Abstract
Background
Recent guidelines on dual antiplatelet therapy (DAPT) duration after percutaneous coronary intervention (PCI) balance the subsequent risks of major bleeding with ischemic events. Although generally favoring shorter DAPT duration with second‐generation drug‐eluting stents, the effects on long‐term outcomes in the wider population are uncertain.
Methods and Results
We tracked all patients having PCI with second‐generation drug‐eluting stents in the Veterans Affairs Healthcare System between 2006 and 2016 for death, myocardial infarction, stroke, and major bleeding up to 13 years. We compared these outcomes with 4 DAPT durations of 1 to 5, 6 to 9, 10 to 12, and 13 to 18 months after the index PCI using hazard ratios (HRs) and 95% CIs from Cox proportional hazards models adjusted by inverse probability weighting. A total of 40 882 subjects with PCI were followed up for a median of 4.3 (25%–75%: 2.4–6.5) years. DAPT discontinuation was rare early after PCI (5.8% at 1–5 months and 6.3% at 6–9 months) but increased (19% and 44%) >9 months. The risk of cardiovascular and noncardiovascular death was higher (HR, 2.03–3.41) with DAPT discontinuation <9 months, likely reflecting premature cessation from factors related to early death. DAPT discontinuation after 9 months following PCI was associated with lower risks of death (HR, 0.93 [95% CI, 0.88–0.99]), cardiac death (HR, 0.79 [95% CI, 0.70–0.90]), myocardial infarction (HR, 0.75 [95% CI, 0.69–0.82]), and major bleeding (HR, 0.82 [95% CI, 0.74–0.91]). Results were similar with an index PCI for an acute coronary syndrome.
Conclusions
Stopping DAPT after 9 months is associated with lower long‐term risks of adverse ischemic and bleeding events and supports recent guidelines of shorter duration DAPT after PCI with second‐generation drug‐eluting stents.
Keywords: drug‐eluting stents, dual antiplatelet therapy, duration, outcomes, percutaneous coronary interventions
Subject Categories: Percutaneous Coronary Intervention, Stent, Pharmacology, Treatment, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- DAPT
dual antiplatelet therapy
- VA
Veterans Affairs
Clinical Perspective.
What Is New?
Long‐term risks of ischemic and major bleeding events are lower in patients who discontinue dual antiplatelet therapy (DAPT) after 9 months following percutaneous coronary intervention (PCI) with second‐generation drug‐eluting stents.
These results also occur in patients having PCI for acute coronary syndromes.
Together, these results support recent guidelines for shorter‐duration DAPT after PCI.
What Are the Clinical Implications?
Long‐term ischemic and bleeding risks may be lower by stopping DAPT after 9 months following PCI rather than extending DAPT beyond this duration.
Patients having PCI for acute coronary syndromes may also have shorter‐duration DAPT.
Studies using older stent designs show that extending dual antiplatelet therapy (DAPT) by >12 months after percutaneous coronary intervention (PCI) lowers the risk of myocardial infarction (MI) but increases the risk of major bleeding. 1 , 2 , 3 Studies with second‐generation drug‐eluting stents suggest shorter‐duration DAPT may offer a more favorable benefit/risk ratio in patients with lower risk of ischemic events or higher risks of bleeding. Randomized trials and meta‐analyses of DAPT duration show that DAPT duration of 6 versus 12 months in selected groups offers similar ischemic risk with a lower bleeding risk. 4 , 5 , 6 , 7 , 8 , 9 As a result, current guidelines suggest a minimum of 6 months DAPT after PCI for stable coronary syndromes and 12 months for acute coronary syndromes (ACSs). 1 , 3 In patients at high bleeding risk, more recent studies suggest that 1‐ to 3‐month versus 12‐month DAPT may lower the risk of major bleeding after PCI. 10 , 11 , 12 , 13 , 14 , 15 , 16
Many of the studies supporting the guidelines examined outcomes up to 1 to 2 years after PCI, but the balance of cardiovascular, noncardiovascular, and bleeding outcomes may change over longer follow‐up. It is uncertain if the current guidelines avoiding long DAPT duration reflect optimal risk over longer follow‐up in a wider population than the clinical trials. We sought to describe the relationships of death, cause‐specific death, MI, and major bleeding after PCI with second‐generation drug‐eluting stents and relate these to DAPT duration in all patients in the Veterans Affairs (VA) Healthcare System over a time frame that preceded the current guidelines.
METHODS
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Dr Scott Kinlay at the VA Boston Healthcare System. We identified all patients having PCI with second‐generation drug‐eluting stents in the VA Healthcare System from 2006 until 2016 from the VA Clinical Assessment Reporting and Tracking program. 17 The Clinical Assessment Reporting and Tracking program records all PCIs in the VA Healthcare System and includes procedural data, such as the dimension and name of stents implanted. These data were imported into the secure VA Informatics and Computing Infrastructure server, and patient data were linked to the VA Corporate Data Warehouse, which provided baseline demographic data, clinical history information, duration of medication prescriptions, and the diagnoses for readmissions. We also linked data on non‐VA hospital admission diagnoses from the VA Information Resource Center, which links data from the Centers for Medicare and Medicaid Services database (Medicaid inpatient, and Medicare Provider Analysis and Review, files). Death was ascertained from the VA Death Index and cause of death from the Joint VA and Department of Defense Center of Excellence for Mortality Data Repository linked to the National Death Index. The study was approved by the VA Boston Institutional Review Board with consent waived.
Subjects were excluded if they had missing or incongruent data, no record of DAPT use after PCI, ticlopidine use, or missing stent dimensions (Figure 1). We also excluded patients with death in the first 14 days after PCI, as it is uncertain if this related to salvage cases or technical issues with the index PCI.
Figure 1. Flowchart of exclusions and the final cohort.
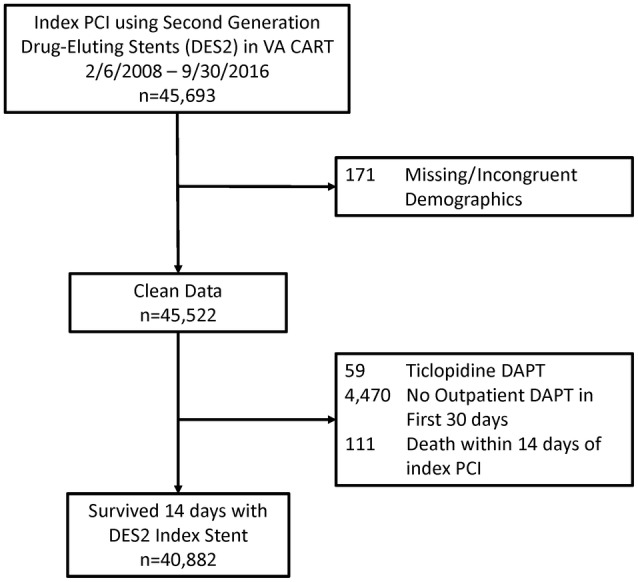
CART indicates Clinical Assessment Reporting and Tracking; DAPT, dual antiplatelet therapy; DES2, second‐generation drug‐eluting stent; PCI, percutaneous coronary intervention; and VA, Veterans Affairs.
DAPT Use and Procedural Data
Aspirin purchased over the counter outside the VA is not recorded in the pharmacy database and is a common practice among veterans who are required to pay copayments for VA drugs (ie, veterans without catastrophic disabilities, who do not have a service‐related disability of at least 50%, and who are considered able to afford copayments). Aspirin use is a VA quality assessment measure, and other studies at the beginning of the time period in our study show high use of aspirin in VA patients with coronary artery disease. 18
We tracked prescriptions for P2Y12 inhibitors (clopidogrel, ticlopidine, and prasugrel) over the duration of follow‐up. Because VA prescriptions are usually written for 90‐day time periods, if a P2Y12 prescription lapsed by >90 days from the last day of supply, the patient was considered not on DAPT. We structured the data with 18 different starting points for each of the first 18 months after the index PCI. Each month, patients were categorized as on DAPT for the entire month or discontinued DAPT during the month. Because people who discontinue DAPT early generally stop because of an increased risk of an event, we could not directly compare people based on their discontinuation month. Instead, we created a starting point at each of the 18 months following PCI and ran monthly comparisons of those on DAPT to those who discontinued DAPT during each starting month. Baseline medications were defined by their use in the month before each start month.
