Abstract
Isolated major aortopulmonary collateral artery (MAPCA), in the absence of evidence of structural heart disease, is a very rare observation. This anomaly usually appears in preterm newborns. In the majority of babies, isolated MAPCAs cause no symptoms and regress spontaneously after birth and their conservative management is usually sufficient. We report a case of an asymptomatic full-term 5-month-old infant presenting with heart murmur as the only sign during clinical evaluation. Echocardiography revealed a dilated left ventricle, with no pulmonary hypertension. Computed tomography angiogram showed a large MAPCA arising from the descending thoracic aorta and supplying blood to the left lower lobe. The condition was managed successfully by percutaneous obliteration with Amplatzer vascular plugs. Isolated MAPCA is usually a benign anomaly, presenting no clinical finding and requiring no specific treatment. However, in a small minority of infants, this congenital disorder may progress, with detrimental impacts on cardiac structure before clinical symptoms appear. Early intervention may be required to prevent irreversible sequelae.
Keywords: Isolated major aortopulmonary collateral artery, Amplatzer vascular plugs, Closure, Asymptomatic
Introduction
Major aortopulmonary collateral arteries (MAPCAs) are anomalous blood vessels that develop from the aorta or its main branches and are connected to pulmonary arteries. They are commonly seen in congenital heart disease with reduced pulmonary blood flow, such as tetralogy of Fallot or pulmonary atresia [1].
In rare cases, MAPCAs may present with no evidence of congenital heart disease, especially in preterm neonates; they are usually small and rarely symptomatic and often regress on their own [2]. Symptomatic isolated MAPCAs have been described as having features of left-to-right shunt and present as recurrent respiratory tract infections, pulmonary hypertension, congestive cardiac failure, bronchopulmonary dysplasia, or hemoptysis [3], [4], [5], [6], [7]. Very few isolated MAPCAs have been recorded in full-term babies, and even fewer have been reported to need intervention [8].
We discuss the case of an infant presenting with heart murmur as the only clinical finding, but on echocardiographic examination showing a dilated left ventricle secondary to an isolated MAPCA. Embolization procedure was performed successfully by inserting 2 Amplatzer vascular plugs.
Case report
A 5-month-old infant, weighing 6.4 kg, was found to have a heart murmur at birth. She was delivered at full term, with a birth weight of 2.7 kg and no noticeable perinatal complications. The baby showed normal mental and physical development. At the age of 4 months, the parents brought her to a clinic because she had fever. No serious infection was recorded, but the heart murmur was consistent; therefore, she was transferred to our hospital, which specializes in heart diseases. There was no abnormal finding at presentation, except for a left sternal continuous III/VI murmur on clinical examination. Chest X-ray showed cardiomegaly with pulmonary plethora, mainly in the left lower lobe, but no evidence of pulmonary sequestration. Echocardiography revealed left ventricular dilation with no pulmonary hypertension or hypokinesis (Fig. 1).
Fig. 1.
Echocardiography showing the dilated left ventricle.
Abdominal aortic Doppler showed pan-diastolic flow reversal. An abnormal artery, 6 mm in diameter, was found arising from the descending aorta and coursing tortuously, likely toward the left lung (Fig. 2). No other structural abnormality was seen.
Fig. 2.
Abnormal artery originating from the aorta.
A computed tomography angiogram showed a large MAPCA, originating from the D7 to D8 vertebral levels of the descending aorta and supplying blood to the left lower lobe (Fig. 3).
Fig. 3.
Computed tomography angiogram showing a MAPCA, arising from the aorta and connected to the left lower lobe (arrow). MAPCA, major aortopulmonary collateral artery.
