Abstract
Cardiomyocyte death is one of the major mechanisms contributing to the development of myocardial infarction (MI) and myocardial ischemia/reperfusion (MI/R) injury. Due to the limited regenerative ability of cardiomyocytes, understanding the mechanisms of cardiomyocyte death is necessary. Pyroptosis, one of the regulated programmed cell death pathways, has recently been shown to play important roles in MI and MI/R injury. Pyroptosis is activated by damage-associated molecular patterns (DAMPs) that are released from damaged myocardial cells and activate the formation of an apoptosis-associated speck-like protein containing a CARD (ASC) interacting with NACHT, LRR, and PYD domains-containing protein 3 (NLRP3), resulting in caspase-1 cleavage which promotes the activation of Gasdermin D (GSDMD). This pathway is known as the canonical pathway. GSDMD has also been shown to be activated in a non-canonical pathway during MI and MI/R injury via caspase-4/5/11. Suppression of GSDMD has been shown to provide cardioprotection against MI and MI/R injury. Although the effects of MI or MI/R injury on pyroptosis have previously been discussed, knowledge concerning the roles of GSDMD in these settings remains limited. In this review, the evidence from in vitro, in vivo, and clinical studies focusing on cardiac GSDMD activation during MI and MI/R injury is comprehensively summarized and discussed. Implications from this review will help pave the way for a new therapeutic target in ischemic heart disease.
Key words: Pyroptosis, Gasdermin D, Heart, Ischemia, Ischemia–reperfusion injury, Myocardial infarction
Graphical abstract
Myocardial infarction (MI) and myocardial ischemia/reperfusion (MI/R) injury induce GSDMD-mediated pyroptosis in both canonical and non-canonical pathways. Inhibition of GSDMD-mediated pyroptosis by several interventions can provide cardioprotection against MI and I/R injury.
1. Introduction
Myocardial infarction (MI) is a major cause of death and disability and has been a challenging issue for clinicians and researchers for several decades1. To treat the condition, timely reperfusion is the treatment of choice. However, restoration of the blood flow to the ischemic myocardium can cause further tissue damage, a phenomenon known as myocardial ischemia/reperfusion (MI/R) injury2. Many mechanisms contribute to the injury and death of cardiac cells from MI and MI/R injury, however, one of the most well–known pathways is the overproduction of reactive oxygen species (ROS) during timely reperfusion3. ROS can induce the opening of mitochondrial permeability transition pores (mPTP), contributing to intracellular Ca2+ overload, lipid peroxidation of the cell membrane, and oxidative damage to the DNA3. In addition, neutrophils also accumulate in response to ROS overgeneration which can independently induce the multiple programmed cell death pathways in the acutely ischemic myocardium3. Since cardiomyocytes have poor regenerative ability4, and the amount of cardiomyocyte death and infarct size are correlated with the prognosis and the risk of developing heart failure5, 6, 7, understanding the mechanisms of cell death and a potential way to limit cardiomyocyte death might provide a critical benefit in the outcome of MI patients.
In the last decade, several types of cell death pathways have been demonstrated to play a key role in the progression of MI and MI/R injury8,9. Necrosis has been considered a major type of cardiac cell death during MI due to deficiencies in the oxygen and nutrition10, resulting in the release of danger signals that trigger potent inflammation9,11. However, apoptosis caused by oxidative stress also plays a damaging role in MI and MI/R injury8. Furthermore, a caspase-independent mode of programmed necrotic cell death called necroptosis was shown to contribute to significant myocardial damage following I/R injury12. Last but not least, several recent studies have focused on a new potential molecular mechanism of cell death related to MI and MI/R injury known as “pyroptosis”.
Pyroptosis is a type of regulated cell death that is critically dependent on the formation of pores in the plasma membrane instigated by members of the gasdermin protein family, which frequently occurs as a result of the inflammatory caspase activation13. It has been reported that prolonged lack of oxygen or cell starvation following MI contributes to a loss of cell membrane integrity and leads to the release of damage-associated molecular patterns (DAMPs) (e.g., alarmins [high-mobility group box 1 (HMGB1), heat shock proteins (HSP), oxidative stress, adenosine triphosphate (ATP)]14, which mediate the upregulation of several downstream signaling proteins, including NOD-like receptor (NLR), caspase-1, caspase-4/5/11, Gasdermin D (GSDMD), and Interleukin (IL)-1β and IL-1815. Apart from that, MI or MI/R-induced pyroptosis is also associated with the formation of NACHT, LRR, and PYD domains containing protein 3 (NLRP3) inflammasome, which is a multimeric protein complex that causes caspase-1 to self-cleave into an active form, and leads to pyroptosis by cleaving GSDMD14,16. The modification of pyroptosis has been proposed as a potential strategy for combating MI or MI/R17. Inhibiting major proteins in the pyroptosis pathway has been shown to exert effective cardioprotection, potentially leading to increased functional outcomes and reduced cardiac injury after MI and MI/R conditions18,19. Although pyroptosis has been acknowledged as another potential cell death pathway following myocardial injury induced by ischemia, the potential roles of pyroptosis and its therapeutic targets for intervention in cases of MI and MI/R still need to be explored.
This review aims to summarize and discuss the mechanisms of the pyroptosis pathway of greatest importance, focusing on in vitro, in vivo, and clinical studies into MI and MI/R injury, and identify whether pyroptosis could be a target for an effective cardioprotective intervention against MI and MI/R injury. Collectively, this information encourages further investigation and paves the way for the development of new therapeutic strategies to improve the clinical outcome of patients with MI and MI/R injury.
2. The roles of GSDMD and regulation of the pyroptosis signaling pathway in MI and MI/R injury
Gasdermin is an executioner protein that mediates membrane pore formation in pyroptosis. There are several forms of gasdermin found in the heart such as GSDMD, and gasdermin E (GSDME)20. However, only GSDMD is responsible for pore formation during MI and MI/R21,22, while GSDME participated in pyroptosis caused by viral infection and chemotoxicity20. Pyroptosis, which is mediated by GSDMD, is characterized by plasma membrane blebbing, chromatin condensation (while the nucleus remains intact), cell swelling, and finally pore formation up till cellular rupture with the release of cytokines14,23.
Pyroptosis participates in many cardiovascular diseases, including atherosclerosis, diabetes, MI, arrhythmia, and cardiac hypertrophy since it is strongly associated with oxidative stress and inflammation24. Focusing on MI and MI/R, pyroptosis is divided into two individual pathways, specifically canonical and non-canonical pathways25,26. Both pyroptosis pathways have been reported to implicate cardiomyocyte death and infarction of cardiac tissue21,27. Caspase-1-mediated canonical pyroptosis is the most common pathway following MI and MI/R10,14,28. Mechanistically, MI or MI/R induces the release of DAMPs which bind to their complementary membrane protein receptors (e.g., the toll-like receptor (TLR) and NLR), known as the pattern recognition receptor (PRR)14. Apart from the DAMPs mentioned before, mitochondrial DNA (mtDNA) released from damaged mitochondria has recently been found to be one of the powerful DAMPs mediating cardiac pyroptosis29, 30, 31. The roles of mtDNA DAMPs in the regulation of cardiac pyroptosis have been reported in two studies29,30. Mitochondria are abundantly found in the heart for the ATP production; however, a large amount of DAMPs are released from the mitochondria during pathological stress31. Recently, mtDNA, that is liberated from damaged mitochondria to the cytosol or extracellular space, is recognized as important DAMPs to trigger various inflammatory or degenerative diseases31. Mechanically, mtDNA DAMP activates TLR9/NLRP3/GSDMD pathways32, then, IL-1β and IL-18 are released from the injured cells32. Despite this pathway, activation of TLR could induce innate and adaptive immune systems in cardiac cells. Releasing of IL-1, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) also recruits neutrophils and monocytes from the circulation to the injury site. Then, the migration of cardiac B cells and T cells to the damaged myocardium occurs, leading to cardiac inflammation and heart failure33. A previous study has shown that mtDNA exacerbates odontoblast inflammation through GSDMD-mediated pyroptosis32. Consistent with the odontoblast study, mtDNA was increased in HL1 cardiomyocytes after 12 h of hypoxia, along with an upregulation of GSDMD N-terminal fragment (GSDMD-N)30. Moreover, application of palmitic acid (400 μmol/L) for 24 h promoted mtDNA released, followed by an activation of cyclic guanosine monophosphate-adenosine monophosphate synthetase-stimulator of interferon gene/NLRP3/GSDMD-N29.
On binding to PRRs, DAMPs mediate the activation of nuclear factor kappa B (NF-κB) and lead to the transcription of hundreds of pro-inflammatory genes, including the components of the inflammasome pathways. Among these inflammasomes, the NLRP3 inflammasome has emerged as an almost ubiquitous PRR that is in an activated form. The NLRP3 inflammasome consists of the NLRP3 protein, the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC), and pro-caspase-116. The assembly of the NLRP3 inflammasome leads to the self-cleavage of pro-caspase-1 into the active form of caspase-116. Active caspase-1 cleaves pro-IL into active IL and cleaves GSDMD into a GSDMD-N and GSDMD C-terminal fragment (GSDMD-C). GSDMD-N then forms pores in the plasma membrane, causing pyroptotic cell death and the release of IL-1β and IL-18 to induce more inflammation14.
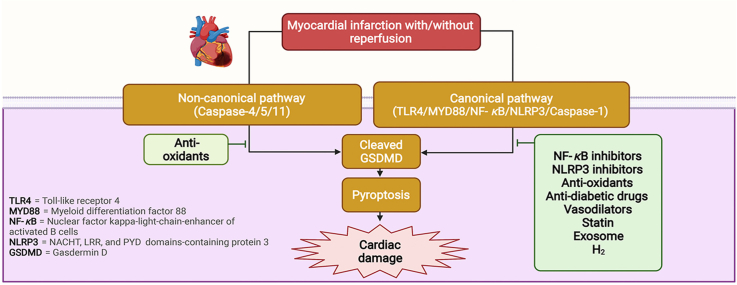
In addition to the canonical pathway, non-canonical human caspase-4/5 (murine caspase-11), the caspase-3/8-mediated pathway, and granzyme-mediated pathway have been recently reported as playing a significant role in various cell types and diseases, particularly cardiac pathologies15,34. Focusing on MI and MI/R injury, caspase-4/5/11 has been shown to contribute to pyroptosis in the setting of conventional or unconventional inflammasome signaling26. Caspase-4/5/11 itself can cleave GSDMD and trigger pyroptotic cell death, or it can induce the NLRP3 inflammasome/caspase-1 signaling pathway which leads to pyroptosis26. However, in the setting of MI and MI/R-induced pyroptosis, there is limited research available about the non-canonical pathway. Only the non-canonical caspase 4/5/11/GSDMD pathway has been reported as being associated with these pathologies21,22. Figures summarizing the pyroptosis pathways in MI and MI/R injury are shown in Figure 1, Figure 2, respectively.
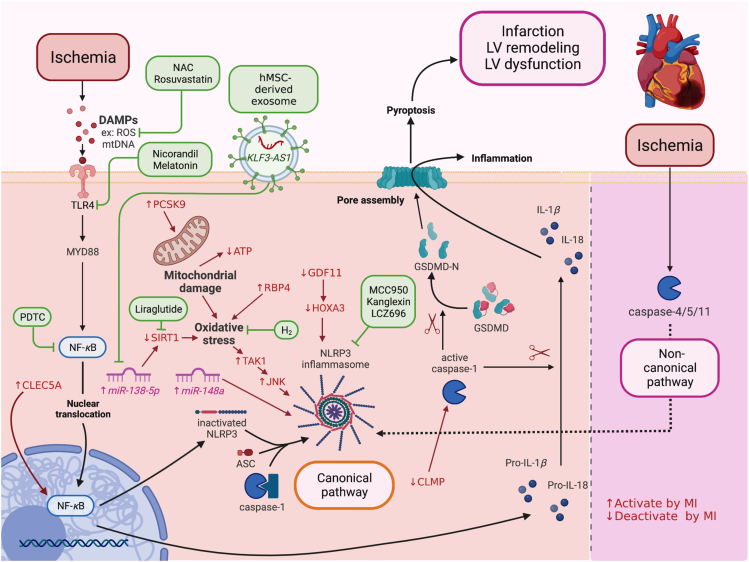
Figure 1.
The canonical and non-canonical pathways of MI-induced pyroptosis and potential interventions. DAMPs such as ROS, mtDNA bind to TLR4 and activate MYD88/NF-κB/ASC/caspase-1/NLRP3 pathways in the canonical pathway. In the non-canonical pathway, MI activates caspase-4/5/11 and NLRP3 signaling. After the formation of the NLRP3 inflammasome, caspase-1 becomes active caspase-1, which cleaves pro-IL-1β to IL-1β, pro-IL-18 to IL-18, GSDMD to GSDMD-N. GSDMD-N then forms pores in the plasma membrane, causing pyroptotic cell death and secretion of IL-1β, IL-18 to cause more inflammation. NAC, rosuvastatin, nicorandil, melatonin, PDTC, lncRNA KLF3-AS1, liraglutide, H2, MCC950, kanglexin, and LCZ696 reduce cardiomyocyte/myocardial damage against MI by targeting the canonical pathway of pyroptosis, leading to the reduction of GSDMD. ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; DAMPs, damage-associated molecular patterns; GSDMD, Gasdermin D; GSDMD-N, Gasdermin D N-terminal fragment; IL, interleukin; lnc-RNA, long non-coding ribonucleic acid; MI, myocardial infarction; mtDNA, mitochondrial DNA; MYD88, myeloid differentiation factor 88; NAC, N-acetyl cysteine; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; NLRP3, NACHT, LRR, and PYD domains-containing protein 3; PDTC, pyrrolidine dithiocarbamate; ROS, reactive oxygen species; TLR4, toll-like receptor 4.
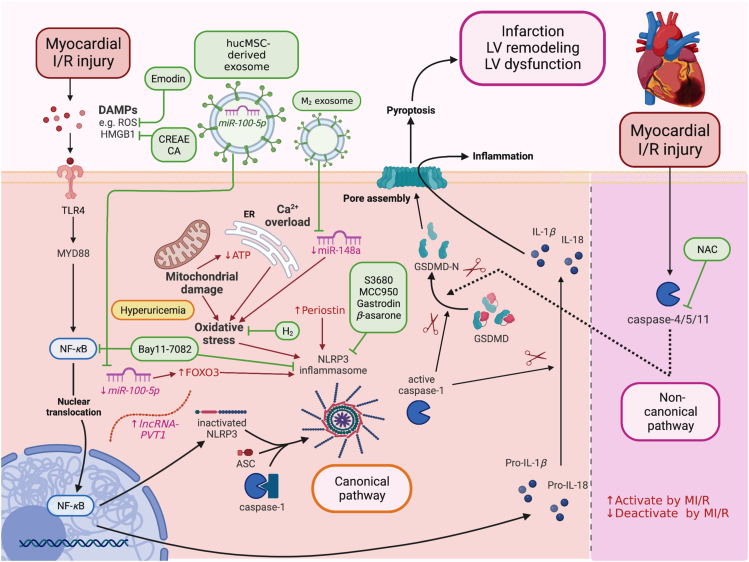
Figure 2.