To assess the risks of discontinuing DAPT, we collapsed the monthly data into 4 time periods after the index PCI, corresponding to the recommended duration in current guidelines and 2 intermediate time intervals. The time intervals were 1 to 5, 6 to 9, >9 to 12, and >12 to 18 months after PCI.
Demographic and Comorbidity Data
Data from the index PCI included patient sex, race, prior MI, prior PCI, prior coronary artery bypass grafting, prior stroke, prior major bleed, and smoking status. Smoking was defined as “never smoked” or “current or former smoking” using a probabilistic algorithm validated in the Million Veterans Program. 19 Age was assessed at the start of each month after PCI. We defined comorbidities based on International Classification of Diseases, Ninth Revision (ICD‐9), and International Classification of Diseases, Tenth Revision (ICD‐10), in the 5 years before PCI (Table S1). Baseline comorbidities were assessed from 5 years before the index PCI until the start of each month after PCI. An ACS at the index PCI was defined from 7 days before until 14 days after the index PCI. ACS and major bleed were also defined from 15 days after index PCI to the start of each month after PCI.
Outcomes
We determined the clinical outcomes of death, MI, stroke, and major bleeding starting 14 days after the initial PCI using ICD‐9 and ICD‐10 codes (Table S1). We excluded events within 14 days as prior studies using the VA Clinical Assessment Reporting and Tracking database show that these may reflect admission diagnoses leading to hospitalization and the index PCI or relate to procedural complications. 20 Cause of death was grouped by cardiac death, noncardiac vascular death, and noncardiovascular death using criteria described in the DAPT trial (Table S1). 2
Statistical Analysis
The 4 time periods (1–5, 6–9, >9–12, and >12–18 months after the index PCI) were considered separate cohorts containing subjects who stopped DAPT within the time period and subjects who continued DAPT during the time period. Clinical outcomes were followed from the time period until the end of follow‐up up to a maximum 13 years after the index stent and a short‐term risk assessed outcomes up to 2 years after the time period. Subjects were censored at the end of the follow‐up period (February 29, 2020), 18 months after the last medical record in the VA electronic medical record, the date they resumed DAPT after stopping DAPT for >90 days, or the date of death (for nondeath outcomes).
Baseline demographics, comorbidities, and stent dimensions were described as means and SDs or frequencies and percentages, as appropriate. Event curves for the outcomes for each time period compared subjects who discontinued DAPT from those who continued DAPT during the time period. Hazard ratios (HRs) and 95% CIs for each clinical outcome were estimated from Cox proportional hazards regression models. We used stabilized inverse probability of treatment weighting based on the propensity to stop DAPT within a given time period to adjust the HRs for potential confounders. The propensity weights were developed separately for each of the 4 time periods from regression models of characteristics at the beginning of each time period (Table S2). We assessed statistical significance at the P<0.01 level because of multiple end points.
Sensitivity analyses included interaction terms for subjects who had PCI for an ACS versus no ACS, anticoagulation versus no anticoagulation, and event curves by the subgroups of clopidogrel and ticagrelor or prasugrel. All programming used SAS statistical software. Data are available on request to the authors.
RESULTS
From 2006 to 2016, 70 635 unique patients had a PCI in the VA Healthcare System. Of these patients, 40 882 had PCI with a second‐generation drug‐eluting stent, survived at least 14 days after their PCI, and had at least 1 outpatient prescription in the first month after PCI (Figure 1). Follow‐up ended in February 2020, and the median duration of follow‐up was 4.3 (interquartile range, 2.4–6.5) years.
Figure 2 shows the number and percentage of patients discontinuing DAPT at each of the 4 time periods after the index PCI. A small proportion of patients (5%–6%) discontinued DAPT in each of the first 2 time periods, but this increased over time to nearly half of subjects stopping DAPT after 1 year. Table 1 shows the baseline characteristics of subjects for each of the 4 time periods grouped by whether they discontinued or continued DAPT within that time frame. The mean age at the index PCI was 65 to 66 years, and patients with an ACS more likely discontinued DAPT at later time periods. Other comorbidities and medical treatments were similar between the groups, with high uses of statins (>90%), angiotensin‐converting enzyme inhibitors (48%–65%), and angiotensin receptor blockers (12%–17%). Aspirin use prescribed by the VA Healthcare System was high soon after discharge in the first time period but decreased to approximately half the subjects in later time periods and likely reflects the common use of over‐the‐counter aspirin by veterans over the time frame of the study. 18
Figure 2. Number and proportion of patients discontinuing dual antiplatelet therapy in the 4 time periods after the index PCI. PCI indicates percutaneous coronary intervention.
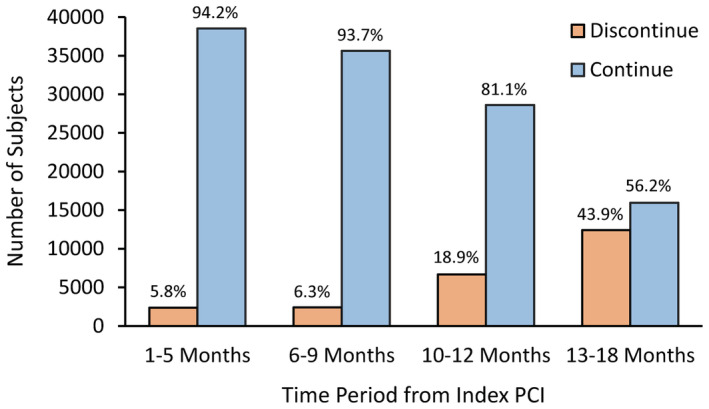
Table 1.
