Abstract
Background
Transarterial radioembolization (TARE) is increasingly used as an alternative to transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma (HCC). We aimed to perform an overall and individual patient data (IPD) meta‐analysis of studies comparing TACE and TARE.
Methods
We performed a systematic literature search using pre‐specified keywords with the aid of an informationist for articles from inception to 3/2020. The primary endpoint was overall survival (OS), and the secondary endpoint was time to progression (TTP).
Results
Seventeen studies met inclusion criteria with 2465 unique patients, with one randomized trial, 4 prospective studies and 12 retrospective studies. Barcelona Clinic Liver Cancer (BCLC) stage B (42.8%) was the most common stage followed by BCLC A (30.3%) and BCLC C (29.0%). There was no difference in OS between the two modalities (−0.55 months, 95% CI −1.95 to 3.05). In three studies with available TTP data, TARE resulted in a longer TTP than TACE (mean TTP 17.5 vs. 9.8 months; mean TTP difference 4.8 months, 95% CI 1.3–8.3 months). IPD‐level meta‐analysis of 311 patients from three studies showed no difference in overall OS between the two modalities including among subgroups stratified by tumor stage and liver function. Limitations of the current literature include inconsistent length of follow‐up, inconsistency in response criteria, and safety reporting.
Conclusions
Current data suggest TARE provides significantly longer TTP than TACE, although the two treatments do not significantly differ in terms of OS. Given limitations of the current data, there is rationale for prospective studies comparing these modalities.
Keywords: HCC, locoregional therapy, TACE, Y‐90
In this overall and individual level meta‐analysis, patients with hepatocellular carcinoma treated with transarterial chemoembolization and transarterial radioembolization (TARE) showed similar survival. Patients treated with TARE however, had a longer time to tumor progression.
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer related to mortality worldwide, largely owing to ineffective early detection strategies, competing risk from comorbid cirrhosis, and historical lack of effective therapies for intermediate and advanced stage disease. 1 Curative therapies are typically reserved for patients with early‐stage HCC, resulting in >70% 5‐year survival, whereas intermediate and advanced disease have marked decrements in survival. 2
Furthermore, there are no well‐established treatment algorithms, with significant variation in treatment application. 3 This is due in part to the lack of head‐to‐head data comparing many treatment modalities leading to variance in treatment allocation. For example, in patients with early intermediate‐stage disease, transarterial chemoembolization (TACE) and transarterial radioembolization (TARE) are two commonly used loco‐regional therapies (LRT) which aim to prolong survival by slowing tumor progression, or to bridge to more definitive therapies. However, there is a lack of robust comparative effectiveness data comparing these treatments.
TACE has the highest quality of evidence among LRTs, supported by several randomized controlled trials. 4 , 5 , 6 TARE recently attained Food and Drug Administration premarket approval with publication of recent large observational study showing excellent efficacy in the treatment of unifocal HCC in a multicenter cohort 7 ; however, due to lack of robust controlled data, TARE has not been widely adopted as a frontline treatment in guidelines. 8 , 9 A single center randomized controlled trial with 45 patients comparing these modalities showed no difference in overall survival (OS), but showed longer time to progression with TARE, although generalizability remains limited due to the overall design and sample size. 10 Existing observational data have been similarly limited by sample size, patient selection, and lack of correlates for treatment efficacy. While other meta‐analyses comparing TACE and TARE have been completed, we lack multicenter individual level data comparing TARE and TACE and correlates of survival. Our aim was to perform a meta‐analysis, including individual patient data, comparing TACE and TARE to determine the comparative effectiveness of these treatments.
2. METHODS
2.1. Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRIMSA) guidelines were used to guide study selection and data collection. 11 Additionally, the study was registered in PROSPERO (CRD42019129117) prior to its initiation. A systemic literature search using PubMed, Medline, EMBASE, Scopus, Web of Science, and ClinicalTrials.gov was performed using pre‐specified keywords with the aid of an informationist for articles from inception to March 2020. The search strategy was included in Appendix S1. Inclusion criteria were studies that directly compared TACE and TARE for the treatment of HCC. Exclusion criteria included study of the use of additional treatment modalities (e.g. other locoregional or systemic therapies), liver tumors besides primary HCC, and studies published only in abstract form. Only full text English language studies were included. W combined modalities of drug eluting bead (DEB) TACE and conventional TACE (cTACE) and the modalities of therasphere TARE and SIRS‐Sphere TARE due to the lack of data showing significant differences in outcomes between modalities. 12 , 13
2.2. Data extraction
The search yielded 1784 unique articles that were screened for inclusion by two independent reviewers (AB and IK). Conflicts were resolved with the assistance of a third reviewer (NP). Studies to be included were more closely analyzed by the authors and selected for appropriateness for data extraction. Data were extracted by each reviewer using standardized forms. Forms collected demographic, liver function, cancer staging, unadjusted survival, time to progression data, and adverse events when available. Study quality assessment was performed with Newcastle‐Ottawa Scale (NOS).
2.3. Outcomes
The primary endpoint was overall survival (OS), and the secondary endpoint was time to progression (TTP).
