Abstract
Background
Heart failure with recovered ejection fraction (HFrecEF) has been a newly recognized entity since 2020. However, the concept has primarily focused on left ventricular ejection fraction improvement, with less focus on the recovery of the left atrium. In this study, we investigated changes in left atrial (LA) echocardiographic indices in HFrecEF.
Methods and Results
An inpatient cohort with heart failure with reduced ejection fraction (HFrEF) was identified retrospectively and followed up prospectively in a single tertiary hospital. The enrolled patients were classified into HFrecEF and persistent HFrEF groups. Alternations in LA parameters by echocardiography were calculated. The primary outcome was a composite of cardiovascular death or heart failure rehospitalization. A total of 699 patients were included (HFrecEF: n=228; persistent HFrEF: n=471). Compared with persistent HFrEF, the HFrecEF group had greater reductions in LA diameter, LA transverse diameter, LA superior–inferior diameter, LA volume, and LA volume index but not in LA sphericity index. Cox regression analysis showed that the HFrecEF group experienced lower risks of prespecified end points than the persistent HFrEF group after adjusting for confounders. Additionally, 136 (59.6%) and 62 (13.0%) patients showed LA reverse remodeling (LARR) for the HFrecEF and persistent HFrEF groups, respectively. Among the HFrecEF subgroup, patients with LARR had better prognosis compared with those without LARR. Multivariate logistic analysis demonstrated that age and coronary heart disease were 2 independent negative predictors for LARR.
Conclusions
In HFrecEF, both left ventricular systolic function and LA structure remodeling were improved. Patients with HFrecEF with LARR had improved clinical outcomes, indicating that the evaluation of LA size provides a useful biomarker for risk stratification of heart failure.
Keywords: heart failure with recovered ejection fraction, left atrium, remodeling, reverse
Subject Categories: Heart Failure, Remodeling
Clinical Perspective.
What Is New?
Compared with patients with persistent heart failure with reduced ejection fraction, both left ventricular systolic function and left atrial structure were improved in patients with heart failure with recovered ejection fraction.
In the heart failure with recovered ejection fraction subgroup, patients with left atrial reverse remodeling had improved clinical outcomes
What Are the Clinical Implications?
The evaluation of left atrial size provides a useful biomarker for the risk stratification of heart failure
Older age and the presence of coronary heart disease are 2 negative predictors for left atrial reverse remodeling, highlighting the importance of treating the cause for the management of heart failure.
Nonstandard Abbreviations and Acronyms
- CRT
cardiac resynchronization therapy
- E/e′
mitral Doppler early velocity/mitral annular early velocity
- GDMT
guideline‐directed medical therapy
- HFrecEF
heart failure with recovered ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LARR
left atrial reverse remodeling
Left ventricular ejection fraction (LVEF) is the main parameter used for the classification of heart failure (HF). The latest European Society of Cardiology HF guideline recommended that HF was divided into 3 distinct phenotypes: HF with reduced ejection fraction (HFrEF, LVEF <40%), HF with mildly reduced ejection fraction (LVEF 40%–49%), and HF with preserved ejection fraction (LVEF ≥50%) based on LVEF measurement. 1 However, LVEF is not static but can vary dynamically. Deterioration of LVEF occurs during sudden cardiac injury, abnormal neurohormonal factors, sustained cardiac pressure, or volume overload, whereas the recovery of LVEF presents when eliminating the risk factors, receiving guideline‐directed medical therapy (GDMT), or after invasive or surgical management. 2 Therefore, just measuring LVEF once at 1 time point may be far from adequate, and ongoing evaluation in the trajectory of LVEF over time is an important predictor of adverse outcomes. 3
Previous studies have focused on the trajectory of LVEF to detect the incidence, predictors, and prognosis of LVEF changes in patients with HF. 4 , 5 , 6 The 2020 Journal of the American College of Cardiology Scientific Expert Panel recommended a novel working definition of heart failure with recovered ejection fraction (HFrecEF), which includes the following 2 : (1) documentation of a decreased LVEF <40% at baseline, (2) ≥10% absolute improvement in LVEF, and (3) a second measurement of LVEF >40%. An additional diagnostic criterion for HFrecEF is that the second echocardiographic examination must be at least 3 to 6 months after the baseline assessment to avoid acute changes because of heart rate and cardiac load. Extensive studies have proved that HFrecEF is generally associated with a better clinical outcome, but improvement in LVEF does not imply full myocardial recovery or normalization of LV function. 7 , 8 Moreover, the concept of HFrecEF is mainly focused on the improvement of cardiac systolic function, characterized by raised LVEF, with less attention paid to the recovery of left atrial (LA) structure. In this study, we investigated the clinical characteristics, prognosis, and improvement of LA structure in patients with HFrecEF.
Methods
Data and study materials are available upon reasonable request through the Department of Cardiology, The First Affiliated Hospital of Dalian Medical University.
Study Population and Grouping
This study was approved by the institutional review board of The First Affiliated Hospital of Dalian Medical University. The procedures were conducted in accordance with the Declaration of Helsinki and its amendments. All subjects provided informed consent. No identifiable data were included in the database extracted for this study.
This cohort, which included patients with HFrEF hospitalized at The First Affiliated Hospital of Dalian Medical University between January 1, 2015 and October 31, 2019, was identified retrospectively and followed prospectively. Patients with end‐stage renal failure, in‐hospital death, missing echocardiography data, and who were lost to follow‐up were excluded from the study. Enrolled patients underwent at least 2 echocardiographic examinations. When >2 tests were available, the first and last assessments were used to calculate the changes in echocardiographic indices. The time interval between the 2 echocardiography examinations was at least 3 months. According to the recovery of LVEF, the cohort was divided into the HFrecEF group and persistent HFrEF group. The subjects who met the HFrecEF criteria were included in the HFrecEF group, whereas the others were enrolled in the persistent HFrEF group. Baseline demographics, laboratory data, echocardiogram findings, and medications were collected from Yidu Cloud, which is one of the largest medical databases in China.
