Abstract
Changes in behaviour over the lifetime of single-cell organisms have primarily been investigated in response to environmental stressors. However, growing evidence suggests that unicellular organisms undergo behavioural changes throughout their lifetime independently of the external environment. Here we studied how behavioural performances across different tasks vary with age in the acellular slime mould Physarum polycephalum. We tested slime moulds aged from 1 week to 100 weeks. First, we showed that migration speed decreases with age in favourable and adverse environments. Second, we showed that decision making and learning abilities do not deteriorate with age. Third, we revealed that old slime moulds can recover temporarily their behavioural performances if they go throughout a dormant stage or if they fuse with a young congener. Last, we observed the response of slime mould facing a choice between cues released by clone mates of different age. We found that both old and young slime moulds are attracted preferentially toward cues left by young slime moulds. Although many studies have studied behaviour in unicellular organisms, few have taken the step of looking for changes in behaviour over the lifetime of individuals. This study extends our knowledge of the behavioural plasticity of single-celled organisms and establishes slime moulds as a promising model to investigate the effect of ageing on behaviour at the cellular level.
This article is part of a discussion meeting issue ‘Collective behaviour through time’.
Keywords: ageing, learning, decision making, Physarum polycephalum, slime moulds
1. Introduction
One aim of this special issue of Philosophical Transactions is to understand collective behaviour over the lifetime of individuals. In unicellular organisms, changes in collective behaviour through time have been studied almost exclusively in response to environmental stressors. For instance, it has been shown that bacteria [1,2] and cellular slime moulds [3–5] transit to new collective states such as coordinated cellular migration or aggregation when nutrients become scarce [6]. On the contrary, how collective behaviour changes with correlates of age in unicellular organisms independently of the external environment remains an open question. The main reason for this might be that single-cell organisms were mistakenly believed to be short-lived and immune to ageing under optimum growth conditions [7–11]. It has now been demonstrated that some unicellular organisms, such as bacteria Escherichia coli and Caulobacter crescentus, ciliates Paramecium and Tetrahymena, yeast Saccharomyces cerevisiae, and cellular and acellular slime moulds Dictyostelium discoideum and Physarum polycephalum, undergo intrinsic changes over time that affect cell behaviour and physiology [11–13]. In this paper, our aim is to understand how cell behaviour varies over the lifetime of individuals in the acellular slime mould P. polycephalum. In contrast with the cellular slime mould D. discoideum, in which the vegetative state consists of solitary amoebas that divide about every 4 h when food is plentiful, in P. polycephalum the vegetative state is a unique large cell containing a large collection of nuclei that divide about every 8 h and share a common cytoplasm. Acellular slime moulds are particularly attractive organisms in which to study collective behaviour changes over time in unicellular organisms for several reasons.
First of all, although they are not collective per se, acellular slime moulds embrace most of the key principles of collective behaviour (reviewed in [14]): synchronization and coordination [15–18], positive and negative feedbacks [19], variability [20–22], symmetry breaking [20,23], phase transition [24], redundancy [14], information transfer [25,26], and capacity to make complex decisions [27–30]. In addition, owing to their internal dynamics, they are often regarded as a collection of nonlinear oscillators that are interconnected, often coupled and partially dependent on each other (reviewed in [16]). Furthermore, similarly to more traditional model systems in collective behaviour such as ant colonies or fish schools, their behaviours have successfully been simulated using a multi-agent approach [31,32]. In these models, their behaviours rely on particle-like agents that follow simple rules but are capable collectively to generate self-organized amoeboid movement and to construct optimized networks [31,32].
Second, despite being unicellular, acellular slime moulds can produce seemingly complex behaviours. Indeed, since the seminal contribution of Toshiyuki Nakagaki and colleagues more than 20 years ago [33], P. polycephalum has become an essential model organism for studying problem-solving in non-neural systems [32–37]. Past experiments have shown that acellular slime moulds can find the shortest path in a maze [33,38], build optimized networks to connect several food sources [34], anticipate events [39], learn to ignore irrelevant stimuli [40,41], encode memory in their environment [42] or in their morphology [43], interact with their congeners [21,44], optimize nutrient intake [28,45], make optimal decisions [27,29,46], etc. Physarum polycephalum's behaviour relies on its self-organized internal architecture, which consists of a transport network of interconnected tubes [47]. The tubes contract and relax periodically, causing the cytoplasm to flow back and forth, a phenomenon termed ‘shuttle streaming’ [48]. Physarum polycephalum can migrate at a speed of up to few millimetres per hour through the interplay of intracellular flow, adhesion and rhythmic contractions of the tubes [48–50]. The frequency and the amplitude of the contractions depend on external stimuli, and, as a result, P. polycephalum is capable of altering its shape and motion as a function of a variety of external stimuli such as chemicals [51–53], light [54], temperature [55–57], humidity [44], gravity [58] or substrate distortion [46].
Third, acellular slime moulds are easy to track through time and relatively long-lived. In unicellular organisms, lifetime can either be chronological if we consider the time a cell remains viable before dividing, or replicative if we consider the number of times a cell divides before it dies [9]. Tracking division is usually difficult as it requires following and distinguishing individual cells. Physarum polycephalum offers the possibility to consider both chronological and replicative time simultaneously. During its development, the zygote undergoes synchronous mitotic divisions every 8–12 h but without cytokinesis to form a single multi-nucleated cell, called a plasmodium [59,60]. Hence, the cell grows over time but there is no cell division. A plasmodium reared on solid medium by routine serial subculture under laboratory conditions can be maintained from a few weeks to several months [61,62]. The lifespan of the plasmodium is in part genetically determined as different strains have different lifespans, and subclones derived from a single plasmodium age in a coordinated manner, i.e. death occurs at approximately the same time [61,63,64].
Fourth, P. polycephalum provides unique possibilities for experimental manipulations [65,66]. The plasmodium can extend up to hundreds of square centimetres. It can be severed into viable and structurally similar yet smaller plasmodia. Upon contact, genetically identical plasmodia can fuse with each other to form a single plasmodium. In response to environmental stressors, the plasmodium can enter a resting stage called sclerotium and remain dormant for years without deterioration. During the sclerotization process, the plasmodia lose 50% of their total protein content together with 40% of their DNA and 65% of their RNA [59]. After transfer to a humid and nutritive medium, a sclerotium can revert to a mobile plasmodium within 24 h. Plasmodial cultures can be easily initiated from sclerotia after up to 3 years. Interestingly, numerous authors have reported that a sclerotium issued from a seemingly ageing plasmodium may produce a vigorous plasmodium again, a form of ‘rejuvenescence’. Following a period of growth, this secondary plasmodium will undergo senescence again. This cycle of growth and senescence can be repeated several times and, thus, cultures can be maintained over many generations.
Last, P. polycephalum displays senescence, which is defined as a loss of fitness during ageing. A plasmodium can be maintained for months, during which no change is apparent until a reproducible decline in viability occurs [61]. Senescence in the plasmodial stage is characterized by morphological change [61], reduction of growth rate [61,67], reduced cytoplasmic flow [63], loss of yellow pigment [63], accumulation of polyploid nuclei [68], high level of mitochondrial DNA fragmentation [67], decreased replication of mitochondrial DNA [62], and increase in nuclear size and DNA content [68,69]. Senescence ends with the fragmentation of the plasmodium into several small spherical structures, with a concomitant lysis of the plasmodium [63]. Longevity in slime moulds is not affected by most environmental factors, with the exception of high temperature [70]. Senescence in slime moulds has been mainly studied at the cellular level. The potential effects of ageing on the plasmodium behaviour remained to be investigated.
In this paper, we studied how P. polycephalum behaviour changes over time. First, we explored the effect of age on migration speed in nutritive and adverse environments. Second, we investigated if decision making and learning performances changed with age. Third, we tested if older slime moulds were able to recover after a dormant period or after fusing with younger clones. Lastly, we examined if slime moulds were able to discriminate cues from congeners of different age.
2. Methods
(a) . Species studied and rearing conditions
Physarum polycephalum, an acellular slime mould, belongs to the supergroup Amoebozoa and the class Myxomycetes. In nature, slime moulds are found on organic substrates like tree bark or forest soil, where they feed on microorganisms such as bacteria or fungi. The P. polycephalum vegetative morph is a vast multi-nucleated cell named a plasmodium. We used the LU352 strain of P. polycephalum, kindly provided by Professor Dr Wolfgang Marwan (Max Planck Institute for Dynamics of Complex Technical Systems, Magdeburg, Germany). Eighty large plasmodia were initiated from sclerotia at different times to obtain slime moulds of different age. In this paper, by 'age' of the slime mould, we understand the time that has elapsed since the moment of inoculation of a sclerotium onto a nutritive medium. The oldest plasmodia were kept after this study and are still being cultivated in our laboratory. They are currently more than 2 years old and none of them has died so far. In previous reports, plasmodium lifespan has been shown to vary from one month to 1 year, depending on the strain [61–63]. Hence, we considered as ‘old’, slime moulds that were over 1 year old and ‘very-old’ slime moulds were those that were almost 2 years old.
Plasmodia were reared on a 1% w/v (weight/volume) agar medium with rolled oat flakes (Quaker Oats Company) in Petri dishes (90 mm Ø). They were kept in the dark in a thermoregulated chamber at a temperature of 20°C and a humidity of 80% from Monday to Friday. They were transferred every day onto a new agar medium on which was spread a single layer of rolled oat flakes. During the weekends, slime moulds were unfed and kept at 12°C. One day before each experiment, all plasmodia were transferred onto a food medium consisting of finely ground oat flakes (5% w/v) mixed with 1% agar gel, hereafter referred as ‘oat gel’.
