Abstract
Objective
There is not a known elevated prevalence of delirium in older adult cancer patients. However, it is unknown if the incidence of delirium varies by cancer type among older adult patients. Therefore, this study aimed to examine the association between the incidence of delirium and cancer type among older adult patients using a Japanese hospital‐based administrative claims database.
Methods
A total of 76,868 patients over 65 years of age or older, first diagnosed with cancer on an initial date of hospitalization between April 2008 and December 2019, were included in this retrospective longitudinal study. Delirium was defined by the World Health Organization's International Statistical Classification of Diseases and Related Health Problems (ICD‐10) codes or antipsychotic medication use. Cox proportional hazard models were performed to estimate the risk of delirium incidence according to 22 cancer types during the one‐year hospitalization period.
Results
The incidence rates of delirium were 17.1% for men and 15.3% for women. Compared to gastric cancer, the risk of delirium was significantly higher for pancreatic cancer (HR: 1.26, 95% CI: 1.11–1.42 for men; HR: 1.27, 95% CI: 1.11–1.45 for women), leukemia (HR: 1.24, 95% CI: 1.09–1.41 for men; HR: 1.20, 95% CI: 1.03–1.41 for women), and oropharyngeal cancer (HR: 1.30, 95% CI: 1.10–1.54 for men; HR: 1.32; 95% CI: 1.02–1.72 for women) after adjusting for age, initial hospitalization year, antipsychotic medications, and surgery.
Conclusions
As compared to gastric cancer, patients with pancreatic cancer, leukemia, oropharyngeal cancer were found to have a higher risk of developing delirium. Our study findings suggested that the risk of delirium incidence may vary by cancer type.
Keywords: cancer, delirium, longitudinal study
The incidence rates of delirium were 17.1% for men and 15.3% for women with cancer. Compared to gastric cancer, the risk of delirium was significantly higher for pancreatic cancer, leukemia, and oropharyngeal cancer.

1. INTRODUCTION
Delirium is a common symptom in patients with cancer, regardless of the stage. The prevalence ranges from 10% to 31% at in‐patient admission. 1 One previous study reported that up to 42% of advanced cancer patients were diagnosed with delirium at hospital admission, and 45% developed delirium while admitted. 2 However, the incidence and prevalence of delirium among cancer patients has not been well established. 3
Delirium is a neuropsychiatric syndrome characterized by an acute onset of deficits in attention, consciousness, and cognition. 4 Predisposing factors for developing delirium include advanced age, surgery, cognitive impairment, low albumin level, dehydration, infection, hypoxemia, bone metastases, hematological malignancies, and usage of medications such as steroids, opioids, or benzodiazepines. 4 , 5 Although the pathophysiological mechanisms of the development of delirium through these predisposing factors are not clear, 6 patients with advanced stages of cancer are at an increased risk of developing delirium due to surgery, medications, cancer progression, and comorbidities. 2
Additionally, delirium may be caused by an elevation of cytokines (i.e., interleukin [IL]‐1, IL‐6, and IL‐8), interferon, and tumor necrosis factor. 7 Levels of IL‐6 may promote hyperactive delirium 8 and are significantly elevated in lung, breast, 9 and colorectal cancers. 10 Immunohistochemistry assays have also shown that IL‐8 is significantly expressed in gastric cancer tissues. 11 The onset of delirium depends on the type of cancer due to distinct differences in the expression of inflammatory markers. Furthermore, ApoE2 contributes to cell growth in colorectal cancer patients and is a potential contributor to the development of delirium. 7 , 12 Diabetes mellitus is also a known risk factor for both pancreatic cancer and delirium. 7 , 13 Thus, the risk of developing delirium may also differ by cancer type via metabolic disorders or levels of ApoE.
Dehydration and prescription drugs use, such as steroids and opioids, are common among patients with cancer. 4 , 14 Dehydration is often caused by chemotherapy and is a known risk factor of delirium. 15 Steroids are included in many chemotherapy regimens, 16 and opioids are administered to patients with moderate to severe pain. 17 Additionally, opioids including morphine, hydromorphone, oxycodone, fentanyl, and methadone are often used for pain management and can induce neurotoxicity that may, in turn, lead to delirium. 18 , 19
Advanced age is a significant risk factor for developing delirium due to age‐related decline in regional cerebral blood flow, neuronal loss, and neurotransmitter systems. 20 The prevalence of delirium among people living in the community is low overall (1%–2%), but the risk increases among the older adult community population (8%–17%). 7 The prevalence of delirium in older adult patients with advanced lung cancer was found to be much higher (43%). 21 A meta‐analysis has provided evidence that delirium in older adult patients is associated with poor health outcomes such as mortality, institutionalization, and dementia. 22 Older adult patients with cancer were found to have a higher prevalence of delirium, placing a heavy burden on the patients themselves and their families. 23
However, to our knowledge, only one study has examined the association of delirium with cancer type and advanced age. 5 In Japan, the proportion of the population aged 65 years or older was 28.6% by 2021 (https://www.stat.go.jp/english/info/news/20211228.html). Therefore, it is important to examine the risk of delirium symptoms developing in older patients, according to cancer type. If the incidence of delirium can be stratified by cancer type, this study will provide useful information to assist healthcare professionals in preventing the onset of delirium symptoms among the growing number of elderly cancer patients. Based on a prior study showing that the risk of delirium development differs according to sex, 24 the present analyses were stratified by sex. The data were drawn from the largest nationwide database of administrative claims in Japanese hospitals (Medical Data Vision databases [MDV], https://www.mdv.co.jp).
