Abstract
Background & Aims:
The presence of perioperative diabetes may lead to increased mortality risks following liver transplant (LT) in patients with non-alcoholic steatohepatitis (NASH). This risk factor was evaluated using a UNOS-STAR national database.
Methods:
The UNOS-STAR liver transplant registry 2005–2019 was used to select patients with NASH (including cryptogenic liver disease). The following populations were excluded: those younger than 18 years old and those with living donors/dual transplants. Selected patients were stratified into those with and without pre-LT diabetes and compared to the individual mortality endpoints using iterative Cox analyses.
Results:
6324 recipients with and 8251 without diabetes were selected. The median follow-up time was 3.07 years. Those with diabetes were older (58.50 vs. 54.50 years, p<0.001), were more likely to be Hispanic or Asian, and had higher BMI than the non-diabetics (31.10 vs. 29.70 kg/m2 p<0.001); however, there was no difference in gender (female 41.9 vs. 43.1% p=0.170). Compared to non-diabetics, recipients with diabetes had a higher rate of all-cause mortality (61.68 vs. 47.80 per 1000 person-years). In multivariate iterations, pre-LT diabetes was associated with all-cause mortality (aHR 1.19 95% CI 1.11–1.27) as well as deaths due to cardiac (p=0.014 aHR 1.24 95% CI 1.04–1.46) and renal causes (p=0.039 aHR 1.38 95% CI 1.02–1.87).
Conclusion:
The presence of pre-LT diabetes is associated with all-cause mortality and deaths due to cardiac and renal causes following LT. The findings warrant an early preoperative screening procedure to ensure that patients with diabetes have their metabolic risk factors optimized prior to LT.
Keywords: pre-LT diabetes, liver transplant prognosis, metabolic risk factors, STAR-UNOS registry, specific-causes of death
Introduction
Currently, there is a growing incidence of NASH due to the concurrent rise in obesity and metabolic syndromes (1). In particular, insulin resistance and diabetes have been mechanistically linked to the growth of NASH in the United States (2). From a physiologic level, the development of NASH is spurred by hormonal disruptions and metabolic irregularities in the liver that are caused by the desensitization of hepatic insulin receptors and insulin-mediated pathways (3), which result in dysregulated lipogenesis and steatotic changes (4). Once steatosis and hepatic inflammation occur, fibrosis can ensue and culminate in liver failure and end-stage liver disease (5).
For patients with NASH liver failure and hepatic decompensation, liver transplant can provide curative therapy by replacing the diseased liver (6). This operative maneuver effectively normalizes the hepatic and extrahepatic manifestations of portal hypertension and liver failure and consequently improves patient survival (7). However, with the high prevalence of diabetes found in these patients, it is important to consider the short and long-term effects of diabetes on post-LT patient survival and surgical complication risks (8). While the prognostic link between pre-LT diabetes and mortality risks has been ascertained from prior studies (9), the connection between pre-LT diabetes and post-LT cardiovascular outcomes is less known. This is critical to evaluate, given that the association of diabetes with a significantly elevated risk of developing myocardial infarction and other adverse cardiovascular outcomes is well established in non-transplant literature (10, 11). Given these observations, it is plausible that a similar mechanistic relationship can be present in patients who undergo LT, with the preoperative presence of diabetes mediating post-LT cardiovascular outcomes.
Therefore, in this study, we used the UNOS US-transplant database to evaluate the clinical implications of recipient diabetes on post-LT outcomes, specifically evaluating mortality due to cardiovascular causes.
Methods
Database
The UNOS STAR database is a compilation of patient data aggregated from the UNOS data. This data connects cases to patient outcomes and other longitudinal follow-up information. This study utilizes the UNOS STAR database and patient access files for analysis. The database contains health information gathered from the UNOS registry from 2005 to June 2019. It is rigorously maintained in confidentiality and de-identification status through safety mechanisms shared DUA-agreements, and the Health Resources and Services Administration Contract 234–2005-370011C. The content of this study is the responsibility of the authors alone and does not necessarily reflect the view or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Study population and variables
This study used a stepwise and iterative approach to select patients from a predetermined raw sum, isolating the discrete NASH population without significant confounders or comorbidities. The final study population was then stratified using the presence of pre-LT diabetes, which was defined using the presence of diabetes present prior to LT. Various covariates and medical conditions were included to define the baseline characteristics of the study population, and the variables included the patient demographics, etiologic causes of liver disease, immunosuppressant medications, relevant hepatic biomarkers, critical care and life-supporting assistive devices, and variables pertaining to donor characteristics.
For the study endpoints, the primary outcomes encompassed all-cause mortality (as a composite sum of causes of deaths due to various etiologies) and graft failure. The secondary endpoints delineated the etiologic reasons for a patient’s death using the specific causes encoded in the database, and the causes of death included deaths due to cardiovascular reasons, deaths due to renal causes, and deaths due to graft rejection. All endpoints were derived from UNOS, including the outcome variables as well as the censoring (negative) events.
