Abstract
Dihydromyricetin (DHM), a dihydroflavonoid compound, exhibits a variety of biological activities, including antitumor activity. However, the effects of DHM on mammalian reproductive processes, especially during early embryonic development, remain unclear. In this study, we added DHM to porcine zygotic medium to explore the influence and underlying mechanisms of DHM on the developmental competence of parthenogenetically activated porcine embryos. Supplementation with 5 μM DHM during in vitro culture (IVC) significantly improved blastocyst formation rate and increased the total number of cells in porcine embryos. Further, DHM supplementation also improved glutathione levels and mitochondrial membrane potential; reduced natural reactive oxygen species levels in blastomeres and apoptosis rate; upregulated Nanog, Oct4, SOD1, SOD2, Sirt1, and Bcl2 expression; and downregulated Beclin1, ATG12, and Bax expression. Collectively, DHM supplementation regulated oxidative stress during IVC and could act as a potential antioxidant during in vitro porcine oocytes maturation.
Keywords: Dihydromyricetin, In vitro culture, Oxidative stress, Porcine oocyte
Variations between in vivo environments and in vitro culture systems are manifested as differences in maturation and survival rates of in vitro culture (IVC). In vitro embryo production (IVP) procedures, including in vitro oocyte maturation (IVM) and in vitro embryo culture, have gained attention due to their applications in various fields such as agricultural production and scientific research. Porcine is an important livestock for IVP. However, compared to other livestock embryos, such as that of cattle and sheep, porcine embryos are more sensitive to reactive oxygen species (ROS), osmotic pressure, temperature, pH, humidity, and other environmental factors [1]. Therefore, optimizing porcine IVP is a focus of many studies.
In vitro production of mammalian embryos has potentially significant applications, not only to understand biological processes, but also to produce transgenic porcine for generating disease models or xenotransplantation [2, 3]. During in vitro culture, oocytes and embryos are subjected to various oxidative stresses that may result in programmed cell death processes such as apoptosis and autophagy, affecting the production efficiency of embryos. Therefore, it is important to protect oocytes and embryos from oxidative stress to maintain the efficiency and quality of in vitro embryo production [4, 5].
Adding antioxidants or hormones to the culture medium can prevent mitochondrial dysfunction and oxidative damage by promoting the production of glutathione (GSH) with antioxidant properties in cells or supporting an appropriate pro/antioxidant balance to protect oocytes and embryos. Antioxidants also improve embryo development rate and quality [6,7,8] and regulate the expression of various genes such as apoptosis-related genes (Caspase-3, Bcl-2, Bax) [9]. Antioxidants upregulate pluripotency-related genes (Nanog, Oct4, Sox2) [10, 11] and antioxidant-related genes (SOD, CAT, Sirt1) [12,13,14], while downregulating autophagy-related genes (Beclin1, ATG12, LC3) [15, 16]. Collectively, adding antioxidants to culture media enhances overall embryo quality [17]. For example, ferulic acid [18], lycopene [12], and melatonin [19] have been shown to improve the quality of early embryonic development. Nevertheless, discovering new antioxidant additives to further improve embryo quality is an interesting research avenue.
Dihydromyricetin [(2R,3R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one] (DHM) is a flavanol extracted from Ampelopsis grossedentata. It exerts anti-inflammatory, antitussive, antioxidant, antibacterial, antitumor, antihypertensive, and hepatoprotective actions [20,21,22,23,24,25,26] with almost no toxicity and excellent safety profiles [27]. DHM is widely used to prevent alcoholic liver disease (ALD) and cancer as well as to inhibit fat formation. Additionally, DHM improves antioxidant capacity and mitochondrial function, enhances glucose uptake, and reduces inflammation and oxidative stress [28,29,30]. DHM has also shown great potential in improving hippocampal lipid peroxidation and protecting human umbilical vein endothelial cells (HUVEC) from oxidative damage [23, 31, 32]. Moreover, DHM can protect endothelial cells from oxidative stress damage in the mitochondrial pathway [22]. However, its effects on mammalian reproductive processes, especially on ovarian development and early embryonic development, are still unclear.
Although DHM has well-known protective properties, its effect on mammalian oocytes has not yet been studied. In the current study, we hypothesized that supplementation with DHM during IVC could enhance the developmental potential of early porcine embryos by reducing oxidative stress.
Materials and Methods
Chemicals and reagents
Unless stated otherwise, all chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Medium 199 (TCM-199), Dulbecco’s phosphate-buffered saline (DPBS), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine-iodide dye (JC-1), 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (CMF2HC), and penicillin-streptomycin (PS) were obtained from Thermo Fisher Scientific (Waltham, MA, USA). BeyoClick EdU-488 cell proliferation detection kit and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) were purchased from Beyotime (Shanghai, China). The in-situ apoptosis detection kit was purchased from Roche (Mannheim, Germany). DHM was dissolved in dimethyl sulfoxide (DMSO), with DMSO content ≤ 0.1%, and then diluted to specific concentrations used for the experiment.
Oocyte collection and in vitro maturation (IVM)
Ovaries of prepubertal gilts were obtained from a local slaughterhouse and transferred to the laboratory in 0.9% saline at 30–35ºC. A 10 ml syringe was used to aspirate the cumulus-oocyte complexes (COCs) from 3 to 6 mm follicles using an 18-gauge needle. Oocytes with at least three layers of cumulus cells were washed twice with Tyrode’s lactate HEPES (TL-HEPES) washed again thrice with IVM medium. Approximately 80 COCs were transferred to 500 µl of mineral oil-covered IVM medium (medium 199 supplemented with 10% porcine follicular fluid, 0.57 mM L-cysteine, 20 ng/ml epidermal growth factor, 1% PS, 0.2 mM sodium pyruvate, 10 IU/ml follicle stimulating hormone (Ningbo No. 2 Hormone Factory, China), 10 IU/ml luteinizing hormone (Ningbo No. 2 Hormone Factory, China)) and cultured at 38.5ºC in 5% CO2 and 100% humidity for 45 h.