2. METHODS
2.1. Data source
Data from patients with cancer under inpatient care were extracted from claim forms of the MDV database in Japan between April 2008 and December 2019. The database contains data from about 24 million in‐ and outpatients (about 19% of the Japanese population) from 360 large hospitals that have adopted the Diagnostic Procedure Combination/Per‐Diem Payment System, which comprise about a fifth of all hospitals in Japan. The MDV contains anonymized patient characteristics, diagnoses, medical expenses, procedures, and drug prescriptions. Patient information from within the same hospital can be traced over time. The MDV data were provided by Medical Data Vision, a commercial data service. As the data were anonymized, informed consent was not required, in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan.
2.2. Inclusion and exclusion criteria
Patients aged 65 years or older who were first diagnosed with cancer on an initial date of hospitalization between April 2008 and December 2019 and were diagnosed with only one type of cancer were included in the present study. Patients diagnosed with a psychiatric disorder or dementia before the initial date of hospitalization or with multiple cancers during the study period were excluded from the study.
2.3. Definition of delirium and cancer
Delirium was defined as any condition identified using any of the relevant World Health Organization International Statistical Classification of Diseases and Related Health Problems (ICD‐10) codes: A812, E512, F050, F101, F102, F109, F111, F112, F119, F121, F122, F129, F131, F132, F139, F141, F142, F149, F151, F152, F159, F161, F162, F169, F181, F182, F189, F191, F192, F199, G92, G934, I673, I674, I678, J108, J118, or P916 (Table S1). Alternatively, we identified delirium based on the prescription of antipsychotic medications (risperidone, quetiapine, haloperidol, olanzapine, perospirone, or tiapride) during the hospital stay (Table S2). Some of the antipsychotic medications (quetiapine, haloperidol, olanzapine) were included based on previous studies. 25 , 26 The remainder were included based on the Japanese clinical practice guidelines. 4
According to a previous study, cancer types were defined using ICD‐10 criteria: bladder cancer (C67), brain and other nervous system cancers (C70, C71, C72), breast cancer (C50), colorectal cancer (C18, C19, C20), esophageal cancer (C15), gallbladder and bile duct cancer (C23, C24), gastric cancer (C16), hepatic cancer (C22), laryngeal cancer (C32), leukemia (C91, C92, C93, C94, C95), lung cancer (C33, C34), malignant lymphoma (C81, C82, C83, C84, C85, C96), multiple myeloma (C88, C90), oral and pharyngeal cancer (C00, C01, C02, C03, C04, C05, C06, C07, C08, C09, C10, C11, C12, C13, C14), ovary cancer (C56), pancreatic cancer (C25), prostatic cancer (C61), renal cancer (C65, C66, C68), skin cancer (C43, C44), thyroid cancer (C73), uterine cancer (C53, C54, C55), and other cancers (C17, C21, C26, C30, C31, C37, C38, C40, C41, C45, C46, C47, C48, C49, C51, C52, C57, C58, C60, C62, C63, C69). 27
2.4. Study design
This study was designed as a retrospective longitudinal observational cohort study (Figure 1). The data used include those for patients admitted via the emergency ward as well as ambulatory patients. The follow‐up period, in person‐years, was calculated for each type of cancer for a one‐year hospitalization period (365.25 days), from the date of initial hospitalization to the date of delirium onset. The patients were censored at the date of delirium onset, discharge, or after reaching 365 days without delirium, whichever came first. There were no data available for the period after discharge.
FIGURE 1.

A framework of the present study
2.5. Statistical analysis
The patients' demographic characteristics at initial hospitalization were summarized. Kaplan–Meier survival analysis and Cox regression analyses were performed to estimate the hazard ratio (HR) and 95% confidence interval (CI) of delirium incidence during the study period according to cancer type.
All analyses were stratified by sex. The multivariate analysis included age (year), calendar year of initial hospitalization date (year), surgery (yes/no), opioid use (yes/no), benzodiazepine as an antianxiety or hypnotic treatment (yes/no), non‐benzodiazepines (yes/no), and steroid use (yes/no) as potential confounders. Therefore, Model 1 was adjusted for age and calendar year on initial date of hospitalization. Model 2 was further adjusted for the history of surgery. Finally, Model 3 was further adjusted for opioid, benzodiazepine as antianxiety or hypnotics, non‐benzodiazepine and steroid. All analyses were stratified by sex using SAS version 9.4 software (SAS Institute Inc.) and SPSS (IBM). The study protocol was approved by the Juntendo Human Research Ethics Committee (#2020250).
3. RESULTS
Data from a total of 1,004,185 older adult patients with cancer in ambulatory care and without psychiatric disorders or dementia diagnoses were obtained from the MDV database between April 2008 and December 2020. After applying the inclusion criteria, 76,868 patients (44,971 men and 31,897 women) diagnosed with cancer at the initial date of hospitalization were included in the study (Figure 2).
FIGURE 2.
