Abstract
Background & Aims
The response of patients with chronic liver disease (CLD) to COVID-19 vaccines remains unclear. Our aim was to assess the humoral immune response and efficacy of two-dose COVID-19 vaccines among patients with CLD of different aetiologies and disease stages.
Methods
A total of 357 patients were recruited in clinical centres from six European countries, and 132 healthy volunteers served as controls. Serum IgG (nM), IgM (nM), and neutralising antibodies (%) against the Wuhan-Hu-1, B.1.617, and B.1.1.529 SARS-CoV-2 spike proteins were determined before vaccination (T0) and 14 days (T2) and 6 months (T3) after the second-dose vaccination. Patients fulfilling inclusion criteria at T2 (n = 212) were stratified into ‘low’ or ‘high’ responders according to IgG levels. Infection rates and severity were collected throughout the study.
Results
Wuhan-Hu-1 IgG, IgM, and neutralisation levels significantly increased from T0 to T2 in patients vaccinated with BNT162b2 (70.3%), mRNA-1273 (18.9%), or ChAdOx1 (10.8%). In multivariate analysis, age, cirrhosis, and type of vaccine (ChAdOx1 > BNT162b2 > mRNA-1273) predicted ‘low’ humoral response, whereas viral hepatitis and antiviral therapy predicted ‘high’ humoral response. Compared with Wuhan-Hu-1, B.1.617 and, further, B.1.1.529 IgG levels were significantly lower at both T2 and T3. Compared with healthy individuals, patients with CLD presented with lower B.1.1.529 IgGs at T2 with no additional key differences. No major clinical or immune IgG parameters associated with SARS-CoV-2 infection rates or vaccine efficacy.
Conclusions
Patients with CLD and cirrhosis exhibit lower immune responses to COVID-19 vaccination, irrespective of disease aetiology. The type of vaccine leads to different antibody responses that appear not to associate with distinct efficacy, although this needs validation in larger cohorts with a more balanced representation of all vaccines.
Impact and Implications
In patients with CLD vaccinated with two-dose vaccines, age, cirrhosis, and type of vaccine (Vaxzevria > Pfizer BioNTech > Moderna) predict a ‘lower’ humoral response, whereas viral hepatitis aetiology and prior antiviral therapy predict a ‘higher’ humoral response. This differential response appears not to associate with SARS-CoV-2 infection incidence or vaccine efficacy. However, compared with Wuhan-Hu-1, humoral immunity was lower for the Delta and Omicron variants, and all decreased after 6 months. As such, patients with CLD, particularly those older and with cirrhosis, should be prioritised for receiving booster doses and/or recently approved adapted vaccines.
Keywords: Chronic liver disease, Cirrhosis, COVID-19 vaccine, Humoral immunity, SARS-CoV-2
Graphical abstract
Highlights
-
•
Two-dose vaccines increase Wuhan-Hu-1 IgG, IgM, and neutralisation levels in patients with CLD.
-
•
Cirrhosis, older age, and type of vaccine predict lower Wuhan-Hu-1 IgG titres.
-
•
Compared with Wuhan-Hu-1, humoral immunity was lower for the B.1.617 and B.1.1.529 variants, and all decreased after 6 months.
-
•
No major clinical or immune IgG parameters associated with SARS-CoV-2 infection rates or vaccine efficacy.
Introduction
Patients with chronic liver disease (CLD), particularly those with cirrhosis, are at higher risk of developing more severe COVID-19 and display higher mortality rates than patients without CLD.1 As such, both the EASL and the American Association for the Study of Liver Diseases recommend that providers advocate for prioritising patients with CLD for vaccination against COVID-19, particularly those with cirrhosis or liver cancer.[2], [3], [4] Nevertheless, certain patients with advanced CLD develop cirrhosis-associated immune dysfunction (CAID), a distinctive spectrum of immune alterations associated with the course of end-stage liver disease.5 These have been suggested to explain some of the complications of COVID-19 observed in patients with cirrhosis, as well as potential impaired immunological responses to SARS-CoV-2 vaccination.6 Moderna/NIAID mRNA-1273 and BioNTech-Pfizer BNT162b2 mRNA vaccines, as well as the AstraZeneca/University of Oxford ChAdOx1 adenoviral vectored vaccine, have each reported excellent safety profiles, have marked efficacy in preventing symptomatic COVID-19 (62–95%), and have all gained rapid regulatory approval.[7], [8], [9] Despite the high number of study participants (∼100,000), only a few patients with underlying liver disease were included in the trials (0.6% for mRNA-1273, 0.6% for BNT162b2, and 1.6% for ChAdOx1), and patients with immunosuppressive conditions were excluded.[7], [8], [9] As such, it is essential to examine the effect of COVID-19 vaccines in patients with CLD. In this regard, recent studies have demonstrated good safety and immunogenicity of COVID-19 vaccination in patients with liver disease, although responses appear to be attenuated in patients with cirrhosis.[10], [11], [12], [13], [14], [15], [16], [17] However, some of these studies were either single centre, had a small sample size, did not include controls, and/or did not evaluate RNA vaccines. In particular, long-term (at least 6-month) immune responses of patients receiving two-dose mRNA vaccines, before receiving the third/boost dose, are largely unknown. Similarly, very few studies have evaluated correlations between serology responses to vaccines and COVID-19 infection in patients with CLD.18,19 In this multicentric study, we evaluated humoral responses to SARS-CoV-2 vaccination in a large cohort of patients with CLD, at 2 weeks and 6 months after second-dose vaccination. Results were compared with those of heathy individuals. Predictors of low and high response to vaccination were identified, and humoral immunity of patients with CLD to SARS-CoV-2 variants was assessed. Finally, association between COVID-19 infection rates and vaccine efficacy with clinical and serological parameters in patients with CLD was sought.
Patients and methods
Study population
We established a collaborative prospective registry of non-pregnant adult patients (≥18 years old) with CLD vaccinated against COVID-19, at the pan-European level. The definition of CLD was based on clinical, radiological, or histological evidence of liver disease. Patients with previous liver transplantation were excluded. Patients with CLD were extensively characterised at enrolment (anthropometric, clinical, and biochemical data), as reported in the Supplementary methods. Adults with no history of liver disease (≥18 years) vaccinated for COVID-19 were recruited in the Lisbon area. These were homogenously distributed between vaccine types regarding sex and age.
Registry and sample collection
Blood samples and clinical data were collected between February 2021 and February 2022. Data were stored in the HEPCOVIVac Registry using a de-identified format in an electronic case report form, using ‘Research Electronic Data Capture’ (REDCap™) hosted at the National Center for Data Registries in Gastroenterology, Sociedade Portuguesa de Gastroenterologia (https://www.spg.pt), a non-profit scientific and medical society focused on gastroenterology research.
The HEPCOVIVac Registry is an investigator-reported, prospective, international, multicentre, and observational registry, not interfering with the usual clinical routine and treatment of patients. Case report forms included general information about the patient (e.g. sex, age, and demographics), liver disease aetiology and severity, comorbidities and risk factors, clinical parameters and therapy, and SARS-CoV-2 infection (symptoms, date of onset and resolution of symptoms, history of PCR testing, or other) and vaccination details (type of vaccine, date of administration, adverse effects, or other).
The study protocol consisted of collecting blood samples and clinical data from patients with CLD at different time points, namely T0 (baseline; before vaccination), T1 (2 weeks after first-dose vaccination; optional time point), T2 (2 weeks after second-dose vaccination or after a single dose vaccine – completed vaccination), and T3 (6 months after the start of vaccination). Case report forms were filled at each visit by clinicians. Venous blood samples were collected by trained nurses using appropriate personal protective equipment at participating clinical centres (for patients with CLD) or clinical analysis laboratories (for healthy volunteers). Samples were immediately processed for plasma collection and stored at -80 °C.
Before enrolment, all participants gave written informed consent and a disclosure form according to the EU personal/patient data act.