Oocyte parthenogenetic activation (PA) and in vitro culture
Oocytes matured and cultured in vitro for 45 h were gently treated with 0.1% hyaluronidase to remove the outer cumulus cells of the COCs. Denuded mature oocytes were parthenogenetically activated using two direct-current pulses of 120 V for 60 µsec in 300 mM mannitol containing 0.5 mM HEPES, 0.1 mM MgSO4·7H2O, 0.05 mM CaCl2·2 H2O, 0.01% polyvinyl alcohol (PVA), and 0.1% (w/v) bovine serum albumin (BSA). Next, the oocytes were cultured in bicarbonate-buffered porcine zygote medium 5 (PZM-5) [33] containing 4 mg/ml BSA and 7.5 µg/ml cytochalasin B for 3 h to suppress extrusion of the pseudo-second polar body. Finally, the oocytes were washed and cultured in 50 μl of bicarbonate-buffered PZM-5 containing 4 mg/ml BSA with or without DHM for another 7 days and covered with mineral oil at 38.5°C in 5% CO2 without changing the medium.
Cell counting and 5-ethynyl-2ʹ-deoxyuridine (EdU) assay
Cell proliferation was assessed using the BeyoClick EdU-488 cell proliferation detection kit according to the manufacturer’s instructions. Briefly, on day 6, the embryos were incubated in IVC medium (pre-balanced at 38.5°C for more than 3 h) containing 10 μM EdU for 8 h. Next, blastocysts were fixed with 3.7% paraformaldehyde in phosphate-buffered saline with 0.1% polyvinyl alcohol (PBS-PVA) for 30 min and then permeabilized by incubation in 0.3% triton X-100 for 30 min at room temperature, followed by incubation with 1% BSA in PBS-PVA for 1 h at 37ºC. After washing thrice with PBS-PVA, the embryos were incubated with BeyoClick additive solution at room temperature in the dark for 60 min. The embryos were then incubated with 10 μg/ml Hoechst 33342 for 15 min at room temperature to label the nuclei. A fluorescence microscope (Eclipse Ti2; Nikon, Tokyo, Japan) and Image J software (NIH, Bethesda, MD, USA) were used to analyze the number of EdU-positive cells and the total cells. The proliferation rate was calculated as the ratio of EdU-positive cell number to total cell number.
Cell counting and TUNEL assays
The apoptosis rate of blastocyst cells was determined using an in-situ apoptosis detection kit (cat #11684795910, Roche). The day-7 blastocysts were fixed in 3.7% formalin (FA) at room temperature for 30 min. Then, they were incubated in 0.3% triton X-100 at room temperature for 30 min, followed by blocking in 0.1% PBS-PVA for 30 min, and incubation in 1% BSA in PBS-PVA for 1 h at 37°C. Fluorescein coupled dUTP and terminal deoxynucleotidyl transferase were incubated in the dark at 37ºC for 1 h. The cells were then washed three times with 0.1% PBS-PVA for 3 times. The embryos were then treated with 10 μg/ml Hoechst 33342, incubated in the dark at 37ºC for 15 min, washed thrice in 0.1% PBS-PVA, and fixed on a slide. Fluorescence microscopy and ImageJ software were used to analyze the fluorescence intensities, number of apoptotic nuclei, and total number of nuclei. Apoptosis was evaluated based on the percentage of apoptotic nuclei in the blastocysts.
ROS and GSH level assays
After culturing for 48 h, intracellular ROS and GSH levels in the blastomeres were determined by incubating the 4-cell-stage embryos in PBS-PVA medium containing 10 µM H2DCFDA and 10 µM CMF2HC for 15 min and 30 min at 37ºC, respectively. After washing thrice with PBS-PVA, fluorescence microscopy and ImageJ software were used to analyze the fluorescence intensities.
Determination of mitochondrial membrane potential (MMP, ΔΨm)
Mitochondrial function was assessed by measuring the ΔΨm. Briefly, 4-cell-stage embryos were washed thrice with PBS-PVA and incubated in PBS-PVA containing 2.5 μM JC-1 at 37ºC for 30 min. After washing thrice with PBS-PVA, red and green fluorescence intensities were captured using a fluorescence microscope. The average ΔΨm values of all 4-cell-stage embryos were then calculated as the ratio of red fluorescence intensity (corresponding to active mitochondria) to green fluorescence intensity (corresponding to inactive mitochondria) using the ImageJ software.
RNA extraction and real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
Blastocysts were collected from day-7 embryos, and DynaBeads mRNA direct kit (Cat #61012; Dynal Asa, Oslo, Norway) was used to extract the mRNA according to manufacturer’s instructions. Next, the DynaBeads mRNA direct kit (Life Technologies, Carlsbad, CA, USA) was used to reverse transcribe the extracted RNA to obtain cDNA. qPCR was performed using the CFX connect optics module PCR instrument and Kapa kit. The 20 µl PCR reaction solution included 2 µl cDNA and 1 µl primer (including forward primer and reverse primer), 10 µl SYBR green, and 7 µl ddH2O. The following settings were used for the thermal cycler: initial denaturation at 95ºC for 3 min, denaturation at 95ºC for 3 sec, annealing at 60ºC for 30 sec, and extension at 72ºC for 20 sec. The reaction was run for 40 cycles. The target genes were Bax, Bcl2, Nanog, Oct4, Beclin1, ATG12, SOD1, SOD2, and Sirt1. GAPDH was used as an internal control. The primers used to amplify each gene are listed in Table 1. Quantitative RNA data were obtained using the 2 −ΔΔCT method.