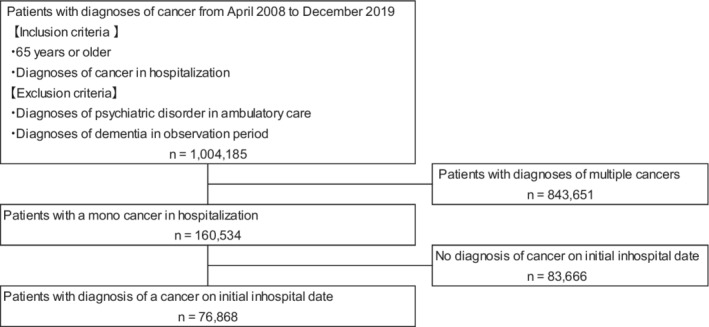
A flow diagram of study patients
The demographic characteristics of patients were presented in Table 1. The median age of patients was 79 years (interquartile range = 72–84) for men and 81 years (interquartile range = 74–87) for women. The incidence rates of delirium during the follow‐up were 17.1% for men and 15.3% for women. The most common cancers were prostatic cancer (18.2%), gastric cancer (14.6%), and colorectal cancer (14.2%) for male patients; and colorectal cancer (18.5%), gastric cancer (11.6%), and breast cancer (11.3%) for female patients.
TABLE 1.
The baseline characteristics of patients according to sex
| Total (N = 76,868) | Men (N = 44,971) | Women (N = 31,897) | p‐value a | ||||
|---|---|---|---|---|---|---|---|
| Number | % or median (Inter quartile range) | Number | % or median (Inter quartile range) | Number | % or median (Inter quartile range) | ||
| Age, y | ‐ | 79 (72–84) | ‐ | 81 (74–87) | <0.0001 | ||
| 65–69 | 10,180 | 13.2 | 6600 | 14.7 | 3580 | 11.2 | |
| 70–74 | 12,883 | 16.7 | 8345 | 18.6 | 4538 | 14.2 | |
| 75–79 | 14,950 | 19.4 | 9341 | 20.8 | 5609 | 17.6 | |
| 80–84 | 16,107 | 20.9 | 9534 | 21.2 | 6573 | 20.6 | |
| 85–89 | 13,700 | 17.8 | 7387 | 16.4 | 6313 | 19.8 | |
| 90–94 | 6905 | 8.9 | 3050 | 6.8 | 3855 | 12.1 | |
| 95–99 | 1895 | 2.4 | 649 | 1.4 | 1246 | 3.9 | |
| ≧100 | 248 | 0.3 | 65 | 0.1 | 183 | 0.6 | |
| Delirium | 12,561 | 16.3 | 7686 | 17.1 | 4875 | 15.3 | <0.0001 |
| Bladder cancer | 2787 | 3.6 | 1961 | 4.4 | 826 | 2.6 | <0.0001 |
| Brain and other nervous system | 939 | 1.2 | 492 | 1.1 | 447 | 1.4 | 0.0001 |
| Breast cancer | 3632 | 4.7 | 25 | 0.1 | 3607 | 11.3 | <0.0001 |
| Colorectal cancer | 12,296 | 16.0 | 6382 | 14.2 | 5914 | 18.5 | <0.0001 |
| Esophageal cancer | 1744 | 2.2 | 1412 | 3.1 | 332 | 1.0 | <0.0001 |
| Gallbladder and Bile duct cancer | 3700 | 4.8 | 1794 | 4.0 | 1906 | 6.0 | <0.0001 |
| Gastric cancer | 10,265 | 13.3 | 6571 | 14.6 | 3694 | 11.6 | <0.0001 |
| Hepatic cancer | 5450 | 7.0 | 3370 | 7.5 | 2080 | 6.5 | <0.0001 |
| Lanryngeal cancer | 412 | 0.5 | 382 | 0.8 | 30 | 0.1 | <0.0001 |
| Leukemia | 2906 | 3.7 | 1566 | 3.5 | 1340 | 4.2 | <0.0001 |
| Lung cancer | 7332 | 9.5 | 5023 | 11.2 | 2309 | 7.2 | <0.0001 |
| Malignant Lymphoma | 2831 | 3.6 | 1413 | 3.1 | 1418 | 4.4 | <0.0001 |
| Multiple Myeloma | 1736 | 2.2 | 815 | 1.8 | 921 | 2.9 | <0.0001 |
| Oral and pharyngeal cancer | 1102 | 1.4 | 783 | 1.7 | 319 | 1.0 | <0.0001 |
| Other | 3609 | 4.7 | 1986 | 4.4 | 1623 | 5.1 | <0.0001 |
| Ovary cancer | 382 | 0.5 | ‐ | ‐ | 382 | 1.2 | <0.0001 |
| Pancreatic cancer | 3344 | 4.3 | 1491 | 3.3 | 1853 | 5.8 | <0.0001 |
| Prostatic cancer | 8185 | 10.6 | 8181 | 18.2 | ‐ | ‐ | <0.0001 |
| Renal cancer | 1384 | 1.8 | 820 | 1.8 | 564 | 1.8 | 0.56 |
| Skin cancer | 785 | 1.0 | 345 | 0.8 | 440 | 1.4 | <0.0001 |
| Thyroid cancer | 807 | 1.0 | 159 | 0.4 | 648 | 2.0 | <0.0001 |
| Uterine cancer | 1240 | 1.6 | ‐ | ‐ | 1240 | 3.9 | <0.0001 |
Chi‐square test.