The canonical and non-canonical pathways of MI/R-induced pyroptosis and potential interventions. DAMPs such as ROS and HMGB1 bind to TLR4 and activate the MYD88/NF-κB/ASC/caspase-1/NLRP3 pathway in the canonical pathway. After the formation of the NLRP3 inflammasome, caspase-1 becomes active caspase-1, which cleaves pro-IL-1β to IL-1β, pro-IL-18 to IL-18, GSDMD to GSDMD-N. In the non-canonical pathway, MI/R activates caspase-4/5/11, and caspase-4/5/11 directly cleaves GSDMD to GSDMD-N. GSDMD-N then forms pores in the plasma membrane, causing pyroptosis and secretion of IL-1β, IL-18. Emodin, CREAE, CA, Bay 11-7082, miR-100-5p, M2 exosome, H2, S3680, MCC950, Gastrodin, β-asarone, and NAC reduce cardiomyocyte/myocardial damage against MI/R by targeting canonical and non-canonical pathway of pyroptosis, leading to the reduction of GSDMD. ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; CA, cinnamic acid; CREAE, ethyl acetate extract of Cinnamomi ramulus; DAMPs, damage-associated molecular patterns; GSDMD, Gasdermin D; GSDMD-N, Gasdermin D N-terminal fragment; HMGB1, high-mobility group box 1; IL, interleukin; M2 exosome, M2 macrophage-derived exosomes; MI/R, myocardial ischemia/reperfusion injury; miR, microRNA; MYD88, myeloid differentiation factor 88; NAC, N-acetyl cysteine; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NACHT, LRR, and PYD domains-containing protein 3; ROS, reactive oxygen species; TLR4, toll-like receptor 4.
For the mechanisms involved in heart failure (HF), MI causes cardiomyocytes death and infarction, subsequently leads to left ventricular (LV) dysfunction, and it is a major cause of HF35. Acute phase of HF occurs within 1–7 days after MI36. During this phase, ROS activate the canonical pyroptosis pathway where the inflammatory mediators are recruited37. Then, the inflammatory cytokines induce extracellular matrix protein accumulation37, causing fibroblast activation, and resulting in cardiac fibrosis during the chronic phase of HF, which usually occurs a few weeks after MI36.
3. Evidence of pyroptosis in MI and MI/R injury: Reports from clinical studies
Several clinical reports showed that serum and cardiac tissue GSDMD levels could be a potential marker for pyroptosis in MI or MI/R injury21,30. Patients with chronic MI had higher levels of serum caspase-1, GSDMD, IL-1β, IL-18, and Proprotein convertase subtilisin/kexin type 9 (PCSK9) than healthy controls30. Additionally, expression of caspase-1, GSDMD, IL-1β, and PCSK9 were increased in the border zone rather than the remote zone of MI hearts, implying that PCSK9 and pyroptosis occurred under MI conditions30. In another clinical report, there was no significant difference in the levels of serum GSDMD following percutaneous coronary intervention in acute ST-elevated MI (STEMI) patients, in comparison to stable coronary artery disease patients21. However, serum GSDMD levels in acute STEMI patients were significantly increased after 1 h of percutaneous coronary intervention treatment and remained elevated for the following 24 h21. It indicates that, in addition to MI, pyroptosis might play a role in reperfusion injury in the human heart and occurs in the semi-early phase after reperfusion, which may give us time to intervene at the time of revascularization in acute STEMI patients. While GSDMD was implicated in human MI and MI/R injury, there was no evidence of GSDMD-N elevation in acute MI patients and no evidence of intervention modulating GSDMD in MI or MI/R patients. All of these clinical reports are summarized in Table 121,30.
Table 1.
Evidence of pyroptosis in MI and MI/R injury: reports from clinical studies.
| Patients | Control group | Pyroptotic biomarker |
Inflammatory marker | Other relevant finding | Interpretation | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| GSDMD | Caspase-1 | Other | ||||||
| Acute STEMI patients underwent PCI treatment Age: 58.4 (8.4) N = 19 (Serum collected at different time point after PCI) PCI-0 h |
Stable CAD patients serum Age: 58 (10) N = 15 |
↔ | – | – | – | – | GSDMD levels were detectable after PCI in patient sera, suggested that cardiomyocytes pyroptosis might occur at reperfusion period. | 21 |
| PCI-1 h | ↑ | – | – | – | – | |||
| PCI-12 h | ↑ | – | – | – | – | |||
| PCI-24 h | ↑ | – | – | – | – | |||
| CMI patients' serum Age: 64.5 (8.3) N = 21 |
Healthy subjects serum Age: 66.8 (7.1) N = 20 |
↑ | ↑ | – | ↑ IL-1β ↑ IL-18 |
↑ PCSK9 ↑ LDH |
CMI patients had higher level of caspase-1/GSDMD-mediated pyroptosis and PCSK9 levels in serum, as well as in the MI heart. | 30 |
| Border zone of human heart died from MI within 1 week Age 65–80 year N = 5 |
Remote zone of human heart died from MI within 1 week Age 65–80 year N = 5 |
↑ | ↑ | – | ↑ IL-1β | ↑ PCSK9 | ||
CAD: coronary artery disease; CMI: chronic myocardial ischemia; GSDMD: Gasdermin D; I/R: ischemia/reperfusion; IL: interleukin; LDH: lactate dehydrogenase; MI: myocardial ischemia; PCI: percutaneous coronary intervention; PCI-x h: post PCI x hour(s); PCSK9: protein convertase subtilisin/Kexin type 9; STEMI: ST elevation myocardial infarction.
4. Pyroptosis and GSDMD-mediated cardiomyocyte death under hypoxia: Evidence from in vitro studies
As shown in Table 218,21,27,30,38, 39, 40, 41, 42, 43, 44, hypoxic conditions could induce upregulation of NLRP3/caspase-1/GSDMD-mediated pyroptosis. Hypoxia-induced cardiomyocyte pyroptosis was related to activation of inflammation and oxidative stress pathways through the TLR4/NF-κB signaling cascade38, together with an increase in intracellular ROS levels40. In addition, glutathione peroxidase (GSH-PX) and superoxide dismutase (SOD) were suppressed following hypoxia, indicating a decrease in antioxidant capacity40. Cellular injury and death of cardiac cells were observed with the detection of NLRP3/caspase-1-mediated pyroptosis, thereby decreasing cell viability in hypoxic cardiomyocytes18,27,38, 39, 40, 41. Furthermore, cardiomyoblast hypoxia caused by TNF-α co-incubation to mimic an ischemic environment led to cardiomyoblast pyroptosis by increasing NLRP3/caspase-1 dependent pyroptosis, and ROS overproduction, and decreasing cell viability42,43.
Table 2.
Evidence of pyroptosis in MI: reports from in vitro studies.
| Condition/Hypoxia (h) | Model | Pyroptotic marker |
Inflammatory marker /ROS |
Cell viability /Toxicity |
Other cell death marker/Relevant finding | Interpretation | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| GSDMD |
Caspase-1 | Others | ||||||||
| Active form | Full form | |||||||||
| 2 | H9C2 cells | ↑ | – | ↑ | ↑ NLRP3 | ↑ TLR4 ↑ NF-κB ↑ IL-1β ↑ IL-18 |
↑ LDH | – | Hypoxia activated GSDMD in canonical pathway, leading to cardiomyoblast pyroptosis. | 38 |
| 4 | NRCMs | ↑ GSDMD-N per GSDMD-FL ratio | ↑ | ↑ ASC ↑ NLRP3 |
↑ IL-1β | ↓ Cell viability ↑ LDH |
↑ PI | Hypoxia activated GSDMD in canonical pathway, leading to cardiomyocyte pyroptosis. | 18 | |
| 6 | H9C2 cells | ↑ | – | ↑ | ↑ ASC ↑ NLRP3 |
↑ IL-1β ↑ IL-18 |
↓ Cell viability | ↑ TUNEL | Hypoxia activated GSDMD in canonical pathway, leading to cardiomyoblast pyroptosis. | 39 |
| 6 | NMVCMs | ↑ | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
↑ IL-18 ↑ MDA ↑ ROS ↓ GSH-PX ↓ SOD |
↓ Cell viability ↑ LDH |
↑ RBP4 ↑ PI |
Upregulation of RBP4 involved in GSDMD activation in canonical pathway inducing cardiomyocyte injury and pyroptosis during hypoxia, which was confirmed by both genetic and pharmacological inhibition. | 40 |
| NMVCMs + RBP4 overexpression | ↑↑ | ↑↑ | ↑↑ | ↑↑ ASC ↑↑ NLRP3 |
↑↑ IL-18 ↑↑ MDA ↑↑ ROS ↓↓ GSH-PX ↓↓ SOD |
↓↓ Cell viability ↑↑ LDH |
↑↑ RBP4 ↑↑ PI |
|||
| NMVCMs + sh-RBP4 vs. hypoxia | ↓ | ↓ | ↓ | ↓ ASC ↓ NLRP3 |
↓ IL-18 ↓ MDA ↓ ROS ↑ GSH-PX ↑ SOD |
↑ Cell viability ↓ LDH |
↓ RBP4 ↓ PI |
|||
| NMVCMs + RBP4 overexpression + si-NLRP3 vs. RBP4 overexpression + hypoxia | ↓ | ↓ | ↓ | ↓ ASC ↓ NLRP3 |
↓ IL-18 | ↓ LDH | ↔ RBP4 ↓ PI |
|||
| NMVCMs + RBP4 overexpression + MCC950 (NLRP3 inhibitor) vs. RBP4 overexpression + hypoxia | ↓ | ↓ | ↓ | ↓ ASC ↓ NLRP3 |
↓ IL-18 | ↓ LDH | ↔ RBP4 ↓ PI |
|||
| 12 | NMCMs | ↑ | – | ↑ | ↑ ASC ↑ NLRP3 |
– | ↓ Cell viability | ↓ GDF11 ↓ HOXA3 |
Downregulation of GDF11/HOXA3 involved in GSDMD activation in canonical pathway to induce cardiomyocyte injury and pyroptosis during hypoxia, which was confirmed by genetic overexpression. | 41 |
| NMCMs + GDF11 overexpression vs. hypoxia | ↓ | – | ↓ | ↓ ASC ↓ NLRP3 |
– | ↑ Cell viability | – | |||
| NMCMs + HOXA3 overexpression vs. hypoxia | ↓ | – | ↓ | ↓ ASC ↓ NLRP3 |
– | – | – | |||
| 24 | NMCMs | ↑ | ↑ | ↑ | ↑ NLRP3 | ↑ IL-1β ↑ IL-18 |
– | ↑ TUNEL ↑ PI |
Hypoxia activated GSDMD in canonical pathway, leading to cardiomycyte pyroptosis. | 27 |
| 48 h | Primary cardiomyocyte | – | – | – | – | – | ↓ Cell viability | ↑ PCSK9 | Upregulation of PCSK9 induced oxidative stress which led to GSDMD activation in canonical pathway to induce cardiomyocyte injury and pyroptosis during hypoxia, which was confirmed by genetic inhibition. | 30 |
| PCSK9−/− HL-1 cells |
↓ | – | ↓ | ↓ ASC ↓ NLRP3 |
↓ IL-1β ↓ ROS |
↓ LDH | – | |||
| HL-1 cells + hrPCSK9 vs. hypoxia | ↑ | – | ↑ | ↑ ASC ↑ NLRP3 |
↑ IL-1β ↑ ROS |
↑ LDH | – | |||
| HL-1 cells + PCSK9CRISPRavs. hypoxia | ↑ | – | ↑ | ↑ ASC ↑ NLRP3 |
↑ IL-1β ↑ ROS |
↑ LDH | – | |||
| 12 (+TNF-α) | H9C2 cells | ↑ | – | ↑ | ↑ NLRP3 | ↑ NOX4 ↑ ROS |
↓ Cell viability ↑ LDH |
↓ SIRT1 ↑ PI |
Hypoxia with TNF-α increased oxidative stress, and reduced SIRT1 to activate GSDMD in canonical pathway resulting in cardiomyoblast injury and pyroptosis. | 42,43 |
| ↑ GSDMD-N per GSDMD-FL ratio | ||||||||||
| OGD 36 h |
H9C2 cells | ↑ | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
↑ p-NF-κB ↑ ROS ↓ SOD |
↑ LDH | – | OGD increased oxidative stress to activate GSDMD in canonical pathway, leading to cardiomyoblast pyroptosis. | 42, 43 |
| H2O2 1, 2, 3 h |
AMVCMs | ↑ | ↑ | – | – | – | – | – | Oxidative stress induced the activation of GSDMD, which is an executioner of cardiomyocyte pyroptosis. | 42, 43 |
AMVCMs: adult mouse ventricular cardiomyocytes; ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; GDF11: growth differentiation factor 11; GSDMD: Gasdermin D; GSDMD-FL: full-length Gasdermin D; GSDMD-N: N-terminal Gasdermin D fragment; GSH-PX: glutathione peroxidase; H: hypoxia; H/R: hypoxia/reoxygenation; H2O2: hydrogen peroxide; HCEMCs: human cardiac microvascular endothelial cells line; HOXA3: homeobox A3; IL: interleukin; LDH: lactate dehydrogenase; MDA: malondyaldehyde; NF-κB: nuclear factor κ-light-chain-enhancer of activated B cells; NLRP3: NACHT, LRR and PYD domains-containing protein 3; NMCMs: neonatal mouse cardiomyocytes; NMVCMs: neonatal mouse ventricular cardiomyocytes; NOX4: nicotinamide adenine dinucleotide phosphate oxidase 4; NRCMs: neonatal rat cardiomyocytes; OGD: oxygen–glucose deprivation; PCSK9: proprotein convertase subtilisin kexin type 9; PI: propidium iodide; R: reoxygenation; RBP4: retinol-binding protein 4; ROS: reactive oxygen species; sh: short hairpin; si-: short interfering; SIRT: sirtuin; SOD: superoxide dismutases; TLR4: toll-like receptor 4; TNF-α: tumor necrosis factor alpha; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
In addition to normal hypoxic experiments, oxygen-glucose deprivation (OGD)44 and H2O2-induced myocardial injury models have been studied with regard to their roles in the pyroptosis pathway21. Oxidative stress seems to be one of the main triggers of this pyroptosis30,40,42, 43, 44, and H2O2 alone can upregulate GSDMD-N, the instigator of the pyroptosis21. Overall, GSDMD/NLRP3/caspase-1-mediated pyroptosis occurred in conjunction with overgeneration of ROS together with NF-κB activation44. Elevated levels of IL-1β and IL-18 were also found in cardiomyocytes and cardiomyoblasts as a result of GSDMD pore formation-induced pyroptotic cell death18,27,30,38, 39, 40. Collectively, all of these findings supported the idea that NLRP3/caspase-1-mediated pyroptosis was one of the mechanisms of cell death that occurred in the hypoxic cardiac cells.
Some proteins were found to play a key role in the regulation of pyroptotic cell death of hypoxic cardiac cells including retinol-binding protein 4 (RBP4), growth differentiation factor 11 (GDF11), homeobox A3 (HOXA3), and PCSK930,40,41. Previous studies demonstrated that RBP4 and PCSK9 promoted ROS generation to induce NLRP3 activation in the cardiomyocytes30,40.This finding was confirmed by a study in hypoxic PCSK9 knockout HL-1 cardiomyocytes that showed lower ROS, lactate dehydrogenase (LDH), and pyroptotic protein expression levels30. On the other hand, 12 h of hypoxia particularly downregulated the expression of GDF11-HOXA3 signaling proteins, leading to activation of cardiomyocyte pyroptosis41. As a result, cardiomyocyte/cardiomyoblast hypoxia could induce oxidative stress and inflammation and lead to canonical NLRP3/caspase-1-mediated pyroptosis.
