Abstract
Oligodendroglioma, the third most common glioma, accounts for 5% of primary brain tumors and around 20% of all glial neoplasms. They are quite uncommon in children. Here, we aimed to show an unusual case of a 9-year-old boy developing a huge anaplastic oligodendroglioma. A high-grade astrocytoma-like supratentorial tumor was discovered by a sophisticated brain scan employing magnetic resonance imaging. The tumor was identified by histopathology as an anaplastic oligodendroglioma. Anaplastic oligodendroglioma should be considered while making the differential diagnosis of high-grade astrocytoma notwithstanding its rarity.
Keywords: Anaplastic oligodendroglioma, Giant, Children, Magnetic resonance imaging
Introduction
About 5% of initial intracranial tumors are oligodendrogliomas, a kind of diffusely infiltrating glioma that frequently affects the cortical gray matter and is most frequently seen in the frontal lobes [1]. Historically, the histologic characteristics of the tumor were used to diagnose oligodendroglioma. Oligodendrogliomas are typically low-grade WHO grade II neoplasms that are slow-growing tumors and have a positive therapeutic response when compared to other gliomas. However, in 2016, the WHO updated the criteria for the categorization of central nervous system (CNS) tumors to incorporate both phenotypic and genotypic study [2]. The word "anaplastic" has been dropped from the fifth edition of the WHO classification of CNS tumors (2021), which now only assigns tumors a grade of 2 or 3 [3]. Grade III anaplastic oligodendroglioma (AO) is a more aggressive type of the tumor with a worse prognosis that can develop independently or as a result of lower grade oligodendroglioma degeneration [4]. Oligodendrogliomas often affect middle-aged people and are more prevalent in the fourth and fifth decades of life than grade 3 tumors are. There is a minor preference for men. In children, they are exceptionally unusual [5]. In this article, we purposed to describe an uncommon case of giant, AO in a child.
Case presentation
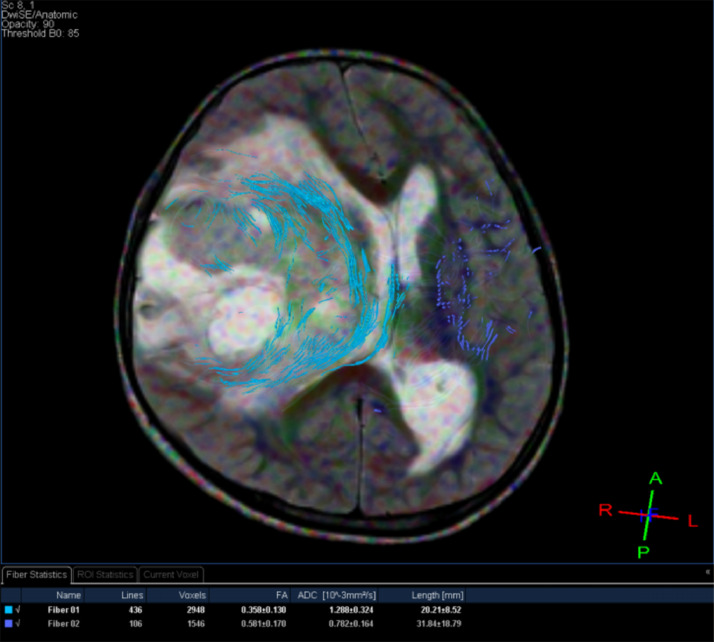
A 9-year-old male, suffering from 2-month headache, nausea, and vomiting, was admitted to our hospital. Her medical profile manifested no abnormalities. Neurologic deficits were not detected during clinical evaluation. The routine laboratory tests and tumor markers were within normal ranges. Brain magnetic resonance imaging (MRI) with contrast agent was immediately indicated for him. A heterogeneous mass with central necrosis inside the structure (80 mm × 82 mm × 80 mm), was noticed in the right parietal lobe with surrounding vasogenic edema, on T2-weighted and Fluid-attenuated inversion recovery (FLAIR) images (Fig. 1). The tumor triggered midline shift and mass effect. There was no hemorrhage or calcification observed inside the lesion. The mean apparent diffusion coefficient (ADC) values for the solid component of mass was 0.67 × 10−3 mm2/s (Fig. 2). The cerebral blood flow value of solid component of mass was very high at 91.91 (Fig. 3). The fractional anisotrophy value of the tumor was 0.35 (Fig. 4). On MRI spectroscopy, the choline/N-acetyl aspartate ratio of the solid part of mass was 2.28 (Fig. 5). The tumor was enhanced heterogeneously on T1-weighted image with contrast agent (Fig. 6). The initial diagnosis, relied on the clinical and subclinical information, was a high-grade astrocytoma. The patient adopted gross-total tumor resection. The histopathological evaluation of the excised tumor tissues displayed an AO. Four weeks later, the patient was released and prompted to adopt adjuvant chemo- and radio-therapy at a distinct oncological center.
