Abstract
Renal oncocytomas are commonly reported in association with Birt-Hogg-Dube (BHS) syndrome, while BHD-associated oncocytomas of the parotid gland are rare. To date, there have been only 11 cases of BHD-associated parotid gland oncocytoma, without a reported case of malignant transformation. We present the first reported case of oncocytic carcinoma of the parotid gland associated with BHD, with radiologic and histologic correlation. This case establishes that BHD-associated parotid oncocytic lesions, previously identified only as benign oncocytomas in the literature to date, can undergo malignant transformation, and should potentially be regarded with a higher index of suspicion and lower threshold for aggressive management.
Keywords: BHS, Oncocytoma, Parotid oncocytoma, Oncocytic carcinoma, Birt-Hogg-Dube
Introduction
Birt-Hogg-Dube (BHS) syndrome is an autosomal dominant disorder characterized by mutations in the tumor suppressor gene FLCN, and classically manifests as pulmonary cysts, cutaneous fibrofolliculomas, and renal tumors [1,2]. Although initially known for its cutaneous tumors when the syndrome was first described in 1977 [1], BHD is now known to predispose to more serious complications, such as oncocytomas and renal cell carcinomas [3,4]. Oncocytomas are of particular interest in BHD – Pavlovich et al. [4] histologically identified microscopic renal oncocytic lesions in most patients with BHD, further postulating that these lesions may develop into renal cell cancers. Although renal oncocytomas have been frequently reported in association with BHD, parotid gland oncocytoma is rare [4], with only 11 reported cases of BHD-associated parotid gland oncocytoma [5], [6], [7], [8], [9], [10], [11], [12], and imaging analysis in only one case [6]. To date, there have been no known cases of BHD-associated parotid gland oncocytic carcinoma, for which the prognosis and treatment approach are drastically different compared to benign oncocytomas. Here we present the first reported case of oncocytic carcinoma of the parotid gland associated with BHD. This case highlights the role of imaging and histology in arriving at a timely and accurate diagnosis and the prognostic and treatment implications of oncocytic carcinoma in association with BHD.
