Abstract
Purpose
In recent years, immune checkpoint inhibitors have become a major therapeutic method for the treatment of metastatic colorectal cancer (mCRC). Growing evidence indicates that tumour-infiltrating lymphocytes (TILs) in the tumour microenvironment are a prerequisite for the effectiveness of PD-1/PD-L1 blockade therapy. In this study, we aimed to compare PD-L1 expression and cluster of differentiation 4 (CD4) and CD8 TIL infiltration in primary tumours and paired metastases.
Patients and methods
Altogether, 111 patients with mCRC who underwent surgery at our hospital were included. PD-L1, CD4, and CD8 expression were detected by immunohistochemistry in a tissue microarray. PD-L1 expression was assessed using the combined positivity score (CPS), and a score ≥1 was judged as positive. The area proportion of TILs with positive staining ≥10% was classified as “high”, while <10% was classified as “low”.
Results
We observed the discordance of PD-L1 expression between primary tumours and paired metastases in 35/111 (31.5%) patients (κ = 0.137, P = 0.142). This heterogeneity was significantly correlated with discordance of CD8 TIL infiltration between primary tumours and paired metastases (P = 0.003). Compared with corresponding colorectal cancer tumours, lung metastases showed more CD8 TIL infiltration (P = 0.022, median: 8.5% vs. 5.0%), whereas liver metastases exhibited less CD8 TIL infiltration (P = 0.028, median: 3.0% vs. 5.0%). Area proportion of CD4+ and CD8+ TIL infiltration in lung metastases were all higher than those in liver metastases (P = 0.005, median: 15.0% vs. 9.0%; P = 0.001, median: 8.5% vs. 3.0%). Compared with p MMR (MSI-L/MS-S) subgroup, area proportion of CD8 TIL infiltration in primary tumours and CD4, CD8 TIL infiltration in paired metastases were all higher in d MMR (MSI-H) group (P = 0.026, median: 15.0% vs 5.0%; P = 0.039, median: 15.0% vs 9.0%; P = 0.015, median: 15.0% vs 5.0%). Preoperative chemo/radiotherapy may increase CD8 TIL infiltration in primary tumours (P = 0.045, median: 10.0% vs. 5.0%). CD8 TIL infiltration in primary tumours was an independent predictive factor for overall survival (HR 0.28, 95% CI 0.09–0.93, P = 0.038).
Conclusion
Heterogeneity in PD-L1 expression and CD8 TIL infiltration was found between primary tumours and paired metastases in mCRC. CD8 TIL infiltration in primary tumours could independently forecast the overall survival of patients with mCRC.
Keywords: Metastatic colorectal cancer (mCRC), Programmed death-ligand 1 (PD-L1), CD8 tumour infiltrating lymphocytes (TILs), Heterogeneity, Prognosis
Abbreviations: PD-L1, programmed death-ligand 1; CD8, cluster of differentiation 8; TILs, tumour infiltrating lymphocytes; mCRC, metastatic colorectal cancer; CPS, combined positivity score; MSI-H, microsatellite instability-high; dMMR, deficient mismatch repair; MSI-L, microsatellite instability-low; MS-S, microsatellite stability; pMMR, proficient mismatch repair
1. Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the world and the second leading cause of cancer-related deaths [1]. A previous study showed that approximately half of CRC patients develop distant metastases, especially in the lung or liver [2]. Immune checkpoint inhibitor therapy, which targets the programmed cell death-ligand 1 (PD-L1) and programmed cell death-1 (PD-1) pathways, has been increasingly used in the treatment of metastatic colorectal cancer (mCRC) [3].
As a therapeutic target, PD-L1 expression in primary tumours and distant metastases jointly dictates the response to immunotherapy. However, the expression pattern and prognostic value of PD-L1 can be altered during tumour progression and metastasis [[4], [5], [6], [7]]. Discordance of PD-L1 expression in metastatic and primary tumours has been verified in a variety of cancers, including breast cancer, endometrial cancer and clear-cell renal cell carcinoma [[8], [9], [10]]. Wei et al. also reported that PD-L1 expression in simultaneous liver metastases was significantly greater than that in corresponding colorectal tumours [11]. However, whether this inconsistency of PD-L1 expression exists in other metastatic sites of mCRC has not been investigated. The prognostic value of PD-L1 expression in primary tumours of mCRC has been widely investigated, but the conclusion was quite conflicting [12,13]. Whether PD-L1 expression in metastases affects the prognosis of patients with mCRC has rarely been reported.