5. Pyroptosis and GSDMD-mediated cardiomyocyte death in H/R injury: Evidence from in vitro studies
Pyroptosis is also shown to have a role in the hypoxia/reoxygenation (H/R) model, which involves upregulation of the pro-inflammatory signaling pathway (Table 312,19,21,45, 46, 47, 48, 49, 50, 51). H/R injury activated thioredoxin-interacting protein (TXNIP), which triggered the TLR4/myeloid differentiation factor 88 (MYD88)/NF-κB signaling pathway, and caused NLRP3 inflammasome/caspase-1-mediated pyroptosis45,46. In addition to inflammation, ROS also play a role in the activation of NLRP3 inflammasome/caspase-1-mediated pyroptosis45,47. Not only did H/R injury cause pyroptosis in cardiomyocytes and cardiomyoblasts, but it also caused NLRP3/caspase-1-mediated pyroptosis in human cardiac microvascular endothelial cell lines (HCMECs) with evidence of vascular endothelial proliferation19. However, a study by Shi et al.21 showed occurrence of a non-canonical pathway of pyroptosis in an adult mouse ventricular cardiomyocyte (AMVCMs) H/R model. As expected, caspase-11 acts as the major activator of GSDMD-N without an increase in NLRP3, ASC, or caspase-1 expression. Moreover, caspase-11 knockout AMVCMs in the H/R model caused a reduction in GSDMD-N, LDH, propidium iodide (PI) positive cells and an increase in cellular ATP levels21. In contrast, caspase-1 knockout AMVCMs had no discernible impact on these. These findings suggest that caspase-11 predominantly mediates inflammation-mediated pyroptosis during H/R injury21.
Table 3.
Evidence of pyroptosis in MI/R injury: reports from in vitro studies.
| Condition |
Model | Pyroptotic marker |
Inflammatory marker ROS |
Cell viability Toxicity |
Other cell death marker Relevant finding |
Interpretation | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H (h) | R (h) | GSDMD |
Caspase-1 | Others | |||||||
| Active form | Full form | ||||||||||
| 1 | 2 | NRCMs | ↑ | ↔ | ↑ | ↑ ASC ↑ NLRP3 |
↑ TLR4 ↑ MYD88 ↑ p-IκBα/IκBα ↑ p-NF-κB/NF-κB ↑ IL-1β ↑ ROS |
↓ Cell viability ↑ LDH |
– | H/R increased oxidative stress to activate GSDMD in canonical pathway, leading to cardiomyocyte pyroptosis. | 42, 43 |
| 2 | 24 | NRCMs | ↑ | ↔ | – | ↑ ASC ↑ NLRP3 |
↑ TLR4 ↑ MYD88 ↑ p-IκBα ↑ p-NF-κB ↑ IL-1β ↑ IL-18 ↑ TXNIP |
↓ Cell viability ↑ LDH ↑ CK ↑ CK-MB |
↓ miR-148a ↑ Annexin V/PI ↑ IP3R ↑ SERCA2a ↑ Ca2+ overload |
H/R reduced miRNA-148a, then TXNIP was increased to promote oxidative stress and calcium overload, leading to the activation of GSDMD in canonical pathway to induce pyroptosis. | 42, 43 |
| 4 | 2 | NMCMs | – | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
↑ IL-1β ↑ ROS |
↓ Cell viability ↑ LDH |
↑ Caspase-3 ↑ TUNEL |
H/R increased oxidative stress to activate GSDMD in canonical pathway, leading to cardiomyocyte pyroptosis and apoptosis. | 42, 43 |
| 4 | 2 | AC16 cells | ↑ | – | ↑ | ↑ NLRP3 | ↑ IL-1β ↑ IL-18 |
↓ Cell viability ↑ LDH |
↓ miR-100-5p ↑ PI |
H/R suppressed miR-100-5p, leading to an activation of GSDMD mediated cardiomyocyte pyroptosis. | 42, 43 |
| 4 | 3 | H9C2 cells | ↑ | ↔ | ↑ | – | ↑ IL-1β | ↓ Cell viability ↑ LDH ↑ CK-MB |
↑ lncRNA Pvt 1 ↑ Apoptotic rate |
LncRNA Pvt 1 play a crucial role in caspase-1/GSDMD-mediated cardiomyoblast pyroptosis and apoptosis. | 42, 43 |
| H9C2 cells + sh-Pvt 1 vs. H/R | ↓ | ↔ | ↓ | – | ↓ IL-1β | ↑ Cell viability ↓ LDH ↓ CK-MB |
↓ lncRNA Pvt 1 ↓ Apoptotic rate |
||||
| 4 | 4 | H9C2 cells | – | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
↑ IL-1β ↑ IL-18 |
↓ Cell viability | ↑ Periostin | H/R 4/8 h had higher level of pyroptotic markers than the other groups, and it was mediated by periostin to induce canonical pyroptosis pathway. | 42, 43 |
| 8 | – | ↑↑ | ↑↑ | ↑↑↑ ASC ↑↑ NLRP3 |
↑↑ IL-1β ↑↑↑ IL-18 |
↓ Cell viability | ↑ Periostin | ||||
| 16 | – | ↑ | ↑ | ↑↑ ASC ↑ NLRP3 |
↑↑ IL-1β ↑↑ IL-18 |
↓ Cell viability | ↑ Periostin | ||||
| 4 | 8 | H9C2 cells + si-periostin vs. H/R | – | ↓ | ↓ | ↓ ASC ↓ NLRP3 |
↓ IL-1β ↓ IL-18 |
↑ Cell viability | – | ||
| H9C2 cells + periostin overexpression vs. H/R | – | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
↑ IL-1β ↑ IL-18 |
↓ Cell viability | – | ||||
| 0.5 | 3 | AMVCMs | ↔ | ↑ | ↔ | ↔ ASC ↔ NLRP3 ↑ Caspase-11 ↔ GSDME-FL |
– | – | – | Cardiomyocyte pyroptosis occurred after 6 h of reoxygenation through the non-canonical pathway. | 42, 43 |
| 6 | ↑ | ↑ | ↔ | ↔ ASC ↔ NLRP3 ↑ Caspase-11 ↔ GSDME-FL |
– | – | – | ||||
| 12 | ↑ | ↑ | ↔ | ↔ ASC ↔ NLRP3 ↑ Caspase-11 ↔ GSDME-FL |
– | – | – | ||||
| 24 | ↑ | ↑ | ↔ | ↔ ASC ↔ NLRP3 ↑ Caspase-11 ↔ GSDME-FL |
↔ IL-1β ↑ IL-18 |
↑ LDH | ↑ PI ↓ ATP |
||||
| 0.5 | 24 | CASP1−/− AMVCMs vs. H/R | ↔ | – | ↓ | – | ↔ IL-18 | ↔ LDH | ↔ PI ↔ ATP |
||
| CASP11−/− AMVCMs vs. H/R | ↓ | – | – | ↓ Caspase-11 | ↓ IL-18 | ↓ LDH | ↓ PI ↑ ATP |
||||
| GSDMD−/− AMVCMs vs. H/R |
– | – | – | – | ↓ IL-18 | ↓ LDH | ↓ PI ↑ ATP |
||||
| GSDMD-FL overexpression AMVCMs vs. H/R |
– | ↑ | – | – | – | ↔ LDH | ↔ PI ↔ ATP |
||||
| GSDMD-N overexpression AMVCMs vs. H/R |
↑ | – | – | – | – | ↑ LDH | ↑ PI ↓ Cell ATP |
||||
| GSDMD-C overexpression AMVCMs vs. H/R |
– | – | – | ↑ GSDMD-C | – | ↔ LDH | ↔ PI ↔ ATP |
||||
| 2 | 1 | NMCMs | ↔ | ↔ | ↑ | ↔ ASC ↑ NLRP3 |
↑ TXNIP | – | ↓ GATA4 ↓ Bcl-2/BAX ↑ Caspase-3 ↑ Annexin V/PI |
A short duration of reoxygenation led to oxidative stress and apoptosis, but the duration was inadequate to induce pyroptosis. | 42, 43 |
| 2 | 2 | HCMECs | – | ↑ | ↑ | ↑ NLRP3 | ↑ IL-1β | – | ↑ VEGF | H/R activated GSDMD in canonical pathway, leading to endothelial cells pyroptosis. However, angiogenesis was increased in this setting. | 42, 43 |
AMVCMs: adult mouse ventricular cardiomyocytes; ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; ATP: adenosine triphosphate; BAX: Bcl 2 associated X protein; Bcl-2: B-cell lymphoma 2; CASP1−/−: caspase-1 knockout; CASP11−/−: caspase-11 knockout; CK: creatine kinase; CK-MB: creatine kinase myocardial band; GATA4: GATA binding protein 4; GSDMD: Gasdermin D; GSDMD−/−: cardiac specific Gasdermin D knockout; GSDMD-C: C-terminal Gasdermin D fragment; GSDMD-FL: full-length Gasdermin D; GSDMD-N: N-terminal Gasdermin D fragment; GSDME-FL: full-length Gasdermin E; H: hypoxia; H/R: hypoxia/reoxygenation; HCEMCs: human cardiac microvascular endothelial cells line; IκBα: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; IL: interleukin; IP3R: type 3 inositol 1,4,5-trisphosphate receptor; LDH: lactate dehydrogenase; lncRNA: long non-coding RNA; miR: microRNA; MYD88: myeloid differentiation factor 88; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3: NACHT, LRR and PYD domains-containing protein 3; NMCMs: neonatal mouse cardiomyocytes; NRCMs: neonatal rat cardiomyocytes; p-IκBα: phosphorylated IκBα; PI: propidium iodide; Pvt 1: plasmacytoma variant translocation 1; R: peoxygenation; ROS: reactive oxygen species; SERCA2a: sarco/endoplasmic reticulum Ca2+-ATPase 2a; sh-: short hairpin; siRNA: small interfering RNA; TLR4: toll-like receptor 4; TXNIP: thioredoxin-interacting protein; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling; VEGF: vascular endothelial growth factor.
GSDMD plays a key role as a pyroptosis executor in the H/R model since no change in GSDME was observed after H/R injury21. In cardiac-specific GSDMD knockout AMVCMs, the findings showed a decrease in LDH, PI-positive cells, and an increase in cell ATP level21. Overexpression of GSDMD-N could aggravate LDH production, the number of PI-positive cells and further decrease cellular ATP levels. In contrast, overexpression of full-length GSDMD (GSDMD-FL) and GSDMD-C showed no change in association with cell death and injury21. According to some studies which measured both GSDMD-FL and GSDMD-N, only GSDMD-N increased following H/R induction, whereas GSDMD-FL remained constant45,46. IL-1β and IL-18 were the cytokines released following the H/R-induced pyroptosis46,48,49. However, after pyroptosis, IL-18 increased, whereas IL-1β remained unchanged in AMVCMs21.
In experiments with different time points of reoxygenation, pyroptosis seems to occur prominently in the semi-early phase after reoxygenation. The expression levels of pyroptotic proteins at the time of the lowest cell viability was highest at 8 h after reoxygenation and decreased at 16 h after reoxygenation49. GSDMD-N was also found to be increased significantly at 6 h post-reoxygenation, peaked at 12 h post-reoxygenation, and remained relatively stable at 24 h post-reoxygenation21. In neonatal mouse cardiomyocytes (NMCMs), 2 h of hypoxia followed by 1 h of reoxygenation could induce the expression of NLRP3 and caspase-1, but not GSDMD, indicating that pyroptosis either did not occur during the early phase of H/R or could be due to the low expression of GSDMD in NMCMs50.
Recently, several non-coding ribonucleic acid (RNA), including microRNA (miR) and long non-coding RNA (lncRNA), have been studied and found to be associated with H/R injury and pyroptosis46,48,51. H/R significantly led to pyroptotic death by decreasing miR-148a and miR-100-5p expression while increasing expression of lncRNA plasmacytoma variant translocation 1 (Pvt 1)46,48,51. However, cardiomyoblasts transfected with short hairpin Pvt1 (sh-Pvt1) exhibited a reduction in pyroptotic and apoptotic proteins, and also cardiac enzymes51. Additionally, it has been demonstrated that periostin, an osteoblast-specific factor 2, could induce pyroptosis in H/R-induced H9C2 cells via NLRP3/caspase-1-mediated pyroptosis as periostin overexpression increased production of pyroptotic proteins and decreased cell viability49. In contrast, antiperiostin, small interfering (si)RNA transfection, had the opposite effect49. All of these findings indicated that H/R injury can induce inflammatory signals and oxidative stress in cardiac cells, resulting in GSDMD-N-mediated pyroptosis during the semi-early phase of reoxygenation, with evidence for both canonical and non-canonical pathways. It is important, however, as noted previously, to recognize that other proteins and non-coding RNAs may also play a role in the modulation of pyroptosis.
6. Evidence of pyroptosis in MI and MI/R injury: Reports from in vivo studies
MI is known to cause cardiomyocyte death via oxidative stress, inflammation, and mitochondria-dependent pathways11. Focusing on pyroptosis, MI was shown to result in the upregulation of the expression of NLRP3-mediated IL-1β and IL-18 activation from 6 h up to 4 weeks in mouse/rat models with either left anterior descending coronary artery (LAD) ligation27,38, 39, 40, 41,52, 53, 54 or left coronary artery ligation18,30. Consistent with reports in in vitro models, MI also increased oxidative stress via NLRP3-mediated pyroptosis and the NLRP3 inflammasomes via the TLR4/MYD88/NF-κB pathway18,38,40,52,53. Furthermore, oxidative stress activated transforming growth factor β-activated kinase 1 (TAK1) and its downstream factor, c-Jun N-terminal kinase (JNK), resulting in an inflammatory response, pyroptotic cell death, and fibrosis53.
Focusing on the relevant mechanisms of MI-induced pyroptosis, MI increased the expression of RBP4 in the heart tissue without changing serum RBP4 and retinol or retinyl ester levels, whereas knockdown of RBP4 alleviated pyroptosis-mediated cardiac injury and dysfunction40. In addition, MI also reduced the expression of GDF11 and HOXA3 proteins, while GDF11 overexpressed mice manifested cardioprotection via the lowering of pyroptotic protein expression levels and reducing mitochondrial damage, resulting in improved LV function41. Interestingly, PCSK9 has been reported to regulate pyroptosis in a mouse model30. As mentioned earlier, PCSK9 was associated with the upregulation of cellular ROS to induce NLRP3-mediated pyroptosis30. PCSK9 knockout mice showed less MI-induced cardiac injury and lower levels of NLRP3-mediated pyroptosis proteins30. In addition to PCSK9, C-type lectin member 5 A (CLEC5A), a recognition receptor for some pathogenic microorganisms, and CXADR-like membrane protein (CLMP) have been shown to increase after MI54,55. Silencing CLEC5A significantly reduced the activation of inflammatory cascades, macrophages, NLRP3-mediated pyroptosis, and improved LV function in mouse models of MI54. However, CLMP knockdown mice appeared to have more serious myocardial injury resulting from the promotion of non-NLRP3 dependent pyroptosis, leading to greater neutrophil accumulation, LV dysfunction, and higher mortality after MI55. These findings indicated that ischemia-induced CLMP expression was vital for the balance of the regulation of pyroptosis and the inflammatory response. In the experiment with different time points of MI, pyroptosis was initially observed at 6 h after MI as indicated by the elevation of GSDMD and other pyroptotic proteins (e.g., NLRP3, caspase-1, caspase-4, and IL-1β), and increased in a time-dependent manner (measured until 72 h)22.
When pyroptosis occurred, there was evidence of an upregulation of caspase-4, one of the caspases that activates pyroptosis22, suggesting that the NLRP3/caspase-1 canonical pathway was not the only one that plays a role in MI-induced pyroptosis. Similarly, one of the causes of coronary microvascular dysfunction after MI is coronary microembolization (CME) from atherosclerotic plaque rupture debris56. In mouse models, CME led to mitochondrial disruption, ROS generation, and evidence of NLRP3/caspase-1-mediated pyroptosis43, suggesting that pyroptosis might also be one of the mechanisms responsible for CME-induced cardiac injury. All of these findings are comprehensively summarized in Table 418,22,27,30,38, 39, 40, 41,43,52, 53, 54, 55.
