Abstract
Oral squamous cell carcinoma (OSCC) is the 11th most prevalent tumor worldwide. Despite advantages of therapeutic approaches, the 5-year survival rate of patients with OSCC is less than 50%. It is urgent to elucidate mechanisms underlying OSCC progression for developing novel treatment strategies. Our recent study has revealed that Keratin 4 (KRT4) suppresses OSCC development, which is downregulated in OSCC. Nevertheless, the mechanism downregulating KRT4 in OSCC remains unknown. In this study, touchdown PCR was utilized to detect KRT4 pre-mRNA splicing, while m6A RNA methylation was identified by methylated RNA immunoprecipitation (MeRIP). Besides, RNA immunoprecipitation (RIP) was used to determine RNA-protein interaction. Herein, this study indicated that intron splicing of KRT4 pre-mRNA was suppressed in OSCC. Mechanistically, m6A methylation of exon-intron boundaries prevented intron splicing of KRT4 pre-mRNA in OSCC. Besides, m6A methylation suppressed the binding of splice factor DGCR8 microprocessor complex subunit (DGCR8) to exon-intron boundaries in KRT4 pre-mRNA to prohibit intron splicing of KRT4 pre-mRNA in OSCC. These findings revealed the mechanism downregulating KRT4 in OSCC and provided potential therapeutic targets for OSCC.
Keywords: Oral squamous cell carcinoma, Keratin 4, m6A methylation, Pre-mRNA splicing
Introduction
Oral squamous cell carcinoma (OSCC) is the 11th most prevalent tumor worldwide, which accounts for 2–3% of all cancers (Ferlay et al., 2010; Hasegawa et al., 2021). It is estimated that there are around 350,000 new cases and 170,000 deaths from OSCC annually (Bray et al., 2018; Yang et al., 2021). Despite advantages of therapeutic approaches, the 5-year survival rate of patients with OSCC is less than 50% due to high rates of recurrence and metastasis (Bloebaum et al., 2014; Panzarella et al., 2014). Therefore, it is essential to elucidate mechanisms underlying OSCC progression for developing novel treatment strategies.
Keratin 4 (KRT4) is a member of the type II keratin family (Zhang et al., 2018). Previous studies have indicated that KRT4 expression is downregulated in OSCC (Lallemant et al., 2009; Toruner et al., 2004; Ye et al., 2008). Our recent study has also revealed that KRT4 expression is decreased in OSCC cells and KRT4 suppresses autophagy related 4B cysteine peptidase (ATG4B)-mediated autophagy to inhibit OSCC development (Li et al., 2022). Nevertheless, the mechanism downregulating KRT4 mRNA in OSCC remains unknown.
Dysregulation of pre-mRNA splicing would lead to the downregulation of mRNA (Han et al., 2011; Lee & Rio, 2015). Growing evidence has demonstrated that RNA methylation contributes to proper pre-mRNA splicing and subsequent mRNA expression. For instance, N6-methyladenosine (m6A) reader YTH domain containing 1 (YTHDC1) associates with m6A modified pre-mRNA and facilitates exon inclusion during pre-mRNA splicing by recruiting serine and arginine rich splicing factor 3 (SRSF3) whereas blocking SRSF mRNA binding (Xiao et al., 2016). In addition, the m6A methylation of 3′ spice site in S-adenosylmethionine (SAM) synthetase pre-mRNA prevents RNA splicing through inhibiting binding of splicing factor U2 small nuclear RNA auxiliary factor 1 (U2AF1) to the 3′ spice site (Mendel et al., 2021). Yet the role of m6A methylation in KRT4 pre-mRNA splicing has not been reported.
DGCR8 microprocessor complex subunit (DGCR8) is an essential splicing factor for microRNA (miRNA) processing (Guo & Wang, 2019; Michlewski & Caceres, 2019). Besides, DGCR8 is also involved in RNA methylation-mediated miRNA processing. A previous study has indicated that m6A methylation enhances the binding of DGCR8 and primary miR-19a to facilitate miRNA processing in cardiovascular endothelial cell (Zhang et al., 2020a). Except regulating miRNA splicing, DGCR8 contributes to mRNA processing as well. In mouse embryonic stem cells, DGCR8 interacts with transcription factor 7 like 1 (Tcf7l1) pre-mRNA to promote the splicing of Tcf7l1 pre-mRNA (Cirera-Salinas et al., 2017). However, the role of DGCR8 in RNA methylation-mediated KRT4 pre-mRNA splicing is largely unknown.
Therefore, the primary aim of the current study was to investigate the effects of m6A methylation and DGCR8 on KRT4 pre-mRNA splicing in OSCC.
Materials and Methods
Cell culture
Normal oral keratinocytes (NOK) and OSCC cell line HN6 cells were obtained from Cell Bank at the Chinese Academy of Sciences (Shanghai, China) and cultured as previously described (Li et al., 2022).
Cell transfection
Cells were transfected with METTL3, METTL14 or DGCR8 siRNA and siRNA negative control (NC) by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) as previously described (Li et al., 2022). Then cells were collected for following experiments at 48 h after transfection. Sequences of siRNAs were listed in Table 1.
