Abstract
Gout and hyperuricemia are present in 25% and 60% of patients with chronic kidney disease (CKD), respectively. Despite the common association, the role of uric acid in the progression of kidney disease and in metabolic complications remains contested. Some authorities argue that the treatment of asymptomatic hyperuricemia in CKD is not indicated, and some have even suggested hyperuricemia may be beneficial. Here, we review the various arguments both for and against treatment. The weight of the evidence suggests asymptomatic hyperuricemia is likely injurious, but it may primarily relate to subgroups, those who have systemic crystal deposits, those with frequent urinary crystalluria or kidney stones, and those with high intracellular uric acid levels. We recommend carefully designed clinical trials to test if lowering uric acid in hyperuricemic subjects with cardiometabolic complications is protective.
Keywords: chronic kidney disease, gout, hyperuricemia, metabolic syndrome, systemic inflammation
Hyperuricemia (defined as a serum uric acid level >7 mg/dl in males and >6 mg/dl in women) is common in CKD. This is because hyperuricemia is common in type 2 diabetes and hypertension, which are conditions that cause CKD, and also because CKD results in reduced urinary excretion of uric acid. As a consequence, the prevalence of gout increases from 1% to 2% of adults with normal kidney function to 32% of those with stage 4 CKD. Hyperuricemia prevalence also increases from 11% among those with normal kidney function to 80% among those with stage 4 CKD.1 The converse is also true. CKD stage 2 or higher is present in 70% of subjects with gout and 50% of those with hyperuricemia.2
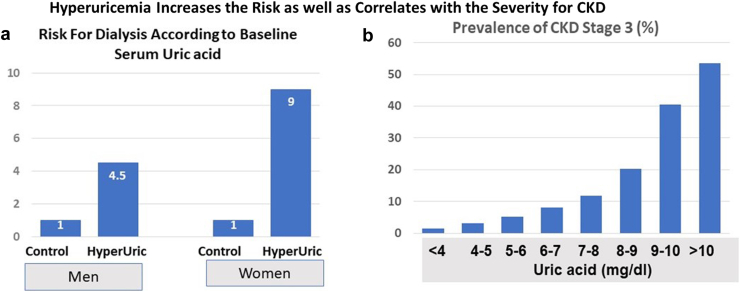
It is therefore important to understand if high uric acid levels modify kidney or metabolic outcomes. This is especially true because hyperuricemia is an independent predictor of CKD and metabolic diseases,3, 4, 5 including in subjects who are healthy without any morbidities.6 There is also a direct relationship of serum uric acid level with prevalence of hypertension, diabetes, and CKD (Figure 1).7,8
Figure 1.
Relationship of Serum Uric acid with CKD. (a) A study in which more than 48,000 Japanese that were 20 years or older who were followed for 7 years. After controlling for baseline serum creatinine and other variables, the presence or absence of baseline hyperuricemia (defined as >7 mg/dl in men and >6 mg/dl in women) markedly increased the risk for developing end stage kidney disease requiring dialysis. (b) A figure based on the study of 5707 participants aged 20 years and older from the National Health and Nutrition Examination Survey 2007–2008. There is an exponential relationship of serum uric acid levels with CKD. (a) Adapted from Iseki et al.7 and (b) Adapted from Zhu et al.8 CKD, chronic kidney disease; HyperUric, hyperuricemia.
Today, there remains controversy over the role of uric acid in CKD and cardiometabolic outcomes. Several groups have suggested that asymptomatic hyperuricemia in CKD is benign and should not be treated, or may even be beneficial.9, 10, 11, 12 Here, we present our countering viewpoint with recommendations for how to move forward. For purposes of the discussion, our analysis will be separately about those who have gout and CKD, and those with asymptomatic hyperuricemia and CKD.
Gout and CKD
Gout is classically treated with urate-lowering agents to reduce the risk for recurrent arthritic attacks and joint damage.13 It has remained controversial whether lowering serum uric acid in gout has an effect on kidney disease or cardiovascular events. However, there are at least 3 arguments that strongly suggest that lowering uric acid in subjects with gout may be beneficial for CKD and/or cardiovascular events. All these are based on the fact that urate crystals are known to be very proinflammatory, and known to induce local inflammation that is mediated by activation of inflammasomes and the release of interleukin-1.14
The first argument is that urate crystals are known to deposit not only in joints, but also in other tissues,15 and one of the favored sites is in the collecting ducts of the kidney.16 As urine concentrates, it also acidifies, and this can lead to urate crystallization. Some urate crystals adhere to the tubular epithelium, where they can cause local inflammation that leads to rupture of the tubular wall with the crystals escaping into the interstitium.17 This can be associated with marked local inflammation with macrophage infiltration.18 Subjects with gout have reduced fractional excretion of uric acid. Nevertheless, they excrete large amounts of uric acid, especially following ingestion of a purine-rich19 or fructose-rich20 meal.
Indeed, in the days before urate-lowering treatment was available, autopsies of patients with gout showed an almost universal presence of kidney disease, characterized by arteriolosclerosis, focal segmental glomerulosclerosis, and chronic tubulointerstitial disease,16,21 and as many as 20% or 25% of gouty subjects would have markedly reduced kidney function.22 Most strikingly, urate crystals were found in 90% of autopsies, always concentrated in the outer medulla.21 In contrast, kidney biopsies rarely document urate crystals23 because the biopsies are of the cortex where uric acid crystals are sparse. In addition, urate crystals are often washed out during the fixation process unless special techniques, such as using alcohol fixation and the De Galantha stain, are used.