Case report
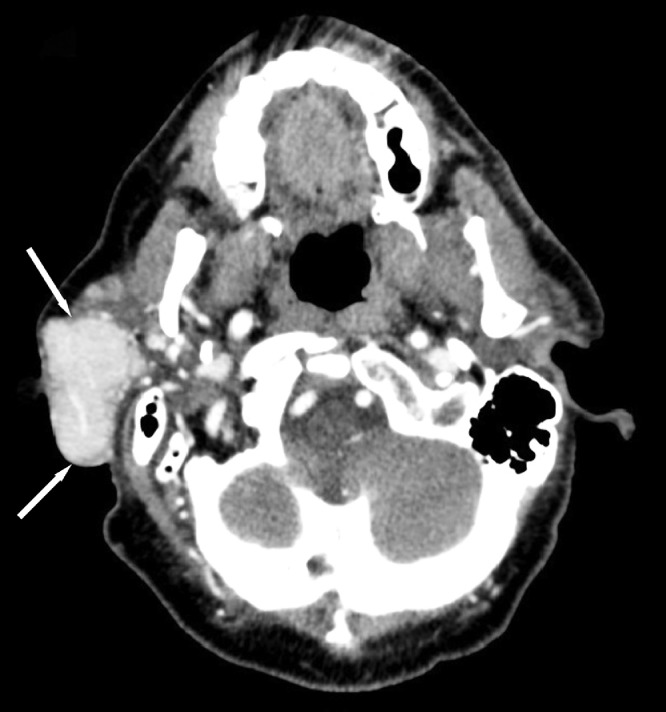
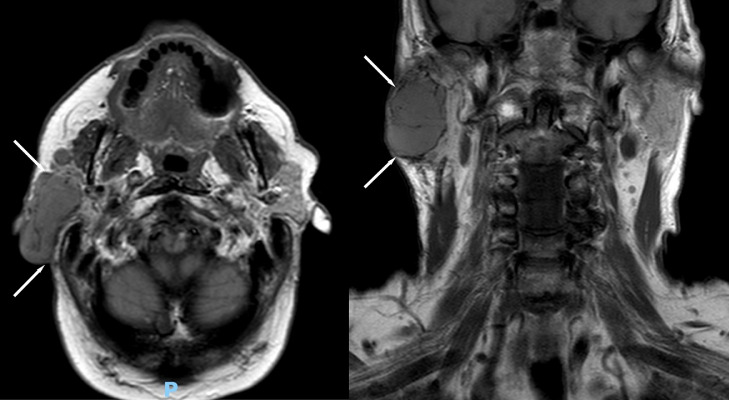
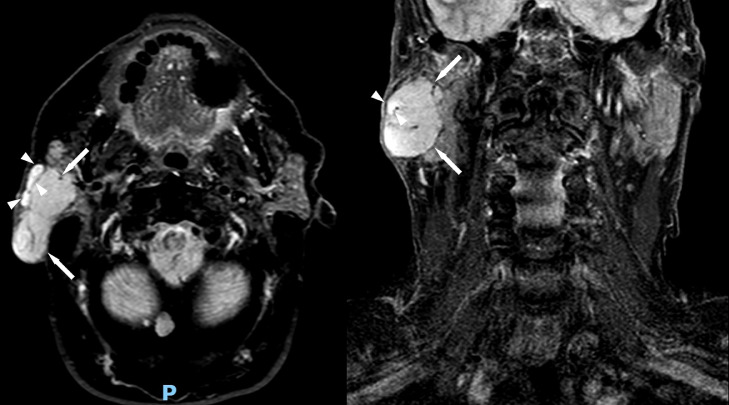
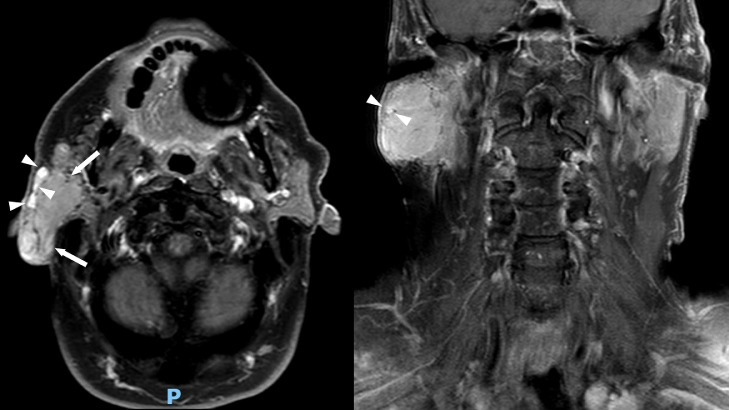
A 68-year-old man presented with a slow growing, nontender right face mass over 3-4 years. His past medical history and family history were unremarkable. Physical exam was notable for numerous white papules scattered throughout his face and violaceous skin changes overlying the right parotid region. A computed tomography (CT) of his neck (Fig. 1) demonstrated a well-circumscribed enhancing mass in his right parotid gland. Follow-up magnetic resonance imaging (MRI) of the neck was performed for further characterization which demonstrated the parotid mass to be hypointense on T1 and mildly hyperintense on STIR compared to the normal parotid gland (Figs. 2 and 3). Along the lateral aspect of the mass, there is a component of the mass that is more hyperintense compared to the remainder of the mass. On fat-saturated T1-weighted postgadolinium sequences, the mass was predominantly isointense with a hyperintense component along its lateral border corresponding to the STIR hyperintense component (Fig. 4). He then underwent total right parotidectomy, right cervicofacial advancement flap, and submental rotational flap reconstruction.
Fig. 1.
Contrast-enhanced computed tomography axial images of the neck demonstrate an enhancing mass within the right parotid gland (white arrows).
Fig. 2.
T1-weighted axial (left) and coronal (right) magnetic resonance images demonstrate a hypointense mass within the right parotid gland (white arrows).
Fig. 3.
STIR axial (left) and coronal (right) magnetic resonance images demonstrate a mildly hyperintense mass compared to the normal parotid gland representing the oncocytoma component of the mass (white arrows). The lateral component of this mass demonstrates more hyperintense signal compared to the remainder of the mass representing the carcinoma component (white arrowheads).
Fig. 4.