Table 1. Primer sequences.
| Gene | Primer sequences (5'-3') |
|---|---|
| GAPDH | F: TTCCACGGCACAGTCAAG |
| R: ATACTCAGCACCAGCATCG | |
| OCT4 | F: GTGAGAGGCAACCTGGAGAG |
| R: TCGTTGCGAATAGTCACTGC | |
| Bax | F: GATCGAGCAGGGCGAATG |
| R: GGGCCTTGAGCACCAGTTTA | |
| ATG12 | F: CAACTGCTGCTGAGGGCGATG |
| R: CACCGGCAGGTTCTTCTGTTCC | |
| NANOG | F: AAGTACCTCAGCCTCCAGCA |
| R: GTGCTGAGCCCTTCTGAATC | |
| Bcl2 | F: GCCGAAATGTTTGCTGAC |
| R: GCCGATCTCGAAGGAAGT | |
| SOD2 | F: CTGGACAAATCTGAGCCCTAAC |
| R: GACGGATACAGCGGTCAACT | |
| SOD1 | F: TGACTGCTGGCAAAGATGGT |
| R: TTTCCACCTCTGCCCAAGTC | |
| SIRT1 | F: ATCGTCACCAATGGTTTCCA |
| R: GGATCTGTGCCAATCATGAG | |
| BECLIN1 | F: AGGAGCTGCCGTTGTACTGTTCT |
| R: TGCTGCACACAGTCCAGGAA |
The annealing temperature for all reactions was 60ºC. F: forward primer; R: reverse primer.
Statistical analysis
Results are presented as mean ± standard deviation (SD). The total number of embryos used (n) and the number of independent repeats (R) for each experiment are shown in the figures. Data for the two groups were compared using Student’s t-test. Three or more means were analyzed using one-way analysis of variance (Tukey-Kramer). All statistical analyses were performed using SPSS version 17.0 (IBM, Chicago, IL, USA). Significant differences are represented by * (P < 0.05), ** (P < 0.01) and *** (P < 0.001).
Results
Different DHM concentrations affect porcine early embryonic development
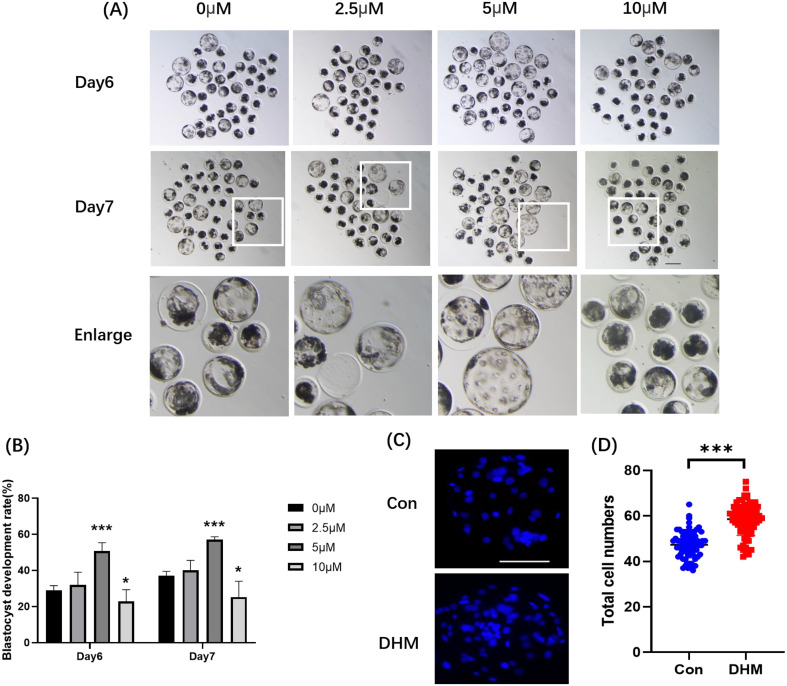
After parthenogenetic activation, one-cell-stage parthenotes were cultured in the presence of various concentrations of DHM. We examined the blastocyst rate at different concentrations of DHM (0 (control), 2.5, 5, 10 μM). The results revealed that on day 6, the blastocyst formation rates were 29.06 ± 2.48%, 31.89 ± 7.14%, 50.74 ± 4.65%, and 22.83 ± 6.50% with 0 μM, 2.5 μM, 5 μM, and 10 μM DHM, respectively. On day 7, the rates were 37.00 ± 2.48%, 40.11 ± 5.47%, 57.04 ± 1.60%, and 25.32 ± 8.73%, respectively (Figs. 1A, B). On day 6, the total blastocyst cell numbers in the control and 5 μM DHM-treated group were 47.32 ± 5.85% and 58.56 ± 7.22%, respectively (Figs. 1C, D). Based on these results, a concentration of 5 μM was selected for subsequent experiments.
Fig. 1.
Effects of various DHM concentrations (0 μM, 2.5 μM, 5 μM, and 10 μM) on porcine embryo development. (A) Embryo development on different days. Scale bar = 200 μm. (B) Blastocyst formation rates on different days. Numbers of oocytes (n) used in this experiment were 189, 173, 191, and 175 with 0 μM, 2.5 μM, 5 μM, and 10 μM DHM, respectively. R = 5. (C) On the seventh day, the blastocysts were stained with Hoechst 33342. Scale bar = 100 μm. (D) Total cell number of blastocysts on day 7 in the Con (n = 72) and DHM-treated (n = 80) groups. R = 4. Data are presented as the mean ± SD. Significant differences are represented by *(P < 0.05), **(P < 0.01), and ***(P < 0.001).
