Abstract
Many animal behaviours exhibit complex temporal dynamics, suggesting there are multiple timescales at which they should be studied. However, researchers often focus on behaviours that occur over relatively restricted temporal scales, typically ones that are more accessible to human observation. The situation becomes even more complex when considering multiple animals interacting, where behavioural coupling can introduce new timescales of importance. Here, we present a technique to study the time-varying nature of social influence in mobile animal groups across multiple temporal scales. As case studies, we analyse golden shiner fish and homing pigeons, which move in different media. By analysing pairwise interactions among individuals, we show that predictive power of the factors affecting social influence depends on the timescale of analysis. Over short timescales the relative position of a neighbour best predicts its influence and the distribution of influence across group members is relatively linear, with a small slope. At longer timescales, however, both relative position and kinematics are found to predict influence, and nonlinearity in the influence distribution increases, with a small number of individuals being disproportionately influential. Our results demonstrate that different interpretations of social influence arise from analysing behaviour at different timescales, highlighting the importance of considering its multiscale nature.
This article is part of a discussion meeting issue ‘Collective behaviour through time’.
Keywords: leadership, collective behaviour, movement ecology, timescales, schooling, flocking
1. Introduction
Spectacular displays of coordinated group movement are widespread across the animal kingdom—from fish schools [1,2] exhibiting near-instantaneous changes in their direction, to locust swarms [3,4] marching across the desert, to pigeon [5,–8] and starling [9–11] flocks weaving through the skies to escape predators. A hallmark of such collective response is that the movement—and especially changes in movement—of each individual has the potential to influence the behaviour of other group members, which in turn can trigger behavioural changes in other group members, and so on. A key aspect in understanding collective behaviour, therefore, is establishing the degree to which each individual has the capacity to influence others, often referred to in the literature as ‘leadership’ [8,12] or ‘social influence’ [13].
Within most animal groups influence changes dynamically over time, and is thus distributed among members of the group. All individuals, over a course of time, either directly or indirectly influence each other's movement to some degree. However, within-group heterogeneity in various traits has been found to give some individuals more influence over the group's movement direction than others. For example, body size [14], relative speed [15,16], navigational experience [17], sociability [15,18], motivation [18], social status [19], informational status [2] and spatial position [19–21] within the group have all been found to be correlated with social influence within groups. Driven by such a diverse range of mechanisms, collective behaviour inherently exhibits complex and multiscale temporal dynamics. Furthermore, when animals interact with one another they can mutually, and recursively, influence each others' behaviour. This itself can introduce further timescales not present when individuals are in isolation. Thus, although considerable progress has been made in recent years in the quantification of behaviour [15,22–26] and influence [13], there still exist many challenges in correctly identifying and interpreting the multiscale nature of both individual and collective animal behaviour [27].
Here, we analyse fine-scale collective movement of two very different model systems, schooling golden shiner fish (Notemigonus crysoleucas) and flocking homing pigeons (Columba livia), at multiple timescales. First, we obtain position and kinematic properties—speed and acceleration—of each individual relative to every other individual in the group (figure 1a). Second, we use the time-lagged directional cross-correlation technique [8,28] to analyse leader–follower relationships between pairs of individuals (figure 1b). While numerous other methods have previously been used to quantify social influence [13], we chose this approach as it is simple and also agnostic to the exact nature of the social interactions in which individuals engage. Third, we combine the above metrics at different timescales to explore the predictive power of relative position and kinematic properties in predicting leadership (relative social influence) in groups (figure 1c). Taken together, our work reveals that the strength of predictions made by the different variables (positional or kinematic) on leadership, and the inferred distribution of leadership across members of the group, can change considerably depending on the timescale of analysis.
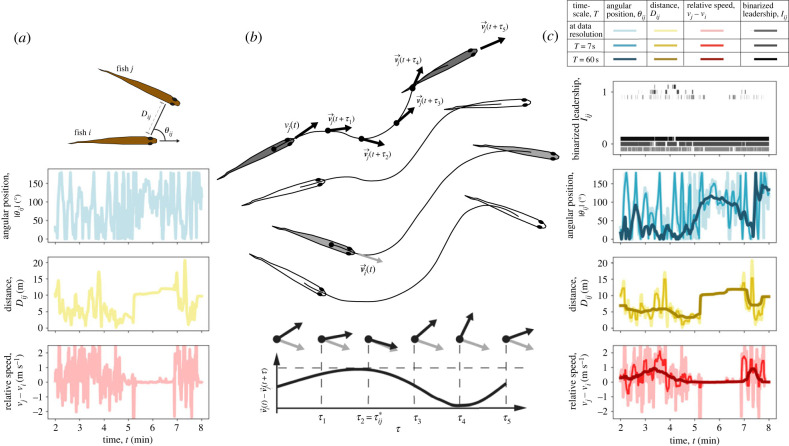
Figure 1.
Summary of the leader–follower analyses conducted over multiple timescales. (a) Temporal dynamics of some features used to predict social influence in animal groups. The top panel is an illustration of angular position (θij) and metric distance (Dij) for two neighbouring fish. The following three plots in (a) show short time segments of angular position (in blue), distance (in yellow) and difference in movement speed of a pair of individuals (in red) at the resolution at which data were recorded. (b) A schematic of the time-lagged directional cross-correlation technique (adapted from [8]) used to analyse leader–follower relationships between pairs of individuals. For each pair (i ≠ j), the directional correlation function is , where 〈…〉 denotes a time average at the specified timescale. Below is a visualization of the dot product of the normalized velocity of individual i at time t and that of individual j at time t + τ (modified from [8]). (c) Illustration of the leadership scores over multiple timescales for the same sample period shown in (a) along with angular position, distance and relative speed at corresponding timescales.