The T lymphocyte-mediated adaptive immune response plays an important role in antitumour processes. As the main effector lymphocytes, CD8 tumour infiltrating lymphocytes (TILs) are responsible for killing tumour cells, and CD4 TILs play an auxiliary role by secreting cytokines [4]. Growing evidence indicates that abundant TILs in the immune environment are a prerequisite for the effectiveness of immunotherapy [[14], [15], [16]]. Greater than half of dMMR/MSI-H mCRC patients could benefit from immunotherapy due to higher levels of neoantigens, which attract more infiltration of CD8+ TILs in the tumour microenvironment [17]. A small proportion of MMR-proficient colon cancers achieving pathological response to neoadjuvant immunotherapy also exhibited a high density of CD8 TILs in tumours [18]. Previous literature has found that liver metastases are less sensitive to immunotherapy in metastatic melanoma and non-small-cell lung cancer [19]. Similar results were also observed in mCRC patients, indicating that patients with liver metastases exhibited significantly lower objective response rates and shorter median progression-free survival than those with extrahepatic metastases [20,21]. This phenomenon may be attributed to the unique immune microenvironment of liver metastases [11].
Based on the critical role of PD-L1 expression as well as TILs in predicting clinical benefits from immunotherapy, we investigated the heterogeneity of PD-L1 expression and CD4 and CD8 TIL infiltration in primary tumours and paired metastases as well as their impacts on prognosis.
2. Materials and methods
2.1. Patients and samples
Altogether, 111 patients with mCRC who underwent surgery at the Fourth Hospital of Hebei Medical University between June 2009 and October 2019 were included in this study. Primary and paired metastatic tumour specimens of these patients were collected. Completed clinicopathological information of all patients was obtained through the electronic case database of the Fourth Hospital of Hebei Medical University. An informed consent form was signed for the utilization of the postoperative pathology specimens.
2.2. Tissue microarrays and immunohistochemistry
Postoperative paraffin blocks were collected from each patient and then classified into primary tumour foci and paired metastases. Three representative areas in each paraffin block were selected for tissue microarrays (RP- 20, Servicebio, Wuhan, China). PD-L1, CD4, CD8 and DNA mismatch repair protein (MLH1, MSH2, MSH6, PMS2) were detected by immunohistochemistry. Briefly, paraffin sections were dewaxed and rehydrated. Primary antibodies (rabbit antibodies against PD-L1 (1:2000, Agilent Dako, California, USA), CD4 (1:500, Maixin Biotech, Fuzhou, China), CD8 (1:800, Maixin Biotech, Fuzhou, China), MLH1 (1:200, Abcam, Cambridge, England), MSH2 (1:50, Roche, Mannheim, Germany), MSH6 (1:100, Roche, Mannheim, Germany), PMS2(1:100, Abcam, Cambridge, England)) were added and incubated overnight at 4 °C after endogenous peroxidase blocking and antigen restoration. Secondary antibody was added after washing with PBS and incubating for 20 min at room temperature. DAB staining was performed, and the nuclei were counterstained with haematoxylin. The sections were sealed with neutral gum and observed under a microscope.
2.3. Scoring of immunohistochemistry
Immunohistochemical results were scored by two highly experienced pathologists separately. PD-L1 expression was assessed using the combined positivity score (CPS) as described previously [22]. Tumour cells with any intensity of membrane staining and lymphocytes and macrophages with membrane or cytoplasm staining were all judged to be positive, and a score ≥1 was recorded as positive. CD4 and CD8 expression were evaluated to assess the tumour-infiltrating immune cells as previously described [23,24]. TILs density is defined as the area proportion of TILs infiltration in the whole tumour area.The area proportion of immune cells with positive staining ≥10% was classified as “high”, whereas <10% was classified as “low” [11]. Simultaneous expression of the four mismatch repair proteins (MLH1, MSH2, MSH6, PMS2) was considered as proficient DNA mismatch repair (p MMR). Otherwise, the absence of at least one of the above four indexes is considered to be deficient DNA mismatch repair (d MMR).
