Abstract
Introduction
Baricitinib, a Janus kinase (JAK) 1/2 inhibitor, is an approved treatment for rheumatoid arthritis (RA), atopic dermatitis (AD), and alopecia areata (AA). Further characterisation of adverse events of special interest (AESI) for JAK inhibitors in at-risk populations will improve benefit–risk assessment for individual patients and diseases.
Methods
Data were pooled from clinical trials and long-term extensions in moderate-to-severe active RA, moderate-to-severe AD, and severe AA. Incidence rates (IR) per 100 patient-years of major adverse cardiovascular event (MACE), malignancy, venous thromboembolism (VTE), serious infection, and mortality were calculated for patients with low risk (younger than 65 years with no specified risk factors), and patients at risk (≥ 1 of: aged 65 years or older, atherosclerotic cardiovascular disease, diabetes mellitus, hypertension, current smoking, HDL cholesterol < 40 mg/dL, BMI ≥ 30 kg/m2, poor mobility on EQ-5D, or history of malignancy).
Results
Datasets included baricitinib exposure up to 9.3 years with 14,744 person-years of exposure (PYE) (RA), 3.9 years with 4628 PYE (AD), and 3.1 years with 1868 PYE (AA). In patients with low risk (RA: 31%, AD: 48%, AA: 54%), IRs for MACE (0.05, 0.04, 0), malignancies (0.20, 0.13, 0), VTE (0.09, 0.04, 0), serious infection (1.73, 1.18, 0.57), and mortality (0.04, 0, 0) in the RA, AD, and AA datasets, respectively, were low. In patients at risk (RA: 69%, AD: 52%, AA: 46%), IRs were for MACE (0.70, 0.25, 0.12), malignancies (1.23, 0.45, 0.35), VTE (0.66, 0.08, 0.12), serious infection (2.95, 2.30, 1.17), and mortality (0.78, 0.16, 0) for RA, AD, and AA datasets, respectively.
Conclusion
Populations with low risk have low incidence of the examined JAK inhibitor-related AESI. In the dermatologic indications, incidence is also low for patients at risk. Considering individual disease burden, risk factors, and response to treatment is relevant to make informed decisions for individual patients treated with baricitinib.
Keywords: Adverse events, Baricitinib, JAK inhibitors, MACE, Malignancy, Mortality, Safety, Serious infection, VTE
Key Summary Points
| Why carry out this study? |
| Janus kinase (JAK) inhibitors have been linked to a possible class effect associated with particular adverse events, such as major adverse cardiovascular event (MACE), venous thromboembolism (VTE), and malignancy. |
| Further characterisation of adverse events of special interest (AESI) for JAK inhibitors in at-risk populations will improve benefit–risk assessment for individual patients and diseases. |
| This analysis examines AESI in the baricitinib clinical programmes for rheumatoid arthritis, atopic dermatitis, and alopecia areata for patients with low risk (younger than 65 years with no specified risk factors), and patients at risk (at least one of: aged 65 years or older, atherosclerotic cardiovascular disease, diabetes mellitus, hypertension, current smoking, HDL cholesterol <40 mg/dL, BMI ≥ 30 kg/m2, poor mobility on EQ-5D, or history of malignancy). |
| What was learned from this study? |
| Incidence of AESI in patients treated with baricitinib who were younger than 65 years without risk factors were reduced compared with the population of patients with risk factors for all indications, and the number of AESI is also minimal for patients with atopic dermatitis and alopecia areata who have risk factors. |
| Individual disease burden, risk factors, and response to treatment should be considered to make informed decisions for individual patients treated with baricitinib. |
Introduction
Patients with chronic, systemic, immune-mediated inflammatory conditions have elevated rates of other comorbidities that increase over time with longer disease duration and in cases of refractory disease. Rheumatoid arthritis (RA) is recognised as an independent risk factor for major adverse cardiovascular events (MACE) [1], and has been associated with venous thromboembolism (VTE) [2], certain types of malignancy (lymphoma, lung cancer) [3], and infections [4] among other comorbidities. For patients with atopic dermatitis (AD), the most common comorbidities include other atopic disorders, such as asthma or allergic rhinitis [5], depression and anxiety [5, 6], and AD patients are more prone to infections, including serious infections [7, 8]. AD is associated with a moderate increase in MACE, which may be associated with comorbidities and lifestyle factors or disease severity [9], and has also been linked to an upregulation of atherosclerosis and cardiovascular risk proteins [10, 11]. While an increased risk has been described for VTE in moderate-to-severe AD [12–14], this was not consistently reported for malignancies, except lymphoma [15]. Common comorbidities in alopecia areata (AA) include atopic disorders, depression, anxiety, overweight/obesity, and thyroid disease [16, 17]. Risk of MACE and VTE are not increased in AA [13, 18], but the risks of infection and malignancy are uncertain [19–21].
Post-marketing safety studies are important to better characterise and establish the safety profile of medications. There have been few post-marketing safety studies for anti-inflammatory medications following the uncovering of possible adverse events associated with certain treatments, such as tuberculosis, malignancies [non-melanoma skin cancer (NMSC)], congestive cardiac failure, or demyelinating disease for tumour necrosis factor-α inhibitors (TNFi) [22–24] or diverticulitis, gastrointestinal perforations, and cardiovascular events with the interleukin 6 receptor antagonist tocilizumab [25, 26]. JAK inhibitors (JAKi) have recently been in the spotlight due to a possible class effect associated with particular adverse events, such as MACE, VTE, and malignancy. However, lack of controlled comparative data has led to clinical difficulty in making relative benefit–risk assessments among alternative treatment options with different mechanisms of action.
Baricitinib, an oral selective JAK 1/2 inhibitor, is approved for the treatment of adults with moderately-to-severely active RA, moderate-to-severe AD, severe AA, and, in the United States, hospitalised patients with coronavirus disease 2019 (COVID-19). This report complements the recently published cross-indication safety review of baricitinib [28] by expanding on the safety of baricitinib for adverse events of special interest (AESI) for JAKi in populations with risk factors for these AESI, using integrated clinical trial datasets for the approved systemic inflammatory indications of RA, AD, and AA. These safety events, including MACE, VTE, malignancy, serious infections, and mortality, were investigated for patients aged 65 years or older, or with at least one of eight specified risk factors: history of atherosclerotic cardiovascular disease (ASCVD), hypertension, diabetes mellitus, current smoking, high-density lipoprotein (HDL) cholesterol less than 40 mg/dL, history of malignancy, body mass index (BMI) 30 kg/m2 or greater, and severe mobility impairment on the EuroQol-5 Dimension (EQ-5D).