Clinical Definitions
HFrecEF was defined according to the 2020 Journal of the American College of Cardiology HFrecEF Expert Consensus. The diagnostic criteria were: (1) documentation of a decreased LVEF <40% at baseline, (2) ≥10% absolute improvement in LVEF, and (3) a second measurement of LVEF >40%. End‐stage renal failure was defined by estimated glomerular filtration rate <30 mL/min per 1.73 m2.
Changes in LA Echocardiographic Indices
In this study, 3 indices were calculated to evaluate LA structure remodeling, including LA volume index, LA sphericity index, and LA reverse remodeling (LARR). LA volume index, measured by 2‐dimensional echocardiography, is an accurate descriptor of LA volume and could reflect LV diastolic dysfunction. 9 LA sphericity index is a novel index to assess the agreement between LA shape and a perfect sphere, which is more sensitive and changes earlier than LA volume index when exposed to varying stressors. 10 It can be obtained by calculating the ratio of the transverse and longitudinal diameters of the left atrium. LARR is defined as a reduction >15% in the LA end‐systolic volume. 11 Other relevant indicators, such as LA diameter, LA transverse diameter, LA superior–inferior diameter, and LA volume were also recorded.
Clinical Outcomes on Follow‐Up
The adverse end points were the composite of cardiovascular death or HF‐related admission, cardiovascular death, and HF‐related admission. All enrolled subjects were encouraged to return to the outpatient clinic regularly. If the patients did not attend their scheduled clinic appointments, they would be contacted by telephone. The deadline for follow‐up was October 31, 2020 or the occurrence of prespecified end points, whichever was earlier.
Statistical Analysis
Statistical analysis was performed with Statistical Package for Social Sciences, version 24.0 (IBM, Armonk, NY). Categorical variables were expressed as percentage, whereas continuous variables were presented as median (interquartile range) (nonnormal distribution) or mean±SD (normal distribution). Descriptive characteristics were compared between the 2 groups using χ2, Kruskal‐Wallis, and independent‐sample t tests for categorical, nonnormally distributed, and normally distributed variables, respectively. Kaplan‐Meier analysis was performed to calculate the incidence of adverse end points, with a log‐rank test assessing the differences. Cox regression analysis was constructed to compare the risks of adverse events. Covariates in the multivariate analysis included age, body weight, systolic blood pressure, heart rate, coronary artery disease, diabetes, hemoglobin, urea, BNP (B‐type natriuretic peptide), high‐sensitivity troponin I, left ventricular end‐diastolic diameter, mitral Doppler early velocity/mitral annular early velocity, time interval between echocardiograms, spironolactone, loop diuretic, aspirin, statins, nitrate, cardiac resynchronization therapy, which were statistically different at baseline between the groups. Additional covariates adjusted for clinically relevant characteristics, including atrial fibrillation, β‐blockers, and renin‐angiotensin‐aldosterone system blockers. For the model comparing the risks of adverse outcomes between the LARR and no‐LAAR groups, covariates included age, sex, BNP, and LVEF. Hazard ratio (HR) with 95% CI were presented. Logistic regression analysis was used to identify the independent factors that predict LA reverse remodeling. A 2‐sided P value <0.05 was considered to be statistically different.
Results
Demographic and Clinical Characteristics
A total of 1037 patients with HFrEF were initially included. Of these, 338 cases were excluded to meet the exclusion criteria. Consequently, the remaining 699 patients were eventually involved in our study (Figure 1). Based on LVEF recovery, 228 (32.6%) patients were assigned into the HFrecEF group and 471 (67.4%) to the persistent HFrEF group. Their baseline characteristics are shown in Table 1. Overall, compared with the persistent HFrEF group, patients in the HFrecEF group were younger, had higher blood pressure and faster heart rate, lower frequency of diabetes and coronary heart disease, and lower levels of BNP and high‐sensitivity troponin I. Moreover, they showed lower left ventricular end‐diastolic diameter and mitral Doppler early velocity/mitral annular early velocity, and were less likely to receive medications, such as spironolactone, loop diuretic, aspirin, statins, nitrate, and cardiac resynchronization therapy. Notably, the interval between 2 echocardiography tests was longer for the persistent HFrEF group.
Figure 1.
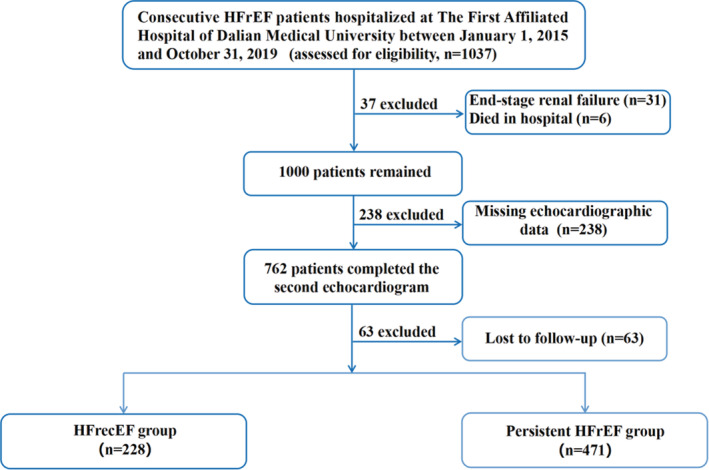
Flow diagram of inclusion and exclusion of study subjects.
HFrecEF indicates heart failure with recovered ejection fraction; and HFrEF, heart failure with reduced ejection fraction.
Table 1.