Table 1. Sequences of siRNA used in this study.
| SiRNA | Sequences (5′–3′) | |
|---|---|---|
| METTL3 siRNA | Sense | CACAGAGTGTCGGAGGTGATTC |
| Antisense | CTGTAGTACGGGTATGTTGAGCC | |
| METTL14 siRNA | Sense | GACCTTGGAAGAGTGTGTTTACG |
| Antisense | CTTTGATCCCCATGAGGCAGT | |
| DGCR8 siRNA | Sense | ACAUCUUGGGCUUCUUUCGAG |
| Antisense | CGAAAGAAGCCCAAGAUGUCC | |
| siRNA NC | Sense | UUCUCCGAACGUGUCACGU |
| Antisense | ACGUGACACGUUCGGAGAA |
Bioinformatics analysis
RMBase v2.0 database (https://rna.sysu.edu.cn/rmbase/) and RMVar database (https://rmvar.renlab.org/) were used to explore potential m6A modification sites in or nearby KRT4 pre-mRNA splicing sites. Besides, ENCORI database (https://starbase.sysu.edu.cn/index.php) was utilized to mine RNA-protein interactions.
Touchdown polymerase chain reaction (PCR)
Touchdown PCR was utilized to detect KRT4 pre-mRNA splicing. First, total RNA was extracted from cells by TRIzol reagent (Invitrogen) followed by cDNA synthesis using PrimeScript RT Reagent Kit (Takara, Dalian, Liaoning, China). Subsequently, touchdown PCR was performed in a volume of 50 µL using Phanta Max Buffer (Vazyme, Nanjing, Jiangsu, China) as follows: 95 °C for 3 min, 95 °C for 15 s, 74 °C for 90 s for 5 cycles; 95 °C for 15 s, 72 °C for 90 s for 5 cycles; 95 °C for 15 s, 70 °C for 90 s for 5 cycles; 95 °C for 15 s, 68 °C for 90 s for 25 cycles followed by 68 °C for 5min. Sequences of primers used for touchdown PCR were listed in Table 2.
Table 2. Sequences of primers for PCR used in this study.
| Genes | Sequences (5′–3′) | |
|---|---|---|
| KRT4 pre-mRNA (E1/E2) | Forward | CTCCTCAACAACAAGTTTGCCTC |
| Reverse | CTTTGTCATTGCCCAAGGTATC | |
| KRT4 pre-mRNA (E2/E3) | Forward | AGCCCCTCTTTGAGACCTACC |
| Reverse | TCATTCTCGGCTGCTGTGC | |
| KRT4 pre-mRNA (E3/E4) | Forward | GCACAGCAGCCGAGAATGAC |
| Reverse | TGTTCAGGTAGGCAGCATCCAC | |
| KRT4 pre-mRNA (E4/E5) | Forward | GGATGCTGCCTACCTGAACAAG |
| Reverse | TTGGTCTGGTACAGGGCTTCAG | |
| KRT4 pre-mRNA (E5/E6) | Forward | CGAGGAGATTGCCCAGAGGA |
| Reverse | CAGCCTCTGGATCATCCTGTTG | |
| KRT4 pre-mRNA (E6/E7) | Forward | GATCTCGGTTGACCAACATGG |
| Reverse | TCCTGGTACTCACGCAGCATT | |
| KRT4 pre-mRNA (E7/E9) | Forward | AAGATGCCCACAGCAAGCG |
| Reverse | AGACACTGCCACCAAACCCA |
Quantitative real-time polymerase chain reaction (qRT-PCR)
After RNA extraction and cDNA synthesis, qRT-PCR was performed by the ABI7300 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) using TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) (Takara) as previously described (Li et al., 2022). Sequences of primers used for qRT-PCR were listed in Table 3.
Table 3. Sequences of primers for qRT-PCR used in this study.
| Genes | Sequences (5′–3′) | |
|---|---|---|
| KRT4 pre-mRNA (E2/E3) | Forward | AGCCCCTCTTTGAGACCTACC |
| Reverse | TCATTCTCGGCTGCTGTGC | |
| KRT4 pre-mRNA (E3/E4) | Forward | GCACAGCAGCCGAGAATGAC |
| Reverse | TGTTCAGGTAGGCAGCATCCAC | |
| KRT4 pre-mRNA (E5/E6) | Forward | CGAGGAGATTGCCCAGAGGA |
| Reverse | CAGCCTCTGGATCATCCTGTTG | |
| KRT4-E2(3) | Forward | TGCAACTAATTACGTGGATA |
| Reverse | TTCTTTAGGACCACAAAGTC | |
| KRT4-E3(4) | Forward | AGAGGAGATCAACAAACGCACAG |
| Reverse | AACCCATGACTTCAGCCAAAGA | |
| KRT4-E5(6) | Forward | CCAGATGCAGACCCATGTCAG |
| Reverse | GCTTGAGCTAATGATCACCTGTTC | |
| KRT4 mRNA | Forward | CATTGATCGCTGGGGTTGA |
| Reverse | ATACCCTTGACCGAAGACCG | |
| METTL3 | Forward | CACAGAGTGTCGGAGGTGATTC |
| Reverse | CTGTAGTACGGGTATGTTGAGCC | |
| METTL14 | Forward | GACCTTGGAAGAGTGTGTTTACG |
| Reverse | CTTTGATCCCCATGAGGCAGT | |
| DGCR8 | Forward | CAAGATGCACCCACAAAGAAAG |
| Reverse | GATCCGTAAGTCACACCATCAA | |
| GAPDH | Forward | AACGGATTTGGTCGTATTGGG |
| Reverse | CCTGGAAGATGGTGATGGGAT |
Methylated RNA immunoprecipitation (MeRIP)
NOK and HN6 cells were collected and lysed. Then nucleic acid fragments were interrupted by ultrasound. Next, cell lysate was incubated with 1 µL m6A antibody (1:500, #ab208577, Abcam, Cambridge, MA, USA) overnight at 4 °C. Subsequently, m6A antibody and methylated RNA fragments were captured by avidin magnetic beads, and the level of methylated RNA was detected by qRT-PCR.