Statistical methods
The baseline characteristics were summarized using either mean-based or nominal-based statistics, which included Fisher’s or Chi-square comparisons. The non-nominal variables were evaluated for kurtosis and skewness/parametricity and were further analyzed using either parametric or non-parametric testing with the Whitney U test. Iterative models were built using the comorbidity set into several sequential iterations (a variant of forward selection method), from which multivariate cox regression was performed using predefined outcome variables as the regression endpoints or dependent variables. The iterations included the following models: model 1 – unadjusted; model 2 – adjusted with the inclusion of age, gender, race, and BMI, model 3 – additional inclusion of comorbidities, functional status, live etiologies, and medications data (both induction and maintenance data), model 4 - additional inclusion of with biomarker data, and model 5 – additional inclusion of donor information. Standard 95% CI two-tail confidence intervals with p-values of 0.05 were used to indicate statistical significance. For the all-cause mortality endpoint, Kaplan-Meier survival and hazard-event analyses were performed to derive the log-rank statistics, using the prespecified strata to evaluate the comparative outcomes. Furthermore, additional subanalyses were performed using strata-specific constructions of subgroups as determined by various relevant and potentially meaningful patient characteristics. These included a series of strata-specific analyses that pertained to recipient age (categorized as a nominal selection of increasing age-intervals), recipient gender, and recipient race. Furthermore, to estimate the relevance of longevity of survival on the prognostic relationship between pre-LT diabetes and outcomes, strata-specific analyses were performed using the survival time periods with the following thresholds: <1 year, 1–5 years, >5 years. Also, given that the definition of NASH continued to evolve over the last two decades, the annualized timepoints of the transplant dates were used to segregate the cohort into the following strata: those who underwent LT between the years 2005–2009, 2010–2014, and 2015–2019.
As for the competing risk analysis (graft failure versus mortality), competing risk regression using the proportional sub-distribution hazards model, delineated in the cumulative incidence function by Fine and Gray (14), was used to expose the regression representations for each hazard. This was also iterated using the sequential iterative modeling mentioned above, spanning from the unadjusted model to model 5. Interactions were evaluated using interaction plots prior to regression analyses. Aside from exclusionary variables (i.e., endpoints), variable terms were graphically analyzed to assess missingness patterns and consequently underwent multiple imputation procedures via multiple imputations using chained equations (MICE) method to improve the statistical power and viability (15).
All tests were conducted using RStudio version 1.2.5042 with R code version 3.6.3.
Results
Patient Selection
A series of inclusion and exclusion criteria were applied to the data cohort to finalize a selection of patients eligible for the study; in this process, the initial selection of patients comprised of a total 99987 patients registered in the UNOS-STAR data, who received their LT from the years 2005 to 2019. Following, those who either were loss to follow-up (n=3445) or had undergone retransplantation (n=4310) were excluded. Furthermore, those with impossible biological values (ie creatinine < 0) (n=5), those under the age of 18 (n=7538) (12, 13), those with grafts from non-heart beating donors (n=4500), those with living donor transplantation (n=3116), those with partial and incomplete grafts (non-whole liver transplantations) (n=1030), those with multi-organ transplants (n=7174), and those with hepatocellular carcinoma as a primary indication for LT (n=16519) were excluded. Lastly, recipients without the registration/admission diagnosis of NASH were excluded (n=37775) to result in a final selection of patients with NASH undergoing LT (n=14575). The configuration of the selection process and the final representation of the study populations is shown in Figure 1.
Figure 1: Patient Selection.
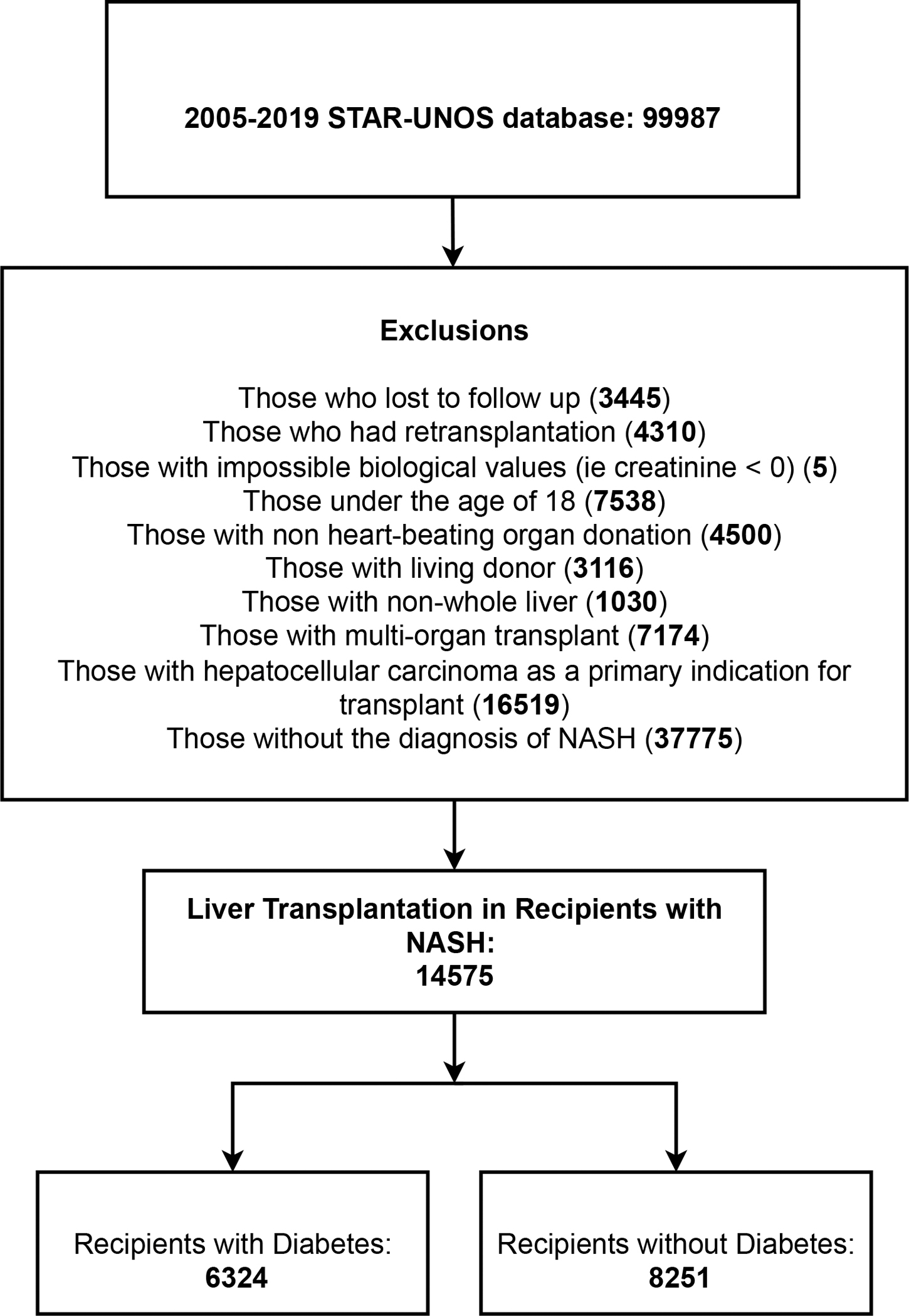
This figure describes the patient selection process for the study.