2.4. Individual patient data (IPD) meta‐analysis
Authors of all included studies were contacted for data sharing to include patient‐level data to allow IPD meta‐analysis, with the authors of three studies responding. These studies included papers by Moreno‐Luna et al., 14 Soydal et al., 15 and Massani et al. 16 Prior to data transfer, we obtained University of Michigan and local site institutional review board approval, and deidentified data were transferred using data use agreements.
2.5. Statistical analysis
OS and TTP were reported in four different ways: (1) mean and standard deviation, (2) mean and standard error, (3) median and interquartile range, or (4) median and 95% confidence interval. To facilitate meta‐analysis, we harmonized all outcome reporting into mean and standard deviation per (1). For (2), we used reported mean and calculated . For (3), we estimated mean as and standard deviation as , where T represents time (OS or TTP), Q1 represents the 25th percentile time, med the median time, Q3 the 75th percentile time, the upper x‐th percentile, and n the number of patients receiving that specific treatment. For (4), we estimated mean as and standard deviation as , where T represents time, either OS or TTP, L represents the lower bound of the confidence interval, med the median time, and H the upper bound of confidence interval. 17
Overall meta‐analysis was performed comparing TACE and TARE using a random effects model incorporating mean OS/TTP, standard deviation, and n for TACE and TARE recipients. We evaluated mean difference of both OS and TTP. Preplanned subgroup analyses stratifying studies by study quality. We assessed publication bias visually using a funnel plot and numerically with an Egger test.
IPD meta‐analysis was performed using data from three studies. The primary outcome was OS, as data on TTP were not available in the studies in which IPD were available. We generated Cox proportional hazard survival models rather than comparing difference in mean survival as these models are more informative. We conducted pre‐specified subgroup analyses based on BCLC stage and CP class, in which we separately evaluated hazard ratio for OS in each study and subgroup, and then meta‐analyzed across studies and within each subgroup (cluster random effects meta‐analysis) using a random effects meta‐analysis similar to as described above. Finally, we generated in each cohort a multivariable Cox proportional hazard survival model with outcome of OS and predictor of treatment type (TARE vs. TACE), adjusting for age, sex, CP class, and BCLC stage, and meta‐analyzed across the three cohorts as above.
p < 0.05 was used for significance in all analyses. All analyses were performed in R version 3.5.1.
3. RESULTS
3.1. Overall cohort characteristics
In total, our initial search revealed 1784 studies, 17 of which met inclusion criteria including 2465 unique patients (Figure S1). Twelve of the included studies were retrospective cohort studies and the remainder consisted of one randomized trial and four prospective cohort studies. 10 , 14 , 15 , 16 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 The mean patient age was 62.1 years, and the majority were male (77.0%) and white (74.3%). Approximately two‐thirds of patients had compensated cirrhosis (Child Pugh [CP] A 65.4%) and the remainder were decompensated (CP B: 30.9%; CP C: 2.1%). Barcelona Clinic Liver Cancer (BCLC) stage B (42.1%) was the most common stage followed by BCLC C (29.0%) and BCLC A (30.3%) (Table 1).
TABLE 1.
Descriptive statistics of overall meta‐analysis stratified by treatment type
| Parameter | Overall (n = 2465) | TACE (n = 1657) | TARE (n = 808) |
|---|---|---|---|
| Mean age (years) | 62.1 | 60.8 | 66.5 |
| Male (%) | 77.0 | 77.0 | 76.9 |
| Race/Ethnicity | |||
| White (%) | 74.3 | 71.8 | 76.9 |
| Black (%) | 12.0 | 11.7 | 12.3 |
| Hispanic (%) | 6.8 | 8.0 | 5.6 |
| Asian (%) | 8.7 | 9.2 | 8.3 |
| Other race (%) | 6.9 | 10.0 | 3.6 |
| Etiology of cirrhosis | |||
| Alcohol (%) | 27.2 | 26.7 | 28.2 |
| Hepatitis C (%) | 31.2 | 29.4 | 35.2 |
| Hepatitis B (%) | 10.7 | 11.5 | 8.7 |
| Non‐alcoholic steatohepatitis (%) | 6.0 | 5.7 | 6.2 |
| Other etiology (%) | 22.8 | 23.0 | 22.6 |
| Child‐Pugh class | |||
| A (%) | 65.4 | 66.0 | 64.6 |
| B (%) | 30.9 | 29.2 | 33.3 |
| C (%) | 2.1 | 2.0 | 2.3 |
| Barcelona Clinic Liver Cancer Stage | |||
| A (%) | 30.3 | 33.1 | 27.4 |
| B (%) | 42.8 | 41.8 | 43.8 |
| C (%) | 29.0 | 27.3 | 30.7 |
| D (%) | 3.1 | 3.7 | 2.5 |
Abbreviation: BCLC, Barcelona Clinic Liver Cancer.
Patients receiving TARE were significantly more likely to have chronic hepatitis C as the etiology of their liver disease (35.2% vs. 29.4%; p = 0.008), but otherwise there were no differences between the groups.
