Abstract
Background
Developments in medical technology are resulting in continuous decreases in the cancer mortality rate of patients with gallbladder cancer, while non‐cancer deaths in cancer patients are becoming more common. The main cause of this is cardiovascular mortality (CVM). The purpose of this study was to determine the CVM risk in patients with primary gallbladder cancer (PGC).
Methods
We extracted information on patients in the SEER database who were diagnosed with PGC from 2004 to 2015, compared CVM in patients with PGC with the general United States population, and calculated standardized mortality rates (SMRs) and the absolute excess risk. A competing risks model was used to identify and analyze the independent risk factors for cardiovascular death in patients with PGC.
Results
This study included 5925 patients, 247 of whom died from cardiovascular disease. The SMR of cardiovascular death in patients with PGC was 15.84 (95% confidence interval: 15.83–15.85), and the SMR was slightly lower in male than female patients. The competing risks analysis indicated that age, marital status, cancer cell differentiation, chemotherapy status, and year of diagnosis were risk factors for cardiovascular death in patients with PGC.
Conclusions
The CVM risk is considerably higher in patients with PGC than in the general population. It is therefore very necessary to apply cardioprotective interventions to patients with PGC.
Keywords: cancer education, cancer risk factors, cholangiocarcinoma, clinical cancer research, epidemiology
Using patient data from the SEER database to assess risk factors for cardiovascular death in Primary gallbladder carcinoma patients, the article adopts a competing risk model approach that can provide a clearer understanding of the link between Primary gallbladder carcinoma and cardiovascular disease. It can better help clinicians make relevant clinical decisions.
1. BACKGROUND
Gallbladder cancer (primary gallbladder cancer [PGC]) is a relatively rare tumor clinically, but it is the most common tumor of the biliary system, accounting for 80%–95% of all tumors of that system. 1 , 2 In the United States, nearly 4000 people are diagnosed with PGC each year, and about 2000 die from it. 1 Because the early stage of the disease is no different from a normal condition, it is often diagnosed in its late stage, and it has a poor prognosis. 3 With recent improvements in cancer surgery strategies, increased safety awareness of doctors, and changes in daily life, outcomes for patients with PGC have significantly improved. 4 , 5 A recent study found that the 5‐year mortality rate of patients with PGC in the United States was about 20.2%. 6
In 2019, 18.6 million people died from cardiovascular disease (CVD) worldwide, accounting for one‐third of all deaths, and making it the most common cause of mortality. 7 In the United States, many patients who die of CVD are cancer survivors. 8
The mortality rate of patients with cancer has recently been decreasing, 9 and non‐cancer deaths in patients with cancer are also becoming increasingly common, of which CVD is the most common cause. 10 Cardio‐oncology has recently become a hot topic, with cancers such as colorectal, testicular, and endometrial cancer all being associated with cardiovascular mortality (CVM). 11 , 12 , 13 There is no study that we know of that investigated the relationship between patients with PGC and CVD. We therefore assessed the risk factors for CVD among patients with PGC with the aim of identifying those who require earlier screening and treatment, and to establish preventive measures for CVD in patients with PGC.
2. MATERIALS AND METHODS
2.1. Data source
The SEER database collects data on cancer diagnoses, treatments, and prognoses for approximately 30% of the United States population, and it is an important population‐based data set that can be used to study the implications of pathological diagnoses across demographics, geographic regions, and time. It is freely available to the public and does not require informed consent from patients or institutional review board approval. It also includes data on chemotherapy and radiotherapy, for which SEER software is licensed. 14 , 15
2.2. Research variables
We searched the SEER database for all PGC cases using the ICD‐O‐3 tumor site diagnostic code C23.9, and then obtained information on 5925 patients with PGC older than 18 years according to the inclusion and exclusion criteria (Figure 1). The extracted variables included year of diagnosis, age, sex, ethnicity, marital status, TNM stage, surgery, chemotherapy, and radiotherapy statuses, and cause of death.