RNA immunoprecipitation (RIP)
RIP was performed by RNA-Binding Protein Immunoprecipitation Kit (Merck Millipore, Billerica, MA, USA). Briefly, HN6 cells were collected and lysed. Then nucleic acid fragments were interrupted by ultrasound. Subsequently, cell lysate was incubated with 1 µL METTL3 (#15073-1-AP, Proteintech; Rosemont, IL, USA), METTL14 (#26158-1-AP; Proteintech) or DGCR8 antibody (#60084-1-Ig; Proteintech) at 4 °C overnight. Next, protein-binding RNA fragments were captured by avidin magnetic beads, and the level of protein-binding RNA was identified by qRT-PCR.
Statistical analysis
Quantitative data of the current study were present as mean ± standard deviation (SD) and statistical differences were analyzed by SPSS 20 software (SPSS Inc., Chicago, IL, USA) as previously described (Li et al., 2022). Besides, P < 0.05 was considered as statistically significant.
Results
Intron splicing of KRT4 pre-mRNA is suppressed in OSCC
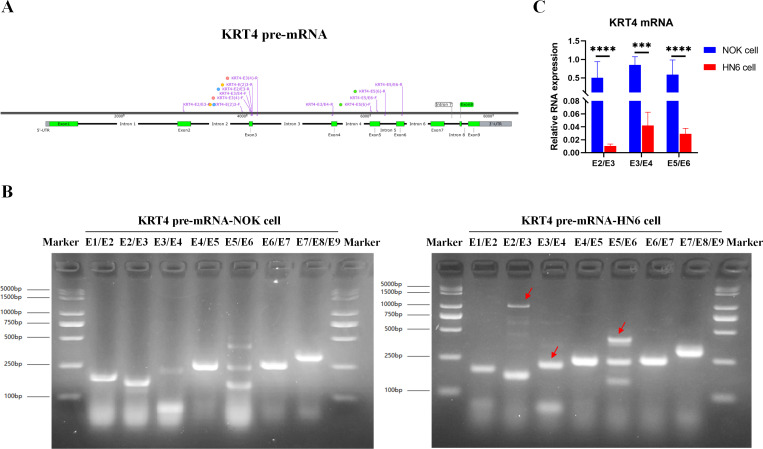
To explore the mechanism downregulating KRT4 in OSCC, splicing of KRT4 pre-mRNA was detected by touchdown PCR. Compared to NOK cell, splicing of intron 2 between exon 2 and 3 (E2/E3), intron 3 between exon 3 and 4 (E3/E4), and intron 5 between exon 5 and 6 in KRT4 pre-mRNA (E5/E6) was inhibited in HN6 cells (Figs. 1A and 1B). Red arrows indicated fragments of KRT4 pre-mRNA containing exon-intron structures (Figs. 1A and 1B). Moreover, results of qRT-PCR confirmed that junctions of exon 2-exon 3 (E2/E3), exon 3-exon 4 (E3/E4) and exon 5-exon 6 (E5/E6) in KRT4 mRNA were dramatically decreased in HN6 cells compared to those in NOK cells, respectively (Fig. 1C). Therefore, these data suggested that intron splicing of KRT4 pre-mRNA was suppressed in OSCC.
Figure 1. Intron splicing of KRT4 pre-mRNA is suppressed in OSCC.
(A) Schematic diagram of KRT4 pre-mRNA. (B) Fragments of KRT4 pre-mRNA containing exon-intron structures or KRT4 mRNA detected by PCR in NOK cells and HN6 cells. Red arrows indicated fragments of KRT4 pre-mRNA containing exon-intron structures. (C) Levels of junctions of exon 2-exon 3 (E2/E3), exon 3-exon 4 (E3/E4) and exon 5-exon 6 (E5/E6) in KRT4 mRNA detected by qRT-PCR in NOK cells and HN6 cells. E, exon. ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
m6A levels of exon-intron boundaries in KRT4 pre-mRNA is increased in OSCC
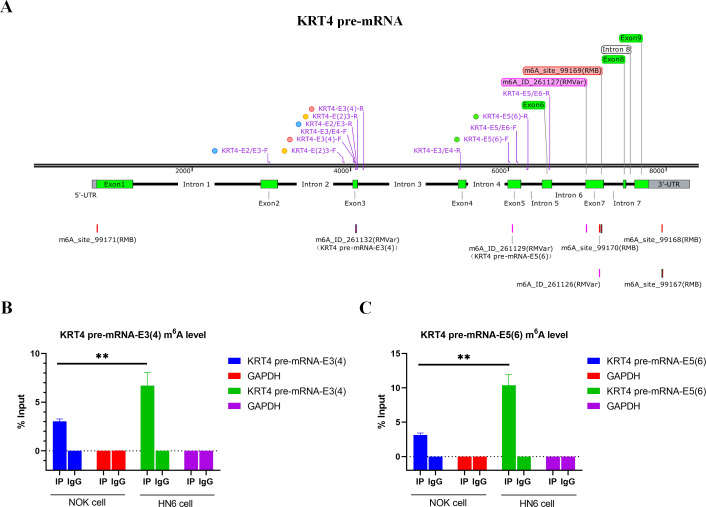
Bioinformatics analysis showed that there were m6A sites in exon 3 (KRT4-E3(4)) and exon 5 (KRT4-E5(6)) of KRT4 pre-mRNA nearby exon-intron boundaries (Fig. 2A). Besides, results of MeRIP found that m6A levels of m6A sites in exon 3 (KRT4-E3(4)) and exon 5 (KRT4-E5(6)) of KRT4 pre-mRNA were significantly elevated in HN6 cell compared to those in NOK cells (Figs. 2B and 2C). Thus, m6A methylation might be involved in the regulation of KRT4 pre-mRNA splicing in OSCC.