DHM supplementation can reduce oocyte apoptosis and increase the level of proliferation
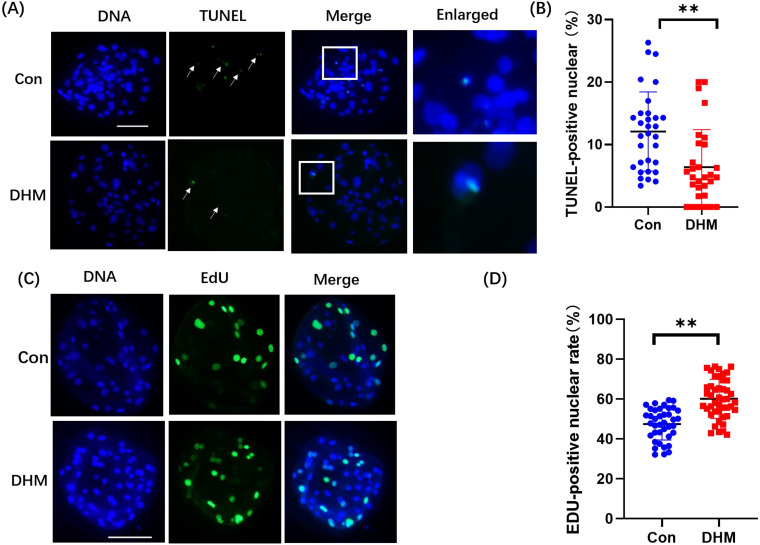
To explore how 5 μM DHM treatment improved the quality of porcine PA embryos, we first evaluated cell proliferation ability and apoptosis in blastocyst cultures with and without DHM treatment. The relative number of EdU-positive nuclei in DHM-treated PA embryos increased by 12.66 ± 1.84-fold compared to that in the control group (Figs. 2A, B). The ratio of TUNEL-positive nuclei in the DHM-treated PA embryos and control embryos were 12.08 ± 6.35% and 6.38 ± 6.01%, respectively (Figs. 2C, D).
Fig. 2.
Effects of DHM on proliferation and apoptosis of blastocyst cells. (A) TUNEL staining of blastocysts on day 7. Scale bar = 100 μm. (B) The proportion of apoptotic cells in the Con (n = 31) and DHM-treated (n = 30) groups. R = 3. (C) Representative EdU stained images of blastocysts. (D) Proportion of proliferating cells in embryos treated with DHM (n = 39) and not treated with DHM (n = 45) on day 7. R = 4. Data are presented as the mean ± SD. Significant differences are represented by *(P < 0.05), **(P < 0.01), and ***(P < 0.001).
DHM enhanced the oxidation resistance of porcine early-stage embryos
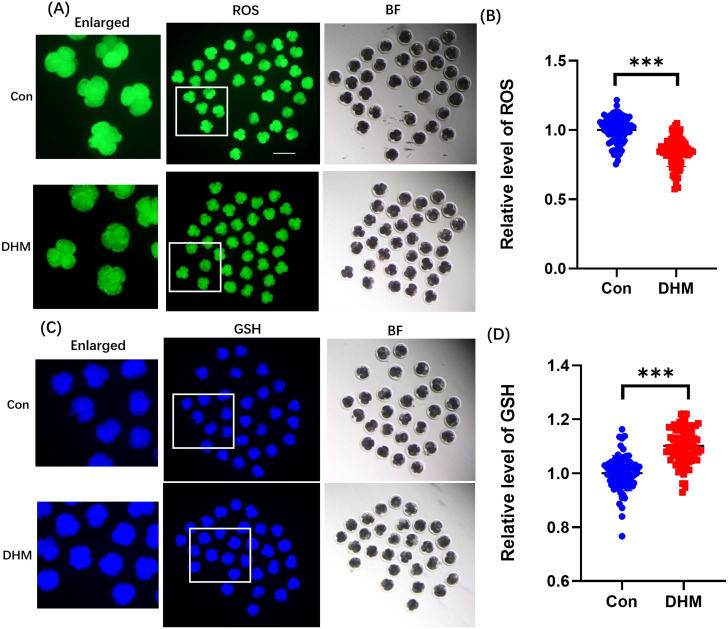
Early embryos are easily perturbed by oxidative stress during development, which results in developmental arrest. Therefore, ROS levels were measured in this study. The ROS levels in early embryos in the DHM-treated group were significantly lower than those in the control group (Fig. 3A, 0.20 ± 0.76-fold). As GSH levels vary in different cells, and GSH exhibits strong antioxidant properties, the GSH levels were measured. The GSH levels of early embryos in the DHM-treated group were significantly higher than those in the control group (Fig. 3B, 0.12 ± 0.01-fold).
Fig. 3.
Effects of DHM on ROS and GSH levels in 4-cell embryos. (A) Representative ROS staining images of 4-cell-stage embryos. Scale bar = 200 μm. (B) Relative changes in ROS fluorescence intensity levels of 4-cell-stage embryos in the Con (n = 83) and DHM-treated (n = 89) groups. ROS fluorescence intensity in DHM-treated 4-cell stage embryos was significantly decreased to 0.16 ± 0.07-fold compared to that in the Con group. Blue and red dots represent the measurement distributions in the control and DHM groups, respectively. R = 3. (C) Representative GSH-stained images of 4-cell-stage embryos. Scale bar = 200 μm. (D) Relative GSH fluorescence intensity levels of 4-cell-stage embryos in the Con (n = 76) and DHM-treated (n = 85) groups. DHM treatment of 4-cell stage embryos led to a 0.11 ± 0.01-fold increase in GSH fluorescence intensity levels. R = 3. Data are presented as mean ± SD. Significant differences are represented by *(P < 0.05), **(P < 0.01), and ***(P < 0.001).