FIGURE 1.
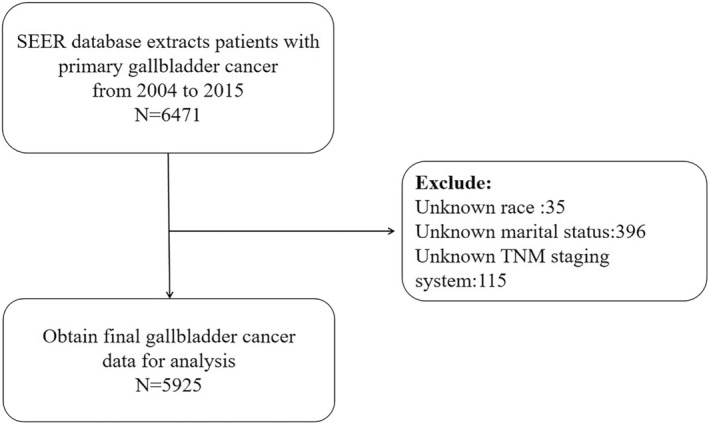
Patient extraction flowchart.
The primary outcome of our study was CVD, including the following six causes of death from the SEER database: hypertension without heart disease, cerebrovascular disease, heart disease, atherosclerosis, aortic aneurysm and dissection, and other arterial diseases in the arterioles, capillaries, and vascular system. 12
2.3. Statistical analysis
The article extracted the CVM rate of the general population from 2004 to 2015 from the United States Centers for Disease Control and Prevention, and then compared it with that of patients with PGC to calculate the standardized mortality rate (SMR). Absolute excess risk (AER) was also calculated as (observed death − expected deaths)/(person‐years of observation). The cumulative incidence function was used to estimate the cumulative incidence of time‐point‐specific death, and Nelson‐Aalen estimator curves were plotted for each factor. 16 Univariate analyses were performed using Gray's test, and factors that were statistically significant in these analyses were then included in the Fine‐Gray subdistribution hazard model in the analysis to identify independent prognostic factors and calculate the hazard ratio (HR) and 95% confidence interval (CI) of each prognostic factor.
The above statistical methods were all implemented using R software, all tests were two‐sided, and the significance threshold was α = 0.05.
3. RESULTS
3.1. Patient characteristics
The information of 6471 patients with PGC was collected after applying the inclusion criteria, and according to the exclusion criteria, we excluded 35 patients without race information, 396 without marital information, and 115 without TNM stage information. Finally, 5925 patients were included in the statistical analysis. The patient details are listed in Table 1, where DSW indicates divorced, separated, or widowed.
TABLE 1.
Characteristics of the patient cohort.
| N = 5925 | |
|---|---|
| Age | |
| <60 | 1433 (24.2) |
| ≥60 | 4492 (75.8) |
| Sex | |
| Male | 1713 (28.9) |
| Female | 4212 (71.1) |
| Marital status | |
| Marital | 3077 (51.9) |
| Single | 868 (14.6) |
| DSW | 1980 (33.4) |
| Race | |
| White | 4516 (76.2) |
| Black | 730 (12.3) |
| Other | 679 (11.5) |
| Year of diagnosis | |
| 2004–2007 | 1878 (31.7) |
| 2008–2011 | 1897 (32.0) |
| 2012–2015 | 2150 (36.3) |
| Grade | |
| Well | 810 (13.7) |
| Moderate | 2434 (41.1) |
| Poor | 2523 (42.6) |
| Undifferential | 158 (2.7) |
| Surgery | |
| Yes | 905 (15.3) |
| No/unknown | 5020 (84.7) |
| Radiotherapy | |
| Yes | 1027 (17.3) |
| No/unknown | 4898 (82.7) |
| Chemotherapy | |
| Yes | 2100 (35.4) |
| No/unknown | 3825 (64.6) |
| Lymph node metastasis | |
| Yes | 1700 (28.7) |
| No/unknown | 4225 (71.3) |
| Distant metastasis | |
| Yes | 1743 (29.4) |
| No/unknown | 4182 (70.6) |
3.2. SMR and AER
The SMR of cardiovascular death in patients with PGC was 15.84 (95% CI, 15.83–15.84), and the AER was 202.7/10,000. The stratified analysis of the different variables indicated that the SMR and AER of cardiovascular death were higher in patients with PGC. Regarding age stratification, the older the age, the closer the cardiovascular risk of patients with PGC was to that of the general population. For the year of diagnosis, the later the year, the closer the SMR value was to the normal population. Among sexes, females had slightly higher SMRs than males (Table 2).