Methods
Data were analysed from the integrated RA [29], AD [30], and AA [31] baricitinib trials, which make up the regulatory submission package for the clinical development programmes sponsored by Eli Lilly and Company, to yield the largest possible dataset of patients with reliable data of exposure to baricitinib to give the best guidance for future patients. These analysis sets are not placebo-controlled but provide reliable estimates for adverse event incidence within the baricitinib clinical programme, which is particularly relevant for less common event types.
RA Analysis Set
Safety data from nine randomised clinical trials [Phase 1b: I4V-MC-JADB; Phase 2: NCT01185353, NCT00902486; NCT01469013 (Japan); Phase 3: NCT01710358 (RA-BEAM), NCT01721044 (RA-BEACON), NCT01721057 (RA-BUILD), NCT01711359 (RA-BEGIN), NCT02265705 (RA-BALANCE)] and one completed long-term extension (LTE) trial [NCT01885078 (RA-BEYOND)] running from May 2009–November 2020 were pooled. The all-bari-RA analysis set includes data of all patients who received at least one dose of baricitinib (doses ranged from 1 to 15 mg daily, with 2 mg and 4 mg daily doses used in the phase 3 and long-term extension trials) using all available data after the first dose without censoring for rescue or dose change.
AD Analysis Set
Safety data are included from six double-blinded, randomised clinical studies [Phase 2: NCT02576938; Phase 3: NCT03334396 (BREEZE-AD1), NCT03334422 (BREEZE-AD2), NCT03428100 (BREEZE-AD4), NCT03435081 (BREEZE-AD5), NCT03733301 (BREEZE-AD7)], one double-blinded, randomised LTE [NCT03334435 (BREEZE-AD3)], and one open-label LTE [NCT03559270 (BREEZE-AD6)], with completed studies running from November 2017–August 2019 and data cut-offs of 15 December 2021 for BREEZE-AD4, and 3 November 2021 (BREEZE-AD3) and 21 December 2021 (BREEZE-AD6) in the ongoing LTE studies. The all-bari-AD analysis set includes data for all patients who received at least one dose of baricitinib (1 mg, 2 mg, or 4 mg) from any of the eight clinical trials at any time.
AA Analysis Set
Safety data are included from Phase 2 and Phase 3 cohorts of BRAVE-AA1 (NCT03570749) and Phase 3 BRAVE-AA2 (NCT03899259), with data cut-offs of 11 November 2021 and 5 November 2021, respectively. The all-bari-AA analysis set combines all patients exposed to any baricitinib dose (1 mg, 2 mg, or 4 mg) at any time up to the data cut-off.
Compliance with Ethics Guidelines
All studies were conducted in accordance with ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and the research protocols were approved by each centre’s institutional review board or ethics committee. All patients provided written informed consent.
Statistical Analysis
Each analysis set was reviewed for AESI, defined as MACE [positively adjudicated events of myocardial infarction (MI), stroke and cardiovascular deaths combined), MI separately, malignancies (excluding NMSC), VTE (deep vein thrombosis (DVT) or pulmonary embolism (PE)], serious infections, and mortality.
For AESI, the incidence rate (IR) was calculated as standard (number of patients with an event per 100 patient-years at risk (PYR)], including follow-up time censored at event onset date. Poisson distribution was used to calculate 95% CI.
The IR for AESI was evaluated in subgroups of patients aged over 65 years or with at least one of eight specified risk factors [history of ASCVD, hypertension, diabetes mellitus, current smoking, HDL cholesterol less than 40 mg/dL, history of malignancy, BMI 30 kg/m2 or greater, and severe mobility impairment on EQ-5D (i.e., responding either ‘I have severe problems in walking about’ or ‘I am unable to walk about’)] and patients aged below 65 years without any of the specified risk factors. Past smoking was not systematically documented in all studies but was checked for in case histories of patients with an event. This IR was calculated as 100 times the number of patients experiencing the event divided by PYR (exposure time up to the event for patients with the AESI and exposure time up to the end of the exposure and 1-month follow-up for patients without the AESI) in years in the subgroup of the specific factor. To account for ageing of the cohort, the number of expected malignancies (excluding NMSC) were calculated using age-specific malignancy data from the Surveillance, Epidemiology, and End Results 17 (SEER17), 2013–2017 US population cancer rates [32] and compared to the number of observed malignancies in the baricitinib clinical programme.
The baricitinib clinical trials for RA did not exclude patients with any of the above-mentioned risk factors, except for recent comorbidities such as MI or stroke within 12 weeks, active or recent (within 30 days) serious infection, or malignant disease [33]. Clinical trials studying baricitinib in AD and AA also excluded patients with MI, stroke, or VTE events within 12 weeks of screening, active or recent (within 30 days) serious infection, or malignant disease, as well as those who had history of, or are considered at high risk for, VTE [34, 35].
Results
In clinical trials for RA, AD, and AA, 7709 patients received baricitinib and the data include patients with long-term baricitinib exposure (Table 1). In the RA dataset, the mean age of patients at baseline was 53 years and exposure lasted up to 9.3 years, with a median exposure of 4.6 years, for a total of 14,744 person-years of exposure (PYE) [29]. The AD dataset includes exposure up to 3.9 years with a median exposure of 1.6 years, for a total of 4628 PYE with a mean age of patients at baseline of 37 years. As the most recently-approved indication for baricitinib, the AA dataset includes a limited follow-up period (median 1.5 years, maximum 3.1 years), for a total of 1868 PYE with mean age of patients at baseline of 38 years [31]. The proportion of each population with each specified risk factor at baseline is given in Table 1.
Table 1.