Table 4.
Evidence of pyroptosis in MI: reports from in vivo studies.
| Disease Condition |
Model | Pyroptotic marker |
IS% | LV function | Cardiac injury | Inflammatory marker/ROS | Other cell death marker/Relevant findings | Interpretation | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ischemia | GSDMD |
Caspase-1 | Others | |||||||||
| Active form | Full form | |||||||||||
| 10 min | C57BL/6 mice | – | ↔ | ↔ | ↔ NLRP3 ↔ Caspase-4 |
– | – | – | ↔ IL-1β ↔ IL-18 |
↔ CF genes ↔ DEGs, DEPs of immune and apoptosis ↔ Caspase-3 |
GSDMD was activated in both canonical and non-canonical pathways, other molecular changes were adapted after 6 h of MI, with inflammatory cells infiltration. They continued to increase in a time dependent manner, leading to cardiac fibroblast activation at 72 h. | 42, 43 |
| 1 h | – | ↔ | ↑ | ↔ NLRP3 ↔ Caspase-4 |
– | – | – | ↔ IL-1β ↔ IL-18 |
↔ CF genes ↔ DEGs, DEPs of immune and apoptosis ↔ Caspase-3 |
|||
| 6 h | – | ↑ | ↑ | ↑ NLRP3 ↑↑ Caspase-4 |
– | – | – | ↑↑ IL-1β ↔ IL-18 ↑ Inflammatory cells ↑ %CD8 |
↔ CF genes ↑ DEGs, DEPs of immune and apoptosis ↔ Caspase-3 |
|||
| 24 h | – | ↑ | ↑ | ↑ NLRP3 ↑ Caspase-4 |
– | ↓ %EF | – | ↑↑ IL-1β ↑ IL-18 ↑ Inflammatory cells ↑ %CD8 ↑ Monocyte ↑ M0- macrophage |
↔ CF genes ↑ DEGs, DEPs of immune and apoptosis ↑ Caspase-3 |
|||
| 72 h | – | ↑↑ | ↑↑ | ↑ NLRP3 ↑↑ Caspase-4 |
– | ↓ %EF | – | ↑ IL-1β ↑↑ IL-18 ↑↑Inflammatory cells ↑ %CD8 ↑ Monocyte ↑ M0- Macrophage |
↑ CF genes ↑ DEGs, DEPs of immune and apoptosis ↑↑ Caspase-3 |
|||
| 12 h | C57BL/6 mice | ↑ | – | ↑ | ↑ ASC ↑ NLRP3 |
– | ↓ %EF ↓ %FS ↑ LVIDd ↑ LVIDs |
– | ↑ IL-1β ↑ IL-18 |
↓ GDF11 ↓ HOXA3 ↑ Mitochondrial damage |
MI activated GSDMD through GDF11/HOXA3 in canonical pathway, leading to pyroptosis, mitochondrial damage, and LV dysfunction. | 42, 43 |
| C57BL/6 mice + GDF11 overexpression vs. WT-MI |
↓ | – | ↓ | ↓ ASC ↓ NLRP3 |
– | ↑ %EF ↑ %FS ↓ LVIDd ↓ LVIDs |
– | ↓ IL-1β ↓ IL-18 |
↑ Myofibrils arrangement ↓ Mitochondrial damage |
|||
| 24 h | C57BL/6 mice | ↑ | ↑ | ↑ | ↑ NLRP3 | ↑ | ↓ %EF ↓ %FS |
↑ LDH | ↑ IL-1β ↑ IL-18 |
↑ DNA fragmentation ↑ Mitochondrial swelling ↑ Cytoplasmic membrane pore formation |
MI activated GSDMD in canonical pathway, leading to pyroptosis, mitochondrial damage, and LV dysfunction. | 42, 43 |
| 24 h | C57BL/6 mice | ↑ | – | ↑ | ↑ NLRP3 | ↑ | ↓ %EF ↓ %FS |
↑ LDH | ↑ TLR4 ↑ NF-κB ↑ IL-1β ↑ IL-18 |
– | MI activated GSDMD in canonical pathway, leading to pyroptosis, and LV dysfunction. | 42, 43 |
| 1 day | SD rats | ↑ | ↑ | ↑ | ↑ NLRP3 | ↑ | ↓ %EF ↓ %FS ↑ LVIDd ↑ LVIDs |
↑ ROS ↑ TAK1 ↑ p-JNK ↑ IL-1β ↑ IL-18 |
↔ CVF | MI increased oxidative stress, activated TAK1/JNK pathway, and activated GSDMD in canonical pathway, resulting in pyroptosis and LV dysfunction. The deterioration of LV function was in a time dependent manner, and increase of collagen volume was observed only at 7 days post–MI. | 42, 43 | |
| 3 days | ↑ | ↑ | ↑ | ↑ NLRP3 | ↑↑ | ↓↓ %EF ↓↓ %FS ↑↑ LVIDd ↑↑ LVIDs |
↑↑ ROS ↑ TAK1 ↑ p-JNK ↑ IL-1β ↑ IL-18 |
↔ CVF | ||||
| 7 days | ↑ | ↑ | ↑ | ↑ NLRP3 | ↑↑↑ | ↓↓↓ %EF ↓↓↓ %FS ↑↑↑ LVIDd ↑↑↑ LVIDs |
↑↑↑ ROS ↑ TAK1 ↑ p-JNK ↑ IL-1β ↑ IL-18 |
↑ CVF | ||||
| 72 h | SD rats | ↑ | ↔ | ↑ | ↑ ASC ↑ NLRP3 |
↑ | ↓ %EF ↓ %FS ↑ LVIDd ↑ LVIDs |
↑ cTnI ↑ LDH |
↑ TLR4 ↑ MYD88 ↑ NF-κB ↑ IL-1β ↑ IL-18 |
↓ ATP ↓ ADP ↓ AMP ↓ ATP/ADP ratio ↓ Total adenine nucleotide |
MI activated GSDMD in canonical pathway, leading to pyroptosis, ATP depletion, and LV dysfunction. | 42, 43 |
| 3 days | C57BL/6 mice | ↑ | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
– | ↓ %EF ↓ %FS ↑ LVIDd ↑ LVIDs ↑ LVvold ↑ LVvols |
– | ↑ MDA ↑ ROS ↓ GSH-PX ↓ SOD ↑ IL-1β ↑ IL-18 |
↑ Heart RBP4 ↔ Serum RBP4 ↔ Retinol ↔ Retinyl ester |
MI increased oxidative stress to activate GSDMD through RBP in canonical pathway, leading to pyroptosis and LV dysfunction. However, serum RBP4, retinol or retinyl ester did not change following MI. | 42, 43 |
| C57BL/6 mice + sh-RBP4 vs. WT + MI | ↓ | ↓ | ↓ | ↓ ASC ↓ NLRP3 |
↓ | ↑ %EF ↑ %FS ↓ LVIDd ↓ LVIDs ↓ LVvold ↓ LVvols |
↓ ANP ↓ BNP ↓ MHC-7 ↓ LDH |
↓ IL-1β ↓ IL-18 |
↓ Heart RBP4 | |||
| 3 days | WT-FVB/NJ mice | ↑ | – | ↑ | – | ↑ | ↓ %EF ↓ %FS ↑ LVIDs ↑ LVvols (3 days) ↑ LVIDd ↑ LVvold (7 days) |
– | – | ↑ CLMP | MI increased CLMP expression to prevent pyroptosis and LV dysfunction following MI. Knockdown CLMP led to more serious myocardial injury through promoting non-NLRP3-dependent pyroptosis but not necroptosis or parthanatos. |
42, 43 |
| CLMP+/− FVB/NJ mice vs. WT + MI | ↑↑ | – | ↑↑ | ↔ NLRP3 | ↑↑ | ↓↓ %EF ↓↓ %FS (14 days) ↑↑ LVIDd ↑↑ LVIDs ↑↑ LVvols ↑↑ LVvold (7 days) |
↑ LDH | ↑ IL-1β ↑ MPO ↑ Ly6g |
↓ CLMP ↔ RIPK3 ↔ CaMKII ↔ PARP |
|||
| 1 week | C57BL/6 mice | ↑ | – | ↑ | ↑ ASC ↑ NLRP3 |
↑ | – | – | ↑ IL-1β ↑ IL-18 |
↑ PCSK9 | Upregulation of PCSK9 involved in the activation of GSDMD in canonical pathway to induce cardiac pyroptosis, which was confirmed by genetic inhibition. | 42, 43 |
| PCSK9−/− C57BL/6 mice vs. WT-MI |
↓ | ↔ | ↓ | ↓ ASC ↓ NLRP3 |
– | – | ↓ LDH | ↓ IL-1β ↓ IL-18 |
– | |||
| 1 week | C57BL/6 mice | ↑ GSDMD-N per GSDMD-FL ratio | ↑ | ↑ ASC ↑ NLRP3 |
↑ | ↓ %EF ↓ %FS ↑ LVIDd ↑ LVIDs ↑ LVvols ↑ LVvold |
↑ p-NF-κB/NF-κB ↑ nuNF-κB ↓ cytoNF-κB ↑ IL-1β ↑ IL-18 ↑ IL-6 ↑ TNF-α ↑ iNOS ↑ MAC-3+ CLEC5A+ ↑ MAC-3+ iNOS+ |
↑ CLEC5A | Upregulation of CLEC5A involved in the activation of GSDMD in canonical pathway to induce cardiac pyroptosis, cytokine release, and LV dysfunction, which was confirmed by genetic inhibition. | 54 | ||
| C57BL/6 mice + sh-CLEC5A vs. WT-MI |
↓ GSDMD-N per GSDMD-FL ratio | ↓ | ↓ ASC ↓ NLRP3 |
↓ | ↑ %EF ↑ %FS ↓ LVIDd ↓ LVIDs ↓ LVvols ↓ LVvold |
↓ p-NF-κB/NF-κB ↓ nuNF-κB ↑ cytoNF-κB ↓ IL-1β ↓ IL-18 ↓ IL-6 ↓ TNF-α ↓ iNOS ↓ MAC-3+ CLEC5A ↓ MAC-3+ iNOS+ |
↓ CLEC5A | |||||
| 1 week | F344 rats | ↑ | – | ↑ | ↑ ASC ↑ NLRP3 |
↑ | ↓ %EF ↑ LVIDd ↑ Heart weight/tibial length |
– | ↑ IL-1β ↑ IL-18 |
↑ TUNEL ↑ Cell swelling↑ Irregular nuclear arrangement ↑ Neutrophil infiltration |
MI activated GSDMD in canonical pathway, leading to pyroptosis, LV dysfunction, and LV remodeling. | 42, 43 |
| 4 weeks | SD rats | ↑ GSDMD-N per GSDMD-FL ratio | ↑ | ↑ ASC ↑ NLRP3 |
– | ↓ %EF ↓ %FS ↑ LVIDd ↑ LVIDs |
↑ BNP | ↑ MDA ↑ •OH ↑ IL-1β |
↑ Mitochondrial damage ↑ Fibrosis |
MI increased oxidative stress to activate GSDMD in canonical pathway, leading to pyroptosis, mitochondrial damage, LV remodeling, and LV dysfunction. | 18 | |
| CME induction (Post CME 3 days) | C57BL/6 mice | ↑ GSDMD-N per GSDMD-FL ratio | ↑ | ↑ NLRP3 | ↑ | ↓ %EF ↓ %FS |
↑ LDH | ↑ SDHA ↑ SDHB ↑ ROS ↑ IL-1β |
↑ Collagen deposit ↑ Vacuolated and malformed mitochondria |
CME increased oxidative stress and GSDMD in canonical pathway, leading to pyroptosis, mitochondrial dysfunction, LV remodeling, and LV dysfunction. | 43 | |
·OH: hydroxyl radical; ADP: adenosine di-phosphate; AMP: adenosine monophosphate; ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; ATP: adenosine triphosphate; BNP: B-type natriuretic peptide; CaMKII: calcium/calmodulin-dependent protein kinase II; CD8: T cell CD8; CF: cardiac fibroblasts; CLEC5A: C-type lectin membrane 5 A; CLMP: CXADR-like membrane protein; CME: coronary microembolization; CVF: collagen volume fraction; cyto NF-κB: the expression of NF-κB in cytosol; DEGs: differentially expressed genes; DEPs: differentially expressed proteins; DNA: deoxyribonucleic acid; %EF: left ventricular ejection fraction; %FS: left ventricular fractional shortening; GDF11: growth differentiation factor 11; GSDMD: Gasdermin D; GSDMD-FL: full-length Gasdermin D; GSDMD-N: N-terminal Gasdermin D fragment; HOXA3: homeobox A3; I: ischemia by coronary artery ligation; IL: interleukin; iNOS: inducible Nitric oxide synthase; IS%: infarct size/area at risk; LDH: lactate dehydrogenase; LV: left ventricle; LVIDd: LV internal dimension at end-diastole; LVIDs: LV internal dimension at end-systole; LVvold: LV volume at end-diastole; LVvols: LV volume at end-systole; Ly6g: lymphocyte antigen six complex locus G6D; MAC-3: macrophage marker MAC-3; MDA: malondialdehyde; MI: myocardial ischemia; MPO: myeloperoxidase; MYD88: myeloid differentiation factor 88; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; nu NF-κB: the expression of NF-κB in nuclues; NLRP3: NACHT, LRR and PYD domains-containing protein 3; nu p65: nuclear NF-κB-p65; p-JNK: phosphorylated Jun N-terminal kinase; PARP: Poly (ADP-ribose) polymerase; PCSK9: proprotein convertase subtilisin/Kexin type 9; PCSK9−/−: PCSK9 knockdown; R: reperfusion; RBP4: retinol-binding protein 4; RIPK3: receptor-interacting protein kinase 3; ROS: reactive oxygen species; SD rats: Sprague–Dawley rats; SDHA: succinate dehydrogenase complex flavoprotein subunit A; SDHB: succinate dehydrogenase complex flavoprotein subunit B; SOD: superoxide dismutases; TAK1: transforming growth factor-β-activated kinase 1; TLR4: toll-like receptor 4; TNF-α: tumor necrosis factor alpha; TXNIP: thioredoxin-interacting protein; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling; WT: wild type.
In the case of MI/R injury, there is evidence to confirm that NLRP3-inflammasome-mediated pyroptosis did occur as shown in Table 519,21,45, 46, 47,49,51,57,67,70. In a mouse model, LAD ligation-induced MI/R injury could upregulate HMGB1 protein (one of the potent alarmins)57 and TXNIP, and activate the TLR4/MYD88/NF-κB signaling pathway, which promoted NLRP3-mediated pyroptosis46. However, similar to the in vitro reports which reported that the canonical inflammasome components (e.g., ASC, NLRP3, and caspase-1) were barely expressed in the cardiomyocytes21, caspase-11 was found to play a major role in MI/R-induced pyroptosis, which occurred after 3 h of post-reperfusion without the involvement of the GSDME21. This finding demonstrated the importance of the non-canonical caspase-11-mediated pyroptotic pathway and the timing of I/R-mediated myocardial injury from pyroptosis cell death. Additionally, lncRNA Pvt 1, an lncRNA that has been linked to the pathogenesis of cardiovascular disease58, was also shown to have a role in MI/R injury, as it was upregulated during the MI/R period, while knockdown lncRNA Pvt1 showed less cytokine release, cardiac injury, apoptosis, and NLRP3-mediated pyroptosis51. In summary, pyroptosis plays a part in MI and MI/R injury in the in vivo settings, which could be activated by both the canonical NLRP3 pathway and the non-canonical caspase 4/11 pathway. This also appears to occur in the semi-early phase, similar to that observed in in vitro studies.