3.2. TACE and TARE modalities
Conventional TACE (cTACE) was the most commonly used modality in the included studies (8/17). Five of the studies used drug‐eluting bead transarterial chemoembolization (DEB‐TACE), while three studies used both cTACE and DEB‐TACE. One study used TACE with degradable starch microspheres (DSM). Each study had a mix of lobar treatment and segmental treatments.
With regard to TARE, the majority of the studies (10/17) used TheraSphere while six studies used SIR‐sphere, and one study used both (Table 2). Notably, no study specified the use of individual dosimetry for TARE delivery, although four used selective catheterization of the tumor vessels for treatment delivery.
TABLE 2.
Hepatocellular carcinoma treatment modalities used by the included studies
| Study | Year | TACE n | TACE modality | TACE treatment | TARE n | TARE modality | TARE treatment |
|---|---|---|---|---|---|---|---|
| Akinwande 24 | 2016 | 28 | DEB‐TACE | Lobar and selective | 20 | TheraSphere | Lobar |
| Akinwande 21 | 2015 | 291 | DEB‐TACE | Lobar and selective | 67 | TheraSphere | Lobar |
| Auer 30 | 2021 | 18 | DSM‐TACE | Lobar | 18 | SIR‐Sphere | Lobar |
| Biederman 22 | 2018 | 877 | DEB‐TACE | Selective | 534 | TheraSphere | Lobar |
| Carr 29 | 2010 | 691 | cTACE | Lobar and selective | 99 | TheraSphere | Lobar |
| El Fouly 20 | 2014 | 42 | cTACE | Selective | 44 | TheraSphere | Lobar |
| Kooby 19 | 2010 | 44 | cTACE | Selective | 27 | SIR‐Sphere | Lobar |
| Lance 18 | 2011 | 35 | cTACE and DEB‐TACE | Selective | 38 | TheraSphere and SIR‐Sphere | Lobar and Selective |
| Massani 16 | 2017 | 82 | cTACE and DEB‐TACE | Lobar and Selective | 39 | SIR‐Sphere | Lobar |
| McDevitt 27 | 2017 | 24 | DEB‐TACE | NS | 26 | TheraSphere | NS |
| Moreno‐Luna 14 | 2012 | 55 | cTACE | Lobar and Selective | 61 | TheraSphere | Lobar |
| Padia 26 | 2017 | 77 | cTACE and DEB‐TACE | Selective | 101 | TheraSphere | Selective |
| Pitton 25 | 2014 | 12 | DEB‐TACE | Selective | 12 | SIR‐Sphere | Lobar |
| Salem 23 | 2011 | 122 | cTACE | NS | 123 | TheraSphere | NS |
| Salem 10 | 2016 | 21 | cTACE | NS | 24 | TheraSphere | NS |
| She 28 | 2014 | 16 | cTACE | Lobar and Selective | 16 | SIR‐Sphere | Selective |
| Soydal 15 | 2016 | 40 | cTACE | Selective | 40 | SIR‐Sphere | Selective |
Abbreviations: cTACE, conventional transarterial chemoembolization; DEB, Drug eluting bead; DSM, degradable starch microsphere; NS, not specified; TACE, Transarterial chemoembolization; TARE, transarterial radioembolization.
3.3. Outcomes
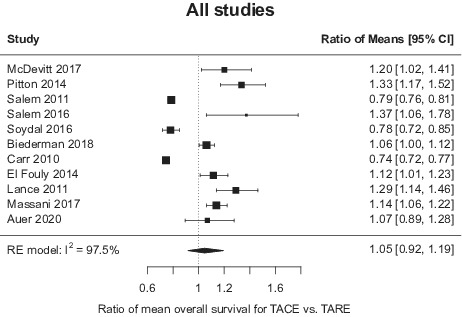
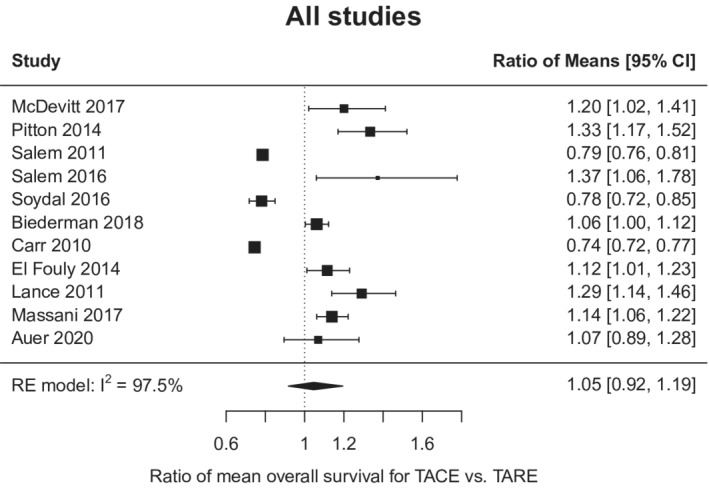
There was no difference in OS between the two modalities with absolute difference −0.55 months, 95% CI −1.95 to 3.05 (for TARE relative to TACE) (Figure 1); however, there was notable heterogeneity among the studies (I 2 97.5%; p < 0.001). In the three studies with available TTP data, TARE resulted in a significantly longer TTP than TACE (mean TTP 17.5 vs. 9.8 months; mean TTP difference 4.8 months, 95% CI 1.3–8.3 months) (Figure 2). Similarly, heterogeneity was again seen among the studies reporting the TTP outcome (I 2 > 97%; p < 0.001).