Baseline Demographics and Clinical Characteristics of Patients at the Time of First Echocardiography
| Variables | HFrecEF group, n=228 | Persistent HFrEF group, n=471 | P value |
|---|---|---|---|
| Age, y | 58.87±15.02 | 65.04±12.76 | <0.001 |
| Men, n (%) | 177 (77.6) | 339 (72.0) | 0.111 |
| SBP, mm Hg | 136.07±24.33 | 128.56±22.28 | <0.001 |
| DBP, mm Hg | 85.27±17.45 | 79.27±14.36 | <0.001 |
| Heart rate, bpm | 89.27±21.86 | 82.72±18.79 | <0.001 |
| Smoking, n (%) | 84 (36.8) | 184 (39.0) | 0.571 |
| Alcohol consumption, n (%) | 51 (22.3) | 111 (23.5) | 0.725 |
| QRS duration, ms | 108.02±28.77 | 118.71±36.41 | <0.001 |
| QTc interval, ms | 480.58±42.21 | 479.18±56.16 | 0.725 |
| Height, m | 1.71±0.08 | 1.69±0.08 | 0.105 |
| Weight, kg | 76.39±16.07 | 73.19±15.03 | 0.012 |
| BSA, kg/m2 | 1.96±0.22 | 1.93±0.23 | 0.180 |
| NYHA class III/IV, n (%) | 54 (23.7) | 125 (26.5) | 0.417 |
| Coronary heart disease (n,%) | 59 (25.9) | 220 (46.7) | <0.001 |
| Hypertension, n (%) | 147 (64.5) | 289 (61.4) | 0.425 |
| Atrial fibrillation, n (%) | 87 (38.2) | 150 (31.8) | 0.299 |
| Diabetes, n (%) | 67 (29.4) | 169 (35.9) | <0.001 |
| Cerebrovascular disease, n (%) | 20 (8.8) | 58 (12.3) | 0.163 |
| Laboratory values | |||
| White blood cells, 109/L | 6.91 (5.68–8.25) | 6.77 (5.38–8.44) | 0.671 |
| Neutrophil, % | 62.8 (55.9–70.3) | 65.1 (57.0–72.1) | 0.100 |
| Neutrophil, 109/L | 4.28 (3.25–5.63) | 4.27 (3.25–5.68) | 0.790 |
| Hemoglobin, g/L | 140.52±21.82 | 135.74±20.45 | 0.005 |
| Platelet, 109/L | 199 (160–246) | 191 (159–232) | 0.215 |
| BNP, pg/mL | 692.2 (355.9–1427.9) | 900.1 (451.3–1650.0) | 0.010 |
| D‐dimer, μmol/L | 670 (285–1235) | 680 (330–1373) | 0.406 |
| Glucose, mmol/L | 5.35 (4.72–6.57) | 5.40 (4.76–7.09) | 0.160 |
| Triglyceride, mmol/L | 1.18 (0.86–1.53) | 1.11 (0.87–1.52) | 0.634 |
| Cholesterol, mmol/L | 4.10 (3.48–5.23) | 4.22 (3.46–5.04) | 0.944 |
| LDL‐C, mmol/L | 2.29 (1.89,3.04) | 2.41 (1.87–3.02) | 0.842 |
| HDL‐C, mmol/L | 1.00 (0.79–1.22) | 1.01 (0.82–1.20) | 0.608 |
| hs‐TnI, μg/L | 0.044 (0.020–0.116) | 0.058 (0.027–0.198) | 0.009 |
| Urea, mmol/L | 7.54 (5.67–9.47) | 7.94 (6.33–10.31) | 0.020 |
| Creatinine, mmol/L | 85 (72–101) | 87 (74–112) | 0.145 |
| Uric acid, mmol/L | 463 (361–583) | 445 (366–567) | 0.873 |
| Serum sodium, mmol/L | 141 (139–144) | 141 (138–143) | 0.053 |
| Serum potassium, mmol/L | 3.98±0.49 | 3.98±0.51 | 0.902 |
| Echocardiography findings | |||
| LVEDD, mm | 60.29±7.78 | 62.03±9.38 | 0.010 |
| LVEF | 30.05±6.14 | 30.59±6.12 | 0.275 |
| IVS, mm | 10.63±1.70 | 10.17±1.94 | 0.002 |
| LVPWT, mm | 10.00 (9.75, 11.00) | 10.0 (9.00, 11.00) | <0.001 |
| LAD, mm | 45.76±7.58 | 44.81±6.32 | 0.080 |
| LATD, mm | 49.23±8.07 | 48.52±6.52 | 0.209 |
| LASID, mm | 62.68±8.85 | 62.21±8.77 | 0.509 |
| E/e′ | 12.3 (10.3, 16.0) | 14.3 (11.0, 19.0) | 0.002 |
| EDT, mms | 150 (120, 190) | 150 (127, 190) | 0.465 |
| LAV, mL | 70.54 (56.09, 84.81) | 68.12 (54.19, 87.71) | 0.381 |
| LAVI, mL/m2 | 44.55 (32.32, 64.83) | 42.50 (31.03, 67.07) | 0.640 |
| LASI | 0.786±0.067 | 0.783±0.066 | 0.592 |
| Time interval, mo | 13.5 (7.0, 26.0) | 17.0 (8.0, 31.0) | 0.007 |
| Medications | |||
| β‐Blockers, n (%) | 221 (96.9) | 450 (95.5) | 0.380 |
| RAAS blockers, n (%) | 181 (79.4) | 366 (77.7) | 0.614 |
| Spironolactone, n (%) | 134 (58.8) | 339 (72.0) | <0.001 |
| Digoxin, n (%) | 66 (28.9) | 128 (27.2) | 0.624 |
| Loop diuretic, n (%) | 78 (34.2) | 241 (51.2) | <0.001 |
| Aspirin, n (%) | 74 (32.5) | 219 (46.5) | <0.001 |
| Statins, n (%) | 102 (44.7) | 269 (57.1) | 0.002 |
| Nitrates, n (%) | 53 (23.2) | 181 (38.4) | <0.001 |
| Warfarin, n (%) | 70 (30.7) | 140 (29.7) | 0.792 |
| ICD, n (%) | 2 (0.9) | 13 (2.8) | 0.107 |
| CRT, n (%) | 5 (2.2) | 29 (6.2) | 0.022 |