Table 5.
Evidence of pyroptosis in MI/R injury: rreports from in vivo studies.
| Disease Condition |
Model | Pyroptotic marker |
IS% | LV function | Cardiac injury | Inflammatory marker/ROS | Other cell death markers/Relevant findings | Interpretation | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | R | GSDMD |
Caspase-1 | Others | |||||||||
| Active form | Full form | ||||||||||||
| 0.5 h | 2 h | Kunming mice | – | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
↑ | – | ↑ CK-MB ↑ LDH |
↑ IL-1β | ↑ TUNEL ↑ Caspase-3 |
I/R activated GSDMD in canonical pathway, leading to pyroptosis, apoptosis, cardiac injury and infarction. | 42, 43 |
| 0.5 h | 2 h | SD rats | ↑ | ↔ | – | – | ↑ | – | – | ↑ IL-1β | ↑ Disordered and swollen myocardial cells | I/R activated GSDMD-N, leading to pyroptosis, and infarction. | 42, 43 |
| 0.5 h | 2 h | SD rats | ↑ | ↔ | – | ↑ ASC ↑ NLRP3 |
↑ | – | ↑ CK ↑ CK-MB ↑ LDH |
↑ TXNIP ↑ TLR4 ↑ MYD88 ↑ p-IκBα ↑ p-NF-κB ↑ IL-1β ↑ IL-18 |
↓ miR-148a ↑ IP3R ↑ SERCA2a ↑ Ca2+ overload ↑ Disordered and swollen cells |
I/R increased oxidative stress through miR-148a/TXNIP axis to activate GSDMD in canonical pathway, resulting in pyroptosis, calcium overload, and infarction. | 42, 43 |
| 0.5 h | 2 h | SD rats | ↑ | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
↑ | ↓ %EF ↓ %FS ↑ E/A |
– | ↑ IL-1β ↑ IL-6 ↑ TNF-α |
↑ HMGB1 | Upregulation of HMGB1 induced by I/R activated GSDMD in canonical pathway, leading to pyroptosis, cytokine release, infarction, and LV dysfunction. | 42, 43 |
| 0.5 h | 24 h | SD rats | – | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
↑ | – | – | ↑ IL-1β ↑ IL-18 |
↑ TUNEL ↑ Periostin ↑ Inflammatory cells infiltration ↑ Myocardial ell swelling & degeneration |
I/R activated GSDMD in canonical pathway, leading to pyroptosis, inflammatory cells in filtration, and infarction. | 42, 43 |
| 45 min | 6 h | C57BL/6 mice | – | ↑ | ↑ | ↑ NLRP3 | ↑ | – | ↑ CK ↑ CK-MB ↑cTnI ↑ LDH |
– | ↑ TUNEL ↑ Neutrophils and macrophages infiltration ↓ %Survival rate |
I/R activated GSDMD in canonical pathway, leading to pyroptosis, cardiac injury and infarction. | 42, 43 |
| 45 min | 24 h | SD rats | ↑ | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
– | ↓ %EF ↓ %FS ↑ LVIDd ↑ LVIDs |
↑ cTNT ↑ MPO |
↑ IL-1β | – | I/R activated GSDMD in canonical pathway, leading to pyroptosis, cardiac injury and LV dysfunction. | 42, 43 |
| 45 min | 48 h | C57BL/6 mice | ↑ | ↔ | ↑ | ↑ NLRP3 | – | – | ↑ BNP ↓ α-MHC ↑ β-MHC ↑ CK-MB ↑ cTnI ↑ LDH |
↑ TLR4 ↑ MYD88 ↑ p-NF-κB/NF-κB ↑ IL-1β ↑ IL-6 ↑ TNF-α |
↑ lncRNA Pvt 1 ↑ BAX ↓ Bcl-2 ↑ c-Caspase-3 |
I/R increased lncRNA Pvt 1 to activate GSDMD in canonical pathway, leading to pyroptosis, apoptosis, cytokine release, and cardiac injury, which was confirmed by genetic inhibition. | 42, 43 |
| C57BL/6 mice + sh-Pvt1 vs. WT-I/R | ↓ | ↔ | ↓ | ↓ NLRP3 | – | – | ↓ BNP ↑ α-MHC ↓ β-MHC ↓ CK-MB ↓ cTnI ↓ LDH |
↓ TLR4 ↓ MYD88 ↓ p-NF-κB/NF-κB ↓ IL-1β ↓ IL-6 ↑ TNF-α |
↓ lncRNA Pvt 1 ↓ Bax ↑ Bcl-2 ↓ c-Caspase-3 |
||||
| 1 h | 24 h | SD rats | ↑ | – | ↑ | ↑ ASC ↑ NLRP3 |
– | ↓ %EF ↓ %FS |
↑ cTnI | ↑ IL-1β ↑ ROS ↑ MDA ↑ 8-OHDG |
↑ Mitochondrial damage | I/R increased oxidative stress to activate GSDMD in canonical pathway, leading to pyroptosis, mitochondrial damage, cardiac injury, and LV dysfunction, | 42, 43 |
| 0.5 h | 3 h | C57BL/6 mice | ↔ | ↔ | – | ↔ GSDME ↔ Caspase-11 |
– | – | – | – | – | I/R activated GSDMD in non-canonical pathway, leading to pyroptosis in the early phase of reperfusion. However, GSDME had no role in I/R. | 42, 43 |
| 12 h | ↑ | ↑ | – | ↔ GSDME ↑ Caspase-11 |
– | – | – | – | – | ||||
| 24 h | ↑ | ↑ | – | ↔ GSDME ↑ Caspase-11 |
– | – | – | – | – | ||||
Abbreviations: 8-OHDG: 8-hydroxy-2′-deoxyguanosine; ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; BAX: Bcl-2 associated X protein; Bcl-2: B-cell lymphoma-2; BNP: B-type natriuretic peptide; c-caspase: cleaved caspase; CK: creatine kinase; CK-MB: creatine kinase myocardial band; cTnI: cardiac Troponin I; cTnT: cardiac Troponin T; E/A: mitral early diastolic flow/late diastolic flow velocity; %EF: left ventricular ejection fraction; FS%: left ventricular fractional shortening; GSDMD: Gasdermin D; GSDME: Gasdermin E; HMGB1: high mobility group box 1 protein; I: ischemia by coronary artery ligation; I/R: ischemia/reperfusion; IκBα: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; IL: interleukin; IP3R: type 3 inositol 1,4,5-trisphosphate receptor; %IS: %infarct size/area at risk; LDH: lactate dehydrogenase; LncRNA: long non-coding RNA; LV: left ventricle; LVIDd: LV internal dimension at end-diastole; LVIDs: LV internal dimension at end-systole; MDA: malondialdehyde; MHC: myosin heavy chain; MI: myocardial ischemia; miR: microRNA; MYD88: myeloid differentiation factor 88; NF-κB: nuclear factor κ-light-chain-enhancer of activated B cells; NLRP3: NACHT, LRR and PYD domains-containing protein 3; p-IκBα: phosphorylated IκBα; p-NF-κB: phosphorylated NF-κB; Pvt 1: plasmacytoma variant translocation 1; R: reperfusion; RIPK3: receptor-interacting protein kinase 3; ROS: reactive oxygen species; SD rats: Sprague–Dawley rats; SERCA2a: sarco/endoplasmic reticulum Ca2+-ATPase 2a; sh-: short hairpin; TLR4: toll-like receptor 4; TNF-α: tumor necrosis factor alpha; TXNIP: thioredoxin-interacting protein TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling; WT: wild-type.
7. Potential interventions against pyroptosis-mediated cell death in MI: Reports from in vitro and in vivo studies
A number of pharmacological agents and interventions have been investigated to assess their potential to attenuate pyroptosis, myocardial injury, and enhance LV function following MI, as summarized in Tables 618,27,38,39,42, 43, 44 and 718,19,21,27,38,39,43,45,47,52,53,57,67,70. It is well established that the pyroptosis pathway is involved in the process of cell death following MI18,27,38,39,42, 43, 44,52,53. Fortunately, it has been demonstrated that several pharmacological agents could inhibit the canonical pyroptosis signaling components and exert cytoprotective effects against cardiomyocyte hypoxia/ischemia17,59. In vitro experiments (Table 6) revealed that cardiomyocytes treated with either an NLRP3 inhibitor (MCC950) during ischemia, or pyrrolidine dithiocarbamate (PDTC, NF-κB inhibitor) following OGD, effectively reduced GSDMD-N-mediated pyroptosis, resulting in increased cell viability18,44. Since ROS is one of the most potent DAMPs for the induction of oxidative stress in cells60, targeting ROS may be a strategy for providing cytoprotection during an ischemic insult. It has been shown that N-acetyl cysteine (NAC) effectively reduces ROS since it is a ROS scavenger61. NAC suppressed the NF-κB/NLRP3/caspase-1 pathway in both the OGD and the ischemic mimic models, resulting in decreased GSDMD-N levels and thus increased cell viability44,52. Together with NAC, other pharmacological agents such as melatonin, liraglutide, rosuvastatin, and sacubitril/valsartan (LCZ696) have been shown to reduce pyroptosis by reducing any increase in oxidative stress following MI38,42,43,53.
Table 6.
Evidence of interventions to modify pyroptosis in MI: Reports from in vitro studies.
| Condition/Hypoxia (h) | Model | Intervention | Pyroptotic marker |
Inflammatory marker/ROS | Cell viability/Toxicity | Other cell death marker/relevant finding | Interpretation | Ref | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GSDMD |
Caspase-1 | Others | |||||||||
| Active form | Full form | ||||||||||
| 2 | H9C2 cells | Melatonin 10 μmol/L, during hypoxia | ↓ | – | ↓ | ↓ NLRP3 | ↓ TLR4 ↓ NF-κB ↓ IL-1β ↓ IL-18 |
↓ LDH | – | Melatonin reduced pyroptosis through the canonical pathway. | 42, 43 |
| 4 | NRCMs | H2-containing medium, >0.6 mmol/L, during hypoxia | ↓ GSDMD-N per GSDMD-FL ratio | ↓ | ↓ ASC ↓ NLRP3 |
↓ IL-1β | ↑ Cell viability ↓ LDH |
↓ PI | H2 and MCC950 reduced pyroptosis through the canonical pathway. However, combined treatment did not provide further protective effect. | 18 | |
| MCC950 (NLRP3 inhibitor) 10 μmol/L, during hypoxia | ↓ GSDMD-N per GSDMD-FL ratio | ↓ | ↓ ASC ↓ NLRP3 |
↓ IL-1β | ↑ Cell viability ↓ LDH |
↓ PI | |||||
| H2 -containing medium + MCC950, during hypoxia | ↓ GSDMD-N per GSDMD-FL ratio | ↓ | ↓ ASC ↓ NLRP3 |
↓ IL-1β | ↑ Cell viability ↓ LDH |
↓ PI | |||||
| 6 | H9C2 cells | hMSC-derived exosomes | ↓ | ↓ | ↓ | ↓ ASC ↓ NLRP3 |
↓ IL-1β ↓ IL-18 |
↑ Cell viability | ↓ TUNEL | Exosomal lncRNA KLF3-AS1 derived from hMSCs reduced pyroptosis through inhibition of miR-138-5p, and promoted Sirt1, subsequently reduced GSDMD. | 42, 43 |
| KLF3-AS1 overexpression hMSC-derived exosomes | ↓↓ | ↓↓ | ↓↓ | ↓↓ ASC ↓↓ NLRP3 |
↓↓ IL-1β ↓↓ IL-18 |
↑↑ Cell viability | ↓↓ TUNEL | ||||
| hMSC-derived exosomes + miR-138-5p inhibitor cell transfection |
↓↓ | ↓↓ | ↓↓ | ↓↓ ASC ↓↓ NLRP3 |
↓↓ IL-1β ↓↓ IL-18 |
↑↑ Cell viability | ↓↓ TUNEL | ||||
| hMSC-derived exosomes + Sh-Sirt1 cell transfection vs. Hypoxia + exosomes | ↑ | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
↑ IL-1β ↑ IL-18 |
↓ Cell viability | ↑ TUNEL | ||||
| 24 | NMCMs | Kanglexin 10 μmol/L, during hypoxia | ↓ | ↓ | ↓ | ↓ NLRP3 | ↓ IL-1β ↓ IL-18 |
– | ↓ TUNEL ↓ PI |
Kanglexin reduced pyroptosis through the canonical pathway. | 42, 43 |
| 12 (+TNF-α) | H9C2 cells | Liraglutide 100 nmol/L, pretreatment 1 h before hypoxia | ↓ | – | ↓ | ↓ NLRP3 | ↓ NOX4 ↓ ROS |
↑ Cell viability ↓ LDH |
↑ SIRT1 ↓ PI |
Liraglutide pretreatment reduced oxidative stress and promoted SIRT1, subsequently reduced pyroptosis through the canonical pathway. | 42, 43 |
| liraglutide pretreatment + EX527 (SIRT1 inhibitor) 200 nmol/L, pretreatment 1 h before hypoxia vs. hypoxia + liraglutide | ↑ | – | ↑ | ↑ NLRP3 | ↑ NOX4 ↑ ROS |
↓ Cell viability ↑ LDH |
↑ PI | ||||
| 12 (+TNF-α) | H9C2 cells | Rosuvastatin 20 μmol/L, pretreatment 2 h before hypoxia | ↓ GSDMD-N per GSDMD-FL ratio | ↓ | ↓ NLRP3 | ↓ ROS | ↑ Cell viability ↓ LDH |
↓ PI | Rosuvastatin and NAC pretreatment reduced oxidative stress, subsequently reduced pyroptosis through the canonical pathway. | 43 | |
| NAC 5 μmol/L, pretreatment 2 h before hypoxia | ↓ GSDMD-N per GSDMD-FL ratio | ↓ | ↓ NLRP3 | – | ↑ Cell viability ↓ LDH |
↓ PI | |||||
| OGD 36 h | H9C2 cells | NAC 50 μmol/L, after 36 h OGD | ↓ | ↓ | ↓ | ↓ ASC ↓ NLRP3 |
↓ p-NF-κB ↓ ROS ↑ SOD |
↓ LDH | – | NAC and PDTC reduced oxidative stress, subsequently reduced pyroptosis through the canonical pathway. | 42, 43 |
| PDTC 25 μmol/L, after 36 h OGD | ↓ | ↓ | ↓ | ↓ ASC ↓ NLRP3 |
↓ p-NF-κB | ↓ LDH | – | ||||
ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; GSDMD: Gasdermin D; GSDMD-FL: full-length Gasdermin D; GSDMD-N: N-terminal Gasdermin D fragment; H: hypoxia; H/R: hypoxia/reoxygenation; hMSC: human mesenchymal stem cell; IL: interleukin; KLF3-AS1: KLF3 antisense RNA1; LDH: lactate dehydrogenase; lncRNA: long non-coding RNA; miR: microRNA; NAC: N-acetylcysteine; NF-κB: nuclear factor κ-light-chain-enhancer of activated B cells; NLRP3: NACHT, LRR and PYD domains-containing protein 3; NMCMs: neonatal mouse cardiomyocytes; NOX4: nicotinamide adenine dinucleotide phosphate oxidase 4; NRCMs: neonatal rat cardiomyocytes; OGD: oxygen-glucose deprivation; PDTC: pyrrolidine dithiocarbamate; PI: propidium iodide; R: reoxygenation; ROS: reactive oxygen species; sh-Sirt1: short hairpin Sirtuin 1; Sirt1: Sirtuin 1; SOD: superoxide dismutase; TNF-α: tumor necrosis factor alpha; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
Melatonin is an endogenous hormone secreted by the pineal gland. It is widely known for its role in the regulation of circadian rhythms and its action as a potent antioxidant in the presence of severe injury (e.g., MI)62,63. Melatonin treatment during hypoxia reduced TLR4/NF-κB/NLRP3/caspase-1/GSDMD-N expression, thereby reducing cellular injury38. Melatonin administered intragastrically for 14 consecutive days prior to MI also reduced the infarct size and LV dysfunction in mice via the same pathway as verified in the cellular study (Table 8). These findings suggest that melatonin treatment inhibited GSDMD-N exerting cardioprotective effects either pre- or post-ischemic insult38.