FIGURE 1.
Forest plot of mean overall survival for transarterial chemoembolization (TACE) vs transarterial radioembolization (TARE). RE, random effects
FIGURE 2.
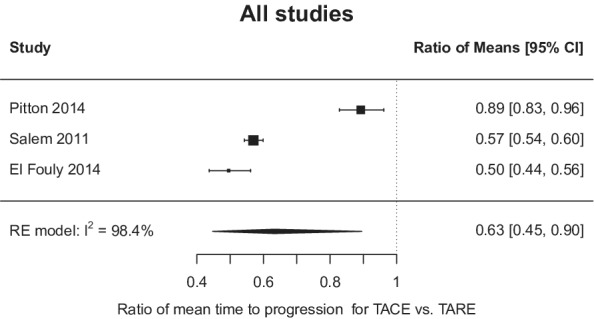
Forest plot of mean time to progression for transarterial chemoembolization (TACE) vs transarterial radioembolization (TARE). RE, random effects
3.4. Individual level meta‐analysis
Individual‐level meta‐analysis included 311 patients, 143 in the TACE group and 168 in the TARE group (Table 3). Mean age was 67.8 years, 81% were male, and 88.5% were white. 61% of patients were BCLC stage A. And 83.2% had CP A liver function, while 16.8% had CP B. The cohort characteristics were similar between the TACE and TARE groups, except for a higher proportion of BCLC A patients in the TACE group (26.4% vs 12.2%; p = 0.012). Overall hazard ratio showed no difference in OS between TARE and TACE (HR: 0.90; 95% CI: 0.70–1.16). The results were consistent in key subgroups stratified by BCLC and CP class (Figures 3 and 4). In multivariate analysis (Table S1), worse OS was associated with male sex (vs. females) (HR: 1.43; 95% CI: 1.07–1.92); Child Pugh B/C cirrhosis (vs. CP A) (HR: 1.36; 95% CI: 1.02–1.82); and more advanced stage HCC (BCLC B vs. A; HR: 1.58; 95% CI: 1.13–2.21; BCLC C/D vs. A; HR: 2.08; 95% CI: 1.46–2.95).
TABLE 3.
Descriptive statistics of individual‐level meta‐analysis stratified by treatment type
| Parameter | Overall | TACE (n = 179) | TARE (n = 168) | p‐value |
|---|---|---|---|---|
| Mean age (years) | 67.8 | 68.6 | 66.6 | 0.86 |
| Male (%) | 81.3 | 81.3 | 81.3 | 1.00 |
| Race/Ethnicity | ||||
| White (%) | 88.5 | 88.0 | 89.0 | 1.00 |
| Black (%) | 2.1 | 0.0 | 4.0 | 0.40 |
| Hispanic (%) | 3.1 | 2.0 | 4.0 | 0.93 |
| Other race (%) | 6.4 | 9.0 | 4.0 | 0.47 |
| Etiology of cirrhosis | ||||
| Alcohol (%) | 36.0 | 36.6 | 35.2 | 0.93 |
| Hepatitis C (%) | 21.6 | 23.5 | 18.9 | 0.50 |
| Hepatitis B (%) | 13.2 | 13.4 | 12.8 | 1.00 |
| Non‐alcoholic steatohepatitis (%) | 12.7 | 18.0 | 8.0 | 0.18 |
| Other etiology (%) | 29.2 | 24.2 | 36.0 | 0.069 |
| Child‐Pugh class | ||||
| A (%) | 83.2 | 82.5 | 84.1 | 0.88 |
| B (%) | 16.8 | 17.5 | 15.9 | |
| Barcelona Clinic Liver Cancer Stage | ||||
| A (%) | 20.4 | 26.4 | 12.2 | 0.012 |
| B (%) | 46.6 | 44.2 | 49.8 | 0.38 |
| C (%) | 31.9 | 30.8 | 33.2 | 0.73 |
FIGURE 3.