The hazard ratios (HRs) and 95% confidence intervals (CIs) for age were 1.03 (95% CI = 1.03–1.04) for men and 1.02 (95% CI = 1.02–1.03) for women, and for surgery these were 1.03 (95% CI = 0.95–1.05) for men and 0.93 (95% CI = 0.87–0.99) for women (not shown in Table 2). Because of its position on the Kaplan–Meier curve, gastric cancer was used as a reference (Figures S1 and S2). Table 2 shows the multivariate HR and CI for each cancer type compared to gastric cancer. After adjusting for age, calendar year of initial hospitalization date, history of surgery, and medications including opioids, benzodiazepines, non‐benzodiazepines, and steroids, the delirium incidences for both men and women were significant as follows: pancreatic cancer (HR = 1.26; 95% CI = 1.11–1.42 for men; HR = 1.27; 95% CI = 1.11–1.45 for women), leukemia (HR = 1.24; 95% CI = 1.09–1.41 for men, HR = 1.20; 95% CI = 1.03–1.41 for women) and oropharyngeal cancer (HR = 1.30; 95% CI = 1.10–1.54 for men, HR = 1.32; 95% CI = 1.02–1.72 for women). Among men, a higher risk of delirium incidence was found in brain and other nervous system cancers (HR = 1.40; 95% CI = 1.14–1.71), lung cancer (HR = 1.31; 95% CI = 1.20–1.43), malignant lymphoma (HR = 1.24; 95% CI = 1.08–1.42), esophageal cancer (HR = 1.21; 95% CI = 1.06–1.39), and hepatocellular carcinoma (HR = 1.15; 95% CI = 1.04–1.27); prostatic cancer had a lower risk of delirium incidence (HR = 0.65; 95% CI = 0.59–0.71). In women, a lower risk of delirium incidence was found for patients with skin cancer (HR = 0.55; 95% CI = 0.39–0.80), breast cancer (HR = 0.69; 95% CI = 0.60–0.79), and bladder cancer (HR = 0.74; 95% CI = 0.59–0.92).
TABLE 2.
The hazard ratios of delirium according to cancer type and sex
| Men | Women | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Person‐years | Number of events | Model 1 | Model 2 | Model 3 | Person‐years | Number of events | Model 1 | Model 2 | Model 3 | |||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||||
| Gastric cancer a | 1141 | 1060 | 1.00 | ‐ | 1.00 | ‐ | 1.00 | ‐ | 606 | 540 | 1.00 | ‐ | 1.00 | ‐ | 1.00 | ‐ |
| Bladder cancer | 341 | 278 | 0.87 | 0.77–1.00 | 0.87 | 0.77–1.00 | 0.90 | 0.79–1.02 | 167 | 91 | 0.72 | 0.58–0.90 | 0.72 | 0.58–0.90 | 0.74 | 0.59–0.92 |
| Brain and other nervous system | 113 | 104 | 1.27 | 1.03–1.55 | 1.27 | 1.04–1.55 | 1.40 | 1.14–1.71 | 106 | 62 | 0.87 | 0.67–1.13 | 0.86 | 0.66–1.12 | 0.95 | 0.73–1.24 |
| Breast cancer | 5 | 3 | 0.66 | 0.21–2.03 | 0.65 | 0.21–2.03 | 0.68 | 0.22–2.10 | 631 | 323 | 0.66 | 0.57–0.75 | 0.65 | 0.57–0.75 | 0.69 | 0.60–0.79 |
| Colorectal cancer | 1169 | 1074 | 1.06 | 0.97–1.15 | 1.06 | 0.97–1.15 | 1.05 | 0,97–1.15 | 1085 | 918 | 1.05 | 0.95–1.17 | 1.06 | 0.95–1.18 | 1.05 | 0.94–1.16 |
| Esophageal cancer | 213 | 259 | 1.24 | 1.08–1.42 | 1.24 | 1.08–1.42 | 1.21 | 1.06–1.39 | 49 | 49 | 1.15 | 0.86–1.54 | 1.14 | 0.85–1.53 | 1.08 | 0.81–1.45 |
| Gallbladder and Bile duct cancer | 333 | 356 | 1.119 | 0.99–1.26 | 1.12 | 0.99–1.26 | 1.01 | 0.90–1.14 | 395 | 349 | 1.11 | 0.97–1.27 | 1.13 | 0.98–1.29 | 1.01 | 0.89–1.16 |
| Hepatic cancer | 640 | 598 | 1.16 | 1.05–1.28 | 1.16 | 1.05–1.28 | 1.15 | 1.04–1.27 | 424 | 303 | 1.01 | 0.87–1.16 | 1.00 | 0.87–1.15 | 0.97 | 0.84–1.12 |
| Laryngeal cancer | 66 | 61 | 1.03 | 0.79–1.33 | 1.03 | 0.79–1.33 | 1.08 | 0.83–1.39 | 7 | 1 | 0.21 | 0.03–1.51 | 0.21 | 0.03–1.50 | 0.23 | 0.03–1.61 |
| Leukemia | 374 | 301 | 1.15 | 1.01–1.31 | 1.15 | 1.01–1.31 | 1.24 | 1.09–1.41 | 291 | 221 | 1.12 | 0.96–1.31 | 1.14 | 0.97–1.33 | 1.20 | 1.03–1.41 |
| Lung cancer | 642 | 988 | 1.37 | 1.25–1.49 | 1.37 | 1.26–1.50 | 1.31 | 1.20–1.43 | 328 | 354 | 1.14 | 1.00–1.30 | 1.12 | 0.97–1.28 | 1.08 | 0.94–1.24 |
| Malignant Lymphoma | 230 | 267 | 1.19 | 1.04–1.37 | 1.20 | 1.05–1.37 | 1.24 | 1.08–1.42 | 230 | 217 | 1.07 | 0.91–1.25 | 1.06 | 0.90–1.24 | 1.09 | 0.93–1.28 |
| Multiple Myeloma | 189 | 155 | 1.11 | 0.93–1.31 | 1.11 | 0.93–1,31 | 1.09 | 0.92–1.29 | 248 | 162 | 1.07 | 0.90–1.28 | 1.07 | 0.90–1.28 | 1.06 | 0.89–1.26 |
| Oral and pharyngeal cancer | 119 | 159 | 1.37 | 1.16–1.62 | 1.37 | 1.16–1.62 | 1.30 | 1.10–1.54 | 45 | 62 | 1.41 | 1.09–1.84 | 1.39 | 1.07–1.81 | 1.32 | 1.02–1.72 |
| Other cancer | 248 | 280 | 1.07 | 0.94–1.22 | 1.07 | 0.94–1.23 | 1.06 | 0.93–1.22 | 226 | 217 | 1.09 | 0.93–1.27 | 1.07 | 0.91–1.25 | 1.05 | 0.90–1.23 |