2.4. Statistical analysis
Statistics were performed using SPSS 21.0 software. The correlation between PD-L1 expression and clinicopathological data was assessed using the chi-square test and Fisher's exact test. The Kappa concordance test was used to analyse the concordance of PD-L1 expression levels in primary and paired metastases. The Wilcoxon matched-pairs signed rank test and Mann–Whitney test were performed to compare the density of CD4- and CD8-infiltrating lymphocytes between different groups. Ordinal logistic model was used for multivariate analysis of factors associated with heterogeneity of CD8 TIL infiltration between primary tumours and paired metastases.
Overall survival (OS) was calculated as the time interval between CRC diagnosis and the death attributed to CRC metastasis or censored at death from other causes or the last follow-up. Single-factor survival analysis was performed using the log rank test, and multifactor survival analysis was performed using the Cox proportional hazard model. The corresponding cumulative survival function curves were plotted using the Kaplan–Meier method. P < 0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
The detailed clinicopathological characteristics of the 111 patients are shown in Table 1. The average age of patients undergoing resection of primary tumours was 58.3 ± 9.9 years old. A total of 23.4% of tumours were found in the right colon, whereas 76.6% were found in the left colon. All primary tumours were histologically identified as adenocarcinoma. In total, 92.8% were moderately differentiated, whereas 7.2% were poorly differentiated. A total of 93.7% of primary tumours were p MMR (MSI-L/MS-S), whereas 6.3% were d MMR (MSI-H). A total of 71.2% of the cases exhibited synchronous metastases, and 28.8% of the cases exhibited metachronous metastases. A total of 71.2% of metastatic sites were liver, and 19.8% were lung. Metastasis in the peritoneum and brain were noted in 7.2% and 1.8% of cases, respectively.
Table 1.
Basic clinicopathologic data for metastatic colorectal cancers.
| Characteristics | Category | N (%) |
|---|---|---|
| Age | Mean [years (SD)] | 58.3 (9.9) |
| Range | 30–79 | |
| Sex | Male | 84 (75.7) |
| Female | 27 (24.3) | |
| ECOG PS | 0 | 17 (15.3) |
| 1 | 53 (47.7) | |
| 2 | 28 (25.2) | |
| 3 | 13 (11.8) | |
| Diameter of primary tumours | Median [cm (IQR)] | 4.0 (2.1) |
| Range | 1.3–10.0 | |
| Diameter of paired metastases | Median [cm (IQR)] | 2.0 (2.0) |
| Range | 0.6–20 | |
| Primary tumour location | Right colon cancer | 26 (23.4) |
| Left colon cancer | 85 (76.6) | |
| Histological type | Adenocarcinoma | 111 (100) |
| Histopathological grade | Moderately differentiated | 103 (92.8) |
| Poorly differentiated | 8 (7.2) | |
| Blood vessel invasion | Positive | 5 (4.5) |
| Negative | 106 (95.5) | |
| Metastatic type | Synchronous metastasis | 79 (71.2) |
| Metachronous metastasis | 32 (28.8) | |
| Metastatic site | Lung | 22 (19.8) |
| Liver | 79 (71.2) | |
| Brain | 2 (1.8) | |
| Peritoneum | 8 (7.2) | |
| K-ras gene | Wild type | 35 (46.1) |
| Mutant type | 41 (53.9) | |
| MMR/MSI status | d MMR (MSI-H) | 7 (6.3) |
| p MMR (MSI-L/MS-S) | 104 (93.7) | |
| Preoperative chemo/radiotherapy for synchronous metastasis | Yes | 13 (16.5) |
| No | 66 (83.5) |
Abbreviations: d MMR, deficient mismatch repair; p MMR, proficient mismatch repair; MSI-H, microsatellite instability-high; MSI-L, microsatellite instability-low; MS-S, microsatellite stability.