T1-weighted contrast-enhanced axial (left) and coronal (right) magnetic resonance images show minimal enhancement in most of the parotid gland mass relative to the parotid gland representing the oncocytoma component (white arrows). In the lateral most aspect, the more avidly enhancing component represents the carcinoma component (white arrowheads).
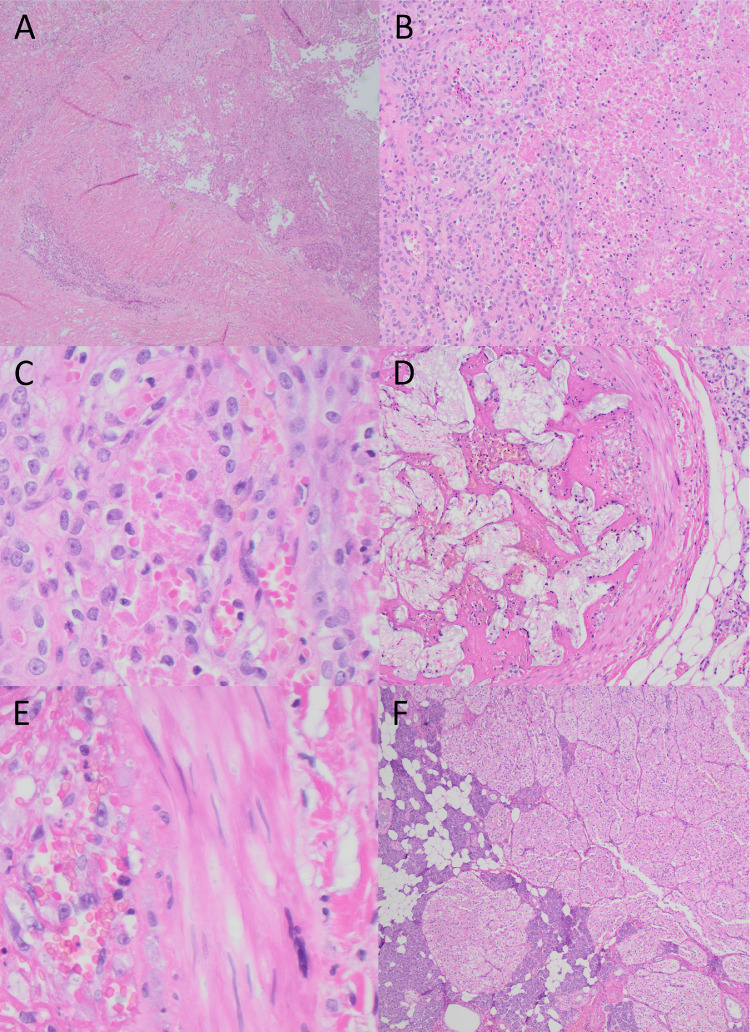
Histologic analysis of the resected mass demonstrated an eosinophilic neoplasm with invasive features approaching the epidermal surface and dermal appendages (Fig. 5A). At higher magnifications, these infiltrative cells were organized into solid sheets and nests of large, atypical oncocytic cells with distinct cell borders surrounding granular, eosinophilic cytoplasm and large, mildly pleomorphic nuclei with irregular nuclear membranes and prominent nucleoli (Figs. 5B and C). Although no mitoses were appreciated, there was associated necrosis and karyorrhectic debris. Vasculature within the sampled tissue demonstrated embolization artifact with luminal red blood cells and histiocytic reaction, but no definitive vascular invasion (Figs. 5D and E). Scattered oncocytomas were identified near uninvolved salivary gland and adipose tissue (Fig. 5F). These findings were consistent with a diagnosis of oncocytic carcinoma, and the patient subsequently underwent neck dissection and a course of adjuvant radiation therapy.
Fig. 5.