TABLE 2.
SMR and AER.
| Observed deaths (%) | Expected deaths | SMR (95% CI) | Excess risk per 10,000 | Persons (%) | |
|---|---|---|---|---|---|
| Total | 247 | 15.59 | 15.84 (15.83–15.85) | 202.66 | 5925 |
| Male | 70 | 4.53 | 15.47 (15.45–15.48) | 213.86 | 1713 |
| Female | 177 | 11.03 | 16.05 (16.03–16.06) | 198.60 | 4212 |
| ≤44 | 2 | 0.02 | 81.19 (80.45–81.19) | 36.09 | 226 |
| 45–49 | 2 | 0.19 | 10.68 (10.64–10.73) | 34.39 | 240 |
| 50–54 | 7 | 0.48 | 14.51 (14.46–14.55) | 67.16 | 377 |
| 55–59 | 10 | 1.14 | 8.77 (8.74–8.80) | 66.25 | 590 |
| 60–64 | 14 | 2.03 | 6.90 (6.89–6.91) | 80.99 | 694 |
| 65–69 | 16 | 3.41 | 4.69 (4.68–4.70) | 72.54 | 784 |
| 70–74 | 28 | 5.65 | 4.96 (4.95–4.97) | 139.12 | 803 |
| 75–79 | 42 | 9.77 | 4.29 (4.29–4.30) | 241.88 | 804 |
| 80–84 | 46 | 16.26 | 2.83 (2.82–2.83) | 264.41 | 732 |
| 85+ | 80 | 39.45 | 2.02 (2.02–2.02) | 534.45 | 675 |
| 2004–2007 | 115 | 5.29 | 21.73 (21.70–21.75) | 263.83 | 1878 |
| 2008–2011 | 76 | 4.84 | 15.68 (15.67–15.70) | 185.54 | 1897 |
| 2012–2015 | 56 | 5.44 | 10.29 (10.28–10.30) | 147.61 | 2150 |
| White | 200 | 12.77 | 15.67 (15.65–15.68) | 217.49 | 4516 |
| Black | 26 | 1.68 | 15.43 (15.41–15.47) | 186.58 | 730 |
| Other | 21 | 0.67 | 31.40 (31.27–31.53) | 134.96 | 679 |
Abbreviations: CI, confidence interval; SMR, standardized mortality rate.
3.3. Univariate analyses of cardiovascular death in patients with PGC
Figure 2 displays all causes of death in patients with PGC using the Fine‐Gray subdistribution hazard model. It shows that cardiovascular death had the lowest cumulative mortality, and the cumulative mortality due to primary site death has always been the highest. Figure 3 displays all subgroups of patients with PGC who died from various causes. According to the univariate analysis results, age, marital status, distant metastasis, radiotherapy and chemotherapy statuses, degree of differentiation, year of diagnosis, and lymph node metastasis were associated with cardiovascular death in patients with PGC. Among them, the associations with sex and marital status were not significant.
FIGURE 2.