Figure 2. m6A levels of exon-intron boundaries in KRT4 pre-mRNA is increased in OSCC.
(A) Potential m6A modification sites in or nearby KRT4 pre-mRNA splicing sites. (B) The m6A level of m6A sites in exon 3 (KRT4-E3(4)) of KRT4 pre-mRNA detected by MeRIP. (C) The m6A level of m6A sites in exon 5 (KRT4-E5(6)) of KRT4 pre-mRNA detected by MeRIP. E, exon. ∗∗P < 0.01.
m6A methylation of exon-intron boundaries prevents intron splicing of KRT4 pre-mRNA in OSCC
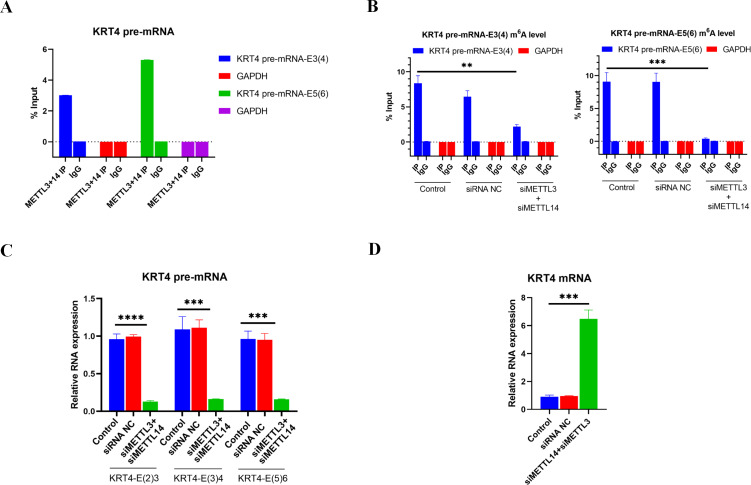
Results of RIP performed by METTL3 and METTL 14 antibodies showed that m6A writers METTL3 and METTL14 associated with m6A sites in exon 3 (KRT4 pre-mRNA-E3(4)) and exon 5 (KRT4 pre-mRNA-E5(6)) of KRT4 pre-mRNA nearby exon-intron boundaries in HN6 cells (Fig. 3A), suggesting that METTL3 and METTL14 should modify m6A levels of m6A sites in exon 3 (KRT4 pre-mRNA -E3(4)) and exon 5 (KRT4 pre-mRNA -E5(6)) of KRT4 pre-mRNA in HN6 cells.
Figure 3. m6A methylation of exon-intron boundaries prevents intron splicing of KRT4 pre-mRNA in OSCC.
(A) Quantification of KRT4 pre-mRNA containing exon-intron structures by qRT-PCR following RIP performed by METTl3 or METTL14 antibody in HN6 cells. (B) The m6A level of m6A sites in exon 3 (KRT4-E3(4)) and exon 5 (KRT4-E5(6)) of KRT4 pre-mRNA detected by MeRIP in HN6 cells. (C) Levels of junctions of intron 2-exon 3 (KRT4-E(2)3), exon 3-intron 3 (KRT4-E(3)4) and exon 5-intron 5 (KRT4-E(5)6) in KRT4 pre-mRNA detected by qRT-PCR in HN6 cells treated with or without METTL3 and METTL14 siRNA. (D) Levels of KRT4 mRNA in HN6 cells treated with or without METTL3 and METTL14 siRNA. NC, negative control; siMETTL3, METTL3 siRNA; siMETTL14, METTL14 siRNA; E, exon. ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
Next, METTL3 and METTL14 were silenced by siRNAs in HN6 cells (Fig. S1) to identify the effect of m6A methylation on intron splicing of KRT4 pre-mRNA in OSCC. Silence of METTL3 and METTL14 decreased m6A levels of m6A sites in exon 3 (KRT4 pre-mRNA -E3(4)) and exon 5 (KRT4 pre-mRNA -E5(6)) of KRT4 pre-mRNA in HN6 cells (Fig. 3B). Then intron splicing of KRT4 pre-mRNA was detected by qRT-PCR in HN6 cells. Results indicated that silence of METTL3 and METTL14 reduced structures of intron 2-exon 3 (KRT4-E(2)3), exon 3-intron 3 (KRT4-E(3)4) and exon 5-intron 5 (KRT4-E(5)6) in KRT4 pre-mRNA (Figs. 1A and 3C), which associated with junctions of exon 2-exon 3, exon 3-exon 4 and exon 5-exon 6 in KRT4 mRNA, respectively. By contrast, silence of METTL3 and METTL14 increased mature KRT4 mRNA level in HN6 cells (Fig. 3D). In addition, siRNA NC had no effect on levels of KRT4 pre-mRNA and mRNA (Figs. 3C and 3D).