Proportion of patients with each of the specified risk factors at baseline from RCT
| RA N = 3770, PYR = 14,744 |
AD N = 2636, PYR = 4628 |
AA N = 1303, PYR = 1868 |
|
|---|---|---|---|
| ASCVD | 119 (3.2) | 25 (0.9) | 12 (0.9) |
| Current smoker | 603 (16.0) | 634 (24.1) | 222 (17.0) |
| Hypertension | 1348 (35.8) | 349 (13.2) | 145 (11.1) |
| HDL < 40 mg/dL | 308 (8.2) | 360 (13.7) | 116 (8.9) |
| Diabetes mellitus | 335 (8.9) | 71 (2.7) | 40 (3.1) |
| ≥ 65 yearsa | 632 (16.8) | 101 (3.8) | 33 (2.5) |
| BMI ≥ 30 kg/m2 | 1100 (29.2) | 496 (18.8) | 265 (20.4) |
| History of malignancy | 52 (1.4) | 27 (1.0) | 17 (1.3) |
| Severe mobility impairment (EQ-5D)b | 468 (12.4) | 51 (1.9) | 4 (0.3) |
| Any of 9 risk factorsc | 2619 (69.5) | 1373 (52.1) | 599 (46.0) |
Data are n (%)
AA alopecia areata; AD atopic dermatitis; ASCVD atherosclerotic cardiovascular disease; BMI body mass index; CV cardiovascular; EQ-5D EuroQol-5 dimension; HDL high-density lipoprotein; MACE major adverse cerebrocardiovascular event; MI myocardial infarction; N number of patients in analysis population; n number of patients in the specified category; PYR patient-years of exposure for risk; RA rheumatoid arthritis
aThe age of participants in AA clinical trials was limited to 60 years or younger for males and 70 years or younger for females to reduce concomitant androgenic alopecia
bSevere mobility impairment indicated by a response of either ‘I have severe problems in walking about’ or ‘I am unable to walk about’
cASCVD, current or past smoking (past smoking was not systematically documented in all trials), hypertension, HDL cholesterol < 40 mg/dL, diabetes mellitus, age 65 years or older, BMI 30 kg/m2, history of malignancy, and severe mobility impairment at EQ-5D baseline
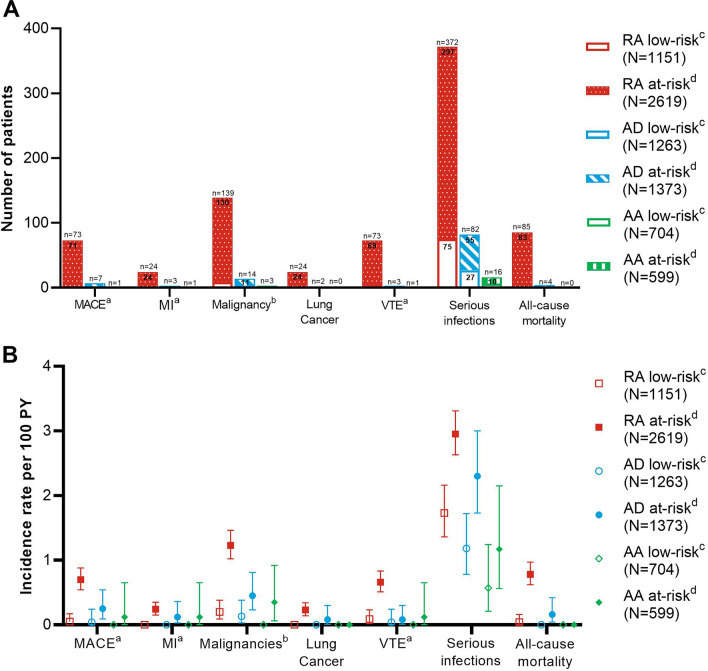
Across indications, in patients younger than 65 years without one of the eight pre-specified risk factors, (low-risk) data show low IRs of MACE, MI, lung cancer, VTE, and overall mortality relative to the literature-reported incidence in these patient populations, and a reduced incidence of overall malignancy and serious infections, compared with the at-risk population (Fig. 1). For AD and AA populations, the incidence of AESI is low irrespective of the presence of risk factors.
Fig. 1.
Overview of occurrence of events of special interest in patients younger than 65 years without risk factors (low-risk) versus patients above 65 years and at least one pre-specified risk factor (at-risk) in integrated baricitinib clinical study datasets. A Number of patients in each risk group recording events of interest. B Incidence rate of events of interest per 100 patient-years of exposure for risk in each risk group. Abbreviations: AA alopecia areata; AD atopic dermatitis; BMI body mass index; CI confidence interval; EQ-5D EuroQol-5 dimension; HDL high-density lipoprotein; MACE major adverse cardiovascular event; MI myocardial infarction; N number of patients in the safety analysis set; n number of patients in the specified category; PYE patient-years of exposure; RA rheumatoid arthritis; VTE venous thromboembolic event. aFor MACE and MI in all-bari-RA: patients with low risk, n = 909; patients at-risk, n = 2342. For MACE, MI, and VTE in all-bari-AD: patients with low risk, n = 1231; patients at-risk, n = 1330. bExcluding non-melanoma skin cancer. cPatients without risk factors and < 65 years. dPatients ≥ 65 years or with at least one of the following risk factors: atherosclerotic cardiovascular disease, diabetes mellitus, hypertension, current smoking (past smoking was not systematically documented in all trials), HDL < 40 mg/dL, BMI ≥ 30 kg/m2, poor mobility on EQ-5D, and history of malignancy. Also including factors only documented in case narrative, such as past smoking
Approximately 31% (n = 1151) of the all-bari-RA dataset were younger than 65 years and did not have any of the specified risk factors (low-risk). Very few patients from this low-risk group had an AESI. There were 2 MACE events (0.2%, IR = 0.05), both were haemorrhagic strokes, and no MI (Fig. 1). VTE was recorded in 4 patients (0.3%, IR = 0.09); all 4 had a DVT and none had a fatal outcome. Serious infection was reported in 75 low-risk patients (6.5%, IR = 1.73) compared with 372 patients (9.9%, IR = 2.58) in the overall study population (Fig. 1). Mortality was observed in 2 patients (0.2%, IR = 0.04) (Fig. 1).
For the 69% (n = 2619) of patients in the all-bari-RA dataset who were ≥ 65 years or with at least one risk factor (at-risk), the IRs of AESI were higher than for the overall all-bari-RA population (Fig. 1). In this at-risk group, 71 patients (IR = 0.70) recorded a MACE event, 24 of whom were ≥ 65 years (IR = 1.19). A total of 69 patients (IR = 0.66) had VTE, with 87 VTEs recorded in total (39 PE and 48 DVT). Of the patients recording VTE, 46 had BMI ≥ 30 kg/m2 (IR = 1.07). There were 130 patients (IR = 1.23) with malignancies (including respiratory, breast, gastrointestinal, reproductive, skin, renal and urinary, lymphomas, and endocrine neoplasms), 55 of whom were smokers (IR = 2.01). Serious infection was reported in 297 patients (IR = 2.95), 113 of whom were ≥ 65 years (IR = 5.49). Mortality was observed in 83 patients (IR = 0.78), 44 of whom were ≥ 65 years (IR = 1.97). The majority (> 90%) of AESI were observed in patients with at least one risk factor, but many patients with an event (60–70%) had two or more risk factors. The most common risk factors in this group were hypertension and BMI ≥ 30 kg/m2. Details on the safety in the all-bari-RA population have been previously published [29].
