Abstract
Arrhythmogenic cardiomyopathy (ACM), a fatal heart disease characterized by fibroadipocytic replacement of cardiac myocytes, accounts for 20% of sudden cardiac death and lacks effective treatment. It is often caused by mutations in desmosome proteins, with Desmoglein-2 (DSG2) mutations as a common etiology. However, the mechanism underlying the accumulation of fibrofatty in ACM remains unknown, which impedes the development of curative treatment. Here we investigated the fat accumulation and the underlying mechanism in a mouse model of ACM induced by cardiac-specific knockout of Dsg2 (CS-Dsg2−/−). Heart failure and cardiac lipid accumulation were observed in CS-Dsg2−/− mice. We demonstrated that these phenotypes were caused by decline of fatty acid (FA) β-oxidation resulted from impaired mammalian target of rapamycin (mTOR) signaling. Rapamycin worsened while overexpression of mTOR and 4EBP1 rescued the FA β-oxidation pathway in CS-Dsg2−/− mice. Reactivation of PPARα by fenofibrate or AAV9-Pparα significantly alleviated the lipid accumulation and restored cardiac function. Our results suggest that impaired mTOR–4EBP1–PPARα-dependent FA β-oxidation contributes to myocardial lipid accumulation in ACM and PPARα may be a potential target for curative treatment of ACM.
Key words: Arrhythmogenic cardiomyopathy, Desmosome, Desmoglein2, Heart failure, Lipid accumulation, mTOR, PPARα, FA oxidation
Graphical abstract
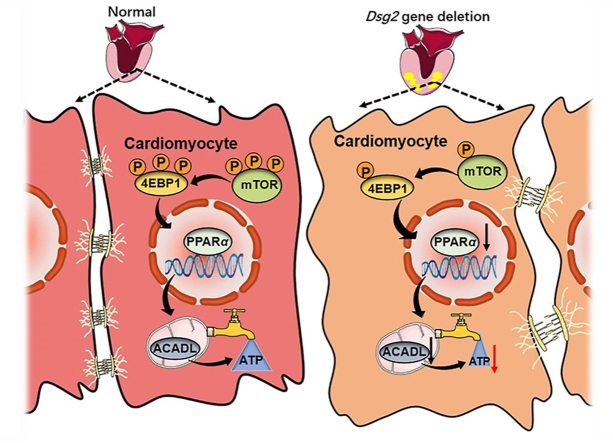
Deletion of Dsg2 in cardiomyocytes leads to downregulation of the mTOR–4EBP1–PPARα signaling pathway, resulting in impaired fatty acid β-oxidation pathway and the development of arrhythmogenic cardiomyopathy.
1. Introduction
Arrhythmogenic cardiomyopathy (ACM) is a heritable cardiac disease associated with ventricular arrhythmias, heart failure (HF), thromboembolism and sudden cardiac death (SCD) especially in young athletes1. It is believed to be responsible for up to 20% of SCD cases2. Up to now, there is no effective treatment for ACM, while the main goal of treatment is the prevention of SCD3. ACM is pathologically characterized by a progressive loss of cardiomyocytes and fibro-fatty tissue replacement predominantly in the right ventricle (RV), with left ventricular (LV) or bilateral ventricular involvement in some cases1,4. More than 50% ACM patients present one or more mutations in genes encoding structural proteins especially those consist the cardiac desmosomes, including desmoglein2 (DSG2)5, desmocollin26, plakoglobin7, desmoplakin8 and plakophilin-29. Desmosomes are specialized and highly ordered membrane structures that mediate cell–cell contact and strong adhesion. Desmosomes contribute to the mechanical integrity of the myocardium by anchoring the intermediate filament system to the plasma membrane in adjacent cells10,11. DSG2 is a major cadherin of the cardiac desmosome and connect neighboring cardiomyocytes with desmocollin2 via interaction of their extracellular domains12,13. Dsg2 mutations are the second common etiology of ACM and associate with worst prognosis14. Transgenic mice overexpressing the mutation of Dsg2 p.N271S exhibit a progressive cardiomyocyte loss and fibroadipocytic replacement, which is associated with severe heart muscle diseases such as ACM15. In another study, mice carrying a deletion of the adhesive extracellular domain of Dsg2 also developed ARVC-like phenotype with fibrotic lesion and lipid droplet detected in the heart more frequently than in wild-type mice16.
Impaired energy supply is regarded as the major cause of cardiac dysfunction in ACM patients17. The cardiac excitation–contraction coupling consumes the majority of the ATP produced by the mitochondria to fuel incessant activity18. Under normal conditions, 60%–90% of the ATP supply in heart relies on fatty acid (FA) oxidation, while the remaining 10%–40% is derived from pyruvate oxidation19. However, in pathological scenarios, development of overt cardiac dysfunction is accompanied by decline in FA oxidation rates, which has been confirmed in different species and models of HF, including ischaemic20 and pacing-induced21 HF, as well as in humans end-stage HF22,23. The decrease in FA oxidation might be explained at least in part by the suppression of peroxisome proliferator-activated receptors α (PPARα) signaling24. PPARα is a nuclear receptor with most abundant expression in the myocardium, where it upregulates the transcription of genes related to FA uptake and oxidation with PPARγ co-activator 1α (PGC1α)25.
Mechanistic target of rapamycin (mTOR), served as an intracellular fuel sensor, is vital for the maintenance of cardiac structure and function in the postnatal period and adulthood26,27. Cardiac-specific mTOR-knockout embryos present a significant reduction in cardiomyocyte proliferation and an increase in apoptosis, which results in cardiac dilation and dysfunction, with signs of terminal HF28. Overexpression of eukaryotic initiation factor 4E (eIF4E) binding protein (4E-BP), one of mTORC1 downstream target, leads to enhanced mitochondrial respiration with increased PGC-1α translation29. Activation of PGC1α/PPARα/mTOR pathway plays a significant role in maintaining the oxidative metabolism/glycolysis balance and improves mitochondrial function in rat models of HF30. These findings inspired the hypothesis that mTOR–4EBP1–PPARα pathway plays a role in ACM-related cardiac dysfunction and lipid accumulation. To test this hypothesis, we examined this pathway and its involvement in FA oxidation and cardiac function in an ACM mouse model induced by cardiac-specific Dsg2 knockout. We show that mTOR–4EBP1–PPARα axis in cardiac muscle is essential for the energy supply by FA oxidation and normal heart function. Furthermore, reactivation of PPARα by fenofibrate (a PPARα agonist) or cardiac-specific overexpression of PPARα rescues the impaired cardiac morphology and function resulted from cardiac Dsg2 deletion, suggesting PPARα is a potential target for ACM treatment.
2. Materials and methods
2.1. Materials
Rapamycin, DMSO and fenofibrate were purchased from Sigma–Aldrich (St. Louis, MO, USA). Rabbit anti-phospho-mTOR (Ser2448), anti-mTOR, anti-phospho-4EBP1 (Thr37/46), anti-4EBP1, anti-PGC1α antibodies and mouse monoclonal anti-β-actin were from Cell Signaling Technology (Beverly, MA, USA). Rabbit anti-DSG2, anti-PPARα, anti-CPT1b, anti-ACADL, anti-GAPDH antibodies were from Abcam Inc. (Cambridge, MA, USA). Horseradish peroxidase-conjugated, donkey anti-rabbit IgG and donkey anti-mouse IgG were from Jackson ImmunoResearch (West Grove, PA, USA). Immobilon western chemiluminescent HRP substrate was purchased from Millipore (Temecula, CA, USA). Trizol reagent and the reverse transcription (RT) system were purchased from Promega Inc. (Madison, WI, USA).