2. Material and methods
(a) . Datasets
In this paper, we re-analysed two datasets from previous studies [29,30], which contained trajectories of freely swimming schooling golden shiners (Notemigonus crysoleucas) [1,31–33] extracted from video data [29], and GPS trajectories of freely flying pigeon (Columba livia) [21,34–38] flocks near their home lofts [30]. Below, we describe some of the details specific to each dataset:
-
(i)
Golden shiners. Groups of 10 and 30 golden shiners (Notemigonus crysoleucas) were allowed to swim freely in a 2.1 × 1.2 m experimental tank containing a 4.5–5 cm depth of water. Fish were filmed at 30 Hz for 2 h using a Sony EX-1 camera placed 2 m above the tank. Three trials were conducted per group size and fish were not reused over multiple trails. Our analyses are focused on 13 min segments from each trial. Chosen segments started 1 h after the onset of the trial to minimize effects of stress on the fish from handling. Fish positions, orientations and body sizes were extracted from videos using SchoolTracker [1] and manual data correction was performed to ensure accuracy of the tracks and to maintain individual identities over time.
-
(ii)
Homing pigeons. GPS data were collected at 10 Hz from free flights of flocks of homing pigeons (Columba livia) around the loft. All 30 pigeons were aged 2.8 ± 1.6 years (mean ± s.d.) and came from two neighbouring lofts at the University of Oxford Field Station. Birds were allocated to three groups of 10 (A, B and C) and were also tested in a combined group of 30 (group ABC). All analyses were conducted on two flights each for groups A, B and C, and one flight for group ABC. We analysed data in two dimensions (using x- and y-coordinates) on flight segments that ranged between 8 and 40 min in duration.
(b) . Data analyses
-
(i)
Processing trajectory data. First, trajectories obtained from the two datasets were used to calculate kinematic properties, i.e. speed and acceleration, of individuals (see electronic supplementary material for the details on calculating these variables). Since our interest is to examine social influence at the dyadic level, we also computed pairwise features from the trajectories. At each frame, we determined the relative position of each individual with respect to every other individual in the group. This was measured as an egocentric vector-based representation of the neighbour i.e. the angular position and distance of a neighbour relative to a focal individual (figure 1a; see electronic supplementary material for the details on calculating positional variables). We also calculated relative kinematics, i.e. the difference in speed and acceleration between a focal individual and its neighbours (figure 1a; see electronic supplementary material for the details on calculating kinematic variables). Once all relevant features were computed for every individual (and pair) across the entire dataset, we used a moving window average to analyse our data at four different timescales—T ∈ {7, 15, 30, 60 s}. These were the timescales of our analysis.
-
(ii) Calculating influence. To calculate the influence of a given individual on its neighbour, we adopted the time-lagged directional correlation technique [8,12,28] with a moving time window—T ∈ {7, 15, 30, 60 s}. Note that we use the terms 'influence' and 'leadership' interchangeably; in doing so, we refer to this time-lagged directional correlation definition. At each time step t, we calculated the time-lagged correlations between directions of pairs of individuals Cij(τ, t),
where denotes the velocity vector of individual i at time t, denotes the unit direction of individual i at time t and 〈…〉 denotes a time average at the specified timescale. For each time step t, the maximum value of the correlation function Cij(τ, t) was determined as and the corresponding time lag τij(t) was determined as (figure 1b). Positive values correspond to being followed—when the directional motion of the focal individual i is ‘copied’ by its neighbour j—while negative values correspond to following—when the focal individual i ‘copies’ directional motion of its neighbour j. To ensure that the correlation value holds meaningful information about directional copying, we only consider periods when the average directional difference (within the analysis timescale) is less than 30° i.e. periods when as leading events. We also disregarded events where the time lag as we considered this time window to be too short for directional copying. As demonstrated by a sensitivity analysis, detailed in the electronic supplementary material, our results are insensitive to the exact values chosen for these two thresholds (angular threshold and time threshold; electronic supplementary material, figures S1–S4). Using these criteria, we simplified the cross-correlation values and classified interactions between all dyads across all frames as being engaged in a leader–follower relationship, or not:2.1
Since in our study cases all individuals in a group are relatively well-aligned, we further filtered the above leadership scores and binarized them (figure 1c) such that, for a given individual i, only a single leader—the one with the largest correlation—is considered [39]:2.2
where lij(t) is the binarized leadership score for dyad i,j at time t and N is the number of individuals in the group. The network of inter-individual interactions obtained from the binarized leadership score lij(t) was then used to calculate the local reaching centrality [40] Rk(t). This measure quantifies the proportion of the group influenced by individual k (both directly and indirectly) at time step t. By aggregating reaching centralities over time, we obtain influence scores Rk for all individuals. An assessment of the distribution of influence across the group is then conducted by calculating the Gini coefficient of the individual influence scores (Rk).2.3 -
(iii)
Predicting influence across timescales. To assess the strength of prediction of positional and kinematic properties on leadership (lij) at each considered timescale, we constructed a balanced dataset—with an equal number of points where pairs of individuals exhibited a leader–follower relationship and where pairs did not exhibit this relationship—following which we fitted a Bayesian GLM to the data. The response variable was Bernoulli distributed and represented whether or not the pair exhibited a leader–follower relationship at that moment in time (lij(t)). To explore the role of the different predictors, we constructed three different models denoted as M1, M2 and M3: M1, with relative position as a predictor—given by distance to, and angular position of, the neighbour relative to the focal individual; M2, with absolute and relative kinematics as predictors—speed, acceleration and difference in speed and acceleration between a focal individual and its neighbour; and M3, with both relative position and kinematics as predictors. Note that M3 combines all predictors used in the above models M1 and M2.
For each model, we estimated the posterior P(θμ|X, y) using the No-U-Turn Sampler (NUTS), a self-tuning variant of the Hamiltonian Monte Carlo (HMC) algorithm implemented in Stan (https://www.jstatsoft.org/article/view/v076i01). We drew HMC samples using four independent Markov chains consisting of 1000 warm-up iterations and 1000 sampling iterations, making a total of 4000 sampling iterations. To speed up sampling, we used a balanced dataset consisting of 5000 data points when fitting each generalized linear model. To balance the dataset, we randomly subsampled from our original dataset such that we had 2500 data points where pairs of individuals engaged in a leader–follower relationship, and 2500 data points where this was not true (see electronic supplementary material, tables S1 and S2 for details regarding the subsampling). Even though the proportion of data used in our GLMs is small (as shown in electronic supplementary material, tables S1 and S2), we ensured that it is representative of the entire dataset by assessing the model fit on five independent subsamples of the data. All models converged without any signs of pathological behaviour, and priors were chosen to be relatively uninformative distributions .