3.2. PD-L1 expression in primary and metastatic tumours
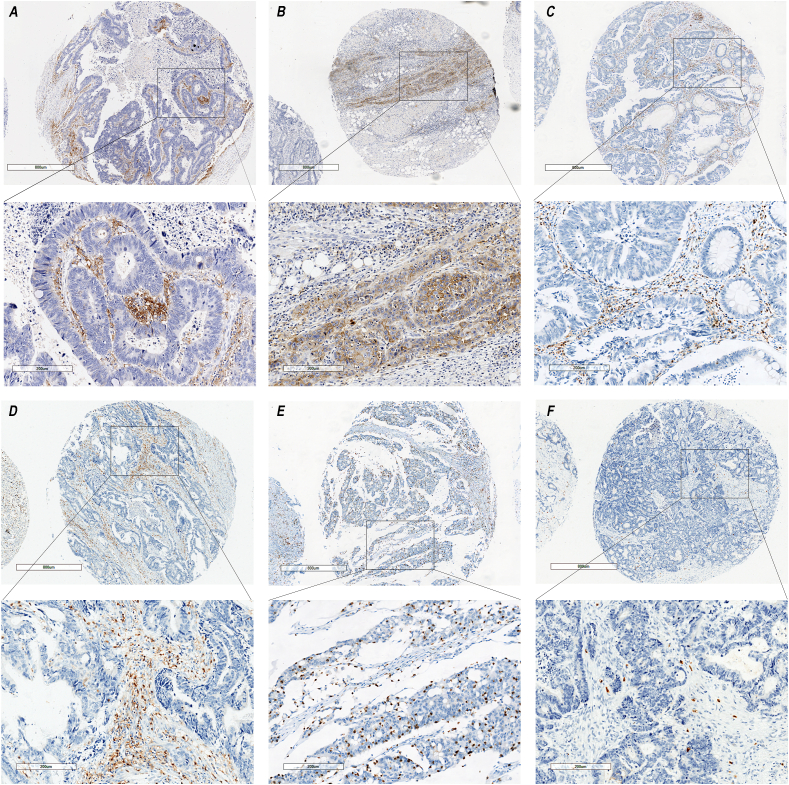
PD-L1 was found to be positive in 20.7% of primary tumours and 27.0% of distant metastases (Fig. 1a and b). PD-L1 expression in primary tumours and paired metastases was associated with CD8 TIL infiltration (P < 0.001, P = 0.003) but not with any other clinicopathological factors. The high CD8+ lymphocyte infiltration group tended to have a higher PD-L1 positive rate (53.8% vs. 10.6%, 45.7% vs. 18.4%) (Table 3).
Fig. 1.
Representative immunostaining of PD-L1, CD4 and CD8. (A) PD-L1 expression in primary tumour with a CPS score of 10.0; (B) PD-L1 expression in corresponding liver metastasis with a CPS score of 30.0; (C) CD4 TIL in primary tumour with area proportion of 15.0%; (D) CD4 TIL in corresponding pulmonary metastasis with area proportion of 15.0%; (E) CD8 TIL in primary tumour with area proportion of 10.0%; (F) CD8 TIL in corresponding liver metastasis with area proportion of 1.0%.
Table 3.
PD-L1 expression in primary tumours compared to their paired distant metastases.
| Distant metastases | Overall (κ = 0.137) | Lung (κ = 0.238) | Liver (κ = 0.112) | |||
|---|---|---|---|---|---|---|
| P valuea | 0.142 | 0.219 | 0.115 | |||
| Primary tumours | Positive | Negative | Positive | Negative | Positive | Negative |
| Positive | 9 (8.1%) | 14 (12.6%) | 3 (13.6%) | 3 (13.6%) | 6 (7.6%) | 9 (11.4%) |
| Negative | 21 (18.9%) | 67 (60.4%) | 4 (18.2%) | 12 (54.6%) | 17 (21.5%) | 47 (59.5%) |
Note:
Kappa test was used.
In our study, 68.5% of patients had concordant PD-L1 expression in primary tumours and corresponding metastases. However, 31.5% of patients were inconsistent. The Kappa test indicated poor consistency in PD-L1 expression between primary and paired metastatic tumours (κ = 0.137, P = 0.142) (Table 2). We also found that this heterogeneity was correlated with discordance of CD8 TIL infiltration between primary tumours and paired metastases (P = 0.003) (Table 3).
Table 2.
