 1). In the current study, we focused on IL-12Rβ2. By using a common MHC-mismatched murine bone marrow transplantation (BMT) model, C57BL/6 to BALB/c, we first compared the ability of WT and IL-12Rβ2 knockout (KO) T cells to induce aGVHD. We found no significant difference in aGVHD severity reflected by survival and body weight maintenance (Figure 1 A, B). Subsequently, we compared the role of IL-12Rβ2 in recipients after allo-HCT using another MHC-mismatched BMT model, FVB to C57BL/6. We found that the IL-12Rβ2KO recipients had significantly improved survival while body weight loss is comparable compared with WT counterparts (Figure 1 C, D). These results suggest that IL-12Rβ2 on recipient, but not donor, contributes to the pathogenesis of aGVHD in MHC-mismatched BMT models.
Figure 1. Recipient IL-12Rβ2 is required for development of aGVHD.
Lethally irradiated BALB/c mice (650cGy) were transplanted with 5×106 T-cell depleted (TCD)-BM alone (BMA, n=5) or plus 0.75–1×106 purified T cells from WT (n=11) and IL-12Rβ2KO (n=10) mice on C57BL/6 (B6) background. Recipients were monitored for survival (A) and body weight loss (B) until 80 days post BMT. Lethally irradiated WT and IL-12Rβ2KO B6 mice (1100cGy) were transplanted with 5×106 TCD-BM alone (n=9) or plus 1×106 purified T cells (n=46) isolated from FVB mice. Recipients were monitored for (C) survival and (D) body weight loss until 80 days post BMT. Data shown are from 2 for A, B or are from 5 repeated experiments for C, D. Log-rank (Mantel-Cox) test (A and C) was used for comparison of survival between groups. Nonparametric Mann-Whitney U test (B and D) was used to compare body weight loss between groups. Statistical data were presented as mean ± 1SD; significance was determined by Student’s t test. *** p < 0.001.
IL-12Rβ2 on recipient non-hematopoietic cells is important for aGVHD development.
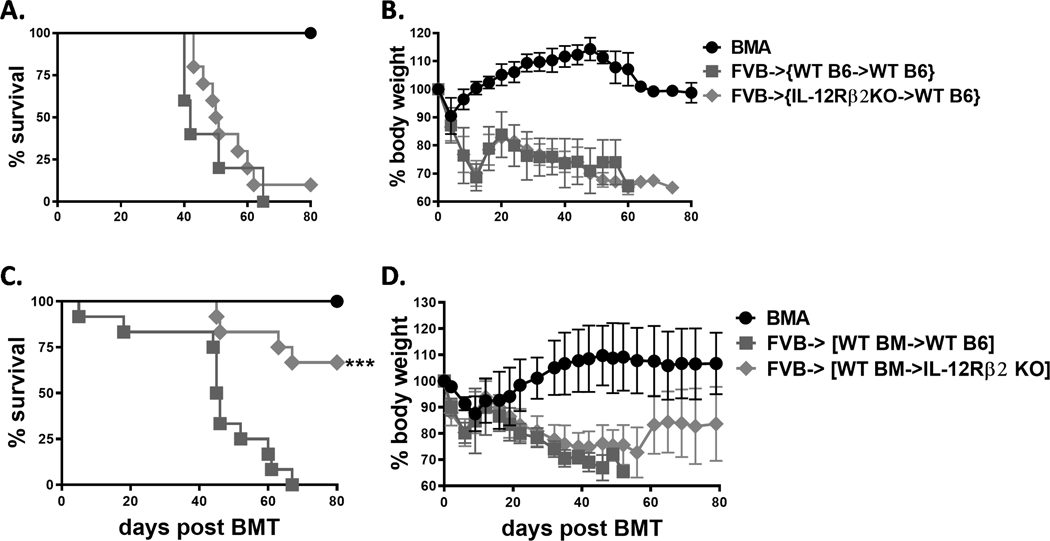
We then sought to determine whether IL-12Rβ2 expressed on the hematopoietic or non-hematopoietic compartment contributes to aGVHD development. To do so, we established BM chimeric mice so that IL-12Rβ2 was either deficient in hematopoietic or non-hematopoietic cells. These BM chimeras were then transplanted with BM and T cells from allogeneic FVB donors. We observed no difference in GVHD severity between WT and IL-12Rβ2 deficient recipients in the hematopoietic compartment (Figure 2 A, B). However, the IL-12Rβ2KO recipients in non-hematopoietic compartment had significantly improved survival and less body weight loss compared with WT controls (Figure 2 C, D). Thus, we conclude that IL-12Rβ2 in recipient non-hematopoietic, but not hematopoietic, cells contribute to GVHD pathogenesis.
Figure 2. IL-12Rβ2 on recipient non-hematopoietic cells is important for aGVHD development.
Lethally irradiated B6 Ly5.1+ mice (1100cGy, n=20) were transplanted with 5×106 TCD-BM from WT and IL-12Rβ2KO (Ly5.2+) B6 mice. After 8–10 weeks, engraftment was confirmed by percentage of CD45.2+ cells in the peripheral blood mononuclear cells. The chimeras were lethally irradiated and transplanted with 5×106 TCD-BM alone (BMA) (n=5) or plus purified T cells (n=20) from FVB donors. Recipients were monitored for survival (A) and body weight loss (B) until 80 days post second BMT. Lethally irradiated WT C57BL/6 and IL-12Rβ2KO mice were transplanted with 5×106 TCD-BM from B6 Ly5.1+ mice. The chimeras were then lethally irradiated and transplanted with 5×106 TCD-BM alone (n=4) or plus 1–1.5 × 106 purified T cells (n=24) from FVB donors as successful engraftment was confirmed. Recipients were monitored for survival (C) and body weight loss (D) until 80 days post second BMT. Data shown are from 2 replicate experiments for A and B or from 4 repeated experiments for C and D. Log-rank (Mantel-Cox) test (A and C) was used for comparison of survival between groups. Nonparametric Mann-Whitney U test (B and D) was used to compare body weight loss between groups. Statistical data were presented as mean ± 1SD; significance was determined by Student’s t test. *** p < 0.001.
Recipient IL-12Rβ2 enhances donor T cell infiltration to target organs and inhibits hematopoiesis and immune reconstitution.