-
(iv)
Temporal dynamics of social influence. The analysis conducted above provides insights into factors that are correlated with leadership. However, to investigate the differences in the strength of prediction between the different timescales, we examined the temporal dynamics of leader–follower events by analysing the process as a time series. To this end, we isolated 40 s segments centred around 2 s where the leadership dynamics changed (pairs of fish exhibit a leader–follower relationship for 1 s followed by a 1 s period where they abandon this relationship, and vice versa). Averaging across all isolated time series segments, we asked: What features predict that an individual will start following a certain neighbour? or What features predict that an individual will stop following its current leader? Statistically, these two time series can be thought of as representing a switch in leadership from a focal follower's perspective. Finally, the isolated time series events were bootstrapped to obtain 95% confidence intervals around the means.
3. Results
We use two approaches to examine social influence among pairs of individuals in two case studies—swimming golden shiner schools and flying homing pigeon flocks. First, we use Bayesian GLMs to identify the relationship between position and kinematic properties of individuals relative to their neighbours, and their tendency to influence the movement direction of these neighbours. Based on their direct and indirect influence on group members, we get an influence metric for each individual in the group, measured here as the aggregate local reaching centrality (Rk) of the individual. We subsequently quantify the distribution of influence across group members by calculating the Gini coefficient of this influence score (Rk). All analyses are conducted at multiple temporal scales, and call attention to the timescale-dependent nature of our results. Second, we conduct a time series analysis of leader–follower events. In this analysis, the temporal sequence of relative positions and kinematics—before, during and after leadership—allow us to evaluate their association with leadership and inform us why we see timescale-dependence in the previous analysis. Below, we discuss results from both these analyses.
(a) . Timescale-dependence of social influence
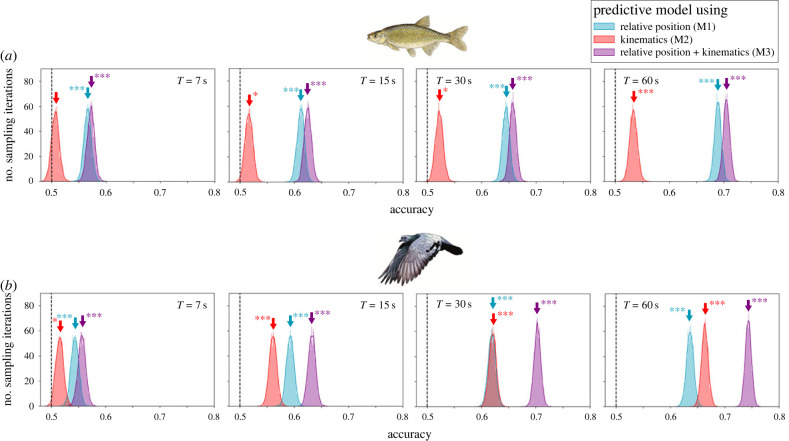
The three different models that we constructed (M1, M2 and M3) to predict leadership based on relative position and kinematics of dyads performed differently across timescales and species. For both species, within the range of timescales analysed, we observe that the predictability of leadership increases when the data are analysed over longer timescales (figure 2; see tables 1 and 2 for prediction accuracies across timescales for golden shiners and homing pigeons respectively). At short timescales (T ≤ 15 s), relative position of the leader with respect to the follower (M1) is found to be a strong predictor of influence, with nearer individuals occupying relatively frontal positions being most influential. Contrary to our expectation [15,16], kinematic properties (M2) are found to have low predictive power (figure 2). At longer timescales (T ≥ 30 s), both relative position and kinematics predict leadership (figure 2). On the one hand, in golden shiners, despite an increase in predictive power of kinematics, we find that relative position is still the strongest predictor of social influence (at T = 60 s mean prediction accuracy 68.88% was higher for model M1 than 53.36% for model M2). On the other hand, in homing pigeons, a reversal in the strength of prediction made by relative position (M1) and kinematics (M2) is observed at longer timescales (median prediction accuracy 63.62% was lower for M1 as compared with 66.36% for M2). At these long timescales, kinematics become a better predictor of leadership than relative position (compare panels in figure 2b). For both species, across timescales, a combined model (M3) using both relative position and kinematics best predicts leadership (figure 2; see tables 1 and 2 for prediction accuracies).
Figure 2.
Timescale-dependence of social influence. Prediction accuracy of three models in a dataset of 5000 randomly selected data points for (a) golden shiners and (b) homing pigeons. The y-axis represents the number of sampling iterations (from a total of 4000) at any given accuracy. The four panels within each species represent four different timescales of analysis—T ∈ {7, 15, 30, 60 s}. Across models, and within the explored timescales, predictability of social influence grows as we analyse data over longer timescales. Coloured arrows represent the median accuracy of corresponding distributions and asterisks next to them indicate which distributions are significantly different from a chance expectation (shown here by the dashed line). The adopted criteria for significance are *p < 0.05, **p < 0.01 and ***p < 0.001.
Table 1.
Prediction accuracies of GLMs for golden shiner fish: mean and 95% confidence intervals on prediction accuracy of the three models M1, M2 and M3 at the considered timescales T ∈ {7, 15, 30, 60 s}.
| timescale | relative position (M1) |
kinematics (M2) |
relative position + kinematics (M3) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| mean | 2.5% | 97.5% | mean | 2.5% | 97.5% | mean | 2.5% | 97.5% | |
| T = 7 s | 56.61 | 55.24 | 58.00 | 50.64 | 49.34 | 52.06 | 57.19 | 55.84 | 58.56 |
| T = 15 s | 61.15 | 59.80 | 62.46 | 51.66 | 50.26 | 53.02 | 62.48 | 61.18 | 63.82 |
| T = 30 s | 64.46 | 63.20 | 65.72 | 52.24 | 50.88 | 53.62 | 65.75 | 64.50 | 67.02 |
| T = 60 s | 68.88 | 67.68 | 70.04 | 53.36 | 51.94 | 54.72 | 70.51 | 69.36 | 71.68 |
Table 2.