Correlation of clinicopathological characteristics with PD-L1 expression in primary tumours and paired metastases.
| Characteristic | Primary tumours(P) |
Paired metastases(M) |
P≠Mb | P valuea | ||
|---|---|---|---|---|---|---|
| PD-L1 (+) | P valuea | PD-L1 (+) | P valuea | |||
| Sex [no (%)] | 0.443 | 0.178 | 0.817 | |||
| Male | 16/84 (19.0) | 20/84 (23.8) | 26/84 (31.0) | |||
| Female | 7/27 (25.9) | 10/27 (37.0) | 9/27 (33.3) | |||
| Age [no (%)] | 0.993 | 0.056 | 0.771 | |||
| ≤60 | 12/58 (20.7) | 11/58 (19.0) | 19/58 (32.8) | |||
| >60 | 11/53 (20.8) | 19/53 (35.8) | 16/53 (30.2) | |||
| Primary tumour location [no (%)] | 0.830 | 0.319 | 0.924 | |||
| Right colon cancer | 5/26 (19.2) | 9/26 (34.6) | 8/26 (30.7) | |||
| Left colon cancer | 8/85 (21.2) | 21/85 (24.7) | 27/85 (31.7) | |||
| Histopathological grade [no (%)] | 0.886 | 1.000 | 1.000 | |||
| Moderately differentiated | 22/103 (21.4) | 28/103 (27.2) | 32/103 (31.1) | |||
| Poorly differentiated | 1/8 (12.5) | 2/8 (25.0) | 3/8 (37.5) | |||
| Blood vessel invasion [no (%)] | 1.000 | 1.000 | 0.940 | |||
| Positive | 1/5 (20.0) | 1/5 (20.0) | 1/5 (20.0) | |||
| Negative | 22/106 (20.8) | 29/106 (27.4) | 34/106 (32.1) | |||
| K-ras gene [no (%)] | 0.518 | 0.352 | 0.835 | |||
| Wild type | 9/35 (25.7) | 7/35 (20.0) | 12/35 (34.3) | |||
| Mutant type | 8/41 (19.5) | 12/41 (29.3) | 15/41 (36.6) | |||
| MMR/MSI status [no (%)] | 0.633 | 0.385 | 0.676 | |||
| d MMR (MSI-H) | 2/7 (28.6) | 3/7 (42.9) | 3/7 (42.9) | |||
| p MMR (MSI-L/MS-S) | 21/104 (20.2) | 27/104 (26.0) | 32/104 (30.8) | |||
| Metastatic type [no (%)] | 0.479 | 0.760 | 0.968 | |||
| Synchronous metastasis | 15/79 (19.0) | 22/79 (27.8) | 25/79 (31.6) | |||
| Metachronous metastasis | 8/32 (25.0) | 8/32 (25.0) | 10/32 (31.3) | |||
| Metastatic site [no. (%)] | 0.582 | 0.806 | 0.923 | |||
| Lung | 6/22 (27.3) | 7/22 (31.8) | 7/22 (36.4) | |||
| Liver | 15/79 (19.0) | 23/79 (29.1) | 26/79 (31.6) | |||
| Preoperative chemo/radiotherapy for synchronous metastasis [no (%)] | 0.425 | 0.551 | 0.120 | |||
| Yes | 4/13 (30.7) | 5/13 (71.4) | 7/13 (30.8) | |||
| No | 11/66 (16.7) | 17/66 (36.9) | 18/66 (31.8) | |||
| CD4 TIL infiltration [no (%)] | 0.091 | 0.192 | ||||
| Low | 8/56 (14.3) | 14/63 (22.2) | ||||
| High | 15/55 (27.3) | 16/48 (33.3) | ||||
| CD8 TIL infiltration [no (%)] | <0.001 | 0.003 | ||||
| Low | 9/85 (10.6) | 14/76 (18.4) | ||||
| High | 14/26 (53.8) | 16/35 (45.7) | ||||
| Discordance of CD4 TIL infiltration between primary and metastatic tumours [no (%)] | 0.736 | |||||
| Yes | 20/66 (30.3) | |||||
| No | 15/45 (33.3) | |||||
| Discordance of CD8 TIL infiltration between primary and metastatic tumours [no (%)] | 0.003 | |||||
| Yes | 16/73 (21.9) | |||||
| No | 19/38 (50.0) | |||||
Note:
Chi-square test or Fisher's exact test was used. Bold indicates p < 0.05.