DHM enhances mitochondrial activity during early porcine embryonic development
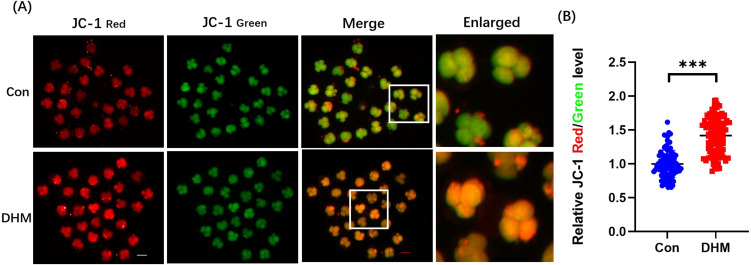
As mitochondria play an important role in maintaining normal cell metabolism, the mitochondrial membrane potential was analyzed. A representative image of JC-1 staining is shown in Fig. 4. Our results showed that the ΔΨm values of embryos in the DHM treatment group were significantly higher (1.34 ± 0.48-fold, P < 0.01) than those of the control embryos. Thus, the ΔΨm increased significantly after DHM treatment, indicating that DHM supplementation enhanced mitochondrial activity.
Fig. 4.
Effects of DHM on mitochondrial function in 4-cell stage embryos. (A) Representative JC-1 staining images of 4-cell stage embryos in the con and DHM-treated groups. Scale bar = 100 μm. (B) Relative fluorescence levels of JC-1 red/green in 4-cell stage embryos with (n = 95) and without DHM treatment (n = 98). R = 3. Four-cell stage embryos with DHM treatment led to a 0.42 ± 0.07-fold increase in JC-1 fluorescence intensity levels. Data are presented as the mean ± SD. Significant differences are represented by *(P < 0.05), **(P < 0.01), and ***(P < 0.001).
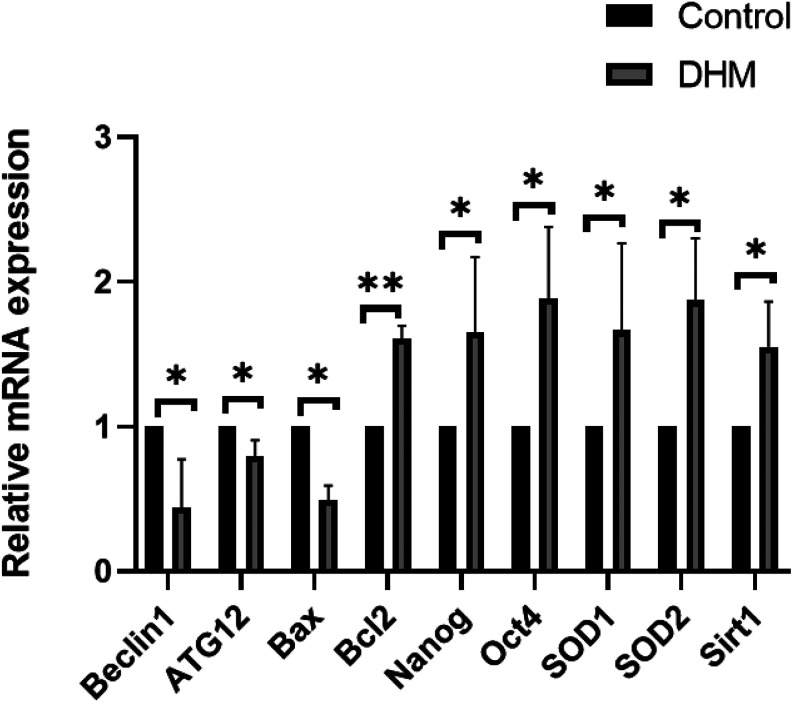
Differential gene expression in blastocysts with or without DHM
To analyze the potential mechanism by which DHM influences embryonic development, the expression levels of pluripotency-related genes (Nanog and Oct4), autophagy-related genes (Beclin1 and ATG12), apoptosis-related genes (Bax and Bcl2), and antioxidant stress-related genes (SOD1, SOD2, and SIRT1) were quantified using qRT-PCR (Fig. 5). The results showed that the expression levels of Nanog (P < 0.05), Oct4 (P < 0.05), SOD1 (P < 0.05), SOD2 (P < 0.05), SIRT1 (P < 0.05), and Bcl2 (P < 0.01) were higher than those of the control group. Conversely, those of Beclin1 (P < 0.05), ATG12 (P < 0.05), and Bax (P < 0.05) were lower in the DHM-supplemented group than those in the control group.
Fig. 5.
Differential gene expression in blastocysts. Gene expression levels were analyzed in porcine blastocysts with or without DHM treatment on day 7. Significant differences are represented by *(P < 0.05) and **(P < 0.01).
Discussion
During IVC, embryos are subjected to various oxidative stressors. Owing to the limited ability of the embryos to alleviate oxidative stress, ROS overproduction could disrupt the existing cellular pre-oxidant/antioxidant balance and lead to serious oxidative stress [34], causing high embryonic mortality and low development rate. Therefore, this study examined whether the addition of the natural antioxidant DHM could reduce the level of oxidative stress to improve embryo quality. Different concentrations of DHM were added to the IVC of embryos for investigation. The experiment revealed that 5 μM DHM effectively improves blastocyst development by stabilizing mitochondrial function, alleviating oxidative stress, and reducing apoptosis and autophagy. These results indicate that DHM can increase the developmental potential of early embryos by reducing oxidative stress.
The in vitro maturation efficiency of oocytes is significantly lower than the in vivo efficiency, and the number of preantral follicular embryos cultured in vitro is very low. This could be attributed to the oxidative stress induced by the imbalance between intracellular antioxidant activity and ROS clearance [35]. Mammalian oocytes are highly sensitive to ROS. However, the maturation process of porcine oocytes is longer than that of other mammalian oocytes [36]. Thus, porcine oocytes are more sensitive to oxidative stress owing to prolonged ROS production. Therefore, ensuring a dynamic balance between ROS and antioxidant levels is essential for normal oocyte development. GSH, the major cellular antioxidant contributing to both non-enzymatic and enzyme-dependent defense against ROS, is an inefficient substrate for SOD1-catalyzed H2O2 formation [37]. The absence of SOD1 thus leads to oxidative stress, which dramatically increases intracellular ROS levels and induces mitochondrial dysfunction [38, 39].