Prediction accuracies of GLMs for homing pigeons: mean and 95% confidence intervals on prediction accuracy of the three models M1, M2 and M3 at the considered timescales T ∈ {7, 15, 30, 60 s}.
| timescale | relative position (M1) |
kinematics (M2) |
relative position + kinematics (M3) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| mean | 2.5% | 97.5% | mean | 2.5% | 97.5% | mean | 2.5% | 97.5% | |
| T = 7 s | 54.48 | 53.12 | 55.86 | 51.48 | 50.12 | 52.88 | 55.72 | 54.34 | 57.18 |
| T = 15 s | 59.26 | 57.96 | 60.60 | 56.08 | 54.68 | 57.46 | 63.26 | 61.88 | 64.56 |
| T = 30 s | 61.90 | 60.56 | 63.18 | 62.04 | 60.68 | 63.40 | 70.30 | 69.06 | 71.48 |
| T = 60 s | 63.62 | 62.32 | 64.90 | 66.36 | 65.12 | 67.54 | 74.34 | 73.24 | 75.48 |
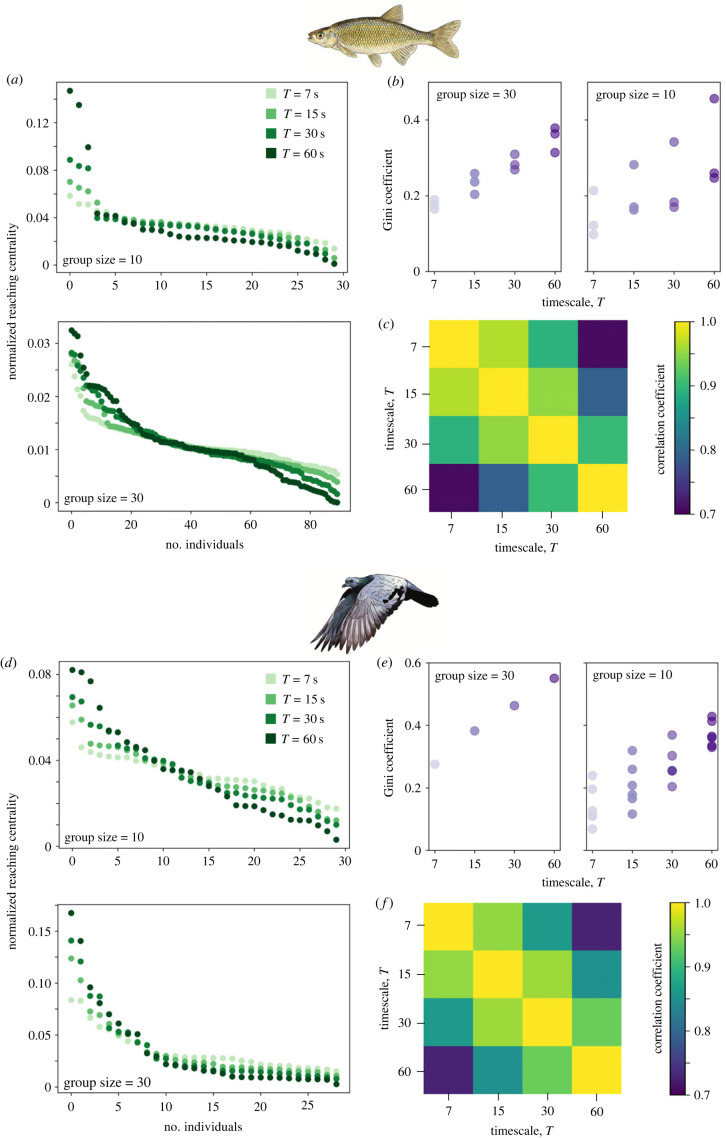
At the individual level, we also found differences in the distribution of influence across the different timescales, with individual influence scores being more similar for shorter timescales (figure 3c,f). For both species, at short timescales the distribution of influence across group members was relatively linearly descending (figure 3a,b,d,e). At longer timescales, between-individual differences aggregate, resulting in a larger asymmetry in the influence distribution across group members (indicated by the increasing Gini coefficients with the analysis timescale in figure 3b,e).
Figure 3.
Distribution of social influence within the group. Distribution of individual influence scores defined as normalized reaching centralities at four different timescales T ∈ {7, 15, 30, 60 s} for groups of 10 and 30 golden shiner fish (a–c) and homing pigeons (d–f). (a,d) At smaller timescales, the distribution of influence is relatively linear within the group, with a smaller slope for the fish compared with the pigeons. However, at larger timescales, between-individual differences in influence add up to result in larger and more consistent inter-individual differences. (b,e) This is evidenced by the increase in the Gini coefficient with the timescale of analysis. Finally, (c,f) we also calculate the Pearson's correlation coefficient between individual influence scores (Rk) across the different timescales of analysis. While influence scores show strong correlations across the different timescales, a drop in the correlation is seen as the magnitude of the difference between timescales increases.