(b) . Migration speed on various substrates as a function of slime moulds' age
The aim of this experiment was to investigate how age affects slime moulds' migration speed on various substrates (figure 1a). Six groups of 10 large plasmodia of different ages (1, 17, 32, 54, 74 and 94 weeks old) were used to conduct this experiment. Circular samples (10 mm Ø) were cut from each plasmodium and gently placed in contact with an oat gel bridge (35 mm long, 10 mm wide) in an experimental arena (Petri dish 12 × 12 mm). The bridge either contained an aversive substance: NaCl (100 mM, 0.6% w/v) (oat + NaCl bridge) or NaNO3 (100 mM, 0.8% w/v) (oat + NaNO3 bridge) or did not (oat). Experimental arenas housed eight bridges and were kept in a thermoregulated chamber at a temperature of 25°C. After about 8 h of incubation, the distance travelled by the slime moulds on each bridge was measured with a ruler. We computed the migration speed as the distance travelled divided by the time spent in contact with the bridge. A minimum of 48 samples were tested for each substrate and each age group, leading to total of 2396 assays.
Figure 1.
Experimental design. (a) Effect of various substrates (nutritive substrate: 5% oat gel; aversive substrates: 0.6% NaCl + oat gel; 0.8% NaNO3 + oat gel) on migration speed as a function of slime mould age (six groups of different ages were tested, from 1 to 94 weeks old). (b) Effect of temperature (standard temperature: 25°C; extreme temperatures: 10 and 38.5°C) on migration speed as a function of slime mould age (five groups of different ages were tested, from 8 to 101 weeks old). (c) Decision making under nutritive (2.5% oat gel versus 5% oat gel) and aversive conditions (0.4% NaCl + 5% oat gel versus 0.6% NaCl + 5% oat gel, 0.4% NaNO3 + 5% oat gel versus 0.6% NaNO3 + 5% oat gel) as a function of slime mould age (five groups of different ages were tested, from 6 to 99 weeks old). (d) Habituation to an aversive substance (0.6% NaCl) as a function of slime mould age (two groups of different ages were tested: young slime moulds of 12 weeks and old ones 74 weeks old). (e) Effect of dormancy on migration speed on a neutral (agar gel) and nutritive substrate (oat gel) as a function of slime mould age (two groups of different ages were tested: old slime moulds of 60 weeks and very old ones 100 weeks old). (f) Fusion ability as a function of slime mould age (two groups of different ages were tested: relatively young slime moulds of 18 weeks and old ones 75 weeks old). (g) Effect of fusion on migration speed on a nutritive substrate (oat gel) as a function of slime mould age (two groups of different ages were tested: young slime moulds of 5 weeks and old ones 54 weeks old). (h) Detection of chemical cues left by conspecifics (young and old) as a function of slime mould age (two groups of different ages were tested: young slime moulds of 3 weeks and old ones 52 weeks old). (Online version in colour.)
(c) . Migration speed at different temperatures as a function of slime moulds' age
Slime moulds have been shown to suffer both from low and from high temperatures, which affect the shuttle streaming and therefore locomotion [71]. The aim of this experiment was to investigate how age affects slime moulds' migration speed under different temperatures (figure 1b). Five groups of 10 large plasmodia of different ages (8, 24, 61, 81 and 101 weeks old) were used to conduct this experiment. Circular samples (10 mm Ø) were cut from each plasmodium and gently placed in contact with an oat gel bridge (35 mm long, 10 mm wide) in an experimental arena (Petri dish 12 × 12 mm). Experimental arenas housed eight bridges and were kept in thermoregulated chambers at a temperature of 10, 25 or 38.5°C. Twenty-five degrees celsius is a standard temperature to rear P. polycephalum, while 10 and 38.5°C represent extreme conditions. After about 8 h of incubation, the distance travelled by the slime moulds on each bridge was measured with a ruler. We computed the migration speed as the distance travelled divided by the time spent in contact with the bridge. Numerous slime moulds did not move at all during the experiments at extreme temperatures (10 or 38.5°C); they were not taken into account to compute speed, but we calculated the proportion of motionless slime moulds for each age group and each temperature. A minimum of 80 samples were tested for each temperature and each age group, leading to total of 3533 assays.
(d) . Decision making ability as a function of slime moulds' age
Slime moulds have been shown to succeed in selecting the best option when offered multiple alternatives [22,27,45,72]. To investigate how age affects slime moulds' ability to make a decision, we studied how a slime mould distributed itself between two agar gel bridges containing or not an aversive substance (NaCl or NaNO3) (figure 1c). Under non-aversive conditions, the slime moulds were offered a choice between a high-quality oat gel (5% w/v) and a low-quality one (2.5% w/v). Under aversive conditions, the slime moulds were given a choice between a high-aversive option (0.6% w/v NaCl or NaNO3) and a low-aversive option (0.4% w/v NaCl or NaNO3). Five groups of 10 large plasmodia of different ages (6, 22, 59, 79 and 99 weeks old) were used to conduct this experiment. Circular samples (10 mm Ø) were cut from each plasmodium, and placed between two bridges (35 mm long, 10 mm wide) in experimental arenas (Petri dish 12 × 12 mm). Experimental arenas housed four binary choices and were kept in a thermoregulated chamber at a temperature of 25°C. After about 8 h of incubation, the distance travelled (in millimetres) on each bridge was measured with a ruler. We computed a decision-making performance as the distance covered on the better bridge (high-quality or low-aversive substrate) divided by the total distance. A minimum of 235 samples were tested for each age and each binary choice, leading to total of 4206 assays.
(e) . Learning ability as a function of slime moulds' age
Slime moulds have been shown to be capable of habituation, a simple form of learning, when repeatedly exposed to an innocuous aversive substance [40,73]. To investigate how age affects slime moulds' ability to learn, we studied how slime moulds habituated to an aversive substance [25,40]. A bridge-crossing experiment adapted from [40] was conducted to habituate the slime mould to an aversive substance (NaCl 100 mM, 0.6% w/v) (figure 1d). We used two groups of 10 large plasmodia of two different ages, 12 and 74 weeks old. Before starting the habituation experiment, to accustom the slime moulds to the experimental set-up, 10 circular samples (10 mm Ø) were cut from each plasmodium and introduced into an experimental arena (Petri dish 12 × 12 mm). The samples were then connected to an oat gel bridge (35 mm long, 10 mm wide). After a day, the slime moulds had covered the bridge. A circular sample was then cut from each bridge and transferred to a new experimental arena to start the habituation. On day 1 of the habituation, half of the samples were offered an oat gel bridge containing NaCl (substrate: oat + NaCl; treatment: habituated; N = 50 for each age group) while the remaining half had to cross a bridge without NaCl (substrate: oat; treatment: control; N = 50 for each age group). The following two days (day 2 and day 3), a circular sample was cut from each bridge daily and transferred to another arena, where it was offered a new bridge. On day 4, once the habituation training was completed, we cut one or two circular samples from each bridge and transferred them to a new arena. Half of the habituated and control samples were offered a bridge containing NaCl (substrate: oat + NaCl) while the remaining half had to cross a bridge without NaCl (substrate: oat). Thus, on day 4, the control group was either facing a bridge containing NaCl for the first time (N = 30 for each age group) or a bridge without NaCl (N = 30 for each age group) while the habituated group was either facing a bridge containing NaCl for the fourth time (N = 30 for each age group) or a bridge without NaCl (N = 30 for each age group). Every day, after about 8 h of incubation, the distance travelled (in millimetres) on each bridge was measured with a ruler. Data collected on day 1 allowed us to confirm that slime moulds showed a clear aversive behaviour toward the NaCl, while data collected on day 4 enabled us to test if slime mould learned to ignore the aversive substance. On day 4, we synthesized the results with an aversion index HI and CI as in [41]:
HON, CON, HO and CO are the habituated (H) and control slime moulds (C) on oat + NaCl (ON) and oat (O), respectively. Using those indexes allowed us to normalize each variable value corresponding to the treatments HON and CON by the mean and the s.d. of their respective controls HO and CO. Values clearly above zero indicate an aversion towards the repellent, whereas values close to zero indicate habituation to the repellent, i.e. slime moulds react the same way to oat + NaCl and oat.
(f) . Recovery ability following dormancy as a function of slime moulds' age
To investigate how age affects slime moulds' ability to recover, we studied how fast old slime moulds migrated after a dormancy period (figure 1e). We used two groups of 10 large plasmodia of two different ages, 60 and 100 weeks old, referred as ‘old’ and ‘very old’. To initiate the transition from plasmodia to sclerotia, we cut two circular samples from each plasmodium (Ø = 20 mm), placed each of them on a moist paper filter (Ø = 145 mm) in a Petri dish (Ø = 145 mm) and kept them in a thermoregulated chamber at a temperature of 30°C for 2 days to dry. The sclerotia were then stored for a week before being reinitiated to obtain new plasmodia. A week later, we then had 40 large plasmodia, 20 were 1 week old and originated from old and very old plasmodia (treatment: dormancy) and 20 were old and very old (treatment: no dormancy). Circular samples (Ø = 10 mm) were cut from each plasmodium and gently placed in contact with an oat gel bridge (nutritive substrate) or an agar gel bridge (agar 1% w/v, non-nutritive substrate) in an experimental arena (Petri dish 12 × 12 mm). Experimental arenas housed eight bridges and were kept in a thermoregulated chamber at a temperature of 25°C. After about 8 h of incubation, the distance travelled by the slime moulds on each bridge was measured with a ruler. We computed the migration speed as the distance travelled divided by the time spent in contact with the bridge. A minimum of 80 samples were tested for each age group (old, very old) and each treatment (dormancy or not), leading to total of 640 assays.