2.2. Animals and treatments
Cardiac-specific Dsg2 knockout mice (CS-Dsg2−/−) were generated using a standard strategy of Cre-LoxP recombination. The targeting vector contained 1.9 kb 5′-homologous arm, flox region, PGK-Neo-polyA marker, 3.3 kb 3′-homologous arm and MC1-TK-polyA negative selection marker. An FRT-flanked Neo cassette was inserted to 3′-homologous arm, and two LoxP sites were introduced to 5′-homologous arm and 3′ of Neo, respectively. Chimeric mice carrying the Neo-floxed Dsg2 allele were crossed with Flp mice (C57BL/6J background) to remove the Neo cassette to obtain F1 mice (Dsg2fl-neo/+). Alternatively, Dsg2fl-neo/+ mice were crossed with Ckmm-Cre mice (C57BL/6J background) to obtain Dsg2+/+ (wild-type; WT), Dsg2+/− (heterozygous; HET) and Dsg2−/− (homozygote; −/−) mice. Animals used in this study were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publications No. 8023, revised 1978). All animal protocols were approved by the Animal Care and Use Committee of Jinan University (Guangzhou, China).
8–14-week mice were kept on a regular chow diet (Research Diets Inc., 10% fat) and allowed ad libitum access to food and water.
To verify the effect of mTOR signaling on myocardial lipid metabolism after Dsg2 knockout, 8–10-week-old WT and CS-Dsg2−/− mice were tail vein injected with dimethyl sulfoxide (DMSO) or rapamycin (1 mg/kg) for 9 consecutive days.
To determine whether PPARα reactivation ameliorate heart lipid metabolism and function after Dsg2 knockout, the PPARα agonist fenofibrate (Sigma–Aldrich, dissolved in 0.75% [w/v] cellulose carrier solution) was administrated by daily oral gavage at a dose of 150 mg per kg body weight for 4 weeks; CS-Dsg2−/− mice were tail vein injected with adeno-associated virus containing green fluorescent protein (AAV9-cTnT-GFP, 5 × 1011 vg per mouse) or adeno-associated virus containing peroxisome proliferator-activated receptors α (AAV9-cTnT-Pparα, 5 × 1011 vg per mouse) 28 days before sacrifice.
2.3. AAV9-cTnT promoter-Pparα construction
To construct the plasmid for cardiomyocyte-specific Pparα over-expression, coding sequence of mouse Pparα was inserted into AAV-cTnT-promoter vector. Then the recombinant plasmid and its control plasmid (AAV-cTnT promoter) were transfected into 293T cells for virus packaging. Virus was purified and concentrated by gradient centrifugation. AAV9 titer was determined by quantitative PCR.
2.4. Transthoracic echocardiography
Echocardiographic measurements were performed on mice, anesthetized with 1%–2% isoflurane gas, using the Vevo2100 High Resolution In Vivo Imaging System (Visual Sonics, Toronto, Canada) with a SL3116 high-frequency linear array probe and a 22 MHz transducer. HR (heart rate), the thickness of left ventricular anterior wall in diastole period (LVAW;d) and in systole period (LVAW;s), the thickness of left ventricular posterior wall in diastole period (LVPW;d) and in systole period (LVPW;s), ejection fraction of left ventricle (EF), the short axis shortening rate of left ventricle (FS), cardiac output per minute of left ventricle (CO), stroke volume of left ventricle (SV), the diameter of left ventricle in systole period (Diameter;s) and in diastole period (Diameter;d), the volume of left ventricle in systole period (V;s) and in diastole period (V;d) were measured digitally on M-mode tracing.
2.5. Histological analysis
Heart samples were harvested, fixed in 4% paraformaldehyde, paraffin-embedded, cut into 3-μm sections and stained with hematoxylin–eosin (H&E) according to standard procedures. For Oil red O staining, frozen sections (6 μm) of heart were stained in 0.5% Oil red O solution for 2 h in a 50 °C oven and then in 85% propylene glycol solution for 5 min. Sections were rinsed in distilled water, stained in Gill's hematoxylin for 2 s, washed, and mounted with aqueous mounting medium.
2.6. Determine of triglyceride and free fatty acid in heart and plasma
The triglyceride and free fatty acid content in heart tissue and plasma were measured according to the manufacturer's instructions (Boxbio, Beijing, China). Values were normalized to protein concentration. For human plasma triglyceride measurement, ACM patients and normal participants were recruited in current study. Participation in this study was voluntary and written informed consent was obtained from each participant. The guidelines of the Declaration of Helsinki of the World Medical Association were followed. All protocols were approved by the Guangdong Medical Institutional Review Board and Medical Ethics Committees [No. GDREC2016001H(R1)].
2.7. Fatty acid β-oxidation rate analysis and quantification of cardiac ATP
Myocardial mitochondria were isolated integrally by Mitochondrial Isolation Kit (Beyontime, Jiangsu, China). Then the mitochondria were used to investigate fatty acid β-oxidation rate according to the Fatty Acid β-oxidation Kit from Genmed Scientifics Inc., USA.
ATP contents were measured in the heart tissues (20 mg) of mice after overnight fasting by using the ATP Colorimetric/Fluorometric Assay Kit (BioVision).
2.8. Cell culture and treatment
The murine atrial cardiac myocyte cell line HL-1 was purchased from Sigma–Aldrich and maintained at 37 °C in an atmosphere of 5% CO2. For transient transfection, cells were plated at optimal densities and grown for 24 h. Cells were then transfected with Dsg2 siRNA using lipofectamine reagent according to the manufacturer's instructions. Twenty-four hours after transfection, the cells were treated with DMSO or fenofibrate (100 μmol/L) for another 24 h, or treated with Gfp or AAV9-Pparα (AAV9-cTnT-Pparα, 1.5 × 1011 vg/mL) for 48 h. To investigate the effect of mTOR signaling on FA oxidation after Dsg2 knockdown, mTOR or 4Ebp1 plasmid was co-transfected with Dsg2 siRNA for 48 h.
2.9. Quantitative real-time PCR
Quantitative real-time PCR was performed as described previously31. Primers used in this study were shown in Supporting Information Table S1. For gene expression analysis, RNA was isolated from mouse tissues using TRIzol and reverse-transcribed into cDNAs using the First-Strand Synthesis System for RT-PCR kit. SYBR Green-based real-time PCR was performed using the Mx3000 multiplex quantitative PCR system (Stratagene, La Jolla, CA, USA).
2.10. Western blot analysis
Protein extracts were electrophoresed, blotted, and then incubated with primary antibodies. The antibodies were detected using 1:10,000 horseradish peroxidase-conjugated, donkey anti-rabbit IgG and donkey anti-mouse IgG (Jackson ImmunoResearch, USA). A Western blotting luminol reagent was used to visualize bands corresponding to each antibody. The band intensities were quantitated by Image J software.