Description of the Cohort by Each of the 4 Time Periods After PCI and by DAPT Use Within 4 Time Periods After PCI
| Variable | Months 1–5 (n=40 882) | Months 6–9 (n=38 041) | Months 10–12 (n=35 274) | Months 13–18 (n=28 361) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAPT discontinued | On DAPT | DAPT discontinued | On DAPT | DAPT discontinued | On DAPT | DAPT discontinued | On DAPT | |||||||||
| (n=2351) | (n=38 531) | (n=2408) | (n=35 633) | (n=6667) | (n=28 607) | (n=12 408) | (n=15 953) | |||||||||
| Age, mean (SD), y | 65.5 | (9.9) | 65.5 | (8.7) | 65.9 | (9.8) | 65.8 | (8.6) | 65.9 | (8.6) | 66.0 | (8.6) | 66.1 | (8.8) | 66.5 | (8.4) |
| Men, n (%) | 2318 | (98.6) | 37 894 | (98.4) | 2367 | (98.3) | 35 043 | (98.3) | 6545 | (98.2) | 28 144 | (98.4) | 12 210 | (98.4) | 15 690 | (98.4) |
| Acute coronary syndrome at index PCI, n (%) | 244 | (10.4) | 5151 | (13.4)* | 435 | (18.1) | 6196 | (17.4) | 1010 | (15.2) | 5916 | (20.7)* | 2400 | (19.3) | 4422 | (27.7)* |
| Current smoker, n (%) | 913 | (38.8) | 12 643 | (32.8)* | 911 | (37.8) | 11 566 | (32.5)* | 2159 | (32.4) | 9297 | (32.5) | 4142 | (33.4) | 5070 | (31.8) |
| Former smoker, n (%) | 1030 | (43.8) | 18 706 | (48.6)* | 1065 | (44.2) | 17 384 | (48.8)* | 3314 | (49.7) | 13 891 | (48.6) | 5906 | (47.6) | 7849 | (49.2) |
| Comorbidities, n (%) | ||||||||||||||||
| Anemia | 611 | (26.0) | 8556 | (22.2)* | 664 | (27.6) | 8273 | (23.2)* | 1457 | (21.9) | 7028 | (24.6)* | 3095 | (24.9) | 4257 | (26.7)† |
| Angina | 980 | (41.7) | 17 952 | (46.6)* | 1158 | (48.1) | 17 201 | (48.3) | 2927 | (43.9) | 14 477 | (50.6)* | 5882 | (47.4) | 8922 | (55.9)* |
| Congestive heart failure | 792 | (33.7) | 10 898 | (28.3)* | 828 | (34.4) | 10 298 | (28.9)* | 1757 | (26.4) | 8622 | (30.1)* | 3538 | (28.5) | 5307 | (33.3)* |
| Chronic kidney disease | 496 | (21.1) | 7721 | (20.0) | 554 | (23.0) | 7324 | (20.6)† | 1244 | (18.7) | 6152 | (21.5)* | 2568 | (20.7) | 3759 | (23.6)* |
| COPD | 848 | (36.1) | 12 755 | (33.1)† | 846 | (35.1) | 12 096 | (34.0) | 2145 | (32.2) | 10 053 | (35.1)* | 4244 | (34.2) | 5962 | (37.4)* |
| Diabetes on insulin | 944 | (40.2) | 15 920 | (41.3) | 1013 | (42.1) | 14 841 | (41.7) | 2536 | (38.0) | 12 239 | (42.8)* | 4926 | (39.7) | 7334 | (46.0)* |
| Diabetes on oral treatment | 906 | (38.5) | 16 397 | (42.6)* | 978 | (40.6) | 15 391 | (43.2)† | 2676 | (40.1) | 12 671 | (44.3)* | 5176 | (41.7) | 7559 | (47.4)* |
| Hypertension | 2180 | (92.7) | 36 340 | (94.3)† | 2259 | (93.8) | 33 852 | (95.0) | 6269 | (94.0) | 27 362 | (95.7)* | 11 801 | (95.1) | 15 446 | (96.8)* |
| Peripheral artery disease | 204 | (8.7) | 2883 | (7.5) | 212 | (8.8) | 2765 | (7.8) | 424 | (6.4) | 2365 | (8.3)* | 798 | (6.4) | 1644 | (10.3)* |
| Cancer and chemotherapy or radiotherapy | 89 | (3.8) | 1667 | (4.3) | 145 | (6.0) | 1574 | (4.4)* | 313 | (4.7) | 1312 | (4.6) | 578 | (4.7) | 777 | (4.9) |
| History, n (%) | ||||||||||||||||
| Prior myocardial infarct | 234 | (10.0) | 2800 | (7.3)* | 217 | (9.0) | 2496 | (7.0)† | 366 | (5.5) | 2052 | (7.2)* | 703 | (5.7) | 1303 | (8.2)* |
| Prior PCI | 204 | (8.7) | 2564 | (6.7)† | 177 | (7.4) | 2337 | (6.6) | 313 | (4.7) | 1984 | (6.9)* | 621 | (5.0) | 1339 | (8.4)* |
| Prior CABG | 85 | (3.6) | 1330 | (3.5) | 78 | (3.2) | 1236 | (3.5) | 186 | (2.8) | 1039 | (3.6)† | 332 | (2.7) | 694 | (4.4)* |
| Prior stroke | 82 | (3.5) | 1081 | (2.8) | 87 | (3.6) | 959 | (2.7) | 128 | (1.9) | 801 | (2.8)* | 305 | (2.5) | 480 | (3.0)† |
| Prior major bleed | 115 | (4.9) | 1402 | (3.6)† | 114 | (4.7) | 1238 | (3.5)† | 208 | (3.1) | 1003 | (3.5) | 405 | (3.3) | 568 | (3.6) |
| Medications, n (%) | ||||||||||||||||
| Aspirin (VA prescribed) | 2271 | (96.6) | 37 222 | (96.6) | 1220 | (50.7) | 19 398 | (54.4)† | 2962 | (44.4) | 14 275 | (49.9)* | 5608 | (45.2) | 8971 | (56.2)* |
| Oral anticoagulant | 227 | (9.7) | 3778 | (9.8) | 265 | (11.0) | 2907 | (8.2)* | 605 | (9.1) | 2169 | (7.6)* | 1220 | (9.8) | 1074 | (6.7)* |
| ACE inhibitor | 1534 | (65.3) | 24 687 | (64.1) | 1221 | (50.7) | 19 601 | (55.0)* | 3264 | (49.0) | 15 093 | (52.8)* | 6031 | (48.6) | 8791 | (55.1)* |
| Angiotensin receptor blocker | 296 | (12.6) | 6090 | (15.8)* | 281 | (11.7) | 5481 | (15.4)* | 936 | (14.0) | 4454 | (15.6)† | 1840 | (14.8) | 2801 | (17.6)* |
| β‐Blocker | 2165 | (92.1) | 35 802 | (92.9) | 1835 | (76.2) | 30 731 | (86.2)* | 5307 | (79.6) | 24 268 | (84.8)* | 9948 | (80.2) | 14 175 | (88.9)* |
| Calcium channel blocker | 743 | (31.6) | 13 672 | (35.5)† | 607 | (25.2) | 10 148 | (28.5)† | 1763 | (26.4) | 8017 | (28.0)† | 3343 | (26.9) | 4944 | (31.0)* |
| Statin | 2256 | (96.0) | 37 506 | (97.3)* | 1956 | (81.2) | 32 902 | (92.3)* | 5831 | (87.5) | 25 806 | (90.2)* | 10 735 | (86.5) | 14 867 | (93.2)* |
| Antilipemic other | 171 | (7.3) | 3777 | (9.8)* | 147 | (6.1) | 2731 | (7.7)† | 417 | (6.3) | 2078 | (7.3)† | 722 | (5.8) | 1388 | (8.7)* |
| Antiarrhythmic | 112 | (4.8) | 1310 | (3.4)† | 81 | (3.4) | 914 | (2.6) | 166 | (2.5) | 691 | (2.4) | 300 | (2.4) | 443 | (2.8) |
| Diuretic | 1007 | (42.8) | 17 407 | (45.2) | 867 | (36.0) | 13 233 | (37.1) | 2195 | (32.9) | 10 524 | (36.8)* | 4218 | (34.0) | 6521 | (40.9)* |
| NSAID | 361 | (15.4) | 7495 | (19.5)* | 229 | (9.5) | 4331 | (12.2)† | 680 | (10.2) | 3094 | (10.8) | 1258 | (10.1) | 2275 | (14.3)* |
| Protein pump inhibitor | 1120 | (47.6) | 20 480 | (53.2)* | 876 | (36.4) | 14 834 | (41.6)* | 2488 | (37.3) | 11 427 | (39.9)* | 4771 | (38.5) | 7002 | (43.9)* |
| Blood values, mean (SD) | ||||||||||||||||
| eGFR, mL/min | 75 | (23.4) | 75 | (20.6) | 73 | (23.4) | 74 | (20.8) | 74 | (20.6) | 73 | (21.0)* | 74 | (21.0) | 72 | (21.1)* |
| Glucose, mg/dL | 133 | (47.7) | 132 | (45.2) | 132 | (55.5) | 131 | (49.6) | 129 | (50.4) | 131 | (52.4)† | 129 | (51.6) | 133 | (49.7)* |
| Hemoglobin, g/dL | 13.3 | (1.8) | 13.5 | (1.6)* | 13.3 | (1.9) | 13.6 | (1.6)* | 13.7 | (1.7) | 13.7 | (1.6) | 13.7 | (1.7) | 13.7 | (1.6)† |
| White cell count, 1000/mm3 | 8.0 | (2.8) | 7.8 | (2.5)* | 7.7 | (2.9) | 7.6 | (2.5) | 7.5 | (2.8) | 7.6 | (2.5) | 7.5 | (2.4) | 7.5 | (2.7) |
| Platelet count, 1000/mm3 | 211 | (64) | 206 | (58)* | 213 | (66) | 208 | (59)† | 211 | (63) | 209 | (60) | 211 | (61) | 209 | (59) |
| LDL cholesterol, mg/dL | 96 | (36) | 88 | (32)* | 89 | (37) | 81 | (31)* | 82 | (32) | 80 | (31)* | 81 | (33) | 78 | (30)* |
| HDL cholesterol, mg/dL | 40 | (12) | 39 | (11) | 40 | (12) | 39 | (11) | 40 | (11) | 40 | (11)* | 40 | (12) | 40 | (11)* |
| Troponin, ng/mL | 3.2 | (12.9) | 2.5 | (9.5)† | 2.2 | (7.8) | 2.2 | (9.1) | 1.9 | (7.8) | 2.0 | (8.8) | 1.9 | (7.9) | 1,8 | (3, 8) |
| N‐terminal ProB‐type natriuretic peptide, pg/mL | 404.7 | (839.6) | 261.8 | (661.1)* | 352.0 | (742.2) | 241.2 | (653.7)* | 240.5 | (827.3) | 232.6 | (644.2) | 213.4 | (451.9) | 238.6 | (776.4) |
ACE indicates angiotensin‐converting enzyme inhibitor; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; PCI, percutaneous coronary intervention; and VA, Veterans Affairs.