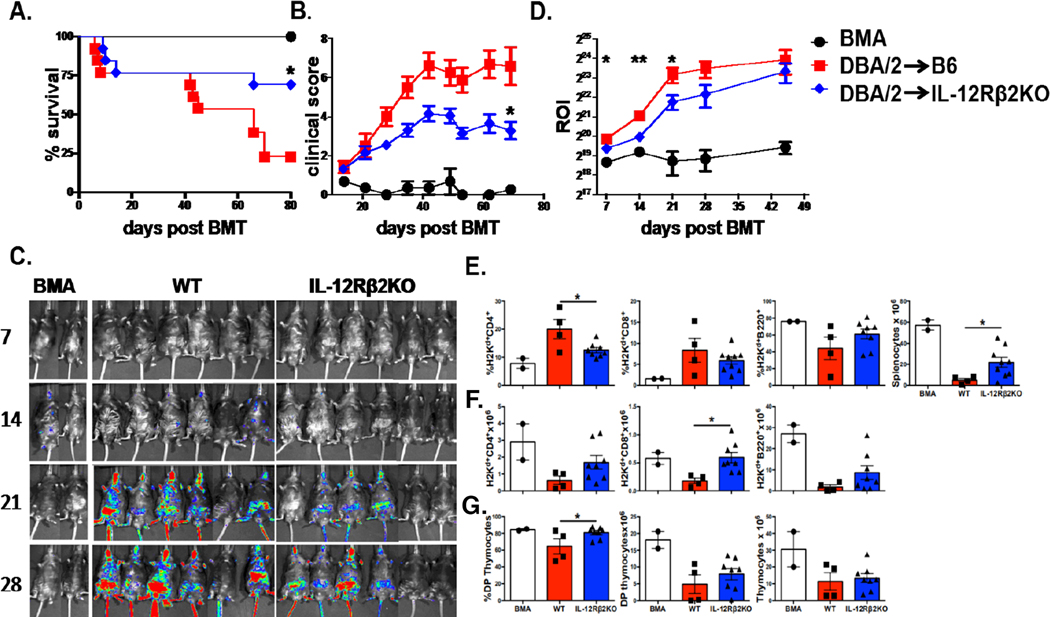
To understand the underlying mechanisms by which recipient IL-12Rβ2 affects donor T-cell expansion and migration, we transplanted donor BM from normal DBA2 mice with donor T cells from β-actin driven luciferase transgenic (Tg) mice on DBA2 background. In this MHC-mismatched BMT model, we confirm that IL-12Rβ2 KO recipients show a significant reduction in GVHD severity compared to WT controls, reflected by improved survival and lower clinical scores (Figure 3 A, B). Given that GVHD is caused by allo-reactive donor T cells that migrate into and attack host tissues, we next wanted to validate how recipient IL-12Rβ2 impacts donor T-cell expansion and trafficking to target organs through bioluminescent imaging (BLI). Signal strengths of BLI were significant lower in IL-12Rβ2KO recipients than in WT controls at day 7, 14, and 21 post BMT (Figure 3 C, D). In a long-term follow-up, we found that the recipients deficient for IL-12Rβ2 had higher numbers of T and B cells among splenocytes as well as double positive (CD4+CD8+) cells in thymus (Figure 3E-G). These data suggest that recipients deficient for IL-12Rβ2 have improved immune reconstitution and thymic recovery, reflecting milder GVHD than WT controls.
Figure 3. Host IL-12Rβ2 enhances donor T-cell infiltration to target organs and inhibits hematopoiesis and immune reconstitution.
WT C57BL/6 and IL-12Rβ2KO mice were irradiated (1100cGy) and transplanted with 5×106 TCD-BM alone (BMA, n=3) or plus 0.5–1×106 β-actin luciferase transgenic T cells (n=17) isolated from DBA/2 mice. Recipients were monitored for survival (A) and body weight loss (B). T-cell migration was monitored using bioluminescent imaging (BLI). Representative macro-photos are shown for BLI of total-body (C) over time with a region of interest (ROI) summary (D). 80 days after BMT, recipient spleen and thymus were harvested for analysis through flow cytometry. Absolute number of splenocytes, average percentages, and absolute counts of H2Kb+CD4+, H2Kb+CD8+, and H2Kb+B220+ cells in spleen are shown (E). Double positive (CD4+CD8+) frequencies, absolute numbers of double positive (CD4+CD8+) cells, and absolute count of thymocytes are shown (G). Data shown are from 2 replicate experiments. Log-rank (Mantel-Cox) test (A) or nonparametric Mann-Whitney U test (B and C) was used to compare between groups. Statistical data are presented as mean ± 1SD; significance was determined by Student’s t test. * p < 0.05 and **p < 0.01.
IL-12Rβ2 sustains donor T cells and promotes target organ damage.
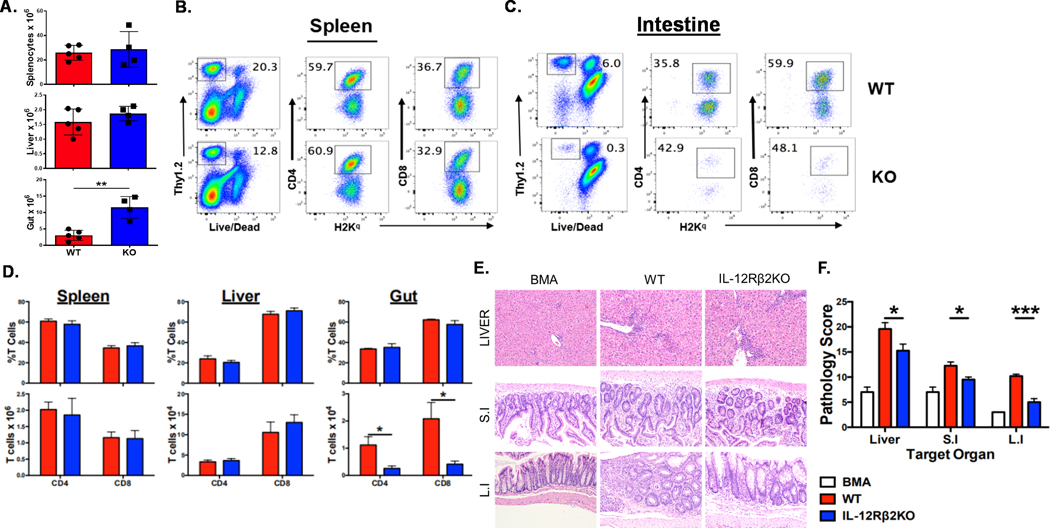
To further determine how IL-12Rβ2 in recipients affects donor T-cell responses, we transplanted donor BM and T cells from FVB mice into WT or IL-12Rβ2KO C57BL/6 recipients. Two weeks after BMT, we found that the absolute numbers of total cells isolated from recipient intestine were significantly increased in IL-12Rβ2KO recipients than in WT controls, whereas the number of splenocytes and liver cells were comparable among the two groups of recipients (Figure 4 A); the percentage and the number of donor T cells were also similar in the spleen and liver (Figure 4 B, D). However, the total number of donor T cells was significantly reduced in the intestine of IL-12Rβ2KO recipients compared to WT controls (Figure 4 D) because the percentage of donor T cells (Thy1.2+) was dramatically reduced in the KO group (Figure 4 C). We examined the proliferation and apoptosis of donor T cells in the intestine and observed that they were comparable in WT and IL-12Rβ2KO recipients (Supplementary Figure 2A, B). We thus interpret that the reduced donor T cell number in the intestine of IL-12Rβ2KO recipient was likely due to decreased T cell migration to the target organ as shown in Figure 3. Pathological analysis showed considerably reduced GVHD damage in liver, small and large intestine in IL-12Rβ2KO recipients compared to that of WT controls (Figure 4E, F).
Figure 4. IL-12Rβ2 sustains donor T cells and promotes target organ damage.
Lethally irradiated (1100cGy) WT and IL-12Rβ2KO B6 mice underwent BMT with 5×106 TCD-BM from FVB mice (Thy1.1) alone (BMA) or plus 0.5×106 purified T cells from FVB mice (Thy1.2). Two weeks after BMT, spleens, livers and intestine of the recipients were harvested and analyzed with flow cytometry. Absolute counts of splenocytes, liver cells and intestinal cells were shown (A) Representative flow cytometry dot plots from spleen (B) and intestine (C), the average frequencies and the absolute counts of CD4+ or CD8+ T cells among gated H2Kq+Thy1.2+ donor cells from spleen, liver and intestine are shown (D). Livers, small intestines and large intestines were collected and analyzed for pathological damage on day 14 post-BMT. Pathology scores of liver, small and large intestine are shown (E). The experiments were repeated 3 independent times. Five mice per group were used for each experiment. Statistical data are presented as mean ± 1SD; significance was determined by Student’s t test. * p < 0.05, **p < 0.01 and ***p < 0.001.