Above results suggested that m6A methylation of exon-intron boundaries prevented intron splicing of KRT4 pre-mRNA in OSCC. Besides, these results also revealed that the m6A modification of m6A sites in exon 3 of KRT4 pre-mRNA nearby the exon-intron boundary (KRT4 pre-mRNA-E3(4)) could modify not only intron 2-exon 3 structure (KRT4-E(2)3) but also exon 3-intron 3 structure (KRT4-E(3)4). Therefore, silence of METTL3 and METTL14 by siRNAs could simultaneously reduce intron 2-exon 3 structure (KRT4-E(2)3) but also exon 3-intron 3 structure (KRT4-E(3)4).
m6A methylation inhibits the binding of DGCR8 to exon-intron boundaries in KRT4 pre-mRNA in OSCC
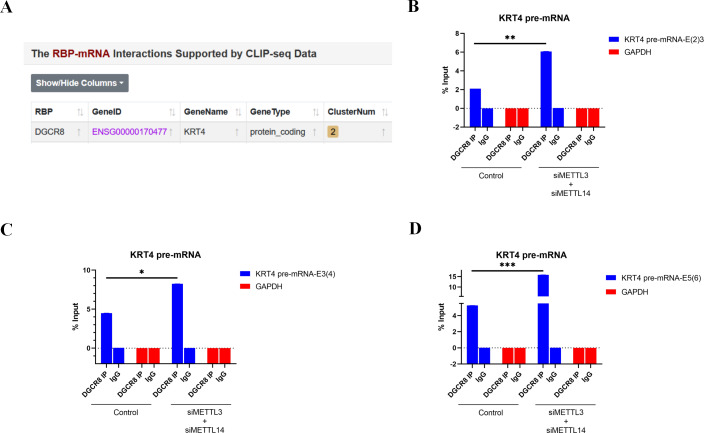
Bioinformatics analysis of CLIP-seq data from ENCORI database further found that splicing factor DGCR8 could bind to KRT4 mRNA in cancer cells (Fig. 4A). Furthermore, results of RIP demonstrated that DGCR8 associated with boundaries of intron 2-exon 3 (KRT4-E(2)3), exon 3-intron 3 (KRT4-E(3)4) and exon 5-intron 5 (KRT4-E(5)6) in KRT4 pre-mRNA in HN6 cells (Figs. 4B–4D), suggesting that DGCR8 might regulate KRT4 pre-mRNA splicing in HN6 cells. Moreover, silence of METTL3 and METTL14 facilitated the binding of DGCR8 to boundaries of intron 2-exon 3 (KRT4-E(2)3), exon 3-intron 3 (KRT4-E(3)4) and exon 5-intron 5 (KRT4-E(5)6) in KRT4 pre-mRNA (Figs. 4B–4D). All these data together suggested that m6A methylation suppressed the binding of DGCR8 to exon-intron boundaries in KRT4 pre-mRNA in OSCC.
Figure 4. m6A methylation inhibits the binding of DGCR8 to exon-intron boundaries in KRT4 pre-mRNA in OSCC.
(A) Potential binding site of DGCR8 in KRT4 mRNA. (B–D) Quantification of KRT4 pre-mRNA containing junctions of intron 2-exon 3 (KRT4-E(2)3, (B), exon 3-intron 3 (KRT4-E(3)4, (C) and exon 5-intron 5 (KRT4-E(5)6, (D) in KRT4 pre-mRNA by qRT-PCR following RIP performed by DGCR8 antibody in HN6 cells. E, exon. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
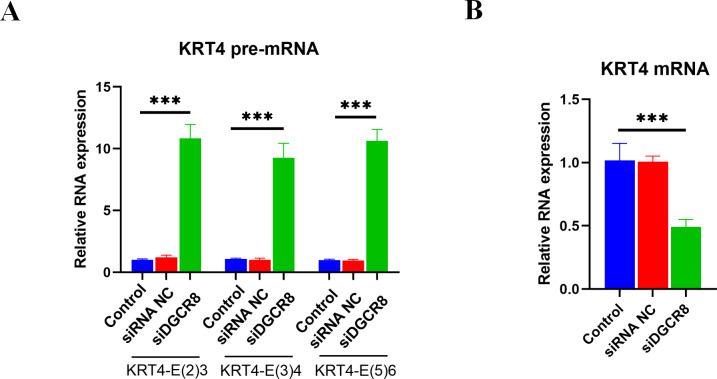
Silence of DGCR8 prohibits intron splicing of KRT4 pre-mRNA in OSCC
Next, the role of DGCR8 in intron splicing of KRT4 pre-mRNA was demonstrated in HN6 cells. Results of qRT-PCR showed that silence of DGCR8 by siRNA (Fig. S1) increased structures of intron 2-exon 3 (KRT4-E(2)3), exon 3-intron 3 (KRT4-E(3)4) and exon 5-intron 5 (KRT4-E(5)6) in KRT4 pre-mRNA (Fig. 5A). In contrast, silence of DGCR8 decreased mature KRT4 mRNA level in HN6 cells (Fig. 5B). Besides, siRNA NC had no effect on levels of KRT4 pre-mRNA and mRNA (Figs. 5A and 5B). Thus, these results indicated that silence of DGCR8 prohibited intron splicing of KRT4 pre-mRNA in OSCC.
Figure 5. Silence of DGCR8 prohibits intron splicing of KRT4 pre-mRNA in OSCC.
(A) Quantification of KRT4 pre-mRNA containing junctions of intron 2-exon 3 (KRT4-E(2)3), exon 3-intron 3 (KRT4-E(3)4) and exon 5-intron 5 (KRT4-E(5)6) in KRT4 pre-mRNA by qRT-PCR in HN6 cells treated with or without DGCR8 siRNA. (B) Levels of KRT4 mRNA in HN6 cells treated with or without DGCR8 siRNA. NC, negative control; siDGCR8, DGCR8 siRNA; E, exon. ∗∗∗P < 0.001.
Discussion
The current study indicated that intron splicing of KRT4 pre-mRNA was suppressed in OSCC. Mechanistically, m6A methylation of exon-intron boundaries prevented intron splicing of KRT4 pre-mRNA in OSCC. Besides, m6A methylation suppressed the binding of DGCR8 to exon-intron boundaries in KRT4 pre-mRNA in OSCC, and silence of DGCR8 prohibited intron splicing of KRT4 pre-mRNA in OSCC.