Approximately 48% (n = 1263) of the all-bari-AD dataset had low risk (Fig. 1). The IR for AESI in this population were in general lower compared to RA, reflecting different baseline risk [28]. In this low-risk population, 1 patient (0.1%, IR = 0.04) reported MACE: a female patient of 39 years who had a haemorrhagic stroke due to a ruptured cerebral aneurysm and hence was not related to a thromboembolic process. Malignancy was reported for 3 patients (0.2%, IR = 0.13), 2 of whom had lymphoma and 1 had testis cancer. Serious infection was reported in 27 low-risk patients (2.1%, IR = 1.18) compared with 82 patients (3.1%, IR = 1.8) in the all-bari-AD population (Fig. 1). There were no deaths in the low-risk AD patient group.
In the at-risk AD population (52%, n = 1373), 6 patients (IR = 0.25) reported MACE, all of whom presented at least one predefined risk factor (Fig. 1). The 3 patients who had an MI (IR = 0.06) all had cardiovascular risk factors at the start of baricitinib treatment. Malignancy was reported in 11 patients at risk (0.8%, IR = 0.45), 5 of whom were 65 years or older, were current smokers, or both, while the remaining 6 patients had another of the specified risk factors. Two of these patients at risk had lung cancer; both were older than 65 years and were current or past smokers. A total of 55 of the 82 (67.1%) cases of serious infection in the all-bari-AD dataset were reported in patients at risk, and included infections associated with AD, such as cellulitis, eczema herpeticum, and erysipelas, as well as pneumonia, sepsis, and COVID-19. None of the infections were fatal. The 4 deaths (IR = 0.16) reported in the all-bari-AD dataset were patients at risk who had presented with at least one of the predefined risk factors. Two of the 4 patients were at least 65 years of age, 2 had a history of hypertension, and 3 were current smokers.
In the all-bari-AD dataset, 3 of 2561 patients (0.1%, IR = 0.06) reported a VTE, all of which were PE. All patients were younger than 65 years and had none of the predefined risk factors. However, both female patients who had a VTE were taking hormonal contraception (as were approximately 30% of female patients in the all-bari-AD dataset that did not have VTEs). These patients also had other risk factors, including 1 past smoker and 1 with no specified risk factor but with family history of coagulation disorder. There were no fatal VTEs. The majority (67–100%) of AESI were observed in patients with at least one risk factor, but many patients with an event (31–75%) had two or more risk factors. The most common risk factors in this group were smoking and BMI ≥ 30 kg/m2. Even among this at-risk population, the IRs fall within, or below, the expected range for patients with AD.
In the all-bari-AA dataset, approximately 54% (n = 704) of the population is low risk for AESI (Fig. 1). There were 6 cases of serious infection reported in this group (IR = 0.57). No other AESI were reported.
The majority of the AESI in the all-bari-AA dataset were reported for patients at risk (46%, n = 599). The single patient with MACE (MI) (IR = 0.12) had multiple cardiovascular risk factors at the start of the baricitinib treatment. All the patients with malignancy (excluding NMSC) had at least one risk factor; there was no patient with lung cancer. The patient with VTE (IR = 0.12) had BMI ≥ 30 kg/m2 and experienced a PE temporally associated with COVID-19 pneumonia; coagulation tests revealed a prothrombin gene heterozygosity that may impart an approximately threefold increased risk of VTE. The IR of serious infections (0.8) was clearly lower than in RA (2.6) and AD (1.8), and there were no deaths. The most common risk factors in this at-risk population were BMI ≥ 30 kg/m2 and smoking. Even among this at-risk population, the IRs fall within, or below, the expected range for a population in this age category. Details on the safety in the all-bari-AA population have been previously published [31].
Discussion
In this analysis of data from the baricitinib clinical trial programme for RA, AD, and AA, patients who were younger than 65 years and without any of the risk factors pre-specified had relatively low incidence of the AESI examined, particularly compared with the incidence reported in literature for these patient populations. In the dermatologic indications, the incidence of AESI is also low for patients aged 65 years or older or with another of the eight specified risk factors; however, the incidence of AESI for patients at risk with RA is increased in comparison with the patients with low risk.
Patients with inflammatory diseases such as RA often have many comorbidities. Systemic inflammation with increased proinflammatory mediators and endothelium damage leads to accelerated atherosclerosis in RA and to some degree for AD [11, 36]. Endothelial injury and hypercoagulability due to chronic inflammation contributes to double risk of VTE in RA when compared with age- and sex-matched controls [37]. Early and adequate treatment of inflammatory conditions can lower the incidence of these comorbidities by halting systemic inflammation. In a randomised trial, a treat-to-target approach of patients with RA and cardiovascular disease risk factors led to reduced subclinical and clinical atherosclerosis compared to usual care of cardiovascular disease [38], and can also reduce the burden of associated comorbidities and normalise survival [39]. Specifically for cardiovascular risk, long-lasting disease, seropositivity, and presence of extra-articular manifestations multiply independent risk of MACE by 1.5-fold [40]. Risk of VTE has been associated with RA disease activity, and in ORAL Surveillance, the risks of MACE, VTE, and non-serious infections excluding herpes zoster were higher in patients with active disease than when in remission, confirming that disease control is critical to prevent adverse events [27]. Among patients with RA, the risk (and risk ratio) increased with increasing RA disease activity, from 0.52% following visits in DAS28 ESR remission to 1.08% in another study following visits with DAS28 ESR high disease activity [41].
However, different anti-inflammatory agents and immune modulators may be linked with different AESI. Many efforts have been made to characterise the risk of AEs with different treatments through both clinical trials and observational studies. The recent randomised study of patients with RA aged 50 years or older enriched for cardiovascular risk factors, ORAL Surveillance, reported a small increased risk of MACE and malignancy, which did not meet non-inferiority criteria, as well as higher incidences of serious infection, VTE, and all-cause mortality in patients being treated with tofacitinib compared with TNFi-treated patients [27]. This trend towards increase in MACE, although not significant, was also observed in a similar population of patients aged 50 years or older with cardiovascular risk factors receiving tofacitinib compared with TNFi analysed from routine care data, but not in the overall RA population treated with tofacitinib in routine care compared with those who received TNFi [42]. The AESI for JAKi, mainly MACEs, VTEs, and malignancies, are most predominant in the RA clinical programme, although these are still uncommon in general, particularly in patients with no risk factors.
MACE in the Baricitinib RA Clinical Programme
In the baricitinib long-term clinical trial dataset, over 60% of patients with RA treated with baricitinib who recorded AESI had at least two risk factors, the most common of which were hypertension and BMI of at least 30 kg/m2. These risk factors are not only easily identifiable in this patient population but can be managed to minimise the risk of AESI in these patients. The proportion of patients with RA at risk, specifically those with long-established disease, was higher than for AD and AA, where the AESI were much lower for both the low-risk and at-risk populations.