Categorical data are presented as percentages, and a χ2 test was used to compare the differences. Continuous variables with nonnormal distribution are expressed as median (interquartile range) and were analyzed using the Kruska‐Wallis test. Continuous variables with normal distribution are presented as mean±SD, and an independent‐sample t test was applied to assess the differences. BNP indicates B‐type natriuretic peptide; BSA, body surface area; CRT, cardiac resynchronization therapy; DBP, diastolic blood pressure; E/e′, mitral Doppler early velocity/mitral annular early velocity; EDT, E peak deceleration time; HDL‐C, high‐density lipoprotein cholesterol; HFrecEF, heart failure with recovered ejection fraction; HFrEF, heart failure with reduced ejection fraction; hs‐TnI, high‐sensitivity troponin I; ICD, implanted cardiac device; IVS, interventricular septal; LAD, left atrium diameter; LASI, left atrial sphericity index; LASID, left atrial superior–inferior diameter; LATD, left atrial transverse diameter; LAV, left atrial volume; LAVI, left atrial volume index; LDL‐C, low‐density lipoprotein cholesterol; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVPWT, left ventricular posterior wall thickness; NYHA, New York Heart Association; RAAS, renin‐angiotensin‐aldosterone system; and SBP, systolic blood pressure.
Changes in Echocardiographic Data
Alterations in echocardiographic parameters from baseline to follow‐up were calculated and compared between the HFrecEF and persistent HFrEF groups. Compared with the persistent HFrEF group, the HFrecEF group had greater reductions in left atrial diameter (median reduction: −4.00 [−7.00 to 0.00] versus 2.00 [−3.00 to 6.00], P<0.001), left atrial transverse diameter (median reduction: −4.00 [−8.00 to 0.00] versus 1.00 [−4.00 to 6.00], P<0.001), left atrial superior–inferior diameter (median reduction: −4.00 [−10.00 to 0.00] versus 1.00 [−5.00 to 7.00], P<0.001), left atrial volume (median reduction: −14.46 [−27.85 to −0.89] versus 4.19 [−11.08 to 22.56], P<0.001), and left atrial volume index (median reduction: −5.20 [−13.64 to 1.59] versus 6.89 [−1.80 to 20.61], P<0.001) but not in left atrial sphericity index (median reduction: 0.000 [−0.058 to 0.043] versus −0.005 [−0.061 to 0.048], P=0.800) (Figure 2).
Figure 2.
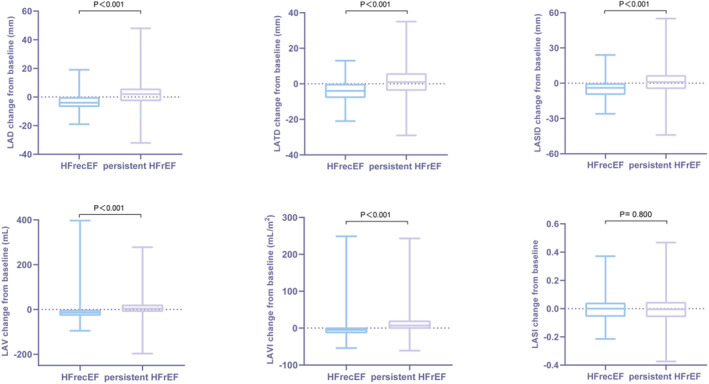
Changes in echocardiographic indices from baseline to follow‐up for HFrecEF and persistent HFrEF groups.
HFrecEF indicates heart failure with recovered ejection fraction; HFrEF, heart failure with reduced ejection fraction; LAD, left atrial diameter; LASI, left atrial sphericity index; LASID; left atrial superior–inferior diameter; LATD, left atrial transverse diameter; LAV, left atrial volume; and LAVI, left atrial volume index.
Adverse Outcomes on Follow‐Up
On follow‐up, 245 patients met the composite end point (HFrecEF: n=34 [14.9%] versus persistent HFrEF: n=211 [44.3%]), of which 106 died from cardiovascular causes (HFrecEF: n=14 [6.1%] versus persistent HFrEF: n=92 [19.5%]), and 166 were rehospitalized for worsening HF (HFrecEF: n=21 [9.2%] versus persistent HFrEF: n=145 [30.7%]). The Kaplan‐Meier survival curve shows that those in the persistent HFrEF group experienced a higher incidence of prespecified adverse end points than the HFrecEF group (Figure 3). Cox regression analysis demonstrated that the persistent HFrEF group had higher risks of composite outcome (HR, 3.479 [95% CI, 2.422–4.999]; P<0.001), HF rehospitalization (HR, 3.454 [95% CI, 2.186–5.460]; P<0.001), and cardiovascular death (HR, 3.606 [95% CI, 2.017–6.448]; P<0.001). The association remained significant for the composite outcome (HR, 2.734 [95% CI, 1.823–4.102]; P<0.001), HF rehospitalization (HR, 2.916 [95% CI, 1.777–4.786]; P<0.001), and cardiovascular death (HR, 2.597 [95% CI, 1.331–5.067]; P=0.005) after adjusting for confounding factors (Table 2).
Figure 3.