The HEPCOViVac Registry study protocol and informed consent were reviewed and approved by the Ethic Committee of the Faculty of Pharmacy, Universidade de Lisboa (Code: 02/2021) and the Lisbon Academic Medical Center (Code: 24/21), as coordinating centres, before study implementation. In addition, each participating centre obtained a local ethical approval. The healthy controls study protocol and informed consent were reviewed and approved by the Ethic Committee of the Faculty of Pharmacy, Universidade de Lisboa (CovidVac Project – immunological response of health professionals and nursing homes residents vaccinated against COVID-19; Code: 03/2021).
Measurement of antibodies
To test reactivity against SARS-CoV-2 antigens, the SARS-CoV-2 reference (Wuhan-Hu-1) spike S1 protein (AcroBiosystems), the SARS-CoV-2 B.1.617 (Delta) spike RBD protein (Sino Biological), or the SARS-CoV-2 B.1.1.529 (Omicron) spike RBD protein (Sino Biological) were used with an ‘in-house’ ELISA, as described in the Supplementary methods.
Surrogate neutralisation assay
For the detection of neutralising antibodies against SARS-CoV-2 RBD, AlphaLISA® technology was used, which allows detection of SARS-CoV-2 binding antibodies on human plasma samples, with the ability to block or inhibit the viral entry into cells through cellular receptor angiotensin-converting enzyme 2. The assay was performed as previously described,20 with minor modifications, as indicated in the Supplementary methods.
Evaluation of infection data
At each time point/visit, patients were asked whether they had been infected with COVID-19 (validated by PCR) and, if so, whether they had any symptoms (i.e. abdominal pain, diarrhoea, nausea, vomiting, fever, coughing, shortness of breath, fatigue, and myalgia) and whether hospitalisation with/without oxygen supply was needed. This information was recorded in the HEPCOVIVac Registry on REDCap™. At each time point/visit, previous COVID-19 infection was confirmed or evaluated for all patients by testing for the SARS-CoV-2 anti-nucleocapsid protein antibody. Briefly, the SARS-CoV-2 nucleocapsid protein (Sino Biologicals) was used in conjugation with a goat anti-human IgG Fc horseradish peroxidase-conjugated antibody (Abcam) in an ELISA. Patients who developed infection before T2 (n = 75) were used to query for associations with demographic or clinical characteristics, whereas patients who developed infection between T2 and T3 (n = 29) were used to measure vaccine efficacy.
Statistical analysis
Patients’ demographic and clinical characteristics were summarised using descriptive statistics. Continuous data were described as median and IQR, whereas categorical variables were summarised as number of patients (n) and probability percentage. The Shapiro–Wilk test was used to test continuous variables for normal distribution. For comparisons between two groups, parametric or non-parametric data were analysed using Student’s t test or the Mann–Whitney test, respectively. For multiple comparisons, parametric or non-parametric data were compared using ANOVA or Kruskal–Wallis tests, respectively, followed by Bonferroni’s or Dunn's post hoc test, respectively. Pairwise comparisons were calculated using the Wilcoxon test for comparisons between two groups or the Friedman test followed by Dunn's post hoc test for multiple comparisons. Pearson’s Chi-square (χ2) test was used to compare categorical variables between the three vaccine developers. Association between two variables was assessed by the Spearman’s correlation coefficient with Gaussian approximation. The odds were estimated by binomial logistic regression analysis based on likelihood ratios carried out including a list of independent variables, defined as p <0.25 in the univariate analysis. Statistical analyses were performed with IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 8.0 for Microsoft Windows (GraphPad Software, La Jolla, CA, USA). All p values were obtained in two-tailed tests, and p <0.05 was considered statistically significant.
Results
Patients’ characteristics
A total of 489 participants were recruited, 132 healthy individuals and 357 patients with CLD. Among the patients with CLD, 55 were excluded owing to lack of blood sample at T2. This was mostly because patients dropped out of the study but also because liver disease was not confirmed, patients underwent liver transplantation, or they died. In addition, given the very low number (n = 15) of patients with CLD vaccinated with vector-based vaccine JNJ-78436735 (Janssen Pharmaceuticals, Johnson & Johnson, New Brunswick, NJ, USA), which further presents a unique administrating scheme compared with the other three vaccines in the study, namely one vs. two dosages, these patients were also excluded from the study. Patients with seropositivity to the SARS-CoV-2 nucleocapsid protein at T2 (n = 75) were also excluded from the short-term (2 weeks post vaccination) follow-up study (Fig. S1).
A total of 212 patients with CLD were analysed at T0 and T2. Table 1 presents the demographical and clinical characteristics of the study population. Their median age was 57 years (IQR 52–64 years), and 56.6% were male. The most prevalent underlying liver disease aetiologies were viral hepatitis (45.3%) and metabolic related fatty liver disease (MRFLD; patients with non-alcoholic fatty liver disease [NAFLD] and/or heavy alcohol consumption; 45.8%), followed by autoimmune and/or cholestatic liver disease (12.7%). Most patients presented with advanced liver fibrosis (F3–F4; 66.5%), and 61.8% presented with cirrhosis. The most common comorbidities were arterial hypertension (31.1%), type 2 diabetes mellitus (25.5%), and obesity (19.8%). Of note, most patients had multiple disease aetiologies and comorbidities. Patients were vaccinated with two doses (T2) of ChAdOx1 (10.8%; Oxford-AstraZeneca; Cambridge, UK), mRNA-1273 (18.9%; Moderna, Cambridge, MA, USA), or BNT162b2 (70.3%; BioN-Tech Manufacturing GmbH/Pfizer, Mainz, Germany). Most variables showed no association with the type of vaccine, with some notable exceptions, including age, viral hepatitis, MRFLD, stage of liver disease, arterial hypertension, and obesity.
Table 1.
Demographic and clinical characteristics of patients with CLD.