Inhibition of intron splicing could lead to exon exclusion and subsequent expression of non-functional proteins. For instance, serine and arginine rich splicing factor 2 (SRSF2) prevents intron splicing to reduce exon 7 inclusion within survival of motor neuron (SMA) mRNA to produce non-functional SMA protein (Cho et al., 2015; Kashima et al., 2007; Moon et al., 2017). Besides, heterogenous ribonucleaoprotein C1 (hnRNP C1) facilitates exon inclusion within Ron mRNA through promoting intron 10 splicing (Moon et al., 2019). However, our recent study has indicated that KRT4 mRNA level is decreased in OSCC (Li et al., 2022). Therefore, inhibition of intron splicing in KRT4 pre-mRNA should not lead to expression of non-functional KRT4 protein in OSCC.
In addition, suppression of intron splicing also results in intron retention (Pendleton et al., 2017). Several studies have demonstrated that intron retention could result in nuclear pre-mRNA degradation. For example, intron retention stimulates nuclear methionine adenosyltransferase 2A (MAT2A) pre-mRNA decay under high S-adenosylmethionine (SAM) condition (Pendleton et al., 2017). Besides, poly(A)-binding protein nuclear 1 (PABPN1) protein negatively modifies its own expression through binding with PABPN1 pre-mRNA to enhance retention of the 3′-terminal intron and induce nuclear PABPN1 pre-mRNA degradation (Bergeron et al., 2015). Thus, inhibition of intron splicing in KRT4 pre-mRNA should result in intron retention and subsequent nuclear KRT4 pre-mRNA degradation in OSCC.
Growing evidence has indicated that m6A methylation plays a crucial role in intron retention. A previous study has revealed that METTL16 increases m6A level of a hairpin of MAT2A pre-mRNA to facilitate intron retention (Pendleton et al., 2017). By contrast, overexpression of alkB homolog 5 RNA demethylase (ALKBH5), which is a m6A-demethylase, enhances intron retention on E6 mRNA of human papillomavirus type 16 (Cui et al., 2022). Nevertheless, the mechanism of m6A methylation regulating intron retention on mRNA remains unclear.
A previous study has demonstrated that DGCR8 also regulates pre-mRNA splicing (Cirera-Salinas et al., 2017). Yet the role of DGCR8 in KRT4 pre-mRNA splicing has not been reported. Our results revealed that silence of DGCR8 prohibited intron splicing of KRT4 pre-mRNA in OSCC. Thus, this study uncovered the effect of DGCR8 on KRT4 pre-mRNA splicing for the first time.
DGCR8 also contributes to m6A methylation-mediated splicing of primary microRNAs (pri-miRNAs). In mammalian cells, METTL3 promotes m6A methylation of pri-miRNAs to enhance the binding of DGCR8 to pri-miRNAs and splicing of pri-miRNAs (Alarcon et al., 2015). By contrast, our data revealed that m6A methylation suppressed the binding of DGCR8 to exon-intron boundaries in KRT4 pre-mRNA to prohibit intron splicing of KRT4 pre-mRNA in OSCC. Therefore, DGCR8 might exert opposite effects on pre-mRNA splicing and miRNA processing.
A recent study has revealed that genes related to RNA methylation are associated with immunology, gene mutation and survival of OSCC patients (Wu, Tang & Cheng, 2022). Besides, METTL3 facilitates tumorigenesis and metastasis of OSCC by enhancing BMI1 m6A methylation (Liu et al., 2020). Moreover, METTL3 promotes OSCC progress by improving m6A methylation of protein arginine methyltransferase 5 (PRMT5) and programmed death-ligand 1 (PD-L1) (Ai et al., 2021). Therefore, above studies and our findings together suggest m6A methylation should facilitating OSCC progress.
The role of DGCR8 in OSCC has not been report. Nevertheless, two recent studies have indicated that DGCR8 enhances radiosensitive of head and neck squamous cell carcinoma (Long et al., 2021; Zhang et al., 2020b). Thus, previous studies and our results suggested that DGCR8 should play the opposite role of m6A methylation in OSCC. More importantly, these studies could further confirm the validity of our findings.
However, there were some limitations of the current study. For example, this study could be strengthened through identifying functional relevance of m6A methylation and DGCR8 to the suppression of OSCC cell growth induced by KRT4. Besides, the findings of this study should be confirmed by in vivo experiments.
Conclusion
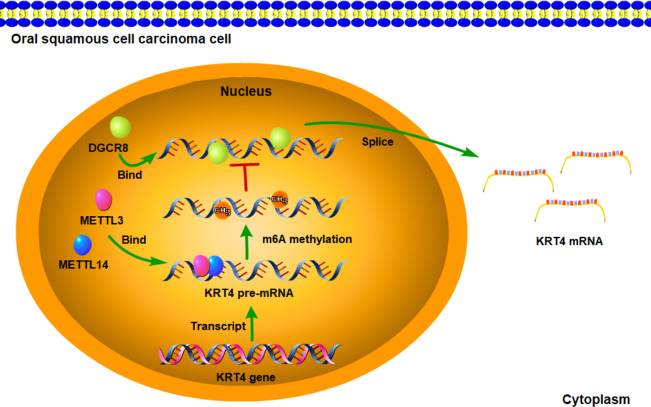
In summary, the current study indicated that intron splicing of KRT4 pre-mRNA was suppressed in OSCC. Mechanistically, m6A methylation of exon-intron boundaries prevented intron splicing of KRT4 pre-mRNA in OSCC. In addition, m6A methylation suppressed the binding of DGCR8 to exon-intron boundaries in KRT4 pre-mRNA to prohibit intron splicing of KRT4 pre-mRNA in OSCC (Fig. 6). These results revealed the mechanism downregulating KRT4 in OSCC and provided potential therapeutic targets for OSCC.