Table 8.
Evidence of interventions to modify pyroptosis in MI/R injury: Reports from in vitro studies.
| Condition |
Model | Intervention | Pyroptotic marker |
Inflammatory marker/ROS | Cell viability/Toxicity | Other cell death marker/relevant finding | Interpretation | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H (h) | R (h) | GSDMD |
Caspase-1 | Others | ||||||||
| Active form | Full form | |||||||||||
| 1 | 2 | NRCMs | Emodin 5, 10 μmol/L, pretreatment 1 h before H/R | ↓ | ↔ | ↓ | ↓ ASC ↓ NLRP3 |
↓ TLR4 ↓ MYD88 ↓ p-IκBα ↓ p-NF-κB ↓ IL-1β ↓ MDA ↑ SOD ↓ DHE |
↓ Cell viability ↓ LDH |
– | Emodin pretreatment reduced oxidative stress, subsequently decreased pyroptosis through the canonical pathway. Inhibition of NF-κB and NLRP3 reduced pyroptosis through the reduction of GSDMD-N. |
42, 43 |
| Bay 11-7082 (NF-κB and NLRP3 inflammasome inhibitor) 5 μmol/L, pretreatment 1 h before H/R |
↓ | ↔ | – | – | ↓ IL-1β | – | – | |||||
| S3680 (NLRP3 inflammasome inhibitor) 10 μmol/L, pretreatment 1 h before H/R | ↓ | ↔ | – | – | ↓ IL-1β | – | – | |||||
| 2 | 24 | NRCMs | M2-exosome, pretreatment 24 h before H/R | – | – | – | ↓ ASC ↓ NLRP3 |
↓ TLR4 ↓ MYD88 ↓ p-IκBα ↓ p-NF-κB ↓ TXNIP |
↑ Cell viability ↓ LDH ↓ CK ↓ CK-MB |
↑ miR-148a ↓ Annexin V/PI ↓ IP3R ↓ SERCA2a ↓ Ca2+ overload |
M2-exosome promoted miR-148a to reduce oxidative stress, calcium overload, subsequently decreased pyroptosis through the canonical pathway. | 42, 43 |
| siTXNIP-M2-exosome, pretreatment 24 h before H/R |
↓ | ↓ | – | – | ↓ IL-1β ↓ IL-18 ↓ TXNIP |
↑ Cell viability | – | |||||
| 4 | 2 | AC16 cells | hucMSC-exo, pretreatment 24 h before H/R | ↓ | – | ↓ | ↓ NLRP3 | ↓ IL-1β ↓ IL-18 |
↑ Cell viability ↓ LDH |
↑ miR-100-5p ↓ PI positive |
Enriched miR-100-5p in hucMSC-exo reduced pyroptosis through suppressed FOXO3, leading to downregulation of the canonical pathway of pyroptosis. | 42, 43 |
| miR-100-5p mimic + hucMSC-exo, pretreatment 24 h before H/R | ↓↓ | – | ↓↓ | ↓↓ NLRP3 | ↓↓ IL-1β ↓↓ IL-18 |
↓↓ LDH | ↑↑ miR-100-5p ↓↓ PI positive |
|||||
| miR-100-5p inhibitor + hucMSC-exo, pretreatment 24 h before H/R vs. H/R + hucMSC-exo pretreatment |
↑ | – | ↑ | ↑ NLRP3 | ↑ IL-1β ↑ IL-18 |
↑ LDH | ↓ miR-100-5p ↑ PI positive |
|||||
| AC16 cells FOXO3 overexpression |
hucMSC-exo, pretreatment 24 h before H/R vs. H/R + hucMSC-exo pretreatment |
↑ | – | ↑ | ↑ NLRP3 | ↑ IL-1β ↑ IL-18 |
↑ LDH | ↑ PI positive | ||||
| 2 | 2 | HCMECs | Gastrodin 40 μmol/L, before reoxygenation | – | ↓ | ↓ | ↓ NLRP3 | ↓ IL-1β | – | – | Gastrodin reduced pyroptosis through the canonical pathway. | 42, 43 |
| MCC950 (NLRP3 inhibitor) 1 μmol/L, before reoxygenation | – | ↓ | ↓ | ↓ NLRP3 | – | – | ||||||
| 0.5 | 24 | AMVCMs | NAC 20 mmol/L, after hypoxia | ↓ | ↓ | – | ↓ Caspase-11 | – | – | NAC, ROS scavengers, reduced pyroptosis through the non-canonical pathway. | 42, 43 | |
| 4 | 2 | NMCMs | Soluble UA 100 mg/L, 24 h before H/R | – | ↑↑ | – | ↑↑ ASC ↑↑ NLRP3 ↑↑ Caspase-1 |
↑↑ ROS ↑↑ IL-1β |
– | ↑↑ TUNEL ↑↑ Caspase-3 |
UA aggravated H/R injury accompanied by an increase in canonical pyroptotic and apoptotic pathways, which were inhibited by NLRP3 inhibitor and ROS scavenger. | 42, 43 |
| Soluble UA + Bay 11-7082, 5 μmol/L, 24 h before H/R |
– | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
↑ ROS ↑ IL-1β |
↓ Cell viability ↑ LDH |
↑ TUNEL ↑ Caspase-3 |
|||||
| Soluble UA + NAC 10 mmol/L,24 h before H/R |
– | ↑ | ↑ | ↑ ASC ↑ NLRP3 |
↑ ROS ↑ IL-1β |
↓ Cell viability ↑ LDH |
↑ TUNEL ↑ Caspase-3 |
|||||
AMVCMs: adult mouse ventricular cardiomyocytes; ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; CK: creatine kinase; CK-MB: creatine kinase myocardial band; DHE: dihydroethidium; FOXO3: Forkhead box O3; GSDMD: Gasdermin D; H: hypoxia; H/R: hypoxia/reoxygenation; HCEMCs: human cardiac microvascular endothelial cells line; hMSC: human mesenchymal stem cell; hucMSC-exo: human umbilical cord mesenchymal stem cell-derived exosomes; IκBα: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; IL: interleukin; M2-exosome: M2 macrophage-derived exosomes; MDA: malondialdehyde; miR: microRNA; MYD88: myeloid differentiation factor 88; NAC: N-acetylcysteine; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3: NACHT, LRR and PYD domains-containing protein 3; NMCMs: neonatal mouse cardiomyocytes; NRCMs: neonatal rat cardiomyocytes; p-IκBα: phosphorylated IκBα; PI: propidium iodide; R: reoxygenation; ROS: reactive oxygen species; SERCA2a: sarco/endoplasmic reticulum Ca2+-ATPase 2a; siTXNIP: small interfering TXNIP transfection; SOD: superoxide dismutase; TLR4: toll-like receptor 4; TNF-α: tumor necrosis factor alpha; TXNIP: thioredoxin-interacting protein; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling; UA: uric acid.
Liraglutide is a glucagon-like peptide-1 (GLP1) analog that has been shown to improve cardiovascular outcomes in type two diabetes mellitus patients in addition to its glucose-lowering effect64. Additionally, pretreatment with liraglutide has been shown to increase the expression of sirtuin 1 (SIRT1), decrease oxidative stress and inhibit pyroptosis via the canonical pathway42. In cardiac cells exposed to ischemic conditions, in CME-induced excessive ROS generation, and in MI rats, rosuvastatin and LCZ696 were shown to effectively suppress ROS production and subsequently inhibit NLRP3-mediated pyroptosis43,53. LCZ696 was also found to reduce NLRP3/caspase-1/GSDMD-N expression levels via the TAK1/JNK signaling pathway, resulting in decreased pyroptosis, cellular injury, infarction, and LV dysfunction53.
Previously thought to be an inert gas with no biological effect, hydrogen gas (H2) was discovered to have an antioxidant effect65. After MI, it has been demonstrated that inhaling 2% H2 for 3 h per day reduced oxidative stress, mitochondrial damage, and pyroptosis via the canonical pathway, resulting in decreased LV dysfunction and remodeling18. These findings indicated that H2 effectively inhibited pyroptosis by lowering ROS levels and reducing the activity of the canonical pyroptosis pathway. In summary, reducing oxidative stress in MI subjects decreased cardiac pyroptosis via the canonical pathway, decreasing cardiac injury and LV dysfunction.
Fortunately, novel interventions such as exosomes can also be used to modulate cardiac pyroptosis in MI39. Exosomes are membrane-bound spherical structures released by mesenchymal stem cells that contain lipids, proteins, and RNAs involved in intercellular communication66. Mao et al.39 discovered that the lncRNA KLF3-AS1 in human mesenchymal stem cell (hMSC)-derived exosomes could alleviate hypoxia-induced NLRP3-mediated pyroptosis by inhibiting miR-138-5p and promoting SIRT1 expression. Similarly, inhibiting NLRP3-mediated cardiac pyroptosis has been reported to exert cardioprotection against MI by attenuating infarction39.
Modulation of GSDMD-N-mediated pyroptosis has also been shown to have cardioprotective benefits following hypoxia or MI. Kanglexin, a novel anthraquinone compound, inhibited the activation of GSDMD-N in both hypoxia and MI via the canonical pathway27. Inhibition of TLR4–GSDMD-N-dependent pyroptosis using nicorandil potentially promoted energy production, improving LV function and reducing infarct size52. These findings suggest that inhibition of TLR4 may be a therapeutic target for the reduction of pyroptosis. The potential interventions against pyroptosis-mediated cell death in MI are illustrated in Fig. 1.
8. Potential interventions against pyroptosis-mediated cell death in MI/R injury: Reports from in vitro and in vivo studies
Several pharmacological agents that inhibit the canonical pathway of pyroptosis have been beneficial in treating MI/R injury (Table 7, Table 8,19,21,45, 46, 47, 48). Pretreatment with an NLRP3 inhibitor (MCC950) reduced NLRP3/caspase-1/GSDMD-mediated pyroptosis in HCMECs19, and attenuated GSDMD-mediated cardiac injury in the MI/R mouse model19. Furthermore, inhibition of either NF-κB (Bay 11-7082) or the NLRP3 inflammasome (S3680) effectively inhibited GSDMD-N-mediated pyroptosis in a H/R rat cardiomyocyte model13,45. It has also been demonstrated that blocking HMGB1, a DAMPs family member, could be an effective strategy for the mitigation of MI/R-induced pyroptosis45,57. Pretreatment with an ethyl acetate extract of C. ramalus (CREAE) and its bioactive compound cinnamic acid significantly reduced HMGB1 and, as a result, pyroptosis, via the NLRP3/caspase-1/GSDMD pathway57.
Table 7.
Evidence of interventions to modify pyroptosis in MI and MI/R injury: Reports from in vivo studies.