| Ovary cancer | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 75 | 64 | 1.21 | 0.94–1.57 | 1.20 | 0.92–1.55 | 1.16 | 0.89–1.51 |
| Pancreatic cancer | 224 | 333 | 1.46 | 1.29–1.65 | 1.46 | 1.29–1.65 | 1.26 | 1.11–1.42 | 270 | 388 | 1.49 | 1.31–1.70 | 1.49 | 1.31–1.70 | 1.27 | 1.11–1.45 |
| Prostatic cancer | 1646 | 888 | 0.62 | 0.57–0.68 | 0.62 | 0.57–0.68 | 0.65 | 0.59–0.71 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Renal cancer | 135 | 128 | 1.00 | 0.84–1.21 | 1.00 | 0.84–1.21 | 1.00 | 0.84–1.21 | 95 | 95 | 1.17 | 0.94–1.46 | 1.17 | 0.94–1.45 | 1.15 | 0.93–1.43 |
| Skin cancer | 58 | 42 | 0.74 | 0.54–1.00 | 0.74 | 0.54–1.00 | 0.81 | 0.60–1.11 | 61 | 31 | 0.51 | 0.35–0.73 | 0.51 | 0.35–0.73 | 0.55 | 0.39–0.80 |
| Thyroid cancer | 26 | 14 | 0.62 | 0.36–1.04 | 0.62 | 0.36–1.04 | 0.65 | 0.38–1.09 | 98 | 60 | 0.74 | 0.57–0.97 | 0.74 | 0.57–0.97 | 0.77 | 0.59–1.01 |
| Uterine cancer | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 211 | 143 | 0.86 | 0.71–1.03 | 0.85 | 0.71–1.02 | 0.87 | 0.72–1.04 |
Notes: Model 1 is adjusted for age, calendar year on initial date of hospitalization. Model 2 is adjusted as in Model 1 + history of sugery. Model 3 is adjusted as in Model 2 + opioid, benzodiazepine as antianxiety or hypnotics, non‐benzodiazepine and steroid.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Reference category for cancer types.
4. DISCUSSION
To our knowledge, this is the first study to report the association between the risk of delirium and type of cancer. In the present study, the risk of developing delirium was higher among those patients with pancreatic cancer, lymphoma, and oropharyngeal cancer than those with gastric cancer, for both men and women. Additionally, when stratified by sex, the risk of developing delirium in men was higher in those with brain and other nervous system cancers, lung cancer, malignant lymphoma, esophageal cancer, and hepatic cancer than in those with gastric cancer. However, similar risks were not found for women.
In the present study, the risk of developing delirium was higher among those patients with cancerous cells close to the central nervous system (CNS), such as brain or oropharyngeal cancer. One of the risk factors for the development of delirium is the presence of intracranial lesions, such as brain tumors. 28 CNS cancer can cause delirium primary to lesions and secondary to edema or inflammation. 28 In our study, the risk of delirium in men with CNS cancer (HR = 1.40; 95% CI = 1.14–1.71) was higher than in those with gastric cancer, with no difference found in women (HR = 0.95; 95% CI = 0.73–1.24), probably because meningiomas are more common in women than in men. Meningiomas are the most common type of primary CNS cancer (24%–30%), most are benign 29 and are considered to have a lower risk of developing delirium.
Overall, all patients with hematological malignancies, such as leukemia, were more likely to develop delirium. Previously, delirium was primarily observed in older adult patients ineligible for intensive chemotherapy regimens. 30 Because this study focused on older adult patients, it is possible that many subjects ineligible for intensive chemotherapy regimens were included in our study population. Our results also suggest that delirium is more common in patients with hematological malignancies, as found in previous studies. 5 Patients with acute myeloid leukemia (AML) are susceptible to infections caused by leukopenia, anemia, and thrombocytopenia due to an overgrowth of leukemia cells in the bone marrow, significantly impairing normal hematopoietic function. 31 In addition, many regimens for hematological malignancies are immunosuppressive and require long‐term treatment, the main side effect of which is infection. 31 As infections have been shown to have a major impact on the development of delirium, 4 , 30 patients treated with an immunosuppressive regimen are more likely to develop delirium due to these side effects. Therefore, surgical treatments for primary gastric cancer are prioritized due to their non‐immunosuppressive methodology, including endoscopic submucosal dissection (ESD), endoscopic mucosal resection (EMR), or gastrectomy. 32 In the present study, we found that the prevalence of infection among patients with leukemia was 41.1%, higher than 15.9% of the total, with multiple myeloma and malignant lymphoma at 36.8% and 26.8%, and gastric cancer at 12.9% (not shown in table).
