To the Editor:
We read with great interest the recent study by van Leeuwen et al.1 regarding the compassionate use of avacopan in difficult-to-treat antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Avacopan is a novel promising treatment for antibody-associated vasculitis targeting the complement system by blocking C5a receptors.2,3 Here, we report the tailored use of avacopan in a case with refractory antibody-associated vasculitis and concominant complement system activation.
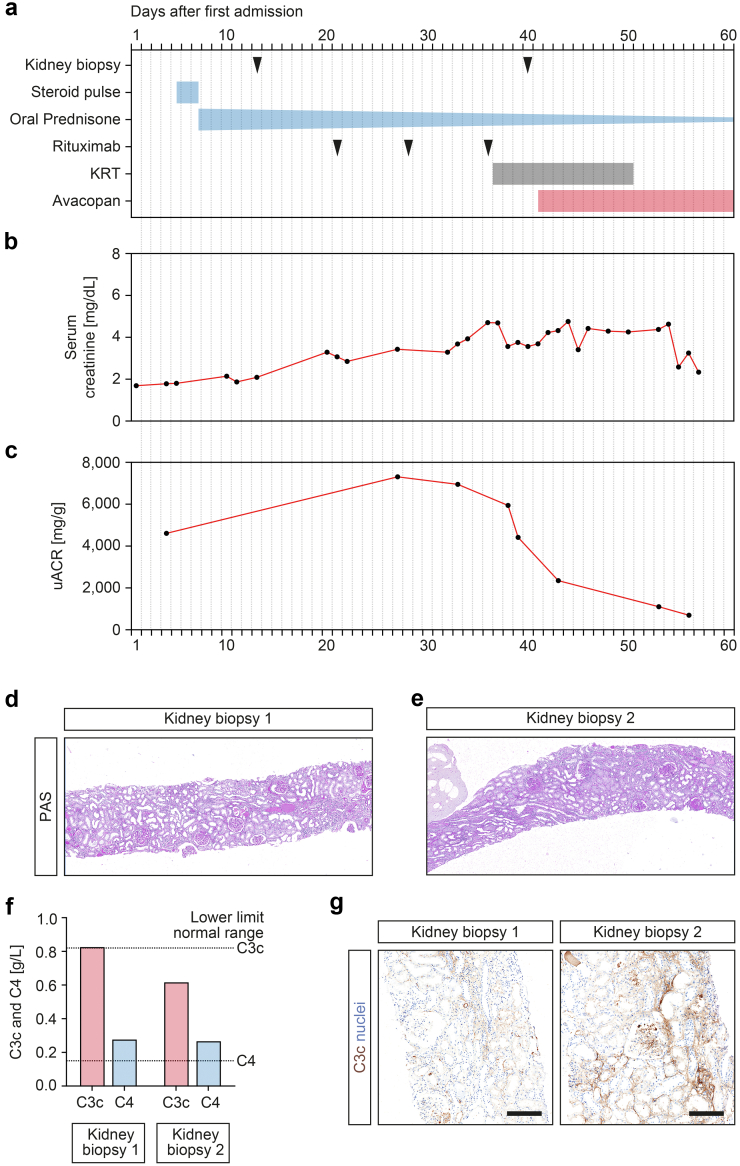
An 84-year-old man with pre-existing hypertension and diabetes presented to our tertiary hospital with lower extremity purpuric papules, acute kidney injury (serum creatinine of 1.69 mg/dl, reference range: 0.5–1 mg/dl; eGFR 40 ml/min per 1.73 m2, reference: >60 ml/min per 1.73 m2), albuminuria (5044 mg/g creatinine, reference: <30 mg/g), and hematuria (Figure 1a–c). A skin biopsy showed dermal vasculitis, and laboratory testing confirmed presence of myeloperoxidase-ANCAs (8.4 IU/ml, reference: <3.5 IU/ml). A kidney biopsy confirmed focal class necrotizing and crescentic ANCA-associated renal vasculitis (Figure 1d). On the basis of these findings, steroid pulse (500 mg i.v. methylprednisone) and rituximab (375 mg/m2, 750 mg) treatment was initiated for remission induction therapy. After a total number of 3 infusions of rituximab, laboratory testing showed depleted peripheral CD19+ B cells (2/μl, reference range: 100−500/μl) and regression of myeloperoxidase-ANCAs (2.2 IU/ml, reference: <3.5 IU/ml). However, kidney function and albuminuria worsened with requirement kidney replacement therapy (Figure 1a–c). A second kidney biopsy confirmed persistence of active ANCA-associated renal vasculitis (Figure 1e). On the basis of complement system activation with serum C3c lowering (0.62 g/l, reference range: 0.82−1.93 g/l) and progressive intrarenal complement C3 deposits that were not present at first presentation (Figure 1f and g), a tailored treatment with avacopan (30 mg twice daily) was initiated. Thereafter, kidney function and albuminuria improved and kidney replacement therapy was no longer required after 10 days of avacopan treatment (Figure 1a–c).
Figure 1.
Avacopan improves treatment response in refractory ANCA-associated renal vasculitis with concominant complement system activation. (a) Time of kidney biopsies and treatment regimens. (b,c) Time course of serum creatinine and uACR levels. (d,e) Representative sections from the first and second kidney biopsy stained with periodic acid-schiff confirmed focal class necrotizing and crescentic ANCA-associated renal vasculitis. (f) Measurements of serum C3c and C4 at time of first and second kidney biopsy, the dotted lines represent the lower limit of the normal range. (g) Representative sections from the first and second kidney biopsy stained for intrarenal complement C3c confirmed progressive deposits (scale bar: 200 μm). ANCA, antineutrophil cytoplasmic antibody; KRT, kidney replacement therapy; PAS, periodic acid-schiff; uACR, Urine albumin to creatinine ratio.
Here, we report real-life practice data on the tailored use of avacopan in a case of refractory antibody-associated vasculitis. In this case, avacopan had additional effects with respect to improved disease control and in line with previous reports.1 Moreover, we provide the first evidence, to the best of our knowledge, that assessment of complement system activation might enable identification of patients that benefit from a complement-targeted therapy.
References
- 1.van Leeuwen J.R., Bredewold O.W., van Dam L.S., et al. Compassionate use of avacopan in difficult-to-treat antineutrophil cytoplasmic antibody-associated vasculitis. Kidney Int Rep. 2022;7:624–628. doi: 10.1016/j.ekir.2021.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayne D.R.W., Bruchfeld A.N., Harper L., et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28:2756–2767. doi: 10.1681/ASN.2016111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayne D.R.W., Merkel P.A., Schall T.J., Bekker P., ADVOCATE Study Group Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. 2021;384:599–609. doi: 10.1056/NEJMoa2023386. [DOI] [PubMed] [Google Scholar]