*P<0.001, † P<0.01 (comparisons within each time frame).
Table S3 shows comparisons of baseline characteristics of patients excluded and included in the study. There were significant differences in many characteristics. Chart review showed that many of the patients without a DAPT prescription within 30 days of the index PCI were discharged to long‐term care and were at higher short‐term risk of adverse events (older age, frailty, and multiple comorbidities), as outlined in our previous study. 19 Patients who died within 14 days also reflected more complex cases (eg, salvage PCI) or possibly mechanical complications related to the index PCI. We excluded these patients as they may have biased the primary goal of assessing the long‐term effects of DAPT duration.
Table 2 shows the procedural characteristics at the index PCI, including the coronary arteries stented, type of stent, stent diameters, total stent length, and post‐PCI P2Y12 inhibitor. For the 10‐ to 12‐ and 13‐ to 18‐month groups, subjects remaining on DAPT were more likely to have left main or graft PCI, use of Promus stents, or multiple stents.
Table 2.
Lesion and Procedure Details for the Index PCI by Treatment Time Period
| Variable | Months 1–5 (n=40 882) | Months 6–9 (n=38 041) | Months 10–12 (n=35 274) | Months 13–18 (n=28 361) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAPT discontinued | On DAPT | DAPT discontinued | On DAPT | DAPT discontinued | On DAPT | DAPT discontinued | On DAPT | |||||||||
| (n=2351) | (n=38 531) | (n=2408) | (n=35 633) | (n=6667) | (n=28 607) | (n=12 408) | (n=15 953) | |||||||||
| PCI arteries, n (%) | ||||||||||||||||
| Left anterior descending | 979 | (41.6) | 16 116 | (41.8) | 1022 | (42.4) | 14 885 | (41.8) | 2825 | (42.4) | 11 925 | (41.7) | 5292 | (42.6) | 6516 | (40.8) |
| Left circumflex | 696 | (29.6) | 11 164 | (29.0) | 707 | (29.4) | 10 312 | (28.9) | 1968 | (29.5) | 8223 | (28.7) | 3575 | (28.8) | 4576 | (28.7) |
| Right coronary | 702 | (29.9) | 12 082 | (31.4) | 704 | (29.2) | 11 252 | (31.6) | 2111 | (31.7) | 9055 | (31.7) | 4065 | (32.8) | 4932 | (30.9) |
| Left main | 91 | (3.9) | 1265 | (3.3) | 79 | (3.3) | 1144 | (3.2) | 122 | (1.8) | 993 | (3.5)* | 244 | (2.0) | 734 | (4.6)* |
| Graft (vein or arterial) | 160 | (6.8) | 2406 | (6.2) | 159 | (6.6) | 2196 | (6.2) | 319 | (4.8) | 1832 | (6.4)* | 572 | (4.6) | 1234 | (7.7)* |
| Stent brand, n (%) | ||||||||||||||||
| Endeavor | 169 | (7.2) | 2319 | (6.0) | 143 | (5.9) | 2149 | (6.0) | 374 | (5.6) | 1754 | (6.1) | 708 | (5.7) | 1032 | (6.5)† |
| Promus | 547 | (23.3) | 9732 | (25.3) | 556 | (23.1) | 9052 | (25.4) | 1516 | (22.7) | 7453 | (26.1)* | 2993 | (24.1) | 4398 | (27.6)* |
| Resolute | 395 | (16.8) | 6276 | (16.3) | 458 | (19.0) | 5710 | (16.0)† | 1079 | (16.2) | 4557 | (15.9) | 2092 | (16.9) | 2424 | (15.2)† |
| Xience | 1330 | (56.6) | 21 661 | (56.2) | 1326 | (55.1) | 20 082 | (56.4) | 3932 | (59.0) | 15 948 | (55.8)* | 7022 | (56.6) | 8787 | (55.1) |
| Procedural details, n (%) | ||||||||||||||||
| 1 Stent | 1452 | (61.8) | 23 863 | (61.9) | 1511 | (62.7) | 22 069 | (61.9) | 4370 | (65.5) | 17 497 | (61.2)* | 7999 | (64.5) | 9366 | (58.7)* |
| 2 Stents | 601 | (25.6) | 9794 | (25.4) | 601 | (25.0) | 9069 | (25.5) | 1605 | (24.1) | 7369 | (25.8) | 3080 | (24.8) | 4219 | (26.4) |
| ≥3 Stents | 298 | (12.7) | 4874 | (12.6) | 296 | (12.3) | 4495 | (12.6) | 692 | (10.4) | 3741 | (13.1) | 1329 | (10.7) | 2368 | (14.8) |
| Maximum stent diameter, mean (SD), mm | 3.0 | (0.5) | 3.0 | (0.5) | 3.0 | (0.5) | 3.0 | (0.5) | 3.0 | (0.5) | 3.0 | (0.5) | 3.0 | (0.5) | 3.0 | (0.5) |
| Total stent length, mean (SD), mm | 30.5 | (19.7) | 31.1 | (21.4) | 31.1 | (21.2) | 31.0 | (21.3) | 26.9 | (19.9) | 31.3 | (21.6)* | 29.7 | (19.9) | 32.5 | (22.6)* |
| Fluoroscopy time, mean (SD), min | 17.9 | (61.3) | 17.3 | (125.3) | 17.1 | (44.5) | 17.4 | (129.9) | 15.9 | (48.9) | 17.7 | (143.0) | 15.6 | (38.2) | 19.2 | (188.2) |
| Total contrast, mean (SD), mL | 197.6 | (111.7) | 208.6 | (836.2) | 197.7 | (100.9) | 209.3 | (869.7) | 199.4 | (99.9) | 211.5 | (969.2) | 202.1 | (400.1) | 218.6 | (1246.6) |
| Post‐PCI P2Y12 inhibitor, n (%) | ||||||||||||||||
| Clopidogrel | 2208 | (93.9) | 36 589 | (95.0) | 2204 | (91.5) | 32 810 | (92.1) | 6149 | (92.2) | 26 295 | (91.9) | 11 384 | (91.8) | 14 774 | (92.6) |
| Ticagrelor | 103 | (4.4) | 1358 | (3.5) | 93 | (3.9) | 996 | (2.8) | 155 | (2.3) | 815 | (2.9) | 335 | (2.7) | 505 | (3.2) |
| Prasugrel | 128 | (5.4) | 2329 | (6.0) | 117 | (4.9) | 2085 | (5.9) | 372 | (5.6) | 1686 | (5.9) | 712 | (5.7) | 988 | (6.2) |
There are multiple counts in some patients. PCI indicates percutaneous coronary intervention. *P<0.001, † P<0.01 (comparisons within each time frame).