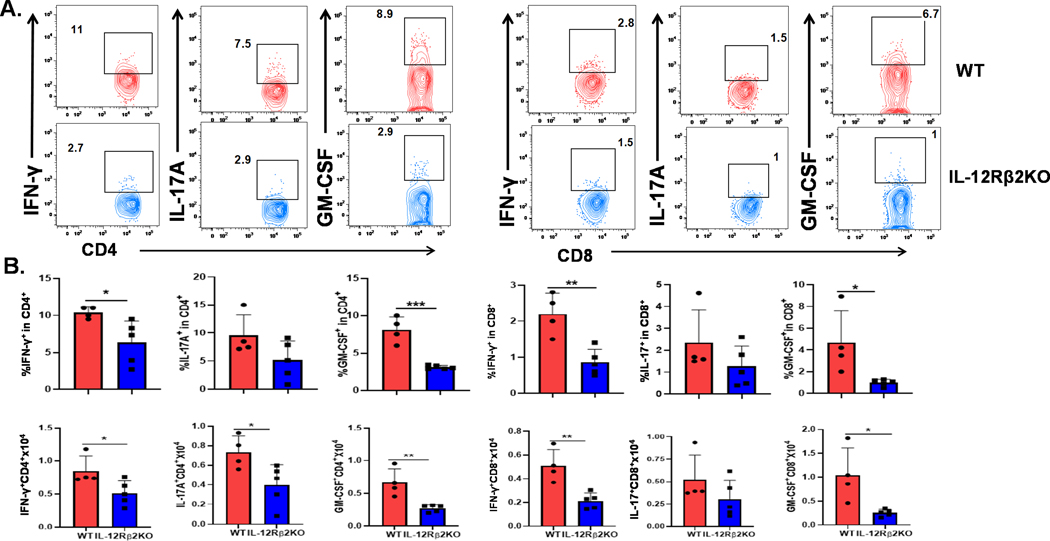
IL-12Rβ2 promotes IFN-γ, IL-17A and GM-CSF production by donor T cells in the GI tract.
Subsequently, we measured cytokine production of donor T cells in different organs at day 14 post BMT. In recipient intestines, IFN-γ and GM-CSF production by CD4+ or CD8+ donor T cells were significantly reduced, but IL-17A only decreased in CD4+ donor T cells in IL-12Rβ2KO recipients (Figure 5 A, B). CD4+GMCSF+ T cell population arises during GVHD development and constitutes a heterogeneous population, which produces GM-CSF alone or GM-CSF in conjunction with IFN-γ or IL-17 (32). We tested co-expression of GM-CSF and IFN-γ or IL-17 and observed that donor CD4+ T cells that expressed GM-CSF alone and co-expressed IFN-γ or IL-17 were significantly reduced in IL-12Rβ2KO recipients (Supplementary Figure 2C, D). Consistent with cytokine profiles in the intestine, there was a significant reduction of IFN-γ, IL17A or GM-CSF production by donor T cells in mesenteric lymph nodes (mLNs) of IL-12Rβ2KO recipients (Supplemental Figure 3A, B). In the spleen, we observed reduced percentage of GM-CSF-producing cells among CD4+ donor T cells, while IFN-γ and IL17A production were similar in both groups (Supplemental Figure 3C, D). We observed no significant difference in cytokine production by donor T cells in the liver between WT and IL12Rβ2 KO cohorts (Data not shown). Taken together, we conclude that recipient IL12Rβ2 contributes to GVHD pathogenesis primarily through regulating donor T-cell expansion and activation in recipient intestines.
Fig. 5. IL-12Rβ2 promotes IFNγ, IL-17A and GM-CSF production by donor T cells in the GI tract.
Lethally irradiated WT and IL-12Rβ2KO B6 mice underwent BMT with 5×106 TCD-BM from FVB mice (Thy1.1+) alone (BMA) or plus splenocytes (equal to 0.5×106 T cells) from FVB mice (Thy1.2+). Recipient intestines were harvested on day 14 after BMT and analyzed with flow cytometry. Representative dot plots, the average frequencies and absolute counts of IFN-γ+, IL17A+, and GM-CSF+ among gated donor CD4+ or CD8+ T cells are shown (A and B). The experiments were repeated 3 independent times. 4–5 mice per group were used for each experiment. Statistical data are presented as mean ± 1SD; significance was determined by Student’s t test. * p < 0.05, **p < 0.01 and ***p < 0.001.
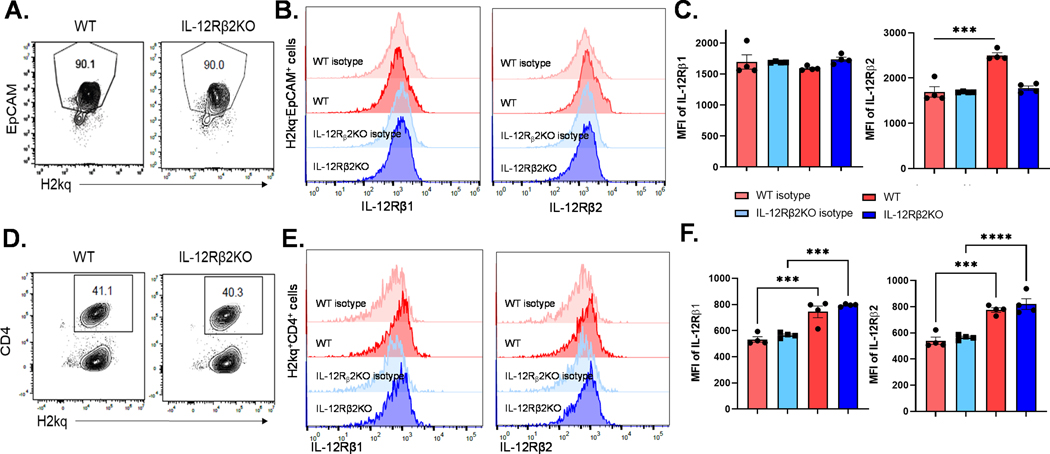
IL-12 receptor on intestinal epithelial cells (IECs) only consists of IL12Rβ2 chain.
It was reported that IL-12 receptor expressed on IECs is formed solely by IL12Rβ2 chain without IL12Rβ1 (33). We measured the IL12Rβ1 and IL12Rβ2 expression on IECs in C57BL/6 WT mice by flow cytometry. In agreement with the above report, we detected IL12Rβ2 expression on H2Kq-EpCAM+ IECs (Figure 6 A-C) and H2Kq+ donor CD4 T cells (Figure 6 D-F). As expected, no IL12Rβ2 expression was seen in IL12Rβ2KO mice IEC (Figure 6 B, C). We were not able to detect IL12Rβ1 on H2Kq-EpCAM+ recipient IEC (Figure 6 A-C) whereas the H2Kq+ donor cells expressed IL12Rβ1 (Figure 6 D-F). We observed IEC preservation in IL12Rβ2KO recipients (Supplementary Figure 4A) and found that IL12Rβ1 was expressed on infiltrating donor T cells, but not on IECs, 14 days after BMT (Supplementary Figure 4 B, C). Moreover, we observed that IL12Rβ2 expression was induced in IECs upon irradiation conditioning (Supplementary Figure 4 D, E). Given IL-23 and IL-12 receptor share the IL-12Rβ1 subunit, we tested the expression of IL-23 receptor and observed that its expression is comparable in unmanipulated WT and IL-12Rβ2 KO mice (Supplementary Figure 4F). However, IL-23R expression is significantly reduced in IL-12Rβ2 KO recipients after irradiation and BMT (Supplementary Figure 4G). These data suggest that IL-23R expression can be regulated by IL-12 signals.