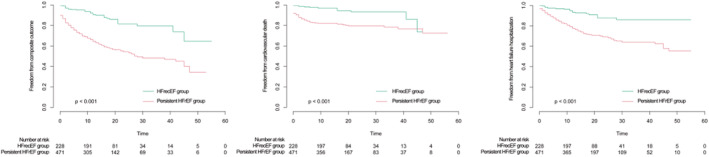
Kaplan‐Meier survival curve for adverse events between the HFrecEF and persistent HFrEF groups.
HFrecEF indicates heart failure with recovered ejection fraction; and HFrEF, heart failure with reduced ejection fraction.
Table 2.
Cox Regression Analysis for Adverse Outcomes Between HFrecEF and Persistent HFrEF Groups
| Clinical outcomes | Unadjusted | Fully adjusted * | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Cardiovascular death or heart failure hospitalization | … | … | … | … | … | … |
| HFrecEF group | 1 | reference | NA | 1 | reference | NA |
| Persistent HFrEF group | 3.479 | 2.422–4.999 | <0.001 | 2.734 | 1.823–4.102 | <0.001 |
| Cardiovascular death | … | … | … | … | … | … |
| HFrecEF group | 1 | reference | NA | 1 | reference | NA |
| Persistent HFrEF group | 3.606 | 2.017–6.448 | <0.001 | 2.597 | 1.331–5.067 | 0.005 |
| Heart failure hospitalization | … | … | … | … | … | … |
| HFrecEF group | 1 | reference | NA | 1 | reference | NA |
| Persistent HFrEF group | 3.454 | 2.186–5.460 | <0.001 | 2.916 | 1.777–4.786 | <0.001 |
HFrecEF indicates heart failure with recovered ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; and NA, not applicable.
Adjusted for age, body weight, systolic blood pressure, heart rate, atrial fibrillation, coronary artery disease, diabetes, hemoglobin, urea, B‐type natriuretic peptide, high‐sensitivity troponin I, left ventricular end‐diastolic diameter, mitral Doppler early velocity/mitral annular early velocity, time interval between echocardiograms, β‐blockers, renin‐angiotensin‐aldosterone system blockers, aspirin, spironolactone, loop diuretic, statins, nitrate, and cardiac resynchronization therapy.
LARR in HFrecEF
In this study, 136 cases (59.6%) in the HFrecEF group and 62 (13.0%) in the persistent HFrEF group showed LARR (P<0.001) (Figure 4). Cox regression analysis showed that patients with HFrecEF with LARR experienced lower risks of the composite outcome (HR, 2.276 [95% CI, 1.149–4.509]; P=0.018) and cardiovascular death (HR, 3.809 [95% CI, 1.194–12.150]; P=0.024), but not HF rehospitalization (HR, 1.700 [95% CI, 0.722–4.005]; P=0.225). The association persisted after adjustment (adjusted HR, 2.745 [95% CI, 1.348–5.589]; P=0.005 for composite outcome; adjusted HR, 3.613 [95% CI, 1.088–11.992]; P=0.036 for cardiovascular death) (Table 3). Multivariate logistic regression analysis revealed that higher systolic blood pressure (odds ratio [OR], 1.014 [95% CI, 1.002–1.025]; P=0.018) and low‐density lipoprotein cholesterol (OR, 1.247 [95% CI, 1.017–1.529]; P=0.034) were associated with LARR. Age (OR, 0.980 [95% CI, 0.967–0.992]; P=0.001) and coronary heart disease (OR, 0.630 [95% CI, 0.434–0.914]; P=0.015) were identified as 2 negative predictors of LARR (Table 4).
Figure 4.
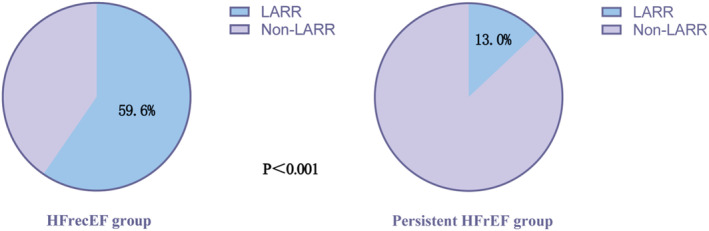
LARR in HFrecEF and persistent HFrEF groups.
HFrecEF indicates heart failure with recovered ejection fraction; HFrEF, heart failure with reduced ejection fraction; and LARR, left atrial reverse remodeling.
Table 3.
Cox Regression Analysis for Adverse Outcomes Between LARR and No LARR Groups Among HFrecEF Subtype
| Clinical outcomes | Unadjusted | Fully adjusted * | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Cardiovascular death or heart failure hospitalization | … | … | … | … | … | … |
| No LARR vs LARR | 2.276 | 1.149–4.509 | 0.018 | 2.745 | 1.348–5.589 | 0.005 |
| Cardiovascular death | … | … | … | … | … | … |
| No LARR vs LARR | 3.809 | 1.194–12.150 | 0.024 | 3.613 | 1.088–11.992 | 0.036 |
| Heart failure hospitalization | … | … | … | … | … | … |
| No LARR vs LARR | 1.700 | 0.722–4.005 | 0.225 | 2.101 | 0.870–5.072 | 0.099 |
HR indicates hazard ratio; and LARR, left atrial reverse remodeling.
Adjusted for age, sex, B‐type natriuretic peptide, and left ventricular ejection fraction.
Table 4.