The IR of MACE in the baricitinib clinical data for the at-risk RA subpopulation of ≥ 65 years old with at least one risk factor was 0.70 per 100 PY and much lower for the RA low-risk population, 0.05 per 100 PY. Both fall within the expected IR of MACE in patients with RA reported in real-world settings (0.27–3.2 per 100 PY) [28]. A retrospective observational study pooling data from disease registries and claims databases, with the French SNDS and Swedish ARTIS data sources contributing the most patients, has investigated the safety of baricitinib compared with TNFi in a matched RA population based on propensity scores for confounding factors. The study showed a numerically, but not significantly, greater risk of MACE in patients receiving baricitinib (incidence rate ratio 1.54, 95% CI 0.93–2.54; incidence rate difference 0.22, 95% CI − 0.07 to 0.52 per 100 PY) [43]. Recently, Hoisnard et al., using data from SNDS after inverse propensity score treatment weighting, showed that there was no difference in risk of MACE (or VTE) between patients initiating a JAKi (60% baricitinib, 40% tofacitinib) and initiating the TNFi adalimumab. A subgroup analysis of patients aged 65 years or older, and with at least one risk factor for cardiovascular disease, also found no evidence of a difference in risk between the JAKi and adalimumab cohorts [44]. Further contextualization of these observational cohort studies with respect to ORAL Surveillance is hard to elucidate due to different study designs and endpoints (Cox model and time to event vs. incidence rates, as well as length of follow-up and methods of addressing confounding factors through propensity scores vs. inverse probabilities).
MACE in the Baricitinib AD and AA Clinical Programmes
For the baricitinib clinical programme in patients with AD, IR for MACE in the at-risk subpopulation (0.25 per 100 PY) lies within the expected rates for the AD patient population reported in real-world settings (IR of 0.15–0.63 per 100 PY), with the low-risk AD baricitinib clinical population having a much lower IR (0.04) [28]. The IRs for patients with AA in the baricitinib clinical studies (subpopulation at-risk and low-risk, 0.12 and 0 per 100 PY, respectively) are the lowest of those observed in the baricitinib studies, and reflect that the general population of patients with AA do not have an increase in risk of MACE.
VTE in the Baricitinib RA Clinical Programme
For VTEs, the IR in the baricitinib clinical programme for the at-risk RA subgroup (0.66 per 100 PY) falls within the expected IR reported in the literature for patients with RA of 0.33–0.79 per 100 PY, with a minimal IR in the low-risk patient population from the baricitinib studies (0.09 per 100 PY). However, a recent study using data from the Swedish Rheumatology Quality Register found a higher incidence of VTE in patients receiving JAKi (IR = 11.33 per 1000 PY) compared with both TNFi (IR = 5.15) and the general RA population (IR = 5.86) [45]. Similarly, Salinas et al. found increased risk of VTE with baricitinib versus TNFi (incidence rate ratio 1.51, 95% CI 1.10–2.08; incidence rate difference 0.26, 95% CI − 0.04 to 0.57 per 100 PY) [43]. However, Salinas et al. made no adjustment based on previous treatment failures or disease activity, nor carried out any analysis on patient subpopulations with or without relevant risk factors. As highlighted before, Hoisnard et al. did not show differences for VTEs in patients treated with JAKi compared with those who received adalimumab using SNDS data, including in a subpopulation similar to the at-risk population of the baricitinib studies presented here (aged 65 years or older with at least one risk factor for cardiovascular disease) [44]. The occurrence of VTE in patients at risk who received baricitinib treatment in the RA clinical programme is thus associated with previously identified independent VTE risk factors, such as previous VTE, older age, obesity, malignancy, and immobility [46]. The risk of VTE is reflected in the current SmPC for baricitinib due to an imbalance in observed events during the placebo-controlled period of RA Phase 3 studies [29].
VTE in the Baricitinib AD and AA Clinical Programmes
In the baricitinib AD and AA clinical trial programmes, the IRs of VTE in at-risk baricitinib treatment groups were low (IR of 0.08 and 0.12 per 100 PY, respectively), as expected due to the younger age and lower vulnerability of these study populations, both in the clinical studies and the general populations of patients with these conditions. These are in line with, or below, the background IR of 0.18–0.24 per 100 PY in patients with AD and 0.09 per 100 PY in patients with AA. Although 2 of the patients with VTE in the AD population were receiving hormonal contraception, overall approximately 30% of female patients were taking hormonal contraceptives during these studies. A risk factor analysis performed on data from the baricitinib RA clinical programme did not identify oral contraceptive use as an independent risk factor in RA [46]. This analysis was not performed for the AD baricitinib data due to the low number of events.
Malignancy in the Baricitinib RA, AD, and AA Clinical Programmes
In this analysis of the baricitinib clinical trial programmes, the IR of malignancies excluding NMSC in the LTE at-risk RA population was 1.23 per 100 PY, which is slightly elevated compared to the all-bari-RA population (IR = 0.92 per 100 PY). When comparing the all-bari-RA population to the expected number of malignancies based on SEER17 data, the age-adjusted standardised IR was 1.07 (95% CI 0.90–1.26) [29], suggesting a modest increase in incidence for these patients at risk. The IR of malignancies excluding NMSC in the at-risk group in AD (0.45) and AA (0.31) baricitinib clinical trials appear to be at the lower end of the reported IR in these disease populations (AD: 0.33–0.85 per 100 PY [47, 48]; and AA: 0.37–0.43 per 100 PY [20]).
Mortality in the Baricitinib RA, AD, and AA Clinical Programmes
The mortality rate for the baricitinib LTE at-risk RA subpopulation (0.78) is lower than expected when compared with the general population calculated from the SEER incidence distribution (2013–2017) (83 observed deaths vs. 105.9 expected) [32]. IRs of mortality for baricitinib at-risk groups in AD and AA clinical studies are low (0.16 and 0, respectively) and in line with the younger age and lower vulnerability of these study populations.
Contextualisation and Clinical Recommendations
The risk factors presented in this analysis are those relevant for the adverse events of special interest investigated, as determined by the recommendation of the safety committee of the European Medicines Agency (EMA) following the results of the ORAL Surveillance study to use JAK inhibitors in the following patients only if no suitable treatment alternatives are available: those aged 65 years or above, those at increased risk of major cardiovascular problems (such as heart attack or stroke), those who smoke or have done so for a long time in the past, and those at increased risk of cancer [49]. The Committee also recommended using JAK inhibitors with caution in patients with risk factors for blood clots in the lungs and in deep veins (VTE). The cardiovascular risk factors included in our analysis were chosen to reflect the entry criteria for the ORAL Surveillance study [27]: risk of VTE is linked to older age, increased BMI [50], and immobility, represented by the surrogate factor of poor mobility on EQ-5D, while risk of cancer was determined by age, history of malignancy, and smoking status as the most reliable predictive factors for cancer risk.