Baseline characteristics
After excluding patients who did not meet the eligibility criteria, there were a total of 14575 patients who were included, of which there were 6324 with diabetes and 8251 without diabetes. The median time period for follow-up was 3.07 (25–75% IQR: 1–7.05) years, with the median time for diabetic recipients being 3.01 (25–75% IQR: 0.99–6.91) years and the median time for non-diabetic recipients being 3.27 (25–75% IQR: 1.02–7.45) years.
The recipients with prior history of diabetes were older (58.80 ± 8.31 vs. 54.50 ± 11.10 years, p<0.001) and were more likely to be Hispanic or Asian compared to other racial/ethnic groups (White: 77.90 vs. 78.60 %; Black: 3.62 vs. 5.16 %; Hispanic: 14.70 vs. 12.80 %; Asian: 2.36 vs. 2.17 %; other 1.44 vs. 1.31%, p<0.001). Furthermore, recipients with diabetes exhibited higher BMI than the non-diabetics (31.10 ± 5.99 vs. 29.70 ± 6.39 kg/m2, p<0.001). Lastly, there was no difference in the gender distributions of the two groups (58.10 vs. 56.90 %, p=0.170).
In terms of additional liver diseases, recipients with diabetes were more likely to have a concurrent diagnosis of hepatitis B (4.06 vs. 5.47%, p<0.001) as well as alcoholic liver disease (27.00 vs. 33.90%, p<0.001) than those without diabetes. All patients in both the study and control groups had cirrhosis as an underlying etiology. In terms of other liver-related variables, recipients with diabetes were more likely to have undergone pre-LT TIPS (14.20 vs 9.73%, p<0.001), but had lower MELD scores (22.90 ± 8.84 vs 24.90 ± 8.96, p<0.001) than the non-diabetics. There were no differences in the severity of ascites (absent: 14.00 vs. 13.80 %; slight: 47.20 vs 47.80 %; moderate: 38.00 vs 38.30 %; p=0.750) and encephalopathy (none: 24.50 vs. 25.30 %; 1–2: 62.20 vs. 61.20 %; 3–4: 13.30 vs. 13.50%; p=0.400) between the groups. Immunosuppressant use was also not different, with diabetics and nondiabetics utilizing the following medications: mycophenolate mofetil (MMF) (82.00 vs. 81.50%, p=0.460); cyclosporine (5.30 vs. 4.79%, p=0.170); tacrolimus (90.00 vs. 90.60 %, p=0.200); sirolimus (2.02 vs. 1.62, p=0.082); and steroids (90.70 vs. 91.20 %, p=0.370).
When evaluating the biomarkers, the diabetic recipients, compared to non-diabetics, had higher levels of albumin (3.07 ± 0.71 vs. 3.03 ± 0.73 mg/dL, p<0.001) and creatinine (1.65 ± 1.13 vs. 1.54 ± 1.09 mg/dL, p<0.001), but lower INR (1.93 ± 0.86 vs. 2.18 ± 1.21, p<0.001) and total bilirubin (8.30 ± 10.50 vs. 10.90 ± 11.30, p<0.001). Considering donor demographics, the diabetic recipients were more likely to receive grafts from donors who were older (44.20 ± 17.30 vs. 42.80 ± 16.90 years, p<0.001) with higher BMI (28.30 ± 6.88 vs. 28.00 ± 6.56 kg/m2, p=0.037); however, there were no differences in donor gender (59.30 vs. 58.90 %, p=0.630) or race (White: 65.40 vs. 65.40 %; Black: 18.90 vs. 18.50 %; Hispanic: 11.20 vs. 12.00 %; Asian: 2.26 vs. 2.33 %; other 2.18 vs. 1.78%; p=0.230).