Figure 3 shows the event curves for outcomes for each of the 4 time periods according to patients who discontinued versus continued DAPT. All‐cause death was higher among subjects who discontinued DAPT in the first 1 to 5 and 6 to 9 months after PCI but was not different among subjects discontinuing DAPT after 9 months. The differences for MI and major bleeding were smaller in the first 2 time periods, but after 9 months, DAPT discontinuation was associated with less bleeding and MI.
Figure 3. Event curves by DAPT use for death, myocardial infarction, and stroke over the 4 time periods after the index percutaneous coronary intervention.
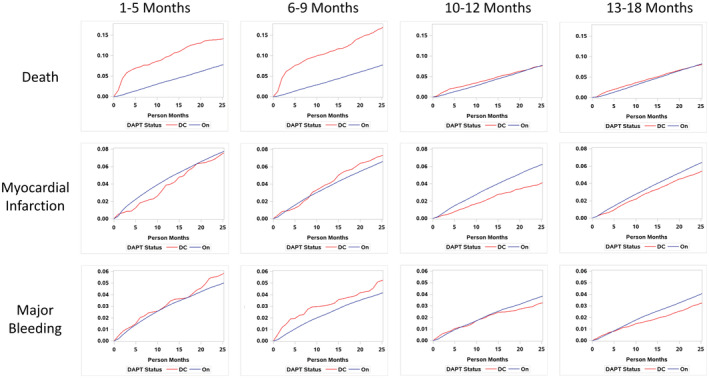
DAPT indicates dual antiplatelet therapy; and DC, discontinued.
Table 3 shows the incidence rates and HRs and 95% CIs for the outcomes for each time period. All‐cause death was significantly higher for patients who discontinued DAPT within 1 to 5 and 6 to 9 months after PCI, with the greatest increase in death occurring within 30 to 60 days of each of these time periods (Figure 3). However, there were no differences in MI or bleeding with stopping DAPT <9 months after PCI. This suggests that the higher all‐cause mortality was unrelated to stopping antithrombotic agents and that other factors related to death were driving DAPT cessation rather than the other way around.
Table 3.
Crude and IPTW HRs and 95% CIs for Outcomes Related to DAPT Discontinuation Within 4 Time Periods After PCI
| Outcome | Time | DAPT | Crude | ITPW‐adjusted | |||
|---|---|---|---|---|---|---|---|
| after PCI, mo | Discontinued | Continued | HR (95% CI) | HR (95% CI) | |||
| Events, n | Incidence* | Events, n | Incidence* | ||||
| Death | |||||||
| 1–5 | 330 | 6.81 | 7624 | 3.56 | 2.17 (1.94–2.43)† | 2.03 (1.81–2.28)† | |
| 6–9 | 422 | 7.35 | 6712 | 3.51 | 2.27 (2.05–2.51)† | 2.21 (1.99–2.45)† | |
| 10–12 | 981 | 3.10 | 5372 | 3.65 | 0.90 (0.85–0.96)‡ | 0.93 (0.88–0.99) | |
| 13–18 | 1941 | 3.41 | 3185 | 3.99 | 0.92 (0.88–0.97)‡ | 0.97 (0.92–1.02) | |
| Cardiac death | |||||||
| 1–5 | 113 | 2.41 | 2458 | 1.19 | 2.10 (1.75–2.53)† | 2.03 (1.68–2.45)† | |
| 6–9 | 130 | 2.37 | 2096 | 1.14 | 2.10 (1.76–2.49)† | 2.03 (1.70–2.42)† | |
| 10–12 | 255 | 0.83 | 1670 | 1.18 | 0.77 (0.68–0.87)† | 0.79 (0.70–0.90)† | |
| 13–18 | 519 | 0.95 | 1035 | 1.35 | 0.78 (0.71–0.85)† | 0.82 (0.75–0.90)† | |
| Noncardiac vascular death | |||||||
| 1–5 | 19 | 0.41 | 284 | 0.14 | 3.20 (2.06–4.97)† | 2.99 (1.91–4.68)† | |
| 6–9 | 25 | 0.46 | 236 | 0.13 | 3.53 (2.40–5.19)† | 3.41 (2.30–5.07)† | |
| 10–12 | 40 | 0.13 | 188 | 0.13 | 1.03 (0.76–1.39) | 1.06 (0.78–1.43) | |
| 13–18 | 75 | 0.14 | 102 | 0.13 | 1.10 (0.85–1.44) | 1.18 (0.91–1.54) | |
| Noncardiovascular death | |||||||
| 1–5 | 170 | 3.63 | 3654 | 1.77 | 2.26 (1.94–2.63)† | 2.04 (1.75–2.38)† | |
| 6–9 | 213 | 3.88 | 3208 | 1.74 | 2.35 (2.05–2.70)† | 2.29 (1.99–2.64)† | |
| 10–12 | 513 | 1.67 | 2520 | 1.78 | 0.98 (0.90–1.07) | 1.02 (0.93–1.11) | |
| 13–18 | 966 | 1.76 | 1438 | 1.88 | 1.01 (0.94–1.08) | 1.05 (0.98–1.13) | |
| Myocardial infarction | |||||||
| 1–5 | 142 | 3.09 | 5995 | 3.00 | 1.09 (0.92–1.27) | 1.05 (0.89–1.24) | |
| 6–9 | 145 | 2.71 | 4448 | 2.50 | 1.11 (0.94–1.30) | 1.07 (0.91–1.26) | |
| 10–12 | 519 | 1.70 | 3413 | 2.52 | 0.74 (0.68–0.80)† | 0.75 (0.69–0.82)† | |
| 13–18 | 1096 | 2.02 | 2001 | 2.81 | 0.84 (0.78–0.89)† | 0.86 (0.81–0.92)† | |
| Major bleeding | |||||||
| 1–5 | 97 | 2.12 | 3640 | 1.78 | 1.18 (0.97–1.44) | 1.13 (0.92–1.38) | |
| 6–9 | 104 | 1.94 | 2844 | 1.56 | 1.25 (1.03–1.51) | 1.16 (0.95–1.41) | |
| 10–12 | 344 | 1.14 | 2162 | 1.55 | 0.80 (0.72–0.89)† | 0.82 (0.74–0.91)† | |
| 13–18 | 650 | 1.20 | 1311 | 1.74 | 0.79 (0.73–0.86)† | 0.82 (0.75–0.89)† | |
| Stroke | |||||||
| 1–5 | 53 | 1.13 | 1996 | 0.95 | 1.27 (0.97–1.67) | 1.26 (0.96–1.66) | |
| 6–9 | 69 | 1.24 | 1653 | 0.89 | 1.46 (1.15–1.84)† | 1.44 (1.14–1.84)† | |
| 10–12 | 227 | 0.73 | 1282 | 0.89 | 0.87 (0.76–0.99) | 0.89 (0.78–1.01) | |
| 13–18 | 449 | 0.81 | 740 | 0.96 | 0.96 (0.86–1.06) | 1.00 (0.90–1.11) | |
DAPT indicates dual antiplatelet therapy; HR, hazard ratio; ITPW, inverse probability of treatment weighting; and PCI, percutaneous coronary intervention.
Incidence=Events/1000 patient months.
P<0.001.
P<0.01.
All‐cause death, MI, and major bleeding were significantly lower for DAPT discontinuation between >9 and 12 or >12 months versus continuing DAPT in these time periods. Adjustment with multiple variables related to the propensity of DAPT discontinuation using inverse probability weighting did not affect these results.
Cause of Death
Event curves for cause of death showed a similar pattern for all‐cause death, with DAPT discontinuation before 9 months associating with a higher risk of cardiac death, vascular death, and noncardiovascular death (Figure 4). Table 3 shows the HRs for the 3 causes of death with discontinuing DAPT. Cardiac death, vascular death, and noncardiovascular death were all significantly associated with DAPT discontinuation in the 1 to 5 and 6 to 9 months after PCI. However, DAPT discontinuation >9 months after PCI only associated with less cardiac death and was not associated with vascular or noncardiovascular death.
Figure 4. Event curves by DAPT use for cardiac death, vascular death, and noncardiovascular death.