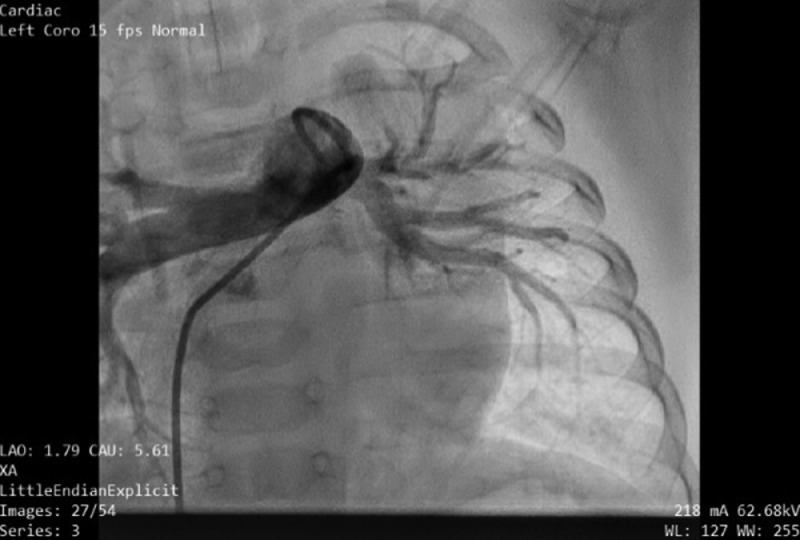
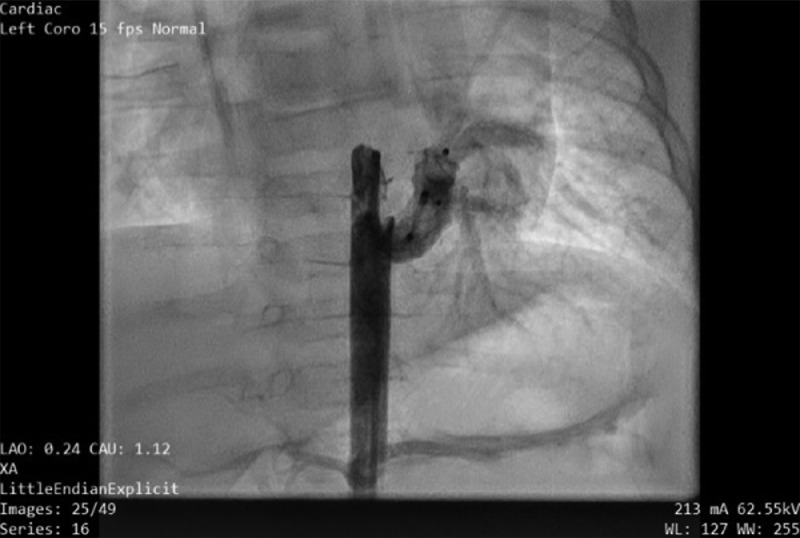
Cardiac catheterization was performed under general anesthesia. Angiography of the descending aorta and subsequent selective anterior–posterior and lateral angiography revealed a large MAPCA (6 mm in diameter) supplying the left lower lobe, with the levophase showing pulmonary venous return to the left atrium (Fig. 4). Pulmonary angiography showed a normal branching pattern, with native arteries supplying blood to the left lower lobe (Fig. 5). The levophase of the pulmonary angiogram showed pulmonary venous drainage to the left atrium, which ruled out lobal sequestration. Two Amplatzer vascular plugs, one 6 mm and the other 8 mm in size, were deployed sequentially and significantly reduced circulation through the MAPCA (Fig. 6). Post-embolization pulmonary angiogram showed remarkably improved flow to the left lower lobe (Fig. 7). No complication was recorded on follow-up after 3 days, and the baby was discharged with a prescription for low-dose furosemide. After 1 month, echocardiography showed only a small residual flow (2 mm in diameter), with reduced left ventricle size.
Fig. 4.
MAPCA supplying blood to the left lower lobe. MAPCA, major aortopulmonary collateral artery.
Fig. 5.
The left lower lobe is supplied with the pulmonary artery.
Fig. 6.
Angiogram of the MAPCA after the procedure, showing a small residual flow. MAPCA, major aortopulmonary collateral artery.
Fig. 7.
Increased flow through native pulmonary arteries to the left lower lobe after the procedure.
Discussion
MAPCAs are very rare abnormal phenomena, mostly recorded in neonates with congenital heart disease, especially in cases of insufficient pulmonary perfusion [1]. They may be congenital variations of embryologic development, representing persisting fetal aortopulmonary anastomosis [9] or incomplete regression of pulmonary–bronchial artery connections [10]. They may also be a response to a stimulus, such as lung parenchymal hypoperfusion or hypoxia [11,12]. MAPCAs can arise directly from the aorta or branches of the aortic arch or as connections between pulmonary and bronchial arteries [13,14]. Although MAPCAs are usually related to congenital heart disease, they may occur in isolation with no underlying abnormality in some newborns, especially premature babies [2]. In most cases, they are asymptomatic and close spontaneously and require no specific treatment. In a minority of newborns, isolated MAPCAs can cause symptoms of cardiac failure, hemoptysis, or recurrent pneumonia, which require intervention. Echocardiogram is the best initial modality for investigation [13], while the gold standard for diagnosis of MAPCAs is computed tomography, magnetic resonance imaging, or cardiac catheterization [15]. The main treatment option is closure, either by endovascular means or cardiac surgery. More recently, coil or device occlusion has become the preferred treatment method, with reports of successful outcomes [16,17].
Most published cases of isolated MAPCAs needed intervention after examination for clinical symptoms of heart failure or recurrent respiratory tract infections. Our case is unique as the isolated MAPCA in a full-term baby did not lead to a serious clinical finding, except for a heart murmur, while the echocardiogram revealed a significantly dilated left ventricle. Percutaneous intervention with deployment of Amplatzer vascular plugs was necessary to prevent further harmful sequelae.
Conclusion
Although isolated MAPCAs in asymptomatic infants, especially full-term babies, may be benign variants requiring no specific treatment [8], there is still a slight chance that they could progress and lead to a harmful effect. Early intervention with percutaneous embolization or surgery may be required to avert potential detrimental impacts on heart function.
Author's contributions
Le XH and Le KT contributed equally to this article as co-first authors. Le XH and Le KT: Case file retrieval and case summary preparation. Le XH and Nguyen MD: preparation of manuscript and editing. All authors read and approved the final manuscript.