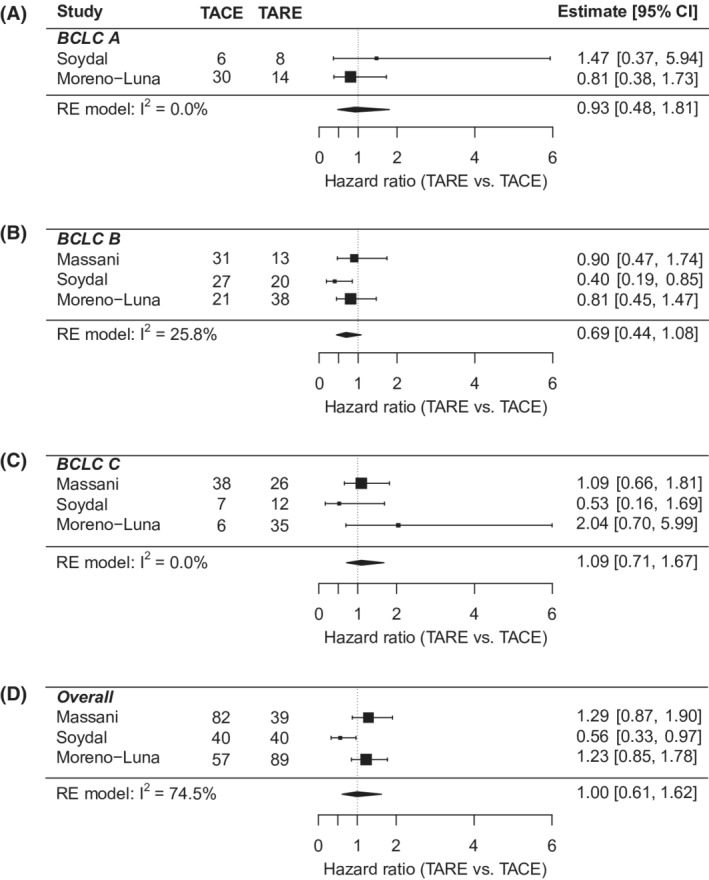
Individual‐level meta‐analysis forest plots for (A) Barcelona Clinic Liver Cancer stage (BCLC) A, (B) B, (C) C; (D) overall. Hazard ratio <1 implies TARE is favored and >1 implies TACE is favored. RE, random effects; TACE, transarterial chemoembolization; TARE, transarterial radioembolization
FIGURE 4.
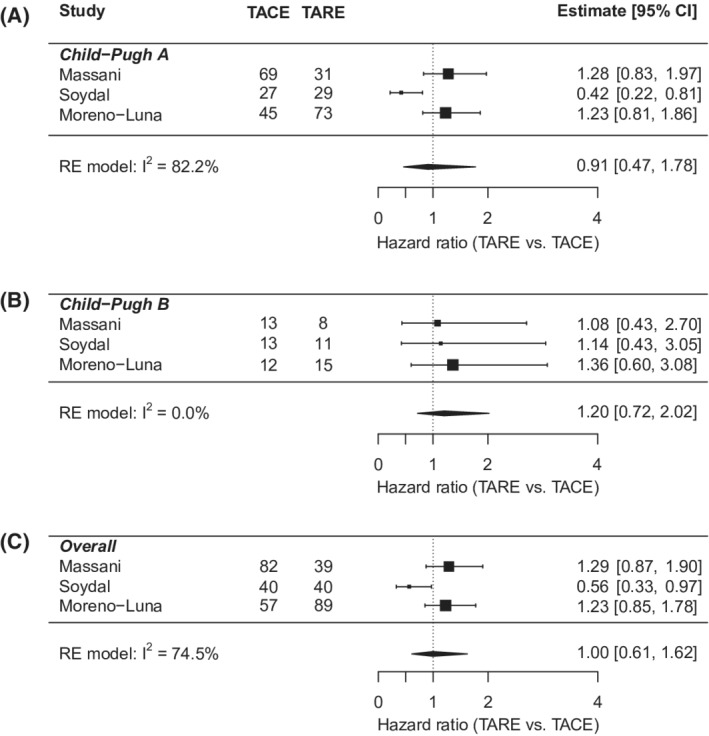
Individual‐level meta‐analysis forest plots for (A) Child Pugh A, (B) Child Pugh B, (C) overall. Hazard ratio <1 implies TARE is favored and >1 implies TACE is favored. RE, random effects. TACE, transarterial chemoembolization. TARE, transarterial radioembolization
3.5. Publication bias and study quality
There was no evidence of publication bias for mean differences for either OS or TTP based on Egger's regression test and rank correlation test (Figure S2).
The mean total quality score for the included studies was 7.2 on NOS. Sixteen studies (84%) were high quality (NOS ≥ 7) while one study was medium quality (NOS <7) (Table S2). There were only two studies classified as medium‐quality. When only high‐quality studies were included, OS with TARE was not significantly higher than with TACE (mean difference 0.27 months; 95% CI: −2.43 to 2.97; I 2: 98.1%.) (Figure S3).