Figure 6. Schematic diagram of molecular mechanisms for the current study.
The current study indicated that intron splicing of KRT4 pre-mRNA was suppressed in OSCC. Mechanistically, m6A methylation of exon-intron boundaries prevented intron splicing of KRT4 pre-mRNA in OSCC. In addition, m6A methylation suppressed the binding of DGCR8 to exon-intron boundaries in KRT4 pre-mRNA to prohibit intron splicing of KRT4 pre-mRNA in OSCC.
Supplemental Information
The level of METTL3 (A), METTL14 (A) and DGCR8 (B) detected by qRT-PCR in HN6 cells treated with or without siRNAs. NC, negative control; siMETTL3, METTL3 siRNA; siMETTL14, METTL14 siRNA; siDGCR8, DGCR8 siRNA. ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 81500864) and Guangdong Basic and Applied Basic Research Foundation (Grant No. 2019A1515011203). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Bin Cheng, Email: chengbin@mail.sysu.edu.cn.
Yun Wang, Email: wangyun23@mail.sysu.edu.cn.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Xiaoxu Li performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Juan Fang performed the experiments, prepared figures and/or tables, and approved the final draft.
Xiaoan Tao performed the experiments, prepared figures and/or tables, and approved the final draft.
Juan Xia analyzed the data, prepared figures and/or tables, and approved the final draft.
Bin Cheng conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Yun Wang conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.
References
- Ai et al. (2021).Ai Y, Liu S, Luo H, Wu S, Wei H, Tang Z, Li X, Lv X, Zou C. METTL3 intensifies the progress of oral squamous cell carcinoma via modulating the m6A amount of PRMT5 and PD-L1. Journal of Immunology Research. 2021;2021:6149558. doi: 10.1155/2021/6149558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon et al. (2015).Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron et al. (2015).Bergeron D, Pal G, Beaulieu YB, Chabot B, Bachand F. Regulated intron retention and nuclear pre-mRNA decay contribute to PABPN1 autoregulation. Molecular and Cellular Biology. 2015;35:2503–2517. doi: 10.1128/MCB.00070-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloebaum et al. (2014).Bloebaum M, Poort L, Bockmann R, Kessler P. Survival after curative surgical treatment for primary oral squamous cell carcinoma. Journal of Cranio-Maxillofacial Surgery. 2014;42:1572–1576. doi: 10.1016/j.jcms.2014.01.046. [DOI] [PubMed] [Google Scholar]
- Bray et al. (2018).Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Cho et al. (2015).Cho S, Moon H, Loh TJ, Jang HN, Liu Y, Zhou J, Ohn T, Zheng X, Shen H. Splicing inhibition of U2AF65 leads to alternative exon skipping. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9926–9931. doi: 10.1073/pnas.1500639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirera-Salinas et al. (2017).Cirera-Salinas D, Yu J, Bodak M, Ngondo RP, Herbert KM, Ciaudo C. Noncanonical function of DGCR8 controls mESC exit from pluripotency. Journal of Cell Biology. 2017;216:355–366. doi: 10.1083/jcb.201606073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui et al. (2022).Cui X, Nilsson K, Kajitani N, Schwartz S. Overexpression of m6A-factors METTL3, ALKBH5, and YTHDC1 alters HPV16 mRNA splicing. Virus Genes. 2022;58:98–112. doi: 10.1007/s11262-022-01889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay et al. (2010).Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Guo & Wang (2019).Guo WT, Wang Y. Dgcr8 knockout approaches to understand microRNA functions in vitro and in vivo. Cellular and Molecular Life Sciences. 2019;76:1697–1711. doi: 10.1007/s00018-019-03020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han et al. (2011).Han J, Xiong J, Wang D, Fu XD. Pre-mRNA splicing: where and when in the nucleus. Trends in Cell Biology. 2011;21:336–343. doi: 10.1016/j.tcb.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa et al. (2021).Hasegawa K, Fujii S, Matsumoto S, Tajiri Y, Kikuchi A, Kiyoshima T. YAP signaling induces PIEZO1 to promote oral squamous cell carcinoma cell proliferation. The Journal of Pathology. 2021;253:80–93. doi: 10.1002/path.5553. [DOI] [PubMed] [Google Scholar]
- Kashima et al. (2007).Kashima T, Rao N, David CJ, Manley JL. hnRNP A1 functions with specificity in repression of SMN2 exon 7 splicing. Human Molecular Genetics. 2007;16:3149–3159. doi: 10.1093/hmg/ddm276. [DOI] [PubMed] [Google Scholar]
- Lallemant et al. (2009).Lallemant B, Evrard A, Combescure C, Chapuis H, Chambon G, Raynal C, Reynaud C, Sabra O, Joubert D, Hollande F, Lallemant JG, Lumbroso S, Brouillet JP. Clinical relevance of nine transcriptional molecular markers for the diagnosis of head and neck squamous cell carcinoma in tissue and saliva rinse. BMC Cancer. 2009;9:370. doi: 10.1186/1471-2407-9-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee & Rio (2015).Lee Y, Rio DC. Mechanisms and regulation of alternative pre-mRNA splicing. Annual Review of Biochemistry. 2015;84:291–323. doi: 10.1146/annurev-biochem-060614-034316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2022).Li X, Wang Y, Fang J, Wang Z, Tao X, Xia J, Cheng B. KRT4 suppresses oral squamous cell carcinoma development by reducing ATG4B-mediated autophagy. Biocell. 2022;46:441–451. doi: 10.32604/biocell.2021.014844. [DOI] [Google Scholar]