In this study, DHM supplementation significantly decreased ROS levels and significantly increased GSH levels in 4-cell-stage embryos. Our results are consistent with the results of other studies [40,41,42]. Simultaneously, the mRNA expression levels of the antioxidant stress genes SOD1, SOD2, and SIRT1 in blastocyst cells were measured. The results showed that the addition of DHM increased the expression of antioxidant stress genes. Collectively, DHM may improve the embryonic development rate by improving the antioxidant capacity of oocytes; however, elucidating the details of this process requires further confirmation.
Mitochondria are important organelles, and their membrane potential affects ATP generation during oxidative phosphorylation [43], which is also a source of ROS in cells. Normal MMP is necessary for mitochondrial oxidative phosphorylation and ATP production. When MMP decreases, mitochondria swell, ATP hydrolysis and ion homeostasis are disrupted, and pro-apoptotic factors (such as cytochrome c) are released to promote apoptosis [44, 45]. Cells and embryos exhibit better growth, proliferation, and growth potential when MMP is high [7, 46, 47]. It has been reported that DHM can promote the phosphorylation of AMPK and ulk1 and improve mitochondrial function [48], which is consistent with our results.
In our study, the cell apoptosis TUNEL and cell proliferation EdU assays indicated that supplementation with DHM effectively reduces apoptosis, and the total cell number and the cell proliferation rate of blastocysts were significantly increased. This is related to the ability of DHM to increase GSH levels and reduce ROS accumulation because high levels of ROS can damage cell membranes and DNA, causing apoptosis [49,50,51]. The downregulation of the apoptotic gene Bax and upregulation of the anti-apoptotic gene Bcl2 in the DHM-supplemented group also supports this hypothesis [52,53,54].
Finally, we examined the expression of pluripotency-related genes (Nanog and Oct4) and autophagy-related genes (Beclin1 and ATG12) to explore the effects of DHM on early embryos. The gene expression of Nanog and Oct4 was upregulated by DHM supplementation, indicating that DHM can maintain pluripotency and self-renewal and sustain the development of pre-implantation embryos [55, 56]. Simultaneously, the expression of autophagy genes Beclin1 and ATG12 decreased, indicating that DHM can protect oocytes from autophagy damage.
Compared to parthenogenesis, in vitro fertilization is associated with a high frequency of polyspermy, which leads to chromosomal abnormalities in embryos [57, 58]. Although many groups have explored various methods to reduce the occurrence of polyspermy in porcine embryos, normal porcine embryos are produced in vitro [57, 59]. This remains a challenge and requires further exploration. However, as of date, no research has been conducted on whether DHM can reduce the occurrence of polyspermy during in vitro fertilization; thus, this topic requires future research and verification.
In summary, adding DHM under in vitro culture conditions after parthenogenesis can improve the quality and developmental competence of early embryos by inhibiting apoptosis, increasing GSH content, enhancing mitochondrial function, and reducing ROS accumulation and autophagy. These findings will help improve in vitro embryo production and the efficiency of assisted reproduction.
Conflict of interests
The authors have no conflicts to declare.
Acknowledgments
This study was supported by Science and Technology Planning Project of Guangdong Provincial Department of Science and Technology (Project No.: 2021B1212040016) and funding for special project in key areas of biomedicine and health of Guangdong Provincial Department of Education (Project No.: 2021ZDZX2046).
References
- 1.Fowler KE, Mandawala AA, Griffin DK, Walling GA, Harvey SC. The production of pig preimplantation embryos in vitro: Current progress and future prospects. Reprod Biol 2018; 18: 203–211. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang H, Han J, Huang Y. Pig cloning using somatic cell nuclear transfer. Methods Mol Biol 2021; 2239: 1–18. [DOI] [PubMed] [Google Scholar]
- 3.Gün G, Kues WA. Current progress of genetically engineered pig models for biomedical research. Biores Open Access 2014; 3: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci 2016; 23: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soto-Heras S, Paramio MT. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res Vet Sci 2020; 132: 342–350. [DOI] [PubMed] [Google Scholar]
- 6.Jiang WJ, Yao XR, Zhao YH, Gao QS, Jin QG, Li YH, Yan AG, Xu YN. L-carnitine prevents bovine oocyte aging and promotes subsequent embryonic development. J Reprod Dev 2019; 65: 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang H, Liang S, Yao XR, Jin YX, Shen XH, Yuan B, Zhang JB, Kim NH. Laminarin improves developmental competence of porcine early stage embryos by inhibiting oxidative stress. Theriogenology 2018; 115: 38–44. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DT, Zhang X, Vanderhyden BC, Khamsi F. Hormonal actions during oocyte maturation influence fertilization and early embryonic development. Ann N Y Acad Sci 1991; 626: 137–158. [DOI] [PubMed] [Google Scholar]