Clinical outcomes
When evaluating the primary endpoints, recipients with diabetes had a higher incidence rate of all-cause mortality (61.68 per 1000 person-years, 95% CI 58.81–64.65) than those without diabetes (47.80 per 1000 person-years, 95% CI 45.67–50); likewise, when determining the crude case incidence rates, those with diabetes had a higher incidence rate of graft failure (9.52 per 1000 person-years, 95% CI 8.38–10.76) than those without diabetes (8.76 per 1000 person-years, 95% CI 7.84–9.75). Upon using the iterative cox regression analysis with an escalating number of variable terms in each model, the final models for the primary endpoints demonstrated following prognostic relationships with recipient diabetes: all-cause mortality (FM [final model]: p<0.001, aHR 1.19 95%CI 1.11–1.27) and graft failure (FM: p=0.306, aHR 1.10 95%CI 0.92–1.30). In the final model, both the recipient and donor variables were included in order to adjust for confounders, and the variables included demographics, etiologies and comorbidities, medications, biomarkers, and donor information as part of the model multivariate term. Figure 2 demonstrates the cumulative hazard curves for those with pre-LT diabetes compared to those without diabetes using all-cause mortality and graft failure as the primary endpoint. These curves show that those with pre-LT diabetes are at a higher cumulative risk for all-cause mortality throughout the follow-up time period. In contrast, the overlap between the curves for graft failure demonstrates a non-elevation in the cumulative risks between the groups. The Supplementary Figures 1 and 2 demonstrates the summarized multivariate forest plot using the pre-LT diabetes as risk variable and either all-cause mortality or graft failure as the primary endpoint.
Figure 2: Cumulative Hazards of All-Cause Death and Graft Failure.
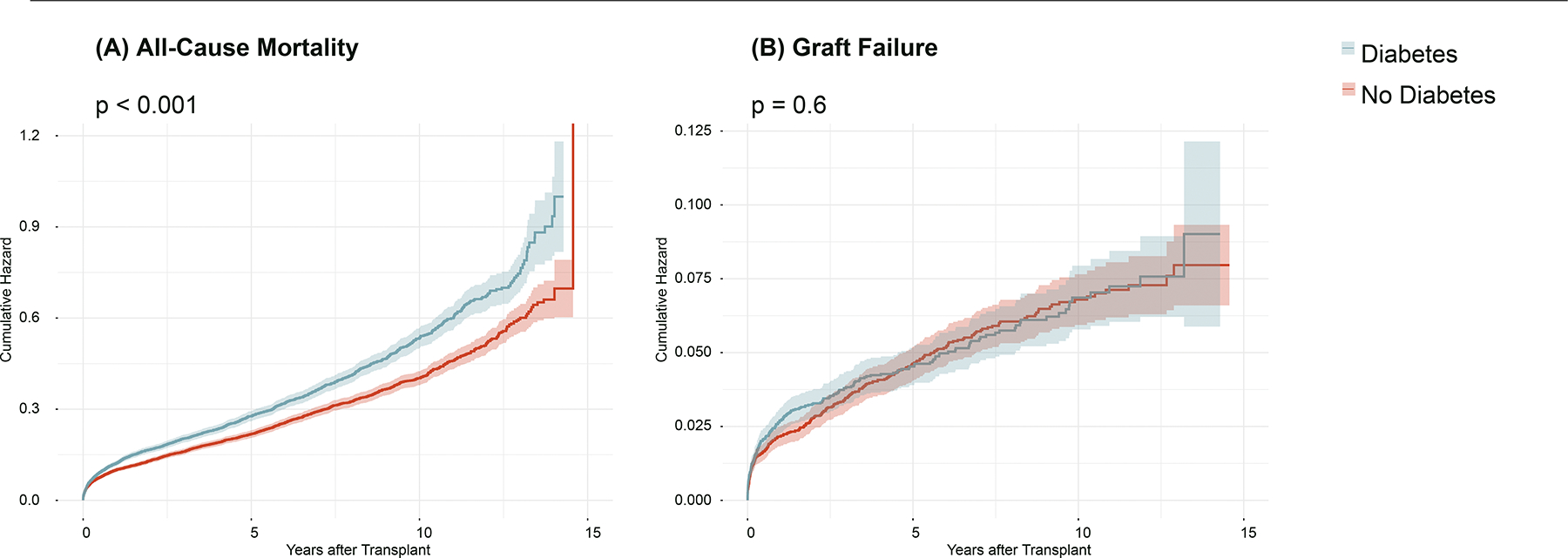
This figure demonstrates the post-transplant cumulative hazards of (A) all-cause mortality and (B) graft failure in patients with and without diabetes. The p-value indicates the respective log-rank p-value for each curve.
When evaluating secondary outcomes using the various causes of death, we found the following mortality-specific endpoints to demonstrate different case-incidence rates as well as a prognostic relationship with pre-LT diabetes; first, the pre-LT diabetes was shown to demonstrate a higher case incidence rate (11.33 per 1000 person-years, 95% CI 10.09–12.68 vs. 7.99 per 1000 person-years, 95% CI 7.11–8.94) and higher risk (p=0.014, aHR 1.24 95% CI 1.04–1.46) with deaths due to cardiac causes. For deaths due to renal causes, those with diabetes had higher case-incidence rates (3.55 per 1000 person-years, 95% CI 2.87–4.34 vs. 2.28 per 1000 person-years, 95% CI 1.83–2.82) as well as higher risk (p=0.039, aHR 1.38 95% CI 1.02–1.87). Interestingly, those with diabetes had lower case-incidence rates (0.49 per 1000 person-years, 95% CI 0.26–0.84 vs. 1.11 per 1000 person-years 95% CI 0.80–1.51) as well as a lower risk (p=0.010, aHR 0.42 95% CI 0.21–0.81) for deaths due to graft rejection. Specifically for the cardiovascular endpoint, the subanalyses that used the survival-time stratified strata showed the cardiovascular risks to climatically increase in the cohort with the longest-survival intervals (>5 years), compared to the moderate (1–5 years) and short-interval surviving cohorts (<1 year). This is delineated in the Supplementary Tables section below.