It is not known how common urate crystals are present in the kidneys of gout patients today. Newer techniques such as the dual energy computed tomography (DECT) scans have been suggested as a method to detect their presence.24 However, there are technical issues in performing and analyzing scans, and adjustments need to be performed for each organ evaluated, and the art of performing DECT scans on kidneys is still being refined. Nevertheless, another approach that has been recommended is the use of renal artery ultrasound to determine if there is enhanced echogenicity in the renal medulla associated with crystal deposition. A hyperechoic medulla by ultrasound is considered strongly suggestive of urate crystal deposition and has been reported to be present in one-third of subjects with gout.25
The second argument to treat gout not just for the risk of recurrent gout attacks is because there is now evidence that urate crystals may be directly involved in the atherosclerotic process. Specifically, DECT scans adjusted for evaluating blood vessels have had the surprising discovery that urate crystals are common in the aorta and coronary arteries of subjects with gout. Indeed, urate crystals are present in the blood vessels of 75% to 86% of patients with gout, with nearly 30% of gout subjects having crystals in their coronary arteries.26,27 The primary sites seem to be in areas of atherosclerotic plaque, which has been confirmed by histologic studies.28, 29, 30 Urate crystals have also been colocalized with sites of vascular calcification.26 Urate crystals are likely to stimulate inflammasomes in the lesions similar to that of cholesterol crystals, and this is expected to increase the risk for plaque extension or rupture.31 These findings could explain why both hyperuricemia and gout are associated with cardiovascular mortality in both epidemiology and Mendelian randomization studies.32, 33, 34
The third argument relates to the observation that urate crystal deposits can result in not just local inflammation but also in systemic inflammation, and the latter is recognized as a contributing factor for progressive kidney disease as well as cardiovascular events.35,36 A striking finding in gout is that resolution of an acute attack is not associated with resolution of urate crystals because they will persist until the next attack (i.e., the “intercritical period”).37 These “silent crystals” can still be associated with evidence for systemic inflammation that may have an indirect role in the progression of kidney disease and cardiovascular events.38 Indeed, both elevated monocyte counts and high levels of highly sensitive C-reactive protein levels are found in patients with a history of gout.39,40 Some studies have found that allopurinol treatment can lower highly sensitive C-reactive protein levels.41 Furthermore, targeting inflammation by giving antibodies to interleukin-1 can reduce highly sensitive C-reactive protein and cardiovascular events in individuals at risk of cardiovascular disease.42,43
We believe these arguments are strong enough to recommend urate-lowering in all subjects who suffer from gout, and treatment could be dietary or involve a nutraceutical or drug; however, the goal would be to lower serum uric acid levels to <6 mg/dl.
Hyperuricemia and CKD
Initially there was strong evidence that hyperuricemia in the absence of gout might be driving CKD. This was supported both by epidemiologic studies,3,44 experimental studies45,46 and pilot clinical trials.47 However, 2 large clinical trials, known as the prevention of early renal loss in type 1 Diabetes and CKD-FIX (Controlled Trial of Slowing of Kidney Disease Progression from the Inhibition of Xanthine Oxidase), were published in the same issue of the New England Journal of Medicine and found no benefit of allopurinol in slowing renal progression.48,49 This initially led several groups, including the Caring for Australians and New Zealanders with Kidney Impairment Guidelines Committee to suggest that there is now conclusive evidence not to treat asymptomatic hyperuricemia in CKD.9,12
We believe this conclusion is premature. The prevention of early renal loss and CKD-FIX studies tested whether lowering uric acid was beneficial but not whether treating hyperuricemia is beneficial, because both studies included large numbers of patients with normal serum uric acid levels. Normal uric acid levels are not expected to significantly increase the risk for CKD (see Figure 1).
Both studies were also intention-to-treat analyses. These analyses count all treated subjects even if they discontinued treatment because of problems with compliance or side effects. In this case, approximately 17.5% to 30% of subjects dropped out before completion of the trial. The fact that this happened in both treatment and placebo groups suggest it was largely not because of the drug but rather because of general characteristics of the population being studied, or perhaps because of general concerns or preset opinions that allopurinol might be associated with high side effects. Therefore, the trial was not actually testing the hypothesis that asymptomatic hyperuricemia might be driving CKD, but rather tested whether allopurinol treatment reduced progression of kidney disease when including issues such as compliance, side effects, and other factors.
The CARI guidelines have some additional problems. For example, the criteria they used in their studies did not require the presence of hyperuricemia (allowing any serum uric acid level), nor did it require the presence of CKD,9 yet the question being addressed was whether treating asymptomatic hyperuricemia in CKD was beneficial. Their conclusion that treatment was not beneficial was also at odds with the meta-analysis they used for their study. The latter had concluded that lowering serum uric acid was beneficial in reducing the decline in estimated glomerular filtration rate and blood pressure.50
More recently, the ALLHEART study was published in which older patients (age >60 years) with a history of ischemic heart disease and no gout were randomized to allopurinol or placebo and followed for almost 5 years without any discernible benefit on subsequent cardiovascular events.51 However, similar to the other trials, patients with normal uric acid levels were included (the mean serum uric acid was 5.6 mg/dl) and gout was excluded. Likewise, there was a large (57%) dropout which were included in the analysis because it was an intention-to-treat study, such that it was not truly testing whether the treatment of hyperuricemia is beneficial on cardiac endpoints.
One might hope that meta-analyses might help resolve the issues, but even here there remains confusion, because some recent studies suggest that treatment of asymptomatic hyperuricemia in CKD does slow progression,52,53 whereas others are mixed or indeterminant,50,54,55 and some are negative.56 Some meta-analyses show benefit with only specific urate-lowering drugs, such as febuxostat.57,58 One possible explanation for the mixed data is that there may be subgroups that particularly benefit from treatment. In the next section, we propose specific groups that we believe might be most likely to respond to urate-lowering therapy.
Subgroups in Which Asymptomatic Hyperuricemia may be Most Likely to Drive CKD and Cardiac Disease
The first group to consider would be patients with asymptomatic hyperuricemia who may harbor “silent” crystals in their joints, blood vessels, or kidneys (similar to the argument provided in the gout section). Approximately 15% of hyperuricemic subjects who do not have gout still have urate deposits in their blood vessels when evaluated by DECT scan.26 Some patients may also harbor crystals silently in their joints or kidneys. Studies that include DECT scanning or renal ultrasound may help identify this subgroup.