Per recent recommendations from committees of the EMA [Pharmacovigilance Risk Assessment Committee (PRAC) and Committee for Medicinal Products for Human Use (CHMP)], the benefit–risk profile of baricitinib for patients with no risk factors remains positive for patients with RA, AD, and AA who have responded inadequately to, or who are intolerant to, cDMARDs [51, 52] or topical treatments [53]. For patients at risk for AESI, benefit–risk shared decision-making discussions should be taken into consideration by the prescriber to evaluate use of baricitinib if no other suitable alternatives are available. Further, PRAC and CHMP recommend the doses of JAK inhibitors should, where possible, be reduced in patient groups who are at risk of VTE, cancer, or major cardiovascular problems [49]. Based on available data across indications for baricitinib, including randomised controlled trials, LTE, and real-world evidence data, a clinically relevant or consistent dose-dependent response with a higher risk for the 4 mg versus the 2 mg dose has not been observed for AESI, except for infections, including herpes zoster, nor for VTE, for which an imbalance between doses was observed during the placebo-controlled period of RA trials [46].
EULAR have recently proposed an updated approach for the management of RA with targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs) and biological DMARDs (bDMARDs), aligned with previous EULAR recommendations in which JAKi remain on the same level as bDMARDs and can be used after conventional DMARD failure in patients who do not have risk factors for cardiovascular disease, VTE, or malignant diseases [54]. EULAR emphasises the importance of shared decision-making, and that, when making recommendations, clinicians give careful consideration to specific risk factors of relevance for the individual patient before initiating JAKi. There are many precedents for the success of mitigation strategies to prevent treatment-associated risks in the case of biologic therapies: for example, screening for latent tuberculosis and hepatitis, cardiac failure, and demyelination risks [55–57]. Furthermore, regardless of medication, RA management recommendations emphasise the importance of careful screening and management of cardiovascular, infection, and malignancy risks [40]. The contribution of specific risk factors to each of these AESIs remains to be further elucidated. However, all of these known risk factors can be identified in routine clinical practice, and therefore health-care providers should follow established guidelines to manage cardiovascular risk, screen for malignancies, and vaccinate patients, as for any other patients with immune-mediated inflammatory conditions.
For AD, it is recommended to limit systemic corticosteroids to use only for 1–2 weeks for severe acute exacerbations, due to the largely unfavourable risk–benefit ratio [58, 59]. Treatment duration with cyclosporine, the first-line systemic option for patients with severe disease, usually varies from 3 months to 1 year due to poor drug survival [58, 59]. Long-term use of cyclosporine is associated with multiple risks, including hyperlipidaemia, hypertension, kidney toxicity, and malignancy. Therefore, it is important to provide access to treatments with a better long-term benefit–risk balance, including dupilumab and JAKi indicated for AD [60, 61]. Baricitinib has been shown in clinical trials to have fast onset of action, especially on the most limiting AD symptom itch, which is also recognised in the recently published EuroGuiDerm 2022 guideline on atopic eczema (atopic dermatitis) [59], and has been shown to be effective on AD signs and other symptoms, such as skin pain and sleep, leading to an overall improved quality of life [62–64]. This treatment algorithm is recommended by the European Guidelines for Treatment of AD, who recommend dupilumab and baricitinib with the same level of evidence “in atopic eczema patients who are candidates for systemic treatment”, while recommending against the long-term use of systemic glucocorticosteroids [59].
In most geographies, for patients with severe AA, there is no licensed treatment alternative to baricitinib. In the baricitinib AA clinical trials, baricitinib treatment demonstrated significant hair regrowth of scalp, eyebrows, and eyelashes compared to placebo up to 36 weeks [35]. Approximately half of the population in the baricitinib AA clinical programme are younger than 65 years, have no cardiovascular or thrombotic risk factors, and have limited risk of malignancy or infection. Published population risks for AA differ from the published background risks for the RA population. For example, in a retrospective cohort study, AA was not associated with an increased risk of heart disease [18]. Additionally, a study involving a heterogeneous AA population in the US found that AA was not associated with an increased risk of stroke and MI [65].
Conclusions
Using data derived from integrated clinical trial safety datasets of baricitinib for RA, AD, and AA, we observed a low IR of MACE, MI, lung cancer, VTE, and overall mortality in patients younger than 65 years without risk factors. Incidence of overall malignancy and serious infections in patients younger than 65 years without risk factors were reduced compared with the population of patients with risk factors. Even for patients with risk factors, the number of AESI are minimal for AD and AA. This raises the question of whether it is clinically useful to extrapolate the risk factors identified in RA patients to AD and AA patient populations.
Risk factors established for the RA population are identifiable through health screening; thus, the physician and patient can make a decision on the appropriateness and acceptability of initiation or continuation of treatment with baricitinib, taking into account the particularities of the individual disease burden, risk factors, and response to treatment.
Acknowledgements
Eli Lilly and Company would like to thank the clinical trial participants and their caregivers, without whom this work would not be possible.
Funding
Sponsorship for this study and its publication, including the journal’s Rapid Service Fee, were funded by Eli Lilly and Company.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Catherine Lynch, PhD, a full-time employee of Eli Lilly and Company.
Author Contributions
Peter C. Taylor, Thomas Bieber, Rieke Alten, Torsten Witte, James Galloway, Walter Deberdt, Maher Issa, Ewa Haladyj, Inmaculada De La Torre, Susanne Grond, and Andreas Wollenberg contributed to the study conception and design. Material preparation, data collection and analysis were performed by Walter Deberdt and Maher Issa. The first draft of the manuscript was written by Inmaculada De La Torre and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Prior Presentation
These data were previously presented in part at the European Academy of Dermatology and Venereology (EADV) Congress; Milan, Italy; 7–10 September 2022 (Bieber T, et al. EADV 2022. Safety of baricitinib for the treatment of atopic dermatitis over a median of 1.6 and up to 3.9 years treatment: an updated integrated analysis of 8 clinical trials. FC03.03).
Disclosures
Peter C. Taylor has been a consultant for or has received grant/research support from AbbVie, Biogen, Celgene, Eli Lilly and Company, Galapagos NV, Gilead Sciences, GlaxoSmithKline, Janssen, Novartis, Pfizer, Sandoz, and UCB Pharma.