Supplementary Tables
Additional analyses using the Fine-Gray sub-distribution hazard model with competing risks are demonstrated in Supplementary Table 1. These models used either all-cause mortality or graft failure as the primary endpoint, while setting the alternative outcome as a competing risk, thus accounting for the sub-distribution probability of developing the competing risk in the models. Supplementary Table 2 demonstrates corresponding incidence rates of primary and secondary endpoints per 1000 person-years. Supplementary Tables 3 - 5 demonstrate the baseline characteristics of the study population from 3 quadrennial time periods: years 2005–2009, 2010–2014, and 2015–2019. In supplementary tables 6–8, sequential regressions of primary and secondary endpoints for each quadrennial sub-group are shown. Supplementary tables 9–11 demonstrate endpoints of the study cohort stratified into 3 groups by the longevity of patient survival: <1 year of survival, 1–5 years of survival, and >5 years of survival. Supplementary tables 15–16 describe gender-stratified sequential iterations. Supplementary tables 17–19 demonstrate race-stratified sequential iterations.
Discussion
The results of the study show that the presence of pre-LT diabetes is associated with increased recipient all-cause mortality as well as deaths due to cardiac and renal causes. Prior single-center and institutional studies also corroborate the current findings and demonstrate that recipients with diabetes develop a higher risk for adverse all-cause mortality and other immediate and subacute post-LT complications (16, 17). However, we expand upon prior studies by including additional organ-specific causes of death as endpoints and evaluating them using the successive Cox iteration analysis, which controls for both recipient and donor covariates in order to enhance the precision of the statistical outputs.
The relationship between diabetes and adverse post-LT outcomes in NASH patients may be explained by the different mechanisms by which diabetes affects recovery. For instance, it is known from surgical literature that diabetes affects the microvasculature and reduces perfusion of capillaries around the surgical site (18), leading to a delayed wound healing response that potentiates post-operational wound complications (19, 20). Furthermore, pre-LT diabetes is known to generate atherosclerotic changes in the coronaries (21) that render the patient susceptible to adverse cardiac events (22); this correlation was evident in the current study that showed the recipients with pre-LT diabetes to experience heightened rates of deaths due to cardiovascular causes (23). Novel to the current study, the evolution of cardiovascular risk appears to apex in the subgroup with the longest follow-up time period, which appears to indicate that this risk gradually reaches a climactic point in the post-LT period, alternative to other postsurgical complications that may manifest immediately during the peri and postoperative phases. This further reinforces the idea that atherosclerotic formations in the coronaries that occur due to pre-LT diabetes, are a gradual process that requires steady growth and maturation, albeit the administration of immunosuppressants may accelerate the transformative process. Similar to the microvascular effects of pre-LT diabetes on the coronary vessels, the presence of pre-LT diabetes is likely to affect the renal vasculature via attacking and degrading the integrity of the renal capillaries (24), which would provide a headway to further destruction and disruption of the renal glomeruli and its functions (25). These types of chronic microvascular changes are likely to undermine the recipient renal functions following LT, which was partially indicated by the recipients with diabetes exhibiting higher levels of creatinine (this presupposes that microvascular damage had likely already occurred) (26). It is quite likely that the escalation of risks evidenced in the cardiovascular and renal mortality endpoints contributed toward a higher general risk of all-cause mortality found in pre-LT diabetic populations. Interestingly, recipient diabetes had minimal effects on the graft itself, and paradoxically, those with diabetes had lower rates of death due to graft rejection. While further studies are needed, we can postulate that the presence of diabetes in the recipient confers an immunosuppressive effect on host immunity (27) that prospectively delays or mitigates the immune response following LT. Prior studies have largely elucidated the various mechanisms of diabetes and how it affected the immune system, which included diminishing the phagocytic activities of macrophages, inhibiting the normal transition and chemotaxis of immune cells, disrupting the intercellular signaling cascades, as well as suboptimizing the pathogen recognition and binding (28, 29). It is likely that these immunologic manifestations of diabetes reduced the mortality risks that are associated with graft complications, as it was observed in the current study.
Overall, the presence of pre-LT diabetes is a risk factor for adverse post-LT outcomes, including all-cause mortality and deaths due to cardiac and renal causes. Given the current finding, this warrants screening of NASH candidates who are pending LT for diabetes. After diagnosing and classifying the severity of diabetes, preoperative strategies should be implemented to attenuate the metabolic risks (30). Such measures may include multidisciplinary efforts with the endocrinology team in tirating antidiabetic medications and providing insulin therapy to reduce insulin resistance and improve metabolic parameters. In addition, further investigative efforts should be made to screen these patients of their cardiac and renal statuses prior to LT, so that cardiac or renal disorders can be amended using appropriate interventional treatments (31). Following LT, patients should follow up longitudinally with surgical and endocrine specialists for proper titration of antidiabetic medications and screening for post-LT complications (32).