Histopathological features by hematoxylin and eosin stain; (A) oncocytic neoplasm with invasion into, but not reaching, epidermis or superficial dermal appendages (4×); (B) oncocytic carcinoma with associated necrosis, ghost cells, and karyorrhectic debris (100×); (C) high-power magnification (400×) of (B) demonstrates large pleomorphic nuclei with occasional nuclear grooves, vesicular nuclei, and prominent nucleoli. Necrotic “ghost” cells and red blood cells are present; (D) embolized blood vessel with histiocytic reaction, indefinite for vascular invasion, along with adjacent adipocytes and salivary gland tissue (100×); (E) high-power magnification (400×) of (D) demonstrates blood vessel wall and lumen filled with red blood cells and histiocytic cells; (F) representative image of associated oncocytoma and surrounding uninvolved salivary gland tissue (40×).
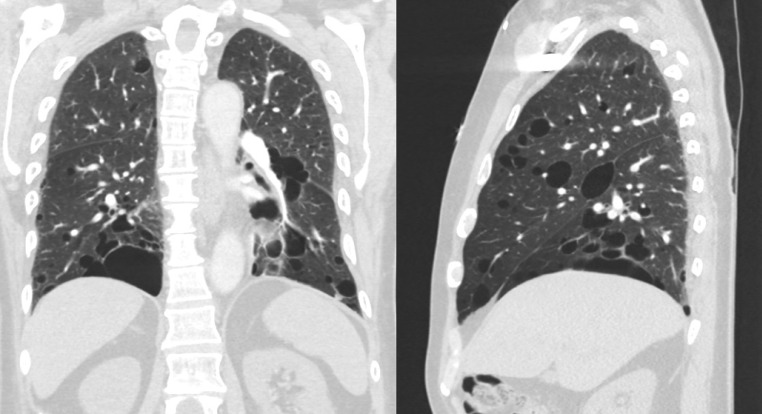
On postoperative day 2 after total right parotidectomy, a CT pulmonary angiogram (Fig. 6) was obtained for suspected pulmonary embolism, which demonstrated multiple cysts in the bilateral lung parenchyma with a slight basilar and subpleural predominance. Due to the combined findings of white papules on the patient's face likely representing fibrofolliculomas, parotid oncocytoma/carcinoma, and characteristic lung cysts, BHD was highly suspected. FLCN genetic testing was performed using peripheral blood sample which confirmed the diagnosis of BHD. Of interest, the patient presented with pneumothorax 1 year later, which was a common complication seen in patients with BHD.
Fig. 6.
Coronal (left) and sagittal (right) computed tomography images demonstrate multiple cysts in the bilateral lung parenchyma with a slight basilar and subpleural predominance.
Discussion
BHD syndrome was initially described in 1977 as a triad of skin manifestations, namely fibrofolliculomas, achordons, and trichodiscomas [1]. Subsequent reports and studies have established an association with multiple systemic manifestations, including pulmonary cysts [13], renal neoplasms [13], colonic polyposis [14,15], lipomas [16], and parathyroid adenomas [16], with the first case of oncocytoma of the parotid gland reported by Liu et al. [17]. Previously, Pavlovich et al. [4] have postulated that oncocytic lesions may be precursors to renal cancers, as most patients with BHD have renal oncocytic cells upon histologic analysis. Our case is the first case of parotid oncocytic carcinoma associated with BHD and establishes that BHD-associated parotid oncocytic lesions, previously identified only as benign oncocytomas in the literature to date, can undergo malignant transformation, and should potentially be regarded with a higher index of suspicion and lower threshold for surgical management.
Oncocytomas are rare benign neoplasms that comprise only 1% of parotid neoplasms [18,19], with oncocytic carcinomas being exceedingly rare, comprising less than 10% of all parotid oncocytic neoplasms in an institutional review [20]. Oncocytes are polygonal cells containing granular, eosinophilic cytoplasm due to an abundance of mitochondria [21]. Oncocytic neoplasms are postulated to result from excess cytoplasmic mitochondria, resulting in oncocytic metaplasia [19]. Previous studies have identified FLCN as the causative gene in BHD [2,22], which encodes the protein folliculin and plays a role in regulating mitochondrial biogenesis, in addition to the mTOR pathway, autophagy, mTORC1 activation [22]. FLCN mRNA is expressed in acinar cells of the parotid gland, in addition to distal renal nephrons, skin, and pulmonary pneumocytes [23]. Recent murine models have shown that FLCN-knockout mice demonstrate increase mitochondrial biogenesis, oxidative phosphorylation, mTOR-S6K upregulation, and nucleotide synthesis [24], [25], [26], which may be the mechanistic link to the observed predisposition to parotid oncocytomas in patients with BHD.