| ChAdOx1 nCoV-19 (n = 23) | mRNA-1273 (n = 40) | BNT162b2 (n = 149) | Total (n = 212) | p value | |
|---|---|---|---|---|---|
| General characteristics | |||||
| Age | 63.0 (57.0–71.0) | 58.0 (53.0–62.5) | 56.0 (50.0–64.0) | 57.0 (52.0–64.0) | <0.01 |
| Male | 14 (60.9) | 26 (65.0) | 80 (53.7) | 120 (56.6) | n.s. |
| Ethnicity | |||||
| Caucasian | 22 (95.7) | 38 (95.0) | 140 (94.0) | 200 (94.3) | n.s. |
| Asian | 1 (4.3) | 0 (0.0) | 3 (2.0) | 4 (1.9) | |
| African | 0 (0.0) | 0 (0.0) | 3 (2.0) | 3 (1.4) | |
| Other | 0 (0.0) | 0 (0.0) | 3 (2.0) | 3 (1.4) | |
| Not reported | 0 (0.0) | 2 (4.9) | 0 (0.0) | 2 (0.9) | |
| Weight | 81.0 (72.0–93.0) | 81.0 (69.0–88.8) | 75.0 (63.0–88.0) | 75.0 (65.0–88.0) | n.s. |
| Height | 168.0 (164.0–175.0) | 175.5 (165.0–179.0) | 169.0 (163.3–175.0) | 170.0 (164.0–176.0) | n.s. |
| BMI (kg/m2) | 28.4 (24.7–34.7) | 26.4 (23.5–30.4) | 25.5 (22.8–29.8) | 26.0 (23.1–30.3) | n.s. |
| Aetiology of liver disease | |||||
| Viral hepatitis | 7 (30.4) | 13 (32.5) | 76 (51.0) | 98 (45.3) | <0.05 |
| Autoimmune and/or cholestatic liver disease | 1 (4.3) | 6 (15.0) | 20 (13.4) | 27 (12.7) | n.s. |
| Metabolic related fatty liver disease | 16 (69.6) | 23 (57.5) | 58 (38.9) | 97 (45.8) | <0.01 |
| Hereditary liver disease | 0 (0.0) | 1 (2.5) | 4 (2.7) | 5 (2.4) | n.s. |
| Cryptogenic liver disease | 0 (0.0) | 0 (0.0) | 1 (0.7) | 1 (0.5) | n.s. |
| Pharmacotherapy | |||||
| Immunosuppressive treatment | 3 (13.0) | 5 (12.5) | 10 (6.7) | 18 (8.5) | n.s. |
| Antiviral therapy | 1 (4.3) | 2 (5.0) | 22 (14.8) | 25 (11.8) | n.s. |
| Metabolic therapy | 10 (43.5) | 10 (25.0) | 50 (33.6) | 70 (33.0) | n.s. |
| Stage of liver disease | |||||
| F3–F4 | 20 (87.0) | 32 (80.0) | 89 (59.7) | 141 (66.5) | <0.01 |
| Liver cirrhosis | 19 (82.6) | 30 (75.0) | 82 (55.0) | 131 (61.8) | <0.01 |
| Hepatocellular carcinoma | 2 (8.7) | 3 (7.5) | 10 (6.7) | 15 (7.1) | n.s. |
| Comorbidities | |||||
| Type 2 diabetes mellitus | 9 (39.1) | 11 (27.5) | 34 (22.8) | 54 (25.5) | n.s. |
| Arterial hypertension | 6 (26.1) | 19 (47.5) | 41 (27.5) | 66 (31.1) | <0.05 |
| Obesity | 9 (39.1) | 7 (17.5) | 26 (17.4) | 42 (19.8) | <0.05 |
| Hypertriglyceridaemia | 3 (13.0) | 2 (5.0) | 9 (6.0) | 14 (6.6) | n.s. |
| Hypercholesterolaemia | 4 (17.4) | 4 (10.0) | 26 (17.4) | 34 (16.0) | n.s. |
| Renal insufficiency | 0 (0.0) | 2 (5.0) | 6 (4.0) | 8 (3.8) | n.s. |
| Asthma | 2 (8.7) | 0 (0.0) | 7 (4.7) | 9 (4.2) | n.s. |
| Heart/cardiovascular disease | 2 (8.7) | 4 (10.0) | 20 (13.4) | 26 (12.3) | n.s. |
| Smoking | 1 (4.3) | 7 (17.5) | 18 (12.1) | 26 (12.3) | n.s. |
| Other | 5 (21.7) | 7 (17.5) | 39 (26.2) | 51 (24.1) | n.s. |
Data are displayed as median (IQR) for continuous variables and n (%) for categorical variables. The Kruskal–Wallis test and Pearson’s Chi-square (χ2) test were used to compare quantitative and qualitative variables, respectively, between the three vaccine developers. A value of p <0.05 was considered statistically significant. Viral hepatitis (hepatitis B [17.9%], C [28.3%], D [1.9%], and/or E [0.9%]); autoimmune and/or cholestatic liver disease (primary sclerosing cholangitis [1.4%], primary biliary cholangitis [5.7%], and/or autoimmune hepatitis [6.6%]); metabolic related fatty liver disease (non-alcoholic fatty liver disease [19.8%] and/or heavy alcohol consumption [28.8%]); hereditary liver disease (Wilson disease [0.9%], haemochromatosis [0.9%] and polycystic liver disease [0.5%]); cryptogenic liver disease (0.5%); immunosuppressive treatment (prednisone [5.2%], tacrolimus [0.5%], mycophenolate mofetil [0.9%], azathioprine [4.7%], budesonide [0.5%], and/or other [1.4%]); antiviral therapy (tenofovir [5.2%], entecavir [5.2%], interferon [0.5%], and/or other [0.5%]); metabolic therapy (ursodeoxycholic acid [8.5%], obeticholic acid [1.9%], fibrates [2.8%], metformin [13.2%], glucagon-like peptide-1 agonists [0.5%], insulin [7.6%], statins [9.9%], and/or other [8.5%]). CLD, chronic liver disease.
The control population included 132 individuals with a negative anti-nucleocapsid IgG test. Median age was 45.5 years (IR 37.0–57.3 years), and 28% were male (Table S1). Healthy controls were vaccinated with two doses (T2) of ChAdOx1 (9.1%), mRNA-1273 (7.6%), or BNT162b2 (83.3%). Patients with CLD were more frequently male (56.6 vs. 28%) and older (median, 57 vs. 45.5 years) compared with controls.
Patients with more severe CLD display lower humoral immunity to COVID-19 vaccines
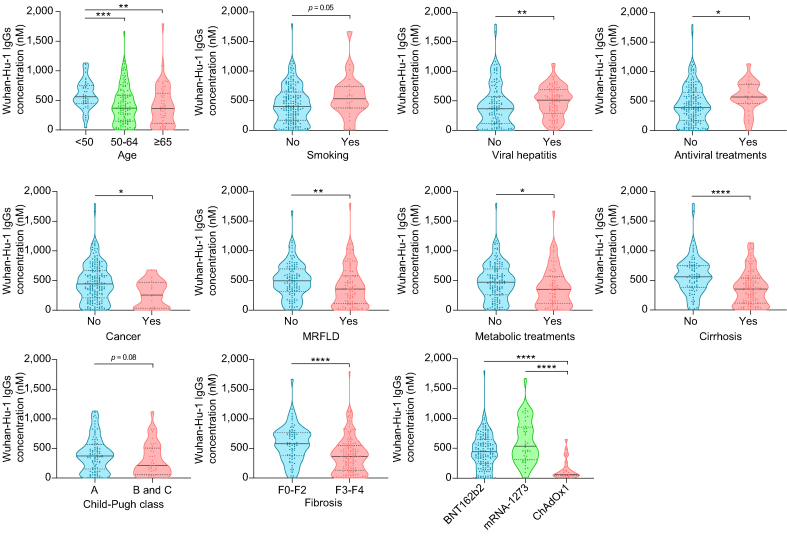
Most patients with CLD (96.2%) responded to COVID-19 vaccination. At T0, Wuhan-Hu-1 IgG, IgM, and % of neutralisation were 0.2 nM (IQR 0.0–0.9 nM), 0.3 nM (IQR 0.1–0.5 nM) and 14.8 (IQR 8.4–21.1), respectively, increasing to 419 nM (188.1–650.0 nM; p <0.0001), 1.4 nM (0.8–2.8 nM; p <0.0001), and 60 (40.3–76.9; p <0.0001) at T2, respectively (Fig. 1A and Table S1). In addition, IgM and neutralisation levels showed a high positive correlation with Wuhan-Hu-1 IgG levels (Fig. 1B). In a subset of patients (n = 44), data for T1 (plasma collected 15 days [median; Q1–Q3, 14–15] after first-dose vaccination) was available (Fig. 1C). IgG, IgM, and neutralisation levels increased by 135-; 3.4-, and 1.5-fold from T0 to T1, respectively, with the biggest increase being observed from T0 to T2 (1,208-; 7.1-, and 2.4-fold, respectively). At T2, IgG levels were significantly higher in patients younger than 50 years, compared with 50- to 64-year-old patients (p <0.001) and in those older than 65 years (p <0.01; Fig. 2). IgG levels were also higher in patients with viral hepatitis and in those receiving antiviral treatments (p <0.01 and p <0.05, respectively). Inversely, Wuhan-Hu-1 IgG levels decreased in patients with cancer (p <0.05), patients with MRFLD (p <0.01), and those receiving metabolic treatments (p <0.05). In terms of liver disease severity, humoral response was lower in patients with CLD with cirrhosis and fibrosis (p <0.0001 for both). A more elevated humoral response was observed for patients with CLD vaccinated with the mRNA-1273 vaccine, followed by the BNT162b2 vaccine and, finally, the ChAdOx1 vaccine (Fig. 2).
Fig. 1.
Wuhan-Hu-1 antibody titres after SARS-CoV-2 vaccination in patients with CLD.