A second group would be subjects with recurrent urate crystalluria or kidney stones. Crystalluria can stimulate inflammasomes in renal tubular cells leading to local inflammation and injury that can accelerate CKD.11,59, 60, 61 One mechanism for the crystalluria would be the presence of a low urinary pH, such as may occur with dehydration, because uric acid is very insoluble in acidic urine. A low urinary pH predicts the development of CKD.62 This may also explain the benefit of bicarbonate therapy to slow CKD, and we reported that bicarbonate therapy can solubilize urate crystals and reduce biomarkers of renal tubular injury in diabetic subjects.63 Heat stress-associated urate crystalluria is also common in subjects with Mesoamerican nephropathy where it might be driving kidney damage,64,65 and uricosuria accompanies rhabdomyolysis where it has been suggested to play an ancillary role.66 Indeed, both allopurinol and bicarbonate therapy are protective in experimental rhabdomyolysis-associated kidney injury.67,68
Although urate crystalluria is largely driven by low urinary pH, some individuals will show “overproduction” uricosuria. Recently, this was shown to be mediated by reduced intestinal uric acid excretion, especially by inhibition of the adenosine triphosphate-binding cassette subfamily G member 2 urate transporter.69,70 The adenosine triphosphate-binding cassette subfamily G member 2 transporter is expressed in both the kidney and intestine, but polymorphisms that are associated with reduced function (such as the Q126X variant [that has near absent function] and the Q141K [which has half-function]) result in reduced intestinal elimination with increased renal excretion.70 New studies suggest that individuals carrying these variants are at higher risk for progression of CKD.69,71 Dietary mechanisms may also be operative. Fructose, for example, also blocks adenosine triphosphate-binding cassette subfamily G member 2 in the intestine, reducing intestinal uric acid excretion,72 while increasing urinary excretion,73 reducing urinary pH,74 and reducing urine volume.75 Dietary intake of high purine foods can also cause transient uricosuria19 that might be important in mediating kidney injury.
Asymptomatic hyperuricemic patients with kidney stones may also be candidates for having recurrent uricosuria. Kidney stones are common in subjects with gout, being present in one-third of subjects when evaluated by helical computerized tomography, although two-thirds of these patients were not aware they had stones. Of interest, the subjects with kidney stones had worse kidney function and lower urine pH than those who did not have stones.76 Therefore, one might consider evaluating subjects with hyperuricemia to determine if they have stones, because this may represent a group with an increased risk for CKD progression.
The importance of urate crystalluria in causing kidney injury was recently elucidated in patients with uricosuria and hypouricemia because of the loss of urate transporters in their kidneys.77,78 In both experimental models and humans, the use of allopurinol to reduce uricosuria is associated with protection from kidney injury.77,79
A third group of subjects might be those who have increased intracellular levels of uric acid occurring in their livers or kidneys. In the liver, intracellular uric acid is thought to mediate oxidative stress to the mitochondria that drives metabolic effects like insulin resistance, hepatic fat accumulation, and elevation in blood pressure.80 Interestingly, this is associated with dietary measures such as intake of fructose or purine-rich foods; howver, it can also occur with block in intestinal uric acid excretion.71 Another condition might by polycystic kidney disease, in which the enlarging cysts are likely stimulating local uric acid generation. In autosomal polycystic kidney disease, serum uric acid levels are high and correlate with progression, whereas pilot studies suggest that lowering serum uric acid may be protective.81,82
A major mechanism by which hyperuricemia induces its effects on the kidney is likely via its vascular effects. Uric acid has been shown experimentally to mediate endothelial dysfunction by both reducing biologically available nitric oxide and inducing oxidative stress.83, 84, 85 Although not all studies are positive, most clinical studies suggest that xanthine oxidase inhibitors can improve endothelial dysfunction.86 Uric acid can also enter into vascular smooth muscle cells via specific transporters,87,88 where it drives proliferation and proinflammatory pathways.87,89,90 Hyperuricemia is especially correlated with disease of the afferent arteriole in both experimental animals45 and in humans.91 Experimentally, this is associated with renal vasoconstriction, altered renal autoregulation, and increased glomerular hydrostatic pressure.92 Serum uric acid also correlates with high renal afferent arteriolar resistance in humans.93
Measuring intracellular uric acid levels is difficult. However, determining plasma xanthine oxidase activity might be an alternative way to identify these patients. Elevated plasma xanthine oxidase activity has been reported to identify subjects with CKD who are at risk for cardiovascular events94 and may be superior to serum uric acid in predicting metabolic disorders.95,96
Other Arguments
Some have argued that uric acid may be beneficial because it can function as an antioxidant.97 However, uric acid is pro-oxidative inside cells because it activates nicotinamide adenine dinucleatide phosphate oxidase and also because it can generate radicals such as hydroperoxide, peroxynitrite-radicals, and myeloperoxidase-based radicals.98,99 When uric acid quells the production of hypochlorous acid by neutrophils, the overall oxidative stress remains the same because of increased production of superoxide.100 Indeed, the overall effects of soluble uric acid are proinflammatory.98,100, 101, 102
Some groups have reported that hyperuricemia induced by inosine may protect against kidney disease.11,103,104 However, inosine has been shown to be anti-inflammatory because it activates adenosine receptors105, 106, 107 and generates hypoxanthine, the latter that can be recycled to IMP and eventually adenosine triphosphate.108 Importantly, in the studies evaluating if uric acid is beneficial in kidney disease, the investigators did not determine if allopurinol treatment would reverse this protection. Other groups have shown that the anti-inflammatory effects of inosine are enhanced by administering allopurinol.109,110
Another issue has been the concern that febuxostat might increase the risk for cardiovascular mortality because it was associated with more cardiovascular events in the CARES trial.111 Other studies could not affirm this association.112 Moreover, there was no placebo group in the CARES study, and studies of allopurinol suggest it may reduce mortality risk in the general population,113 with a trend toward protection in subjects with CKD.114
An additional argument is that most Mendelian randomization studies have not been able to show that genetic polymorphisms that increase serum uric acid translate into increased risk for CKD,115, 116, 117, 118 despite this being shown in other Mendelian randomization studies for hypertension,119 coronary artery disease,34 or cardiovascular events.33 However, serum uric acid is not the critical factor, given that the factors driving kidney disease may relate more to urine uric acid, urine pH, presence or absence of urate crystals in the kidney, and intracellular serum uric acid levels, especially in the liver and kidney. Although intracellular uric acid and serum uric acid levels are often correlated, they can also be dissociated.120 In addition, some urate transporters, such as SLC2A9, may have divergent effects on serum uric acid depending on which target organ is evaluated,121,122 suggesting that genetic polymorphisms altering SLC2A9 function could have opposing biologic effects depending on the target organ that could confound Mendelian randomization studies.