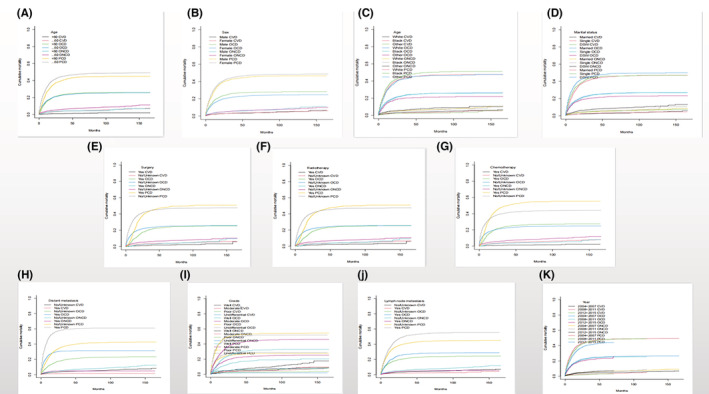
CIF of all causes of death in PGC patients. (A) age, (B) sex, (C) race, (D) marry, (E) surgical, (F) radiotherapy, (G) chemotherapy, (H) distant metastasis, (I) grade, (J) lymph node metastasis, (K) year of diagnosis. CIF, cumulative incidence function; CVD, cardiovascular death; OCDs, other cancer deaths; ONCDs, other non‐cancer deaths; PCD, primary gallbladder cancer death.
FIGURE 3.
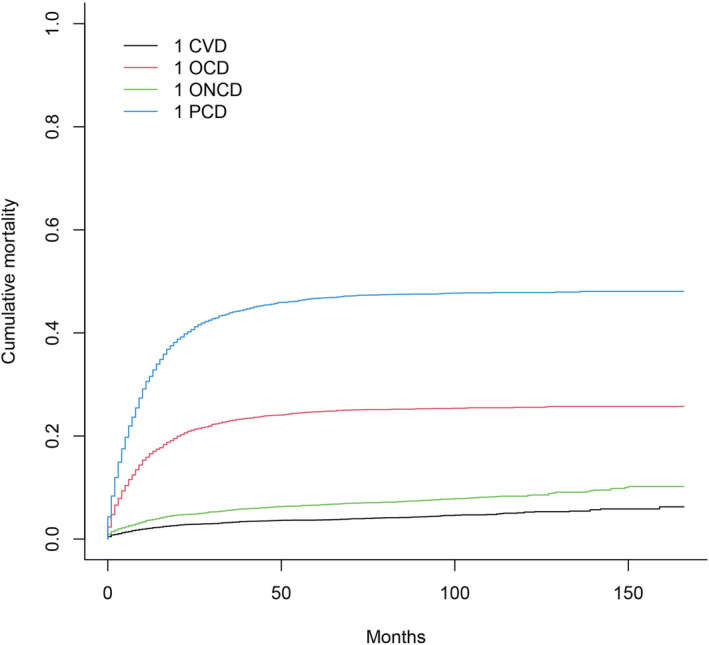
CIF of all subgroups of gallbladder cancer patients dying from all causes. CIF, cumulative incidence function.
3.4. Multivariate analysis of cardiovascular death in patients with PGC
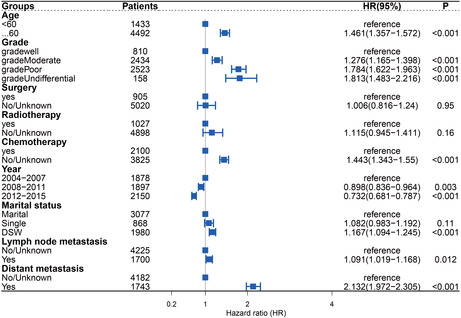
We included the meaningful variables in the univariate analyses in a multivariate competing risks model, and obtained the following factors with a high risk for cardiovascular death in patients with PGC (Figure 4): age ≥60 years (HR: 1.461; 95% CI: 1.357–1.572), DSW (HR: 1.167; 95% CI: 1.094–1.245), moderately differentiated cancer cells (HR: 1.276; 95% CI: 1.165–1.398), poorly differentiated cancer cells (HR: 1.784; 95% CI: 1.622–1.963), undifferentiated cancer cells (HR: 1.813; 95% CI: 1.483–2.216), cancer cells with lymph node metastasis (HR: 1.091; 95% CI: 1.019–1.168), cancer cells with distant metastasis (HR: 2.132; 95% CI: 1.972–2.305), and no chemotherapy (HR: 1.443; 95% CI: 1.343–1.55). The results also indicated that the later the year of diagnosis, the lower the risk of cardiovascular death (2008–2011 [HR: 0.898; 95% CI: 0.836–0.964]; 2012–2015 [HR: 0.732; 95% CI: 0.681–0.787]).