As the clinical presentation and imaging features of benign and malignant oncocytic neoplasms often overlap, histology is crucial for distinguishing benign oncocytomas from malignant oncocytic carcinomas. Due to their high mitochondrial contents, all oncocytes react to histochemical stains for mitochondria, including phosphotungstic acid-hematoxylin [21,27,28]. Oncocytic neoplasms are also stained by cytokeratin markers (KL-1). Whereas benign oncocytomas consist of organized sheets of oncocytes, oncocytic carcinoma exhibits significant cellular atypia, higher mitotic activity, vascular and perineural invasion, and metastases [20,28,29]. In particular, locally infiltrative growth is needed for diagnosing oncocytic carcinoma [27]. Compared to benign oncocytomas, oncocytic carcinoma stains more strongly with MIB-1 and Ki-67 due to higher mitotic activity [28,29].
Although histopathology is invaluable in confirming a final diagnosis, CT and MRI are established first-line imaging modalities in the evaluation of salivary gland tumors, especially in the deep parotid lobe [30,31]. Moreover, CT and MRI imaging can help guide management when surgical management is declined, surgery is relatively contraindicated, or when histopathologic results are inconclusive. Given the association between BHD and oncocytic lesions, the radiologist can play a crucial role in raising oncocytoma as a differential possibility for a parotid mass in a patient with BHD, and vice-versa, to facilitate the clinical diagnosis of BHD in a patient with an oncocytoma.
The imaging findings in this case corroborate much of the existing literature on imaging features of oncocytic neoplasms [30]. Although oncocytic carcinomas have been described as irregularly marginated and heterogeneously enhancing on CT [32], our case demonstrated features typically associated with parotid oncocytomas, which are often well-defined and demonstrate homogenous enhancement. In contrast, pleomorphic adenomas, the most common parotid gland neoplasm, tend to demonstrate less marked early enhancement [33]. Although Warthin tumors and basal cell adenomas also demonstrate early enhancement similar to oncocytomas, these tumors show relatively less enhancement on delayed phases [34,35]. As imaging features of salivary gland tumors overlap on CT, MRI is often used for further characterization.
On MRI, parotid oncocytomas have been described as T1 and T2 hypointense and hyperintense on diffusion-weighted imaging [6,36]. This distinguishes oncocytomas from pleomorphic adenomas, which tend to be T2 hyperintense with heterogeneous nodular enhancement and a T2 dark rim [37]. Warthin tumors, another key diagnostic consideration, are T2 hyperintense and T1 hypointense [36]. The imaging features of parotid oncocytic carcinomas can be variable, and have been described as T1 isointense with central T2 hyperintense signal [32].
Our case of oncocytic carcinoma demonstrated MRI characteristics similar to the BHD-associated oncocytoma reported by Yoshida et al. [6], which was hypointense on contrast-enhanced T1WI relative to the native parotid gland tissue. On the other hand, isolated oncocytomas (without an association with BHD) have been described as iso-intense to native parotid tissue on T1 FS postcontrast and T2 FS WI in a case series by Patel et al. [38], theorized to result from similar lipid compositions of oncocytes relative to normal parotid tissue. In our case, the hypointense signal on T1 FS postcontrast is theorized to result from decreased lipid composition secondary to malignant transformation and/or an association with BHD, as murine models have shown increased lipid metabolism in FLCN-knockout mice [25,26]. A larger sample size is needed to determine the characteristic MR features of oncocytic lesions associated with BHD. Furthermore, morphologic appearance has varied in previous case reports—although many cases, including ours, presented as a solitary parotid mass, other BHD-associated parotid gland oncocytomas have presented as multiple small solid nodules [11,19].