Figure 6. IL-12R was formed solely by the IL12Rβ2 chain without IL12Rβ in the recipients’ GI tract.
WT and IL-12Rβ2KO B6 mice were irradiated (1200cGy) and transplanted with 5 × 106 splenocyte from FVB mice. Four days after BMT, recipients were euthanized, and IECs were isolated for flow cytometry analysis (A).
IL-12Rβ1 and IL-12R2 expression was quantified on H2Kq-EpCAM+ IECs (B and C). Mouse IgG1 PE-conjugated antibody was used as isotype control antibody. H2Kq+ donor CD4 T cells were isolated from spleen (D) and the expression of IL-12Rβ1 and IL-12R2 were detected (E and F). The experiments were repeated 2 independent times. Four mice per were used for each experiment.
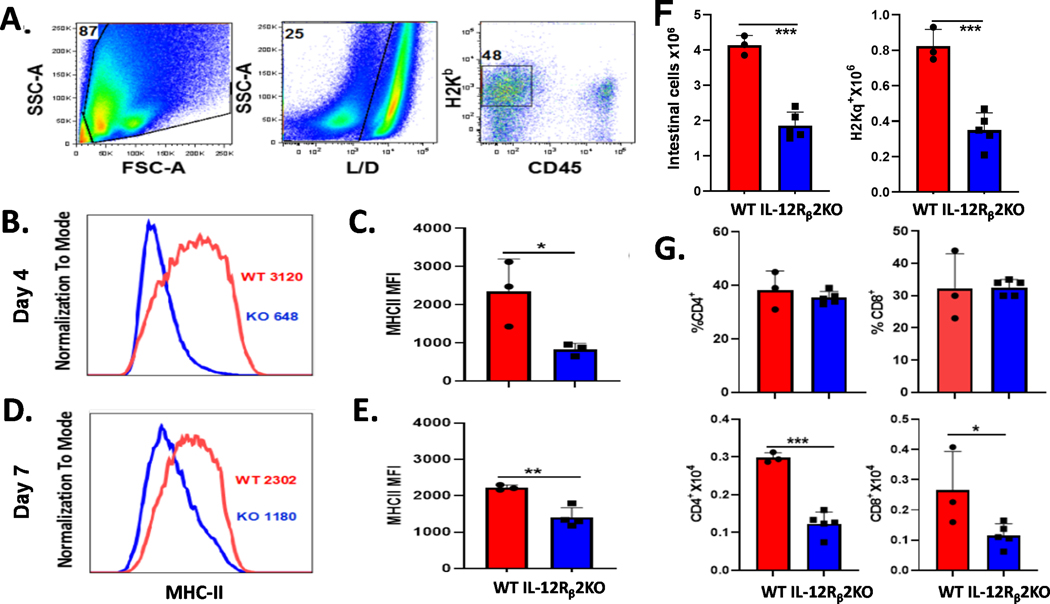
IL-12Rβ2 expression augments MHC class II expression on intestinal cells.
MHC class II expression on IECs is significantly increased after total body irradiation (TBI) and BMT, which presents alloantigen and activates CD4+ donor T cells to initiate GI GVHD (18). Given that the IL-12/IFN-γ axis is required for MHC class II expression on IECs, we hypothesized that IL-12Rβ2 signaling promotes MHC class II expression on IECs and facilitates GI GVHD. To test this hypothesis, we transplanted Thy1.1+ splenocytes to lethally irradiated WT and IL-12Rβ2KO recipient mice. MHC class II expression was significantly reduced on the IECs of IL-12Rβ2KO recipients compared to WT recipients at day 4 (Figure 7 A-C) or day 7 post-BMT (Figure 7 D, E). After 7 days, we found that absolute numbers of total intestinal cells, donor splenocytes, CD4+ and CD8+ donor T cells were significantly reduced in IL-12Rβ2KO recipients, but no differences in percentages of donor CD4+ or CD8+ donor T cells were observed (Figure 7 F, G). These data suggest IL-12Rβ2 on recipient non-hematopoietic cells promotes MHC class II expression after TBI and BMT, which enhance donor T-cell activation and expansion in recipient intestine.
Figure 7. IL-12Rβ2 augments MHC-II expression and donor T cells in recipient GI tract.
WT and IL-12Rβ2KO B6 mice were irradiated (1100cGy) and transplanted with 5×106 splenocytes from FVB mice (Thy1.2). Four days after transfusion, recipient intestines were harvested and IECs were isolated for flow cytometry analysis. Gating strategy for analysis is shown (A). Representative flow histogram (B) and graphical summary (C) for MHC-II expression (MFI) on gated H2Kb+CD45- IECs are shown. WT and IL-12Rβ2KO B6 mice were irradiated (1100cGy) and transplanted with 3×106 splenocytes from FVB mice (Thy1.2). IECs were isolated for flow cytometry analysis on day 7 post transfusion. Representative flow histogram (D) and graphical summary (E) for MHC-II expression (MFI) on gated H2Kb+CD45- IECs are shown. Absolute count of total donor splenocytes, average frequencies and absolute numbers of donor CD4+ or CD8+ are shown (F and G). The experiments were repeated 2 independent times. 3–5 mice per group were used for each experiment. Statistical data are presented as mean ± 1SD; significance was determined by Student’s t test. * p < 0.05, **p < 0.01 and ***p < 0.001.
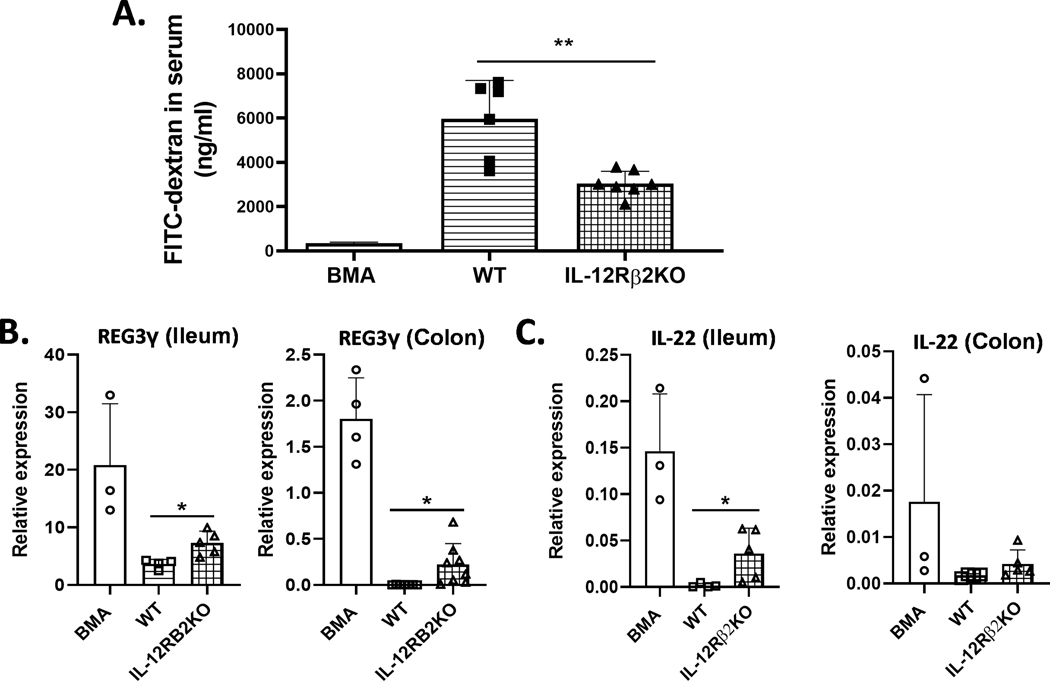
IL-12Rβ2 enhances intestinal permeability and compromises intestine integrity after allo-BMT.