Patients with pancreatic cancer had a higher risk of developing delirium compared to those with gastric cancer. Pancreatic cancer is a complex disease; it involves changes in cancer inflammatory components dominated by IL‐6, 33 which may affect hyperactive delirium. 8 Additionally, pancreatic cancer is not diagnosed early and many patients may have progressed considerably by the time of diagnosis. 34 Thus, patients may be at increased risk of delirium due to a strong inflammatory response.
Results show that male patients with lung cancer have a higher risk of developing delirium than patients with gastric cancer. It has been established that there is a gender difference in histological types for lung cancer; men are more likely to develop squamous cell carcinoma (SqCC), whereas women are more likely to have adenocarcinoma (AC). 35 A Taiwan registry study reported patients with SqCC histology had a lower survival rate than AC patients (univariate: HR = 1.22, CI 95% 1.17–1.28; multivariate: HR = 1.23; CI 95% = 1.17–1.29) and suggested that these lung cancers should be analyzed separately to provide more precise outcomes. 36 A Japanese cohort study of 12,509 patients with non‐small cell lung cancer after resection also reported the overall survival of women was significantly better than that of men for both AC (5‐year survival rates, 77.7% versus 61.9%, p = 0.00) and non‐AC (5‐year survival rates, 59.3% versus 53.1%, p = 0.03). 37 For AC, women have a significantly better prognosis than men for pathologic stage I/II disease. Therefore, men are at higher risk of SqCC, which is more challenging to treat and is often more severe. Additionally, the inflammatory response from tissue invasion may increase the risk of delirium 38 in men.
The low risk for patients with breast, skin, or prostate cancer to develop delirium may be due to early detection. Prostate‐specific antigen (PSA) is used in routine medical checkups for prostate cancer detection, particularly in Japan. 39 Additionally, breast cancer screening and the number of early‐stage patients have increased in Japan. 40 Therefore, we may have found a lower risk of delirium among patients with those types of cancer due to their early detection compared with gastric cancer.
The strength of this study is that it examined the onset of delirium by cancer type using a large hospital database. Diagnosing delirium is complicated, and it is difficult to ensure reliability with the diagnosis of the insurance claims alone, but its reliability could be ensured by citing a study that evaluated the identification of delirium by combining diagnosis (ICD‐10) and medications. 26 Delirium was common in men and in those with hematological malignancies, as seen in previous studies. 5 This study is the first to associate the risk of developing delirium by cancer type. No previous studies have been stratified by cancer type. This study showed that delirium increases in pancreatic cancer, lung cancer, brain tumors, and hematologic cancer.
5. STUDY LIMITATIONS
This study has several limitations. First, it is retrospective and observational in nature and uses only one medical insurance claims database. The MDV database includes patients treated at large acute‐care hospitals in Japan. Therefore, the findings may not be generalizable to patients outside of large hospitals that have adopted the Diagnostic Procedure Combination/Per‐Diem Payment System. Second, because we used the MDV database, there was a lack of data linkage among medical care facilities. Thus, some of the patients included in this study may have been previously diagnosed with cancer at another facility. Third, the severity of the cancer could not be confirmed; thus, it was impossible to know whether the cancer had progressed since diagnosis. Fourth, because of the insurance reimbursement system in use in Japan (https://www.hospital.or.jp/pdf/14_20110928_01.pdf), physicians often use off‐label prescription of antipsychotics to treat delirium symptoms. We therefore identified the onset of delirium symptoms based on both ICD‐10 codes and the administration of antipsychotics. This may limit the reproducibility of this study, since the antipsychotic medications used to treat delirium may differ by country, and their use to treat delirium may be difficult to distinguish from their use to treat other psychotic disorders. Fifth, we were not able to differentiate the subtype of delirium (i.e., hyperactive, hypoactive, mixed motor, or no motor). Finally, the possibility of patients developing psychiatric disorders or dementia after hospital admission could not be completely eliminated, although patients diagnosed with these disorders before the date of initial hospitalization were excluded from the study.
6. CLINICAL IMPLICATIONS
This study examined the risk of the onset of delirium in patients with cancer using a large database of administrative claims in Japanese hospitals. Compared to gastric cancer, men with brain and other nervous system cancers, esophageal cancer, hepatocellular carcinoma, leukemia, malignant lymphoma, lung cancer, oropharyngeal cancer, and pancreatic cancer were more likely to develop delirium. Women with leukemia, oropharyngeal cancer, and pancreatic cancer were at increased risk of developing delirium. Patients with pancreatic cancer, leukemia, oropharyngeal cancer had a higher risk of developing delirium overall. The risk of developing delirium depends on the type of cancer. A previous study report has suggested that the risk of delirium can be reduced among hospitalized elderly when the risk factor specific presentative strategies were introduced. 5 Our study suggested that it is important to consider sex‐ and cancer‐specific preventions for delirium in elderly patients with cancer.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception of the study design; KY, AI, ME, KK and TT to the data analysis and interpretation; KY, AI to drafting of the manuscript; and KY, AI, ME, RF, KK, KI, KN and TT to revising the manuscript for important intellectual content. All authors reviewed and approved the final draft of this manuscript and agree to be accountable for all aspects of the work.