P≠M indicates discordant PD-L1 expression in primary tumours and paired metastases.
3.3. CD4 and CD8 TIL infiltration in primary and metastatic tumours
In this study, the discrepancy in CD4 and CD8 TIL infiltration between primary tumours and paired metastases was investigated (Fig. 1c–f). Compared to corresponding primary tumours, area proportion of CD8 TIL infiltration was found to be higher in lung metastases (P = 0.022, median: 5.0% vs. 8.5%) and lower in liver metastases (P = 0.028, median: 5.0% vs. 3.0%) (Table 4). After adjusting for diameter of primary tumours and paired metastases, metastatic type, preoperative chemo/radiotherapy, MMR/MSI status, metastatic site was an independent factor associated with heterogeneity of CD8 TIL infiltration between primary tumours and paired metastases (OR 3.51, 95% CI 1.10–9.11, P = 0.033) (Table 5). No differences were observed in CD4 TIL infiltration between primary tumours and corresponding lung (P = 0.060, median: 8.5% vs. 15.0%) or liver metastases (P = 0.387, median: 8.0% vs. 9.0%). Analysis was not performed in peritoneum and brain metastases due to the limited case numbers (Table 4).
Table 4.
Comparison of CD4 and CD8 TIL infiltration in primary tumours and paired metastases.
| Characteristic | Area proportion of CD4 TIL (median, %) P valueb |
Area proportion of CD8 TIL (median, %) P valueb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primary tumours | P valuea | Paired metastases | P valuea | Primary tumours | P valuea | Paired metastases | P valuea | |||
| Overall | 8.0 |
9.0 | 0.113 | 5.0 |
5.0 | 0.346 | ||||
| Sex | 0.200 | 0.272 | 0.994 | 0.906 | ||||||
| Male | 9.0 | 9.0 | 0.440 | 5.0 | 5.0 | 0.328 | ||||
| Female | 5.0 | 10.0 | 0.054 | 5.0 | 5.0 | 0.855 | ||||
| Age | 0.104 | 0.126 | 0.128 | 0.417 | ||||||
| ≤60 | 9.0 | 9.0 | 0.394 | 5.5 | 5.0 | 0.298 | ||||
| >60 | 7.0 | 7.0 | 0.166 | 5.0 | 5.0 | 0.844 | ||||
| Primary tumour location | 0.382 | 0.620 | 0.792 | 0.794 | ||||||
| Right colon | 8.0 | 10.0 | 0.128 | 5.0 | 5.0 | 0.963 | ||||
| Left colon | 7.0 | 9.0 | 0.559 | 5.0 | 5.0 | 0.265 | ||||
| Histopathological grade | 0.639 | 0.762 | 0.376 | 0.880 | ||||||
| Moderately differentiated | 8.0 | 9.0 | 0.157 | 5.0 | 5.0 | 0.467 | ||||
| Poorly differentiated | 8.5 | 15.5 | 0.612 | 9.0 | 5.0 | 0.233 | ||||
| Blood vessel invasion | 0.284 | 0.977 | 0.123 | 0.242 | ||||||
| Positive | 15.0 | 6.0 | 1.000 | 6.0 | 3.0 | 0.082 | ||||
| Negative | 8.0 | 9.5 | 0.109 | 5.0 | 5.0 | 0.609 | ||||
| K-ras gene | 0.272 | 0.986 | 0.248 | 0.287 | ||||||
| Wild type | 8.0 | 10.0 | 0.054 | 5.0 | 5.0 | 0.436 | ||||
| Mutant type | 9.0 | 12.0 | 0.261 | 8.0 | 5.0 | 0.095 | ||||
| MMR/MSI status | 0.728 | 0.039 | 0.026 | 0.015 | ||||||
| d MMR (MSI-H) | 10.0 | 15.0 | 0.344 | 15.0 | 15.0 | 0.813 | ||||
| p MMR (MSI-L/MS-S) | 8.0 | 9.0 | 0.171 | 5.0 | 5.0 | 0.288 | ||||
| Diameter of primary tumours | 0.392 | 0.889 | 0.863 | 0.639 | ||||||
| ≤4 cm | 9.0 | 9.0 | 0.617 | 5.0 | 5.0 | 0.334 | ||||
| >4 cm | 8.0 | 10.0 | 0.090 | 5.0 | 5.0 | 0.803 | ||||