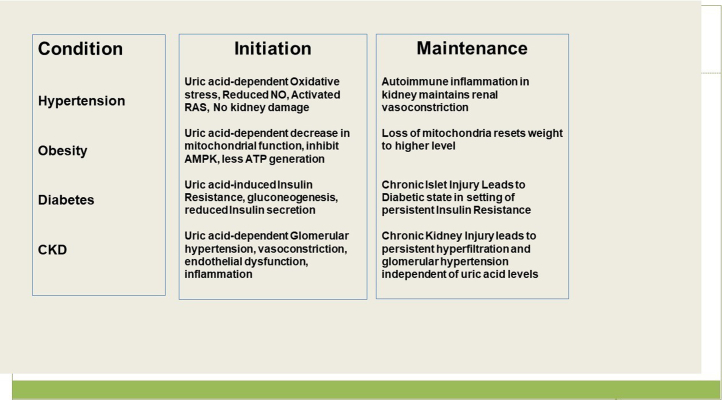
Finally, an important consideration is that what initiates disease might be different from what drives it (Figure 2). For example, experimental studies suggest that experimental hyperuricemia causes a rise in blood pressure because of effects of uric acid; however, over time there is the induction of an autoimmune inflammatory response in the kidney that maintains the hypertension.123 Likewise, experimental hyperuricemia is associated with glomerular hypertension and renal vasoconstriction that is dependent on uric acid levels, but as CKD develops, glomerular hypertension is driven by a reduction in nephron number.124 Similarly, there is evidence that fructose-induced hyperuricemia initially causes a reversible insulin resistance; however, overtime there is progressive injury to the islets resulting in islet dysfunction and a loss in insulin secretory ability.125 There is also evidence that longstanding mitochondrial oxidative stress may lead to a loss of mitochondria that may have persistent effects.126 Thus, the timing for when uric acid is lowered may be important. Similarly, to see a benefit in established disease, one might have to treat for a long time, and there is some evidence that the benefit of lowering uric acid is greatest if treatment is prolonged for 2 years or more.127
Figure 2.
Uric acid may be more Important in the Initiation of Metabolic Diseases Rather than the Maintenance. AMPK, adenosine monophosphatase-activated protein kinas; ATP, adenosine trisphosphate; CKD, chronic kidney disease; NO, nitric oxide; RAS, renin angiotensin system.
Summary
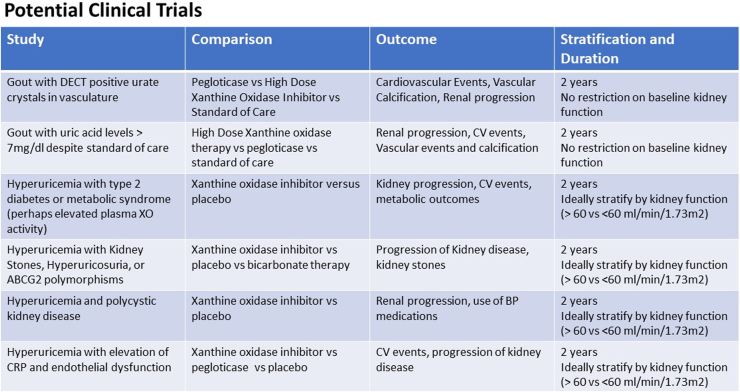
Asymptomatic hyperuricemia is common in subjects with CKD. Although the CKD-FIX and prevention of early renal loss studies did not report benefit of allopurinol in slowing the progression of kidney disease, they did not specifically address the role of hyperuricemia. We suggest that there may be some specific subgroups of subjects with asymptomatic hyperuricemia that would benefit, including those with documented crystal deposition in joints, blood vessels, and the kidneys; those with documented recurrent urate crystalluria or with kidney stones; and those who have evidence for elevated liver or kidney uric acid levels (possibly noted by high plasma xanthine oxidase activity). Examples of some proposed trials are shown in Figure 3. The addition of these carefully designed studies are needed to determine the role of uric acid in the progression of CKD.
Figure 3.
Examples of potential clinical trials to investigate the role of uric acid in cardio-renal diseases. BP, blood pressure; CRP, C-reactive protein; CV, cardiovascular; DECT, dual energy computed tomography; XO, xanthine oxidase.
Disclosure
RJJ has equity with Colorado Research Partners LLC, has stock with XORTX Therapeutics, and is a consultant for Horizon Pharma. MAL and LGl have equity with Colorado Research Partners LLC. CB has research grants from Servier and Alfasigma and serves as a consultant for Servier, Novartis, Novo Nordisk, Alfasigma, Sanofi, and Menarini. FP declares no competing interests.
Acknowledgments
Funding
Supported in part by NIH grant DK121496.
References
- 1.Juraschek S.P., Kovell L.C., Miller E.R., 3rd, Gelber A.C. Association of kidney disease with prevalent gout in the United States in 1988–1994 and 2007–2010. Semin Arthritis Rheum. 2013;42:551–561. doi: 10.1016/j.semarthrit.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnan E. Reduced glomerular function and prevalence of gout: NHANES 2009–10. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu P., Liu Y., Han L., et al. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: a meta-analysis of 15 cohort studies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grayson P.C., Kim S.Y., LaValley M., Choi H.K. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63:102–110. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lv Q., Meng X.F., He F.F., et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuwabara M., Niwa K., Hisatome I., et al. Asymptomatic hyperuricemia without comorbidities predicts cardiometabolic diseases: five-year Japanese cohort study. Hypertension. 2017;69:1036–1044. doi: 10.1161/HYPERTENSIONAHA.116.08998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iseki K., Ikemiya Y., Inoue T., et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–650. doi: 10.1016/S0272-6386(04)00934-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y., Pandya B.J., Choi H.K. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125:679–687.e1. doi: 10.1016/j.amjmed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Stanley I.K., Phoon R.K., Toussaint N., et al. Caring for Australians and New Zealanders with kidney improvement guidelines: rapid development of urate lowering therapy guidelines for people with CKD. Kidney Int Rep. 2022;7:2563–2574. doi: 10.1016/j.ekir.2022.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Q., Immler R., Pruenster M., et al. Soluble uric acid inhibits beta2 integrin-mediated neutrophil recruitment in innate immunity. Blood. 2022;139:3402–3417. doi: 10.1182/blood.2021011234. [DOI] [PubMed] [Google Scholar]
- 11.Sellmayr M., Hernandez Petzsche M.R., Ma Q., et al. Only hyperuricemia with crystalluria, but not asymptomatic hyperuricemia, drives progression of chronic kidney disease. J Am Soc Nephrol. 2020;31:2773–2792. doi: 10.1681/ASN.2020040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Martin G., Cano J., Carriazo S., et al. The dirty little secret of urate-lowering therapy: useless to stop chronic kidney disease progression and may increase mortality. Clin Kidney J. 2020;13:936–947. doi: 10.1093/ckj/sfaa236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drug and Therapeutics Bulletin Latest guidance on the management of gout. BMJ. 2018;362 doi: 10.1136/bmj.k2893. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F., Petrilli V., Mayor A., et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 15.Khanna P., Johnson R.J., Marder B., LaMoreaux B., Kumar A. Systemic urate deposition: an unrecognized complication of gout? J Clin Med. 2020;9:3204. doi: 10.3390/jcm9103204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talbott J.H., Terplan K.L. The kidney in gout. Med (Baltim) 1960;39:405–467. doi: 10.1097/00005792-196012000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Cameron J.S., Simmonds H.A. Uric acid, gout and the kidney. J Clin Pathol. 1981;34:1245–1254. doi: 10.1136/jcp.34.11.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y.G., Huang X.R., Suga S., et al. Involvement of macrophage migration inhibitory factor (MIF) in experimental uric acid nephropathy. Mol Med. 2000;6:837–848. doi: 10.1007/BF03401822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clifford A.J., Riumallo J.A., Youn V.R., Scrimshaw N.S. Effect of oral purines on serum and urinary uric acid of normal, hyperuricemic and gouty humans. J Nutr. 1976;106:428–450. doi: 10.1093/jn/106.3.428. [DOI] [Google Scholar]