Logistic Regression Analysis to Identify Predictors of LARR
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 0.971 | 0.959–0.982 | <0.001 | 0.98 | 0.967–0.992 | 0.001 |
| SBP | 1.018 | 1.007–1.029 | 0.001 | 1.014 | 1.002–1.025 | 0.018 |
| Heart rate | 1.012 | 1.004–1.020 | 0.004 | 1.008 | 0.999–1.017 | 0.072 |
| Hemoglobin | 1.014 | 1.005–1.022 | 0.002 | 1.007 | 0.997–1.016 | 0.161 |
| LDL‐C | 1.243 | 1.021–1.513 | 0.031 | 1.247 | 1.017–1.529 | 0.034 |
| LVEF | 0.968 | 0.942–0.0994 | 0.015 | 0.984 | 0.956–1.014 | 0.298 |
| LASID | 1.025 | 1.001–1.048 | 0.037 | 1.017 | 0.992–1.043 | 0.178 |
| Coronary heart disease | 0.566 | 0.398–0.804 | 0.001 | 0.63 | 0.434–0.914 | 0.015 |
LASID indicates left atrial superior–inferior diameter; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; OR, odds ratio; and SBP, systolic blood pressure.
Discussion
The major findings in our study were (1) patients with HFrecEF showed LA structure reverse remodeling and, (2) among the HFrecEF subtype, patients with LARR experienced lower risks of adverse events compared with those without LARR.
The atrium can undergo structural, electrical, and metabolic remodeling in different disease states. Potential molecular mechanisms include myocyte hypertrophy, cell death, inflammation, fibrosis, and capillary density, which are stimulated by various neurohumoral factors. 12 , 13 LARR has been described as the temporal process of a reduction in LA size or a restoration of specific functional parameters after the removal of external stressors. 13 LA volume is generally considered to be an important echocardiogram parameter to evaluate LA structure change, and strain analysis has been extensively used to describe the functional aspects of LARR. Multiple studies have demonstrated that LA dysfunction may precede changes in LA structure. 14 , 15 , 16 , 17 Thus, we speculate that the changes in LA function may be more sensitive than those of LA volume in detecting LA remodeling, which should be investigated in future researche.
Numerous clinical studies have confirmed that LA remodeling could be reversed with GDMT, including angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, mineralocorticoid receptors antagonists, 18 , 19 angiotensin receptor‐neprilysin inhibitors, 20 and with invasive/surgical approaches. 21 In this study, the proportions of patients taking these medications were comparable in both groups (β‐blockers, P=0.38; renin‐angiotensin‐aldosterone system blockers, P=0.61; implanted cardiac device, P=0.11). In contrast, we found that patients with HFrecEF were younger and experienced a lower prevalence of coronary heart disease. Logistic regression analysis also confirmed that younger age and absence of coronary heart disease were 2 independent predictors of LARR. The aging process, representing a complex interaction of biological and environmental risk factors, is strongly associated with cardiovascular morbidity and mortality. The left atrium gradually dilates with age, which may be attributable to age‐related increase in LV myocardial stiffness. 22 Yoshida et al reported that age was an independent predictor of LA phasic strain parameters, including reservoir, conduit, and pump strain, even after full multivariate adjustment. 23 In short, age has been proven to be related to increases in LA volume and decreases in LA reservoir and passive function. Currently, few studies have described the relationship between ischemic cardiomyopathy and atrial remodeling. Ahn et al conducted a cohort study of 105 patients to investigate the effect of myocardial perfusion on LA remodeling and its determinants following primary percutaneous coronary interventionfor acute myocardial infarction. 24 Despite no overall change of left atrial volume, evidence of significant LA reverse remodeling based on myocardial perfusion grade was reported, with LA volume increasing at thrombolysis in myocardial infarction myocardial perfusion grade 0/1, decreasing at thrombolysis in myocardial infarction myocardial perfusion grade 3, and demonstrating no change at thrombolysis in myocardial infarction myocardial perfusion grade 2. Moreover, perfusion grade and anterior location of myocardial infarction were independent determinants of LA remodeling. Overall, although HF therapies were similar between the 2 groups, the lower prevalence of coronary heart disease and younger age may account for more LARR in patients with HFrecEF.
LVEF has been widely regarded as the most prognostic index for HFrEF. 25 Comparatively, the left atrium has received less focus, although it is used for assessing cardiac diastolic function in HFpEF. In this study, we found that patients with HFrecEF with LARR had better clinical outcomes, characterized by lower risks of cardiovascular death and composite outcome. Other studies have also reported that patients with LARR would have improved clinical end points. A prospective longitudinal follow‐up study from Japan indicated that LARR was associated with a lower cumulative 6‐month incidence of composite of all‐cause death or hospitalization for HF, and the effect was based on LV reverse remodeling. 26 The multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy trial demonstrated that the majority of patients with LV dysfunction and mild HF symptoms selected for cardiac resynchronization therapy–defibrillator therapy experienced a ≥20% reduction in LA volume, whereas the implanted cardiac device–only group had a significantly lower LA response at 1 year (median left atrial volume reduction: 29% [20%–36%] versus 10% [5%–14%]). 27 Consequently, patients who showed a favorable LA response to cardiac resynchronization therapy–defibrillator therapy experienced lower risks of atrial tachycardia, HF events, and death. Cardiac electrophysiological studies have reported that LARR identified by a shrinking LA volume was associated with a reduction of atrial fibrillation recurrence in patients with HF. 28 , 29 , 30 Although several small studies have reported that LARR could significantly improve outcomes, large multicenter trials with stronger quality are needed to confirm these findings.
The 2022 American College of Cardiology/American Heart Association/Heart Failure Society of America HF Guideline recommended a new classification of HF, adding a new type of HF with improved ejection fraction. 7 Compared with HFrecEF, the definition of HF with improved ejection fraction is relatively loose, with previous LVEF ≤40% and a follow‐up measurement of LVEF >40%, omitting the cutoff value of improvement in LVEF. In our opinion, HF with improved ejection fraction is not the same as HFrecEF, and the improvement in LVEF does not mean HF has recovered. In HFrecEF, the trajectory in LVEF is relatively stable and less likely to have deterioration in LVEF and relapse of HF. Because of lack of evidence, the current guidelines and consensus suggest continuous treatment for HF even with normalizing LVEF and LV size, because these improvements most often represent myocardial remission rather than a true cure of HF. However, in clinical practice, we observed that certain types of the HF population with LVEF recovery, such as HF with younger age, nonischemic cause, shorter duration of disease, and fewer comorbidities, could maintain a non‐HF state for a long time even if weaning or withdrawing GDMT. Currently, there has been only 1 randomized controlled clinical trial to assess the safety of weaning GDMT in patients with nonischemic HFrecEF. 31 Future clinical trials are needed to establish evidence of the feasibility of GDMT withdrawal for various causes and types of HFrecEF.