| Diameter of paired metastases | 0.108 | 0.142 | 0.235 0.380 | 0.380 | ||||||
| ≤2 cm | 10.0 | 10.0 | 0.728 | 8.0 | 5.0 | 0.248 | ||||
| >2 cm | 7.0 | 7.5 | 0.588 | 5.0 | 5.0 | 0.881 | ||||
| Metastatic type | 0.123 | 0.918 | 0.265 | 0.976 | ||||||
| Synchronous metastasis | 8.0 | 10.0 | 0.062 | 5.0 | 5.0 | 0.733 | ||||
| Metachronous metastasis | 9.0 | 9.0 | 0.737 | 7.0 | 5.0 | 0.228 | ||||
| Metastatic site | 0.570 | 0.005 | 0.670 | 0.001 | ||||||
| Lung | 8.5 | 15.0 | 0.060 | 5.0 | 8.5 | 0.022 | ||||
| Liver | 8.0 | 9.0 | 0.387 | 5.0 | 3.0 | 0.028 | ||||
| Preoperative chemo/radiotherapy for synchronous metastasis | 0.053 | 0.128 | 0.045 | 0.163 | ||||||
| Yes | 10.0 | 15.0 | 0.327 | 10.0 | 8.0 | 0.988 | ||||
| No | 8.0 | 9.0 | 0.052 | 5.0 | 3.0 | 0.765 | ||||
Note:
Mann-Whitney test was used to compare area proportion of CD4 and CD8 TIL infiltration between different subgroups.
Wilcoxon matched-pairs signed rank test was used to compare area proportion of CD4 and CD8 TIL infiltration between primary tumours and paired metastases within subgroups. Bold indicates p < 0.05.
Table 5.
Multivariate analysis of factors associated with heterogeneity of CD8 TIL infiltration between primary tumours and paired metastases.
| Characteristic | Estimate (s.e.) | Odds Ratio (95%CI) | P valuea |
|---|---|---|---|
|
Diameter of primary tumours ≤4 cm vs > 4 cm |
0.352 (0.428) | 1.42 (0.63–3.29) | 0.410 |
|
Diameter of paired metastases ≤2 cm vs > 2 cm |
−0.065 (0.414) | 0.94 (0.42–2.11) | 0.875 |
|
Metastatic type Synchronous vs metachronous metastasis |
0.713 (0.523) | 2.04 (0.73–5.69) | 0.173 |
|
Metastatic site Lung vs liver |
1.256 (0.590) | 3.51 (1.10–9.11) | 0.033 |
|
Preoperative chemo/radiotherapy No vs yes |
1.022 (0.665) | 2.78 (0.76–8.22) | 0.124 |
|
MMR/MSI status d MMR (MSI-H) vs p MMR (MSI-L/MS-S) |
0.355 (0.827) | 1.43 (0.28–7.20) | 0.668 |
Note:
Ordinal logistic model was used for multivariate analysis. The heterogeneity of CD8 TIL infiltration was split into 3 groups: 1. P > M, primary tumours (high) and paired metastases (low); 2. P = M, primary tumours (high) and paired metastases (high), primary tumours (low) and paired metastases (low); 3. P < M, primary tumours (low) and paired metastases (high). Bold indicates p < 0.05.
Clinical or pathological parameters that could influence CD4 and CD8 TIL infiltration were also assessed in this study. The data indicated that preoperative chemo/radiotherapy might increase CD8 TIL infiltration in primary tumours (P = 0.045, median: 10.0% vs. 5.0%). Moreover, compared with p MMR (MSI-L/MS-S) subgroup, area proportion of CD8 TIL infiltration in primary tumours and CD4, CD8 TIL infiltration in paired metastases were all higher in d MMR (MSI-H) group (P = 0.026, median: 15.0% vs 5.0%; P = 0.039, median: 15.0% vs 9.0%; P = 0.015, median: 15.0% vs 5.0%). In addition, area proportion of CD4 and CD8 TIL infiltration in lung metastases were all greater than those in liver metastases (P = 0.005, median: 15.0% vs. 9.0%; P = 0.001, median: 8.5% vs. 3.0%) (Table 4).