2.11. Statistical analysis
All data are expressed as mean values ± standard error of mean (SEM). Statistical differences were evaluated by one-way ANOVA followed by Newman-Student-Keuls test or Student's t-test. Differences were considered statistically significant with P values < 0.05.
3. Results
3.1. Cardiac-specific Dsg2 null provokes cardiac dysfunction and lipid accumulation
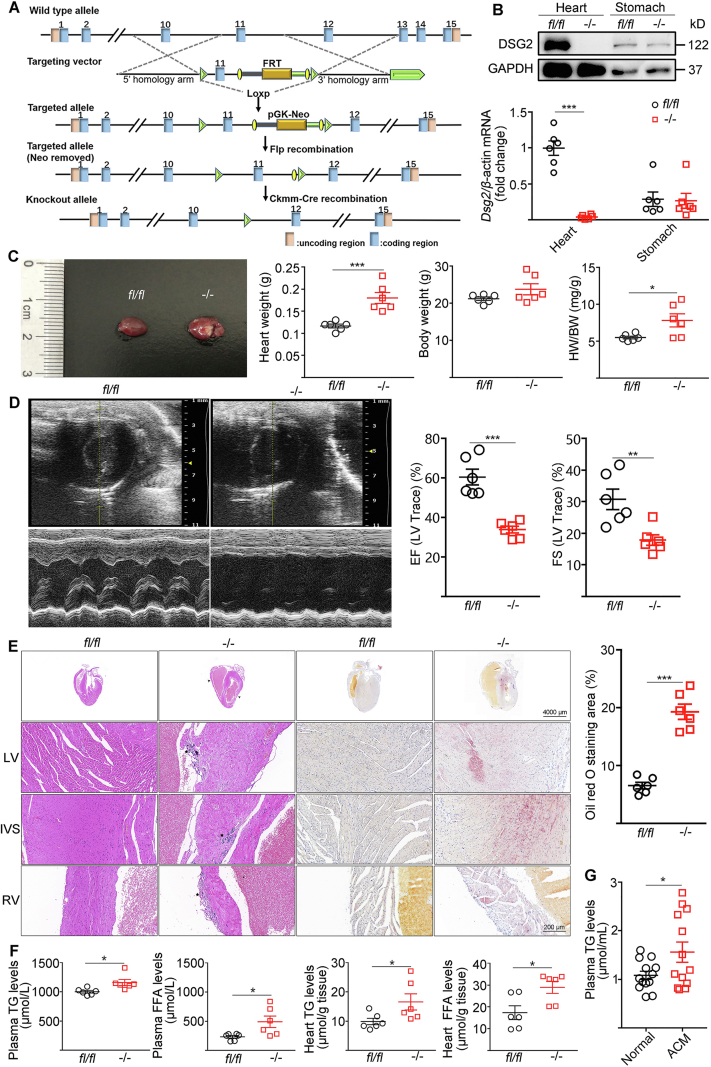
We first generated an ACM mouse model by crossing Dsg2fl-neo/+ mice with Ckmm-Cre mice which resulted in cardiac-specific Dsg2 deletion (CS-Dsg2−/−) (Fig. 1A). Loss of Dsg2 was confirmed in the heart by Western blot but not observed in the stomach of CS-Dsg2−/− mice (Fig. 1B). Cardiac specific Dsg2 deletion cause 13.9% sudden death rate within 3 weeks after birth (Supporting Information Fig. S1). At 12 weeks of age, CS-Dsg2−/− mice showed increased in heart weight by 1.5-fold compared to control mice (Dsg2fl/fl) (0.18 ± 0.01 g for CS-Dsg2−/− mice versus 0.12 ± 0.01 g for controls, P = 0.0007, n = 6) (Fig. 1C). Ventricular tachycardia, atrioventricular block and right bundle branch block were recorded in CS-Dsg2−/− mice (Supporting Information Fig. S2). Dilatation of the right ventricle (RV) and impaired contractile function were observed in 12-week-old CS-Dsg2−/− mice by transthoracic echocardiography (Fig. 1D, Supporting Information Table S2). H&E staining and Oil red O staining also indicated structural disorders and accumulation of lipid in cardiomyocytes after Dsg2 gene deletion (Fig. 1E). No abnormality was observed in other tissues such as brown adipose tissue (BAT), white adipose tissue (WAT), liver, skeletal muscle, pancreas and ileum (Supporting Information Fig. S3). Consistent to the results of Oil red O staining, cardiac specific Dsg2 gene deletion significantly increased plasma and heart triglyceride (TG) levels (Fig. 1F). Similarly, higher TG level was observed in 13 ACM patients than normal controls (Fig. 1G). These results suggest that cardiac-specific Dsg2 deletion was sufficient to provoke cardiac dysfunction and myocardial lipid accumulation.
Figure 1.
Cardiac-specific Dsg2 deletion provoked cardiac dysfunction and lipid accumulation. (A) Strategy used for generating CS-Dsg2−/− mice. (B) mRNA and protein levels of DSG2 were assessed in the heart and stomach of WT (fl/fl) and CS-Dsg2−/− (−/−) mice. (C) Representative heart image, heart weight, body weight and heart/body weight ratio. (D) Representative echocardiographic images (M-Mode). Ejection fraction (EF) and Left ventricular fractional shortening (FS) were calculated from the echocardiographic tracings as previously described. (E) HE staining and Oil red O staining of heart sections. Arrow shows dilated atrium, thinning atrial and ventricular wall, asterisk shows myocardial calcification, fibrosis and atrophy. (F) Plasma triglyceride, plasma free fatty acid, heart triglyceride and free fatty acid levels of WT and CS-Dsg2−/− mice (n = 6, results are expressed as mean values ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 vs. WT mice). (G) Plasma triglyceride levels of normal and ACM patients (n = 13, results are expressed as mean values ± SEM. ∗P < 0.05 vs. normal subjects).
3.2. Impaired FA β-oxidation in CS-Dsg2−/− mice
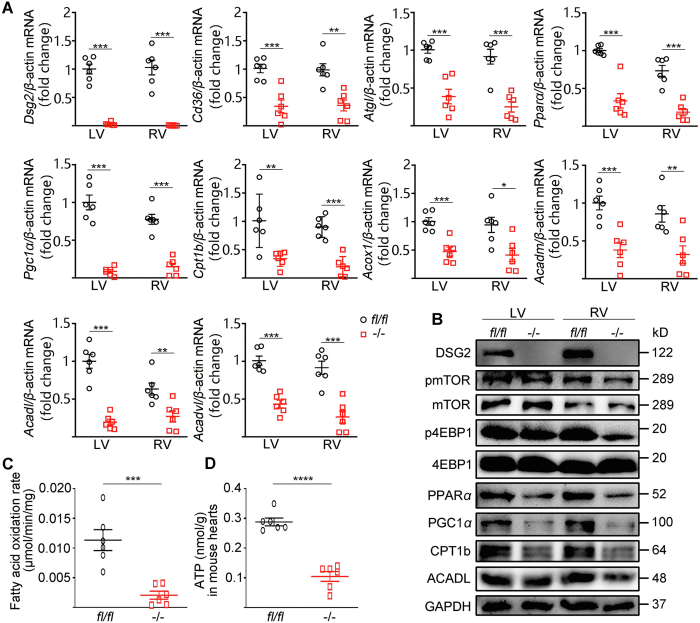
The systolic dysfunction of heart is closely related to myocardial energy supply. A healthy heart relies predominantly (60%–90%) on FA oxidation for ATP production19. Therefore, we next investigated the FA oxidation pathway in heart in CS-Dsg2−/− mice. Downregulation of transcription factors PPARα and PGC1α, as well as enzymes involved in FA oxidation, including carnitine palmitoyltransferase 1b (CPT1b), acyl-CoA oxidase (Acox1), medium-chain acyl-CoA dehydrogenase (ACADM), long-chain acyl-CoA dehydrogenase (ACADL), very long-chain acyl-CoA dehydrogenase (ACADVL) were observed in both LV and RV of CS-Dsg2−/− mice compared to control mice (Fig. 2A and B). We further measured the fatty acid β-oxidation rate of the heart. As shown in Fig. 2C and D, the fatty acid β-oxidation rate and ATP concentration was significantly lower in the heart of CS-Dsg2−/− than controls.