- 20.Raivio K.O., Becker A., Meyer L.J., et al. Stimulation of human purine synthesis de novo by fructose infusion. Metabolism. 1975;24:861–869. doi: 10.1016/0026-0495(75)90133-x. [DOI] [PubMed] [Google Scholar]
- 21.Brown J., Mallory G.K. Renal changes in gout. N Engl J Med. 1950;243:325–329. doi: 10.1056/NEJM195008312430901. [DOI] [PubMed] [Google Scholar]
- 22.Coombs F.S., Pecora L.J., Thorogood E., et al. Renal function in patients with gout. J Clin Invest. 1940;19:525–535. doi: 10.1172/JCI101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barlow K.A., Beilin L.J. Renal disease in primary gout. Q J Med. 1968;37:79–96. [PubMed] [Google Scholar]
- 24.Bardin T., Tran K.M., Nguyen Q.D., et al. Renal medulla in severe gout: typical findings on ultrasonography and dual-energy CT study in two patients. Ann Rheum Dis. 2019;78:433–434. doi: 10.1136/annrheumdis-2018-214174. [DOI] [PubMed] [Google Scholar]
- 25.Bardin T., Nguyen Q.D., Tran K.M., et al. A cross-sectional study of 502 patients found a diffuse hyperechoic kidney medulla pattern in patients with severe gout. Kidney Int. 2021;99:218–226. doi: 10.1016/j.kint.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Klauser A.S., Halpern E.J., Strobl S., et al. Dual-energy computed tomography detection of cardiovascular monosodium urate deposits in patients with gout. JAMA Cardiol. 2019;4:1019–1028. doi: 10.1001/jamacardio.2019.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barazani S.H., Chi W.W., Pyzik R., et al. Quantification of uric acid in vasculature of patients with gout using dual-energy computed tomography. World J Radiol. 2020;12:184–194. doi: 10.4329/wjr.v12.i8.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patetsios P., Song M., Shutze W.P., et al. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am J Cardiol. 2001;88:A186. doi: 10.1016/s0002-9149(01)01621-6. [DOI] [PubMed] [Google Scholar]
- 29.Patetsios P., Rodino W., Wisselink W., et al. Identification of uric acid in aortic aneurysms and atherosclerotic artery. Ann N Y Acad Sci. 1996;800:243–245. doi: 10.1111/j.1749-6632.1996.tb33318.x. [DOI] [PubMed] [Google Scholar]
- 30.Nardi V., Franchi F., Prasad M., et al. Uric acid expression in carotid atherosclerotic plaque and serum uric acid are associated with cerebrovascular events. Hypertension. 2022;79:1814–1823. doi: 10.1161/HYPERTENSIONAHA.122.19247. [DOI] [PubMed] [Google Scholar]
- 31.Strandberg T.E., Kovanen P.T. Coronary artery disease: ‘gout’ in the artery? Eur Heart J. 2021;42:2761–2764. doi: 10.1093/eurheartj/ehab276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo C.F., See L.C., Yu K.H., et al. Significance of serum uric acid levels on the risk of all-cause and cardiovascular mortality. Rheumatol (Oxf Engl) 2013;52:127–134. doi: 10.1093/rheumatology/kes223. [DOI] [PubMed] [Google Scholar]
- 33.Kleber M.E., Delgado G., Grammer T.B., et al. Uric acid and cardiovascular events: a Mendelian randomization study. J Am Soc Nephrol. 2015;26:2831–2838. doi: 10.1681/ASN.2014070660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K., Shi X., Zhu Z., et al. Mendelian randomization analysis of 37 clinical factors and coronary artery disease in East Asian and European populations. Genome Med. 2022;14:63. doi: 10.1186/s13073-022-01067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amdur R.L., Feldman H.I., Gupta J., et al. Inflammation and progression of CKD: the CRIC study. Clin J Am Soc Nephrol. 2016;11:1546–1556. doi: 10.2215/CJN.13121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridker P.M. Targeting interleukin-1 and interleukin-6: the time has come to aggressively address residual inflammatory risk. J Am Coll Cardiol. 2020;76:1774–1776. doi: 10.1016/j.jacc.2020.08.052. [DOI] [PubMed] [Google Scholar]
- 37.Toprover M., Shah B., Oh C., et al. Initiating guideline-concordant gout treatment improves arterial endothelial function and reduces intercritical inflammation: a prospective observational study. Arthritis Res Ther. 2020;22:169. doi: 10.1186/s13075-020-02260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammer H.B., Rollefstad S., Semb A.G., et al. Urate crystal deposition is associated with inflammatory markers and carotid artery pathology in patients with intercritical gout: results from the NOR-Gout study. RMD Open. 2022;8 doi: 10.1136/rmdopen-2022-002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kocaman S.A., Sahinarslan A., Cemri M., et al. Independent relationship of serum uric acid levels with leukocytes and coronary atherosclerotic burden. Nutr Metab Cardiovasc Dis. 2009;19:729–735. doi: 10.1016/j.numecd.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Grainger R., McLaughlin R.J., Harrison A.A., Harper J.L. Hyperuricaemia elevates circulating CCL2 levels and primes monocyte trafficking in subjects with inter-critical gout. Rheumatol (Oxf Engl) 2013;52:1018–1021. doi: 10.1093/rheumatology/kes326. [DOI] [PubMed] [Google Scholar]
- 41.Kanbay M., Huddam B., Azak A., et al. A randomized study of allopurinol on endothelial function and estimated glomerular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6:1887–1894. doi: 10.2215/CJN.11451210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridker P.M., Everett B.M., Thuren T., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 43.Ridker P.M., MacFadyen J.G., Glynn R.J., et al. Inhibition of interleukin-1beta by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol. 2018;71:2405–2414. doi: 10.1016/j.jacc.2018.03.490. [DOI] [PubMed] [Google Scholar]