(b) . Temporal dynamics of social influence
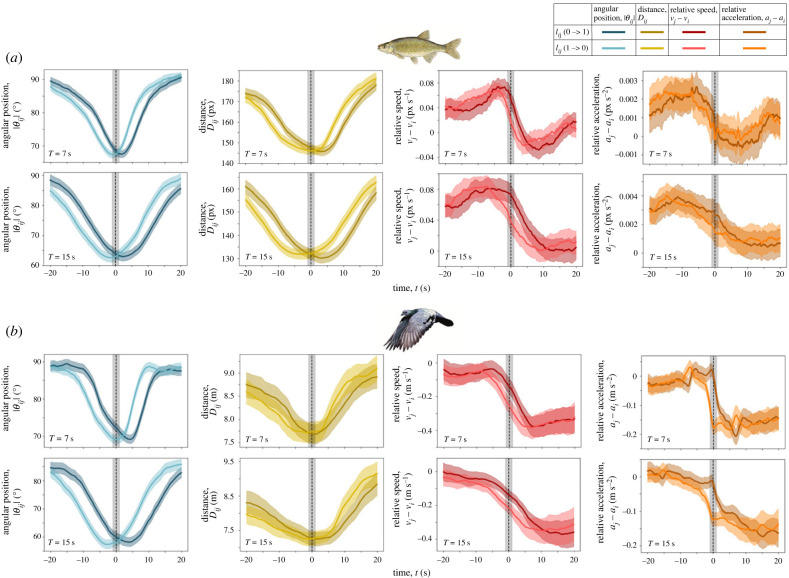
Our time series analysis reveals the temporal sequencing of between-individual relationships, before, during and after periods of leadership. Here, we explore the temporal dynamics in angular position, distance, relative speed and relative acceleration among dyads. Here, relative speeds and relative accelerations are measured as the difference in the magnitudes of the velocity and acceleration vectors of the leader and follower, respectively. We consider transitions of the focal individual both from following a neighbour to not following it (lij(1 → 0)), and from not following to following (lij(0 → 1)). Note that the transition lij(1 → 0) could occur because the focal individual i switched from following neighbour j, to either following a different neighbour k or not following any neighbour. Similarly, lij(0 → 1) could occur because the focal individual i switched from either not following any neighbour or from following some neighbour k to following neighbour j. Since we simplify our analysis such that the focal individual can only be following one other individual at a given time [39], the dashed line in figure 4 represents a timepoint when, statistically, the focal individual switches the neighbour it is following. For both species, we find that individuals follow close-by neighbours (electronic supplementary material, figure S5) that occupy relatively frontal positions. Changes in relative speed and relative acceleration consistently precede these periods of leadership and individuals exert influence in periods when they are slowing down relative to their neighbours (figure 4). In golden shiners, a switch in leadership, from a focal follower's perspective, coincides with the new leader occupying a closer and a more frontal position compared with the previous leader (figure 4a). In homing pigeons, unlike in golden shiners, a switch in the leader is associated only with frontness of the new leader relative to the old one and does not correlate with the difference in closeness of these individuals (figure 4b). For both species, kinematic changes (relative speed and relative acceleration) precede leadership, and are therefore reflected in the GLMs (figure 2) only over longer timescales. Individuals are found to occupy leading positions in periods when they slow down relative to the focal follower. While this could be partially driven by kinematic constraints (e.g. individuals must slow down to make large turns), we observe species-specific differences, which suggests that other factors also contribute to this trend. Additionally, in homing pigeons, unlike golden shiners, leader switches are found to be strongly associated with relative accelerations of the focal follower and the two concerned neighbours, i.e. its old and new leader (figure 4).
Figure 4.
Temporal dynamics of social influence. Temporal dynamics of relative position (distance to, and angular position of, the neighbour relative to the focal individual) and relative kinematics (speed and acceleration difference between a focal individual and its neighbour) that accompany a switch in leader–follower dynamics in (a) golden shiners and (b) homing pigeons. At the black dashed line, the focal individual suddenly decides to follow, or to stop following, its neighbour. The time series are 40 s segments, of which the middle 2 s represent this decision (shown here by the grey-shaded region). The darker lines represent a transition of the focal individual from not following its neighbour to following it (lij(0 → 1)), while the lighter lines represent a transition from following to not following (lij(1 → 0)). Statistically, these two lines (in each plot) can be perceived as the focal individual giving up followership of one neighbour (represented by the lighter lines) to start following another neighbour (represented by the darker lines). All curves show aggregate data from the entire dataset, and the shaded region shows the 95% confidence intervals around the mean.
4. Discussion
In this work we present detailed analyses of collective animal movement that demonstrate timescale-dependence of social influence in fish schools and bird flocks. At relatively short timescales (T ≤ 15 s)—for both golden shiners and homing pigeons—we find that relative position is the strongest predictor of leadership, and contrary to our expectation, little to no relationship was found between kinematics and leadership. At relatively long timescales (T ≥ 30 s), the predictive power of kinematics in relation to leadership increased, but we observed differences between the two study systems. While, in golden shiners, the increase in predictive power of kinematics was modest (and relative position was still the strongest predictor of leadership), in homing pigeons, a reversal in the strength of the different predictors was observed i.e. kinematics became a better predictor of leadership than relative position (figure 2). A time series analysis revealed that this difference in the strength of different predictors at different timescales occurs as a result of the temporal sequencing of behaviour. For both species, changes in leadership were found to coincide with the two individuals coming closer and the leader occupying a more frontal position—seen by the decrease in the values of angular position and distance (figure 4). Changes in kinematics were found to precede leader–follower relationships between dyads, and a decrease in speed was associated with changes in leadership (figure 4). While these changes did not affect individual followership instantaneously, they allowed individuals to occupy influential (frontal and closer) positions, thus increasing their chances of subsequently being followed. This temporal sequencing of kinematic changes preceding leadership is therefore observable only over longer timescales.
Furthermore, our results also suggest that, at short timescales, social influence is relatively linearly distributed across group members, i.e. most or all individuals may exert some influence on group members (figure 3a,b,d,e). However over longer timescales, inequality within the group grows (figure 3b,e), resulting in consistent inter-individual differences in leadership. Our results are in agreement with previous work where context-dependent consistency in leadership has been demonstrated in pigeons [30,36]. Similar results have also been found in golden shiner fish, where it was demonstrated that even when group members did not differ in their knowledge of a food source, individual golden shiner fish differed in their tendency to lead [41] and that only a small proportion of individuals could lead the group to the food source [42].
In general, our work highlights how different interpretations can result from analysing behavioural data at different timescales and demonstrates that analyses at multiple temporal scales are essential for a holistic understanding of the mechanistic and functional underpinnings of collective animal behaviour. We acknowledge that several other approaches have previously been used to quantify social influence—from attraction and alignment force-based approaches [32,43–45] to Bayesian integration [46–48], information theory [49,50] and deep neural network [51] approaches—each with its own set of advantages and limitations. Here, we use the time-lagged directional correlation technique which is agnostic to the exact interaction rules that individuals adopt and has the advantage of directly using movement directions obtained from the data. Our technique not only captures the timescale-dependence of social influence, but also reveals the specificities of leadership that are associated with the different species that we consider. Comparing the two time series considered (lij(1 → 0) and lij(0 → 1)) for each of the predictor variables (angular position, distance, speed difference and acceleration difference), we reveal how positional and kinematic properties are associated (statistically) with a change in leader. In golden shiners, changes in leadership coincided with the new leader occupying a closer and more frontal position compared with the previous leader. In homing pigeons, unlike in golden shiners, leader switches are associated only with changes in relative angular position of the new leader compared with the previous one, and not with distance—this is seen by the strong overlap between the light yellow and dark yellow lines in figure 4b and may result from weak distance-dependence in attraction-like interactions that have been previously described in pigeons [21] and other birds [10]. However, in homing pigeons, a difference in relative acceleration between the old and the new leader also appears to influence leader switches (cf. overlap between the light orange and dark orange lines in figure 4a,b).