Figure 2.
Decreased FA β-oxidation and mTOR–4EBP1 signaling in CS-Dsg2−/− mice. (A) Results of quantitative PCR analysis of Dsg2, Cd36, Atgl, Pparα, Pgc1α, Cpt1b, Acox1, Acadm, Acadl and Acadvl mRNA levels in LV and RV are expressed as fold change of control using β-actin as loading control. (B) Representative Western blots from WT and CS-Dsg2−/− mice. DSG2, pmTOR, p4EBP1, PPARα, PGC1α, CPT1b and ACADL were detected using specific antibodies. mTOR, 4EBP1 and GAPDH were used as loading controls. Cardiac fatty acid oxidation rate (C) and ATP content (D) of WT and CS-Dsg2−/− mice. Results are expressed as mean values ± SEM. n = 6. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 vs. WT mice.
3.3. Downregulation of mTOR–4EBP impairs FA oxidation and cardiac function in CS-Dsg2−/− mice
mTOR–4EBP pathway is essential for the maintenance of cardiac structure and function in the postnatal period and adulthood26,27,32. In our study, decreased phosphorylation of mTOR (Ser2448) and 4EBP1 (Thr37/46) were observed in both LV and RV of CS-Dsg2−/− mice (Fig. 2B and Supporting Information Fig. S4A).
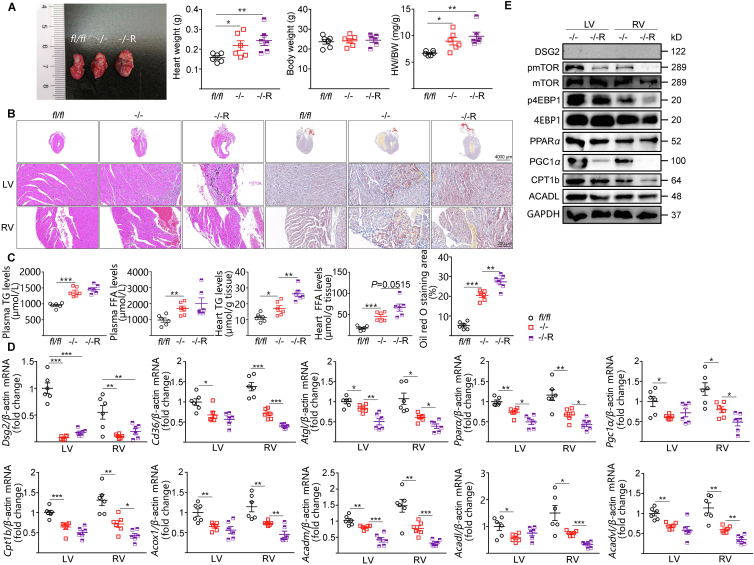
Rapamycin, a well-documented mTORC1 inhibitor, further exacerbated cardiac hypertrophy and lipid accumulation in CS-Dsg2−/− mice (Fig. 3A–C). Rapamycin led to decreased phosphorylation of mTOR and 4EBP1 and suppressions of PPARα and PGC1α, as well as their target genes Cpt1b, Acox1, Acadm, Acadl and Acadvl in the heart (especially the RV) of CS-Dsg2−/− mice (Fig. 3D, E and Fig. S4B).
Figure 3.
Rapamycin exacerbated cardiac function and hypertrophy in CS-Dsg2−/− mice. (A) Representative heart image, heart weight, body weight and heart/body weight ratio of WT, CS-Dsg2−/− and CS-Dsg2−/− mice received rapamycin (–/–R, 1 mg/kg body weight, tail vein injection). (B) HE staining and Oil red O staining of heart sections. (C) Triglyceride and free fatty acid levels in plasma and heart. (D) Results of quantitative PCR analysis for Dsg2, Cd36, Atgl, Pparα, Pgc1α, Cpt1b, Acox1, Acadm, Acadl and Acadvl mRNA levels in mouse heart are expressed as fold change of control using β-actin as loading control. (E) Representative Western blots for DSG2, pmTOR, mTOR, p4EBP1, 4EBP1, PPARα, PGC1α, CPT1b, ACADL and GAPDH. Results are expressed as mean values ± SEM. n = 6. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
We further investigated whether loss of Dsg2 directly affected mTOR and FA β-oxidation in a cardiac myocyte cell line HL-1. Consistent to the in vivo study, Dsg2 siRNA knockdown of Dsg2 led to downregulation of mTOR–4EBP1 and β-oxidation pathway (Supporting Information Fig. S5A, S5B and Fig. S6A), while overexpression of both mTOR and 4EBP1 reversed the decline of β-oxidation in HL-1 cells (Fig. S5C, S5D and Fig. S6B).
3.4. PPARα activation alleviates cardiac dysfunction and lipid accumulation in CS-Dsg2−/− mice
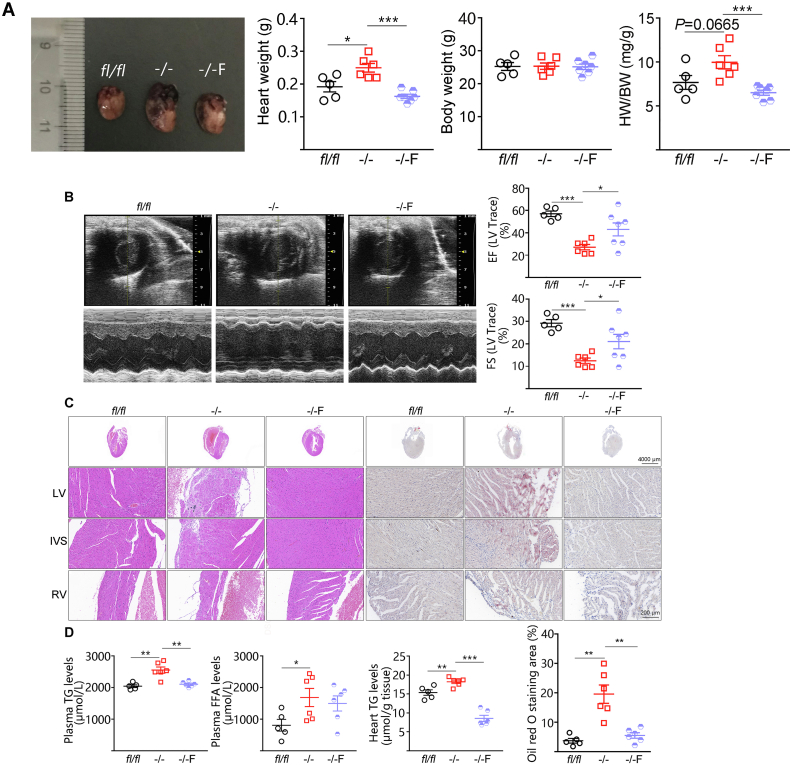
Next, we assessed whether lipid accumulation and cardiac function can be improved by treating CS-Dsg2−/− mice with fenofibrate, a PPARα agonist treating dyslipidemia widely in clinic. Fenofibrate (150 mg/kg/day, for 4 weeks) decreased the size and weight of heart (Fig. 4A). Meanwhile, improved heart function was shown in CS-Dsg2−/− mice after fenofibrate treatment (Fig. 4B, Supporting Information Table S3). Fenofibrate also significantly reversed structural disorders and myocardial accumulation of lipid in CS-Dsg2−/− mice (Fig. 4C and D).