- 44.Johnson R.J., Nakagawa T., Jalal D., et al. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. 2013;28:2221–2228. doi: 10.1093/ndt/gft029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang D.H., Nakagawa T., Feng L., et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 46.Kosugi T., Nakayama T., Heinig M., et al. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Ren Physiol. 2009;297:F481–F488. doi: 10.1152/ajprenal.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato Y., Feig D.I., Stack A.G., et al. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat Rev Nephrol. 2019;15:767–775. doi: 10.1038/s41581-019-0174-z. [DOI] [PubMed] [Google Scholar]
- 48.Doria A., Galecki A.T., Spino C., et al. Serum urate lowering with allopurinol and kidney function in Type 1 diabetes. N Engl J Med. 2020;382:2493–2503. doi: 10.1056/NEJMoa1916624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badve S.V., Pascoe E.M., Tiku A., et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382:2504–2513. doi: 10.1056/NEJMoa1915833. [DOI] [PubMed] [Google Scholar]
- 50.Chen Q., Wang Z., Zhou J., et al. Effect of urate-lowering therapy on cardiovascular and kidney outcomes: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2020;15:1576–1586. doi: 10.2215/CJN.05190420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mackenzie I.S., Hawkey C.J., Ford I., et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): a multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet. 2022;400:1195–1205. doi: 10.1016/S0140-6736(22)01657-9. [DOI] [PubMed] [Google Scholar]
- 52.Luo Q., Cai Y., Zhao Q., et al. Effects of allopurinol on renal function in patients with diabetes: a systematic review and meta-analysis. Ren Fail. 2022;44:806–814. doi: 10.1080/0886022X.2022.2068443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sapankaew T., Thadanipon K., Ruenroengbun N., et al. Efficacy and safety of urate-lowering agents in asymptomatic hyperuricemia: systematic review and network meta-analysis of randomized controlled trials. BMC Nephrol. 2022;23:223. doi: 10.1186/s12882-022-02850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsukamoto S., Okami N., Yamada T., et al. Prevention of kidney function decline using uric acid-lowering therapy in chronic kidney disease patients: a systematic review and network meta-analysis. Clin Rheumatol. 2022;41:911–919. doi: 10.1007/s10067-021-05956-5. [DOI] [PubMed] [Google Scholar]
- 55.Yu X., Gu M., Zhu Y., Zhang L., Kong W., Zou Y. Efficacy of urate-lowering therapy in patients with chronic kidney disease: a network meta-analysis of randomized controlled trials. Clin Ther. 2022;44:723–735.e6. doi: 10.1016/j.clinthera.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Liang X., Liu X., Li D., Qin W., Liu Y. Effectiveness of urate-lowering therapy for renal function in patients with chronic kidney disease: a meta-analysis of randomized clinical trials. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.798150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin T.C., Hung L.Y., Chen Y.C., et al. Effects of febuxostat on renal function in patients with chronic kidney disease: a systematic review and meta-analysis. Med (Baltim) 2019;98 doi: 10.1097/MD.0000000000016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng Y., Sun J. Febuxostat improves uric acid levels and renal function in patients with chronic kidney disease and hyperuricemia: a meta-analysis. Appl Bionics Biomech. 2022;2022 doi: 10.1155/2022/9704862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schepers M.S., van Ballegooijen E.S., Bangma C.H., Verkoelen C.F. Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int. 2005;68:1543–1553. doi: 10.1111/j.1523-1755.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- 60.Shiizaki K., Tsubouchi A., Miura Y., et al. Calcium phosphate microcrystals in the renal tubular fluid accelerate chronic kidney disease progression. J Clin Invest. 2021;131 doi: 10.1172/JCI145693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moe O.W. A generic crystallopathic model for chronic kidney disease progression. J Clin Invest. 2021;131 doi: 10.1172/JCI151858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakanishi N., Fukui M., Tanaka M., et al. Low urine pH Is a predictor of chronic kidney disease. Kidney Blood Press Res. 2012;35:77–81. doi: 10.1159/000330487. [DOI] [PubMed] [Google Scholar]
- 63.Bjornstad P., Maahs D.M., Roncal C.A., et al. Role of bicarbonate supplementation on urine uric acid crystals and diabetic tubulopathy in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20:1776–1780. doi: 10.1111/dom.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wesseling C., Aragon A., Gonzalez M., et al. Heat stress, hydration and uric acid: a cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roncal-Jimenez C., Garcia-Trabanino R., Barregard L., et al. Heat stress nephropathy from exercise-induced uric acid crystalluria: a perspective on Mesoamerican nephropathy. Am J Kidney Dis. 2016;67:20–30. doi: 10.1053/j.ajkd.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 66.Vanholder R., Sever M.S., Erek E., Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553–1561. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez-Lozada L.G., Garcia-Arroyo F.E., Gonzaga G., et al. Kidney injury from recurrent heat stress and rhabdomyolysis: protective role of allopurinol and sodium bicarbonate. Am J Nephrol. 2018;48:339–348. doi: 10.1159/000494663. [DOI] [PubMed] [Google Scholar]
- 68.Gois P.H., Canale D., Volpini R.A., et al. Allopurinol attenuates rhabdomyolysis-associated acute kidney injury: renal and muscular protection. Free Radic Biol Med. 2016;101:176–189. doi: 10.1016/j.freeradbiomed.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 69.Ohashi Y., Kuriyama S., Nakano T., et al. Urate transporter ABCG2 function and asymptomatic hyperuricemia: a retrospective cohort study of CKD progression. Am J Kidney Dis. Forthcoming. 2022 doi: 10.1053/j.ajkd.2022.05.010. [DOI] [PubMed] [Google Scholar]