| Disease Condition |
Model | Intervention | Pyroptotic marker |
IS% | LV function | Cardiac injury | Inflammatory marker/ROS | Other cell death marker/Relevant finding | Interpretation | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | R | GSDMD |
Caspase-1 | Others | ||||||||||
| Active form | Full form | |||||||||||||
| 24 h | – | C57BL/6 mice | Kanglexin 40 mg/kg/day, IG, 7 consecutive days before MI | ↓ | ↓ | ↓ | ↓ NLRP3 | ↓ | ↑ %EF ↑ %FS |
↓ LDH | ↓ IL-1β ↓ IL-18 |
– | Kanglexin pretreatment reduced cardiac injury, infarction, and LV dysfunction by suppressing the canonical pathway. | 42, 43 |
| 24 h | – | C57BL/6 mice | Melatonin 10 mg/kg, IG, 14 consecutive days before MI | ↓ | – | ↓ | ↓ NLRP3 | ↓ | ↓ %EF ↓ %FS |
↓ LDH | ↓ TLR4 ↓ NF-κB ↓ IL-1β ↓ IL-18 |
– | Melatonin pretreatment reduced cardiac injury, infarction, and LV dysfunction by suppressing the canonical pathway. | 42, 43 |
| 72 h | – | SD rats | Nicorandil 15 mg/kg/day, IG, 7 consecutive days before MI | ↓ | ↔ | ↓ | ↓ ASC ↓ NLRP3 |
↓ | ↑ %EF ↑ %FS ↓ LVIDd ↓ LVIDs |
↓ cTnI ↓ LDH |
↓ TLR4 ↓ MYD88 ↓ NF-κB ↓ IL-1β ↓ IL-18 |
↑ ATP ↑ ADP ↑ AMP ↑ ATP/ADP ratio ↑ Total adenine nucleotide |
Nicorandil pretreatment reduced cardiac injury, infarction, and LV dysfunction by suppressing the canonical pathway of pyroptosis and promoted energy production. | 42, 43 |
| 1 week | – | F344 rats | hMSC-derived exosomes 40 μg, IV, before MI |
↓ | ↓ | ↓ | ↓ ASC ↓ NLRP3 ↓ |
↓ | ↓ Heart weight/tibial length |
– | ↓ IL-1β ↓ IL-18 |
↓ TUNEL positive | KLF3-AS1 in hMSC-derived exosomes reduced infarction and LV remodeling byinhibiting the canonical pathway of pyroptosis. | 42, 43 |
| KLF3-AS1 overexpression exosomes, before MI | ↓↓ | ↓↓ | ↓↓ | ↓↓ ASC ↓↓ NLRP3 |
↓↓ | ↓↓ Heart weight/tibial length | – | ↓↓ IL-1β ↓↓ IL-18 |
↓↓ TUNEL positive | |||||
| 1 day | – | SD rats | LCZ696 (Sacubitril/Valsartan) 68 mg/kg/day, IG, daily after MI | ↓ | ↓ | ↓ | ↓ NLRP3 | ↓ | ↔ %EF ↔ %FS ↔ LVIDd ↔ LVIDs |
↓ IL-1β ↓ IL-18 ↓ ROS |
↔ CVF ↓ TAK1 ↓ p-JNK |
Treatment with LCZ696 after MI reduced oxidative stress, TAK1/JNK pathway, subsequently decreased pyroptosis through the canonical pathway, resulting in reduced infarction and LV dysfunction. | 42, 43 | |
| 3 days | – | ↓ | ↓ | ↓ | ↓ NLRP3 | ↓ | ↑ %EF ↑ %FS ↓ LVIDd ↓ LVIDs |
↓ IL-1β ↓ IL-18 ↓ ROS |
↔ CVF ↓ TAK1 ↓ p-JNK |
|||||
| 7 days | – | ↓ | ↓ | ↓ | ↓ NLRP3 | ↓ | ↑ %EF ↑ %FS ↓ LVIDd ↓ LVIDs |
↓ IL-1β ↓ IL-18 ↓ ROS |
↓ CVF ↓ TAK1 ↓ p-JNK |
|||||
| 4 week | – | SD rats | 2% H2 inhalation, 3 h/day, after MI | ↓ GSDMD-N per GSDMD-FL ratio | ↓ | ↓ ASC ↓ NLRP3 |
↑ %EF ↑ %FS ↓ LVIDd ↓ LVIDs |
↓ BNP | ↓ MDA ↓ •OH ↓ IL-1β |
↓ Mitochondrial damage ↓ Fibrosis |
H2 inhalation after MI reduced oxidative stress, mitochondrial damage, cardiac injury, subsequently decreased pyroptosis through the canonical pathway, resulting in decreasing LV dysfunction and cardiac remodeling. Combined H2 with NLRP3 inhibitor further enhanced cardioprotective effect, but could be due to other synergistic mechanisms, not by alleviation of pyroptosis. |
42, 43 | ||
| MCC950 (NLRP3 inhibitor) 30 mg/kg, single dose, IP, after MI | ↓ GSDMD-N per GSDMD-FL ratio | ↓ | ↓ ASC ↓ NLRP3 |
↑ %EF ↑ %FS ↓ LVIDd ↓ LVIDs |
↓ BNP | ↔ MDA ↔ •OH ↓ IL-1β |
↓ Fibrosis | |||||||
| 2% H2 inhalation + MCC950 | ↓ GSDMD-N per GSDMD-FL ratio | ↓ | ↓ ASC ↓ NLRP3 |
↑↑ %EF ↑↑ %FS ↓↓ LVIDd ↓↓ LVIDs |
↓↓ BNP | ↓ MDA ↓ •OH ↓ IL-1β |
↓↓ Fibrosis | |||||||
| CME induction (Post CME 3 days) | C57BL/6 mice | Rosuvastatin 40 mg/kg/day, PO, 3 consecutive days before and after CME induction | ↓ GSDMD-N per GSDMD-FL ratio | ↓ | ↓ NLRP3 | ↓ | ↑ %EF ↑ %FS |
↓ LDH | ↓ IL-1β ↓ SDHA ↓ SDHB ↓ ROS |
↓ Collagen deposit | Treatment with rosuvastatin reduced oxidative stress, cardiac injury, subsequently decreased pyroptosis through the canonical pathway of pyroptosis, resulting in reduced LV dysfunction. | 42, 43 | ||
| 0.5 h | 2 h | SD rats | Emodin 20 mg/kg, IP, 1 h before I/R | ↓ | – | – | – | ↓ | – | – | ↓ IL-1β | ↓ Disordered and swollen cells | Emodin pretreatment reduced infarction by direct suppression of GSDMD-N-mediated pyroptosis. | 42, 43 |
| 0.5 h | 2 h | SD rats | CREAE 74 mg/kg, IG, 7 consecutive days before I/R | ↓ | ↓ | ↓ | ↓ ASC ↑ NLRP3 |
↓ | ↑ %EF ↑ %FS ↓ E/A |
– | ↓ HMGB1 ↓ IL-1β ↓ IL-6 ↓ TNF-α |
– | Pretreatment with CREAE and its bioactive substance CA, reduced infarction, and LV dysfunction by suppressing HMGB1-mediated canonical pathway of pyroptosis. | 42, 43 |
| CA 45 mg/kg, IG, 7 consecutive days before I/R | ↔ protein ↓ mRNA |
↓ | ↓ | ↓ ASC ↔ NLRP3 protein ↓ Nlrp3 mRNA |
↓ | ↑ %EF ↑ %FS ↓ E/A |
– | ↓ HMGB1 ↔ IL-1β protein ↓ Il-1β mRNA ↓ IL-6 ↓ TNF-α |
– | |||||
| 45 min | 6 h | C57BL/6 mice | Gastrodin 100 mg/kg/day, IP, 3 consecutive days before I/R and 15 min before reperfusion | – | ↓ | ↓ | ↓ NLRP3 | ↓ | – | ↓ CK ↓ CK-MB ↓ cTnI ↓ LDH |
– | ↓ TUNEL ↑ %Survival rate ↓ Neutrophil & Macrophage |
Gastrodin pretreatment decreased cardiac injury, and inflammatory cell infiltration through decreasing canonical pathway of pyroptosis. | 42, 43 |
| MCC950 15 mg/kg/day, IP, 3 consecutive days before I/R and 15 min before reperfusion | – | ↓ | – | – | – | – | ↓ CK ↓ cTnI |
– | ↓ TUNEL ↑ %Survival rate ↓ Neutrophil & Macrophage infiltration |
|||||
| 0.5 h | 24 h | C57BL/6 mice Myh6-Cre strain (GSDMD+/+) | NAC 400 mg/kg, IP, 1 h before I/R | ↓ | ↓ | – | ↓ Caspase-11 | ↓ | – | – | – | – | NAC, and ROS scavenger, pretreatment reduced infarct size by inhibiting non-canonical pathway of pyroptosis. | 42, 43 |
| 45 min | 24 h | SD rats | β-Asarone 20 mg/kg, IP, just before reperfusion | ↓ | ↓ | ↓ | ↓ ASC ↓ NLRP3 |
↓ | ↑ %EF ↑ %FS ↓ LVIDd ↓ LVIDs |
↓ cTNT | ↓ MPO ↓ IL-1β |
– | β-Asarone pretreatment reduced infarct size, cardiac injury, and LV dysfunction by inhibiting canonical pathway of pyroptosis. | 42, 43 |
| 1 h | 24 h | SD rats | 2% H2 inhalation, at reperfusion | ↓ | – | ↓ | ↓ ASC ↓ NLRP3 |
↓ | ↑ %EF ↑ %FS |
↓ cTnI | ↓ ROS ↓ MDA ↓ 8-OHDG ↓ IL-1β |
↓ Mitochondrial damage | Hydrogen inhalation at the onset of reperfusion reduced oxidative stress, subsequently decreased pyroptosis through a canonical pathway, resulting in reduction of infarct size, and LV dysfunction. | 42, 43 |
| 0.5 h | 2 h | Kunming mice | Hyperuricemia induction by potassium oxonate + CMC-Na 300 mg/kg/day, IP, 14 consecutive days before I/R | – | ↑↑ | ↑↑ | ↑↑ ASC ↑↑ NLRP3 |
↑↑ | – | ↑↑ CK-MB ↑↑ LDH |
↑↑ IL-1β | ↑↑ TUNEL ↑↑ Caspase-3 |
UA aggravated I/R injury by increasing apoptosis and pyroptosis through a canonical pathway. | 42, 43 |
Abbreviations: 8-OHDG: 8-hydroxy-2′-deoxyguanosine; ·OH: hydroxyl radical; ADP: adenosine di-phosphate; AMP: adenosine monophosphate; ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; ATP: adenosine triphosphate; BNP: B-type natriuretic peptide; CA: cinnamic acid; CK: creatine kinase; CK-MB: creatine kinase myocardial band; CMC-Na: 0.5% sodium carboxymethyl cellulose solvent; CME: coronary microembolization; CREAE: ethyl acetate extract of Cinnamomi ramulus; cTnI: cardiac troponin I; cTnT: cardiac troponin T; CVF: collagen volume fraction; E/A: mitral early diastolic flow/late diastolic flow velocity; EF%: left ventricular ejection fraction; FS%: left ventricular fractional shortening; GSDMD: Gasdermin D; GSDMD+/+: Gasdermin D positive gene; GSDMD-FL: full-length Gasdermin D; GSDMD-N: N-terminal Gasdermin D fragment; HMGB1: high mobility group box 1 protein; hMSC: human mesenchymal cells; I: ischemia by coronary artery ligation; I/R: ischemia/reperfusion; IG: intragastric administration; IL: interleukin; IP: intraperitoneal injection; IS%: infarct size/area at risk; IV: intravenous injection; KATP: ATP-sensitive potassium channel; KLF3-AS1: KLF3 antisense RNA1; LDH: lactate dehydrogenase; LV: left ventricle; LVIDd: left ventricular internal dimension at end-diastole; LVIDs: left ventricular internal dimension at end-systole; Ma: macrophages; MDA: malondialdehyde; MI: myocardial ischemia; MPO: myeloperoxidase; mRNA: messenger RNA; MYD88: myeloid differentiation factor 88; N: meutrophils; NAC: N-acetylcysteine; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3: NACHT, LRR and PYD domains-containing protein 3; p-JNK: phosphorylated Jun N-terminal kinase; PO: peroral; R: reperfusion; ROS: reactive oxygen species; SD rat: Sprague–Dawley rat; SDHA: succinate dehydrogenase complex flavoprotein subunit A; SDHB: succinate dehydrogenase complex flavoprotein subunit B; TLR4: toll-like receptor 4; TNF-α: tumor necrosis factor alpha.
Numerous studies have demonstrated that anti-pyroptotic compounds extracted from Chinese herbs with antioxidant properties, for example Emodin, Gastrodin, and β-asarone, can significantly reduce pyroptosis following H/R or I/R injury19,45,67. Emodin, an anthraquinone derivative derived from Rheum palmatum, has been shown to inhibit ROS production and pyroptosis mediated by TLR4/NF-κB/NLRP3/caspase-1/NLRP3/GSDMD in response to H/R conditions45. Emodin treatment also decreased GSDMD-N and IL-1β expression levels, resulting in a decrease in the infarct size in a MI/R model45. Gastrodin, a monomeric component extracted from Gastrodia elata Blume, was found to inhibit both H/R-induced pyroptosis in HCMECs and MI/R-induced pyroptosis in mouse models19, which could be due to exertion of an anti-oxidant effect68, reducing inflammatory cell infiltration, and alleviating cardiac injury19. Lastly, β-asarone, a significant active component of the Acorus tatarinowii rhizome, also had antioxidant properties69 and has been shown to reduce infarct size, cardiac injury, and cardiac dysfunction via inhibition of the canonical pyroptotic pathway67.
Some specific interventions could also contribute to the modulation of cardiac pyroptosis following MI/R injury by inhibiting oxidative stress. The giving of 2% hydrogen by inhalation during reperfusion effectively reduced oxidative stress, which resulted in alleviation of I/R-induced pyroptosis via the NLRP3/caspase-1/GSDMD pathway, leading to a reduction in cardiac injury enzymes, infarct size and improved LV function70. In addition, pretreatment with M2 macrophage-derived exosomes (M2 exosomes) containing miR-148a markedly decreased oxidative stress in rat cardiomyocytes via the TLR4/NF-κB/NLRP3/caspase-1/GSDMD pathways46. It has been demonstrated that enriched miR-100-5p in human umbilical cord mesenchymal stem cell-derived exosomes (hucMSC-exo) alleviated I/R-induced pyroptosis in human cardiomyocyte cell lines by inhibiting forkhead box O3 (FOXO3), a transcription factor which promotes NLRP3 activity48. Ultimately, NAC administration following hypoxia or before cardiac I/R has been shown to decrease the caspase-11/GSDMD-mediated non-canonical pathway of pyroptosis21.
In addition to interventions for cardiac pyroptosis, the unpleasant condition hyperuricemia exacerbated H/R-induced cardiomyocyte injury via an increase in apoptosis and the canonical pathway of pyroptosis47. These adverse events were blunted when treated with Bay 11-7082 and NAC47. Taken together, these findings suggest that inhibition of DAMPs, oxidative stress, and inflammation may potentially alleviate H/R and I/R-induced cardiac pyroptosis via both canonical and non-canonical pathways. This potential mechanism is illustrated in Fig. 2.
Although various interventions have been tested in the pre-clinical studies, the compound that could be used in the clinical trial should not have been tested and failed before in human studies. Moreover, the compound should be pharmaceutical grade and has high efficacy in reducing infarct size when given during ischemia or right before the reperfusion. In addition, the compounds should be tested in an ischemia/reperfusion model since most STEMI patients require a revascularization71. Among several pharmacological interventions that have been tested in animal studies, NAC exerted a favorable outcome in a preclinical study since it could reduce the infarct size when given during ischemia or at the time of reperfusion72. Moreover, pretreatment with NAC effectively reduced GSDMD in I/R mice model21. In a clinical study, administration of NAC during percutaneous coronary intervention promoted myocardial perfusion in STEMI73. H2 inhalation possibly provides cardioprotective effects in reducing GSDMD in STEMI patients after revascularization since given H2 at the reperfusion exerted beneficial effects with reduced GSDMD in rats70 and enhanced LV stroke volume index in STEMI patients74. Nevertheless, GSDMD was not measured in both clinical trials. Therefore, a measurement of GSDMD is suggested to clarify the potential benefit of NAC or H2 in reducing pyroptosis in the future clinical trial. LCZ696 could reduce GSDMD and had cardioprotective effects in animal study when given after MI53, but the effects of LCZ696 in MI/R injury still need further investigation since revascularization is required in most STEMI patients.
It should be noted that to achieve a successful clinical translation, the efficacy of these interventions needs to be re-evaluated by a consortium for preclinical assessment of cardioprotective therapies (CAESAR) protocol75,76, that will make the favorable therapies in animal studies have the greatest chance of success in clinical studies75,76.
9. Conclusions
Evidence indicates that GSDMD-N is the final executor pyroptotic protein which increased in response to MI and MI/R injury, suggesting that it could be used as a putative biomarker of pyroptosis. By inhibiting pyroptosis via GSDMD signaling pathways, effective cardioprotection against MI and MI/R injury has been demonstrated. These findings indicate that pyroptosis is a potential target for future cardioprotective strategies in the setting of myocardial ischemia and reperfusion injury.
Acknowledgments
This work was supported by the NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (NC), the Senior Research Scholar Grant from the National Research Council of Thailand (SCC), the Chiang Mai University Center of Excellence Award (NC), the National Research Council of Thailand, Fundamental Fund 2022, Chiang Mai University (FF65/044) (CM), and the National Research Council of Thailand (NRCT) (N42A650187) (CM). All figures in this work were created using BioRender.com.