CONFLICT OF INTEREST
KI received personal fees from Eisai, Eli Lilly, Janssen, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Mochida, MSD, Novartis, Otsuka, Shionogi, Sumitomo Pharma, Yoshitomiyakuhin and he received research grant support from Mochida and Sumitomo pharma in the last three years. KN received personal fees from Meiji Seika Pharma, Mochida, Takeda, Yoshitomiyakuhin, Pfizer, Eli Lilly, MSD, Shionogi, Janssen, Eisai, Astellas, Otsuka, Daiichi Sankyo, Nipro, Kissei, Tsumura, Novartis, Mitsubishi Tanabe, and Chugai and he has received research/grant support from Mochida, Takeda, Otsuka, Novartis, Mitsubishi Tanabe, Dainippon Sumitomo, MSD, Eisai, Tsumura, Eli Lilly, GlaxoSmithKline, and Mebix in the last three years. The other authors declare no conflict of interest.
ETHICS STATEMENT
This study used anonymized data. Because the study used anonymized information, informed consent was not required, in accordance with Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. The study protocol was approved by the Juntendo Human Research Ethics Committee (#2020250).
Supporting information
Figure S1
Figure S2
Table S1
Table S2
Yamato K, Ikeda A, Endo M, et al. An association between cancer type and delirium incidence in Japanese elderly patients: A retrospective longitudinal study. Cancer Med. 2023;12:2407‐2416. doi: 10.1002/cam4.5069
DATA AVAILABILITY STATEMENT
The data set used for analysis in this study is available from MDV, but were used under license for the current study. Restrictions thus apply, and the data are not publicly available. For inquiries about access to the data set used in this study, please contact MDV (https://www.mdv.co.jp).
REFERENCES
- 1. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in‐patients: a systematic literature review. Age Ageing. 2006;35:350‐364. doi: 10.1093/ageing/afl005 [DOI] [PubMed] [Google Scholar]
- 2. Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160:786‐794. doi: 10.1001/archinte.160.6.786 [DOI] [PubMed] [Google Scholar]
- 3. Sands MB, Wee I, Agar M, Vardy JL. The detection of delirium in admitted oncology patients: a scoping review. Eur Geriatr Med. 2022;13:33‐51. doi: 10.1007/s41999-021-00586-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsuda Y, Tanimukai H, Inoue S, et al. JPOS/JASCC clinical guidelines for delirium in adult cancer patients: a summary of recommendation statements. Jpn J Clin Oncol. 2020;50(5):586‐593. doi: 10.1093/jjco/hyaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vladan L, Brian K. Risk factors for development of delirium among oncology patients. Gen Hosp Psychiatry. 2003;25:345‐352. doi: 10.1016/s0163-8343(03)00070-7 [DOI] [PubMed] [Google Scholar]
- 6. Maclullich AMJ, Ferguson KJ, Miller T, de Rooij SEJA, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65(3):229‐238. doi: 10.1016/j.jpsychores.2008.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911‐922. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Munster BC, Korevaar JC, Zwinderman AH, Marcel Levi W, Wiersinga J, De Rooij SE. Time‐course of cytokines during delirium in elderly patients with hip fractures. J Am Geriatr Soc. 2008;56(9):1704‐1709. doi: 10.1111/j.1532-5415 [DOI] [PubMed] [Google Scholar]
- 9. Sergei G, Michael K. Autocrine IL‐6 signaling: a key event in tumorigenesis? Cancer Cell. 2008;13(1):7‐9. doi: 10.1016/j.ccr.2007.12.020 [DOI] [PubMed] [Google Scholar]
- 10. Jacqueline B, Timothy CW. Inflammation and cancer: IL‐6 and STAT3 complete the link. Cancer Cell. 2009;15(2):79‐80. doi: 10.1016/j.ccr.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhai J, Shen J, Xie G, et al. Cancer‐associated fibroblasts‐derived IL‐8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37‐43. doi: 10.1016/j.canlet.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 12. Shafi O. Inverse relationship between Alzheimer's disease and cancer, and other factors contributing to Alzheimer's disease: a systematic review. BMC Neurol. 2016;16:236. doi: 10.1186/s12883-016-0765-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liao WC, Tu YK, Wu MS, Lin JT, Wang HP, Chien KL. Blood glucose concentration and risk of pancreatic cancer: systematic review and dose‐response meta‐analysis. BMJ. 2015;350:g7371. doi: 10.1136/bmj.g7371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Price KAR. Hydration in cancer patients. Curr Opin Support Palliat Care. 2010;4(4):276‐280. doi: 10.1097/SPC.0b013e32833e48d1 [DOI] [PubMed] [Google Scholar]
- 15. Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer. Arch Intern Med. 2000;160(6):786‐794. doi: 10.1001/archinte.160.6.786 [DOI] [PubMed] [Google Scholar]
- 16. Ismail MF, Lavelle C, Cassidy EM. Steroid‐induced mental disorders in cancer patients: a systematic review. Future Oncol. 2017;13(29):2719‐2731. doi: 10.2217/fon-2017-0306 [DOI] [PubMed] [Google Scholar]
- 17. King S, Forbes K, Hanks GW, Ferro CJ, Chambers EJ. A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliat Med. 2011;25(5):525‐552. doi: 10.1177/0269216311406313 [DOI] [PubMed] [Google Scholar]
- 18. Gaudreau 1 J‐D, Gagnon P, Harel F, Roy M‐A, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712‐6718. doi: 10.1200/JCO.2005.05.140 [DOI] [PubMed] [Google Scholar]