FIGURE 4.
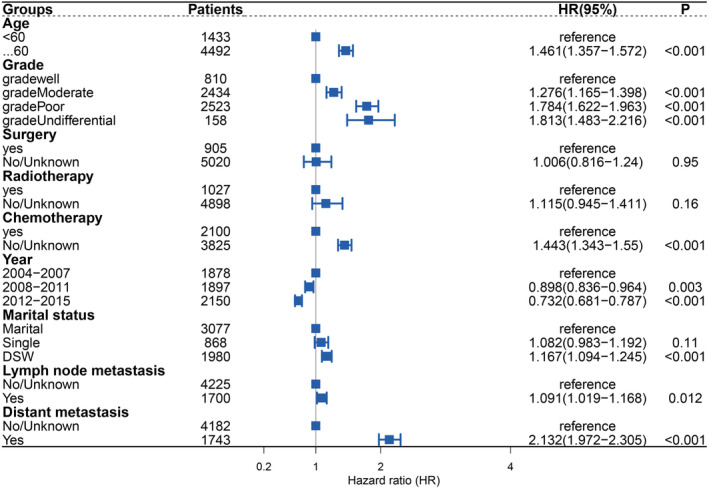
Multifactor competitive risk forest plot.
4. DISCUSSION
Cancer and CVD are two common causes of death in humans. Many recent studies have confirmed that the incidence of cardiovascular death is increasing in patients with cancer, and so many governments strongly support cooperation between these two fields in order to better meet the clinical needs of patients with complex cancer. 17 In a study of 3,234,256 adult patients with cancer, 10 Sturgeon et al. found that 11% of patients died of CVD, which was far higher proportion than in the general United States population.
Our study found that during 2004–2015, the cardiovascular death rate of patients with PGC in the United States was approximately 4101.7 per 100,000 person‐years, compared with 263.1 per 100,000 person‐years in the general United States population, with an SMR of 15.84 (95% CI, 15.83–15.85). After stratifying by year of diagnosis, we found that the SMR during 2004–2007 was 21.73 (95% CI, 21.70–21.75), and that the SMR became smaller over time, mostly due to population differences. 18 Regarding age stratification, the SMR continuously decreased as age increased, but generally patients with PGC had a significant advantage over the general population regardless of age group.
We comprehensively assessed the risk of all‐cause death in patients with PGC, and the competing risks analysis revealed that the cumulative mortality rate of cardiovascular death was lower than that of cancer death, other‐cancer death, and other‐cause death. We then incorporated influencing factors identified in the competing risks univariate analyses into a multivariate model, which revealed age at diagnosis, year of diagnosis, marital status, degree of differentiation, chemotherapy status, distant metastasis, and lymph node metastasis as independent factors for cardiovascular death in patients with PGC.