(A) Violin plots representing IgG, IgM, and neutralisation levels before (T0) and 2 weeks after (T2) the second vaccine dose (n = 212). (B) Correlation between IgM and neutralisation levels with IgG levels at T2. (C) IgG, IgM, and neutralisation levels before (T0) and 2 weeks after the first (T1) and second (T2) vaccine doses (n = 44). Black horizontal lines indicate the median, and grey dotted lines indicate the IQR. Pairwise comparisons were calculated using the Wilcoxon test for comparisons between two groups or the Friedman test followed by Dunn's post hoc test for multiple comparisons. Association between two variables was assessed by Spearman’s correlation coefficient with Gaussian approximation. ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001; ∗∗∗∗p <0.0001. CLD, chronic liver disease; NAb, neutralising antibodies.
Fig. 2.
Wuhan-Hu-1 IgG levels 2 weeks after the second SARS-CoV-2 vaccine dose (T2) in patients with CLD according to demographic and clinical characteristics, and type of vaccine.
Violin plots representing IgG levels. Black horizontal lines indicate the median, and grey dotted lines indicate the IQR. For comparisons between two groups, parametric or non-parametric data was analysed using Student’s t test or the Mann–Whitney test, respectively. For multiple comparisons, parametric or non-parametric data were compared using ANOVA or the Kruskal–Wallis test, respectively, followed by Bonferroni’s or Dunn’s post hoc test. ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001; ∗∗∗∗p <0.0001. CLD, chronic liver disease; MRFLD, metabolic-related fatty liver disease.
In healthy participants, T2 IgG, IgM, and neutralisation levels were 409.6 nM (254.3–576.3 nM), 1.0 nM (0.3–2.2 nM), and 57.6% (43.8–75.1%), respectively (Table S1). Notably, Wuhan-Hu-1 IgG and neutralisation levels were not significantly different between healthy individuals and patients with CLD, with IgM levels being more elevated in patients with CLD (p <0.05; Fig. S2). Stratifying patients with CLD by disease aetiology or severity had no impact on these results, yet patients with cirrhosis or advanced liver fibrosis (F3–F4) displayed lower IgG levels compared with healthy controls (not statistically significant; data not shown).
Liver cirrhosis is a predictor of lower immune response to COVID-19 vaccines in patients with CLD
To identify independent variables that associated with differential antibody response, patients were divided into low or high responders according to their IgG antibodies at T2, using 418.95 nM (median) as the cut-off value (Fig. 3A). As somewhat expected, Wuhan-Hu-1 IgG high responders also presented with increased IgM and neutralisation levels, compared with low responders (p <0.0001 for both; Fig. 3B). In univariate analysis (Table 2), viral hepatitis (odds ratio [OR] 2.34, 95% CI 1.35–4.07) and antiviral therapy (OR 4.70, 95% CI 1.69–13.05) predicted ‘high’ response, whereas older age (OR 0.95, 95% CI 0.92–0.98), MRFLD (OR 0.41, 95% CI 0.24–0.72), and metabolic drugs (OR 0.46, 95% CI 0.26–0.82) predicted ‘low’ response. In terms of liver disease severity, the presence of F3–F4 fibrosis (OR 0.28, 95% CI 0.15–0.51) or cirrhosis (OR 0.28, 95% CI 0.15–0.50) associated with a lower IgG response. Nonetheless, no statistical differences were found when stratifying patients with cirrhosis into Child–Pugh B + C vs. A.
Fig. 3.
Wuhan-Hu-1 IgG, IgM, and neutralisation levels, and B.1.617 (Delta) and B.1.1.529 (Omicron) IgG titres in vaccinated patients with CLD and healthy volunteers at T2 and T3.
(A) Violin plots representing IgG levels at T2. Patients with IgG antibody titres above or below 418.95 nM (median; red horizontal line) were defined as a high or low responders, respectively(B) Violin plots representing IgM and neutralisation levels in high and low responders. (C) Violin plots representing Wuhan-Hu-1, B.1.617, and B.1.1.529 IgG titres in patients with CLD at T2 and T3 (left), and B.1.617 and B.1.1.529 IgG levels in high and low responders (right). (D) Wuhan-Hu-1, B.1.617, and B.1.1.529 IgG titres in healthy controls and patients with CLD at T2 and T3. Black horizontal lines indicate the median, and grey dotted lines indicate the IQR. Pairwise comparisons between different variants or different time points were calculated using the Wilcoxon test for comparisons between two groups or Friedman test followed by Dunn's post hoc test for multiple comparisons. For comparisons between two independent groups, parametric or non-parametric data were analysed using Student’s t test or the Mann–Whitney test, respectively. ∗∗∗p <0.001; ∗∗∗∗p <0.0001. CLD, chronic liver disease; NAb, neutralising antibodies.
Table 2.
Variables associated with antibody response to COVID-19 vaccination in patients with CLD 2 weeks after the second SARS-CoV-2 vaccine dose (T2).
| Low responders∗ (n = 106) | High responders∗ (n = 106) | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |||
| Age | 59.0 (53.8–65.3) | 56.0 (47.0 –62.3) | 0.95 (0.92–0.98) | <0.0001 | 0.97 (0.93–0.99) | 0.042 |
| Sex | ||||||
| Male | 61 (57.5) | 59 (55.7) | 1 (Ref.) | — | ||
| Female | 45 (42.5) | 47 (44.3) | 1.08 (0.63–1.86) | 0.782 | ||
| BMI (kg/m2) | 27.0 (24.2–30.7) | 24.9 (22.5–29.4) | 0.95 (0.90–1.01) | 0.075 | ||
| Aetiology of liver disease, yes (vs. no) | ||||||
| Viral hepatitis | 37 (34.9) | 59 (55.7) | 2.34 (1.35–4.07) | 0.003 | 2.15 (1.04–4.45) | 0.039 |
| Autoimmune and/or cholestatic liver disease | 15 (14.2) | 12 (11.3) | 0.77 (0.34–1.74) | 0.537 | ||
| Metabolic related fatty liver disease | 60 (56.6) | 37 (34.9) | 0.41 (0.24–0.72) | 0.002 | ||
| Hereditary liver disease | 1 (0.9) | 4 (3.8) | 4.12 (0.45–37.47) | 0.209 | ||
| Cryptogenic liver disease | 1 (0.9) | 0 (0.0) | — | — | ||
| Immunosuppressive treatment, yes (vs. no) | 10 (9.4) | 8 (7.5) | 0.78 (0.30–2.07) | 0.623 | ||
| Antiviral therapy, yes (vs. no) | 5 (4.7) | 20 (18.9) | 4.70 (1.69–13.05) | 0.003 | 2.77 (0.83–9.26) | 0.098 |
| Metabolic therapy, yes (vs. no) | 44 (41.5) | 26 (24.5) | 0.46 (0.26–0.82) | 0.009 | ||
| Stage of liver disease, yes (vs. no) | ||||||
| F0–F2 | 21 (19.8) | 50 (47.2) | 1 (Ref.) | <0.0001 | ||
| F3–F4 | 85 (80.2) | 56 (52.8) | 0.28 (0.15–0.51) | |||
| Liver cirrhosis | 81 (76.4) | 50 (47.2) | 0.28 (0.15–0.50) | <0.0001 | 0.33 (0.16–0.68) | 0.003 |
| Child–Pugh score class, A (vs. B + C) | 55 (67.9) | 38 (76.0) | 1.50 (0.67–3.33) | 0.322 | ||
| Hepatocellular carcinoma | 11 (10.4) | 4 (3.8) | 0.34 (0.11–1.11) | 0.072 | ||
| Comorbidities, yes (vs. no) | ||||||
| Type 2 diabetes mellitus | 30 (28.3) | 24 (22.6) | 0.74 (0.40–1.38) | 0.345 | ||
| Arterial hypertension | 34 (32.1) | 32 (30.2) | 0.92 (0.51–1.64) | 0.77 | ||
| Obesity | 21 (19.8) | 21 (19.8) | 1.0 (0.51–1.97) | 1.0 | ||
| Hypertriglyceridaemia | 8 (7.5) | 6 (5.7) | 0.74 (0.25–2.20) | 0.58 | ||
| Hypercholesterolaemia | 22 (20.8) | 12 (11.3) | 0.49 (0.23–1.05) | 0.065 | ||
| Renal Insufficiency | 5 (4.7) | 3 (2.8) | 0.59 (0.14–2.53) | 0.476 | ||
| Asthma | 3 (2.8) | 6 (5.7) | 2.06 (0.50–8.46) | 0.316 | ||
| Heart/cardiovascular disease | 10 (9.4) | 16 (15.1) | 1.71 (0.74–3.96) | 0.213 | ||
| Smoking | 9 (8.5) | 17 (16.0) | 2.06 (0.87–4.85) | 0.099 | ||
| Other | 30 (28.3) | 21 (19.8) | 0.63 (0.33–1.18) | 0.15 | ||
| Vaccine type (vs. mRNA-1273) | ||||||
| mRNA-1273 | 13 (12.3) | 27 (25.5) | 1 (Ref.) | |||
| ChAdOx1 nCoV-19 | 21 (19.8) | 2 (1.9) | 0.05 (0.01–0.24) | <0.0001 | 0.04 (0.01–0.23) | <0.0001 |
| BNT162b2 | 72 (67.9) | 77 (72.6) | 0.52 (0.24–1.06) | 0.077 | 0.26 (0.11–0.59) | 0.001 |
Data are displayed as median (IQR) for continuous variables and n (%) for discontinuous variables. Data from the logistic regression model presented with OR, 95% CI, and p values. Covariates with p <0.25 in the univariate analysis (n = 14) were entered in a multivariate logistic analysis model (method stepwise rewards). CLD, chronic liver disease; OR, odds ratio; Ref., reference.