(g) . Fusion as a function of slime moulds' age
Fusion constitutes a defining feature of the lifestyle of slime moulds. To investigate how age affects slime moulds' ability to fuse, we studied how fast two slime moulds fused as function of their respective age (figure 1f). We used two groups of 10 large plasmodia of two different ages, 18 and 75 weeks old, referred as ‘young’ and ‘old’. Circular samples (10 mm Ø) were cut from each plasmodium and gently placed in contact with an agar gel bridge (15 mm long, 15 mm wide) in an experimental arena (Petri dish 12 × 12 mm). We placed a sample at each extremity of the bridge and tested the following pairs: young/young (N = 220), young/old (N = 330) and old/old (N = 220). Experimental arenas housed eight pairs of slime moulds and were kept in a thermoregulated dark room at a temperature of 21°C. We conducted behavioural scan observations using a flash light to record the latency to membrane and vein fusion. Membranes were considered as fused when we could not see the delimitation between the two slime moulds, while veins were considered as fused when we could discern a vein going from one slime mould to the other. The latency between two scans was less than 5 min. Pairs that did not fuse after 9 h or that fused with their neighbouring slime moulds in the arenas were removed from the analysis (young/young: 26 out of 220; young/old: 25 out of 330; old/old: 18 out of 220).
(h) . Recovery ability following fusion as a function of slime moulds' age
Slime moulds' fusion could provide the potential for cell recovery through resource sharing. Recently, it was shown that a learned behaviour can be transferred during fusion [25]. In this experiment, we measured migration speed after fusion as a function of slime moulds' age (figure 1g). We used two groups of 10 large plasmodia of two different ages, 5 and 54 weeks old, referred as ‘young’ and ‘old’. Circular samples (10 mm Ø) were cut from each plasmodium and gently placed by pairs in an experimental arena (Petri dish 12 × 12 mm). Each experimental arena housed four pairs. We tested the following pairs: young/young (N = 100), young/old (N = 100) and old/old (N = 100). Fusion was allowed by bringing the slime moulds into contact for 3 h (see [25]). After the fusion, the slime moulds were gently separated at the point of contact using a spatula. Then, all slime moulds were required to cross an oat gel bridge (35 mm long, 10 mm wide). Experimental arenas housed eight bridges and were kept in a thermoregulated chamber at a temperature of 25°C. After about 8 h of incubation, the distance travelled by the slime moulds on each bridge was measured with a ruler.
(i) . Detection of conspecifics as a function of slime moulds' age
Slime moulds can sense and respond to chemical cues released in the environment by conspecifics in a foraging context [21,44]. They release calcium while foraging, which is attractive to other slime moulds [21]. In this experiment, we monitored the directional movement response evoked in slime moulds in the presence of substrates previously explored by clone mates of different ages (figure 1h). We used two groups of 10 large plasmodia of two different ages, 3 and 52 weeks old, referred as ‘young’ and ‘old’. Circular Petri dishes (Ø = 90 mm) containing a layer of 1% w/v agar gel were used as experimental arenas. Once the agar in the Petri dish had set, three holes were punched (Ø = 10 mm, distance apart = 20 mm). One hole was filled with a young or an old slime mould (Ø = 10 mm) sitting on 5% w/v oat gel. The other two holes were filled with experimental substrates (Ø = 10 mm), which consisted of agar gels explored for 24 h by well-fed young and old slime moulds. To obtain the experimental substrates, a slime mould was allowed to cover completely an agar gel while being fed with oat flakes (making sure that the food was never in contact with the agar substrate; see [21] for details). Then we removed the slime mould, and rinsed the agar substrate with distilled water just before the experiment. During the experiment, the slime mould would typically explore its environment by expanding in all directions for a short distance, to finally migrate in a specific direction, eventually contacting one experimental substrate. The experimental substrate that was reached first was taken to imply a positive response (i.e. a relative preference for the cue enclosed in the experimental substrate over the alternative). For each assay, we recorded which substrate was contacted first and the latency to reach each substrate. We replicated each binary choice 300 times with old and young slime moulds. Experiments were recorded using digital cameras (EOS 70D, Canon), which took a picture every 5 min.
(j) . Statistical analyses
All statistics were performed with RStudio (version 1.2.1335). To assess the difference in the various parameters measured between the treatments, we used linear mixed models (LMMs) or generalized mixed models (GLMMs) (function lmer or glmer). The package lme4 [74] was used for all mixed models. The lmerTest and Car packages were then used to run Type III analysis of variance on the calculated LMMs and GLMMs, extracting p-values for F-tests and Wald-tests. Assumptions for all LMMs were checked using standard procedure: diagnostic of quantile–quantile normal plots and Shapiro–Wilk test. The models were fitted by specifying the fixed effects (explanatory variables) depending on the experiment and a random effect: the plasmodium identity. Dependent variables that did not fit linear model requirements were transformed using the ‘bestNormalize’ function (bestNormalize package). Continuous variables were centred and scaled if needed. The outcomes of all the models are presented in the electronic supplementary material, tables S1–S13.
3. Results
(a) . Migration speed on various substrates as a function of slime moulds' age
In the first experiment, we investigated if slime moulds' migration speed on different substrates is affected by age. Slime moulds migrated faster on plain oat gel than on oat gels containing an aversive substance (LMM, substrate F = 67.01, p < 0.001; electronic supplementary material, table S1; figure 2). For all substrates, migration speed decreased as age increased (LMM, age F = 80.52, p < 0.001; electronic supplementary material, table S1; figure 2). On plain oat gel, migration speed declined drastically until the slime moulds were 30 weeks old, then decreased gently. On oat gel containing NaNO3, migration speed reached a plateau when the slime moulds were over 30 weeks old. On oat gel containing NaCl, migration speed declined continuously with age (LMM age × substrate F = 28.08, p < 0.001; electronic supplementary material, table S1; figure 2). Hence, NaNO3 was the more aversive substance for young slime moulds while for old slime moulds it was NaCl.
Figure 2.
Mean migration speed as a function of age and substrate. Six groups of 10 large plasmodia of different ages (1, 17, 32, 54, 74 and 94 weeks old) were used to conduct this experiment. The substrates were made of oat gel (5% w/v) containing one of two aversive substances (oat + NaCl (0.6% w/v), oat + NaNO3 (0.8% w/v)) or not (oat). N > 48 for each age and each substrate. Total number of assays = 2396. Error bars indicate 95% CI. (Online version in colour.)
(b) . Migration speed at different temperatures as a function of slime moulds' age
In the second experiment, we examined if slime moulds' migration speed was affected by age at different temperatures. Slime moulds migrated faster at standard temperature than at cold or hot temperature (GLMM, temperature χ2 = 4724.85 p < 0.001; electronic supplementary material, table S2; figure 3). As previously, at standard temperature, migration speed declined drastically with age at the beginning then decreased gently when the slime moulds were over 25 weeks old. At both low and high temperatures, the speed was the lowest for young slime moulds (8 weeks old), then increased until the slime moulds reached an intermediate age between 24 and 61 weeks old and finally decreased slightly with age (GLMM, age χ2 = 5.67 p = 0.017, age × temperature χ2 = 43.26 p < 0.001; electronic supplementary material, table S2; figure 3). The proportion of motionless slime moulds was also affected by age (GLMM, age χ2 = 10.11 p < 0.001; electronic supplementary material, table S3; figure 3) and temperature (GLMM, temperature χ2 = 200.47 p < 0.001; electronic supplementary material, table S3; figure 3). The youngest (8 weeks old) and oldest slime moulds (101 weeks old) remained most often motionless at both extreme temperatures, with the youngest being more vulnerable to cold and the oldest to heat (GLMM, age × temperature χ2 = 39.58 p < 0.001; electronic supplementary material, table S3; figure 3).
Figure 3.
Mean migration speed and mean proportion of motionless slime moulds as a function of age and temperature. Five groups of 10 large plasmodia of different ages (8, 24, 61, 81 and 101 weeks old) were used to conduct this experiment. Three temperatures were tested: a standard one (25°C) and two extremes (10 and 38.5°C). The substrates were made of oat gel (5% w/v). All the slime moulds at 25°C migrated on the bridge, so the proportion of motionless slime moulds was equal to zero for all age groups. N > 80 for each age and each temperature. Total number of assays = 3533. Error bars indicate 95% CI. (Online version in colour.)