3.6. Adverse events
The included studies featured a wide range of adverse events (AE) data. Only four studies reported data on AEs; however, there was significant heterogeneity in the reporting. Any AE associated with TACE patients was 10.8–73%, while any AE for TARE patients was 10%–44%. 18 , 19 , 21 , 24 In those same studies, grade 3–4 AEs were 4.5%–36% and 4%–30% for patients receiving TACE and TARE, respectively. 18 , 19 , 21 , 24 One study reported nausea and vomiting rates to be 16.5% versus 55% 30 for TACE versus TARE; while another reported 38% versus 0% for TACE versus TARE. 20 Two studies reported higher rates of abdominal pain in TACE (73%–83%) vs TARE (5%–33%). 20 , 27 Rates of diarrhea were 21% versus 0% in TACE versus TARE in one study. 10 One study described a higher rate of post‐embolization syndromes in TACE (20%) compared to TARE (2.6%). 18
4. DISCUSSION
In our updated meta‐analysis, we have demonstrated lack of a difference in overall survival in patients receiving TACE versus TARE; however, time to HCC progression was significantly longer in patients receiving TARE therapy. These findings are concordant with the only randomized data comparing TACE and TARE. 10 In an individual meta‐analysis, we were able to confirm the lack of superiority of either modality for overall survival in subgroups of patients stratified by tumor stage and liver function. Notably, TARE had similar associated survival outcomes despite having a higher proportion of patients with more advanced stage liver disease (CP B) and advanced stage HCC (BCLC C). While there have been previous published meta‐analyses comparing these modalities, 31 , 32 , 33 our study includes several additional contemporaneous studies, in addition to individual level meta‐analysis including data including 311 patients.
In the subgroup analyses, there was no difference in overall survival in patients regardless of the modality received. In studies stratifying TACE or TARE by BCLC class, there is consistent decrements in survival with advancing stages. Similarly, several studies have shown the significant decrements in TACE and TARE effectiveness and safety in CP B and C disease. TARE has also been compared to sorafenib in BCLB B and C disease in two randomized trials and was not associated with a survival benefit, 34 , 35 but was associated with superior quality of life and appears to be cost‐effective. 36 In the multivariate analysis of TACE versus TARE from the individual level meta‐analysis, several known correlates of worse survival were significant, including liver function and tumor burden. When controlling for these, TACE and TARE still had similar survival. With the advent of more efficacious therapies for unresectable HCC, the utility of locoregional therapies in more advanced stage HCC deserves further study. While previous adjuvant therapy trials involving TACE and sorafenib in unresectable disease have not shown a survival benefit, there are several ongoing trials pairing inter‐arterial therapies with immunotherapy‐based systemic regimens. However, the majority of these trials are in combination with TACE due to the limited data behind TARE. 37
Nevertheless, TACE and TARE remain primary treatment options for a significant proportion of patients with HCC and our analysis supports the efficacy of both. TARE was associated with an increased TTP compared to TARE in a subset of the included studies; however, there are notable deficiencies in radiographic interpretation of tumor progression after radiation therapy that may have contributed to this finding. 38 While this deserves further scrutiny due to the observed heterogeneity in the data and nonstandardized radiographic interpretation across studies, the increased TTP is a meaningful outcome for a bridging population while awaiting more definitive therapies, such as liver transplantation.
Our study had several strengths and limitations. First, TACE and TARE technique for administration and patient selection were not standardized across the studies. Most of the studies included were retrospective in design, thus bias such as confounding by indication, differences in imaging interpretation, or unmeasured confounders may have contributed to the results of the analysis. Furthermore, treatment with TARE has evolved with several recent studies including personalized dosimetry as the most effective method for TARE delivery; however, most of the included studies utilized lobar treatment or standardized dosimetry. 37 , 39 Similarly, TACE is increasingly delivered in a selective fashion, whereas several of the studies included lobar delivery of TACE. There was also significant heterogeneity in the meta‐analysis results, likely reflecting the differences in study design, patient selection, and treatment administration, however we did confirm the primary findings of the study in the individual level meta‐analysis. While our study focused on efficacy, we were limited in our ability to adequately compare the safety profiles of the treatments due to lack of consistent inclusion of safety data in the included studies. The safety data we were able to derive from the studies indicated a trend toward favorable adverse event profile for TARE compared to TACE. This is consistent with the limited data available on quality of life after TACE and TARE treatment. 40 , 41 Finally, we were unable to control for therapies received prior to and after the TACE or TARE in the included studies and differences in the follow‐up care of these patients may have been mediators of overall survival. The survival reporting was heterogeneous among studies as well, with inconsistent reporting of survival using adjusted HR or censored Kaplan Meier survival. In a subgroup analysis of studies reporting HR only, the results were similar. These weaknesses are balanced by the strengths of an updated comprehensive meta‐analysis and systematic review including thousands of patients showing consistent effects across several subgroup analyses and individual patient level meta‐analysis.
5. CONCLUSIONS
Current data suggest TARE can provide significantly longer TTP than TACE, although the two treatments do not significantly differ in terms of overall survival in both our overall and individual patient level meta‐analysis. Safety profiles appeared to favor TARE; however, these data deserve further prospective confirmation. Given the limitations of the current data, there is rationale for comparing these modalities in larger prospective analyses to allow granular comparison of survival, progression, and safety data.
AUTHOR CONTRIBUTIONS
Parikh is the guarantor of this article. Roles: (a) Concept: Parikh; (b) Analysis: Parikh, Chen; (c) Data acquisition: All authors; (d) Writing: Parikh, Brown, Kassab, Singal; (e) Critical revision: All authors.