Surgical resection is the first-line treatment for parotid tumors, in part because histopathologic confirmation is essential [30]. In prior case reports of BHD-associated parotid oncocytomas in which management is described, surgical resection was performed in the majority [9,10], with one case opting for close surveillance for changes in the parotid oncocytomas [11]. Whereas surgery typically entails superficial parotidectomy for benign oncocytic carcinomas, oncocytic carcinomas are treated with radical parotidectomy and neck dissection depending on stage, as aggressive surgical management has demonstrated more favorable outcomes [20,28,39,40]. Surgical resection is often combined with adjuvant radiotherapy, and long-term follow-up is indicated due to the risk of distant metastases and local recurrence [40,41]. Because oncocytic carcinomas are exceedingly rare, treatment guidelines and prognosis remain unestablished, although existing case reports have reported a relatively poor prognosis [39], with one case series reporting a 54.9% 5-year survival and local recurrence found in 7 of 12 patients [28]. Other cases of short-term (<1 year) follow-up after surgical management did not identify recurrence [20,41].
In summary, we report the first case of oncocytic carcinoma of the parotid gland in a patient with BHD, with radiologic and histologic correlation. This case establishes the possibility of oncocytic carcinoma in the differential of a parotid gland mass in a patient with BHD, which indicates more aggressive management and surveillance compared to the previously reported cases of benign oncocytoma. The radiologist can play a crucial role in raising oncocytic neoplasm in the differential of a parotid gland mass in a patient with BHD and facilitating the diagnosis of BHD in a patient with an oncocytoma. While overlap and ambiguity exists regarding the imaging features and optimal management of oncocytic carcinoma, this case highlights that radiologic and histologic identification of key features can facilitate timely diagnosis and the potential for growth in establishing characteristic imaging features and optimal management.
Patient consent
Written informed consent for publication of this case report was obtained from the patient.
Footnotes
Funding: The Rohrmann Endowment of the University of Washington, Department of Radiology provided financial support towards the open access publishing fee for this article.
Competing Interests: The authors declare that they have no conflict of interest.
References
- 1.Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113(12):1674–1677. [PubMed] [Google Scholar]
- 2.Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn G Turner ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell 2002;2(2):157–164. doi: 10.1016/S1535-6108(02)00104-6. [DOI] [PubMed]
- 3.Zbar B, Alvord WG, Glenn G, Turner M, Pavlovich CP, Schmidt L, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dubé syndrome. Cancer Epidemiol Biomark Prev. 2002;11(4):393–400. [PubMed] [Google Scholar]
- 4.Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, et al. Renal tumors in the Birt-Hogg-Dubé syndrome. Am J Surg Pathol. 2002;26(12):1542–1552. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hasal E, Baskan EB, Gul S, Dilektasli AG, Sag SO, Adim SB, et al. Birt-Hogg-Dubé syndrome: diagnostic journey of three cases from skin to gene. Ann Dermatol. 2022;34(1):66. doi: 10.5021/ad.2022.34.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida K, Miyagawa M, Kido T, Ide K, Sano Y, Sugawara Y, et al. Parotid oncocytoma as a manifestation of Birt-Hogg-Dubé syndrome. Case Rep Radiol. 2018;2018:1–7. doi: 10.1155/2018/6265175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindor NM, Kasperbauer J, Lewis JE, Pittelkow M. Birt-Hogg-Dube syndrome presenting as multiple oncocytic parotid tumors. Hered Cancer Clin Pract. 2012;10(1):13. doi: 10.1186/1897-4287-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toro JR, Wei MH, Glenn GM, Weinreich M, Toure O, Vocke C, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dube syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45(6):321–331. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu V, Kwan T, Page EH. Parotid oncocytoma in the Birt-Hogg-Dubé syndrome. J Am Acad Dermatol. 2000;43(6):1120–1122. doi: 10.1067/mjd.2000.109288. [DOI] [PubMed] [Google Scholar]