Figure 4.
Fenofibrate improved cardiac dysfunction and lipid accumulation in CS-Dsg2−/− mice. (A) Representative heart image, heart weight, body weight and heart/body weight ratio of WT, CS-Dsg2−/− and CS-Dsg2−/− mice received fenofibrate (–/–F). (B) Representative echocardiographic images (M-Mode), EF and FS. (C) H&E staining and Oil red O staining of heart sections. (D) Plasma triglyceride, plasma free fatty acid, and heart triglyceride levels. Results are expressed as mean values ± SEM. n = 5–7. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Improvement of cardiac function and morphology by fenofibrate was associated with increased FA β-oxidation (Fig. 5, and Fig. S4C). Furthermore, fenofibrate also reversed lipid accumulation and reactivated FA β-oxidation in Dsg2 knockdown HL-1 cells (Supporting Information Fig. S7A–S7C and S6C).
Figure 5.
Fenofibrate rescued the declined FA β-oxidation relative pathway in CS-Dsg2−/− mice. (A) Results of quantitative PCR analysis for Dsg2, Cd36, Atgl, Pparα, Pgc1α, Cpt1b, Acox1, Acadm, Acadl and Acadvl mRNA levels are expressed as fold change of control using β-actin as loading control. (B) Representative Western blots of DSG2, pmTOR, mTOR, p4EBP1, 4EBP1, PPARα, PGC1α, CPT1b, ACADL and GAPDH. Fatty acid oxidation rate (C) and ATP content (D) of heart. Results are expressed as mean values ± SEM. n = 5–7. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
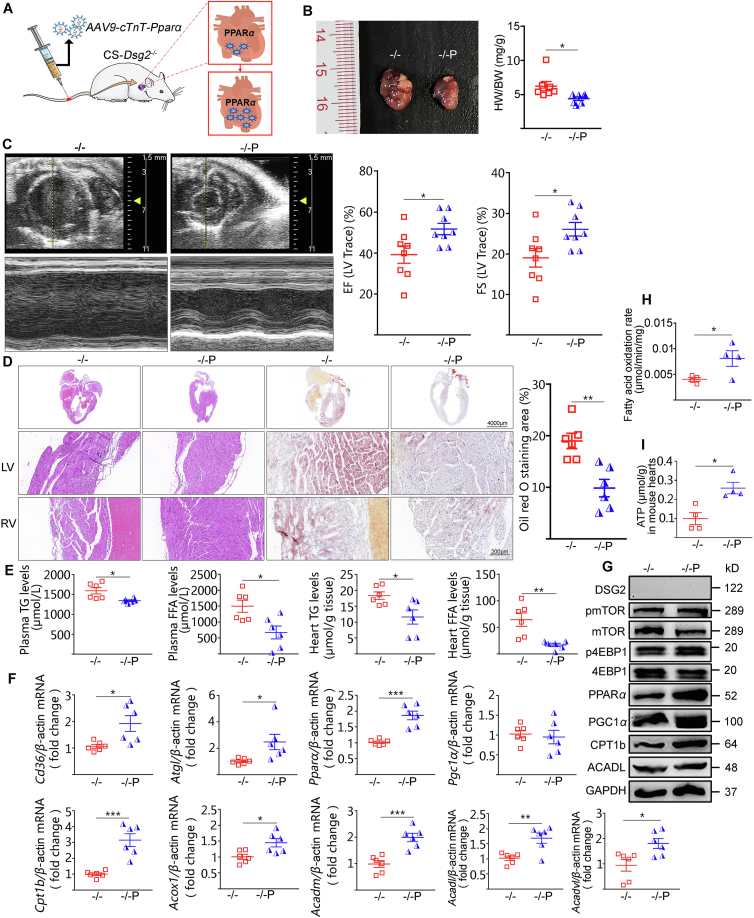
Finally, we examined whether cardiac-specific reactivation of PPARα could relief lipid accumulation and restore cardiac function in Dsg2−/− mice. Tail vein injection of AAV9-cTnT promoter-Pparα into CS-Dsg2−/− mice significantly decreased the size and weight of heart (Fig. 6A and B). Ameliorative LV systolic function and reduced myocardial lipid accumulation were observed in CS-Dsg2−/− mice after administration of AAV9-Pparα (Fig. 6C–E, Supporting Information Table S4). Moreover, most of FA β-oxidation related genes expression and the fatty acid β-oxidation rate were restored after Pparα overexpression (Fig. 6F–I). Consistent with the in vivo results, increased mRNA and protein level associated with FA β-oxidation was observed upon PPARα activation in Dsg2 siRNA-treated HL-1 cells (Supporting Information Fig. S8A, S8B and Fig. S6D).
Figure 6.
Reactivation of cardiac PPARα by AAV9-Pparα improved cardiac dysfunction and lipid accumulation in CS-Dsg2−/− mice. (A) Cardiac-specific PPARα overexpression in mice. (B) Representative heart image, heart weight, body weight and heart/body weight ratio of WT, CS-Dsg2−/− and CS-Dsg2−/− mice treated with AAV9-Pparα (–/–P). (C) Representative echocardiographic images (M-Mode), EF and FS. (D) HE staining and Oil red O staining of heart sections. (E) Triglyceride and free fatty acid levels in plasma and heart. (F) Results of quantitative PCR analysis of Cd36, Atgl, Pparα, Pgc1α, Cpt1b, Acox1, Acadm, Acadl and Acadvl mRNA levels in heart are expressed as fold change of control using β-actin as loading control. (G) Representative Western blots for DSG2, pmTOR, mTOR, p4EBP1, 4EBP1, PPARα, PGC1α, CPT1b, ACADL and GAPDH. Fatty acid oxidation rate (H) and ATP content (I) of heart. Results are expressed as mean values ± SEM. n = 5–8. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 vs. CS-Dsg2−/−.
4. Discussion
First systematically described in 1980s, ACM is demonstrated to be a common cause of SCD among young adults33,34. Mutations in desmosomal genes account for ∼50% ACM patients in different cohorts, among which DSG2 mutation is a common type35. DSG2 mutation can induce many clinical cardiomyopathy phenotypes, including ACM, dilated cardiomyopathy and left ventricular noncompaction, while ACM is most fatal and prone to cause HF and SCD in young adults36,37. Global Dsg2 knockout is embryonically lethal in mice38. In our present study, we generated an ACM mouse model by cardiac-specific Dsg2 knockout using Cre-loxp system. As it is expected, cardiac fibrosis and lipid accumulation, as well as impaired contractile function were observed in CS-Dsg2−/− which recapitulated the ACM phenotypes in human.