Thomas Bieber was speaker or consultant or Investigator for AbbVie, Affibody, Allmiral, AnaptysBio, Arena, Asana Biosciences, ASLAN pharma, Bayer Health, BioVerSys, Böhringer-Ingelheim, Bristol-Myers Squibb, Connect Pharma, Dermavant, Domain Therapeutics, EQRx, Eli Lilly and Company, Galderma, Glenmark, GSK, Incyte, Innovaderm, IQVIA, Janssen, Kirin, Kymab, LEO, LG Chem, L´Oréal, MSD, Novartis, Numab, OM-Pharma, Pfizer, Pierre Fabre, Q32bio, RAPT, Sanofi/Regeneron, UCB. He is founder and chairman of the board of the non-profit biotech “Davos Biosciences”.
Rieke Alten has been a consultant for or received grant or research support from AbbVie, Bristol Myers Squibb, Celltrion, Chugai, Eli Lilly and Company, Galapagos NV, Gilead Sciences, Janssen, Novartis, Pfizer, Roche, and UCB Pharma.
Torsten Witte has received grant/research support or received honoraria or support for attending meetings or participated on a data safety monitoring board or advisory board for AbbVie, Chugai, Eli Lilly and Company, Galapagos, Medac, Janssen, Novartis, Pfizer, Roche, and UCB Pharma.
James Galloway has been a consultant for or has received grant/research support or received honoraria from AbbVie, Eli Lilly and Company, Galapagos, and Pfizer.
Walter Deberdt, Maher Issa, Ewa Haladyj, Inmaculada De La Torre, and Susanne Grond are employees and shareholders of Eli Lilly and Company.
Andreas Wollenberg has served as an advisor or paid speaker for or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Beiersdorf, Bioderma, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, Eli Lilly and Company, Galapagos, Galderma, Glenmark, Janssen-Cilag, L’Oreal, LEO Pharma, Maruho, MedImmune/AstraZeneca, Merck, Novartis, Pfizer, Pierre Fabre, Regeneron, Sanofi-Aventis/Genzyme, and UCB.
Compliance with Ethics Guidelines
All studies were conducted in accordance with ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and the research protocols were approved by each centre’s institutional review board or ethics committee. All patients provided written informed consent.
Data Availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- 1.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524–9. [DOI] [PubMed] [Google Scholar]
- 2.Kim SC, Schneeweiss S, Liu J, Solomon DH. Risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65:1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93. [DOI] [PubMed] [Google Scholar]
- 5.Bruin-Weller MD, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32:164–73. [DOI] [PubMed] [Google Scholar]
- 6.Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren Z, Silverberg JI. Association of atopic dermatitis with bacterial, fungal, viral, and sexually transmitted skin infections. Dermatitis. 2020;31:157–64. [DOI] [PubMed] [Google Scholar]
- 8.Wan J, Shin DB, Syed MN, Abuabara K, Lemeshow AR, Gelfand JM. Risk of herpesvirus, serious and opportunistic infections in atopic dermatitis: a population-based cohort study*. Br J Dermatol. 2022;186:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen YMF, Egeberg A, Gislason GH, Hansen PR, Skov L, Thyssen JP. Risk of myocardial infarction, ischemic stroke, and cardiovascular death in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:310-2.e3. [DOI] [PubMed] [Google Scholar]
- 10.Ascott A, Mulick A, Yu AM, et al. Atopic eczema and major cardiovascular outcomes: a systematic review and meta-analysis of population-based studies. J Allergy Clin Immunol. 2019;143:1821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He H, Del Duca E, Diaz A, et al. Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J Allergy Clin Immunol. 2021;147:1369–80. [DOI] [PubMed] [Google Scholar]
- 12.Meyers KJ, Silverberg JI, Rueda MJ, et al. Risk of venous thromboembolism among patients with atopic dermatitis: A cohort study in a US administrative claims database. Dermatol Ther (Heidelb). 2021;11:1041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneeweiss MC, Kim SC, Wyss R, et al. Incidence of venous thromboembolism in patients with dermatologist-diagnosed chronic inflammatory skin diseases. JAMA Dermatol. 2021;157:805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaheen MS, Silverberg JI. Association of inflammatory skin diseases with venous thromboembolism in US adults. Arch Dermatol Res. 2021;313:281–9. [DOI] [PubMed] [Google Scholar]
- 15.Paller A, Jaworski JC, Simpson EL, et al. Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821–38. [DOI] [PubMed] [Google Scholar]
- 16.Andersen YMF, Nymand L, DeLozier AM, Burge R, Edson-Heredia E, Egeberg A. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12: e053137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Lee H, Lee CH, Lee W-S. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:466-77.e16. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Kim YC, Choi JW. Alopecia areata is not a risk factor for heart diseases: a 10-year retrospective cohort study. PLoS ONE. 2021;16: e0250216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C-C, Chang Y-T, Liu H-N, Chen Y-J. Cancer risk in patients with alopecia areata: a nationwide population-based matched cohort study. Cancer Med. 2018;7:2153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JH, Song Y, Do Han K, et al. Cancer risk by the subtype of alopecia. Sci Rep. 2018;8:9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mostaghimi A, Qureshi S, Joyce C, Guo Y, Huang KP. Reduced incidence of skin cancer in patients with alopecia areata: a retrospective cohort study. Cancer Epidemiol. 2016;41:129–31. [DOI] [PubMed] [Google Scholar]
- 22.Khanna D, McMahon M, Furst DE. Safety of tumour necrosis factor-α antagonists. Drug Saf. 2004;27:307–24. [DOI] [PubMed] [Google Scholar]
- 23.Colombel J-F, Loftus EV, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn’s disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126:19–31. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–7. [DOI] [PubMed] [Google Scholar]
- 25.Strangfeld A, Richter A, Siegmund B, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis. 2017;76:504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giles JT, Sattar N, Gabriel S, et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 2020;72:31–40. [DOI] [PubMed] [Google Scholar]
- 27.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–26. [DOI] [PubMed] [Google Scholar]
- 28.Bieber T, Feist E, Irvine AD, et al. A review of safety outcomes from clinical trials of baricitinib in rheumatology, dermatology and COVID-19. Adv Ther. 2022;39:4910–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor PC, Takeuchi T, Burmester GR, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis. 2022;81:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bieber T, Katoh N, Simpson EL, et al. Safety of baricitinib for the treatment of atopic dermatitis over a median of 1.6 years and up to 3.9 years of treatment: an updated integrated analysis of eight clinical trials. J Dermatolog Treat. 2022. 10.1080/09546634.2022.2161812:1-35. [DOI] [PubMed] [Google Scholar]
- 31.King B, Mostaghimi A, Shimomura Y, et al. Integrated safety analysis of baricitinib in adults with severe alopecia areata from two randomized clinical trials. Br J Dermatol. 2022. 10.1093/bjd/ljac059. [DOI] [PubMed] [Google Scholar]
- 32.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2018, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, Apr 2021.