- 10.Vinit J, Friedel J, Bielefeld P, Muller G, Goudet P, Besancenot JF. Syndrome de Birt-Hogg-Dubé et tumeurs multiples récidivantes. Rev Méd Intern. 2011;32(3):e40–e42. doi: 10.1016/j.revmed.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt LS, Nickerson ML, Warren MB, Glenn GM, Toro JR Merino MJ, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dubé syndrome. Am J Hum Genet 2005;76(6):1023–1033. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed]
- 12.Al-Shinnag M, Marfan H, Susman R, Wakeling J, Gustafson S, Wood S, et al. Birt-Hogg-Dubé syndrome and hereditary leiomyomatosis and renal cell carcinoma syndrome: an effective multidisciplinary approach to hereditary renal cancer predisposing syndromes. Front Oncol. 2021;11:738822. doi: 10.3389/fonc.2021.738822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med. 2007;175(10):1044–1053. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Guyadec T, Dufau JP, Poulain JF, Vaylet F, Grossin M, Lanternier G. Multiple trichodiscomas associated with colonic polyposis. Ann Dermatol Venereol. 1998;125(10):717–719. [PubMed] [Google Scholar]
- 15.Rongioletti F, Hazini R, Gianotti G, Rebora A. Fibrofolliculomas, tricodiscomas and acrochordons (Birt-Hogg-Dube) associated with intestinal polyposis. Clin Exp Dermatol. 1989;14(1):72–74. doi: 10.1111/j.1365-2230.1989.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 16.Chung JY, Ramos-Caro FA, Beers B, Ford MJ, Flowers F. Multiple lipomas, angiolipomas, and parathyroid adenomas in a patient with Birt-Hogg-Dube syndrome. Int J Dermatol. 1996;35(5):365–366. doi: 10.1111/j.1365-4362.1996.tb03642.x. [DOI] [PubMed] [Google Scholar]
- 17.Giordano G, Gabrielli M, Gnetti L, Ferri T. Oncocytic carcinoma of parotid gland: a case report with clinical, immunohistochemical and ultrastructural features. World J Surg Oncol. 2006;4(1):54. doi: 10.1186/1477-7819-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and Ge-netics of Head and Neck Tumours. Kleihues P, Sobin LH, series eds. World Health Organization Classification of Tumours. IARC Press; Lyon, France: 2005. [Google Scholar]; (Paperback, ISBN:9283224l 75, Sl ID, www.iarc.fr).
- 19.Shellenberger TD, Williams MD, Clayman GL, Kumar AJ. Parotid gland oncocytosis: CT findings with histopathologic correlation. Am J Neuroradiol. 2008;29(4):734–736. doi: 10.3174/ajnr.A0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capone RB, Ha PK, Westra WH, Pilkington TM, Sciubba JJ, Koch WM, et al. Oncocytic neoplasms of the parotid gland: a 16-year institutional review. Otolaryngol Neck Surg. 2002;126(6):657–662. doi: 10.1067/mhn.2002.124437. [DOI] [PubMed] [Google Scholar]
- 21.Hamed G, Shmookler BM, Ellis GL, Punja U, Feldman D. Oncocytic mucoepidermoid carcinoma of the parotid gland. Arch Pathol Lab Med. 1994;118(3):313–314. [PubMed] [Google Scholar]
- 22.Schmidt LS, Linehan WM. FLCN: the causative gene for Birt-Hogg-Dubé syndrome. Gene. 2018;640:28–42. doi: 10.1016/j.gene.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warren MB, Torres-Cabala CA, Turner ML, Merino MJ, Matrosova VY, Nickerson ML, et al. Expression of Birt–Hogg–Dubé gene mRNA in normal and neoplastic human tissues. Mod Pathol. 2004;17(8):998–1011. doi: 10.1038/modpathol.3800152. [DOI] [PubMed] [Google Scholar]