- 9.Abbasi B, Dong Y, Rui R. Resveratrol Hinders Postovulatory Aging by Modulating Oxidative Stress in Porcine Oocytes. Molecules 2021; 26: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng YX, Chen CZ, Luo D, Yu WJ, Li SP, Xiao Y, Yuan B, Liang S, Yao XR, Kim NH, Jiang H, Zhang JB. Carnosic acid improves porcine early embryonic development by inhibiting the accumulation of reactive oxygen species. J Reprod Dev 2020; 66: 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Gao L, Li J, Yuan Y, Wang R, Tian Y, Lei A. α-Ketoglutarate improves meiotic maturation of porcine oocytes and promotes the development of pa embryos, potentially by reducing oxidative stress through the Nrf2 pathway. Oxid Med Cell Longev 2022; 2022: 7113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury MMR, Mesalam A, Khan I, Joo MD, Lee KL, Xu L, Afrin F, Kong IK. Improved developmental competence in embryos treated with lycopene during in vitro culture system. Mol Reprod Dev 2018; 85: 46–61. [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Jeong PS, Joo YE, Kang HG, Kim MJ, Lee S, Song BS, Kim SU, Cho SK, Sim BW. Luteolin orchestrates porcine oocyte meiotic progression by maintaining organelle dynamics under oxidative stress. Front Cell Dev Biol 2021; 9: 689826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almubarak AM, Kim E, Yu IJ, Jeon Y. Supplementation with Niacin during in vitro maturation improves the quality of porcine embryos. Theriogenology 2021; 169: 36–46. [DOI] [PubMed] [Google Scholar]
- 15.Xu YN, Shen XH, Lee SE, Kwon JS, Kim DJ, Heo YT, Cui XS, Kim NH. Autophagy influences maternal mRNA degradation and apoptosis in porcine parthenotes developing in vitro. J Reprod Dev 2012; 58: 576–584. [DOI] [PubMed] [Google Scholar]
- 16.Hale BJ, Li Y, Adur MK, Keating AF, Baumgard LH, Ross JW. Characterization of the effects of heat stress on autophagy induction in the pig oocyte. Reprod Biol Endocrinol 2021; 19: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal A, Majzoub A. Role of antioxidants in assisted reproductive techniques. World J Mens Health 2017; 35: 77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanihara F, Hirata M, Nhien NT, Hirano T, Kunihara T, Otoi T. Effect of ferulic acid supplementation on the developmental competence of porcine embryos during in vitro maturation. J Vet Med Sci 2018; 80: 1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Jin JX, Taweechaipaisankul A, Kim GA, Lee BC. Synergistic effects of resveratrol and melatonin on in vitro maturation of porcine oocytes and subsequent embryo development. Theriogenology 2018; 114: 191–198. [DOI] [PubMed] [Google Scholar]
- 20.Murakami T, Miyakoshi M, Araho D, Mizutani K, Kambara T, Ikeda T, Chou WH, Inukai M, Takenaka A, Igarashi K. Hepatoprotective activity of tocha, the stems and leaves of Ampelopsis grossedentata, and ampelopsin. Biofactors 2004; 21: 175–178. [DOI] [PubMed] [Google Scholar]
- 21.Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang C, Li M, Bao S, Zhu R. Dihydromyricetin reduced Bcl-2 expression via p53 in human hepatoma HepG2 cells. PLoS One 2013; 8: e76886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou X, Tong Q, Wang W, Xiong W, Shi C, Fang J. Dihydromyricetin protects endothelial cells from hydrogen peroxide-induced oxidative stress damage by regulating mitochondrial pathways. Life Sci 2015; 130: 38–46. [DOI] [PubMed] [Google Scholar]
- 23.Hou XL, Tong Q, Wang WQ, Shi CY, Xiong W, Chen J, Liu X, Fang JG. Suppression of inflammatory responses by dihydromyricetin, a flavonoid from ampelopsis grossedentata, via inhibiting the activation of NF-κB and MAPK signaling pathways. J Nat Prod 2015; 78: 1689–1696. [DOI] [PubMed] [Google Scholar]
- 24.Meng G, Yang S, Chen Y, Yao W, Zhu H, Zhang W. Attenuating effects of dihydromyricetin on angiotensin II-induced rat cardiomyocyte hypertrophy related to antioxidative activity in a NO-dependent manner. Pharm Biol 2015; 53: 904–912. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Luo HQ, Sun LL, Xu MT, Yu J, Liu LL, Zhang JY, Wang YQ, Wang HX, Bao XF, Meng GL. Dihydromyricetin attenuates myocardial hypertrophy induced by transverse aortic constriction via oxidative stress inhibition and SIRT3 pathway enhancement. Int J Mol Sci 2018; 19: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Chen Y, Luo H, Sun L, Xu M, Yu J, Zhou Q, Meng G, Yang S. Recent update on the pharmacological effects and mechanisms of dihydromyricetin. Front Pharmacol 2018; 9: 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semwal DK, Semwal RB, Combrinck S, Viljoen A. Myricetin: a dietary molecule with diverse biological activities. Nutrients 2016; 8: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng X, Yang J, Hu O, Huang J, Ran L, Chen M, Zhang Y, Zhou X, Zhu J, Zhang Q, Yi L, Mi M. Dihydromyricetin ameliorates nonalcoholic fatty liver disease by improving mitochondrial respiratory capacity and redox homeostasis through modulation of SIRT3 signaling. Antioxid Redox Signal 2019; 30: 163–183. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Zhou M, Lang H, Zhou Y, Mi M. Dihydromyricetin enhances glucose uptake by inhibition of MEK/ERK pathway and consequent down-regulation of phosphorylation of PPARγ in 3T3-L1 cells. J Cell Mol Med 2018; 22: 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Q, Liu L, Yu J, Zhang J, Xu M, Sun L, Luo H, Feng Z, Meng G. Dihydromyricetin attenuated Ang II induced cardiac fibroblasts proliferation related to inhibitory of oxidative stress. Eur J Pharmacol 2017; 807: 159–167. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Li Q, Liu Z, Yang K, Chen Z, Cheng Q, Wu L. The versatile effects of dihydromyricetin in health. Evid Based Complement Alternat Med 2017; 2017: 1053617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshioka K. Development and application of a chemically defined medium for the in vitro production of porcine embryos. J Reprod Dev 2011; 57: 9–16. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki C, Yoshioka K, Sakatani M, Takahashi M. Glutamine and hypotaurine improves intracellular oxidative status and in vitro development of porcine preimplantation embryos. Zygote 2007; 15: 317–324. [DOI] [PubMed] [Google Scholar]