Although it is clear that mutations of desmosome proteins can cause ACM, the mechanism underlying the fatty deposition remain elusive. The cardiac excitation-contraction coupling consumes the majority of ATP to fuel the incessant activity. To sustain sufficient ATP generation, the heart acts as an “omnivore” and can use a variety of different carbon substrates as energy sources if available, among which the FA β-oxidation provides 60%–90% of total ATP consumption18. In our study, we observed downregulation of adipose triglyceride lipase (Atgl), FA transport protein CD36, CPT1b, and FA oxidation enzymes Acox1, ACADM, ACADL, ACADVL in CS-Dsg2−/− mice. The cardiac FA β-oxidation rate was significantly impaired after Dsg2 deletion. This is consistent to a recent finding that ACM patients have a different metabolome compared to healthy controls, and mainly affected beta oxidation of fatty acids17. It is also regarded that lipid accumulation in the heart is associated with arrhythmogenicity39, 40, 41, 42. The amount of adipose tissue that accumulates around the atria is associated with the risk, persistence, and severity of atrial fibrillation (AF). Antidiabetic drugs and lipid-lowing and surgical ablation of epicardial adipose tissue have been demonstrate to improve AF39,40. Pericardial fat accumulation is also associated with alterations in heart rate variability41. Very long-chain acyl-CoA dehydrogenase catalyzes the first reaction of mitochondrial β-oxidation. A longer QTc interval and increased risk of sudden cardiac death were observed in very long-chain acyl-CoA dehydrogenase null mice42. Thus, impaired FA β-oxidation results in not only insufficient ATP production to support cardiac activity, but also lipid accumulation in cardiomyocytes, which explain the fatty deposition, arrhythmogenicity and HF seen in ACM patients.
Despite not referred to as “metabolic organ” generally, the heart is the organ that consumes the highest amount of energy in our body, which relies on highly sophisticated regulatory mechanisms to optimize energy use and protein turnover19,43. mTOR is known as intracellular fuel sensor, which plays a crucial role in lipid homeostasis by controlling both anabolic and catabolic pathways44. The anabolic processes of lipid metabolism include fatty acid synthesis, adipogenesis and esterification. The catabolic processes of lipid metabolism include hydrolysis of triglyceride to yield glycerol and FFA, followed by β-oxidation of FFA to yield acetyl CoA26. mTORC1 has been shown to promote de novo lipogenesis in hepatocytes by activating the transcription factor, sterol regulatory element binding protein (SREBP), which in turn activates ACC45, FAS46, and SCD47 enzymes involved in lipogenesis. S6K1 is found to mediate the upregulation of SREBP by mTORC1 in hepatocytes47. The role of mTOR is extremely complicated in the heart. Doxycycline-induced cardiac-specific knockout of mTOR results in decreased FA β-oxidation and impaired cardiac function. mTOR-KO mice show marked dilation and fibrosis in heart and died in ten weeks after doxycycline induction48. Similar results were found in cardiomyocyte-specific deletion of mTORC1 component raptor49. Reduced FA oxidation but enhanced glucose oxidation was found in Raptor cKO mice, accompanied by downregulation of genes related to FA oxidation, such as PPARα, PGC1α and their downstream targets, which resulted in dilated cardiomyopathy with high mortality49. These indicated an essential role of mTOR pathway in cardiac function by promoting FA oxidation. In contrast to the liver, 4EBP1, but not S6K1, is the critical mediator of mTOR in the heart26,50. In concordance to these studies, we found decrement in mTOR–4EBP1–PPARα signaling pathway results in impaired FA oxidation and cardiac function in the Dsg2 deficient ACM mouse model. Inhibition of this pathway by rapamycin caused declined FA β-oxidation as well as deteriorated cardiac dysfunction, while overexpression of mTOR and 4EBP1 did the reverse. These findings suggested that the impaired FA β-oxidation in cardiac-specific Dsg2 knockout mice was a result of defective mTOR–4EBP1–PPARα signaling pathway, which deprived energy supply from FA β-oxidation and ultimately accelerated the development of cardiac dysfunction and lipid accumulation. Interestingly, although mTOR is vital to cardiac development and function from embryonic stage to adulthood, partial inhibition of mTOR exerts beneficial effects during aging and appear to increase cardiomyocyte resistance to aging stress50. Moreover, mTOR is found to be activated in pressure-induced cardiac hypertrophy caused by transverse aortic constriction (TAC) or hypertension and rapamycin attenuates pressure-induced cardiac hypertrophy51, 52, 53. mTOR is found to be inhibited during cardiomyocyte energy deprivation and ischemia54, but activated during chronic myocardial infarction as a consequence of increased pressure load55. Therefore, lines of evidences suggest that mTOR plays different roles in the heart in different circumstances and stages and use of rapamycin in cardiac dysfunction should be disease-dependent.
It should be noted that other metabolic pathway changes may also be involved in ACM. Kim et al.56 generated an in vitro model of ARVD/C using patient-derived induced pluripotent stem cell-derived cardiomyocytes, which displayed abnormal activation of PPARγ and extensive lipogenesis in two ARVD/C patients with plakophilin-2 mutations. Song et al.57 found elevated ketone metabolic enzymes in the RV of ACM patients with different genetic etiologies, suggesting higher ketone metabolism in ACM. Higher ketone metabolism was also validated in the patient-derived induced pluripotent stem cell-derived cardiomyocytes in vitro. Different alteration in metabolic pathways in ACM suggests heterogeneity in pathogenesis.
The most encouraging finding of our study was that reactivation of PPARα, either by AAV-mediated overexpression or a PPARα agonist fenofibrate, alleviated the lipid accumulation and improved the cardiac function in the Dsg2 deletion-induced ACM. Currently, there is no curative or effective treatment for ACM. Treatments such as medication and lifestyle management are used to reduce and control symptoms, and reduce the risk of complications. Angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, beta receptor inhibitors and anti-arrhythmic medication are commonly used for improving the pumping of heart, controlling arrhythmias and reducing the risk of complications58. Fenofibrate is widely used in dyslipidemia treatment, which transcriptionally regulates the expression of critical genes in lipid and carbohydrate metabolism, leading to an increase in muscle β-oxidation and resulting in a reduction of plasma TG levels consequently59,60. Fenofibrate effectively alleviates HF in the isoproterenol-induced rat model via promoting the FA oxidation61. In our present study, fenofibrate ameliorated cardiac dysfunction and lipid accumulation in CS-Dsg2−/− mice through stimulating PPARα-mediated FA β-oxidation. Meanwhile, targeting PPARα activation by AAV9-Pparα also reversed Dsg2 deletion-induced cardiac dysfunction and lipid accumulation. Our results suggests that PPARα is a promising target for ACM treatment by alleviating the fatty deposition and improving cardiac function.