Patients with IgG antibody titres above or below 418.95 nM (median) were defined as a high or low responders, respectively.
Curiously, among all ChAdOx1-vaccinated patients, only 8.6% were high responders, with this number increasing to 51.7% and 67.5% for the BNT162b2 and mRNA-1273 vaccines, respectively. In multivariable model, age, cirrhosis, and type of vaccine (ChAdOx1 > BNT162b2 > mRNA-1273) were the only independent predictors of ‘low’ response, whereas viral hepatitis and antiviral therapy continued as independent predictors of ‘high’ response.
Vaccinated patients with CLD display low humoral immunity against SARS-CoV-2 variants with antibodies waning at 6 months
We next evaluated the humoral immunity of patients with CLD to the B.1.617 (Delta) and B.1.1.529 (Omicron) variants, prevalent variants of concern at the time of the study, at 2 weeks (T2) and 6 months (T3) post vaccination. During this period, 48 patients and 30 healthy volunteers dropped out from the study, whereas 29 patients and 18 healthy controls tested positive for COVID-19 and presented with anti-nucleocapsid IgGs. As such, a total of 84 healthy individuals and 135 patients with CLD were included in the long-term follow-up study (Fig. S1).
Compared with T2 IgG levels against Wuhan-Hu-1, T2 IgG levels against the B.1.617 variant were lower (p <0.0001) and further decreased for the B.1.1.529 variant (p <0.0001; Fig. 3C, left). This differential pattern was maintained after 6 months (T3), but with significantly lower antibody titres for all variants (p <0.0001; Fig. 3C, left). In both time points, Wuhan-Hu-1 IgG high responders also displayed increased levels of B.1.617 and B.1.1.529 IgG antibodies, compared with low responders (p <0.0001; Fig. 3C, right).
At both T2 and T3, humoral immune responses between healthy individuals and patients with CLD were comparable, except for patients with CLD presenting with lower B.1.1.529 IgG levels at T2 (p <0.01; Fig. 3D). Nevertheless, when data stratified by vaccine type were analysed, patients with CLD administered the ChAdOx1 nCoV-19 vaccine exhibited lower B.1.617 (p <0.05) and B.1.1.529 (p <0.01) IgG antibodies at T2 compared with healthy participants, followed by those administered the mRNA-1273 vaccine and, finally, the BNT162b2 vaccine (Table S2). At 6 months, patients with CLD presented with overall lower IgG levels across all vaccine types, compared with healthy volunteers (Table S2).
As described in the literature21 and in accordance with our data, sex and age are important confounders of the immune response to COVID-19 vaccination. We evaluated differences in antibody response between healthy volunteers and patients with CLD, adjusting by age and sex (independent variables), but failed to find any statistical differences (data not shown). As such, we next performed a match case–control sub-study (Table 3). Matching tolerance was 3 for age and 0 for sex; median age was 50 years (IQR 43–62 years) for healthy volunteers and 51 years (IQR 44–63 years) for patients with CLD. Both groups included 37.3% males. Patients vaccinated with ChAdOx1 nCoV-19 continued to exhibit lower Wuhan-Hu-1, B.1.617, and B.1.1.529 IgG levels, compared with healthy individuals, whereas patients vaccinated with BNT162b2 appeared to have a slightly increased humoral immune response. Notwithstanding, no statistical differences were found when considering the entire cohorts.
Table 3.
Antibody response to COVID-19 vaccination in age- and sex-matched healthy volunteers and patients with CLD 2 weeks after the second SARS-CoV-2 vaccine dose (T2).
| Antibody titre in healthy volunteers | ChAdOx1 nCoV-19 (n = 9) | mRNA-1273 (n = 6) | BNT162b2 (n = 68) | Total (N = 83) | p value |
|---|---|---|---|---|---|
| IgG Wuhan-Hu-1 (nM) | 113.4 (54.1–349.5) | 709.6 (545.2–851.5) | 409.6 (244.2–535.1) | 409.2 (237.1–557.5) | <0.001 |
| IgG B.1.617 (nM) | 77.9 (35.8–285.0) | 408.3 (223.1–485.9) | 269.4 (137.8–375.2) | 269.8 (135.6–381.2) | n.s. |
| IgG B.1.1.529 (nM) | 89.3 (20.4–194.6) | 298.5 (107.3–473.3) | 125.5 (80.1–213.9) | 126.1 (78.8–219.8) | n.s. |
| IgM (nM) | 0.4 (0.2–1.6) | 11.3 (3.5–17.6) | 0.9 (0.3–1.9) | 0.9 (0.3–2.1) | <0.01 |
| % neutralisation |
39.3 (31.6–50.4) |
83.5 (63.2–91.9) |
58.7 (46.7–74.2) |
57.0 (45.1–74.2) |
<0.001 |
|
Antibody titre in patients with CLD |
ChAdOx1 nCoV-19 (n = 6) |
mRNA-1273 (n = 16) |
BNT162b2 (n = 61) |
Total (N = 83) |
pvalue |
| IgG Wuhan-Hu-1 (nM) | 31.9 (17.7–56.7)∗ | 496.2 (312.0–1,104.310) | 534.0 (299.2–707.1)∗ | 494.2 (231.4–713.0) | <0.01 |
| IgG B.1.617 (nM) | 20.9 (3.9–43.7)∗ | 352.8 (195.7–785.7) | 300.2 (179.2–507.2) | 296.0 (136.1–524.0) | <0.01 |
| IgG B.1.1.529 (nM) | 3.9 (1.2–6.7)† | 158.6 (85.1–358.8) | 143.0 (67.0–231.6) | 132.3 (56.3–238.3) | <0.001 |
| IgM (nM) | 0.7 (0.5–2.2) | 3.7 (1.6–8.5) | 1.5 (0.9–3.1)† | 1.6 (0.9–3.7) | <0.01 |
| % neutralisation | 47.3 (41.3–67.3) | 50.8 (27.5–94.8) | 65.6 (50.7–81.6) | 63.1 (43.7–81.5) | n.s. |
Data are displayed as median (IQR). The Kruskal–Wallis test was used to compare the variables between the three vaccine developers, and the Mann–Whitney test to compare between healthy volunteers and patients with CLD. A value of p <0.05 was considered statistically significant. CLD, chronic liver disease.