- 34.Polte T, Tyrrell RM. Involvement of lipid peroxidation and organic peroxides in UVA-induced matrix metalloproteinase-1 expression. Free Radic Biol Med 2004; 36: 1566–1574. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005; 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalous J, Tetkova A, Kubelka M, Susor A. Importance of ERK1/2 in regulation of protein translation during oocyte meiosis. Int J Mol Sci 2018; 19: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chi L, Ke Y, Luo C, Gozal D, Liu R. Depletion of reduced glutathione enhances motor neuron degeneration in vitro and in vivo. Neuroscience 2007; 144: 991–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer LR, Li Y, Asress SA, Jones DP, Glass JD. Absence of SOD1 leads to oxidative stress in peripheral nerve and causes a progressive distal motor axonopathy. Exp Neurol 2012; 233: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tafuri F, Ronchi D, Magri F, Comi GP, Corti S. SOD1 misplacing and mitochondrial dysfunction in amyotrophic lateral sclerosis pathogenesis. Front Cell Neurosci 2015; 9: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mu S, Li Y, Liu B, Wang W, Chen S, Wu J, OuYang L, Zhu Y, Li K, Zhan M, Liu Z, Jia Y, Ma Y, Lei W. Dihydromyricetin ameliorates 3NP-induced behavioral deficits and striatal injury in rats. J Mol Neurosci 2016; 60: 267–275. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Liu J, Lin J, Wang T, Huang J, Lin Y, Chen D. Protective effects of dihydromyricetin against •OH-induced mesenchymal stem cells damage and mechanistic chemistry. Molecules 2016; 21: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu P, Dong Y, Li B, Kang XJ, Gu C, Zhu T, Luo YY, Pang MX, Du WF, Ge WH. Dihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol Lett 2017; 274: 31–41. [DOI] [PubMed] [Google Scholar]
- 43.Klingenberg M. The ADP-ATP translocation in mitochondria, a membrane potential controlled transport. J Membr Biol 1980; 56: 97–105. [DOI] [PubMed] [Google Scholar]
- 44.Cecchino GN, Seli E, Alves da Motta EL, García-Velasco JA. The role of mitochondrial activity in female fertility and assisted reproductive technologies: overview and current insights. Reprod Biomed Online 2018; 36: 686–697. [DOI] [PubMed] [Google Scholar]
- 45.Bentov Y, Casper RF. The aging oocyte—can mitochondrial function be improved? Fertil Steril 2013; 99: 18–22. [DOI] [PubMed] [Google Scholar]
- 46.Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod 2001; 16: 909–917. [DOI] [PubMed] [Google Scholar]
- 47.Wilding M, De Placido G, De Matteo L, Marino M, Alviggi C, Dale B. Chaotic mosaicism in human preimplantation embryos is correlated with a low mitochondrial membrane potential. Fertil Steril 2003; 79: 340–346. [DOI] [PubMed] [Google Scholar]
- 48.Wu B, Lin J, Luo J, Han D, Fan M, Guo T, Tao L, Yuan M, Yi F. Dihydromyricetin protects against diabetic cardiomyopathy in streptozotocin-induced diabetic mice. Biomed Res Int 2017; 2017: 3764370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod 1998; 13: 998–1002. [DOI] [PubMed] [Google Scholar]
- 50.Bian YH, Xu J, Zhao WY, Zhang ZZ, Tu L, Cao H, Zhang ZG. Targeting mTORC2 component rictor inhibits cell proliferation and promotes apoptosis in gastric cancer. Am J Transl Res 2017; 9: 4317–4330. [PMC free article] [PubMed] [Google Scholar]
- 51.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 2014; 94: 909–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen K, WuWong DJ, Wong S, Matsuyama M, Matsuyama S. Pharmacological inhibition of Bax-induced cell death: Bax-inhibiting peptides and small compounds inhibiting Bax. Exp Biol Med (Maywood) 2019; 244: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hattori T, Ookawa N, Fujita R, Fukuchi K. Heterodimerization of Bcl-2 and Bcl-X(L) with Bax and Bad in colorectal cancer. Acta Oncol 2000; 39: 495–500. [DOI] [PubMed] [Google Scholar]
- 54.Li W, Sang H, Xu X, Zhang Y, Meng X, Chen B. Protective effect of dihydromyricetin on vascular smooth muscle cell apoptosis induced by hydrogen peroxide in rats. Perfusion 2022; 2676591211059901. [DOI] [PubMed] [Google Scholar]
- 55.Zhang L, Luo YB, Bou G, Kong QR, Huan YJ, Zhu J, Wang JY, Li H, Wang F, Shi YQ, Wei YC, Liu ZH. Overexpression Nanog activates pluripotent genes in porcine fetal fibroblasts and nuclear transfer embryos. Anat Rec (Hoboken) 2011; 294: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 56.Pfeiffer MJ, Balbach ST, Esteves TC, Crosetto N, Boiani M. Enhancing somatic nuclear reprogramming by Oct4 gain-of-function in cloned mouse embryos. Int J Dev Biol 2010; 54: 1649–1657. [DOI] [PubMed] [Google Scholar]
- 57.Nagai T, Funahashi H, Yoshioka K, Kikuchi K. Up date of in vitro production of porcine embryos. Front Biosci 2006; 11: 2565–2573. [DOI] [PubMed] [Google Scholar]
- 58.Koo DB, Kim YJ, Yu I, Kim HN, Lee KK, Han YM. Effects of in vitro fertilization conditions on preimplantation development and quality of pig embryos. Anim Reprod Sci 2005; 90: 101–110. [DOI] [PubMed] [Google Scholar]
- 59.Grupen CG. The evolution of porcine embryo in vitro production. Theriogenology 2014; 81: 24–37. [DOI] [PubMed] [Google Scholar]