Compared with patients with PGC diagnosed during 2012–2015, those diagnosed during 2004–2007 were more likely to die from CVD, mostly because of advances in medical technology and the emphasis on the convergence of cancer and CVDs. 19 Most of the patients in the present study were elderly, and the results indicated that the CVM rate was 46% for the elderly patients than for the younger patients. Recent studies have pointed out that the risk factors for cancer and CVD include age, 20 mostly due to the decline of physical function in elderly patients, various physiological functions not being perfect, and their poor living habits, all of which are associated with CVD occurrence. Being single, divorced, separated, or widowed were also risk factors for cardiovascular death in patients with PGC. Previous studies have found that living alone and social disengagement exert important effects on CVD, 21 and compared with married patients, single patients may receive less attention and have worse economic, health, and social statuses. 22 , 23
Lymph node and distant metastases of cancer cells are present in patients at an advanced stage. 24 Our study found that lymph node and distant metastases are both risk factors for cardiovascular death in patients with PGC, mostly because cancer cell metastasis can cause symptoms such as inflammation and fever, and also have a greater psychological impact on patients. These can lead to death from CVD. 25 In addition, the better the degree of differentiation of cancer cells, the lower the likelihood of developing CVD since the cancer cells are closer to normal tissue cells. Data on chemotherapy, radiotherapy, and surgery were included in the competing risks model, which found that patients with PGC who did not receive chemotherapy had a higher risk of CVD. Previous studies have found that existing anticancer drugs such as anthracyclines and trastuzumab are associated with a cumulative heart failure incidence, 26 , 27 which was in conflict with our findings, so it is necessary to further verify the relationship between chemotherapy status and patients with PGC. Surgery and radiotherapy are not independent predictors of patients with PGC, and there is a lack of detailed treatment plans for radiotherapy and surgery in the SEER database. Further research on the effects of radiotherapy and surgery on CVM in patients with PGC is therefore needed.
This study had some limitations. First, the SEER database does not guarantee the accuracy of all‐cause death records and may overestimate the probability of cardiovascular death. 28 Second, the database does not include chemotherapy and radiotherapy doses and other factors associated with CVD. Third, there may have been some minor biases among the study participants due to the retrospective design. Finally, the SEER database lacks certain patient specific information, which inevitably causes some errors to the research ending.
5. CONCLUSION
This study found that patients with PGC had a significantly higher risk of CVM than the general United States population, and a competing risks analysis was used to identify independent predictors of CVM in patients with PGC. The age of diagnosis, year of diagnosis, marriage status, degree of differentiation, chemotherapy status, distant metastases, and lymph node metastasis are all independent factor for cardiovascular death in PGC patients. When encountering patients diagnosed with PGC, CVD should be screened as soon as possible, and its risk factors should be controlled.
AUTHOR CONTRIBUTIONS
Jun Lyu conceptualized the research aims, planned the analyses. Chong Chen and Fengshuo Xu guided the literature review. Shiqi Yuan, Xuenuo Zhao, and Didi Han extracted the data from the SEER database. Shiqi Yuan and Mengmeng Qiao participated in data analysis and interpretation. Chong Chen and Fengshuo Zhao wrote the first draft of the paper and the other authors provided comments and approved the final manuscript.
FUNDING INFORMATION
The study was supported by Guangdong Provincial Key Laboratory of Traditional Chinese Medicine Informatization (2021B1212040007).
CONFLICTS OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICS STATEMENT
The data of this study are obtained from the SEER database. The patient's data are public and anonymous, so this study does not require ethical approval and informed consent.
INFORMED CONSENT STATEMENT
Not applicable.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Permit.