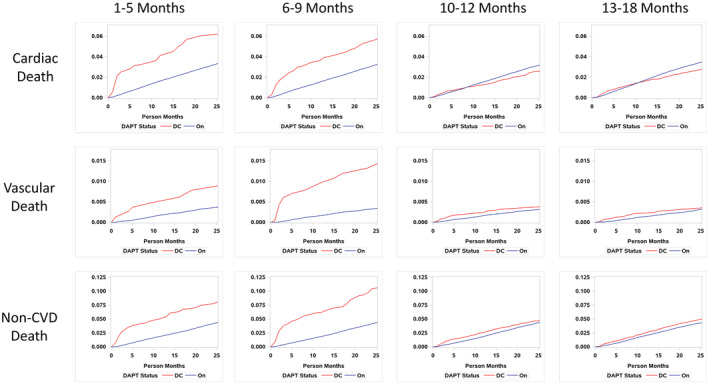
CVD indicates cardiovascular disease; DAPT, dual antiplatelet therapy; and DC, discontinued.
Sensitivity Analyses
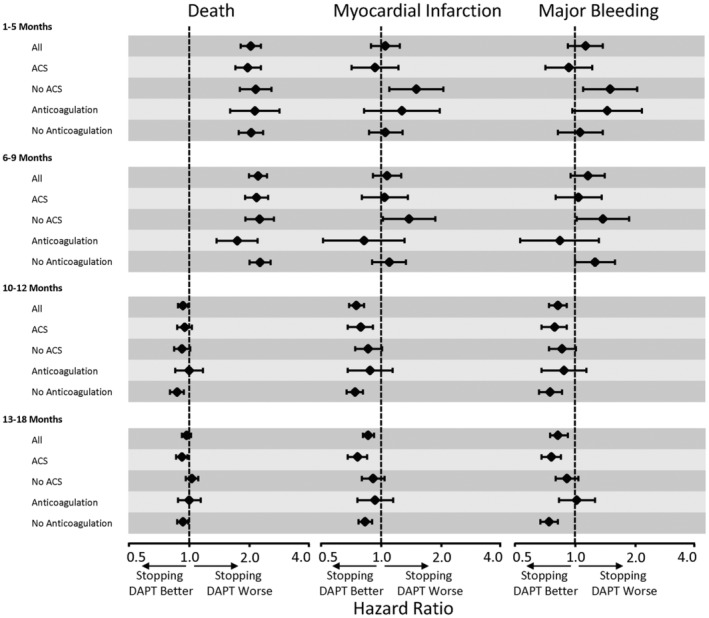
Sensitivity analyses restricting the outcomes to a shorter time frame up to 2 years after PCI accentuated the main findings based on events occurring up until the end of follow‐up at a maximum of 13 years after PCI (Table S4). We also used additional models with interaction terms to assess the relationships of discontinuing DAPT within the clinically important subgroups of patients having PCI for an ACS or the use of anticoagulants. There were some significant interactions for ACS or use of anticoagulants at the index PCI (Figure 5 and Tables S5 and S6); however, the interactions were not consistent across all time periods, and the magnitude of the differences in the point estimates for HRs was directionally similar to the main results and did not affect the overall conclusions. Event curves based on subgroups of clopidogrel versus the more potent ticagrelor or prasugrel showed similar results (Figure S1).
Figure 5. Hazard ratios for DAPT cessation on death, myocardial infarction, and major bleeding in the subgroups of ACSs and use of anticoagulation. ACSs indicates acute coronary syndromes; and DAPT, dual antiplatelet therapy.
DISCUSSION
This study differs from our earlier reports by examining late in addition to early outcomes after PCI and exclusively with second‐generation drug‐eluting stents. 19 , 21 , 22 , 23 , 24 In this VA cohort, the proportion of patients discontinuing DAPT in the first 1 to 5 and 6 to 9 months was small, at 6% in each time period. This reflects the PCI guidelines over most of the study period, which recommended a minimum of 6 to 12 months DAPT after PCI, 1 , 25 and possibly high adherence by ex‐military patients in a relatively low‐cost health care system. 26 Nonveteran populations may show different behaviors in the months shortly after PCI.
In the small numbers of patients who did discontinue DAPT at 1 to 5 and 6 to 9 months, all‐cause death was higher. However, the increased risk of noncardiovascular death as well as cardiac and vascular death occurred early in these time periods and did not affect MI, suggesting a lack of specificity for coronary events that would be expected if early discontinuation of DAPT caused death from stent thrombosis or MI. Thus, it is more likely that the high early death rate, even after adjustment for confounders, was a consequence of factors related to increased risk of death from any cause that drove DAPT cessation. Our prior analysis of an earlier cohort of veterans after PCI, 19 and other short‐term studies, 27 , 28 , 29 , 30 , 31 shows that patients who prematurely discontinued DAPT have greater frailty, lower use of statins, less education, avoidance of health care, and other social factors related to increased risk of adverse outcomes.
More recently randomized trials assessing deescalating DAPT to single antiplatelet aspirin or P2Y12 inhibitors 1 to 3 months after PCI in patients at high bleeding risk show less major bleeding and no significant increase in ischemic events. 10 , 11 , 12 , 13 , 14 , 15 , 16 Single‐arm studies comparing monotherapy with P2Y12 therapy after a short course of DAPT following PCI also show less bleeding compared with historical controls. 32 , 33 , 34 However, there is some inconsistency with these short‐DAPT regimens as the risk of MI was similar to longer DAPT for ticagrelor monotherapy 1 to 3 months but higher with clopidogrel monotherapy 1 to 2 months after PCI for an ACS. 13 , 35 , 36 Thus, shorter courses of DAPT are increasingly justified after PCI, particularly for patients at high bleeding risk (eg, elderly patients, patients using anticoagulants, and patients with prior major bleeding), especially with coexisting low risks of ischemic events (eg, noncomplex coronary anatomy). 37
This cohort was more likely to provide informative data on the value of extending DAPT beyond 6 to 12 months after PCI with second‐generation drug‐eluting stents. Compared with subjects who continued DAPT, subjects who discontinued DAPT after 9 months following PCI had lower rates of all‐cause death, and cardiac death, but not vascular or noncardiovascular death. This pattern of lower cardiac but no relationship to noncardiac causes of death suggests the effect of DAPT discontinuation after this time period is specific to cardiac events and is relatively safe. Discontinuation of DAPT after 9 months was also associated with lower rates of major bleeding and MI. These relationships were stronger in analyses restricting the outcomes to the shorter 2‐year time frame and again support a more direct effect of DAPT discontinuation on lower risk of adverse events. Our results were similar in the clinically important subgroups of PCI for ACSs and patients on anticoagulants at a time when single antiplatelet therapy was not widely embraced.
Our findings are more consistent with smaller randomized trials and observational studies assessing the value of ≥12 months of DAPT after PCI. Randomized trials of 6 versus 12 months of DAPT after PCI generally show similar risks of MI and ischemic end points with 6 months DAPT, 4 , 5 , 6 , 9 with 1 study showing higher rates of MI and less major bleeding. 38 We did find significantly lower risk of cardiac death and MI with stopping DAPT after 9 months. This could reflect the effect of unknown confounders related to lower MI risk and DAPT discontinuation after this time period but overall does not indicate harm from shorter durations of DAPT up to 10 years after PCI. Our study of >40 000 patients having PCI with second‐generation drug‐eluting stents and followed up for an average of 4.3 years finds similar conclusions to meta‐analyses of these studies with 1 to 2 years follow‐up. 39 , 40 Our results support shorter‐duration DAPT followed by single antiplatelet therapy in patients with second‐generation drug‐eluting stents regardless of a presentation of acute or stable coronary syndromes and without concerns of a late catch‐up in the risk of MI.