Conditioning-related damage and activated donor T cells contribute at early stages of GI GVHD development that depend on increased transcellular permeability, leading to intestinal barrier dysfunction (34, 35). We next asked whether IL-12Rβ2 impacts GI tract permeability using a fluorescein isothiocyanate (FITC)-dextran assay. Indeed, we found that GI tract permeability was significantly reduced in IL-12Rβ2KO as compared to WT recipients (Figure 8A). Given that IL-22 and Reg3γ play significant roles in the maintenance of intestinal homeostasis, we further tested whether IL-12Rβ2 affects Reg3γ and IL-22 in recipient intestines. Quantitative RT-PCR analysis revealed significantly increased relative expression of IL-22 in the small intestine, but not large intestine, of IL12Rβ2KO recipients compared to WT controls (Figure 8 B, C). IL-22 producing innate lymphoid cells (ILCs) were shown to be eradicated by alloreactive T cells during GVHD development (20). Thus, these results suggest that increased IL-22 and Reg3γ levels were associated with the preserved gut epithelium due to decreased T cell infiltration and inflammatory cytokines in IL-12Rβ2-deficient recipients.
Figure 8. IL-12Rβ2-deficiency reduces intestinal permeability and protects intestine integrity after BMT.
WT (n=6) and IL-12Rβ2KO B6 mice (n=7) were irradiated (1100cGy) and transplanted with 3×106 splenocytes from FVB mice (Thy1.2). On day 7 after transfusion, the recipients were gavaged with 200 μl of 80 mg/ml FITC dextran (4kDa). Intestinal permeability was assessed by FITC-Dextran concentration in serum after additional four hours (A). Lethally irradiated WT and IL-12Rβ2KO B6 mice underwent BMT with 5×106 TCD-BM from FVB mice (Thy1.1) alone or plus splenocytes (equal to 0.5×106 T cells) from FVB mice (Thy1.2). Relative expression of REG3γ (B) and IL-22 (C) in ileum or colon are shown. The experiments were repeated 2 independent times. 4–5 mice per group were used. Statistical data are presented as mean ± 1SD; significance was determined by Student’s t test. * p < 0.05, **p <0.01.
Discussion
The interactions between donor T cells and host in GVHD pathogenesis is still not fully understood (1, 36, 37). In the clinic, recipient age, sex, human leukocyte antigens (HLA) mismatch, and genetic variation are associated with severe GVHD after allo-HCT (38–40). Data from murine BMT models indicate host hematopoietic and non-hematopoietic APCs, neutrophils, innate lymphoid cells, as well as other recipient-derived cells are involved at early stages of aGVHD pathogenesis (12, 41–43). In the current study, we observed significantly improved aGVHD in recipient mice deficient for IL-12Rβ2 on host non-hematopoietic cells after allo-BMT, while IL-12Rβ2 on donor or host hematopoietic cells is not required for GVHD development. Furthermore, our data showed that MHC-II antigen presentation and GI tract permeability were alleviated in the recipients deficient for IL-12Rβ2, which resulted in reduced donor T-cell migration and inflammatory cytokine production.
GI aGVHD is the most common and serious complication in initiating systemic GVHD and determines the outcome of allo-HCT; GI damage to recipients usually occurs after lethal dose of TBI or high dose chemotherapy conditioning prior to transplantation (44). TBI damage increases inflammatory cytokines and MHC-II expression on recipient APCs to activate donor T cells after BMT (45). Although IL-12/IFN-γ axis from hematopoietic cells has been suggested to induce MHC-II expression on intestinal epithelial cells (18), the contribution of IL-12 receptor signaling was not clear. In the current study, we focused on the contribution of IL-12 receptor subunits on recipient cells to MHC-II expression and GVHD development. Our findings reveal that IL-12Rβ2 is solely induced in IECs by TBI (Figure 6) and important not only in GI GVHD but also in early TBI damage. As non-hematopoietic APCs, intestinal epithelial cells present MHC-II antigen to donor CD4+ T cells and initiates GVHD influenced by microbiota (18). Consistent with this study, we observed that IL-12Rβ2 on host non-hematopoietic cells increased MHC-II expression on IECs (Figure 7), increased the Tc/Th1, Tc/Th17 and GM-CSF expressed T cells in mLN and GI tract (Figure 5), promoted T-cell infiltration into intestinal tissue, and enhanced the severity of GI GHVD.
GI tract damage by TBI conditioning and allogeneic T cells attack is characterized as compromised barrier function by assessing the intestinal permeability (46, 47). In this study, we showed increased paracellular intestinal permeability in aGVHD models compared to TCD-BM alone control (Figure 8 A). IL-12Rβ2 on host non-hematopoietic cells increased permeability by enhancing T cell migration to the intestinal track and inflammatory cytokine production.
IL-22 is a member of the IL-10 family of cytokines produced by several hematopoietic populations, including Th cells and ILCs (20, 48). IL-22 has been shown to have protective and inflammatory properties in the pathophysiology of GVHD (49, 50). On the protective side, IL-22 enhances intestinal epithelial regeneration and barrier function, consequently improving aGVHD (20, 51). IL-22 is also known as a regulator of REG3γ that protects intestinal stem cells and paneth cells against apoptosis (48). Regenerating islet-derived 3α (REG3α) produced by paneth cells is widely used in the clinic as a specific biomarker, since REG3α serum level was upregulated in patients with severe GI GVHD (52, 53). It has been also reported that REG3γ (the mouse homolog of REG3α) production in GI tract decreased as aGVHD progresses is inversely correlated with the elevated level in circulation (54). IL-22 is produced by radio-resistant host ILCs in response to pre-conditioning yet diminishes in the GI tract during GVHD development due to ILC destruction by donor derived alloreactive T cells (20, 51, 54). Our data showed a significant increase in IL-22 in the ileum of IL-12Rβ2 deficient recipient, while we observed a significant increase of REG3γ in the ileum and colon (Figure 8 B, C). We also found that REG3γ expression in the colon was much lower than that in ileum due to a reduced presence of paneth cells in the colon (55). We thus propose that increased IL-22 production in IL-12Rβ2 deficient recipients likely resulted from protection of intestinal epithelium, ILCs and others due to reduced T cell infiltration (Figure 3) and proinflammatory cytokines (Figure 5). Consequently, IL-22 and REG3γ produced by these protected cell types contributed to the intestinal stem cell (ISC) and paneth recovery in the ileum and colon during GVHD.