5. Conclusions
Our study generated an ACM model by cardiac-specific Dsg2 knockout and suggested that impaired FA β-oxidation through inhibition of mTOR–4EBP1–PPARα signaling pathway might underlie the pathogenesis of ACM (Supporting Information Fig. S9). Targeting PPARα activation may be an effective means to combat excessive cardiac lipid deposition and impaired contractile function in ACM patients.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (82170818, 81770794, 31401001), the Fundamental Research Funds for the Central Universities (21620423, China), the Science and Technology Project of Zhuhai (20191210E030072, China).
Author contributions
Yubi Lin, Ruonan Liu and Geyang Xu designed the research. Yubi Lin, Ruonan Liu, Yanling Huang, Zhe Yang, Jianzhong Xian, Jingmin Huang, Zirui Qiu, Xiufang Lin, Mengzhen Zhang and Jiana Huang performed the experiments. Yubi Lin, Ruonan Liu, Jingmin Huang, Hui Chen, Huadong Wang and Geyang Xu analyzed the data and edited the figures. Geyang Xu and Hui Chen wrote the manuscript. All authors contributed to the discussion and revised the article and all approved the final versions of the manuscript. Geyang Xu is responsible for the integrity of the work as a whole.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.05.018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Corrado D., Link M.S., Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376:61–72. doi: 10.1056/NEJMra1509267. [DOI] [PubMed] [Google Scholar]
- 2.Corrado D., Basso C., Pavei A., Michieli P., Schiavon M., Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296:1593–1601. doi: 10.1001/jama.296.13.1593. [DOI] [PubMed] [Google Scholar]
- 3.Corrado D., Wichter T., Link M.S., Hauer R., Marchlinski F., Anastasakis A., et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J. 2015;36:3227–3237. doi: 10.1093/eurheartj/ehv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maron B.J., Towbin J.A., Thiene G., Antzelevitch C., Corrado D., Arnett D., et al. Contemporary definitions and classification of the cardiomyopathies: an american heart association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 5.Pilichou K., Nava A., Basso C., Beffagna G., Bauce B., Lorenzon A., et al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–1179. doi: 10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- 6.Syrris P., Ward D., Evans A., Asimaki A., Gandjbakhch E., Sen-Chowdhry S., et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet. 2006;79:978–984. doi: 10.1086/509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKoy G., Protonotarios N., Crosby A., Tsatsopoulou A., Anastasakis A., Coonar A., et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 8.Alcalai R., Metzger S., Rosenheck S., Meiner V., Chajek-Shaul T. A recessive mutation in desmoplakin causes arrhythmogenic right ventricular dysplasia, skin disorder, and woolly hair. J Am Coll Cardiol. 2003;42:319–327. doi: 10.1016/s0735-1097(03)00628-4. [DOI] [PubMed] [Google Scholar]
- 9.Gerull B., Heuser A., Wichter T., Paul M., Basson C.T., McDermott D.A., et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 10.Al-Amoudi A., Diez D.C., Betts M.J., Frangakis A.S. The molecular architecture of cadherins in native epidermal desmosomes. Nature. 2007;450:832–837. doi: 10.1038/nature05994. [DOI] [PubMed] [Google Scholar]
- 11.Tariq H., Bella J., Jowitt T.A., Holmes D.F., Rouhi M., Nie Z., et al. Cadherin flexibility provides a key difference between desmosomes and adherens junctions. Proc Natl Acad Sci U S A. 2015;112:5395–5400. doi: 10.1073/pnas.1420508112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieding M., Debus J.D., Kerkhoff R., Gaertner-Rommel A., Walhorn V., Milting H., et al. Arrhythmogenic cardiomyopathy related DSG2 mutations affect desmosomal cadherin binding kinetics. Sci Rep. 2017;7:13791. doi: 10.1038/s41598-017-13737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison O.J., Brasch J., Lasso G., Katsamba P.S., Ahlsen G., Honig B., et al. Structural basis of adhesive binding by desmocollins and desmogleins. Proc Natl Acad Sci U S A. 2016;113:7160–7165. doi: 10.1073/pnas.1606272113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermida A., Fressart V., Hidden-Lucet F., Donal E., Probst V., Deharo J.C., et al. High risk of heart failure associated with desmoglein-2 mutations compared to plakophilin-2 mutations in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur J Heart Fail. 2019;21:792–800. doi: 10.1002/ejhf.1423. [DOI] [PubMed] [Google Scholar]
- 15.Pilichou K., Remme C.A., Basso C., Campian M.E., Rizzo S., Barnett P., et al. Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J Exp Med. 2009;206:1787–1802. doi: 10.1084/jem.20090641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kant S., Krull P., Eisner S., Leube R.E., Krusche C.A. Histological and ultrastructural abnormalities in murine desmoglein 2-mutant hearts. Cell Tissue Res. 2012;348:249–259. doi: 10.1007/s00441-011-1322-3. [DOI] [PubMed] [Google Scholar]
- 17.Volani C., Rainer J., Hernandes V.V., Meraviglia V., Pramstaller P.P., Smárason S.V., et al. Metabolic signature of arrhythmogenic cardiomyopathy. Metabolites. 2021;11:195. doi: 10.3390/metabo11040195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertero E., Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol. 2018;15:457–470. doi: 10.1038/s41569-018-0044-6. [DOI] [PubMed] [Google Scholar]
- 19.Stanley W.C., Recchia F.A., Lopaschuk G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 20.Heather L.C., Cole M.A., Lygate C.A., Evans R.D., Stuckey D.J., Murray A.J., et al. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc Res. 2006;72:430–437. doi: 10.1016/j.cardiores.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Osorio J.C., Stanley W.C., Linke A., Castellari M., Diep Q.N., Panchal A.R., et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 22.Dávila-Román V.G., Vedala G., Herrero P., de las Fuentes L., Rogers J.G., Kelly D.P., et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 23.Bedi K.C., Jr., Snyder N.W., Brandimarto J., Aziz M., Mesaros C., Worth A.J., et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaimoto S., Hoshino A., Ariyoshi M., Okawa Y., Tateishi S., Ono K., et al. Activation of PPAR-alpha in the early stage of heart failure maintained myocardial function and energetics in pressure-overload heart failure. Am J Physiol Heart Circ Physiol. 2017;312:H305–H313. doi: 10.1152/ajpheart.00553.2016. [DOI] [PubMed] [Google Scholar]
- 25.Haemmerle G., Moustafa T., Woelkart G., Büttner S., Schmidt A., van de Weijer T., et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L., Brink M. mTOR, cardiomyocytes and inflammation in cardiac hypertrophy. Biochim Biophys Acta. 2016;1863:1894–1903. doi: 10.1016/j.bbamcr.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Sciarretta S., Forte M., Frati G., Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122:489–505. doi: 10.1161/CIRCRESAHA.117.311147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y., Pires K.M., Whitehead K.J., Olsen C.D., Wayment B., Zhang Y.C., et al. Mechanistic target of rapamycin (Mtor) is essential for murine embryonic heart development and growth. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai S., Sitzmann J.M., Dastidar S.G., Rodriguez A.A., Vu S.L., McDonald C.E., et al. Muscle-specific 4E-BP1 signaling activation improves metabolic parameters during aging and obesity. J Clin Invest. 2015;125:2952–2964. doi: 10.1172/JCI77361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q., Chen L., Jian J., Li J., Zhang X. The mechanism behind BAF60c in myocardial metabolism in rats with heart failure is through the PGC1α–PPARα–mTOR signaling pathway. Biochem Cell Biol. 2020:1–11. doi: 10.1139/bcb-2019-0450. [DOI] [PubMed] [Google Scholar]