The study may be limited by the current lack of data regarding the severity of recipient diabetes (30) since the data is not available in the original dataset. Thus, further prospective studies that stratify the diabetic severity using identifiable and definable metabolic parameters are needed to characterize the relationship between the severity of diabetes and the outcomes. Furthermore, in the patient selection process, those with a primary diagnosis of hepatocellular carcinoma (as an indication for LT) were excluded, which omitted those with a probable secondary or non-primary diagnosis of hepatocellular carcinoma from this exclusionary process. Lastly, as with other retrospective studies, the application of inclusion/exclusion criteria to the selection of the study population predisposes the study to selection bias.
Conclusion
The presence of pre-LT diabetes is associated with increased deaths and higher incidence rates in all-cause mortality, cardiovascular and renal causes after LT.
Supplementary Material
Supplementary Figure 1. Multivariate forest plot for all-cause mortality. This figure represents the multivariate forest plot using all-cause mortality as the primary endpoint and a list of covariates (including the exposure of pre-LT diabetes) as independent variables in the model set.
Supplementary Figure 2. Multivariate forest plot for graft failure. This figure represents the multivariate forest plot using graft failure as the primary endpoint and a list of covariates (including the exposure of pre-LT diabetes) as independent variables in the model set.
Table 1:
Baseline Characteristics of Liver Transplant Recipients Stratified Using Recipients Diabetes
| With Diabetes | Without Diabetes | ||
|---|---|---|---|
| Recipient Variables | n = 6324 (43.39 %) | n = 8251 (56.61 %) | p-value |
|
| |||
| Recipient Demographics | |||
| Age, mean ± SD, y | 58.50 (8.31) | 54.50 (11.10) | < 0.001 |
| Male sex, n (%) | 58.10 (%) | 56.90 (%) | 0.170 |
| Race, n (%) | < 0.001 | ||
| White | 77.90 (%) | 78.60 (%) | |
| Black | 3.62 (%) | 5.16 (%) | |
| Hispanic | 14.70 (%) | 12.80 (%) | |
| Asian | 2.36 (%) | 2.17 (%) | |
| Other | 1.44 (%) | 1.31 (%) | |
| BMI, mean ± SD, kg/m2 | 31.10 (5.99) | 29.70 (6.39) | < 0.001 |
| Comorbidities | |||
|
| |||
| Hepatitis B, n (%) | 4.06 (%) | 5.47 (%) | |
| Hepatitis C, n (%) | 14.50 (%) | 15.00 (%) | |
| Alcoholic Liver Disease, n (%) | 27.00 (%) | 33.90 (%) | |
| Hepatic Variables | |||
|
| |||
| Ascites, n (%) | 0.750 | ||
| Absent | 14.00 (%) | 13.80 (%) | |
| Slight | 47.20 (%) | 47.80 (%) | |
| Moderate | 38.80 (%) | 38.30 (%) | |
| Encephalopathy, n (%) | 0.400 | ||
| None | 24.50 (%) | 25.30 (%) | |
| 1–2 | 62.20 (%) | 61.20 (%) | |
| 3–4 | 13.30 (%) | 13.50 (%) | |
| TIPS Procedure, n (%) | 14.20 (%) | 9.73 (%) | < 0.001 |
| MELD Scores, mean ± SD | 22.90 (8.84) | 24.90 (8.96) | < 0.001 |
| Immunosuppressants | |||
|
| |||
| Mycophenolate Mofetil, n (%) | 82.00 (%) | 81.50 (%) | 0.460 |
| Cyclosporine, n (%) | 5.30 (%) | 4.79 (%) | 0.170 |
| Tacrolimus, n (%) | 90.00 (%) | 90.60 (%) | 0.200 |
| Sirolimus, n (%) | 2.02 (%) | 1.62 (%) | 0.082 |
| Steroids, n (%) | 90.70 (%) | 91.20 (%) | 0.370 |
| Laboratory Markers | |||
|
| |||
| Albumin (mg/dL) | 3.07 (0.71) | 3.03 (0.73) | < 0.001 |
| Creatinine (mg/dL) | 1.65 (1.13) | 1.54 (1.09) | < 0.001 |
| INR | 1.93 (0.86) | 2.18 (1.21) | < 0.001 |
| Total Bilirubin (mg/dL) | 8.30 (10.50) | 10.90 (11.30) | < 0.001 |
| Critical Care and Life Support | |||
|
| |||
| Artificial liver devices, n (%) | 0.03 (%) | 0.05 (%) | 0.700 |
| Primary inotropic agent, n (%) | 0.770 | ||
| dobutamine, n (%) | 2.10 (%) | 2.11 (%) | |
| dopamine, n (%) | 16.20 (%) | 16.30 (%) | |
| epinephrine, n (%) | 0.89 (%) | 1.12 (%) | |
| levophed, n (%) | 16.50 (%) | 16.00 (%) | |
| neosynephrine, n (%) | 15.90 (%) | 15.60 (%) | |
| none, n (%) | 46.10 (%) | 46.60 (%) | |
| other, n (%) | 2.29 (%) | 2.13 (%) | |
| Secondary inotropic agent, n (%) | 0.980 | ||
| dobutamine, n (%) | 0.49 (%) | 0.56 (%) | |
| dopamine, n (%) | 1.11 (%) | 1.09 (%) | |
| epinephrine, n (%) | 0.54 (%) | 0.64 (%) | |