- 70.Ichida K., Matsuo H., Takada T., et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun. 2012;3:764. doi: 10.1038/ncomms1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson R.J. Intestinal hyperuricemia as a driving mechanism for CKD. Am J Kidney Dis. Forthcoming. 2022 doi: 10.1053/j.ajkd.2022.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Kaneko C., Ogura J., Sasaki S., et al. Fructose suppresses uric acid excretion to the intestinal lumen as a result of the induction of oxidative stress by NADPH oxidase activation. Biochim Biophys Acta Gen Subj. 2017;1861:559–566. doi: 10.1016/j.bbagen.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 73.Sahebjami H., Scalettar R. Effects of fructose infusion on lactate and uric acid metabolism. Lancet. 1971;1:366–369. doi: 10.1016/s0140-6736(71)92208-2. [DOI] [PubMed] [Google Scholar]
- 74.Johnson R.J., Perez-Pozo S.E., Lillo J.L., et al. Fructose increases risk for kidney stones: potential role in metabolic syndrome and heat stress. BMC Nephrol. 2018;19:315. doi: 10.1186/s12882-018-1105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson R.J., Stenvinkel P., Andrews P., et al. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J Intern Med. 2020;287:252–262. doi: 10.1111/joim.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimizu T., Kitada H., Umeyama M., et al. Novel evaluation of nephrolithiasis as a complication of gout: a cross-sectional study using helical computerized tomography. J Urol. 2013;189:1747–1752. doi: 10.1016/j.juro.2012.11.076. [DOI] [PubMed] [Google Scholar]
- 77.Hosoya T., Uchida S., Shibata S., et al. Xanthine oxidoreductase inhibitors suppress the onset of exercise-induced AKI in high HPRT activity Urat1-Uox double knockout mice. J Am Soc Nephrol. 2022;33:326–341. doi: 10.1681/ASN.2021050616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kikuchi Y., Koga H., Yasutomo Y., et al. Patients with renal hypouricemia with exercise-induced acute renal failure and chronic renal dysfunction. Clin Nephrol. 2000;53:467–472. [PubMed] [Google Scholar]
- 79.Bhasin B., Stiburkova B., De Castro-Pretelt M., et al. Hereditary renal hypouricemia: a new role for allopurinol? Am J Med. 2014;127:e3–e4. doi: 10.1016/j.amjmed.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 80.Lanaspa M.A., Sanchez-Lozada L.G., Choi Y.J., et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han M., Park H.C., Kim H., et al. Hyperuricemia and deterioration of renal function in autosomal dominant polycystic kidney disease. BMC Nephrol. 2014;15:63. doi: 10.1186/1471-2369-15-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Helal I., McFann K., Reed B., et al. Serum uric acid, kidney volume and progression in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant. 2013;28:380–385. doi: 10.1093/ndt/gfs417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu M.A., Sanchez-Lozada L.G., Johnson R.J., Kang D.H. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28:1234–1242. doi: 10.1097/HJH.0b013e328337da1d. [DOI] [PubMed] [Google Scholar]
- 84.Sanchez-Lozada L.G., Lanaspa M.A., Cristobal-Garcia M., et al. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121:e71–e78. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi Y.J., Yoon Y., Lee K.Y., et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014;28:3197–3204. doi: 10.1096/fj.13-247148. [DOI] [PubMed] [Google Scholar]
- 86.Kanbay M., Siriopol D., Nistor I., et al. Effects of allopurinol on endothelial dysfunction: a meta-analysis. Am J Nephrol. 2014;39:348–356. doi: 10.1159/000360609. [DOI] [PubMed] [Google Scholar]
- 87.Kang D.H., Han L., Ouyang X., et al. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am J Nephrol. 2005;25:425–433. doi: 10.1159/000087713. [DOI] [PubMed] [Google Scholar]
- 88.Price K.L., Sautin Y.Y., Long D.A., et al. Human vascular smooth muscle cells express a urate transporter. J Am Soc Nephrol. 2006;17:1791–1795. doi: 10.1681/ASN.2006030264. [DOI] [PubMed] [Google Scholar]
- 89.Kanellis J., Watanabe S., Li J.H., et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 90.Kang D.H., Park S.K., Lee I.K., Johnson R.J. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 91.Myllymaki J., Honkanen T., Syrjanen J., et al. Uric acid correlates with the severity of histopathological parameters in IgA nephropathy. Nephrol Dial Transplant. 2005;20:89–95. doi: 10.1093/ndt/gfh584. [DOI] [PubMed] [Google Scholar]
- 92.Sanchez-Lozada L.G., Tapia E., Santamaria J., et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67:237–247. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 93.Uedono H., Tsuda A., Ishimura E., et al. Relationship between serum uric acid levels and intrarenal hemodynamic parameters. Kidney Blood Press Res. 2015;40:315–322. doi: 10.1159/000368507. [DOI] [PubMed] [Google Scholar]
- 94.Gondouin B., Jourde-Chiche N., Sallee M., et al. Plasma xanthine oxidase activity is predictive of cardiovascular disease in patients with chronic kidney disease, independently of uric acid levels. Nephron. 2015;131:167–174. doi: 10.1159/000441091. [DOI] [PubMed] [Google Scholar]
- 95.Furuhashi M., Matsumoto M., Tanaka M., et al. Plasma xanthine oxidoreductase activity as a novel biomarker of metabolic disorders in a general population. Circ J. 2018;82:1892–1899. doi: 10.1253/circj.CJ-18-0082. [DOI] [PubMed] [Google Scholar]
- 96.Furuhashi M., Mori K., Tanaka M., et al. Unexpected high plasma xanthine oxidoreductase activity in female subjects with low levels of uric acid. Endocr J. 2018;65:1083–1092. doi: 10.1507/endocrj.EJ18-0127. [DOI] [PubMed] [Google Scholar]
- 97.Ames B.N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patricio E.S., Prado F.M., da Silva R.P., et al. Chemical characterization of urate hydroperoxide, a pro-oxidant intermediate generated by urate oxidation in inflammatory and photoinduced processes. Chem Res Toxicol. 2015;28:1556–1566. doi: 10.1021/acs.chemrestox.5b00132. [DOI] [PubMed] [Google Scholar]