- 31.Xu G., Li Z., Ding L., Tang H., Guo S., Liang H., et al. Intestinal mTOR regulates GLP-1 production in mouse L cells. Diabetologia. 2015;58:1887–1897. doi: 10.1007/s00125-015-3632-6. [DOI] [PubMed] [Google Scholar]
- 32.Jin B., Shi H., Zhu J., Wu B., Geshang Q. Up-regulating autophagy by targeting the mTOR–4EBP1 pathway: a possible mechanism for improving cardiac function in mice with experimental dilated cardiomyopathy. BMC Cardiovasc Disord. 2020;20:56. doi: 10.1186/s12872-020-01365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiene G., Nava A., Corrado D., Rossi L., Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–133. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 34.Marcus F.I., Fontaine G.H., Guiraudon G., Frank R., Laurenceau J.L., Malergue C., et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 35.Chen L., Rao M., Chen X., Chen K., Ren J., Zhang N., et al. A founder homozygous DSG2 variant in East Asia results in ARVC with full penetrance and heart failure phenotype. Int J Cardiol. 2019;274:263–270. doi: 10.1016/j.ijcard.2018.06.105. [DOI] [PubMed] [Google Scholar]
- 36.Bhonsale A., Groeneweg J.A., James C.A., Dooijes D., Tichnell C., Jongbloed J.D., et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J. 2015;36:847–855. doi: 10.1093/eurheartj/ehu509. [DOI] [PubMed] [Google Scholar]
- 37.Lin Y., Huang J., Zhao T., He S., Huang Z., Chen X., et al. Compound and heterozygous mutations of DSG2 identified by whole exome sequencing in arrhythmogenic right ventricular cardiomyopathy/dysplasia with ventricular tachycardia. J Electrocardiol. 2018;51:837–843. doi: 10.1016/j.jelectrocard.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Eshkind L., Tian Q., Schmidt A., Franke W.W., Windoffer R., Leube R.E. Loss of desmoglein 2 suggests essential functions for early embryonic development and proliferation of embryonal stem cells. Eur J Cell Biol. 2002;81:592–598. doi: 10.1078/0171-9335-00278. [DOI] [PubMed] [Google Scholar]
- 39.Hatem S.N., Redheuil A., Gandjbakhch E. Cardiac adipose tissue and atrial fibrillation: the perils of adiposity. Cardiovasc Res. 2016;109:502–509. doi: 10.1093/cvr/cvw001. [DOI] [PubMed] [Google Scholar]
- 40.Zhou M., Wang H., Chen J., Zhao L. Epicardial adipose tissue and atrial fibrillation: possible mechanisms, potential therapies, and future directions. Pacing Clin Electrophysiol. 2020;43:133–145. doi: 10.1111/pace.13825. [DOI] [PubMed] [Google Scholar]
- 41.Uceda D.E., Zhu X.Y., Woollard J.R., Ferguson C.M., Patras I., Carlson D.F., et al. Accumulation of pericardial fat is associated with alterations in heart rate variability patterns in hypercholesterolemic pigs. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gélinas R., Thompson-Legault J., Bouchard B., Daneault C., Mansour A., Gillis M.A., et al. Prolonged QT interval and lipid alterations beyond β-oxidation in very long-chain acyl-CoA dehydrogenase null mouse hearts. Am J Physiol Heart Circ Physiol. 2011;301:H813–H823. doi: 10.1152/ajpheart.01275.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carley A.N., Taegtmeyer H., Lewandowski E.D. Matrix revisited: mechanisms linking energy substrate metabolism to the function of the heart. Circ Res. 2014;114:717–729. doi: 10.1161/CIRCRESAHA.114.301863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown N.F., Stefanovic-Racic M., Sipula I.J., Perdomo G. The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metabolism. 2007;56:1500–1507. doi: 10.1016/j.metabol.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Peng T., Golub T.R., Sabatini D.M. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22:5575–5584. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mauvoisin D., Rocque G., Arfa O., Radenne A., Boissier P., Mounier C. Role of the PI3-kinase/mTOR pathway in the regulation of the stearoyl CoA desaturase (SCD1) gene expression by insulin in liver. J Cell Commun Signal. 2007;1:113–125. doi: 10.1007/s12079-007-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Düvel K., Yecies J.L., Menon S., Raman P., Lipovsky A.I., Souza A.L., et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shende P., Plaisance I., Morandi C., Pellieux C., Berthonneche C., Zorzato F., et al. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- 50.Sciarretta S., Volpe M., Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014;114:549–564. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shioi T., McMullen J.R., Tarnavski O., Converso K., Sherwood M.C., Manning W.J., et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 52.McMullen J.R., Sherwood M.C., Tarnavski O., Zhang L., Dorfman A.L., Shioi T., et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 53.Sciarretta S., Zhai P., Shao D., Maejima Y., Robbins J., Volpe M., et al. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation. 2012;125:1134–1146. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buss S.J., Muenz S., Riffel J.H., Malekar P., Hagenmueller M., Weiss C.S., et al. Beneficial effects of mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54:2435–2446. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 55.Völkers M., Konstandin M.H., Doroudgar S., Toko H., Quijada P., Din S., et al. Mechanistic target of rapamycin complex 2 protects the heart from ischemic damage. Circulation. 2013;128:2132–2144. doi: 10.1161/CIRCULATIONAHA.113.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim C., Wong J., Wen J., Wang S., Wang C., Spiering S., et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature. 2013;494:105–110. doi: 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song J.P., Chen L., Chen X., Ren J., Zhang N.N., Tirasawasdichai T., et al. Elevated plasma beta-hydroxybutyrate predicts adverse outcomes and disease progression in patients with arrhythmogenic cardiomyopathy. Sci Transl Med. 2020;12:2132–2144. doi: 10.1126/scitranslmed.aay8329. [DOI] [PubMed] [Google Scholar]
- 58.Towbin J.A., McKenna W.J., Abrams D.J., Ackerman M.J., Calkins H., Darrieux F.C.C., et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy: executive summary. Heart Rhythm. 2019;16:e373–e407. doi: 10.1016/j.hrthm.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 59.Balakumar P., Sambathkumar R., Mahadevan N., Muhsinah A.B., Alsayari A., Venkateswaramurthy N., et al. Molecular targets of fenofibrate in the cardiovascular-renal axis: a unifying perspective of its pleiotropic benefits. Pharmacol Res. 2019;144:132–141. doi: 10.1016/j.phrs.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 60.Pettersen J.C., Pruimboom-Brees I., Francone O.L., Amacher D.E., Boldt S.E., Kerlin R.L., et al. The PPARalpha agonists fenofibrate and CP-778875 cause increased beta-oxidation, leading to oxidative injury in skeletal and cardiac muscle in the rat. Toxicol Pathol. 2012;40:435–447. doi: 10.1177/0192623311431945. [DOI] [PubMed] [Google Scholar]
- 61.Li P., Luo S., Pan C., Cheng X. Modulation of fatty acid metabolism is involved in the alleviation of isoproterenol-induced rat heart failure by fenofibrate. Mol Med Rep. 2015;12:7899–7906. doi: 10.3892/mmr.2015.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.