| levophed, n (%) | 3.38 (%) | 3.35 (%) | |
| neosynephrine, n (%) | 4.78 (%) | 4.78 (%) | |
| none, n (%) | 88.10 (%) | 87.90 (%) | |
| other, n (%) | 1.64 (%) | 1.73 (%) | |
| Ternary inotropic agent, n (%) | 0.440 | ||
| dobutamine, n (%) | 0.16 (%) | 0.16 (%) | |
| dopamine, n (%) | 0.06 (%) | 0.08 (%) | |
| epinephrine, n (%) | 0.24 (%) | 0.11 (%) | |
| levophed, n (%) | 0.47 (%) | 0.39 (%) | |
| neosynephrine, n (%) | 0.51 (%) | 0.51 (%) | |
| none, n (%) | 98.20 (%) | 98.20 (%) | |
| other, n (%) | 0.38 (%) | 0.52 (%) | |
| ICU admission, n (%) | 13.93 (%) | 17.28 (%) | |
| Ventilator Support, n (%) | 4.63 (%) | 6.53 (%) | < 0.001 |
| Donor Demographics | |||
|
| |||
| Donor Age, mean ± SD, y | 44.20 (17.30) | 42.80 (16.90) | < 0.001 |
| Donor Male sex, n (%) | 59.30 (%) | 58.90 (%) | 0.630 |
| Donor Race, n (%) | 0.230 | ||
| White | 65.40 (%) | 65.40 (%) | |
| Black | 18.90 (%) | 18.50 (%) | |
| Hispanic | 11.20 (%) | 12.00 (%) | |
| Asian | 2.26 (%) | 2.33 (%) | |
| Other | 2.18 (%) | 1.78 (%) | |
| Donor BMI, mean ± SD, kg/m2 | 28.30 (6.88) | 28.00 (6.56) | 0.037 |
| Donor Laboratory Markers | |||
|
| |||
| Donor Creatinine, mean ± SD, mg/dL | 1.72 (1.78) | 1.70 (1.79) | 0.240 |
| Donor Total Bilirubin, mean ± SD, mg/dL | 0.91 (0.94) | 0.90 (0.86) | 0.770 |
Assistance variable was created through combining the multileveled activity and functional status scales into three dependence categories.
Table 2:
Sequential Cox Regression Analysis Using Diabetes as a Prognostic Risk Factor for All-Cause Mortality, Graft Failure, and Specific Causes of Death
|
All-cause Mortality
|
Graft Failure
|
||||||
| Sequential Cox Regressions | Sequential Cox Regressions | ||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI |
| 1 | < 0.001 | 1.21 | (1.13 – 1.29) | 1 | 0.49 | 1.06 | (0.90 – 1.26) |
| 2 | < 0.001 | 1.19 | (1.12 – 1.28) | 2 | 0.58 | 1.05 | (0.88 – 1.25) |
| 3 | < 0.001 | 1.18 | (1.10 – 1.26) | 3 | 0.57 | 1.05 | (0.88 – 1.25) |
| †FM | < 0.001 | 1.18 | (1.10 – 1.26) | †FM | 0.61 | 1.05 | (0.88 – 1.25) |
|
Death due to General Cardiac Causes
|
Death due to Graft Rejection
|
||||||
| Sequential Cox Regressions | Sequential Cox Regressions | ||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI |
| 1 | 0.02 | 1.22 | (1.04 – 1.43) | 1 | 0.02 | 0.47 | (0.25 – 0.89) |
| 2 | 0.02 | 1.23 | (1.04 – 1.44) | 2 | 0.02 | 0.47 | (0.25 – 0.89) |
| 3 | 0.02 | 1.21 | (1.03 – 1.43) | 3 | 0.02 | 0.45 | (0.24 – 0.85) |
| †FM | 0.03 | 1.21 | (1.02 – 1.42) | †FM | 0.02 | 0.46 | (0.24 – 0.87) |
|
Death due to General Renal Causes
|
|||||||
| Sequential Cox Regressions | |||||||
| Model | p-value | aHR | 95% CI | ||||
| 1 | 0.03 | 1.39 | (1.03 – 1.88) | ||||
| 2 | 0.03 | 1.39 | (1.03 – 1.88) | ||||
| 3 | 0.06 | 1.34 | (0.99 – 1.81) | ||||
| †FM | 0.06 | 1.34 | (0.99 – 1.82) | ||||
FM indicates Final Model
Model 1 includes VOI (variable of interest) and demographics, model 2 includes VOI, demographics, comorbidities, and medications, model 3 includes VOI, demographics, comorbidities, medications, and biomarkers, model 4 includes VOI, demographics, comorbidities, medications, biomarkers, and donor information
Funding:
This study was funded by NIH NIDDK T32 DK067872-17.
Footnotes
Conflict of Interest: The authors of this manuscript certify they share no affiliation or involvement with any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. None declared.
Animal Research (Ethics): This study did not involve animals.
Consent to Participate (Ethics): As this study used a publicly available registry, there was no consent procedure required in the study as no candidates were enrolled.
Consent to Publish (Ethics): On behalf of the authors, I, the corresponding author, give consent to the publishers to publish the manuscript as a journal article.
Plant Reproducibility: The methodologies contained and delineated in the manuscript outlines the study design and the data collection methods implemented in the paper, in order that the reproducibility of the paper can be optimized.
Clinical Trials Registration: This paper did not involve a clinical trial.