Limitations
There were several limitations in this study. First, considering the retrospective nature of this study, selection bias is inevitable. The present study population comprised 699 patients of 1037 initially identified cases, with 338 participants being excluded, resulting in a significant selection bias that may affect the accuracy of the study results. Second, because of the relatively small single‐center sample size, the findings need to be confirmed in larger multicenter clinical studies. Last, LARR contains structure, function, and electricity reverse remodeling. Nevertheless, in this study, we mainly focused on exploring LA structure reverse remodeling in HFrecEF, with little data about function (strain analysis) and electricity (atrial fibrillation) remodeling.
Conclusions
In patients with HFrecEF, improvement in both LV systolic function and LA structure remodeling was observed. Patients with HFrecEF with LARR had improved clinical outcomes, indicating that the evaluation of LA size provides a useful biomarker for the risk stratification of HF.
Sources of Funding
This study was supported by the National Natural Science Foundation of China (grant numbers U1908209 and 82170385).
Disclosures
None.
Acknowledgments
All authors listed have made a substantial, direct, and intellectual contribution to this work.
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Gary Tse, Email: garytse86@gmail.com, Email: gary.tse@kmms.ac.uk.
Ying Liu, Email: yingliu.med@gmail.com.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Wilcox JE, Fang JC, Margulies KB, Mann DL. Heart failure with recovered left ventricular ejection fraction: JACC scientific expert panel. J Am Coll Cardiol. 2020;76:719–734. doi: 10.1016/j.jacc.2020.05.075 [DOI] [PubMed] [Google Scholar]
- 3. Zhang X, Sun Y, Zhang Y, Chen F, Zhang S, He H, Song S, Tse G, Liu Y. Heart failure with midrange ejection fraction: prior left ventricular ejection fraction and prognosis. Front Cardiovasc Med. 2021;8:697221. doi: 10.3389/fcvm.2021.697221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gu J, Yin ZF, Zhang HL, Fan YQ, Zhang JF, Wang CQ. Characteristics and outcomes of transitions among heart failure categories: a prospective observational cohort study. ESC Heart Fail. 2020;7:616–625. doi: 10.1002/ehf2.12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savarese G, Vedin O, D'Amario D, Uijl A, Dahlstrom U, Rosano G, Lam CSP, Lund LH. Prevalence and prognostic implications of longitudinal ejection fraction change in heart failure. JACC Heart Fail. 2019;7:306–317. doi: 10.1016/j.jchf.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 6. Zhang X, Sun Y, Zhang Y, Chen F, Dai M, Si J, Yang J, Li X, Li J, Xia Y, et al. Characteristics and outcomes of heart failure with recovered left ventricular ejection fraction. ESC Heart Fail. 2021;8:5383–5391. doi: 10.1002/ehf2.13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Writing Committee Members, & ACC/AHA Joint Committee Members . 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Card Fail. 2022;2022:e1–e167. doi: 10.1016/j.cardfail.2022.02.010 [DOI] [PubMed] [Google Scholar]
- 8. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Bohm M, Butler J, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27:387–413. doi: 10.1016/j.cardfail.2021.01.022 [DOI] [PubMed] [Google Scholar]
- 9. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, et al. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641 [DOI] [PubMed] [Google Scholar]
- 10. Mulder MJ, Kemme MJB, Visser CL, Hopman L, van Diemen PA, van de Ven PM, Gotte MJW, Danad I, Knaapen P, van Rossum AC, et al. Left atrial sphericity as a marker of atrial remodeling: comparison of atrial fibrillation patients and controls. Int J Cardiol. 2020;304:69–74. doi: 10.1016/j.ijcard.2020.01.042 [DOI] [PubMed] [Google Scholar]
- 11. Castrichini M, Manca P, Nuzzi V, Barbati G, De Luca A, Korcova R, Stolfo D, Lenarda AD, Merlo M, Sinagra G. Sacubitril/valsartan induces global cardiac reverse remodeling in long‐lasting heart failure with reduced ejection fraction: standard and advanced echocardiographic evidences. J Clin Med. 2020;9:906. doi: 10.3390/jcm9040906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen YC, Voskoboinik A, Gerche A, Marwick TH, McMullen JR. Prevention of pathological atrial remodeling and atrial fibrillation: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;77:2846–2864. doi: 10.1016/j.jacc.2021.04.012 [DOI] [PubMed] [Google Scholar]
- 13. Thomas L, Abhayaratna WP. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging. 2017;10:65–77. doi: 10.1016/j.jcmg.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 14. Minamisawa M, Inciardi RM, Claggett B, Cuddy SAM, Quarta CC, Shah AM, Dorbala S, Falk RH, Matsushita K, Kitzman DW, et al. Left atrial structure and function of the amyloidogenic V122I transthyretin variant in elderly African Americans. Eur J Heart Fail. 2021;23:1290–1295. doi: 10.1002/ejhf.2200 [DOI] [PubMed] [Google Scholar]