∗p <0.05.
†p <0.01 comparing with healthy volunteers.
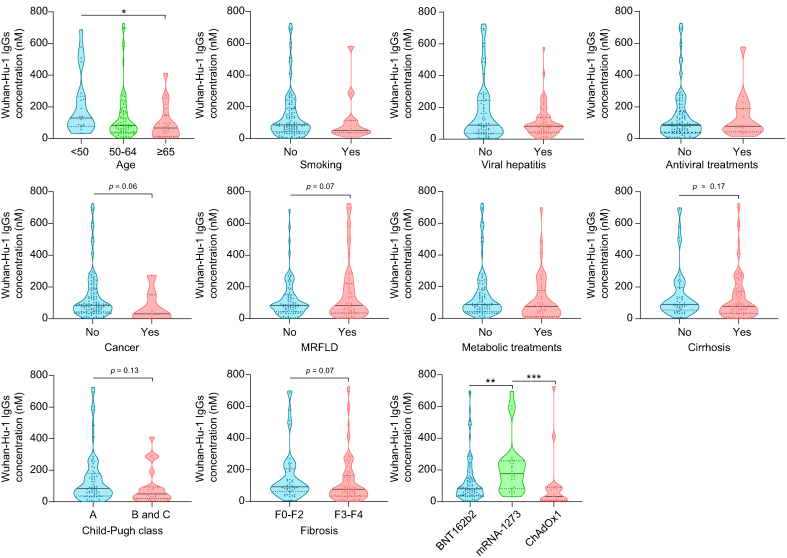
Compared with findings at T2, fewer demographic and clinical characteristics of patients with CLD associated with IgG levels at T3, including viral hepatitis and antiviral treatments (Fig. 4). Nevertheless, older patients continued to exhibit lower IgG levels, with more severe patients with CLD (presence of fibrosis or cirrhosis, as well as Child–Pugh B + C vs. A) displaying a tendency towards lower IgG levels. Differential IgG expression to the distinct mRNA vaccines was also maintained (Fig. 4).
Fig. 4.
T3 Wuhan-Hu-1 IgG levels 6 months after the start of vaccination (T3) in patients with CLD according to demographic and clinical characteristics, and type of vaccine.
Violin plots representing IgG levels. Black horizontal lines indicate the median, and grey dotted lines indicate the IQR. For comparisons between two groups, parametric or non-parametric data were analysed using Student’s t test or the Mann–Whitney test, respectively. For multiple comparisons, parametric or non-parametric data were compared using ANOVA or the Kruskal–Wallis test, respectively, followed by Bonferroni’s or Dunn's post hoc test. ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001. CLD, chronic liver disease; MRFLD, metabolic related fatty liver disease.
SARS-CoV-2 infection and vaccine efficacy do not associate with major clinical and serological parameters in patients with CLD
We next compared patients who developed COVID-19 between T2 and T3 (n = 29), to investigate whether vaccine efficacy was associated with any variable under study (Table 4). All patients were asymptomatic or presented with mild symptoms. Interestingly, vaccine efficacy appeared to be slightly lower in patients with higher weight and height. No correlation was found between the type of vaccine and SARS-CoV-2 infection rates. Similarly, no association was found between IgG titres in T2 and vaccine efficacy. In fact, Wuhan-Hu-1, B.1.617, and B.1.1.529 IgG levels were very similar between SARS-CoV-2-infected and noninfected patients (Table 4). We next investigated whether SARS-CoV-2 infection could potentially associate with demographic or clinical characteristics of patients with CLD. For this, we compared patients who presented with anti-nucleocapsid-IgG before T2 (n = 75) and compared them with noninfected patients with CLD. COVID-19-infected patients presented with unnoticeable to mild symptoms, including fever (75.0%), fatigue (43.8%), coughing (31.3%), myalgia (25.0%), headache (18.8%), sore throat (18.8%), shortness of breath (12.5%), abdominal pain (6.3%), diarrhoea (6.3%), and vomiting (6.3%). Only one patient needed hospitalisation for oxygen administration. Results showed no significant associations between clinical variables and COVID-19 infection rates (Table S3) or infection severity. As somewhat expected, patients with CLD who had been previously infected with COVID-19 exhibited higher Wuhan-Hu-1, B.1.617, and B.1.1.529 IgG levels than did noninfected patients at both T2 and T3 (Fig. S3).
Table 4.
Demographic, clinical, and serological characteristics of patients with CLD infected and noninfected with COVID-19 between two-dose vaccination (T2) and 6 months (T3).
| Noninfected patients (n = 135) | SARS-CoV-2 infection (n = 29) | p value | |
|---|---|---|---|
| General characteristics | |||
| Age | 57.0 (52.0–64.0) | 58.0 (54.0–65.0) | n.s. |
| Male | 75 (55.6) | 18 (62.1) | n.s. |
| Ethnicity | |||
| Caucasian | 127 (94.1) | 27 (93.1) | n.s. |
| Asian | 4 (3.0) | 0 (0.0) | |
| African | 1 (0.7) | 1 (3.4) | |
| Other | 2 (1.5) | 0 (0.0) | |
| Not reported | 1 (0.7) | 1 (3.4) | |
| Weight | 75.0 (64.0–86.0) | 82.7 (70.5–95.4) | <0.05 |
| Height | 168.0 (162.0–175.0) | 174.0 (168.5–180.5) | <0.01 |
| BMI (kg/m2) | 25.5 (23.1–29.8) | 27.3 (24.7–32.2) | n.s. |
| Aetiology of liver disease | |||
| Viral hepatitis | 64 (47.4) | 14 (48.3) | n.s. |
| Autoimmune and/or cholestatic liver disease | 19 (14.1) | 2 (6.9) | n.s. |
| Metabolic related fatty liver disease | 57 (42.2) | 15 (51.7) | n.s. |
| Hereditary liver disease | 4 (3.0) | 1 (3.4) | n.s. |
| Cryptogenic liver disease | 1 (0.7) | 0 (0.0) | n.s. |
| Pharmacotherapy | |||
| Immunosuppressive treatment | 10 (7.4) | 1 (3.4) | n.s. |
| Antiviral therapy | 16 (11.9) | 4 (13.8) | n.s. |
| Metabolic therapy | 45 (33.3) | 13 (44.8) | n.s. |
| Stage of liver disease | |||
| F3–F4 | 90 (66.7) | 23 (79.3) | n.s. |
| Cirrhosis | 87 (64.4) | 20 (69.0) | n.s. |
| Hepatocellular carcinoma | 7 (5.2) | 4 (13.8) | n.s. |
| Comorbidities | |||
| Type 2 diabetes mellitus | 32 (23.7) | 9 (31.0) | n.s. |
| Arterial hypertension | 43 (31.9) | 10 (34.5) | n.s. |
| Obesity | 28 (20.7) | 7 (24.1) | n.s. |
| Hypertriglyceridaemia | 9 (6.7) | 2 (6.9) | n.s. |
| Hypercholesterolaemia | 25 (18.5) | 6 (20.7) | n.s. |
| Renal insufficiency | 1 (0.7) | 2 (6.9) | <0.05 |
| Asthma | 4 (3.0) | 2 (6.9) | n.s. |
| Heart/cardiovascular disease | 16 (11.9) | 4 (13.3) | n.s. |
| Smoking | 13 (9.6) | 3 (10.3) | n.s. |
| Other | 28 (20.7) | 10 (34.5) | n.s. |
| Vaccine type | |||
| mRNA-1273 | 25 (18.5) | 8 (27.6) | n.s. |
| ChAdOx1 nCoV-19 | 18 (13.3) | 0 (0.0) | |
| BNT162b2 | 92 (68.1) | 21 (72.4) | |
| Response to vaccine at T2 | |||
| Low response | 65 (48.1) | 17 (58.6) | n.s. |
| Negative serologic response | 7 (5.2) | 0 (0.0) | n.s. |
| Antibody titre at T2 | |||
| IgG Wuhan-Hu-1 (nM) | 390.1 (127.0–649.1) | 408.0 (267.5–643.5) | n.s. |
| IgG B.1.617 (nM) | 241.9 (76.8–390.6) | 286.7 (188.7–428.2) | n.s. |
| IgG B.1.1.529 (nM) | 103.1 (38.3–188.6) | 94.4 (60.3–204.5) | n.s. |
Data are displayed as median (IQR) for continuous variables and n (%) for categorical variables. The Mann–Whitney test and Fishers’ exact test were used to compare quantitative and qualitative variables, respectively. A value of p <0.05 was considered statistically significant. Viral hepatitis (hepatitis B [17%], C [31.9%], and/or E [0.7%]); autoimmune and/or cholestatic liver disease (primary sclerosing cholangitis [1.5%], primary biliary cholangitis [6.7%], and/or autoimmune hepatitis [6.7%]); metabolic related fatty liver disease (non-alcoholic fatty liver disease [20.7%] and/or heavy alcohol consumption [25.2%]); hereditary liver disease (Wilson disease [1.5%], haemochromatosis [0.7%], and polycystic liver disease [0.7%]); cryptogenic liver disease (0.7%); immunosuppressive treatment (prednisone [5.2%], mycophenolate mofetil [1.5%], azathioprine [3.7%], and/or other [1.5%]); antiviral therapy (tenofovir [4.4%], entecavir [5.2%], interferon [0.7%], and/or other [0.7%]); metabolic therapy (ursodeoxycholic acid [10.4%], obeticholic acid [2.2%], fibrates [3%], metformin [11.9%], GLP-1 agonists [0.7%], insulin [8.9%], statins [11.1%], and/or other [8.2%]). CLD, chronic liver disease.