Chen C, Xu F, Yuan S, et al. Competing risk analysis of cardiovascular death in patients with primary gallbladder cancer. Cancer Med. 2023;12:2179‐2186. doi: 10.1002/cam4.5104
Chong Chen and Fengshuo Xu contributed equally to this study and should be considered co‐first author.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute at https://seer.cancer.gov/.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591‐1602. [DOI] [PubMed] [Google Scholar]
- 4. Akhtar‐Danesh N, Akhtar‐Danseh GG, Seow H, Shakeel S, Finley C. Treatment modality and trends in survival for gallbladder cancer: a population‐based study. J Gastrointest Cancer. 2021;52:256‐262. [DOI] [PubMed] [Google Scholar]
- 5. Chang Y, Li Q, Wu Q, et al. Impact of surgical strategies on the survival of gallbladder cancer patients: analysis of 715 cases. World J Surg Oncol. 2020;18:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Y, Huang C, Bai Q, Yu J. Ribophorin II promotes cell proliferation, migration, and invasion in esophageal cancer cells in vitro and in vivo. Biosci Rep. 2019;39:BSR20182448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982‐3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leopold JA, Loscalzo J. Emerging role of precision medicine in cardiovascular disease. Circ Res. 2018;122:1302‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363‐385. [DOI] [PubMed] [Google Scholar]
- 10. Sturgeon KM, Deng L, Bluethmann SM, et al. A population‐based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889‐3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaitanidis A, Spathakis M, Tsalikidis C, Alevizakos M, Tsaroucha A, Pitiakoudis M. Risk factors for cardiovascular mortality in patients with colorectal cancer: a population‐based study. Int J Clin Oncol. 2019;24:501‐507. [DOI] [PubMed] [Google Scholar]
- 12. Fung C, Fossa SD, Milano MT, Sahasrabudhe DM, Peterson DR, Travis LB. Cardiovascular disease mortality after chemotherapy or surgery for testicular nonseminoma: a population‐based study. J Clin Oncol. 2015;33:3105‐3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol. 2012;126:176‐179. [DOI] [PubMed] [Google Scholar]
- 14. Wu WT, Li YJ, Feng AZ, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res. 2021;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang J, Li Y, Liu Q, et al. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med. 2020;13:57‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC, Fine JP. Practical recommendations for reporting Fine‐gray model analyses for competing risk data. Stat Med. 2017;36:4391‐4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. GBD 2017 Causes of Death Collaborators . Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alvarez‐Cardona JA, Ray J, Carver J, et al. Cardio‐oncology education and training: JACC council perspectives. J Am Coll Cardiol. 2020;76:2267‐2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoang G, Nguyen K, Le A. Metabolic intersection of cancer and cardiovascular diseases: opportunities for cancer therapy. Adv Exp Med Biol. 2021;1311:249‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fang F, Fall K, Mittleman MA, et al. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med. 2012;366:1310‐1318. [DOI] [PubMed] [Google Scholar]
- 21. Bu F, Steptoe A, Fancourt D. Relationship between loneliness, social isolation and modifiable risk factors for cardiovascular disease: a latent class analysis. J Epidemiol Community Health. 2021;75:749‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niu Q, Lu Y, Wu Y, et al. The effect of marital status on the survival of patients with bladder urothelial carcinoma: a SEER database analysis. Medicine (Baltimore). 2018;97:e11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang L, Wilson SE, Stewart DB, Hollenbeak CS. Marital status and colon cancer outcomes in US surveillance, epidemiology and end results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol. 2011;35:417‐422. [DOI] [PubMed] [Google Scholar]
- 24. Oh SH, Choi SY, Choi HJ, et al. The emerging role of xanthine oxidase inhibition for suppression of breast cancer cell migration and metastasis associated with hypercholesterolemia. FASEB J. 2019;33:7301‐7314. [DOI] [PubMed] [Google Scholar]
- 25. Liddane AG, McNamara CA, Campbell MC, Mercier I, Holaska JM. Defects in emerin‐nucleoskeleton binding disrupt nuclear structure and promote breast cancer cell motility and metastasis. Mol Cancer Res. 2021;19:1196‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long‐term follow‐up study. Eur J Cancer. 2006;42:3191‐3198. [DOI] [PubMed] [Google Scholar]
- 27. Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463‐475. [DOI] [PubMed] [Google Scholar]
- 28. Lloyd‐Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020‐1026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute at https://seer.cancer.gov/.