(c) . Decision-making ability as a function of slime moulds' age
In the third experiment, we estimated slime moulds' ability to make a decision as a function of age and substrate. As previously, slime moulds migrated faster on plain oat gels than on oat gels containing an aversive substance (GLMM, substrate χ2 = 451.80 p < 0.001; electronic supplementary material, table S4) and for all substrates migration speed decreased as age increased (GLMM, age χ2 = 5.91 p < 0.001, age × substrate χ2 = 18.88 p < 0.001; electronic supplementary material, table S4). On average, slime moulds had better performances under non-aversive conditions (GLMM, substrate χ2 = 141.34 p < 0.001; electronic supplementary material, table S5; figure 4). However, under aversive conditions, decision-making performance increased with age, while it remained constant under non-aversive conditions (GLMM, age χ2 = 4.22 p = 0.040, age × substrate χ2 = 111.82 p < 0.001; electronic supplementary material, table S5; figure 4). Hence, young slime moulds had better performances under non-aversive conditions, while it was the contrary for old slime moulds.
Figure 4.
Decision making as a function of age and substrate. Five groups of 10 large plasmodia of different ages (6, 22, 59, 79 and 99 weeks old) were used to conduct this experiment. Under the non-aversive condition (oat), the slime moulds were offered a choice between a high-quality oat gel (5% w/v) and a low-quality one (2.5% w/v). Under aversive conditions (oat + NaCl or Oat + NaNO3), the slime moulds were given a choice between a high-aversive option (0.6% w/v) and a low-aversive option (0.4% w/v). Decision-making performance was computed as the distance covered on the better bridge divided by the total distance. A value equal to 0.5 means that the slime mould distribution is symmetric between the two bridges; a value equal to 1 or 0 means that the slime moulds migrated only on the betert or the worse bridge, respectively. N > 235 for each age and each condition. Total number of assays = 4206. Error bars indicate 95% CI. (Online version in colour.)
(d) . Learning ability as a function of slime moulds' age
In the fourth experiment, we investigated if slime moulds' ability to habituate to an aversive substance depended on age. On day 1, slime moulds migrated faster on plain oat gel than on oat gels containing NaCl (LMM, substrate F = 75.86 p < 0.001; electronic supplementary material, table S6; figure 5a), and migration speed was higher for young than for old slime moulds (LMM, age F = 15.27 p = 0.001, age × substrate F = 3.76 p = 0.054; electronic supplementary material, table S6; figure 5a). On day 4, habituated and control slime moulds were tested for habituation and were required to cross a bridge containing NaCl. Control slime moulds showed strong aversive behaviour and a high aversion index. By contrast, habituated slime moulds, encountering NaCl for the fourth time, showed no aversive behaviour and an aversion index close to zero (LMM, treatment F = 23.54 p < 0.001; electronic supplementary material, table S7; figure 5b). Hence, they learned to ignore the aversive substance. Slime moulds age did not affect their learning performance (LMM, age F = 2.15 p = 0.145, age × treatment F = 1.92 p = 0.169; electronic supplementary material, table S7; figure 5b).
Figure 5.
Learning as a function of age. (a) Migration speed on day 1. Two groups of 10 large plasmodia of two different ages, 12 and 74 weeks old, were used to conduct this experiment. Slime moulds were required to migrate on an oat gel bridge with an aversive substance NaCl (oat + NaCl) (0.6% w/v, habituated treatment) or a plain oat gel bridge (oat) without the repellent (control treatment). Low values of speed indicate an aversive response. (b) Aversion index on day 4 computed after the habituation phase. On day 4, all slime moulds (habituated and control) had to migrate on bridges either with the aversive substance or without. An aversion index close to zero indicates habituation while values clearly above zero indicate an aversion to the repellent. N = 50 for each age (young and old) and each treatment (habituated and control). Total number of assays = 200. Error bars indicate 95% CI. (Online version in colour.)
(e) . Recovery ability as a function of slime moulds' age
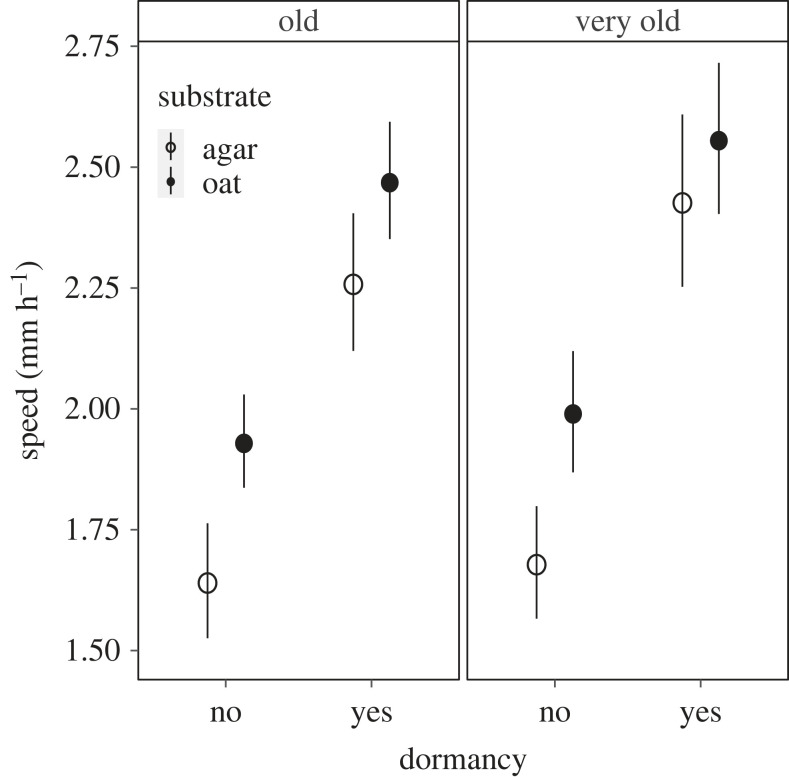
In the fifth experiment, we tested if slime moulds recovered their original migration speed after a dormancy period. Slime moulds migrated faster on oat gels than on agar gels (LMM, substrate F = 30.10 p < 0.001; electronic supplementary material, table S8; figure 6) regardless of age and treatment (LMM, substrate × age F = 0.11 p = 0.741, substrate × treatment F = 2.54 p = 0.111; electronic supplementary material, table S8; figure 6). Slime moulds that were turned into a dormant state and reinitiated were significantly faster than their counterparts that had remained as plasmodia (LMM, treatment F = 59.52 p < 0.001; electronic supplementary material, table S8; figure 6). Age of the dormant slime mould had no effect on speed following dormancy (treatment × age F = 0.09 p = 0.772; electronic supplementary material, table S8; figure 6).
Figure 6.
Mean migration speed following a dormancy period. Two groups of 10 large plasmodia of two different ages, 60 (old) and 100 (very old) weeks old, were used to conduct this experiment. Before the migration speed assays, slime moulds were either turned into sclerotia for a week and reinitiated (dormancy: yes) or kept as plasmodia (dormancy: no). The substrates were made of oat gel 5% w/v (oat) or plain agar gel 1% w/v (agar). N = 80 for each age (old and very old) and each treatment (dormancy yes or no). Total number of assays = 640. Error bars indicate 95% CI.
(f) . Fusion as a function of slime moulds' age
In the sixth experiment, we examined if the fusion process was affected by age. The latency for the membrane to fuse was shorter for pairs of young slime moulds or mixed pairs (young/old) than for pairs of old ones (LMM, age F = 3.63 p = 0.034; electronic supplementary material, table S9; figure 7a). Vein fusion, following membrane fusion, also occurred earlier in pairs of young slime moulds than in the other pairs (LMM, age F = 4.21 p = 0.030, electronic supplementary material, table S10; figure 7b).
Figure 7.
Fusion as a function of age. Two groups of 10 large plasmodia of two different ages, 18 (young) and 75 (old) weeks old, were used to conduct this experiment. Young (old) slime moulds were paired with an individual of the same age or older (younger). The bridge separating the two slime moulds within a pair was made of plain agar gel 1% w/v. (a) Latency to membrane fusion as a function of age. The time of membrane fusion was recorded for each pair. (b) Latency to vein fusion as a function of age. The time of vein connection following membrane fusion was recorded for each pair. N = 198, N = 305 and N = 202 for young/young, young/old and old/old, respectively. Error bars indicate 95% CI. (Online version in colour.)
(g) . Recovery ability following fusion as a function of slime moulds' age
In the fifth experiment, we tested if old slime moulds recovered their original migration speed after fusing with young ones. Young slime moulds were faster than old slime moulds when they were allowed to fuse with a slime mould of the same age (LMM, age F = 19.68.52 p < 0.001; electronic supplementary material, table S11; figure 8). However, old slime moulds that were allowed to fuse with a young one were significantly faster than their counterparts that fused with an old one (LMM, age × pair type F = 33.84 p < 0.001; electronic supplementary material, table S11; figure 8). On the contrary, young ones that were allowed to fuse with an old slime mould were slower than their counterparts that fused with a young one.
Figure 8.
Mean migration speed following fusion. Two groups of 10 large plasmodia of two different ages, 5 (young) and 54 (very old) weeks old, were used to conduct this experiment. Before the migration speed assays, slime moulds were allowed to fuse either with a slime mould of the same age or with a slime mould of a different age. The substrates were made of oat gel 5% w/v (oat). N = 100 for each pair type (young/young, young/old, old/old). Total number of assays = 600. Error bars indicate 95% CI. (Online version in colour.)
(h) . Detection of conspecifics as a function of slime moulds' age
In the last experiment, we investigated if slime moulds could perceive a difference in cues left by slime moulds of different ages. As previously shown, young slime moulds were faster than old ones (GLMM, age χ2 = 14.44 p < 0.001; electronic supplementary material, table S12; figure 9). Both young and old slime moulds contacted first the substrate explored by a young slime mould significantly more often than the substrate explored by an old one (GLMM, observed versus expected: χ2 = 5.67 p = 0.017; electronic supplementary material, table S13; figure 9).