Author contributions
Siriporn C. Chattipakorn and Nipon Chattipakorn: conceptualization; Panat Yanpiset, Chayodom Maneechote, Sirawit Sriwichaiin, and Natthaphat Siri-angkul: writing of the manuscript-original draft; Siriporn C. Chattipakorn and Nipon Chattipakorn: writing of the manuscript-review and editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., Callaway C.W., et al. Heart disease and stroke statistics—2021 update: a report from the American heart association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Frank A., Bonney M., Bonney S., Weitzel L., Koeppen M., Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin CardioThorac Vasc Anesth. 2012;16:123–132. doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashmi S., Al-Salam S. Acute myocardial infarction and myocardial ischemia–reperfusion injury: a comparison. Int J Clin Exp Pathol. 2015;8:8786–8796. [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W., Bian W., Zhou Y., Zhang J. Cardiac fibroblasts and myocardial regeneration. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.599928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone G.W., Selker H.P., Thiele H., Patel M.R., Udelson J.E., Ohman E.M., et al. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol. 2016;67:1674–1683. doi: 10.1016/j.jacc.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 6.Zuurbier C.J., Abbate A., Cabrera-Fuentes H.A., Cohen M.V., Collino M., De Kleijn D.P.V., et al. Innate immunity as a target for acute cardioprotection. Cardiovasc Res. 2019;115:1131–1142. doi: 10.1093/cvr/cvy304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibbons R.J., Valeti U.S., Araoz P.A., Jaffe A.S. The quantification of infarct size. J Am Coll Cardiol. 2004;44:1533–1542. doi: 10.1016/j.jacc.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 8.Konstantinidis K., Whelan R.S., Kitsis R.N. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012;32:1552–1562. doi: 10.1161/ATVBAHA.111.224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whelan R.S., Kaplinskiy V., Kitsis R.N. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 10.Del Re D.P., Amgalan D., Linkermann A., Liu Q., Kitsis R.N. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev. 2019;99:1765–1817. doi: 10.1152/physrev.00022.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frangogiannis N.G. Pathophysiology of myocardial infarction. Compr Physiol. 2015;5:1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 12.Ying L., Benjanuwattra J., Chattipakorn S.C., Chattipakorn N. The role of RIPK3-regulated cell death pathways and necroptosis in the pathogenesis of cardiac ischaemia–reperfusion injury. Acta Physiol. 2021;231 doi: 10.1111/apha.13541. [DOI] [PubMed] [Google Scholar]
- 13.Galluzzi LVitale IAaronson SAAbrams JMAdam DAgostinis P., et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauro A.G., Bonaventura A., Mezzaroma E., Quader M., Toldo S. NLRP3 inflammasome in acute myocardial infarction. J Cardiovasc Pharmacol. 2019;74:175–187. doi: 10.1097/FJC.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 15.Jia C., Chen H., Zhang J., Zhou K., Zhuge Y., Niu C., et al. Role of pyroptosis in cardiovascular diseases. Int Immunopharm. 2019;67:311–318. doi: 10.1016/j.intimp.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi M. Cell-specific roles of NLRP3 inflammasome in myocardial infarction. J Cardiovasc Pharmacol. 2019;74:188–193. doi: 10.1097/FJC.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 17.Olsen M.B., Gregersen I., Sandanger Ø., Yang K., Sokolova M., Halvorsen B.E., et al. Targeting the inflammasome in cardiovascular disease. JACC Basic Transl Sci. 2022;7:84–98. doi: 10.1016/j.jacbts.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie C., Zou R., Pan S., A R., Gao Y., Yang H., et al. Hydrogen gas inhalation ameliorates cardiac remodelling and fibrosis by regulating NLRP3 inflammasome in myocardial infarction rats. J Cell Mol Med. 2021;25:8997–9010. doi: 10.1111/jcmm.16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun W., Lu H., Lyu L., Yang P., Lin Z., Li L., et al. Gastrodin ameliorates microvascular reperfusion injury-induced pyroptosis by regulating the NLRP3/Caspase-1 pathway. J Physiol Biochem. 2019;75:531–547. doi: 10.1007/s13105-019-00702-7. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs S.B., Miao E.A. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27:673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi H., Gao Y., Dong Z., Yang J., Gao R., Li X., et al. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ Res. 2021;129:383–396. doi: 10.1161/CIRCRESAHA.120.318629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W., Shen J., Li Y., Wu J., Luo X., Yu Y., et al. Pyroptosis inhibition improves the symptom of acute myocardial infarction. Cell Death Dis. 2021;12:852. doi: 10.1038/s41419-021-04143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorgensen I., Miao E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X., Tian P.C., Wang K., Wang M., Wang K. Pyroptosis: role and mechanisms in cardiovascular disease. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.897815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu P., Zhang X., Liu N., Tang L., Peng C., Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Targeted Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayagaki N., Stowe I.B., Lee B.L., O'Rourke K., Anderson K., Warming S., et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 27.Bian Y., Li X., Pang P., Hu X.L., Yu S.T., Liu Y.N., et al. Kanglexin, a novel anthraquinone compound, protects against myocardial ischemic injury in mice by suppressing NLRP3 and pyroptosis. Acta Pharmacol Sin. 2020;41:319–326. doi: 10.1038/s41401-019-0307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauf A., Shah M., Yellon D.M., Davidson S.M. Role of caspase 1 in ischemia/reperfusion injury of the myocardium. J Cardiovasc Pharmacol. 2019;74:194–200. doi: 10.1097/FJC.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 29.Yan M., Li Y., Luo Q., Zeng W., Shao X., Li L., et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS–STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Dis. 2022;8:258. doi: 10.1038/s41420-022-01046-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X., Li X., Liu S., Brickell A.N., Zhang J., Wu Z., et al. PCSK9 regulates pyroptosis via mtDNA damage in chronic myocardial ischemia. Basic Res Cardiol. 2020;115:66. doi: 10.1007/s00395-020-00832-w. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama H., Otsu K. Mitochondrial DNA as an inflammatory mediator in cardiovascular diseases. Biochem J. 2018;475:839–852. doi: 10.1042/BCJ20170714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y.F., Zhou L., Mao H.Q., Yang F.H., Chen Z., Zhang L. Mitochondrial DNA leakage exacerbates odontoblast inflammation through gasdermin D-mediated pyroptosis. Cell Death Dis. 2021;7:381. doi: 10.1038/s41420-021-00770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adamo L., Rocha-Resende C., Prabhu S.D., Mann D.L. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269–285. doi: 10.1038/s41569-019-0315-x. [DOI] [PubMed] [Google Scholar]
- 34.Shi J., Gao W., Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Cahill T.J., Kharbanda R.K. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: mechanisms, incidence and identification of patients at risk. World J Cardiol. 2017;9:407–415. doi: 10.4330/wjc.v9.i5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altara R., Manca M., Sabra R., Eid A.A., Booz G.W., Zouein F.A. Temporal cardiac remodeling post-myocardial infarction: dynamics and prognostic implications in personalized medicine. Heart Fail Rev. 2016;21:25–47. doi: 10.1007/s10741-015-9513-8. [DOI] [PubMed] [Google Scholar]
- 37.Chai R., Xue W., Shi S., Zhou Y., Du Y., Li Y., et al. Cardiac remodeling in heart failure: role of pyroptosis and its therapeutic implications. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.870924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen L., Wang M., Luo P., Meng X., Zhao M. Melatonin exerts cardioprotective effects by inhibiting NLRP3 inflammasome-induced pyroptosis in mice following myocardial infarction. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/5387799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao Q., Liang X.L., Zhang C.L., Pang Y.H., Lu Y.X. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 2019;10:393. doi: 10.1186/s13287-019-1522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang K.Z., Shen X.Y., Wang M., Wang L., Sun H.X., Li X.Z., et al. Retinol-binding protein 4 promotes cardiac injury after myocardial infarction via inducing cardiomyocyte pyroptosis through an interaction with NLRP3. J Am Heart Assoc. 2021 doi: 10.1161/JAHA.121.022011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z., Xu H., Liu X., Hong Y., Lou H., Liu H., et al. GDF11 inhibits cardiomyocyte pyroptosis and exerts cardioprotection in acute myocardial infarction mice by upregulation of transcription factor HOXA3. Cell Death Dis. 2020;11:917. doi: 10.1038/s41419-020-03120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen A., Chen Z., Xia Y., Lu D., Yang X., Sun A., et al. Liraglutide attenuates NLRP3 inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS pathway in H9c2 cells. Biochem Biophys Res Commun. 2018;499:267–272. doi: 10.1016/j.bbrc.2018.03.142. [DOI] [PubMed] [Google Scholar]
- 43.Chen A., Chen Z., Zhou Y., Wu Y., Xia Y., Lu D., et al. Rosuvastatin protects against coronary microembolization-induced cardiac injury via inhibiting NLRP3 inflammasome activation. Cell Death Dis. 2021;12:78. doi: 10.1038/s41419-021-03389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei Q., Yi T., Chen C. NF-κB–gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Mon Int Med J Exp Clin Res. 2018;24:6044–6052. doi: 10.12659/MSM.908529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye B., Chen X., Dai S., Han J., Liang X., Lin S., et al. Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Dev Ther. 2019;13:975–990. doi: 10.2147/DDDT.S195412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai Y., Wang S., Chang S., Ren D., Shali S., Li C., et al. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome via signaling pathway. J Mol Cell Cardiol. 2020;142:65–79. doi: 10.1016/j.yjmcc.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Shen S., He F., Cheng C., Xu B., Sheng J. Uric acid aggravates myocardial ischemia–reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110990. [DOI] [PubMed] [Google Scholar]
- 48.Liang C., Liu Y., Xu H., Huang J., Shen Y., Chen F., et al. Exosomes of human mbilical cord MSCs protect against hypoxia/reoxygenation-induced pyroptosis of cardiomyocytes the miRNA-100-5p/FOXO3/NLRP3 Pathway. Front Bioeng Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.615850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao L., Song J., Meng X.W., Ge J.Y., Du B.X., Yu J., et al. Periostin aggravates NLRP3 inflammasome-mediated pyroptosis in myocardial ischemia–reperfusion injury. Mol Cell Probes. 2020;53 doi: 10.1016/j.mcp.2020.101596. [DOI] [PubMed] [Google Scholar]
- 50.Mao C., Li D., Zhou E., Gao E., Zhang T., Sun S., et al. Extracellular vesicles from anoxia preconditioned mesenchymal stem cells alleviate myocardial ischemia/reperfusion injury. Aging (Albany NY) 2021;13:6156–6170. doi: 10.18632/aging.202611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li C., Song H., Chen C., Chen S., Zhang Q., Liu D., et al. LncRNA PVT1 knockdown ameliorates myocardial ischemia reperfusion damage via suppressing gasdermin D-mediated pyroptosis in cardiomyocytes. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.747802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen F., Chen Z.Q., Zhong G.L., Zhu J.J. Nicorandil inhibits TLR4/MyD88/NF-κB/NLRP3 signaling pathway to reduce pyroptosis in rats with myocardial infarction. Exp Biol Med (Maywood) 2021;246:1938–1947. doi: 10.1177/15353702211013444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen J., Fan Z., Sun G., Qi G. Sacubitril/valsartan (LCZ696) reduces myocardial injury following myocardial infarction by inhibiting NLRP3-induced pyroptosis via the TAK1/JNK signaling pathway. Mol Med Rep. 2021;24:676. doi: 10.3892/mmr.2021.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X., Hu Y., Wang Y., Shen D., Tao G. CLEC5A knockdown protects against cardiac dysfunction after myocardial infarction by suppressing macrophage polarization, NLRP3 inflammasome activation, and pyroptosis. Biochem Cell Biol. 2021;99:655–665. doi: 10.1139/bcb-2020-0672. [DOI] [PubMed] [Google Scholar]
- 55.Han X., Zhao Z.A., Yan S., Lei W., Wu H., Lu X.A., et al. CXADR-like membrane protein protects against heart injury by preventing excessive pyroptosis after myocardial infarction. J Cell Mol Med. 2020;24:13775–13788. doi: 10.1111/jcmm.15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camici P.G., d'Amati G., Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12:48–62. doi: 10.1038/nrcardio.2014.160. [DOI] [PubMed] [Google Scholar]
- 57.Peng L., Lei Z., Rao Z., Yang R., Zheng L., Fan Y., et al. Cardioprotective activity of ethyl acetate extract of Cinnamomi ramulus against myocardial ischemia/reperfusion injury in rats via inhibiting NLRP3 inflammasome activation and pyroptosis. Phytomedicine. 2021;93 doi: 10.1016/j.phymed.2021.153798. [DOI] [PubMed] [Google Scholar]
- 58.Li S., Zhao X., Cheng S., Li J., Bai X., Meng X. Downregulating long non-coding RNA PVT1 expression inhibited the viability, migration and phenotypic switch of PDGF-BB-treated human aortic smooth muscle cells via targeting miR-27b-3p. Hum Cell. 2021;34:335–348. doi: 10.1007/s13577-020-00452-5. [DOI] [PubMed] [Google Scholar]
- 59.Buckley L.F., Libby P. Inhibiting NLRP3 inflammasome activity in acute myocardial infarction: a review of pharmacologic agents and clinical outcomes. J Cardiovasc Pharmacol. 2019;74:297–305. doi: 10.1097/FJC.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 60.Land W.G. The role of damage-associated molecular patterns (DAMPs) in human diseases: part II: DAMPs as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ Med J. 2015;15:e157–e170. [PMC free article] [PubMed] [Google Scholar]
- 61.Zhitkovich A. N-Acetylcysteine: antioxidant, aldehyde scavenger, and more. Chem Res Toxicol. 2019;32:1318–1319. doi: 10.1021/acs.chemrestox.9b00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galano A., Reiter R.J. Melatonin and its metabolites vs oxidative stress: from individual actions to collective protection. J Pineal Res. 2018;65 doi: 10.1111/jpi.12514. [DOI] [PubMed] [Google Scholar]
- 63.Fu Z., Jiao Y., Wang J., Zhang Y., Shen M., Reiter R.J., et al. Cardioprotective role of melatonin in acute myocardial infarction. Front Physiol. 2020;11:366. doi: 10.3389/fphys.2020.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A., et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 66.Karantalis V., Hare J.M. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–1430. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao B., Huang X., Wang Q., Wu Y. β-Asarone alleviates myocardial ischemia–reperfusion injury by inhibiting inflammatory response and NLRP3 inflammasome mediated pyroptosis. Biol Pharm Bull. 2020;43:1046–1051. doi: 10.1248/bpb.b19-00926. [DOI] [PubMed] [Google Scholar]
- 68.Jiang T., Chu J., Chen H., Cheng H., Su J., Wang X., et al. Gastrodin inhibits H2O2-induced ferroptosis through its antioxidative effect in rat glioma cell line C6. Biol Pharm Bull. 2020;43:480–487. doi: 10.1248/bpb.b19-00824. [DOI] [PubMed] [Google Scholar]
- 69.Saki G., Eidi A., Mortazavi P., Panahi N., Vahdati A. Effect of β-asarone in normal and β-amyloid-induced Alzheimeric rats. Arch Med Sci. 2020;16:699–706. doi: 10.5114/aoms.2020.94659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nie C., Ding X., A R., Zheng M., Li Z., Pan S., et al. Hydrogen gas inhalation alleviates myocardial ischemia–reperfusion injury by the inhibition of oxidative stress and NLRP3-mediated pyroptosis in rats. Life Sci. 2021;272 doi: 10.1016/j.lfs.2021.119248. [DOI] [PubMed] [Google Scholar]
- 71.Bhatt D.L., Lopes R.D., Harrington R.A. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327:662–675. doi: 10.1001/jama.2022.0358. [DOI] [PubMed] [Google Scholar]
- 72.Senturk T., Cavun S., Avci B., Yermezler A., Serdar Z., Savci V. Effective inhibition of cardiomyocyte apoptosis through the combination of trimetazidine and N-acetylcysteine in a rat model of myocardial ischemia and reperfusion injury. Atherosclerosis. 2014;237:760–766. doi: 10.1016/j.atherosclerosis.2014.10.091. [DOI] [PubMed] [Google Scholar]
- 73.Nozari Y., Eshraghi A., Talasaz A.H., Bahremand M., Salamzadeh J., Salarifar M., et al. Protection from reperfusion injury with intracoronary N-acetylcysteine in patients with STEMI undergoing primary percutaneous coronary intervention in a cardiac tertiary center. Am J Cardiovasc Drugs. 2018;18:213–221. doi: 10.1007/s40256-017-0258-8. [DOI] [PubMed] [Google Scholar]
- 74.Katsumata Y., Sano F., Abe T., Tamura T., Fujisawa T., Shiraishi Y., et al. The effects of hydrogen gas inhalation on adverse left ventricular remodeling after percutaneous coronary intervention for ST-elevated myocardial infarction- first pilot study in humans. Circ J. 2017;81:940–947. doi: 10.1253/circj.CJ-17-0105. [DOI] [PubMed] [Google Scholar]
- 75.Jones S.P., Tang X.L., Guo Y., Steenbergen C., Lefer D.J., Kukreja R.C., et al. The NHLBI-sponsored consortium for preclinical assessment of cardioprotective therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res. 2015;116:572–586. doi: 10.1161/CIRCRESAHA.116.305462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernandez-Jimenez R., Ibanez B. CAESAR: one step beyond in the construction of a translational bridge for cardioprotection. Circ Res. 2015;116:554–556. doi: 10.1161/CIRCRESAHA.115.305841. [DOI] [PubMed] [Google Scholar]