- 19. Bush SH, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;14(10):1039‐1049. doi: 10.1634/theoncologist.2009-0122 [DOI] [PubMed] [Google Scholar]
- 20. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1191‐1222, . doi: 10.1016/j.jagp.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 21. Uchida M, Okuyama T, Ito Y, et al. Prevalence, course and factors associated with delirium in elderly patients with advanced cancer: a longitudinal observational study. Jpn J Clin Oncol. 2015;45:934‐940. doi: 10.1093/jjco/hyv100 [DOI] [PubMed] [Google Scholar]
- 22. Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304:443‐451. doi: 10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- 23. Partridge JSL, Martin FC, Harari D, Dhesi JK. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry. 2013;28(8):804‐812. doi: 10.1002/gps.3900 [DOI] [PubMed] [Google Scholar]
- 24. Cobb JL, Glantz MJ, Nicholas PK, et al. Delirium in patients with cancer at the end of life. Cancer Practice. 2000;8(4):172‐177. doi: 10.1046/j.1523-5394.2000.84006.x [DOI] [PubMed] [Google Scholar]
- 25. Kim DH, Lee J, Kim CA, et al. Evaluation of algorithms to identify delirium in administrative claims and drug utilization database. Pharmacoepidemiol Drug Saf. 2017;26(8):945‐953. doi: 10.1002/pds.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Memtsoudis S, Cozowicz C, Zubizarreta N, et al. Risk factors for postoperative delirium in patients undergoing lower extremity joint arthroplasty: a retrospective population‐based cohort. Reg Anesth Pain Med. 2019;44:934‐943. doi: 10.1136/rapm-2019-100700 [DOI] [PubMed] [Google Scholar]
- 27. Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan . Vital statistics of Japan. https://ganjoho.jp/reg_stat/statistics/data/dl/en.html
- 28. Andrew E, Yesne A. Diagnosing and managing delirium in cancer patients. Oncology (Williston Park). 2017;31(9):686‐692. III. [PubMed] [Google Scholar]
- 29. Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A. Meningioma. In: Louis DN, Ohgaki H, Wiestler OD, Caenee WK, eds. WHO classification of tumours of the central nervous system. 3rd ed. WHO Press; 2007:164‐172. [Google Scholar]
- 30. Atkins S, He F. Chemotherapy and beyond: infections in the era of old and new treatments for hematologic malignancies. Infect Dis Clin North Am. 2019;33(2):289‐309. doi: 10.1016/j.idc.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 31. Miyawaki S. JSH guideline for tumors of hematopoietic and lymphoid tissues: leukemia 1. Acute myeloid leukemia (AML). Int J Hematol. 2017;106(3):310‐325. doi: 10.1007/s12185-017-2303-4 [DOI] [PubMed] [Google Scholar]
- 32. Gotoda T, Iwasaki M, Kusano C, Seewald S, Oda I. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010;97:868‐871. doi: 10.1002/bjs.7033 [DOI] [PubMed] [Google Scholar]
- 33. Pop V‐V, Seicean A, Lupan I, Samasca G, Burz C‐C. Immunology letters. IL‐6 roles ‐ molecular pathway and clinical implication in pancreatic cancer ‐ a systemic review. Immunol Lett. 2017;181:45‐50. doi: 10.1016/j.imlet.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 34. Ueda J, Tanaka M, Ohtsuka T, Tokunaga S, Shimosegawa T, Research Committee of Intractable Diseases of the Pancreas . Surgery for chronic pancreatitis decreases the risk for pancreatic cancer: a multicenter retrospective analysis. Surgery. 2013;153(3):357‐364. doi: 10.1016/j.surg.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 35. Travis WD, Lubin J, Ries L, Devesa S. United States lung carcinoma incidence trends: declining for most histologic types among males, increasing among females. Cancer. 1996;77:2464‐2470. doi: 10.1002/(SICI)1097-0142(19960615) [DOI] [PubMed] [Google Scholar]
- 36. Wang B‐Y, Huang J‐Y, Chen H‐C, et al. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J Cancer Res Clin Oncol. 2020;146(1):43‐52. doi: 10.1007/s00432-019-03079-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sakurai H, Asamura H, Goya T, et al. Survival differences by gender for resected non‐small cell lung cancer: a retrospective analysis of 12,509 cases in a Japanese Lung Cancer Registry study. J Thorac Oncol. 2010;5:1594‐1601. doi: 10.1097/JTO.0b013e3181f1923b [DOI] [PubMed] [Google Scholar]
- 38. Gomes M, Teixeira AL, Coelho A, Araújo A, Medeiros R. The role of inflammation in lung cancer. Adv Exp Med Biol. 2014;816:1‐23. doi: 10.1007/978-3-0348-0837-8_1 [DOI] [PubMed] [Google Scholar]
- 39. Fujita K, Nonomura N. Urinary biomarkers of prostate cancer. Int J Urol. 2018;25(9):770‐779. doi: 10.1111/iju.13734 [DOI] [PubMed] [Google Scholar]
- 40. Toyoda Yasuhiro, Tabuchi Takahiro, Hama Hitomi, Morishima Toshitaka, Miyashiro Isao. Trends in clinical stage distribution and screening detection of cancer in Osaka, Japan: stomach, colorectum, lung, breast and cervix. PLoS One 2020;15(12):e0244644. 10.1371/journal.pone.0244644 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Table S1
Table S2
Data Availability Statement
The data set used for analysis in this study is available from MDV, but were used under license for the current study. Restrictions thus apply, and the data are not publicly available. For inquiries about access to the data set used in this study, please contact MDV (https://www.mdv.co.jp).