Figure 9.
Detection of conspecifics as a function of age. Two groups of 10 large plasmodia of two different ages, 3 (young) and 52 (old) weeks old, were used to conduct this experiment. Slime moulds were offered a choice between two substrates previously explored by individuals of different ages. We computed the proportion of slime moulds that reached each substrate first. A value equal to 0.5 means that the slime moulds contacted the substrate explored by a young individual as often as the substrate explored by an old individual (random choice). N = 300 for each age. The CI of the random choice is delineated by the shaded area. Error bars indicate 95% CI. (Online version in colour.)
4. Discussion
In many organisms, ageing is accompanied by deficits in behavioural performance. In this study, we were able to maintain a culture of slime moulds for more than 2 years and track their behaviour throughout time. We revealed that old slime moulds moved slower than young ones on neutral, nutritive and aversive substrates in all our experiments. We confirmed that aversive substrates slowed down slime moulds regardless of age [25,41,75,76]. Sodium is known to decrease migration rate in slime moulds via a depolarization of the membrane potential [77]. An interesting observation was that sodium chloride had a stronger effect than sodium nitrate on old slime moulds while it was the opposite for young slime moulds. In many eukaryotic cells, high extracellular NaCl increases reactive oxygen species (ROS), causes DNA damage and promotes senescence [78,79]. Given that old slime moulds already suffered from molecular damage due to senescence (effects listed in the introduction [61–63,67–69]), NaCl might have amplified that damage. Interestingly, old slime moulds were better at avoiding high concentration of NaCl or NaNO3 than young slime moulds, suggesting that they were indeed more susceptible to chemical stressors and actively avoided them. As a matter of fact, we did not observe any significant differences in decision abilities as a function of age when slime moulds were facing two food sources of different quality.
We then revealed that migration speed depended on age under extreme temperatures. Temperature is known to be one of the main factors affecting the shuttle streaming in slime moulds. The contraction period decreases from 13 min at 1°C to 1.26 min at 30°C and then increases above 32°C [56,57,80,81]. At 42°C, the shuttle streaming is totally arrested and the membrane exhibits alterations [80]. In our experiment, all slime moulds were slowed down by extreme temperatures, and this effect increased with age when slime moulds' ages ranged from 25 to 100 weeks. Temperature is known to contribute to senescence in most living organisms [82], including slime moulds [70]. Susceptibility to thermal stress increases as an organism ages owing to more molecular damage being generated and accumulated over time [83]. Yet, surprisingly, the effect of temperature was strongest for the youngest slime moulds, which were 8 weeks old. As our culture alternated between 25°C during the week and 12°C during the weekend, it might be possible that thermotolerance was indirectly induced over time. Signs of habituation to low temperature have already been observed in slime moulds [84]. In P. polycephalum, as in most living organisms, expression of heat-shock proteins (HSPs) increases after exposure to a moderate thermal stress [85]. HSPs function as molecular chaperones; they reduce molecular damage induced by extreme temperatures, allowing the organism to cope with this stressor [86–89]. Usually, how much HSPs an organism produces correlates with the level of stress to which it is exposed. Therefore, in our experiment, we can presume that the level of expression of HSPs was higher in older slime moulds than in younger ones, as they experienced more periodic changes in temperature over time. Thus, older slime moulds might have been more susceptible to thermal stress owing to their age but also more thermotolerant owing to their past experiences than younger slime moulds.
Recently [40,73], it was revealed that slime moulds are capable of habituation, a simple form of learning, when they are repeatedly exposed to a chemical stimulus. In animals, learning performance displays a progressive decrease with age. However, when it comes to habituation, it was observed that older organisms habituate more rapidly than young ones in Aplysia californica [90] and Caenorhabditis elegans [91] or as fast as young ones in Drosophila melanogaster [92], mice [93] and rats [94]. Here, we showed that habituation performance is preserved in old slime moulds as well. In animals, it was shown that the response threshold to the stimulus was higher in old animals than in young ones, which could explain their performance [90,93]. By contrast, in our experiment, we showed that the aversion level toward NaCl did not vary with age. On the contrary, in our first experiment, older individuals seemed more susceptible to NaCl than younger ones. Habituation to NaCl in slime moulds relies, in part, on NaCl uptake [41]. In plants, yeast and cellular slime moulds, sodium is carried into the cell passively through non-selective cationic channels [95–97]. Therefore, it is possible that this passive transport of sodium throughout the membrane is not affected by age.
In slime moulds, sclerotia have been found to survive under adverse environmental conditions for long periods of up to 3 years. In the slime mould Didymimim iridis, a related species, the ageing process stops when the plasmodium differentiates into a sclerotium [98]. Once the sclerotium is revived, the plasmodium then lives the remainder of its characteristic lifespan [98]. By contrast, in this study we observed a recovery phenomenon when aged slime moulds went through a dormant stage. After dormancy, old slime moulds migrated as fast as young slime moulds and faster than old slime moulds that had remained in a plasmodial stage. Past work has shown that oxidative stress defences are involved in the transition from plasmodium to sclerotium in P. polycephalum [99,100]. However, cell senescence is in part due to oxidative damage to DNA, RNA and proteins by mitochondrial ROS, which accumulate with age [101]. Hence, one hypothesis might be that oxidative stress defences expressed during differentiation were responsible for the elimination of harmful ROS and, as a consequence, the behavioural recovery observed in old slime moulds after dormancy. However, we only recorded the behaviour of the slime moulds 1 week after they were reinitiated from sclerotia and we cannot rule out that this rejuvenation phenomenon was only transient and did not affect the plasmodium lifespan.
In slime moulds, cell fusion is extremely common. In a related species, Didymium nigripes, it was shown that slime moulds lose the ability to fuse over time [102]. In this study, in contrast, we showed that fusion occurred regardless of slime mould age. As old slime moulds were slower than their younger counterparts, their latency to fuse was simply longer. Vein fusion also took a longer time in old slime moulds than in young ones, but fusion was never aborted. We also revealed that an old slime mould could recover its migration speed performance after fusing with a young counterpart. Resource sharing between cells is often observed as a strategy to cope with environmental stress. For instance, cells can exchange learned information [25], mitochondria [103] or membrane material [104] via cell fusion. It is well know that cells accumulate DNA, protein and metabolite damage over time [105]. In young cells, most of the damage is cleared by maintenance processes [105]. However, as not all damage can be repaired, irreparable damage rises in the cell through time [105]. In aged cells, the maintenance systems can themselves be damaged, amplifying the burden of damage [105]. In our experiment, it might be possible that the damage accumulated over time in an old slime mould were cleared or repaired by the maintenance system of its younger counterpart. Interestingly, in a related species, Didymimim iridis, when age-hybrid slime moulds were formed by the fusion of young and old individuals, 58% of the new slime moulds acquired the remaining lifespan of the older individuals, 22% died concurrently with the younger individuals and 20% died at an intermediate age [106]. The authors concluded that it is the oldest slime mould that determines the lifespan of the age-hybrid slime mould [98,107]. In our experiment, we did not follow the age-hybrid slime moulds' behaviour over time. Hence, even though we observed a form of recovery process in old slime moulds after fusion, we cannot rule out that they would have died concurrently with slime moulds of the same age that did not undergo fusion.
As P. polycephalum migrates on the substrate, it leaves behind extracellular slime [42,108]. Past studies have shown that previously explored substrate might be repulsive or attractive depending on the physiological status of the slime mould that had explored the substrate [21,44]. A substrate previously explored by a starved, irradiated or poisoned slime mould is actively avoided [44], while a substrate that has been explored by a well-fed slime mould is attractive [21]. Calcium has been identified as the main chemical that mediates attraction [21], whereas the substances responsible for the aversion remain to be identified. In this study, we showed that slime moulds were able to use age-related cues released by conspecifics and make a decision accordingly. We revealed that slime moulds, regardless of their age, were more attracted by cues released by younger slime moulds than by older ones. As we did not notice any fusion delay when a young slime mould was facing an old one in the fusion experiment, we believe that the substrate explored by an old slime mould was not aversive but only less attractive. The information supplied by these excretions would appear to be crucial for both young and old slime mould. A young slime mould would benefit from avoiding fusion with an aged slime mould as it could inherit its damage. Indeed, we noticed that after fusing with an old counterpart, a young slime mould was somewhat slower. By contrast, an old slime mould would gain from fusing with a young slime mould, as it would enable it to recover. In many animals, from flies to humans [109–113], as an organism ages, the effect of senescence on both the perception and processing of information might affect its response to the presence of others. Here, we did not observe any effect of age on social interactions. since older individuals were still able to fuse with others (although more slowly) and detect conspecifics.
Although many studies have explored behaviour in single-cell organisms, few have looked for changes in behaviour over the lifetime of individuals. This study extends our knowledge of the behavioural plasticity of unicellular organisms and establishes acellular slime moulds as a promising model to investigate the effect of ageing on behaviour at the cellular level. Their ability to fuse with congeners and their peculiar life cycle might help us to understand how cellular metabolic activity and cellular defences are regulated and coordinated, and how a cell can counteract the effects of ageing.
Acknowledgements
We thank Celia Hay, Oceane de Vanssay, Marion Prodeo, Laure Sirere and Eloise Paulet for assistance in slime mould maintenance.