Limitations
The limitations of our cohort study include the observational design and the potential for other factors confounding the relationships of increased risks of cause‐specific deaths in the small proportion of patients who discontinued DAPT before 9 months. We have studied this group previously and found greater frailty and other less optimal health habits that likely drive this risk of death from cardiac and noncardiac sources rather than DAPT discontinuation per se. As a result, we cannot assess the relationship of DAPT duration of <9 months with this cohort. We have more confidence in our finding of a lower risk of death, cardiac death, MI, and major bleeding with stopping DAPT after 9 months, even in patients with ACSs. This finding is consistent with smaller clinical trials showing no benefit for extending DAPT to ≥12 months in patients receiving second‐generation drug‐eluting stents and extends this observation beyond prior studies up to 13 years after PCI. We were unable to assess the risk of single antiplatelet therapy with P2Y12 inhibitors, but this practice became more in vogue after our follow‐up period. Although there were 670 women in our cohort, our results reflect the predominantly male population in the VA Healthcare System.
In conclusion, we find that patients who had PCI with second‐generation drug‐eluting stents had lower long‐term risks of death, cardiac death, MI, and bleeding by stopping DAPT after 9 months following PCI, and the results do not support extending DAPT beyond this time frame. More recent randomized trials support even shorter durations of DAPT with second‐generation drug‐eluting stents, particularly in patients at high bleeding risk and low ischemic risk. Our results support current guidelines toward shorter DAPT duration in a wider national population of patients followed up for >10 years after PCI with second‐generation drug‐eluting stents.
Sources of Funding
This work was supported by a Veterans Affairs Clinical Science Research and Development Award 1/01CX001549.
Disclosures
None.
Supporting information
Tables S1–S6
Figure S1
Acknowledgments
Veterans Affairs (VA)/Centers for Medicare and Medicaid Services data provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, and VA Information Resource Center (project numbers SDR 02‐237 and 98‐004). Mortality data provided by the Center of Excellence for Mortality Data Repository and Joint Department of Veterans Affairs and Department of Defense Suicide Data Repository–National Death Index.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027055
See Editorial by Mourikis and Polzin.
For Sources of Funding and Disclosures, see page 12.
References
- 1. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of st‐elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134:e123–e155. doi: 10.1161/CIR.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 2. Mauri L, Kereiakes DJ, Yeh RW, Driscoll‐Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, et al. Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med. 2014;371:2155–2166. doi: 10.1056/NEJMoa1409312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 4. Colombo A, Chieffo A, Frasheri A, Garbo R, Masotti‐Centol M, Salvatella N, Oteo Dominguez JF, Steffanon L, Tarantini G, Presbitero P, et al. Second‐generation drug‐eluting stent implantation followed by 6‐ versus 12‐month dual antiplatelet therapy: the security randomized clinical trial. J Am Coll Cardiol. 2014;64:2086–2097. doi: 10.1016/j.jacc.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 5. Gilard M, Barragan P, Noryani AA, Noor HA, Majwal T, Hovasse T, Castellant P, Schneeberger M, Maillard L, Bressolette E, et al. 6‐ versus 24‐month dual antiplatelet therapy after implantation of drug‐eluting stents in patients nonresistant to aspirin: the randomized, multicenter italic trial. J Am Coll Cardiol. 2015;65:777–786. doi: 10.1016/j.jacc.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 6. Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, Han KR, Choi JH, Choi SH, Kang HJ, et al. Six‐month versus 12‐month dual antiplatelet therapy after implantation of drug‐eluting stents: the efficacy of xience/promus versus cypher to reduce late loss after stenting (excellent) randomized, multicenter study. Circulation. 2012;125:505–513. doi: 10.1161/CIRCULATIONAHA.111.059022 [DOI] [PubMed] [Google Scholar]
- 7. Hong SJ, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Her AY, Kim YH, Jang Y, Hong MK, et al. 6‐month versus 12‐month dual‐antiplatelet therapy following long everolimus‐eluting stent implantation: the ivus‐xpl randomized clinical trial. JACC Cardiovasc Interv. 2016;9:1438–1446. doi: 10.1016/j.jcin.2016.04.036 [DOI] [PubMed] [Google Scholar]
- 8. Khan SU, Singh M, Valavoor S, Khan MU, Lone AN, Khan MZ, Khan MS, Mani P, Kapadia SR, Michos ED, et al. Dual antiplatelet therapy after percutaneous coronary intervention and drug‐eluting stents: a systematic review and network meta‐analysis. Circulation. 2020;142:1425–1436. doi: 10.1161/CIRCULATIONAHA.120.046308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulz‐Schupke S, Byrne RA, Ten Berg JM, Neumann FJ, Han Y, Adriaenssens T, Tolg R, Seyfarth M, Maeng M, Zrenner B, et al. Isar‐safe: a randomized, double‐blind, placebo‐controlled trial of 6 vs. 12 months of clopidogrel therapy after drug‐eluting stenting. Eur Heart J. 2015;36:1252–1263. doi: 10.1093/eurheartj/ehu523 [DOI] [PubMed] [Google Scholar]
- 10. Feres F, Costa RA, Abizaid A, Leon MB, Marin‐Neto JA, Botelho RV, King SB 3rd, Negoita M, Liu M, de Paula JE, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus‐eluting stents: the optimize randomized trial. JAMA. 2013;310:2510–2522. doi: 10.1001/jama.2013.282183 [DOI] [PubMed] [Google Scholar]
- 11. Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Park BE, Kang WC, et al. A new strategy for discontinuation of dual antiplatelet therapy: the reset trial (real safety and efficacy of 3‐month dual antiplatelet therapy following endeavor zotarolimus‐eluting stent implantation). J Am Coll Cardiol. 2012;60:1340–1348. doi: 10.1016/j.jacc.2012.06.043 [DOI] [PubMed] [Google Scholar]
- 12. Hahn JY, Song YB, Oh JH, Chun WJ, Park YH, Jang WJ, Im ES, Jeong JO, Cho BR, Oh SK, et al. Effect of p2y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the smart‐choice randomized clinical trial. JAMA. 2019;321:2428–2437. doi: 10.1001/jama.2019.8146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim BK, Hong SJ, Cho YH, Yun KH, Kim YH, Suh Y, Cho JY, Her AY, Cho S, Jeon DW, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the tico randomized clinical trial. JAMA. 2020;323:2407–2416. doi: 10.1001/jama.2020.7580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, et al. Ticagrelor with or without aspirin in high‐risk patients after pci. N Engl J Med. 2019;381:2032–2042. doi: 10.1056/NEJMoa1908419 [DOI] [PubMed] [Google Scholar]
- 15. Valgimigli M, Frigoli E, Heg D, Tijssen J, Juni P, Vranckx P, Ozaki Y, Morice MC, Chevalier B, Onuma Y, et al. Dual antiplatelet therapy after pci in patients at high bleeding risk. N Engl J Med. 2021;385:1643–1655. doi: 10.1056/NEJMoa2108749 [DOI] [PubMed] [Google Scholar]
- 16. Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, et al. Effect of 1‐month dual antiplatelet therapy followed by clopidogrel vs 12‐month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving pci: the STOPDAPT‐2 randomized clinical trial. JAMA. 2019;321:2414–2427. doi: 10.1001/jama.2019.8145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maddox TM, Plomondon ME, Petrich M, Tsai TT, Gethoffer H, Noonan G, Gillespie B, Box T, Fihn SD, Jesse RL, et al. A national clinical quality program for veterans affairs catheterization laboratories (from the veterans affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114:1750–1757. doi: 10.1016/j.amjcard.2014.08.045 [DOI] [PubMed] [Google Scholar]