- 24.Hasumi H, Baba M, Hasumi Y, Huang Y, Oh H, Hughes RM, et al. Regulation of mitochondrial oxidative metabolism by tumor suppressor FLCN. JNCI J Natl Cancer Inst. 2012;104(22):1750–1764. doi: 10.1093/jnci/djs418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isono Y, Furuya M, Kuwahara T, Sano D, Suzuki K, Jikuya R, et al. FLCN alteration drives metabolic reprogramming towards nucleotide synthesis and cyst formation in salivary gland. Biochem Biophys Res Commun. 2020;522(4):931–938. doi: 10.1016/j.bbrc.2019.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan M, Audet-Walsh É, Manteghi S, Dufour CR, Walker B, Baba M, et al. Chronic AMPK activation via loss of FLCN induces functional beige adipose tissue through PGC-1α/ERRα. Genes Dev. 2016;30(9):1034–1046. doi: 10.1101/gad.281410.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Özakkoyunlu Hasçiçek S. Oncocytic lesions of salivary glands with morphological and immunohistochemical finding. SiSli Etfal Hastan Tip Bul Med Bull Sisli Hosp. 2018 doi: 10.14744/SEMB.2018.04935. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou CX, Shi DY, Ma D-q, Zhang J-g, Yu GY, Gao Y. Primary oncocytic carcinoma of the salivary glands: a clinicopathologic and immunohistochemical study of 12 cases. Oral Oncol. 2010;46(10):773–778. doi: 10.1016/j.oraloncology.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Alberty J, August C, Stoll W. Onkozytäre Neoplasien der Parotis. HNO. 2001;49(2):109–117. doi: 10.1007/s001060050719. [DOI] [PubMed] [Google Scholar]
- 30.Tan TJ, Tan TY. CT features of parotid gland oncocytomas: a study of 10 cases and literature review. Am J Neuroradiol. 2010;31(8):1413–1417. doi: 10.3174/ajnr.A2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YYP, Wong KT, King AD, Ahuja AT. Imaging of salivary gland tumours. Eur J Radiol. 2008;66(3):419–436. doi: 10.1016/j.ejrad.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Lubin D, Song S, Zafar HM, Baloch Z. The key radiologic and cytomorphologic features of oncocytic and oncocytoid lesions of the salivary gland. Diagn Cytopathol. 2019;47(6):617–636. doi: 10.1002/dc.24175. [DOI] [PubMed] [Google Scholar]
- 33.Lev MH, Khanduja K, Morris PP, Curtin HD. Parotid pleomorphic adenomas: delayed CT enhancement. AJNR Am J Neuroradiol. 1998;19(10):1835–1839. [PMC free article] [PubMed] [Google Scholar]
- 34.Choi DS, Na DG, Byun HS, Ko YH, Kim CK, Cho JM, et al. Salivary gland tumors: evaluation with two-phase helical CT. Radiology. 2000;214(1):231–236. doi: 10.1148/radiology.214.1.r00ja05231. [DOI] [PubMed] [Google Scholar]
- 35.Yerli H, Teksam M, Aydin E, Coskun M, Ozdemir H, Agildere AM. Basal cell adenoma of the parotid gland: dynamic CT and MRI findings. Br J Radiol. 2005;78(931):642–645. doi: 10.1259/bjr/32453517. [DOI] [PubMed] [Google Scholar]
- 36.Sakai E, Yoda T, Shimamoto H, Hirano Y, Kusama M, Enomoto S. Pathologic and imaging findings of an oncocytoma in the deep lobe of the left parotid gland. Int J Oral Maxillofac Surg. 2003;32(5):563–565. [PubMed] [Google Scholar]
- 37.Zaghi S, Hendizadeh L, Hung T, Farahvar S, Abemayor E, Sepahdari AR. MRI criteria for the diagnosis of pleomorphic adenoma: a validation study. Am J Otolaryngol. 2014;35(6):713–718. doi: 10.1016/j.amjoto.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Patel ND, van Zante A, Eisele DW, Harnsberger HR, Glastonbury CM. Oncocytoma: the vanishing parotid mass. Am J Neuroradiol. 2011;32(9):1703–1706. doi: 10.3174/ajnr.A2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goode RK, Corio RL. Oncocytic adenocarcinoma of salivary glands. Oral Surg Oral Med Oral Pathol. 1988;65(1):61–66. doi: 10.1016/0030-4220(88)90193-4. [DOI] [PubMed] [Google Scholar]
- 40.Huang MW, Zhang JG, Zhang J, Liu SM, Zheng L, Shi Y, et al. Oncocytic carcinoma of the parotid gland. Laryngoscope. 2013;123(2):381–385. doi: 10.1002/lary.23696. [DOI] [PubMed] [Google Scholar]
- 41.Caloglu M, Yurut-Caloglu V, Altaner S, Huseyinova G, Unlu E, Karagol H, et al. Oncocytic carcinoma of the parotid gland. Oncol Res Treat. 2006;29(8–9):388–390. doi: 10.1159/000095029. [DOI] [PubMed] [Google Scholar]