Data accessibility
The datasets supporting this article are provided as electronic supplementary material.
Authors' contributions
A.R.: conceptualization, data curation, investigation, methodology; E.P.: conceptualization, data curation, investigation, methodology; P.M.: conceptualization, data curation, investigation, methodology; C.C.: conceptualization, data curation, investigation, methodology, writing—review and editing; M.D.: methodology; A.D.: conceptualization, formal analysis, methodology, supervision, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
We have no competing interests.
Funding
The research was supported by a grant from the Agence Nationale de la Recherche, reference no. ANR-17-CE02-0019-01 – SMARTCELL, the MITI programme of the CNRS (DBM) and the Toulouse University III Program (Blobaging).
References
- 1.Waters CM, Bassler BL. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319-346. ( 10.1146/annurev.cellbio.21.012704.131001) [DOI] [PubMed] [Google Scholar]
- 2.Whiteley M, Diggle SP, Greenberg EP. 2017. Progress in and promise of bacterial quorum sensing research. Nature 551, 313-320. ( 10.1038/nature24624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross JD, Peacey MJ, Trevan DJ. 1976. Signal emission and signal propagation during early aggregation in Dictyostelium discoideum. J. Cell Sci. 22, 645-656. ( 10.1242/jcs.22.3.645) [DOI] [PubMed] [Google Scholar]
- 4.Bonner JT. 1959. The cellular slime moulds. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Sawai S, Thomason PA, Cox EC. 2005. An autoregulatory circuit for long-range self-organization in Dictyostelium cell populations. Nature 433, 323-326. ( 10.1038/nature03228) [DOI] [PubMed] [Google Scholar]
- 6.Gregor T, Fujimoto K, Masaki N, Sawai S. 2010. The onset of collective behavior in social amoebae. Science 328, 1021-1025. ( 10.1126/science.1183415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell G. 1984. Evolutionary and nonevolutionary theories of senescence. Am. Nat. 124, 600-603. ( 10.1086/284300) [DOI] [Google Scholar]
- 8.Kirkwood TBL, Austad SN. 2000. Why do we age? Nature 408, 233-238. ( 10.1038/35041682) [DOI] [PubMed] [Google Scholar]
- 9.Moger-Reischer RZ, Lennon JT. 2019. Microbial ageing and longevity. Nat. Rev. Microbiol. 17, 679-690. ( 10.1038/s41579-019-0253-y) [DOI] [PubMed] [Google Scholar]
- 10.Ackermann M, Chao L, Bergstrom CT, Doebeli M. 2007. On the evolutionary origin of aging. Aging Cell 6, 235-244. ( 10.1111/j.1474-9726.2007.00281.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Łapińska U, Glover G, Capilla-Lasheras P, Young AJ, Pagliara S. 2019. Bacterial ageing in the absence of external stressors. Phil. Trans. R. Soc. B 374, 20180442. ( 10.1098/rstb.2018.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florea M. 2017. Aging and immortality in unicellular species. Mech. Ageing Dev. 167, 5-15. ( 10.1016/j.mad.2017.08.006) [DOI] [PubMed] [Google Scholar]
- 13.Crane MM, Kaeberlein M. 2018. The paths of mortality: how understanding the biology of aging can help explain systems behavior of single cells. Curr. Opin. Syst. Biol. 8, 25-31. ( 10.1016/j.coisb.2017.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid CR, Latty T. 2016. Collective behaviour and swarm intelligence in slime moulds. FEMS Microbiol. Rev. 40, 798–806. ( 10.1093/femsre/fuw033) [DOI] [PubMed] [Google Scholar]
- 15.Takamatsu A, Fujii T, Endo I. 2000. Time delay effect in a living coupled oscillator system with the plasmodium of Physarum polycephalum. Phys. Rev. Lett. 85, 2026. ( 10.1103/PhysRevLett.85.2026) [DOI] [PubMed] [Google Scholar]
- 16.Boussard A, Fessel A, Oettmeier C, Briard L, Dobereiner HG, Dussutour A. 2020. Adaptive behavior and learning in slime moulds: the role of oscillations. Phil. Trans. R. Soc. Lond. B 376, 20190757. ( 10.1098/rstb.2019.0757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshiyama S, Ishigami M, Nakamura A, Kohama K. 2010. Calcium wave for cytoplasmic streaming of Physarum polycephalum. Cell Biol. Int. 34, 35-40. ( 10.1042/CBI20090158) [DOI] [PubMed] [Google Scholar]
- 18.Alim K, Amselem G, Peaudecerf F, Brenner MP, Pringle A. 2013. Random network peristalsis in Physarum polycephalum organizes fluid flows across an individual. Proc. Natl Acad. Sci. USA 110, 13 306-13 311. ( 10.1073/pnas.1305049110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer B, Ansorge C, Nakagaki T. 2017. The role of noise in self-organized decision making by the true slime mold Physarum polycephalum. PLoS ONE 12, e0172933. ( 10.1371/journal.pone.0172933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel D, Dussutour A, Deneubourg JL. 2018. Symmetry breaking and inter-clonal behavioural variability in a slime mould. Biol. Lett. 14, 20180504. ( 10.1098/rsbl.2018.0504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel D, Nicolis SC, Perez-Escudero A, Nanjundiah V, Sumpter DJT, Dussutour A. 2015. Phenotypic variability in unicellular organisms: from calcium signalling to social behaviour. Proc. R. Soc. B 282, 20152322. ( 10.1098/rspb.2015.2322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dussutour A, Ma Q, Sumpter D. 2019. Phenotypic variability predicts decision accuracy in unicellular organisms. Proc. R. Soc. B 286, 20182825. ( 10.1098/rspb.2018.2825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zabzina N, Dussutour A, Mann RP, Sumpter DJT, Nicolis SC. 2014. Symmetry restoring bifurcation in collective decision-making. PLoS Comput. Biol. 10, e1003960. ( 10.1371/journal.pcbi.1003960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fessel A, Oettmeier C, Bernitt E, Gauthier NC, Döbereiner HG. 2012. Physarum polycephalum percolation as a paradigm for topological phase transitions in transportation networks. Phys. Rev. Lett. 109, 078103. ( 10.1103/PhysRevLett.109.078103) [DOI] [PubMed] [Google Scholar]
- 25.Vogel D, Dussutour A. 2016. Direct transfer of learned behaviour via cell fusion in non-neural organisms. Proc. R. Soc. B 283, 20162382. ( 10.1098/rspb.2016.2382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray SK, Valentini G, Shah P, Haque A, Reid CR, Weber GF, Garnier S. 2019. Information transfer during food choice in the slime mold Physarum polycephalum. Front. Ecol. Evol. 7, 67. ( 10.3389/fevo.2019.00067) [DOI] [Google Scholar]
- 27.Reid CR, Garnier S, Beekman M, Latty T. 2015. Information integration and multiattribute decision making in non-neuronal organisms. Anim. Behav. 100, 44-50. ( 10.1016/j.anbehav.2014.11.010) [DOI] [Google Scholar]
- 28.Reid CR, MacDonald H, Mann RP, Marshall JAR, Latty T, Garnier S. 2016. Decision-making without a brain: how an amoeboid organism solves the two-armed bandit. J. R. Soc. Interface 13, 20160030. ( 10.1098/rsif.2016.0030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beekman M, Latty T. 2015. Brainless but multi-headed: decision making by the acellular slime mould Physarum polycephalum. J. Mol. Biol. 427, 3734-3743. ( 10.1016/j.jmb.2015.07.007) [DOI] [PubMed] [Google Scholar]
- 30.Nakagaki T, Kobayashi R, Nishiura Y, Ueda T. 2004. Obtaining multiple separate food sources: behavioural intelligence in the Physarum plasmodium. Proc. R. Soc. Lond. B 271, 2305-2310. ( 10.1098/rspb.2004.2856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones J. 2015. From pattern formation to material computation. Berlin, Germany: Springer. [Google Scholar]
- 32.Gao C, Liu C, Schenz D, Li X, Zhang Z, Jusup M, Wang Z, Beekman M, Nakagaki T. 2019. Does being multi-headed make you better at solving problems? A survey of Physarum-based models and computations. Phys. Life Rev. 29, 1-26. ( 10.1016/j.plrev.2018.05.002) [DOI] [PubMed] [Google Scholar]
- 33.Nakagaki T, Yamada H, Tóth Á. 2000. Maze-solving by an amoeboid organism. Nature 407, 470. ( 10.1038/35035159) [DOI] [PubMed] [Google Scholar]
- 34.Tero A, Takagi S, Saigusa T, Ito K, Bebber DP, Fricker MD, Yumiki K, Kobayashi R, Nakagaki T. 2010. Rules for biologically inspired adaptive network design. Science 327, 439-442. ( 10.1126/science.1177894) [DOI] [PubMed] [Google Scholar]
- 35.Oettmeier C, Nakagaki T, Döbereiner HG. 2020. Slime mold on the rise: the physics of Physarum polycephalum. J. Phys. D. Appl. Phys. 53, 310201. ( 10.1088/1361-6463/ab866c) [DOI] [Google Scholar]