- 18. Jha AK, Perlin JB, Steinman MA, Peabody JW, Ayanian JZ. Quality of ambulatory care for women and men in the veterans affairs health care system. J Gen Intern Med. 2005;20:762–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kinlay S, Quach L, Cormack J, Morgenstern N, Hou Y, Young M, Sherrod R, Cho K, Faxon DP, Ramadan R, et al. Premature discontinuation of dual antiplatelet therapy after coronary stenting in veterans: characteristics and long‐term outcomes. J Am Heart Assoc. 2021;10:e018481. doi: 10.1161/JAHA.120.018481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsai TT, Stanislawski MA, Shunk KA, Armstrong EJ, Grunwald GK, Schob AH, Valle JA, Alfonso CE, Nallamothu BK, Ho PM, et al. Contemporary incidence, management, and long‐term outcomes of percutaneous coronary interventions for chronic coronary artery total occlusions: insights from the va cart program. JACC Cardiovasc Interv. 2017;10:866–875. doi: 10.1016/j.jcin.2017.02.044 [DOI] [PubMed] [Google Scholar]
- 21. Faxon DP, Lawler E, Young M, Gaziano M, Kinlay S. Prolonged clopidogrel use after bare metal and drug‐eluting stent placement: the veterans administration drug‐eluting stent study. Circ Cardiovasc Interv. 2012;5:372–380. doi: 10.1161/CIRCINTERVENTIONS.111.967257 [DOI] [PubMed] [Google Scholar]
- 22. Siddiqi OK, Smoot KJ, Dufour AB, Cho K, Young M, Gagnon DR, Ly S, Temiyasathit S, Faxon DP, Gaziano JM, et al. Outcomes with prolonged clopidogrel therapy after coronary stenting in patients with chronic kidney disease. Heart. 2015;101:1569–1576. doi: 10.1136/heartjnl-2014-307168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thukkani AK, Agrawal K, Prince L, Smoot KJ, Dufour AB, Cho K, Gagnon DR, Sokolovskaya G, Ly S, Temiyasathit S, et al. Long‐term outcomes in patients with diabetes mellitus related to prolonging clopidogrel more than 12 months after coronary stenting. J Am Coll Cardiol. 2015;66:1091–1101. doi: 10.1016/j.jacc.2015.06.1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wimmer NJ, Dufour AB, Cho K, Gagnon DR, Quach L, Ly S, Do JM, Ostrowski S, Michael Gaziano J, Faxon DP, et al. Long‐term outcomes in patients with acute coronary syndromes related to prolonging dual antiplatelet therapy more than 12 months after coronary stenting. Catheter Cardiovasc Interv. 2017;89:1176–1184. doi: 10.1002/ccd.26831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Task Force on Myocardial Revascularization of the European Society of C, the European Association for Cardio‐Thoracic S, European Association for Percutaneous , Cardiovascular I, Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555.20802248 [Google Scholar]
- 26. Gaffney A, Bor DH, Himmelstein DU, Woolhandler S, McCormick D. The effect of veterans health administration coverage on cost‐related medication nonadherence. Health Aff (Millwood). 2020;39:33–40. doi: 10.1377/hlthaff.2019.00481 [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi T, Glorioso TJ, Armstrong EJ, Maddox TM, Plomondon ME, Grunwald GK, Bradley SM, Tsai TT, Waldo SW, Rao SV, et al. Comparative outcomes after percutaneous coronary intervention among black and white patients treated at us veterans affairs hospitals. JAMA Cardiol. 2017;2:967–975. doi: 10.1001/jamacardio.2017.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathews R, Wang W, Kaltenbach LA, Thomas L, Shah RU, Ali M, Peterson ED, Wang TY. Hospital variation in adherence rates to secondary prevention medications and the implications on quality. Circulation. 2018;137:2128–2138. doi: 10.1161/CIRCULATIONAHA.117.029160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spertus JA, Kettelkamp R, Vance C, Decker C, Jones PG, Rumsfeld JS, Messenger JC, Khanal S, Peterson ED, Bach RG, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the premier registry. Circulation. 2006;113:2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066 [DOI] [PubMed] [Google Scholar]
- 30. Stefanescu Schmidt AC, Steg PG, Yeh RW, Kereiakes DJ, Tanguay JF, Hsieh WH, Massaro JM, Mauri L, Cutlip DE, Investigators D. Interruption of dual antiplatelet therapy within six months after coronary stents (from the dual antiplatelet therapy study). Am J Cardiol. 2019;124:1813–1820. doi: 10.1016/j.amjcard.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 31. Mehran R, Baber U, Steg PG, Ariti C, Weisz G, Witzenbichler B, Henry TD, Kini AS, Stuckey T, Cohen DJ, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (Paris): 2 year results from a prospective observational study. Lancet. 2013;382:1714–1722. doi: 10.1016/S0140-6736(13)61720-1 [DOI] [PubMed] [Google Scholar]
- 32. Kandzari DE, Kirtane AJ, Windecker S, Latib A, Kedhi E, Mehran R, Price MJ, Abizaid A, Simon DI, Worthley SG, et al. One‐month dual antiplatelet therapy following percutaneous coronary intervention with zotarolimus‐eluting stents in high‐bleeding‐risk patients. Circ Cardiovasc Interv. 2020;13:e009565. doi: 10.1161/CIRCINTERVENTIONS.120.009565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krucoff MW, Urban P, Tanguay JF, McAndrew T, Zhang Y, Rao SV, Morice MC, Price MJ, Cohen DJ, Abdel‐Wahab M, et al. Global approach to high bleeding risk patients with polymer‐free drug‐coated coronary stents: the LF II study. Circ Cardiovasc Interv. 2020;13:e008603. doi: 10.1161/CIRCINTERVENTIONS.119.008603 [DOI] [PubMed] [Google Scholar]
- 34. Mehran R, Cao D, Angiolillo DJ, Bangalore S, Bhatt DL, Ge J, Hermiller J, Makkar RR, Neumann FJ, Saito S, et al. 3‐ or 1‐month dapt in patients at high bleeding risk undergoing everolimus‐eluting stent implantation. JACC Cardiovasc Interv. 2021;14:1870–1883. doi: 10.1016/j.jcin.2021.07.016 [DOI] [PubMed] [Google Scholar]
- 35. Vranckx P, Valgimigli M, Juni P, Hamm C, Steg PG, Heg D, van Es GA, McFadden EP, Onuma Y, van Meijeren C, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug‐eluting stent: a multicentre, open‐label, randomised superiority trial. Lancet. 2018;392:940–949. doi: 10.1016/S0140-6736(18)31858-0 [DOI] [PubMed] [Google Scholar]
- 36. Watanabe H, Morimoto T, Natsuaki M, Yamamoto K, Obayashi Y, Ogita M, Suwa S, Isawa T, Domei T, Yamaji K, et al. Comparison of clopidogrel monotherapy after 1 to 2 months of dual antiplatelet therapy with 12 months of dual antiplatelet therapy in patients with acute coronary syndrome: the STOPDAPT‐2 ACS randomized clinical trial. JAMA Cardiol. 2022;7:407–417. doi: 10.1001/jamacardio.2021.5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kinlay S. Post‐pci antithrombotic treatment with high bleeding risk. J Am Coll Cardiol. 2022;80:1238–1240. doi: 10.1016/j.jacc.2022.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hahn JY, Song YB, Oh JH, Cho DK, Lee JB, Doh JH, Kim SH, Jeong JO, Bae JH, Kim BO, et al. 6‐month versus 12‐month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (smart‐date): a randomised, open‐label, non‐inferiority trial. Lancet. 2018;391:1274–1284. doi: 10.1016/S0140-6736(18)30493-8 [DOI] [PubMed] [Google Scholar]
- 39. Giacoppo D, Matsuda Y, Fovino LN, D'Amico G, Gargiulo G, Byrne RA, Capodanno D, Valgimigli M, Mehran R, Tarantini G. Short dual antiplatelet therapy followed by p2y12 inhibitor monotherapy vs. prolonged dual antiplatelet therapy after percutaneous coronary intervention with second‐generation drug‐eluting stents: a systematic review and meta‐analysis of randomized clinical trials. Eur Heart J. 2021;42:308–319. doi: 10.1093/eurheartj/ehaa739 [DOI] [PubMed] [Google Scholar]
- 40. Kuno T, Ueyama H, Takagi H, Fox J, Bangalore S. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome: insights from a network meta‐analysis of randomized trials. Cardiovasc Revasc Med. 2021;28:50–56. doi: 10.1016/j.carrev.2020.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figure S1