Discussion
In this study, patients with CLD displayed increased Wuhan-Hu-1 IgG, IgM, and neutralisation levels at 2 weeks after first-dose vaccination (T1) and further increased levels at 2 weeks after the second-dose vaccination (T2). Viral hepatitis and antiviral therapy were independent predictors of ‘high’ humoral immune response to COVID-19 vaccination. In line with this evidence, a recent study found that at 1, 2, and 3 months after two doses of SARS-CoV-2 inactivated vaccine, IgG seropositivity rates were similar between patients with chronic hepatitis B (CHB) and healthy controls and that IgG titres at 3 months were higher in patients with CHB (in particular, patients with HBeAg+ CHB).22 Nonetheless, it is still unclear why patients with viral hepatitis present with a higher humoral response to COVID-19 vaccines. We further found that IgG levels were decreased in patients with MRFLD, receiving metabolic treatments, or with cancer. Patients with cancer have indeed been described to exhibit suboptimal responses to COVID-19 vaccines, which are highly influenced by the cancer treatments,23 although specific studies on liver cancer are lacking. In parallel, different reports have highlighted NAFLD as an independent risk factor for severe COVID-19,24,25 yet patients with NAFLD were recently shown to develop a positive response to COVID-19 vaccination.12 Our current results suggest that this response might be lower when compared with patients with CLD from different aetiologies. Nevertheless, it should be noted that both MRFLD and metabolic-targeted treatments predicted ‘low’ response in univariate analysis but not in multivariate analysis, and cancer did not associate with humoral immune responses in any of the analyses.
In terms of liver disease severity, the presence of F3–F4 fibrosis (univariate analysis) or cirrhosis (univariate and multivariate analysis) associated with a low response to COVID-19 vaccination. Other studies have similarly suggested that cirrhosis leads to impaired or suboptimal responses in patients with CLD.13,[15], [16], [17] This is a noteworthy finding, which might relate with some level of CAID being present in these patients. In fact, immunodeficiency is a dynamic feature of CAID, resulting from structural distortion of the liver parenchyma and functional impairment of circulating innate and adaptive immune cells, as a result of cirrhosis-associated systemic alterations.5
First-generation COVID-19 vaccines offer short-lived protection against current Omicron strains, although this is partially mitigated after a third/boost vaccine shot.17,26 Consistent with these findings, our results showed that IgG levels against the B.1.617 variant and, further, the B.1.1.529 variant were lower at both 2 weeks and 6 months post second-dose vaccination in patients with CLD, compared with Wuhan-Hu-1 IgG antibodies. Of note, patients with CLD presented lower B.1.1.529 IgG levels at T2, compared with healthy individuals, with most still presenting with very low B.1.1.529 IgG at 6 months, suggesting that liver disease may particularly affect vaccine efficacy against latest COVID-19 variants.27 This is particularly relevant, as it highlights the need for patients with CLD to receive booster shots17 or be prioritised for recently approved adapted vaccines (https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#adapted-covid-19-vaccines-section), to increase the chances of protection against severe or even fatal disease. In fact, recently emerged Omicron variants, particularly BA.4 and BA.5, exhibit high degrees of immune escape, including from originally developed vaccines.[28], [29], [30] Because our results show that patients with CLD may have suboptimal responses to Omicron variants, compared with healthy individuals, studies on the efficacy of adapted vaccines in this vulnerable population are eagerly awaited.
Our study failed to find major associations between clinical parameters of patients with CLD and COVID-19 infection rate at T2, as well as vaccine efficacy at T3, with the notable exception that vaccine efficacy appeared to be slightly lower in patients with higher weight and height, suggesting that vaccine dosages might need adjustment according to body size or volume/area, two main factors influencing pharmacokinetics. It is worth noting that our results suggested that patients with CLD exhibit a higher immunological response to mRNA vaccines than to ChAdOx1 at both 2 weeks and 6 months after two-dose vaccination. Between the two mRNA vaccines, mRNA-1273 induced increased production of IgGs, compared with BNT162b2. Similar results have been found in liver transplant recipients31 and in the general population32 and appear to relate with dosage – with the mRNA-1273 vaccine series being two 100-μg doses and the BNT162b2 vaccine series two 30-μg doses.33 Nevertheless, patients infected with COVID-19 from 2 weeks to 6 months after vaccination were not associated with any vaccine type or with different levels of IgGs at 2 weeks, suggesting that the distinct levels of humoral immunity observed could have little impact on vaccine efficacy. Follow-up studies will be key to assess levels of protection – and duration – by distinct vaccine platforms and distinct levels of humoral immunity.
Lastly, we should highlight some limitations of our study, such as the lack of measurements for recent Omicron strains. Our results also suggested that overall humoral responses of patients with CLD were not much different from those of healthy volunteers. However, these results should be interpreted with caution, given the relatively low number of participants in each vaccine subgroup and the fact that several clinical variables associated with the type of vaccine. In addition, many other factors might influence the overall immune response, including the functional profile of the IgGs and cellular immunity, which were not analysed here. Indeed, patients with cirrhosis were recently shown to exhibit impaired SARS-CoV-2-specific T-cell reactivity after vaccination, compared with healthy controls,15 with a booster dose being able to induce B-cell responses similar to those of controls.16 Given our current results, future studies should additionally take into consideration how viral hepatitis and MRFLD, as well as viral hepatitis treatment, affect vaccine efficacy, so that successful measures to improve immunogenicity to COVID-19 vaccines may be taken.
In summary, this was a multicentric study, with a relative high number of well-characterised patients with CLD, with a follow-up of 6 months (before the third dose/boost vaccine being administered). In terms of safety, no major adverse effects of vaccination were reported in our cohort of patients. As for efficacy, it does appear to be compromised in more severe patients with CLD, which as such constitute a priority disease group for both overall vaccination against COVID-19 and receiving a third dose. The impact of liver disease severity on vaccine efficacy should be convened to patients, so that they can have awareness of their increased risk and continue to take personal protective measures.
Financial support
Partial funds were provided through Fundação para a Ciência e a Tecnologia (grants PTDC/MED-PAT/31882/2017 and EXPL/MED-OUT/1317/2021) and through La Caixa Scientific Foundation (grant HR17-00601).