It has been reported that IL-12 drives GVHD through Th1 activation (56, 57), and the serum level of IL-12 is increased in aGVHD patients compared to the healthy donor (58). Although IL-12/IL-12R signaling plays a critical role in T-cell proliferation, differentiation and effector function after BMT (59), IL-12Rβ2 deficiency in donor cells and in recipient immune cells did not mitigate the severity of aGVHD (Figure 1, 2). A potential explanation could be impaired Treg generation and suppression due to interference of IL-35 signaling in the absence of IL-12Rβ2 (60–62), which remains to be further defined. However, since IL-12Rβ2 deficiency did not impair the function of donor cells, we suppose that inhibition of IL-12/IL12R signaling for GVHD prevention would preserve the GVL activity. We measured IL-12Rβ1 and IL-12Rβ2 on intestinal epithelial cells in naive B6 mice before and after BMT and observed only IL-12β2 was induced by TBI followed by BMT (Figure 6 and supplementary figure 4), which would partially explain an obvious protection on GI GVHD in IL-12β2KO recipients. We also tested the expression of IL-23R since it shares IL-12Rβ1 subunit with the IL-12R and found that IL-23R expression was reduced in IL-12Rβ2 KO recipients. Since IL-12-induced T-bet is known to regulate IL-23R expression for Th17 derivation (63, 64), we suppose that IL-12Rβ2 signaling would also increase IL-23R expression in IECs. The exact molecular mechanisms involved in IL12/IL12Rβ2 signaling of intestinal cells in aGVHD requires further investigation.
In conclusion, we validated that IL-12Rβ2 on recipient non-hematopoietic cells contributes to acute GVHD development, especially GI GVHD. IL-12Rβ2 deficiency in non-hematopoietic cells contributes to the maintenance of intestinal epithelial integrity and consequently preserves IL-22 and REG3γ expression. In turn, IL-12Rβ2 deficiency protects GI tract from donor T-cell mediated injury by attenuating MHC-II expression on IECs. Novel aGVHD preventive and therapeutic strategies have extended to tissue damage repair (65), blockade of cytokine signaling pathways (66, 67), and targeting metabolic pathways (68). If IL-12Rβ2 is expressed in human GI tract as expected, specific blockade of IL-12/IL12R signaling will likely reduce GI GVHD after allo-HCT while not increasing the risk of infection or relapse in patients with malignant hematological diseases.
Supplementary Material
Key Points:
IL-12Rβ2 on recipient non-hematopoietic cells contributes to aGVHD development.
Targeting IL-12Rβ2 may be a potential strategy in the treatment of aGVHD.
Acknowledgments:
The authors are grateful for the technical support provided by the Department of Laboratory Animal Research, Flow Cytometry Core and Imaging Core as part of the Hollings Cancer Center at the MUSC.
This publication was supported, in part by South Carolina SmartState endowment, the MCW Cancer Center Scholar endowment, and R01 grants from the National Institutes of Health including CA169116, HL140953, and R01 CA258440 (X.-Z.Y).
Footnotes
The authors have declared that no conflict of interest exists.
Data and materials availability:
All data associated with this study are present in the paper or Supplementary Figures.
Reference
- 1.Forman Stephen J., R. S. N., Antin Joseph H., Appelbaum Frederick R. 2016. Thomas’ Hematopoietic Cell Transplantation: Stem Cell Transplantation 5th Edition. [Google Scholar]
- 2.Voermans C, and Hazenberg MD 2020. Cellular therapies for graft-versus-host disease: a tale of tissue repair and tolerance. Blood 136: 410–417. [DOI] [PubMed] [Google Scholar]
- 3.Zeiser R, and Blazar BR 2017. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med 377: 2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredricks DN 2019. The gut microbiota and graft-versus-host disease. J Clin Invest 129: 1808–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peled JU, Hanash AM, and Jenq RR 2016. Role of the intestinal mucosa in acute gastrointestinal GVHD. Blood 128: 2395–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naymagon S, Naymagon L, Wong SY, Ko HM, Renteria A, Levine J, Colombel JF, and Ferrara J. 2017. Acute graft-versus-host disease of the gut: considerations for the gastroenterologist. Nat Rev Gastroenterol Hepatol 14: 711–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara JLM, and Chaudhry MS 2018. GVHD: biology matters. Hematology Am Soc Hematol Educ Program 2018: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teshima T, Reddy P, and Zeiser R. 2016. Reprint of: Acute Graft-versus-Host Disease: Novel Biological Insights. Biol Blood Marrow Transplant 22: S3–8. [DOI] [PubMed] [Google Scholar]
- 9.Chakraverty R, and Sykes M. 2007. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood 110: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koyama M, Kuns RD, Olver SD, Raffelt NC, Wilson YA, Don AL, Lineburg KE, Cheong M, Robb RJ, Markey KA, Varelias A, Malissen B, Hammerling GJ, Clouston AD, Engwerda CR, Bhat P, MacDonald KP, and Hill GR 2011. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat Med 18: 135–142. [DOI] [PubMed] [Google Scholar]
- 11.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, and Ferrara JL 2005. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med 11: 1244–1249. [DOI] [PubMed] [Google Scholar]
- 12.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, Ferrara JL, and Teshima T. 2004. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol 172: 7393–7398. [DOI] [PubMed] [Google Scholar]
- 13.Koyama M, and Hill GR 2019. The primacy of gastrointestinal tract antigen-presenting cells in lethal graft-versus-host disease. Blood 134: 2139–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Kaplan DH, Matte-Martone C, Tan HS, Venkatesan S, Johnson K, Demetris AJ, McNiff J, Shlomchik MJ, and Shlomchik WD 2011. Langerhans cells are not required for graft-versus-host disease. Blood 117: 697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B, Branstetter D, Smith J, Paxton RJ, Cerretti DP, Bonham L, Hill GR, and Hume DA 2010. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood 116: 3955–3963. [DOI] [PubMed] [Google Scholar]