Fig. 1.
(A) Axial T2-weighted and (B) FLAIR images show a heterogeneous mass located in the right parietal lobe.
Fig. 2.
(A) Diffusion-weighted image and (B) Apparent diffusion coefficient map of tumor.
Fig. 3.
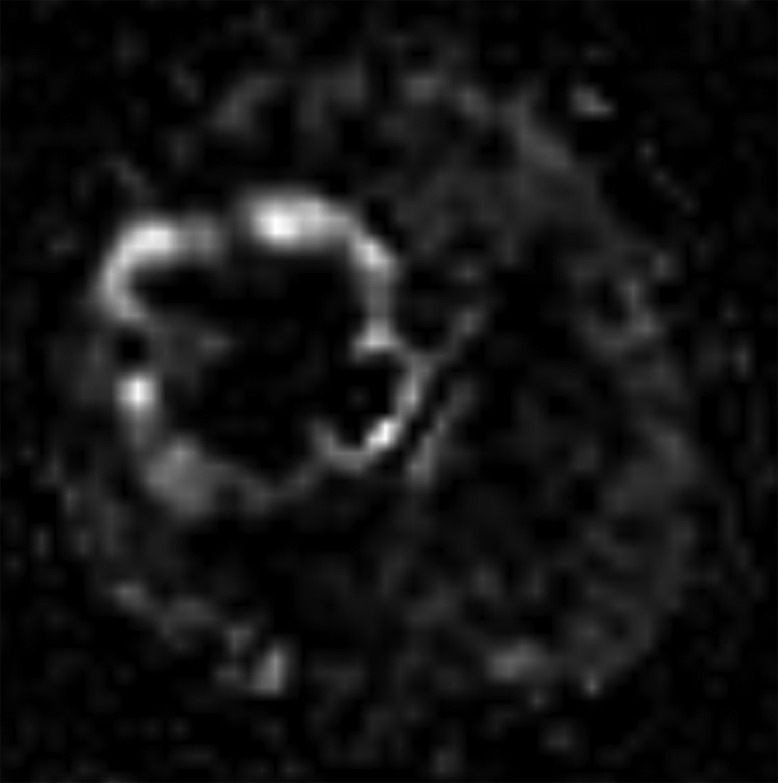
Arterial spin-labeling image of the lesion.
Fig. 4.
Diffusion tensor imaging of the tumor.
Fig. 5.
Magnetic resonance spectroscopy of the lesion.
Fig. 6.

Axial T1-weighted image with contrast agent of the tumor.
Discussion
Bailey and Bucy gave oligodendrogliomas its initial name in 1929 as a result of the fact that, when observed under a microscope, they resembled oligodendrocytes. However, there is conflicting evidence that the tumors originate from mature oligodendrocytes [6]. Instead, it appears that the tumors come from neuroprogenitor cells with glial precursors that further differentiate into oligodendroglial-type cells without the ability to myelinate like oligodendrocytes. The common driver isocitrate dehydrogenase mutation, which is present in a number of diffuse glioma subtypes, lends support to this concept [5,6].
Clinically, oligodendroglioma patients typically report with vague symptoms including headaches. Seizures, on the other hand, are also a highly frequent presenting symptom and are recorded in 35%-85% of people, with some studies reporting a higher participation rate of 91% [7]. The location of the tumor may occasionally cause individuals to suffer localized neurologic impairments. CNS imaging should be performed on any patient who has recent seizures or localized neurologic impairments [8].
Radiologically, MRI provides a more accurate description of the tumor and may be used to determine the oligodendroglioma's real extent and infiltrative nature. T2 weighted sequences show a confined heterogeneously hyperintense tumor, frequently located in the cortical or subcortical region, and may also have a slight peritumoral hyperintense signal. Due to decreased susceptibility signal and greater tumor heterogeneity, there are typically tiny regions of cystic alteration, microhemorrhage, and macroscopic calcifications seen with T2 hypointense signal. On T1 weighted imaging, oligodendrogliomas are hypointense to gray matter. Low-grade tumors frequently exhibit enhancement, which can range from patchy enhancement to significant enhancement. However, enhancement does not always indicate a higher grade oligodendroglioma. On ADC maps, oligodendrogliomas often have hyperintensity and facilitated diffusion as opposed to limited diffusion. Anatomic imaging may be complemented by more sophisticated MRI methods like spectroscopy and perfusion, which may be useful for grading and defining the tumor. Most neoplasms have modestly enhanced choline and reduced N-acetyl aspartate peaks; high-grade gliomas exhibit these changes more prominently than low-grade gliomas due to higher mitotic activity [9].