- 36.Awad A, Pang W, Lusseau D, Coghill GM. 2021. A survey on Physarum polycephalum intelligent foraging behaviour and bio-Inspired applications. arXiv, 2103.00172. ( 10.48550/arXiv.2103.00172) [DOI]
- 37.Oettmeier C, Brix K, Döbereiner HG. 2017. Physarum polycephalum—a new take on a classic model system. J. Phys. D Appl. Phys. 50, 413001. ( 10.1088/1361-6463/aa8699) [DOI] [Google Scholar]
- 38.Reid CR, Beekman M. 2013. Solving the Towers of Hanoi – how an amoeboid organism efficiently constructs transport networks. J. Exp. Biol. 216, 1546-1551. ( 10.1242/jeb.081158) [DOI] [PubMed] [Google Scholar]
- 39.Saigusa T, Tero A, Nakagaki T, Kuramoto Y. 2008. Amoebae anticipate periodic events. Phys. Rev. Lett. 100, 018101. ( 10.1103/PhysRevLett.100.018101) [DOI] [PubMed] [Google Scholar]
- 40.Boisseau RP, Vogel D, Dussutour A. 2016. Habituation in non-neural organisms: evidence from slime moulds. Proc. R. Soc. B 283, 20160446. ( 10.1098/rspb.2016.0446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boussard A, Delescluse J, Pérez-Escudero A, Dussutour A. 2019. Memory inception and preservation in slime moulds: the quest for a common mechanism. Phil. Trans. R. Soc. B 374, 20180368. ( 10.1098/rstb.2018.0368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reid CR, Latty T, Dussutour A, Beekman M. 2012. Slime mold uses an externalized spatial ‘memory’ to navigate in complex environments. Proc. Natl Acad. Sci. USA 109, 17 490-17 494. ( 10.1073/pnas.1215037109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramar M, Alim K. 2021. Encoding memory in tube diameter hierarchy of living flow network. Proc. Natl Acad. Sci. USA 118, e2007815118. ( 10.1073/pnas.2007815118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briard L, Gourjade C, Bousquet C, Dussutour A. 2020. Stress signalling in acellular slime moulds and its detection by conspecifics. Phil. Trans. R. Soc. B 375, 20190470. ( 10.1098/rstb.2019.0470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dussutour A, Latty T, Beekman M, Simpson SJ. 2010. Amoeboid organism solves complex nutritional challenges. Proc. Natl Acad. Sci. USA 107, 4607-4611. ( 10.1073/pnas.0912198107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murugan NJ, et al. 2021. Mechanosensation mediates long-range spatial decision-making in an aneural organism. Adv. Mater. 33, e2008161. ( 10.1002/adma.202008161) [DOI] [PubMed] [Google Scholar]
- 47.Baumgarten W, Hauser MJB. 2013. Functional organization of the vascular network of Physarum polycephalum. Phys. Biol. 10, 026003. ( 10.1088/1478-3975/10/2/026003) [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto K, Takagi S, Nakagaki T. 2008. Locomotive mechanism of Physarum plasmodia based on spatiotemporal analysis of protoplasmic streaming. Biophys. J. 94, 2492-2504. ( 10.1529/biophysj.107.113050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis OL, Zhang S, Guy RD, Del Alamo JC. 2015. Coordination of contractility, adhesion and flow in migrating Physarum amoebae. J. R. Soc. Interface 12, 20141359. ( 10.1098/rsif.2014.1359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumgarten W, Hauser MJB. 2014. Dynamics of frontal extension of an amoeboid cell. Europhys. Lett . 108, 50010. ( 10.1209/0295-5075/108/50010) [DOI] [Google Scholar]
- 51.Durham AC, Ridgway EB. 1976. Control of chemotaxis in Physarum polycephalum. J. Cell Biol. 69, 218-223. ( 10.1083/jcb.69.1.218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyake Y, Tada H, Yano M, Shimizu H. 1994. Relationship between intracellular period modulation and external environment change in Physarum plasmodium. Cell Struct. Funct. 19, 363-370. ( 10.1247/csf.19.363) [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto K, Ueda T, Kobatake Y. 1986. Propagation of phase wave in relation to tactic responses by the plasmodium of Physarum polycephalum. J. Theor. Biol. 122, 339-345. ( 10.1016/S0022-5193(86)80125-4) [DOI] [Google Scholar]
- 54.Marwan W. 2001. Photomovement and photomorphogenesis in Physarum polycephalum: targeting of cytoskeleton and gene expression by light. Compr. Ser. Photosci. 1, 561-587. ( 10.1016/S1568-461X(01)80024-7) [DOI] [Google Scholar]
- 55.Wolf R, Niemuth J, Sauer H. 1997. Thermotaxis and protoplasmic oscillations in Physarum plasmodia analysed in a novel device generating stable linear temperature gradients. Protoplasma 197, 121-131. ( 10.1007/BF01279890) [DOI] [Google Scholar]
- 56.Wohlfarth-Bottermann KE. 1977. Oscillating contractions in protoplasmic strands of Physarum: simultaneous tensiometry of longitudinal and radial rhythms, periodicity analysis and temperature dependence. J. Exp. Biol. 67, 49-59. ( 10.1242/jeb.67.1.49) [DOI] [PubMed] [Google Scholar]
- 57.Halvorsrud R, Laane MM, Giaever I. 1995. A novel electrical method to study plasmodial contractions in Physarum. Synchrony and temperature dependence. Biol. Rhythm Res. 26, 316-330. ( 10.1080/09291019509360345) [DOI] [Google Scholar]
- 58.Block I, Wolke A, Briegleb W. 1994. Gravitational response of the slime mold Physarum. Adv. Space Res. 14, 21-34. ( 10.1016/0273-1177(94)90382-4) [DOI] [PubMed] [Google Scholar]
- 59.Aldrich H. 2012. Organisms, nucleus, and cell cycle. Cell biology of Physarum and Didymium, vol. 1. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 60.Rojas C, Stephenson SL. 2021. Myxomycetes: biology, systematics, biogeography and ecology. New York, NY: Academic Press. [Google Scholar]
- 61.Poulter RTM. 1969. Senescence in the myxomycete Physarum polycephalum. PhD thesis, University of Leicester, UK.
- 62.Nakagawa CC, Jones EP, Miller DL. 1998. Mitochondrial DNA rearrangements associated with mF plasmid integration and plasmodial longevity in Physarum polycephalum. Curr. Genet. 33, 178-187. ( 10.1007/s002940050325) [DOI] [PubMed] [Google Scholar]
- 63.Hu FS, Clark J, Lott T. 1985. Recurrent senescence in axenic cultures of Physarum polycephalum. J. Gen. Microbiol. 131, 811-815. ( 10.1099/00221287-131-4-811) [DOI] [PubMed] [Google Scholar]
- 64.Lott T, Clark J. 1980. Plasmodial senescence in the acellular slime mold Didymium iridis. Exp. Cell Res. 128, 455-457. ( 10.1016/0014-4827(80)90080-4) [DOI] [PubMed] [Google Scholar]
- 65.Adamatzky AI. 2017. Thirty seven things to do with live slime mould. In Advances in unconventional computing (ed. AI Adamatzky), pp. 709-738. Berlin, Germany: Springer. [Google Scholar]
- 66.Keller HW, Everhart SE. 2010. Importance of myxomycetes in biological research and teaching. Pap. Plant Pathol., no. 366. [Google Scholar]
- 67.Abe T, Takano H, Sasaki N, Mori K, Kawano S. 2000. In vitro DNA fragmentation of mitochondrial DNA caused by single-stranded breakage related to macroplasmodial senescence of the true slime mold, Physarum polycephalum. Curr. Genet. 37, 125-135. ( 10.1007/s002940050019) [DOI] [PubMed] [Google Scholar]
- 68.Adler PN, Holt CE. 1974. Change in properties of Physarum polycephalum amoebae during extended culture. J. Bacteriol. 120, 532-533. ( 10.1128/jb.120.1.532-533.1974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mccullough CHR, Cooke DJ, Foxon JL, Sudbery PE, Grant WD. 1973. Nuclear DNA content and senescence in Physarum polycephalum. Nat. New Biol. 245, 263-265. ( 10.1038/newbio245263a0) [DOI] [PubMed] [Google Scholar]
- 70.Clark J, Lott T. 1981. Aging in the acellular slime mold Didymium iridis: temperature and nutritional effects. Exp. Mycol. 5, 369-372. ( 10.1016/0147-5975(81)90043-8) [DOI] [Google Scholar]
- 71.Matsumoto K, Ueda T, Kobatake Y. 1988. Reversal of thermotaxis with oscillatory stimulation in the plasmodium of Physarum polycephalum. J. Theor. Biol. 131, 175-182. ( 10.1016/S0022-5193(88)80235-2) [DOI] [Google Scholar]
- 72.Latty T, Beekman M. 2010. Food quality and the risk of light exposure affect patch-choice decisions in the slime mold Physarum polycephalum. Ecology 91, 22-27. ( 10.1890/09-0358.1) [DOI] [PubMed] [Google Scholar]
- 73.Dussutour A. 2021. Learning in single cell organisms. Biochem. Biophys. Res. Commun. 564, 92-102. ( 10.1016/j.bbrc.2021.02.018) [DOI] [PubMed] [Google Scholar]
- 74.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Scheipl F, Grothendieck G. 2009. Package ‘lme4’. See http://lme4.r-forge.r-project.org.