- Liu et al. (2020).Liu L, Wu Y, Li Q, Liang J, He Q, Zhao L, Chen J, Cheng M, Huang Z, Ren H, Chen J, Peng L, Gao F, Chen D, Wang A. METTL3 promotes tumorigenesis and metastasis through BMI1 m(6)A methylation in oral squamous cell carcinoma. Molecular Therapy. 2020;28:2177–2190. doi: 10.1016/j.ymthe.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long et al. (2021).Long D, Xu L, Deng Z, Guo D, Zhang Y, Liu Z, Zhang C. HPV16 E6 enhances the radiosensitivity in HPV-positive human head and neck squamous cell carcinoma by regulating the miR-27a-3p/SMG1 axis. Infectious Agents and Cancer. 2021;16:56. doi: 10.1186/s13027-021-00397-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel et al. (2021).Mendel M, Delaney K, Pandey RR, Chen KM, Wenda JM, Vagbo CB, Steiner FA, Homolka D, Pillai RS. Splice site m(6)A methylation prevents binding of U2AF35 to inhibit RNA splicing. Cell. 2021;184:3125–3142 e3125. doi: 10.1016/j.cell.2021.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michlewski & Caceres (2019).Michlewski G, Caceres JF. Post-transcriptional control of miRNA biogenesis. RNA. 2019;25:1–16. doi: 10.1261/rna.068692.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon et al. (2017).Moon H, Cho S, Loh TJ, Jang HN, Liu Y, Choi N, Oh J, Ha J, Zhou J, Cho S, Kim DE, Ye MB, Zheng X, Shen H. SRSF2 directly inhibits intron splicing to suppresses cassette exon inclusion. BMB Reports. 2017;50:423–428. doi: 10.5483/bmbrep.2017.50.8.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon et al. (2019).Moon H, Jang HN, Liu Y, Choi N, Oh J, Ha J, Kim HH, Zheng X, Shen H. RRM but not the Asp/Glu domain of hnRNP C1/C2 is required for splicing regulation of Ron exon 11 pre-mRNA. BMB Reports. 2019;52:641–646. doi: 10.5483/BMBRep.2019.52.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzarella et al. (2014).Panzarella V, Pizzo G, Calvino F, Compilato D, Colella G, Campisi G. Diagnostic delay in oral squamous cell carcinoma: the role of cognitive and psychological variables. International Journal of Oral Science. 2014;6:39–45. doi: 10.1038/ijos.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton et al. (2017).Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, Conrad NK. The U6 snRNA m(6)A methyltransferase METTL16 eegulates SAM synthetase intron retention. Cell. 2017;169:824–835 e814. doi: 10.1016/j.cell.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toruner et al. (2004).Toruner GA, Ulger C, Alkan M, Galante AT, Rinaggio J, Wilk R, Tian B, Soteropoulos P, Hameed MR, Schwalb MN, Dermody JJ. Association between gene expression profile and tumor invasion in oral squamous cell carcinoma. Cancer Genetics and Cytogenetics. 2004;154:27–35. doi: 10.1016/j.cancergencyto.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Wu, Tang & Cheng (2022).Wu X, Tang J, Cheng B. Oral squamous cell carcinoma gene patterns connected with RNA methylation for prognostic prediction. Oral Diseases. 2022 doi: 10.1111/odi.14341. Epub ahead of print Aug 7 2022. [DOI] [PubMed] [Google Scholar]
- Xiao et al. (2016).Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, Wang X, Ma HL, Huang CM, Yang Y, Huang N, Jiang GB, Wang HL, Zhou Q, Wang XJ, Zhao YL, Yang YG. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Molecular Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2021).Yang Z, Yan G, Zheng L, Gu W, Liu F, Chen W, Cui X, Wang Y, Yang Y, Chen X, Fu Y, Xu X. YKT6, as a potential predictor of prognosis and immunotherapy response for oral squamous cell carcinoma, is related to cell invasion, metastasis, and CD8+ T cell infiltration. Oncoimmunology. 2021;10:1938890. doi: 10.1080/2162402X.2021.1938890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye et al. (2008).Ye H, Yu T, Temam S, Ziober BL, Wang J, Schwartz JL, Mao L, Wong DT, Zhou X. Transcriptomic dissection of tongue squamous cell carcinoma. BMC Genomics. 2008;9:69. doi: 10.1186/1471-2164-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2020a).Zhang BY, Han L, Tang YF, Zhang GX, Fan XL, Zhang JJ, Xue Q, Xu ZY. METTL14 regulates M6A methylation-modified primary miR-19a to promote cardiovascular endothelial cell proliferation and invasion. European Review for Medical and Pharmacological Sciences. 2020a;24:7015–7023. doi: 10.26355/eurrev_202006_21694. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2020b).Zhang C, Chen H, Deng Z, Long D, Xu L, Liu Z. DGCR8/miR-106 axis enhances radiosensitivity of head and neck squamous cell carcinomas by downregulating RUNX3. Frontiers in Medicine. 2020b;7:582097. doi: 10.3389/fmed.2020.582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang J, Quan J, Ren Y, Chen M, Yang J, Zhang X. Keratin 4 regulates the development of human white sponge nevus. Journal of Oral Pathology & Medicine. 2018;47:598–605. doi: 10.1111/jop.12728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The level of METTL3 (A), METTL14 (A) and DGCR8 (B) detected by qRT-PCR in HN6 cells treated with or without siRNAs. NC, negative control; siMETTL3, METTL3 siRNA; siMETTL14, METTL14 siRNA; siDGCR8, DGCR8 siRNA. ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.