- 16.Matte-Martone C, Wang X, Anderson B, Jain D, Demetris AJ, McNiff J, Shlomchik MJ, and Shlomchik WD 2010. Recipient B cells are not required for graft-versus-host disease induction. Biol Blood Marrow Transplant 16: 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, Ashenberg O, Su CW, Smillie C, Shekhar K, Chen Z, Wu C, Ordovas-Montanes J, Alvarez D, Herbst RH, Zhang M, Tirosh I, Dionne D, Nguyen LT, Xifaras ME, Shalek AK, von Andrian UH, Graham DB, Rozenblatt-Rosen O, Shi HN, Kuchroo V, Yilmaz OH, Regev A, and Xavier RJ 2018. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell 175: 1307–1320 e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama M, Mukhopadhyay P, Schuster IS, Henden AS, Hulsdunker J, Varelias A, Vetizou M, Kuns RD, Robb RJ, Zhang P, Blazar BR, Thomas R, Begun J, Waddell N, Trinchieri G, Zeiser R, Clouston AD, Degli-Esposti MA, and Hill GR 2019. MHC Class II Antigen Presentation by the Intestinal Epithelium Initiates Graft-versus-Host Disease and Is Influenced by the Microbiota. Immunity 51: 885–898 e887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aparicio-Domingo P, Romera-Hernandez M, Karrich JJ, Cornelissen F, Papazian N, Lindenbergh-Kortleve DJ, Butler JA, Boon L, Coles MC, Samsom JN, and Cupedo T. 2015. Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. J Exp Med 212: 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, Ghosh A, Tsai JJ, Rao UK, Yim NL, Smith OM, Velardi E, Hawryluk EB, Murphy GF, Liu C, Fouser LA, Kolesnick R, Blazar BR, and van den Brink MR 2012. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrara JL, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T, Levine JE, Choi SW, Huber E, Landfried K, Akashi K, Vander Lugt M, Reddy P, Chin A, Zhang Q, Hanash S, and Paczesny S. 2011. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood 118: 6702–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, and Spits H. 2018. Innate Lymphoid Cells: 10 Years On. Cell 174: 1054–1066. [DOI] [PubMed] [Google Scholar]
- 23.Srinagesh HK, Ozbek U, Kapoor U, Ayuk F, Aziz M, Ben-David K, Choe HK, DeFilipp Z, Etra A, Grupp SA, Hartwell MJ, Hexner EO, Hogan WJ, Karol AB, Kasikis S, Kitko CL, Kowalyk S, Lin JY, Major-Monfried H, Mielke S, Merli P, Morales G, Ordemann R, Pulsipher MA, Qayed M, Reddy P, Reshef R, Rosler W, Sandhu KS, Schechter T, Shah J, Sigel K, Weber D, Wolfl M, Wudhikarn K, Young R, Levine JE, and Ferrara JLM 2019. The MAGIC algorithm probability is a validated response biomarker of treatment of acute graft-versus-host disease. Blood Adv 3: 4034–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Major-Monfried H, Renteria AS, Pawarode A, Reddy P, Ayuk F, Holler E, Efebera YA, Hogan WJ, Wolfl M, Qayed M, Hexner EO, Wudhikarn K, Ordemann R, Young R, Shah J, Hartwell MJ, Chaudhry MS, Aziz M, Etra A, Yanik GA, Kroger N, Weber D, Chen YB, Nakamura R, Rosler W, Kitko CL, Harris AC, Pulsipher M, Reshef R, Kowalyk S, Morales G, Torres I, Ozbek U, Ferrara JLM, and Levine JE 2018. MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD. Blood 131: 2846–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolic B, Lee S, Bronson RT, Grusby MJ, and Sykes M. 2000. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest 105: 1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastian D, Wu Y, Betts BC, and Yu XZ 2019. The IL-12 Cytokine and Receptor Family in Graft-vs.-Host Disease. Front Immunol 10: 988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai BB, Quinn PM, Wolitzky AG, Mongini PK, Chizzonite R, and Gately MK 1992. IL-12 receptor. II. Distribution and regulation of receptor expression. J Immunol 148: 3125–3132. [PubMed] [Google Scholar]
- 28.Bastian D, Sui X, Nguyen HD, Wu Y, Schutt S, Tian L, Sofi MH, Liu Y, Martin P, Bartee E, and Yu XZ 2021. Interleukin-23 receptor signaling by interleukin-39 potentiates T cell pathogenicity in acute graft-versus-host disease. Am J Transplant 21: 3538–3549. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi O, Yamane H, Imajoh-Ohmi S, and Nariuchi H. 1998. IL-12 receptor (IL-12R) expression and accumulation of IL-12R beta 1 and IL-12R beta 2 mRNAs in CD4+ T cells by costimulation with B7–2 molecules. J Immunol 160: 1638–1646. [PubMed] [Google Scholar]
- 30.Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, Weissman IL, and Contag CH 2004. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A 101: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young JS, Wu T, Chen Y, Zhao D, Liu H, Yi T, Johnston H, Racine J, Li X, Wang A, Todorov I, and Zeng D. 2012. Donor B cells in transplants augment clonal expansion and survival of pathogenic CD4+ T cells that mediate autoimmune-like chronic graft-versus-host disease. J Immunol 189: 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piper C, Hainstock E, Yin-Yuan C, Chen Y, Khatun A, Kasmani MY, Evans J, Miller JA, Gorski J, Cui W, and Drobyski WR 2022. Single-cell immune profiling reveals a developmentally distinct CD4+ GM-CSF+ T-cell lineage that induces GI tract GVHD. Blood Adv 6: 2791–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regoli M, Man A, Gicheva N, Dumont A, Ivory K, Pacini A, Morucci G, Branca JJV, Lucattelli M, Santosuosso U, Narbad A, Gulisano M, Bertelli E, and Nicoletti C. 2018. Morphological and Functional Characterization of IL-12Rbeta2 Chain on Intestinal Epithelial Cells: Implications for Local and Systemic Immunoregulation. Front Immunol 9: 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noth R, Lange-Grumfeld J, Stuber E, Kruse ML, Ellrichmann M, Hasler R, Hampe J, Bewig B, Rosenstiel P, Schreiber S, and Arlt A. 2011. Increased intestinal permeability and tight junction disruption by altered expression and localization of occludin in a murine graft versus host disease model. BMC Gastroenterol 11: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nalle SC, Zuo L, Ong M, Singh G, Worthylake AM, Choi W, Manresa MC, Southworth AP, Edelblum KL, Baker GJ, Joseph NE, Savage PA, and Turner JR 2019. Graft-versus-host disease propagation depends on increased intestinal epithelial tight junction permeability. J Clin Invest 129: 902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blazar BR, Murphy WJ, and Abedi M. 2012. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol 12: 443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Couriel D, Caldera H, Champlin R, and Komanduri K. 2004. Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer 101: 1936–1946. [DOI] [PubMed] [Google Scholar]
- 38.Holler E, Rogler G, Brenmoehl J, Hahn J, Herfarth H, Greinix H, Dickinson AM, Socie G, Wolff D, Fischer G, Jackson G, Rocha V, Steiner B, Eissner G, Marienhagen J, Schoelmerich J, and Andreesen R. 2006. Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem cell transplantation: effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood 107: 4189–4193. [DOI] [PubMed] [Google Scholar]
- 39.Karam E, Laporte J, Solomon SR, Morris LE, Zhang X, Holland HK, Bashey A, and Solh MM 2019. Who Is a Better Donor for Recipients of Allogeneic Hematopoietic Cell Transplantation: A Young HLA-Mismatched Haploidentical Relative or an Older Fully HLA-Matched Sibling or Unrelated Donor? Biol Blood Marrow Transplant 25: 2054–2060. [DOI] [PubMed] [Google Scholar]
- 40.Sobecks RM, Wang T, Askar M, Gallagher MM, Haagenson M, Spellman S, Fernandez-Vina M, Malmberg KJ, Muller C, Battiwalla M, Gajewski J, Verneris MR, Ringden O, Marino S, Davies S, Dehn J, Bornhauser M, Inamoto Y, Woolfrey A, Shaw P, Pollack M, Weisdorf D, Milller J, Hurley C, Lee SJ, and Hsu K. 2015. Impact of KIR and HLA Genotypes on Outcomes after Reduced-Intensity Conditioning Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 21: 1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrara JL, Smith CM, Sheets J, Reddy P, and Serody JS 2017. Altered homeostatic regulation of innate and adaptive immunity in lower gastrointestinal tract GVHD pathogenesis. J Clin Invest 127: 2441–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hulsdunker J, Ottmuller KJ, Neeff HP, Koyama M, Gao Z, Thomas OS, Follo M, Al-Ahmad A, Prinz G, Duquesne S, Dierbach H, Kirschnek S, Lammermann T, Blaser MJ, Fife BT, Blazar BR, Beilhack A, Hill GR, Hacker G, and Zeiser R. 2018. Neutrophils provide cellular communication between ileum and mesenteric lymph nodes at graft-versus-host disease onset. Blood 131: 1858–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markey KA, Kuns RD, Browne DJ, Gartlan KH, Robb RJ, Martins JP, Henden AS, Minnie SA, Cheong M, Koyama M, Smyth MJ, Steptoe RJ, Belz GT, Brocker T, Degli-Esposti MA, Lane SW, and Hill GR 2018. Flt-3L Expansion of Recipient CD8alpha(+) Dendritic Cells Deletes Alloreactive Donor T Cells and Represents an Alternative to Posttransplant Cyclophosphamide for the Prevention of GVHD. Clin Cancer Res 24: 1604–1616. [DOI] [PubMed] [Google Scholar]