- 75.Patino-Ramirez F, Boussard A, Arson C, Dussutour A. 2019. Substrate composition directs slime molds behavior. Scient. Rep. 9, 15444. ( 10.1038/s41598-019-50872-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denbo JR, Miller DM. 1976. Factors affecting the movement of slime mold plasmodia. Comp. Biochem. Physiol. A Physiol. 55, 5-12. ( 10.1016/0300-9629(76)90114-6) [DOI] [PubMed] [Google Scholar]
- 77.Ueda T, Terayama K, Kurihara K, Kobatake Y. 1975. Threshold phenomena in chemoreception and taxis in slime mold Physarum polycephalum. J. Gen. Physiol. 65, 223-234. ( 10.1085/jgp.65.2.223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dmitrieva NI, Burg MB. 2007. High NaCl promotes cellular senescence. Cell Cycle 6, 3108-3113. ( 10.4161/cc.6.24.5084) [DOI] [PubMed] [Google Scholar]
- 79.Dmitrieva NI, Cui K, Kitchaev DA, Zhao K, Burg MB. 2011. DNA double-strand breaks induced by high NaCl occur predominantly in gene deserts. Proc. Natl Acad. Sci. USA 108, 20 796-20 801. ( 10.1073/pnas.1114677108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lomagin AG. 1978. Repair of functional and ultrastructural alterations after thermal injury of Physarum polycephalum. Planta 142, 123-134. ( 10.1007/BF00388203) [DOI] [PubMed] [Google Scholar]
- 81.Bernstam VA, Arndt S. 1974. Effects of supraoptimal temperatures on the myxomycete Physarum polycephalum. Arch. Microbiol. 95, 357-363. ( 10.1007/bf02451777) [DOI] [PubMed] [Google Scholar]
- 82.Keil G, Cummings E, de Magalhaes JP. 2015. Being cool: how body temperature influences ageing and longevity. Biogerontology 16, 383-397. ( 10.1007/s10522-015-9571-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Conti B. 2008. Considerations on temperature, longevity and aging. Cell. Mol. Life Sci. 65, 1626-1630. ( 10.1007/s00018-008-7536-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shirakawa T, Gunji YP, Miyake Y. 2011. An associative learning experiment using the plasmodium of Physarum polycephalum. Nano Commun. Netw. 2, 99-105. ( 10.1016/j.nancom.2011.05.002) [DOI] [Google Scholar]
- 85.Gevers M, Fracella F, Rensing L. 2006. Nuclear translocation of constitutive heat shock protein 70 during S phase in synchronous macroplasmodia of Physarum polycephalum. FEMS Microbiol. Lett. 152, 89-94. ( 10.1111/j.1574-6968.1997.tb10413.x) [DOI] [PubMed] [Google Scholar]
- 86.Lindquist S. 1986. The heat-shock response. Annu. Rev. Biochem. 55, 1151-1191. ( 10.1146/annurev.bi.55.070186.005443) [DOI] [PubMed] [Google Scholar]
- 87.Kregel KC. 2002. Invited review: heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 92, 2177-2186. ( 10.1152/japplphysiol.01267.2001) [DOI] [PubMed] [Google Scholar]
- 88.Sørensen JG, Kristensen TN, Loeschcke V. 2003. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6, 1025-1037. ( 10.1046/j.1461-0248.2003.00528.x) [DOI] [Google Scholar]
- 89.Chen B, Feder ME, Kang L. 2018. Evolution of heat-shock protein expression underlying adaptive responses to environmental stress. Mol. Ecol. 27, 3040-3054. ( 10.1111/mec.14769) [DOI] [PubMed] [Google Scholar]
- 90.Rattan KS, Peretz B. 1981. Age-dependent behavioral changes and physiological changes in identified neurons in Aplysia californica. J. Neurobiol. 12, 469-478. ( 10.1002/neu.480120506) [DOI] [PubMed] [Google Scholar]
- 91.Beck CDO, Rankin CH. 1993. Effects of aging on habituation in the nematode Caenorhabditis elegans. Behav. Process. 28, 145-163. ( 10.1016/0376-6357(93)90088-9) [DOI] [PubMed] [Google Scholar]
- 92.Le Bourg E. 1983. Aging and habituation of the tarsal response in Drosophila melanogaster. Gerontology 29, 388-393. ( 10.1159/000213149) [DOI] [PubMed] [Google Scholar]
- 93.Brennan MJ, Allen D, Aleman D, Azmitia EC, Quartermain D. 1984. Age differences in within-session habituation of exploratory behavior: effects of stimulus complexity. Behav. Neural Biol. 42, 61-72. ( 10.1016/S0163-1047(84)90436-9) [DOI] [PubMed] [Google Scholar]
- 94.Shukitt-Hale B, Casadesus G, Cantuti-Castelvetri I, Joseph JA. 2001. Effect of age on object exploration, habituation, and response to spatial and nonspatial change. Behav. Neurosci. 115, 1059. ( 10.1037/0735-7044.115.5.1059) [DOI] [PubMed] [Google Scholar]
- 95.De Hertogh B, Hancy F, Goffeau A, Baret PV. 2006. Emergence of species-specific transporters during evolution of the hemiascomycete phylum. Genetics 172, 771-781. ( 10.1534/genetics.105.046813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Müller U, Hartung K. 1990. Properties of three different ion channels in the plasma membrane of the slime mold Dictyostelium discoideum. Biochim. Biophys. Acta Biomembr. 1026, 204-212. ( 10.1016/0005-2736(90)90065-V) [DOI] [PubMed] [Google Scholar]
- 97.Demidchik V, Davenport RJ, Tester M. 2002. Nonselective cation channels in plants. Annu. Rev. Plant Biol. 53, 67-107. ( 10.1146/annurev.arplant.53.091901.161540) [DOI] [PubMed] [Google Scholar]
- 98.Lott T, Clark J. 1982. Sclerotization in relation to plasmodial senescence in the acellular slime mould Didymium iridis. Microbiology 128, 1483-1487. ( 10.1099/00221287-128-7-1483) [DOI] [PubMed] [Google Scholar]
- 99.Schreckenbach T, Werenskiold AK. 1986. Gene expression during plasmodial differentiation. In The molecular biology of Physarum polycephalum (eds WF Dove, J Dee, S Hatano, FB Haugli, K-E Wohlfarth-Bottermann), pp. 131-150. Berlin, Germany: Springer. [Google Scholar]
- 100.Allen RG, Newton RK, Sohal RS, Shipley GL, Nations C. 1985. Alterations in superoxide dismutase, glutathione, and peroxides in the plasmodial slime mold Physarum polycephalum during differentiation. J. Cell. Physiol. 125, 413-419. ( 10.1002/jcp.1041250308) [DOI] [PubMed] [Google Scholar]
- 101.López C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153, 1194-1217. ( 10.1016/j.cell.2013.05.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kerr NS, Waxlax JN. 1968. A yellow variant of the eumycetozoan Didymium nigripes which exhibits aging. J. Exp. Zool. 168, 351-361. ( 10.1002/jez.1401680306) [DOI] [PubMed] [Google Scholar]
- 103.Spees JL, Olson SD, Whitney MJ, Prockop DJ. 2006. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl Acad. Sci. USA 103, 1283-1288. ( 10.1073/pnas.0510511103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vassallo CN, Wall D. 2016. Tissue repair in myxobacteria: a cooperative strategy to heal cellular damage. Bioessays 38, 306-315. ( 10.1002/bies.201500132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gladyshev VN, et al. 2021. Molecular damage in aging. Nat. Aging 1, 1096-1106. ( 10.1038/s43587-021-00150-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clark J, Hakim R. 1980. Aging of plasmodial heterokaryons in Didymium iridis. Mol. Gen. Genet. 178, 419-422. ( 10.1007/BF00270493) [DOI] [Google Scholar]
- 107.Clark J, Lott T. 1989. Age heterokaryon studies in Didymium iridis. Mycologia 81, 636-638. ( 10.1080/00275514.1989.12025796) [DOI] [Google Scholar]
- 108.Reid CR, Beekman M, Latty T, Dussutour A. 2013. Amoeboid organism uses extracellular secretions to make smart foraging decisions. Behav. Ecol. 24, 812-818. ( 10.1093/beheco/art032) [DOI] [Google Scholar]
- 109.Almeling L, Hammerschmidt K, Sennhenn-Reulen H, Freund AM, Fischer J. 2016. Motivational shifts in aging monkeys and the origins of social selectivity. Curr. Biol. 26, 1744-1749. ( 10.1016/j.cub.2016.04.066) [DOI] [PubMed] [Google Scholar]
- 110.Brenman-Suttner DB, Yost RT, Frame AK, Robinson JW, Moehring AJ, Simon AF. 2020. Social behavior and aging: a fly model. Genes Brain Behav. 19, e12598. ( 10.1111/gbb.12598) [DOI] [PubMed] [Google Scholar]
- 111.Rosati AG, Hagberg L, Enigk DK, Otali E, Emery Thompson M, Muller MN, Wrangham RW, Machanda ZP. 2020. Social selectivity in aging wild chimpanzees. Science 370, 473-476. ( 10.1126/science.aaz9129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Charles ST, Carstensen LL. 2010. Social and emotional aging. Annu. Rev. Psychol. 61, 383-409. ( 10.1146/annurev.psych.093008.100448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siracusa ER, Higham JP, Snyder-Mackler N, Brent LJN. 2022. Social ageing: exploring the drivers of late-life changes in social behaviour in mammals. Biol. Lett. 18, 20210643. ( 10.1098/rsbl.2021.0643) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting this article are provided as electronic supplementary material.