The between-species differences that we observed could partially arise from differences in the physical media in which these animals move. Birds are relatively constrained in their flight speeds. In air, an individual's movement speed is coupled with its flapping activity, which in turn affects its flying altitude. Hence, relative position for flying species—in our case, homing pigeons—is strongly linked to their preferred flight speeds. Fish, however, are able to use their swim bladder to engage in stop-and-go movement, thus regulating their position within the group and proximity to neighbours with higher precision.
Differential leadership has also been considered in other fields. In business and management theory, three levels of leadership are often referred to: bottom-level supervisors, middle-managers, and top-level leaders [52,53]. These different leaders all lead and direct tasks, but the scope and timescale of their leadership differs. While supervisors may make plans for weeks up to months, middle-managers consider longer time periods in their planning (e.g. 1–2 years), and the top-level leaders such as the CEO and board of directors of a company set the strategic direction of the company, which may span multiple years or even decades [53]. Leadership can be analysed across these multiple levels [54], and in addition may serve functions in different areas, such as transformational leadership, ethical leadership and empowering leadership [55]. Similarly, leadership can be considered in different domains. While here we considered movement decisions, other domains of leadership can be regarding resource acquisition, social learning (e.g. others copy the leader's strategy) and mediating within-group conflict or between-group interactions [56]. These examples underscore the importance of considering the timescale and context to interpret leadership in animal groups.
Leadership at short versus long timescales can represent ‘different types’ of leadership. While leaders at short temporal scales may lead particular turns or changes in direction, leaders at longer timescales may set the more general direction of the group. For example, a short-timescale leader may lead the group to avoid an obstacle, but a long-timescale leader may keep the group in the correct direction to return to the home nest or another goal location. While here we only consider positional and kinematic variables as drivers of leadership, there may be several mechanisms that operate at different timescales. The ability to take long-time recordings, which is currently facilitated by new advances in technology, will allow future work to analyse and compare leadership at different timescales. Combining these advances in technology with new analytical techniques and manipulative experiments may help reveal the mechanisms that drive differences in social influence across timescales. For example, applying the methods developed here to mixed groups composed of satiated and starved individuals may reveal the timescale at which hunger affects social influence, and perhaps the kinematic properties via which this influence is manifested. While previous work has primarily aggregated and used data in a timescale-agnostic manner, our results show the importance of considering different timescales and the possible mechanisms that may drive behaviour at each of these timescales.
Acknowledgements
V.H.S. acknowledges Alison Ashbury for extensive comments on the manuscript, and the International Max Planck Research School (IMPRS) for Organismal Biology for the graduate school community and access to courses and resources. Additionally, all authors thank two anonymous referees for their constructive comments during the review process.
Contributor Information
Vivek H. Sridhar, Email: vivekhsridhar@gmail.com.
Máté Nagy, Email: nagymate@hal.elte.hu.
Iain D. Couzin, Email: icouzin@ab.mpg.de.
Data accessibility
All data to support the findings of this study are available with other published papers. Code availability: all analyses were run using custom code written in CUDA and Python and are available in the GitHub repository (https://doi.org/10.5281/zenodo.7113335).
The data are also provided in the electronic supplementary material.
Authors' contributions
V.H.S.: conceptualization, data curation, formal analysis, investigation, methodology, software, visualization, writing—original draft, writing—review and editing; J.D.D.: methodology, writing—original draft, writing—review and editing; C.R.T.: data curation, software, writing—review and editing; M.M.G.S.: data curation, software, writing—review and editing; M.N.: conceptualization, data curation, formal analysis, methodology, supervision, writing—review and editing; I.D.C.: conceptualization, funding acquisition, methodology, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Open access funding provided by the Max Planck Society.
V.H.S. also acknowledges support from the Collaborative Research Grant funded by the Department for the Ecology of Animal Societies, MPI-AB and the Center for the Advanced Study of Collective Behaviour at the University of Konstanz, Deutsche Forschungsgemeinschaft Centre of Excellence 2117 (ID: 422037984). M.N. acknowledges support from the Hungarian Academy of Sciences (a grant to the MTA-ELTE ‘Lendület’ Collective Behaviour Research Group, grant no. 95152). I.D.C. acknowledges support from the Office of Naval Research (grant ONR, N00014-19-1-2556), the Horizon Europe Marie Sklodowska-Curie Actions (860949) and the Max Planck Society.