Authors’ contributions
Patient recruiting and acquisition of clinical data: APu, ACC, Apo, PZ, IG, DD, TG, IS, ES, SS, MM, MRG, AT, FPR, RS, MB, CM, HCP. Study concept and design: HCP, REC. Acquisition of laboratory data: ALS, CSP, MMH, CA, DAEF, REC. Analysis and interpretation of the data: ALS, CSP, LIS, APu, ACC, IG, CA, JMB, MRG, AT, FPR, RS, MB, CM, JG, HCP, REC. Drafting of the manuscript: ALS, REC. Critical revision of the manuscript for important intellectual content: ALS, CSP, LIS, APu, ACC, Apo, PZ, IG, MMH, CA, DD, TG, IS, ES, SS, MM, DAEF, JMB, MRG, AT, FPR, RS, MB, CM, JG, HCP, REC. Statistical analysis: ALS, CSP, LIS.
Data availability statement
Owing to the nature of this research, participants of this study did not agree for their data to be shared publicly, so data should remain confidential.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank Sociedade Portuguesa de Gastrenterologia (SPG) and Centro Nacional de Registo de Dados em Gastrenterologia (CEREGA) for the support with the REDCap platform. We also thank Dra Elisa Alves, Clinical Analysis Core Laboratory, Faculty of Pharmacy, University of Lisbon, for blood collections.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100697.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Moon A.M., Webb G.J., Aloman C., Armstrong M.J., Cargill T., Dhanasekaran R., et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73:705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fix O.K., Blumberg E.A., Chang K.M., Chu J., Chung R.T., Goacher E.K., et al. American Association for the Study of Liver Diseases expert panel consensus statement: vaccines to prevent coronavirus disease 2019 infection in patients with liver disease. Hepatology. 2021;74:1049–1064. doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornberg M., Buti M., Eberhardt C.S., Grossi P.A., Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marjot T., Eberhardt C.S., Boettler T., Belli L.S., Berenguer M., Buti M., et al. Impact of COVID-19 on the liver and on the care of patients with chronic liver disease, hepatobiliary cancer, and liver transplantation: an updated EASL position paper. J Hepatol. 2022;77:1161–1197. doi: 10.1016/j.jhep.2022.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albillos A., Martin-Mateos R., Van der Merwe S., Wiest R., Jalan R., Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19:112–134. doi: 10.1038/s41575-021-00520-7. [DOI] [PubMed] [Google Scholar]
- 6.Marjot T., Webb G.J., Barritt A.S., Ginès P., Lohse A.W., Moon A.M., et al. SARS-CoV-2 vaccination in patients with liver disease: responding to the next big question. Lancet Gastroenterol Hepatol. 2021;6:156–158. doi: 10.1016/S2468-1253(21)00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and wfficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ai J., Wang J., Liu D., Xiang H., Guo Y., Lv J., et al. Safety and Immunogenicity of SARS-CoV-2 vaccines in patients with chronic liver diseases (CHESS-NMCID 2101): a multicenter study. Clin Gastroenterol Hepatol. 2022;20:1516–1524.e2. doi: 10.1016/j.cgh.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thuluvath P.J., Robarts P., Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75:1434–1439. doi: 10.1016/j.jhep.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J., Hou Z., Liu J., Gu Y., Wu Y., Chen Z., et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021;75:439–441. doi: 10.1016/j.jhep.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruether D.F., Schaub G.M., Duengelhoef P.M., Haag F., Brehm T.T., Fathi A., et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. 2022;20:162–172.e169. doi: 10.1016/j.cgh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider L., Schubert L., Winkler F., Munda P., Winkler S., Tobudic S. SARS-CoV-2 vaccine response in patients with autoimmune hepatitis. Clin Gastroenterol Hepatol. 2022;20:2145–2147.e2. doi: 10.1016/j.cgh.2022.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Dury S., Waern J., Waldenström J., Alavanja M., Saed H.H., Törnell A., et al. Impaired SARS-CoV-2-specific T-cell reactivity in patients with cirrhosis following mRNA COVID-19 vaccination. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giambra V., Piazzolla A.V., Cocomazzi G., Squillante M.M., De Santis E., Totti B., et al. Effectiveness of booster dose of anti SARS-CoV-2 BNT162b2 in cirrhosis: longitudinal evaluation of humoral and cellular response. Vaccines (Basel) 2022;10:1281. doi: 10.3390/vaccines10081281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John B.V., Ferreira R.D., Doshi A., Kaplan D.E., Taddei T.H., Spector S.A., et al. Third dose of COVID-19 mRNA vaccine appears to overcome vaccine hyporesponsiveness in patients with cirrhosis. J Hepatol. 2022;77:1349–1358. doi: 10.1016/j.jhep.2022.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John B.V., Deng Y., Schwartz K.B., Taddei T.H., Kaplan D.E., Martin P., et al. Postvaccination COVID-19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology. 2022;76:126–138. doi: 10.1002/hep.32337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon A.M., Webb G.J., Garcia-Juárez I., Kulkarni A.V., Adali G., Wong D.K., et al. SARS-CoV-2 infections among patients with liver disease and liver transplantation who received COVID-19 vaccination. Hepatol Commun. 2022;6:889–897. doi: 10.1002/hep4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L., et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients' B cells. Cell. 2020;182:73–84.e16. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller L., Andrée M., Moskorz W., Drexler I., Walotka L., Grothmann R., et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73:2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He T., Zhou Y., Xu P., Ling N., Chen M., Huang T., et al. Safety and antibody response to inactivated COVID-19 vaccine in patients with chronic hepatitis B virus infection. Liver Int. 2022;42:1287–1296. doi: 10.1111/liv.15173. [DOI] [PubMed] [Google Scholar]
- 23.Fendler A., de Vries E.G.E., GeurtsvanKessel C.H., Haanen J.B., Wörmann B., Turajlic S., et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol. 2022;19:385–401. doi: 10.1038/s41571-022-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji D., Qin E., Xu J., Zhang D., Cheng G., Wang Y., et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y.J., Zheng K.I., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: a multicenter preliminary analysis. J Hepatol. 2020;73:719–721. doi: 10.1016/j.jhep.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi H., Liu B., Wang X., Zhang L. The humoral response and antibodies against SARS-CoV-2 infection. Nat Immunol. 2022;23:1008–1020. doi: 10.1038/s41590-022-01248-5. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z., Zhang Y., Song R., Wang L., Hu X., Li H., et al. Waning humoral immune responses to inactivated SARS-CoV-2 vaccines in patients with severe liver disease. Signal Transduct Target Ther. 2022;7:174. doi: 10.1038/s41392-022-01032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Y., Yisimayi A., Jian F., Song W., Xiao T., Wang L., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu P., Faraone J., Evans J.P., Zou X., Zheng Y.M., Carlin C., et al. Neutralization of the SARS-CoV-2 Omicron BA.4/5 and BA.2.12.1 subvariants. N Engl J Med. 2022;386:2526–2528. doi: 10.1056/NEJMc2206725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuekprakhon A., Nutalai R., Dijokaite-Guraliuc A., Zhou D., Ginn H.M., Selvaraj M., et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185:2422–2433.e2413. doi: 10.1016/j.cell.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strauss A.T., Hallett A.M., Boyarsky B.J., Ou M.T., Werbel W.A., Avery R.K., et al. Antibody response to severe acute respiratory syndrome-coronavirus-2 messenger RNA vaccines in liver transplant recipients. Liver Transpl. 2021;27:1852–1856. doi: 10.1002/lt.26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Self W.H., Tenforde M.W., Rhoads J.P., Gaglani M., Ginde A.A., Douin D.J., et al. Comparative effectiveness of Moderna, pfizer-BioNTech, and janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions – United States, march–august 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Owing to the nature of this research, participants of this study did not agree for their data to be shared publicly, so data should remain confidential.