Data Availability:
Scientific data used in the study is available upon reasonable request to the corresponding author.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 2.Khan RS, Bril F, Cusi K, Newsome PN. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;70(2):711–724. [DOI] [PubMed] [Google Scholar]
- 3.Kumashiro N, Erion DM, Zhang D, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2011;108(39):16381–16385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wree A, Schlattjan M, Bechmann LP, et al. Adipocyte cell size, free fatty acids and apolipoproteins are associated with non-alcoholic liver injury progression in severely obese patients. Metabolism. 2014;63(12):1542–1552. [DOI] [PubMed] [Google Scholar]
- 5.Haflidadottir S, Jonasson JG, Norland H, et al. Long-term follow-up and liver-related death rate in patients with non-alcoholic and alcoholic related fatty liver disease. BMC Gastroenterol. 2014;14:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555. [DOI] [PubMed] [Google Scholar]
- 7.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5(2):307–313. [DOI] [PubMed] [Google Scholar]
- 8.Tsai MS, Wang YC, Wang HH, Lee PH, Jeng LB, Kao CH. Pre-existing diabetes and risks of morbidity and mortality after liver transplantation: A nationwide database study in an Asian population. Eur J Intern Med. 2015;26(6):433–438. [DOI] [PubMed] [Google Scholar]
- 9.Hoehn RS, Singhal A, Wima K, et al. Effect of pretransplant diabetes on short-term outcomes after liver transplantation: a national cohort study. Liver Int. 2015;35(7):1902–1909. [DOI] [PubMed] [Google Scholar]
- 10.Emerging Risk Factors Collaboration, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anand SS, Dagenais GR, Mohan V, et al. Glucose levels are associated with cardiovascular disease and death in an international cohort of normal glycaemic and dysglycaemic men and women: the EpiDREAM cohort study. Eur J Prev Cardiol. 2012;19(4):755–764. [DOI] [PubMed] [Google Scholar]
- 12.Steggerda JA, Kim IK, Todo T, Malinoski D, Klein AS, Bloom MB. Liver Transplant Survival Index for Patients with Model for End-Stage Liver Disease Score ≥ 35: Modeling Risk and Adjusting Expectations in the Share 35 Era. J Am Coll Surg. 2019;228(4):437–450.e8. [DOI] [PubMed] [Google Scholar]
- 13.Xing M, Kim HS. Independent prognostic factors for posttransplant survival in hepatocellular carcinoma patients undergoing liver transplantation. Cancer Med. 2017;6(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC, Steyerberg EW, Putter H. Fine-Gray subdistribution hazard models to simultaneously estimate the absolute risk of different event types: Cumulative total failure probability may exceed 1. Stat Med. 2021;40(19):4200–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4(2):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuelson AL, Lee M, Kamal A, Keeffe EB, Ahmed A. Diabetes mellitus increases the risk of mortality following liver transplantation independent of MELD score. Dig Dis Sci. 2010;55(7):2089–2094. [DOI] [PubMed] [Google Scholar]
- 17.Volk ML, Hernandez JC, Lok AS, Marrero JA. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transpl. 2007;13(11):1515–1520. [DOI] [PubMed] [Google Scholar]
- 18.Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89(4):1269–1339. [DOI] [PubMed] [Google Scholar]
- 19.Palladino R, Tabak AG, Khunti K, et al. Association between pre-diabetes and microvascular and macrovascular disease in newly diagnosed type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alfonso AR, Kantar RS, Ramly EP, et al. Diabetes is associated with an increased risk of wound complications and readmission in patients with surgically managed pressure ulcers. Wound Repair Regen. 2019;27(3):249–256. [DOI] [PubMed] [Google Scholar]
- 21.Dokken BB. The Pathophysiology of Cardiovascular Disease and Diabetes: Beyond Blood Pressure and Lipids. Diabetes Spectr. 2008;21(3):160–165. [Google Scholar]
- 22.Patel KL. Impact of tight glucose control on postoperative infection rates and wound healing in cardiac surgery patients. J Wound Ostomy Continence Nurs. 2008;35(4):397–404; quiz 405–406. [DOI] [PubMed] [Google Scholar]
- 23.Roccaro GA, Goldberg DS, Hwang WT, et al. Sustained Posttransplantation Diabetes Is Associated With Long-Term Major Cardiovascular Events Following Liver Transplantation. Am J Transplant. 2018;18(1):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21(3):273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell MS, Kotlyar DS, Brensinger CM, et al. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl. 2005;11(9):1048–1055. [DOI] [PubMed] [Google Scholar]
- 27.Price CL, Hassi HOSA, English NR, Blakemore AIF, Stagg AJ, Knight SC. Methylglyoxal modulates immune responses: relevance to diabetes. J Cell Mol Med. 2010;14(6B):1806–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev. 2020;16(5):442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front Immunol. 2020;11:1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallia A, Schmidt K, Oakes DJ, et al. Glycemic Control Reduces Infections in Post-Liver Transplant Patients: Results of a Prospective, Randomized Study. J Clin Endocrinol Metab. 2017;102(2):451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye C, Saincher M, Tandon P, et al. Cardiac work-up protocol for liver transplant candidates: experience from a single liver transplant centre. Can J Gastroenterol. 2012;26(11):806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldwin D Jr, Duffin KE. Rosiglitazone treatment of diabetes mellitus after solid organ transplantation. Transplantation. 2004;77(7):1009–1014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Multivariate forest plot for all-cause mortality. This figure represents the multivariate forest plot using all-cause mortality as the primary endpoint and a list of covariates (including the exposure of pre-LT diabetes) as independent variables in the model set.
Supplementary Figure 2. Multivariate forest plot for graft failure. This figure represents the multivariate forest plot using graft failure as the primary endpoint and a list of covariates (including the exposure of pre-LT diabetes) as independent variables in the model set.
Data Availability Statement
Scientific data used in the study is available upon reasonable request to the corresponding author.