FUNDING INFORMATION
Dr. Singal's research is conducted with support from National Institutes of Health U01 CA230694, R01 MD12565. Dr. Parikh's research is conducted with support from National Institutes of Health U01 CA230669. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
CONFLICT OF INTEREST
Brown: None. Kassab: None. Massani: None. Townsend: None. Singal: Served as a consultant or on advisory boards for Bayer, FujiFilm Wako Diagnostics, Exact Sciences, Roche, Glycotest, and GRAIL. Soydal: None. Moreno‐Luna: None. Roberts: Consults for AstraZeneca, MJH Life Sciences, and Clinical care options; he advises and received grants from Bayer, Exact Sciences, and Gilead; he advises GRAIL, Tavec, QED Therapeutics, Genentech, Envision, and Eisai and received grants from Ariad, BTG International, GylcoTest, RedHill, Ltd Pharma, and Wako Diagnostics. Chen: None. Parikh: Served as a consultant for Bristol Myers‐Squibb, Exact Sciences, Eli Lilly, and Freenome; has served on advisory boards of Genentech, Eisai, Bayer, Exelixis, Wako/Fujifilm; and has received research funding from Bayer, Target RWE, Exact Sciences, Genentech and Glycotest.
ETHICS STATEMENT
The data included in this study were either publicly available or completely de‐identified and were exempt from Institutional Review Board approval.
Supporting information
Appendix S1
Brown AM, Kassab I, Massani M, et al. TACE versus TARE for patients with hepatocellular carcinoma: Overall and individual patient level meta analysis. Cancer Med. 2023;12:2590‐2599. doi: 10.1002/cam4.5125
Vincent L. Chen and Neehar D. Parikh contributed equally to this manuscript and are co‐senior authors.
DATA AVAILABILITY STATEMENT
Data from this study were taken from the published literature which is publicly available. The individual meta‐analysis data can be attained by contacting the authors of the studies used in this analysis.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta‐analysis. PLoS Med. 2014;11(4):e1001624. doi: 10.1371/journal.pmed.1001624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsumoto MM, Mouli S, Saxena P, et al. Comparing real world, personalized, multidisciplinary tumor board recommendations with BCLC algorithm: 321‐patient analysis. Cardiovasc Intervent Radiol. 2021;44(7):1070‐1080. doi: 10.1007/s00270-021-02810-8 [DOI] [PubMed] [Google Scholar]
- 4. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and Management of Hepatocellular Carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723‐750. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 5. Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734‐1739. doi: 10.1016/S0140-6736(02)08649-X [DOI] [PubMed] [Google Scholar]
- 6. Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164‐1171. doi: 10.1053/jhep.2002.33156 [DOI] [PubMed] [Google Scholar]
- 7. Salem R, Johnson GE, Kim E, et al. Yttrium‐90 radioembolization for the treatment of solitary, unresectable hepatocellular carcinoma: the LEGACY Study. Hepatology. 2021;74(5):2342‐2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182‐236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 9. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358‐380. doi: 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 10. Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(6):1155‐1163.e2. doi: 10.1053/j.gastro.2016.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin‐eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(2):255‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Z, Fardanesh MR, Machac J, et al. Comparison of therapeutic response using RECIST criteria: Y‐90 SIR‐spheres and TheraSphere treatment of unresectable hepatocellular carcinoma. Soc Nuclear Med. 2013;54(supplement 2):224. [Google Scholar]
- 14. Moreno‐Luna LE, Yang JD, Sanchez W, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36(3):714‐723. doi: 10.1007/s00270-012-0481-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soydal C, Arslan MF, Kucuk ON, Idilman R, Bilgic S. Comparison of survival, safety, and efficacy after transarterial chemoembolization and radioembolization of Barcelona clinic liver cancer stage B‐C hepatocellular cancer patients. Nucl Med Commun. 2016;37(6):646‐649. doi: 10.1097/MNM.0000000000000486 [DOI] [PubMed] [Google Scholar]
- 16. Massani M. Yttrium‐90 radioembolisation versus transarterial chemoembolisation for unresectable hepatocellular carcinoma: a retrospective comparative analysis according to BCLC classification. Clin Surg. 2017;2:1528. [Google Scholar]
- 17. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lance C, McLennan G, Obuchowski N, et al. Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium‐90 radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22(12):1697‐1705. doi: 10.1016/j.jvir.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 19. Kooby DA, Egnatashvili V, Srinivasan S, et al. Comparison of yttrium‐90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21(2):224‐230. doi: 10.1016/j.jvir.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 20. El Fouly A, Ertle J, El Dorry A, et al. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35(2):627‐635. doi: 10.1111/liv.12637 [DOI] [PubMed] [Google Scholar]