References
- 1.Rosenthal SB, Twomey CR, Hartnett AT, Wu HS, Couzin ID. 2015. Revealing the hidden networks of interaction in mobile animal groups allows prediction of complex behavioral contagion. Proc. Natl Acad. Sci. USA 112, 4690-4695. ( 10.1073/pnas.1420068112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strandburg-Peshkin A, et al. 2013. Visual sensory networks and effective information transfer in animal groups. Curr. Biol. 23, R709-R711. ( 10.1016/j.cub.2013.07.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhl J, Sumpter DJ, Couzin ID, Hale JJ, Despland E, Miller ER, Simpson SJ. 2006. From disorder to order in marching locusts. Science 312, 1402-1406. ( 10.1126/science.1125142) [DOI] [PubMed] [Google Scholar]
- 4.Romanczuk P, Couzin ID, Schimansky-Geier L. 2009. Collective motion due to individual escape and pursuit response. Phys. Rev. Lett. 102, 010602. ( 10.1103/PhysRevLett.102.010602) [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulou M, Hildenbrandt H, Sankey DWE, Portugal SJ, Hemelrijk CK. 2022. Self-organization of collective escape in pigeon flocks. PLoS Comput. Biol. 18, e1009772. ( 10.1371/journal.pcbi.1009772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sankey DW, Shepard EL, Biro D, Portugal SJ. 2019. Speed consensus and the ‘Goldilocks principle'in flocking birds (Columba livia). Anim. Behav. 157, 105-119. ( 10.1016/j.anbehav.2019.09.001) [DOI] [Google Scholar]
- 7.Papadopoulou M, Hildenbrandt H, Sankey DWE, Portugal SJ, Hemelrijk CK. 2022. Emergence of splits and collective turns in pigeon flocks under predation. R. Soc. Open Sci. 9, 211898. ( 10.1098/rsos.211898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagy M, Ákos Z, Biro D, Vicsek T. 2010. Hierarchical group dynamics in pigeon flocks. Nature 464, 890-893. ( 10.1038/nature08891) [DOI] [PubMed] [Google Scholar]
- 9.Cavagna A, Cimarelli A, Giardina I, Parisi G, Santagati R, Stefanini F, Viale M. 2010. Scale-free correlations in starling flocks. Proc. Natl Acad. Sci. USA 107, 11 865-11 870. ( 10.1073/pnas.1005766107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballerini M, et al. 2008. Interaction ruling animal collective behavior depends on topological rather than metric distance: evidence from a field study. Proc. Natl Acad. Sci. USA 105, 1232-1237. ( 10.1073/pnas.0711437105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Procaccini A, et al. 2011. Propagating waves in starling, Sturnus vulgaris, flocks under predation. Anim. Behav. 82, 759-765. ( 10.1016/j.anbehav.2011.07.006) [DOI] [Google Scholar]
- 12.Ákos Z, Beck R, Nagy M, Vicsek T, Kubinyi E. 2014. Leadership and path characteristics during walks are linked to dominance order and individual traits in dogs. PLoS Comput. Biol. 10, e1003446. ( 10.1371/journal.pcbi.1003446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strandburg-Peshkin A, Papageorgiou D, Crofoot MC, Farine DR. 2018. Inferring influence and leadership in moving animal groups. Phil. Trans. R. Soc. B 373, 20170006. ( 10.1098/rstb.2017.0006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bierbach D, Mönck HJ, Lukas J, Habedank M, Romanczuk P, Landgraf T, Krause J. 2020. Guppies prefer to follow large (robot) leaders irrespective of own size. Front. Bioeng. Biotechnol. 8, 441. ( 10.3389/fbioe.2020.00441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A. 2017. Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr. Biol. 27, 2862-2868.e7. ( 10.1016/j.cub.2017.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettit B, Ákos Z, Vicsek T, Biro D. 2015. Speed determines leadership and leadership determines learning during pigeon flocking. Curr. Biol. 25, 3132-3137. ( 10.1016/j.cub.2015.10.044) [DOI] [PubMed] [Google Scholar]
- 17.Watts I, Pettit B, Nagy M, de Perera TB, Biro D. 2016. Lack of experience-based stratification in homing pigeon leadership hierarchies. R. Soc. Open Sci. 3, 150518. ( 10.1098/rsos.150518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conradt L, Krause J, Couzin ID, Roper TJ. 2009. ‘Leading according to need’ in self-organizing groups. Am. Nat. 173, 304-312. ( 10.1086/596532) [DOI] [PubMed] [Google Scholar]
- 19.Averly B, Sridhar VH, Demartsev V, Gall G, Manser M, Strandburg-Peshkin A. 2022. Disentangling influence over group speed and direction reveals multiple patterns of influence in moving meerkat groups. Scient. Rep. 12, 13844. ( 10.1038/s41598-022-17259-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bode NWF, Franks DW, Wood AJ. 2012. Leading from the front? Social networks in navigating groups. Behav. Ecol. Sociobiol. 66, 835-843. ( 10.1007/s00265-012-1331-6) [DOI] [Google Scholar]
- 21.Pettit B, Perna A, Biro D, Sumpter DJT. 2013. Interaction rules underlying group decisions in homing pigeons. J. R. Soc. Interface. 10, 20130529. ( 10.1098/rsif.2013.0529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, Abraira VE, Adams RP, Datta SR. 2015. Mapping sub-second structure in mouse behavior. Neuron 88, 1121-1135. ( 10.1016/j.neuron.2015.11.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephens GJ, Bueno de Mesquita M, Ryu WS, Bialek W. 2011. Emergence of long timescales and stereotyped behaviors in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 108, 7286-7289. ( 10.1073/pnas.1007868108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berman GJ, Choi DM, Bialek W, Shaevitz JW. 2014. Mapping the stereotyped behaviour of freely moving fruit flies. J. R. Soc. Interface 11, 20140672. ( 10.1098/rsif.2014.0672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz Y, Tunstrom K, Ioannou CC, Huepe C, Couzin ID. 2011. Inferring the structure and dynamics of interactions in schooling fish. Proc. Natl Acad. Sci. USA 108, 18 720-18 725. ( 10.1073/pnas.1107583108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbert-Read JE. 2016. Understanding how animal groups achieve coordinated movement. J. Exp. Biol. 219, 2971-2983. ( 10.1242/jeb.129411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacGregor HEA, Ioannou CC. 2021. Collective motion diminishes, but variation between groups emerges, through time in fish shoals. R. Soc. Open Sci. 8, 210655. ( 10.1098/rsos.210655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bumann D, Krause J. 1993. Front individuals lead in shoals of three-spined sticklebacks (Gasterosteus aculeatus) and juvenile roach (Rutilus rutilus). Behaviour 125, 189-198. ( 10.1163/156853993X00236) [DOI] [Google Scholar]