CARE Checklist (2016) statement
The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin
Vietnam.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Our institution does not require ethical approval for reporting individual cases or case series.
Consent for publication
Not applicable.
Patient consent
Informed consent for patient information to be published in this article was obtained.
Footnotes
Funding: None.
Competing Interests: The authors do not report any conflicts of interest.
References
- 1.Wernovsky G, Anderson R., Kumar K, Redington A, Tweddell J, Tweddell J, editors. Anderson's pediatric cardiology. 4th ed. Elsevier; Philadelphia, PA: 2019. editors. [Google Scholar]
- 2.Shaughnessy RD, Reller MD, Rice MJ, McDonald RW. Development of systemic to pulmonary collateral arteries in premature infants. J Pediatr. 1997;131(5):763–765. doi: 10.1016/s0022-3476(97)70110-0. [DOI] [PubMed] [Google Scholar]
- 3.Kunwar BK, Paddalwar S, Ghogare M. Large Isolated major aortopulmonary collateral artery causing severe pulmonary hypertension in an infant: a rare and challenging diagnosis. J Clin Diagn Res. 2017;11(6):OD18–OD20. doi: 10.7860/JCDR/2017/27645.10094. Epub 2017 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patra S, Srinivas SK, Agrawal N, Jayaranganath M. Isolated major aortopulmonary collateral artery in an infant presenting with recurrent lower respiratory tract infection. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tinmaswala MA, Saple PP, Gupta A, Prachi N, Nitinkumar A, Amin K. Isolated major aortopulmonary collateral artery causing CCF in a newborn: a case report. Int J Med Res Health Sci. 2015;4:471–473. [Google Scholar]
- 6.Harshith K, Anoop A, Jineesh V. A rare cause of hemoptysis in west syndrome-isolated aortopulmonary collaterals in structurally normal heart. Indian J Radiol Imaging. 2021;31(3):745–747. doi: 10.1055/s-0041-1735865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covert RF, Drummond WH, Gessner IH. Collateral vessels complicating bronchopulmonary dysplasia. J Pediatr. 1988;113(3):617–618. doi: 10.1016/s0022-3476(88)80672-3. [DOI] [PubMed] [Google Scholar]
- 8.Yu CH, Chen MR. Clinical investigation of systemic-pulmonary collateral arteries. Pediatr Cardiol. 2008;29(2):334–338. doi: 10.1007/s00246-007-9086-y. Epub 2007 Sep 18. [DOI] [PubMed] [Google Scholar]
- 9.Schoenwolf G.C., BSB B.P.R., Francis-West P.H. 4th ed. Churchill; New York, Edinburgh: 2009. Larsen's human embryology. [Google Scholar]
- 10.Liebow AA. Patterns of origin and distribution of the major bronchial arteries in man. Am J Anat. 1965;117:19–32. doi: 10.1002/aja.1001170103. [DOI] [PubMed] [Google Scholar]
- 11.DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE, VanIperen L, Mentink MM. Development of the pharyngeal arch system related to the pulmonary and bronchial vessels in the avian embryo. With a concept on systemic-pulmonary collateral artery formation. Circulation. 1993;87(4):1306–1319. doi: 10.1161/01.cir.87.4.1306. [DOI] [PubMed] [Google Scholar]
- 12.Botenga AS. The significance of broncho-pulmonary anastomoses in pulmonary anomalies: a selective angiographic study. Radiol Clin Biol. 1969;38(5):309–328. [PubMed] [Google Scholar]
- 13.Acherman RJ, Siassi B, Pratti-Madrid G, Luna C, Lewis AB, Ebrahimi M, et al. Systemic to pulmonary collaterals in very low birth weight infants: color Doppler detection of systemic to pulmonary connections during neonatal and early infancy period. Pediatrics. 2000;105(3 Pt 1):528–532. doi: 10.1542/peds.105.3.528. [DOI] [PubMed] [Google Scholar]
- 14.Nakwan N. Congenital unilateral pulmonary atresia with coronary-to-pulmonary collateral artery originating from left circumflex coronary artery. Eur J Cardiothorac Surg. 2015;47(4):744–746. doi: 10.1093/ejcts/ezu223. Epub 2014 May 28. [DOI] [PubMed] [Google Scholar]
- 15.Alex A, Ayyappan A, Valakkada J, Kramadhari H, Sasikumar D, Menon S. Major aortopulmonary collateral arteries. radiol cardiothorac imaging. 2022;4(1):e210157. doi: 10.1148/ryct.210157 [DOI] [PMC free article] [PubMed]
- 16.Padhi SS, Bakshi KD, Shastri RK. Multiple coil closure of isolated aortopulmonary collateral. Ann Pediatr Cardiol. 2010;3(1):65–67. doi: 10.4103/0974-2069.64357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins L, Oliveira RS, Silva P, Marinho J, Sousa G, Castela E. Giant major aortopulmonary collateral artery: a rare cause of heart murmur in newborns. Rev Port Cardiol. 2014;33(7-8):483–485. doi: 10.1016/j.repc.2014.02.002. English, PortugueseEpub 2014 Jul 31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.