- 33.Combe B, Balsa A, Sarzi-Puttini P, et al. Efficacy and safety data based on historical or pre-existing conditions at baseline for patients with active rheumatoid arthritis who were treated with baricitinib. Ann Rheum Dis. 2019;78:1135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bieber T, Thyssen JP, Reich K, et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J Eur Acad Dermatol Venereol. 2021;35:476–85. [DOI] [PubMed] [Google Scholar]
- 35.King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687–99. [DOI] [PubMed] [Google Scholar]
- 36.Raj R, Thomas S, Gorantla V. Accelerated atherosclerosis in rheumatoid arthritis: a systematic review [version 1; peer review: 2 approved, 1 approved with reservations]. F1000Research. 2022;11:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ketfi C, Boutigny A, Mohamedi N, et al. Risk of venous thromboembolism in rheumatoid arthritis. Joint Bone Spine. 2021;88: 105122. [DOI] [PubMed] [Google Scholar]
- 38.Burggraaf B, van Breukelen-van der Stoep DF, de Vries MA, et al. Effect of a treat-to-target intervention of cardiovascular risk factors on subclinical and clinical atherosclerosis in rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2019;78:335–41. [DOI] [PubMed]
- 39.Markusse IM, Akdemir G, Dirven L, et al. Long-term outcomes of patients with recent-onset rheumatoid arthritis after 10 years of tight controlled treatment. Ann Intern Med. 2016;164:523–31. [DOI] [PubMed] [Google Scholar]
- 40.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). Eur Heart J. 2007;28:2375–414. [DOI] [PubMed] [Google Scholar]
- 41.Molander V, Bower H, Frisell T, Askling J. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann Rheum Dis. 2021;80:169–75. [DOI] [PubMed] [Google Scholar]
- 42.Khosrow-Khavar F, Kim SC, Lee H, Lee SB, Desai RJ. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis. 2022;81:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salinas CA, Louder A, Polinski J, et al. Evaluation of VTE, MACE, and serious infections among patients with RA treated with baricitinib compared to TNFi: a multi-database study of patients in routine care using disease registries and claims databases. Rheumatol Ther. 2022. 10.1007/s40744-022-00505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoisnard L, Pina Vegas L, Dray-Spira R, Weill A, Zureik M, Sbidian E. Risk of major adverse cardiovascular and venous thromboembolism events in patients with rheumatoid arthritis exposed to JAK inhibitors versus adalimumab: a nationwide cohort study. Ann Rheum Dis. 2022. 10.1136/ard-2022-222824. [DOI] [PubMed] [Google Scholar]
- 45.Molander V, Bower H, Frisell T, Delcoigne B, Di Giuseppe D, Askling J. Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: a Swedish comparative safety study among patients with rheumatoid arthritis. Ann Rheum Dis. 2022. 10.1136/ard-2022-223050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor PC, Weinblatt ME, Burmester GR, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol. 2019;71:1042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arana A, Wentworth CE, Fernández-Vidaurre C, Schlienger RG, Conde E, Arellano FM. Incidence of cancer in the general population and in patients with or without atopic dermatitis in the U.K. Br J Dermatol. 2010;163:1036–43. [DOI] [PubMed] [Google Scholar]
- 48.Mansfield KE, Schmidt SAJ, Darvalics B, et al. Association between atopic eczema and cancer in England and Denmark. JAMA Dermatol. 2020;156:1086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.European Medicines Agency. EMA confirms measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders. Available from: https://www.ema.europa.eu/en/news/ema-confirms-measures-minimise-risk-serious-side-effects-janus-kinase-inhibitors-chronic. Accessed Jan 16, 2023
- 50.Eichinger S, Hron G, Bialonczyk C, et al. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med. 2008;168:1678–83. [DOI] [PubMed] [Google Scholar]
- 51.Keystone EC, Taylor PC, Tanaka Y, et al. Patient-reported outcomes from a phase 3 study of baricitinib versus placebo or adalimumab in rheumatoid arthritis: secondary analyses from the RA-BEAM study. Ann Rheum Dis. 2017;76:1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376:652–62. [DOI] [PubMed] [Google Scholar]
- 53.Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2022. 10.1136/ard-2022-223356 [DOI] [PubMed]
- 55.Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17–28. [DOI] [PubMed] [Google Scholar]
- 56.Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis—executive summary. Rheumatology (Oxford). 2019;58:220–6. [DOI] [PubMed] [Google Scholar]
- 57.Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford). 2018;57:997–1001. [DOI] [PubMed] [Google Scholar]
- 58.Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34:2717–44. [DOI] [PubMed] [Google Scholar]
- 59.Wollenberg A, Kinberger M, Arents B, et al. EuroGuiDerm guideline on atopic eczema, version 2.0. October 2022. Available from https://guidelines.edf.one//guidelines/living-euroguiderm-guideline-for-the-systemic-treatment-of-atopic-eczema-2 Accessed Nov 28, 2022.
- 60.Dal Bello G, Maurelli M, Schena D, Girolomoni G, Gisondi P. Drug survival of dupilumab compared to cyclosporin in moderate-to-severe atopic dermatitis patients. Dermatol Ther. 2020;33: e13979. [DOI] [PubMed] [Google Scholar]
- 61.Drucker AM, Ellis AG, Bohdanowicz M, et al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol. 2020;156:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buhl T, Rosmarin D, Serra-Baldrich E, et al. Itch and sleep improvements with baricitinib in patients with atopic dermatitis: a post hoc analysis of 3 phase 3 studies. Dermatol Ther (Heidelb). 2021;11:971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242–55. [DOI] [PubMed] [Google Scholar]
- 64.Thyssen JP, Buhl T, Fernández-Peñas P, et al. Baricitinib rapidly improves skin pain resulting in improved quality of life for patients with atopic dermatitis: analyses from BREEZE-AD1, 2, and 7. Dermatol Ther (Heidelb). 2021;11:1599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang KP, Joyce CJ, Topaz M, Guo Y, Mostaghimi A. Cardiovascular risk in patients with alopecia areata (AA): a propensity-matched retrospective analysis. J Am Acad Dermatol. 2016;75:151–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.