Compared to low-grade tumors, higher-grade tumors can exhibit enhanced cerebral blood flow owing to neovascularization on T2* weighted dynamic susceptibility contrast perfusion imaging; nevertheless, there is a large overlap between the two [9,10]. In our situation, the tumor was quite big with surrounding vasogenic edema and core necrosis. T2-weighted and FLAIR images both showed extremely strong signal intensity. For the solid portion of mass, the ADC value was 0.67 × 10−3 mm2/s. The solid component of mass's cerebral blood flow measurement was 91.91, which is quite high. The solid component of the mass had a choline/N-acetyl aspartate ratio of 2.28 on MRI spectroscopy. On the T1-weighted image with contrast agent, the tumor heterogeneously enhanced. All of these features were in line with earlier research on AO [9,10].
Histopathologically, oligodendrogliomas may exhibit identifiable oligodendrocyte and astrocyte cell lineage markers. For instance, myelin basic protein, which is present in Schwann cells and oligodendrocytes that produce myelin, is absent in oligodendrogliomas. They may exhibit glial fibrillary acidic protein, an astrocyte marker, in a positive manner [11]. They may also express oligodendrocyte transcription factor 2, a transcription factor involved in the development of neuronal and/or oligodendrocytic cells, which is a marker for oligodendrocyte progenitor cells [12].
Surgery is the first line of defense against an oligodendroglioma, if at all possible. The purpose of surgery is to acquire tissue in order to identify the kind of tumor and to remove as much of the tumor as is feasible without increasing the patient's symptoms. Following surgery, patients may get radiation, chemotherapy, or clinical trials as treatments [13,14].
Conclusion
An uncommon malignant tumor called AO has characteristics of the oligodendroglial lineage and histologic characteristics that match to WHO grade III. Giant AO in a child is extremely rare. It can mimic a high-grade astrocytoma. Despite its rarity, AO should be considered when determining the differential diagnosis of high-grade astrocytoma.
Authors' contributions
PAT and NMD performed all steps to complete this article.
Ethics approval
This study has been approved by the hospital ethics committee (Ref: 352/NĐ2-CĐT).
Data sharing statement
Not applicable.
Patient consent
Informed consent for patient information to be published in this article was obtained.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing Interests: None of the authors has any potential conflict of interest to disclose in relation to the present article.
Acknowledgments: We are thankful to Children's Hospital 2 owing to their general support and technical help.
References
- 1.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(Suppl. 5):v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson MD, Gilbert MR. Treatment recommendations for anaplastic oligodendrogliomas that are codeleted. Oncology (Williston Park) 2013;27(4) 315-20, 322. [PubMed] [Google Scholar]
- 5.Johnson DR, Diehn FE, Giannini C, Jenkins RB, Jenkins SM, Parney IF, et al. Genetically defined oligodendroglioma is characterized by indistinct tumor borders at MRI. AJNR Am J Neuroradiol. 2017;38(4):678–684. doi: 10.3174/ajnr.A5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairncross G, Jenkins R. Gliomas with 1p/19q codeletion: a.k.a. oligodendroglioma. Cancer J. 2008;14:352–357. doi: 10.1097/PPO.0b013e31818d8178. [DOI] [PubMed] [Google Scholar]
- 7.Engelhard HH. Current diagnosis and treatment of oligodendroglioma. Neurosurg Focus. 2002;12:E2. doi: 10.3171/foc.2002.12.2.3. [DOI] [PubMed] [Google Scholar]
- 8.Minh Thong P, Minh Duc N. The role of apparent diffusion coefficient in the differentiation between cerebellar medulloblastoma and brainstem glioma. Neurol Int. 2020;12:34–40. doi: 10.3390/neurolint12030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smits M. Imaging of oligodendroglioma. Br J Radiol. 2016;89(1060) doi: 10.1259/bjr.20150857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgado AF, Delgado AF. Discrimination between glioma grades II and III using dynamic susceptibility perfusion MRI: a meta-analysis. AJNR Am J Neuroradiol. 2017;38(7):1348–1355. doi: 10.3174/ajnr.A5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesseling P, van den Bent M, Perry A. Oligodendroglioma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):809–827. doi: 10.1007/s00401-015-1424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Meijer DH, Alberta JA, Mehta S, Kane MF, Tien AC, et al. Phosphorylation state of Olig2 regulates proliferation of neural progenitors. Neuron. 2011;69(5):906–917. doi: 10.1016/j.neuron.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinslow CJ, Garton ALA, Rae AI, Marcus LP, Adams CM, McKhann GM, et al. Extent of resection and survival for oligodendroglioma: a U.S. population-based study. J Neurooncol. 2019;144(3):591–601. doi: 10.1007/s11060-019-03261-5. [DOI] [PubMed] [Google Scholar]
- 14.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30(6 Suppl. 19):10–14. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]