- 29.Davidson JD, Sosna MMG, Twomey CR, Sridhar VH, Leblanc SP, Couzin ID. 2021. Collective detection based on visual information in animal groups. J. R. Soc. Interface 18, 20210142. ( 10.1098/rsif.2021.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy M, Vásárhelyi G, Pettit B, Roberts-Mariani I, Vicsek T, Biro D. 2013. Context-dependent hierarchies in pigeons. Proc. Natl Acad. Sci. USA 110, 13 049-13 054. ( 10.1073/pnas.1305552110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jolles JW, Sosna MM, Mazué GP, Twomey CR, Bak-Coleman J, Rubenstein DI, Couzin ID. 2022. Both prey and predator features predict the individual predation risk and survival of schooling prey. eLife 11, e76344. ( 10.7554/eLife.76344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calovi DS, Lopez U, Ngo S, Sire C, Chaté H, Theraulaz G. 2014. Swarming, schooling, milling: phase diagram of a data-driven fish school model. New J. Phys. 16, 015026. ( 10.1088/1367-2630/16/1/015026) [DOI] [Google Scholar]
- 33.Poel W, Daniels BC, Sosna MMG, Twomey CR, Leblanc SP, Couzin ID, Romanczuk P. 2022. Subcritical escape waves in schooling fish. Sci. Adv. 8, eabm6385. ( 10.1126/sciadv.abm6385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usherwood JR, Stavrou M, Lowe JC, Roskilly K, Wilson AM. 2011. Flying in a flock comes at a cost in pigeons. Nature 474, 494-497. ( 10.1038/nature10164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flack A, Guilford T, Biro D. 2014. Learning multiple routes in homing pigeons. Biol. Lett. 10, 20140119. ( 10.1098/rsbl.2014.0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos CD, Neupert S, Lipp H-P, Wikelski M, Dechmann DKN. 2014. Temporal and contextual consistency of leadership in homing pigeon flocks. PLoS ONE 9, e102771. ( 10.1371/journal.pone.0102771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Portugal SJ, Ricketts RL, Chappell J, White CR, Shepard EL, Biro D. 2017. Boldness traits, not dominance, predict exploratory flight range and homing behaviour in homing pigeons. Phil. Trans. R. Soc. B 372, 20160234. ( 10.1098/rstb.2016.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valentini G, Pavlic TP, Walker SI, Pratt SC, Biro D, Sasaki T. 2021. Naïve individuals promote collective exploration in homing pigeons. eLife 10, e68653. ( 10.7554/eLife.68653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jhawar J, Morris RG, Amith-Kumar UR, Raj MD, Rogers T, Rajendran H, Guttal V. 2020. Noise-induced schooling of fish. Nat. Phys. 16, 488-493. ( 10.1038/s41567-020-0787-y) [DOI] [Google Scholar]
- 40.Mones E, Vicsek L, Vicsek T. 2012. Hierarchy measure for complex networks. PLoS ONE 7, e33799. ( 10.1371/journal.pone.0033799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reebs SG, Leblond C. 2006. Individual leadership and boldness in shoals of golden shiners (Notemigonus crysoleucas). Behaviour 143, 1263-1280. ( 10.1163/156853906778691603) [DOI] [Google Scholar]
- 42.Reebs SG. 2000. Can a minority of informed leaders determine the foraging movements of a fish shoal? Anim. Behav. 59, 403-409. ( 10.1006/anbe.1999.1314) [DOI] [PubMed] [Google Scholar]
- 43.Schaerf TM, Herbert-Read JE, Myerscough MR, Sumpter DJT, Ward AJW. 2016. Identifying differences in the rules of interaction between individuals in moving animal groups. arXiv, 1601.08202 [q-bio]. ( 10.48550/arXiv.1601.08202) [DOI]
- 44.Harpaz R, Tkačik G, Schneidman E. 2017. Discrete modes of social information processing predict individual behavior of fish in a group. Proc. Natl Acad. Sci. USA 114, 10 149-10 154. ( 10.1073/pnas.1703817114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang W, et al. 2020. Genetic control of collective behavior in zebrafish. iScience 23, 100942. ( 10.1016/j.isci.2020.100942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mann RP, Perna A, Strömbom D, Garnett R, Herbert-Read JE, Sumpter DJ, Ward AJ. 2013. Multi-scale inference of interaction rules in animal groups using Bayesian model selection. PLoS Comput. Biol. 9, e1002961. ( 10.1371/journal.pcbi.1002961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pérez-Escudero A, de Polavieja GG. 2011. Collective animal behavior from Bayesian estimation and probability matching. PLoS Comput. Biol. 7, e1002282. ( 10.1371/journal.pcbi.1002282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torney CJ, Lamont M, Debell L, Angohiatok RJ, Leclerc LM, Berdahl AM. 2018. Inferring the rules of social interaction in migrating caribou. Phil. Trans. R. Soc. B 373, 20170385. ( 10.1098/rstb.2017.0385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lord WM, Sun J, Ouellette NT, Bollt EM. 2016. Inference of causal information flow in collective animal behavior. IEEE Trans. Mol. Biol. Multi-Scale Commun. 2, 107-116. ( 10.1109/TMBMC.2016.2632099) [DOI] [Google Scholar]
- 50.Twomey CR, Hartnett AT, Sosna MMG, Romanczuk P. 2021. Searching for structure in collective systems. Theory Biosci. 140, 361-377. ( 10.1007/s12064-020-00311-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amornbunchornvej C, Berger-Wolf T. 2021. Framework for inferring leadership dynamics of complex movement from time series. DeepAI. arXiv, 2104.02291v1 [cs.SI]. ( 10.48550/arXiv.2104.02291). See https://deepai.org/publication/framework-for-inferring-leadership-dynamics-of-complex-movement-from-time-series. [DOI]
- 52.Zaccaro SJ, Klimoski RJ. 2001. The nature of organizational leadership: understanding the performance imperatives confronting today's leaders. San Francisco, CA: Jossey-Bass/John Wiley & Sons. [Google Scholar]
- 53.DeChurch LA, Hiller NJ, Murase T, Doty D, Salas E. 2010. Leadership across levels: levels of leaders and their levels of impact. Leadersh. Q. 21, 1069-1085. ( 10.1016/j.leaqua.2010.10.009) [DOI] [Google Scholar]
- 54.Chun JU, Yammarino FJ, Dionne SD, Sosik JJ, Moon HK. 2009. Leadership across hierarchical levels: multiple levels of management and multiple levels of analysis. Leadersh. Q. 20, 689-707. ( 10.1016/j.leaqua.2009.06.003) [DOI] [Google Scholar]
- 55.Lysova EI, Allan BA, Dik BJ, Duffy RD, Steger MF. 2019. Fostering meaningful work in organizations: a multi-level review and integration. J. Vocat. Behav. 110, 374-389. ( 10.1016/j.jvb.2018.07.004) [DOI] [Google Scholar]
- 56.Smith JE, et al. 2016. Leadership in mammalian societies: emergence, distribution, power, and payoff. Trends Ecol. Evol. 31, 54-66. ( 10.1016/j.tree.2015.09.013) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data to support the findings of this study are available with other published papers. Code availability: all analyses were run using custom code written in CUDA and Python and are available in the GitHub repository (https://doi.org/10.5281/zenodo.7113335).
The data are also provided in the electronic supplementary material.