- 21. Akinwande O, Kim D, Edwards J, et al. Is radioembolization ([90]Y) better than doxorubicin drug eluting beads (DEBDOX) for hepatocellular carcinoma with portal vein thrombosis? A retrospective analysis. Surg Oncol. 2015;24(3):270‐275. doi: 10.1016/j.suronc.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 22. Biederman DM, Titano JJ, Korff RA, et al. Radiation segmentectomy versus selective chemoembolization in the treatment of early‐stage hepatocellular carcinoma. J Vasc Interv Radiol. 2018;29(1):30‐37.e2. doi: 10.1016/j.jvir.2017.08.026 [DOI] [PubMed] [Google Scholar]
- 23. Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time‐to‐progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497‐507.e2. doi: 10.1053/j.gastro.2010.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Akinwande O, Philips P, Scoggins C, Martin RC. Radioembolization versus chemoembolization (DEBDOX) for the treatment of unresectable hepatocellular carcinoma: a propensity matched study. Anticancer Res. 2016;36(1):239‐246. [PubMed] [Google Scholar]
- 25. Pitton MB, Kloeckner R, Ruckes C, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug‐eluting bead transarterial chemoembolization (DEB‐TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2015;38(2):352‐360. doi: 10.1007/s00270-014-1012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Padia SA, Johnson GE, Horton KJ, et al. Segmental Yttrium‐90 radioembolization versus segmental chemoembolization for localized hepatocellular carcinoma: results of a single‐center, retrospective, propensity score‐matched study. J Vasc Interv Radiol. 2017;28(6):777‐785.e1. doi: 10.1016/j.jvir.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 27. McDevitt JL, Alian A, Kapoor B, et al. Single‐center comparison of overall survival and toxicities in patients with infiltrative hepatocellular carcinoma treated with Yttrium‐90 radioembolization or drug‐eluting embolic Transarterial chemoembolization. J Vasc Interv Radiol. 2017;28(10):1371‐1377. doi: 10.1016/j.jvir.2017.05.017 [DOI] [PubMed] [Google Scholar]
- 28. She WH, Cheung TT, Yau TC, et al. Survival analysis of transarterial radioembolization with yttrium‐90 for hepatocellular carcinoma patients with HBV infection. Hepatobiliary Surg Nutr. 2014;3(4):185‐193. doi: 10.3978/j.issn.2304-3881.2014.07.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two‐cohort study. Cancer. 2010;116(5):1305‐1314. doi: 10.1002/cncr.24884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Auer TA, Jonczyk M, Collettini F, et al. Trans‐arterial chemoembolization with degradable starch microspheres (DSM‐TACE) versus selective internal radiation therapy (SIRT) in multifocal hepatocellular carcinoma. Acta Radiol. 2021;62(3):313‐321. doi: 10.1177/0284185120926474 [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Li Y, Ji H, Zhao X, Lu H. Transarterial Y90 radioembolization versus chemoembolization for patients with hepatocellular carcinoma: a meta‐analysis. Biosci Trends. 2015;9(5):289‐298. doi: 10.5582/bst.2015.01089 [DOI] [PubMed] [Google Scholar]
- 32. Katsanos K, Kitrou P, Spiliopoulos S, Maroulis I, Petsas T, Karnabatidis D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: a network meta‐analysis of randomized controlled trials. PLoS One. 2017;12(9):e0184597. doi: 10.1371/journal.pone.0184597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Casadei Gardini A, Tamburini E, Inarrairaegui M, Frassineti GL, Sangro B. Radioembolization versus chemoembolization for unresectable hepatocellular carcinoma: a meta‐analysis of randomized trials. Onco Targets Ther. 2018;11:7315‐7321. doi: 10.2147/OTT.S175715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia‐Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36(19):1913‐1921. doi: 10.1200/JCO.2017.76.0892 [DOI] [PubMed] [Google Scholar]
- 35. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium‐90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open‐label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624‐1636. doi: 10.1016/S1470-2045(17)30683-6 [DOI] [PubMed] [Google Scholar]
- 36. Marqueen KE, Kim E, Ang C, Mazumdar M, Buckstein M, Ferket BS. Cost‐effectiveness analysis of selective internal radiotherapy with Yttrium‐90 versus sorafenib in locally advanced hepatocellular carcinoma. JCO Oncol Pract. 2021;17(2):e266‐e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parikh ND, Pillai A. Recent advances in hepatocellular carcinoma treatment. Clin Gastroenterol Hepatol. 2021;19:2020‐2024. [DOI] [PubMed] [Google Scholar]
- 38. Vouche M, Kulik L, Atassi R, et al. Radiological‐pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: imaging analysis from a prospective randomized trial of Y90±sorafenib. Hepatology. 2013;58(5):1655‐1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE‐01): a randomised, multicentre, open‐label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(1):17‐29. [DOI] [PubMed] [Google Scholar]
- 40. Kirchner T, Marquardt S, Werncke T, et al. Comparison of health‐related quality of life after transarterial chemoembolization and transarterial radioembolization in patients with unresectable hepatocellular carcinoma. Abdom Radiol. 2019;44(4):1554‐1561. [DOI] [PubMed] [Google Scholar]
- 41. Salem R, Gilbertsen M, Butt Z, et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. 2013;11(10):1358‐1365.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data from this study were taken from the published literature which is publicly available. The individual meta‐analysis data can be attained by contacting the authors of the studies used in this analysis.