- 99.Imaram W., Gersch C., Kim K.M., et al. Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry. Free Radic Biol Med. 2010;49:275–281. doi: 10.1016/j.freeradbiomed.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carvalho L.A.C., Lopes J., Kaihami G.H., et al. Uric acid disrupts hypochlorous acid production and the bactericidal activity of HL-60 cells. Redox Biol. 2018;16:179–188. doi: 10.1016/j.redox.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kono H., Chen C.J., Ontiveros F., Rock K.L. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120:1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi Y. Caught red-handed: uric acid is an agent of inflammation. J Clin Invest. 2010;120:1809–1811. doi: 10.1172/JCI43132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma Q., Honarpisheh M., Li C., et al. Soluble uric acid is an intrinsic negative regulator of monocyte activation in monosodium urate crystal-induced tissue inflammation. J Immunol. 2020;205:789–800. doi: 10.4049/jimmunol.2000319. [DOI] [PubMed] [Google Scholar]
- 104.Gnemmi V., Li Q., Ma Q., et al. Asymptomatic hyperuricemia promotes recovery from ischemic organ injury by modulating the phenotype of macrophages. Cells. 2022;11:626. doi: 10.3390/cells11040626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Junqueira S.C., Dos Santos Coelho I., Lieberknecht V., et al. Inosine, an endogenous purine nucleoside, suppresses immune responses and protects mice from experimental autoimmune encephalomyelitis: a role for A2A adenosine receptor. Mol Neurobiol. 2017;54:3271–3285. doi: 10.1007/s12035-016-9893-3. [DOI] [PubMed] [Google Scholar]
- 106.da Rocha Lapa F., de Oliveira A.P., Accetturi B.G., et al. Anti-inflammatory effects of inosine in allergic lung inflammation in mice: evidence for the participation of adenosine A2A and A3 receptors. Purinergic Signal. 2013;9:325–336. doi: 10.1007/s11302-013-9351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hasko G., Sitkovsky M.V., Szabo C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25:152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 108.Johnson T.A., Jinnah H.A., Kamatani N. Shortage of cellular ATP as a cause of diseases and strategies to enhance ATP. Front Pharmacol. 2019;10:98. doi: 10.3389/fphar.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kamatani N., Kushiyama A., Toyo-Oka L., et al. Treatment of two mitochondrial disease patients with a combination of febuxostat and inosine that enhances cellular ATP. J Hum Genet. 2019;64:351–353. doi: 10.1038/s10038-018-0558-0. [DOI] [PubMed] [Google Scholar]
- 110.Watanabe H., Hattori T., Kume A., et al. Improved Parkinsons disease motor score in a single-arm open-label trial of febuxostat and inosine. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.White W.B., Saag K.G., Becker M.A., et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378:1200–1210. doi: 10.1056/NEJMoa1710895. [DOI] [PubMed] [Google Scholar]
- 112.Deng H., Zhang B.L., Tong J.D., et al. Febuxostat use and risks of cardiovascular disease events, cardiac death, and all-cause mortality: metaanalysis of randomized controlled trials. J Rheumatol. 2021;48:1082–1089. doi: 10.3899/jrheum.200307. [DOI] [PubMed] [Google Scholar]
- 113.Dubreuil M., Zhu Y., Zhang Y., et al. Allopurinol initiation and all-cause mortality in the general population. Ann Rheum Dis. 2015;74:1368–1372. doi: 10.1136/annrheumdis-2014-205269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wei J., Choi H.K., Neogi T., et al. Allopurinol initiation and all-cause mortality among patients with gout and concurrent chronic kidney disease: a population-based cohort study. Ann Intern Med. 2022;175:461–470. doi: 10.7326/M21-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pfister R., Barnes D., Luben R., et al. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia. 2011;54:2561–2569. doi: 10.1007/s00125-011-2235-0. [DOI] [PubMed] [Google Scholar]
- 116.Jordan D.M., Choi H.K., Verbanck M., et al. No causal effects of serum urate levels on the risk of chronic kidney disease: a Mendelian randomization study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang Q., Kottgen A., Dehghan A., et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3:523–530. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tin A., Marten J., Halperin Kuhns V.L., et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat Genet. 2019;51:1459–1474. doi: 10.1038/s41588-019-0504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gill D., Cameron A.C., Burgess S., et al. Urate, blood pressure, and cardiovascular disease: evidence from Mendelian randomization and meta-analysis of clinical trials. Hypertension. 2021;77:383–392. doi: 10.1161/HYPERTENSIONAHA.120.16547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lanaspa M.A., Kuwabara M., Andres-Hernando A., et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci U S A. 2018;115:3138–3143. doi: 10.1073/pnas.1713837115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.DeBosch B.J., Kluth O., Fujiwara H., et al. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat Commun. 2014;5:4642. doi: 10.1038/ncomms5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dinour D., Gray N.K., Campbell S., et al. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J Am Soc Nephrol. 2010;21:64–72. doi: 10.1681/ASN.2009040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Watanabe S., Kang D.H., Feng L., et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40:355–360. doi: 10.1161/01.hyp.0000028589.66335. aa. [DOI] [PubMed] [Google Scholar]
- 124.Sanchez-Lozada L.G., Tapia E., Johnson R.J., et al. Glomerular hemodynamic changes associated with arteriolar lesions and tubulointerstitial inflammation. Kidney Int Suppl. 2003;(86):S9–S14. doi: 10.1046/j.1523-1755.64.s86.3.x. [DOI] [PubMed] [Google Scholar]
- 125.Roncal-Jimenez C.A., Lanaspa M.A., Rivard C.J., et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism. 2011;60:1259–1270. doi: 10.1016/j.metabol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cristobal-Garcia M., Garcia-Arroyo F.E., Tapia E., et al. Renal oxidative stress induced by long-term hyperuricemia alters mitochondrial function and maintains systemic hypertension. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/535686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu P., Chen Y., Wang B., et al. Allopurinol treatment improves renal function in patients with type 2 diabetes and asymptomatic hyperuricemia: 3-year randomized parallel-controlled study. Clin Endocrinol (Oxf) 2015;83:475–482. doi: 10.1111/cen.12673. [DOI] [PubMed] [Google Scholar]