- 15. Kadappu KK, Abhayaratna K, Boyd A, French JK, Xuan W, Abhayaratna W, Thomas L. Independent echocardiographic markers of cardiovascular involvement in chronic kidney disease: the value of left atrial function and volume. J Am Soc Echocardiogr. 2016;29:359–367. doi: 10.1016/j.echo.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 16. Inciardi RM, Claggett B, Minamisawa M, Shin SH, Selvaraj S, Goncalves A, Wang W, Kitzman D, Matsushita K, Prasad NG, et al. Association of Left atrial structure and function with heart failure in older adults. J Am Coll Cardiol. 2022;79:1549–1561. doi: 10.1016/j.jacc.2022.01.053 [DOI] [PubMed] [Google Scholar]
- 17. Inciardi RM, Claggett B, Gupta DK, Cheng S, Liu J, Echouffo Tcheugui JB, Ndumele C, Matsushita K, Selvin E, Solomon SD, et al. Cardiac structure and function and diabetes‐related risk of death or heart failure in older adults. J Am Heart Assoc. 2022;11:e022308. doi: 10.1161/JAHA.121.022308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Li WM, Gong YT, Li BX, Liu W, Han W, Dong D, Sheng L, Xue JY, Zhang L, et al. The effects of cilazapril and valsartan on the mRNA and protein expressions of atrial calpains and atrial structural remodeling in atrial fibrillation dogs. Basic Res Cardiol. 2007;102:245–256. doi: 10.1007/s00395-007-0641-8 [DOI] [PubMed] [Google Scholar]
- 19. Takemoto Y, Ramirez RJ, Kaur K, Salvador‐Montanes O, Ponce‐Balbuena D, Ramos‐Mondragon R, Ennis SR, Guerrero‐Serna G, Berenfeld O, Jalife J. Eplerenone reduces atrial fibrillation burden without preventing atrial electrical remodeling. J Am Coll Cardiol. 2017;70:2893–2905. doi: 10.1016/j.jacc.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun Y, Song S, Zhang Y, Mo W, Zhang X, Wang N, Xia Y, Tse G, Liu Y. Effect of angiotensin receptor neprilysin inhibitors on left atrial remodeling and prognosis in heart failure. ESC Heart Fail. 2022;9:667–675. doi: 10.1002/ehf2.13691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tops LF, Delgado V, Bertini M, Marsan NA, Den Uijl DW, Trines SA, Zeppenfeld K, Holman E, Schalij MJ, Bax JJ. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2011;57:324–331. doi: 10.1016/j.jacc.2010.05.063 [DOI] [PubMed] [Google Scholar]
- 22. Ronningen PS, Berge T, Solberg MG, Enger S, Nygard S, Pervez MO, Orstad EB, Kvisvik B, Aagaard EN, Rosjo H, et al. Sex differences and higher upper normal limits for left atrial end‐systolic volume in individuals in their mid‐60 s: data from the ACE 1950 study. Eur Heart J Cardiovasc Imaging. 2020;21:501–507. doi: 10.1093/ehjci/jeaa004 [DOI] [PubMed] [Google Scholar]
- 23. Yoshida Y, Nakanishi K, Daimon M, Ishiwata J, Sawada N, Hirokawa M, Kaneko H, Nakao T, Mizuno Y, Morita H, et al. Alteration of cardiac performance and serum B‐type natriuretic peptide level in healthy aging. J Am Coll Cardiol. 2019;74:1789–1800. doi: 10.1016/j.jacc.2019.07.080 [DOI] [PubMed] [Google Scholar]
- 24. Ahn SG, Shin JH, Koh BR, Choi JH, Kang SJ, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ. Impact of myocardial perfusion on left atrial remodeling following primary angioplasty for acute myocardial infarction. Coron Artery Dis. 2006;17:597–603. doi: 10.1097/01.mca.0000236281.74361.d4 [DOI] [PubMed] [Google Scholar]
- 25. Lakhani I, Leung KSK, Tse G, Lee APW. Novel mechanisms in heart failure with preserved, midrange, and reduced ejection fraction. Front Physiol. 2019;10:874. doi: 10.3389/fphys.2019.00874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shiba M, Kato T, Morimoto T, Yaku H, Inuzuka Y, Tamaki Y, Ozasa N, Seko Y, Yamamoto E, Yoshikawa Y, et al. Left atrial reverse remodeling improves risk stratification in patients with heart failure with recovered ejection fraction. Sci Rep. 2022;12:4473. doi: 10.1038/s41598-022-08630-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brenyo A, Link MS, Barsheshet A, Moss AJ, Zareba W, Wang PJ, McNitt S, Huang D, Foster E, Estes M III, et al. Cardiac resynchronization therapy reduces left atrial volume and the risk of atrial tachyarrhythmias in MADIT‐CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy). J Am Coll Cardiol. 2011;58:1682–1689. doi: 10.1016/j.jacc.2011.07.020 [DOI] [PubMed] [Google Scholar]
- 28. Vermes E, Tardif JC, Bourassa MG, Racine N, Levesque S, White M, Guerra PG, Ducharme A. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the studies of left ventricular dysfunction (SOLVD) trials. Circulation. 2003;107:2926–2931. doi: 10.1161/01.CIR.0000072793.81076.D4 [DOI] [PubMed] [Google Scholar]
- 29. Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin‐angiotensin‐aldosterone system (RAAS) for primary prevention of non‐valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2013;165:17–24. doi: 10.1016/j.ijcard.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 30. Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlof B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, et al. Angiotensin II receptor blockade reduces new‐onset atrial fibrillation and subsequent stroke compared to atenolol: the losartan intervention for end point reduction in hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–719. doi: 10.1016/j.jacc.2004.10.068 [DOI] [PubMed] [Google Scholar]
- 31. Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, Jackson R, Rahneva T, Wage R, Smith G, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED‐HF): an open‐label, pilot, randomised trial. Lancet. 2019;393:61–73. doi: 10.1016/S0140-6736(18)32484-X [DOI] [PMC free article] [PubMed] [Google Scholar]