- 44.Zeiser R. 2019. Advances in understanding the pathogenesis of graft-versus-host disease. Br J Haematol 187: 563–572. [DOI] [PubMed] [Google Scholar]
- 45.Hill GR, and Ferrara JL 2000. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 95: 2754–2759. [PubMed] [Google Scholar]
- 46.Chakraverty R, and Teshima T. 2021. Graft-versus-host disease: a disorder of tissue regeneration and repair. Blood 138: 1657–1665. [DOI] [PubMed] [Google Scholar]
- 47.Nalle SC, and Turner JR 2015. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol 8: 720–730. [DOI] [PubMed] [Google Scholar]
- 48.Keir M, Yi Y, Lu T, and Ghilardi N. 2020. The role of IL-22 in intestinal health and disease. J Exp Med 217: e20192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamarthee B, Malard F, Saas P, Mohty M, and Gaugler B. 2016. Interleukin-22 in Graft-Versus-Host Disease after Allogeneic Stem Cell Transplantation. Front Immunol 7: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song Q, Wang X, Wu X, Kang TH, Qin H, Zhao D, Jenq RR, van den Brink MRM, Riggs AD, Martin PJ, Chen YZ, and Zeng D. 2021. IL-22-dependent dysbiosis and mononuclear phagocyte depletion contribute to steroid-resistant gut graft-versus-host disease in mice. Nat Commun 12: 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, Ivanov JA, Fu YY, Takashima S, Hua G, Martin ML, O’Rourke KP, Lo YH, Mokry M, Romera-Hernandez M, Cupedo T, Dow L, Nieuwenhuis EE, Shroyer NF, Liu C, Kolesnick R, van den Brink MRM, and Hanash AM 2015. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528: 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartwell MJ, Ozbek U, Holler E, Renteria AS, Major-Monfried H, Reddy P, Aziz M, Hogan WJ, Ayuk F, Efebera YA, Hexner EO, Bunworasate U, Qayed M, Ordemann R, Wolfl M, Mielke S, Pawarode A, Chen YB, Devine S, Harris AC, Jagasia M, Kitko CL, Litzow MR, Kroger N, Locatelli F, Morales G, Nakamura R, Reshef R, Rosler W, Weber D, Wudhikarn K, Yanik GA, Levine JE, and Ferrara JL 2017. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight 2: e89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solan L, Kwon M, Carbonell D, Dorado N, Balsalobre P, Serrano D, Chicano-Lavilla M, Anguita J, Gayoso J, Diez-Martin JL, Martinez-Laperche C, and Buno I. 2019. ST2 and REG3alpha as Predictive Biomarkers After Haploidentical Stem Cell Transplantation Using Post-transplantation High-Dose Cyclophosphamide. Front Immunol 10: 2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao D, Kim YH, Jeong S, Greenson JK, Chaudhry MS, Hoepting M, Anderson ER, van den Brink MR, Peled JU, Gomes AL, Slingerland AE, Donovan MJ, Harris AC, Levine JE, Ozbek U, Hooper LV, Stappenbeck TS, Ver Heul A, Liu TC, Reddy P, and Ferrara JL 2018. Survival signal REG3alpha prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J Clin Invest 128: 4970–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki N, Sachs N, Wiebrands K, Ellenbroek SI, Fumagalli A, Lyubimova A, Begthel H, van den Born M, van Es JH, Karthaus WR, Li VS, Lopez-Iglesias C, Peters PJ, van Rheenen J, van Oudenaarden A, and Clevers H. 2016. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci U S A 113: E5399–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williamson E, Garside P, Bradley JA, More IA, and Mowat AM 1997. Neutralizing IL-12 during induction of murine acute graft-versus-host disease polarizes the cytokine profile toward a Th2-type alloimmune response and confers long term protection from disease. J Immunol 159: 1208–1215. [PubMed] [Google Scholar]
- 57.Williamson E, Garside P, Bradley JA, and Mowat AM 1996. IL-12 is a central mediator of acute graft-versus-host disease in mice. J Immunol 157: 689–699. [PubMed] [Google Scholar]
- 58.Reddy V, Winer AG, Eksioglu E, Meier-Kriesche HU, Schold JD, and Wingard JR 2005. Interleukin 12 is associated with reduced relapse without increased incidence of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 11: 1014–1021. [DOI] [PubMed] [Google Scholar]
- 59.Hill GR, and Koyama M. 2020. Cytokines and costimulation in acute graft-versus-host disease. Blood 136: 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, and Vignali DA 2007. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450: 566–569. [DOI] [PubMed] [Google Scholar]
- 61.Pylayeva-Gupta Y. 2016. Molecular Pathways: Interleukin-35 in Autoimmunity and Cancer. Clin Cancer Res 22: 4973–4978. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Zhang Y, Wang Q, Li C, Deng H, Si C, and Xiong H. 2019. Interleukin-35 in immune-related diseases: protection or destruction. Immunology 157: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krausgruber T, Schiering C, Adelmann K, Harrison OJ, Chomka A, Pearson C, Ahern PP, Shale M, Oukka M, and Powrie F. 2016. T-bet is a key modulator of IL-23-driven pathogenic CD4(+) T cell responses in the intestine. Nat Commun 7: 11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan NW, Wang S, Ortiz G, Chauhan SK, Chen Y, and Dana R. 2022. Autoreactive memory Th17 cells are principally derived from T-bet(+)RORgammat(+) Th17/1 effectors. J Autoimmun 129: 102816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, Mimura I, Morita H, Sugiyama D, Nishikawa H, Hattori M, Hino Y, Ikegawa S, Yamamoto K, Toya T, Doki N, Koizumi K, Honda K, and Ohashi K. 2016. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 128: 2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Betts BC, Bastian D, Iamsawat S, Nguyen H, Heinrichs JL, Wu Y, Daenthanasanmak A, Veerapathran A, O’Mahony A, Walton K, Reff J, Horna P, Sagatys EM, Lee MC, Singer J, Chang YJ, Liu C, Pidala J, Anasetti C, and Yu XZ 2018. Targeting JAK2 reduces GVHD and xenograft rejection through regulation of T cell differentiation. Proc Natl Acad Sci U S A 115: 1582–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Y, Bastian D, Schutt S, Nguyen H, Fu J, Heinrichs J, Xia C, and Yu XZ 2015. Essential Role of Interleukin-12/23p40 in the Development of Graft-versus-Host Disease in Mice. Biol Blood Marrow Transplant 21: 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sofi MH, Wu Y, Ticer T, Schutt S, Bastian D, Choi HJ, Tian L, Mealer C, Liu C, Westwater C, Armeson KE, Alekseyenko AV, and Yu XZ 2021. A single strain of Bacteroides fragilis protects gut integrity and reduces GVHD. JCI Insight 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or Supplementary Figures.