3.4. Survival analysis
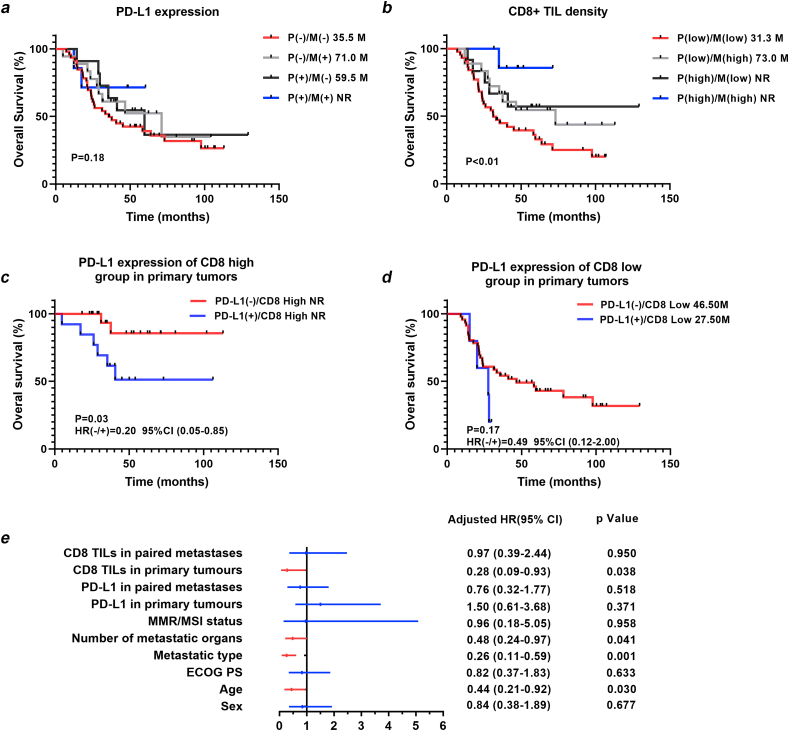
A complete follow-up profile was available for 82 patients with a median follow-up time of 36.5 months. The log rank test revealed no prognostic value of PD-L1 expression in primary and paired metastatic tumours (P = 0.393, P = 0.246) (Table 6, Fig. 2a). More CD8 lymphocyte infiltration in either primary tumours or paired distant metastases predicted a good prognosis (P = 0.036, P = 0.031) (Table 6, Fig. 2b). In multivariate analysis, CD8 TIL density in primary tumours was an independent predictive factor for overall survival (HR 0.28, 95% CI 0.09–0.93, P = 0.038) (Table 6, Fig. 2e).
Table 6.
Univariate and multivariate analysis of overall survival.
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR(95%CI) | P valuea | HR(95%CI) | P value# | |
|
Sex Male vs female |
0.84 (0.41–1.71) | 0.603 | 0.84 (0.38–1.89) | 0.677 |
|
Age ≤60 vs > 60 |
0.44 (0.24–0.79) | 0.005 | 0.44 (0.21–0.92) | 0.030 |
|
ECOG PS ≤2 vs > 2 |
0.43 (0.21–1.14) | 0.034 | 0.82 (0.37–1.83) | 0.633 |
|
Metastatic type Metachronous vs synchronous metastasis |
0.25 (0.14–0.46) | 0.001 | 0.26 (0.11–0.59) | 0.001 |
|
The number of metastatic organs Single vs multi-organ metastasis |
0.45 (0.21–0.95) | 0.009 | 0.48 (0.24–0.97) | 0.041 |
|
MMR/MSI status d MMR (MSI-H) vs p MMR (MSI-L/MS-S) |
0.47 (0.17–1.31) | 0.282 | 0.96 (0.18–5.05) | 0.958 |
|
PD-L1 in primary tumours Positive vs negative |
0.72 (0.36–1.44) | 0.393 | 1.50 (0.61–3.68) | 0.371 |
|
PD-L1 in paired metastases Positive vs negative |
0.66 (0.35–1.25) | 0.246 | 0.76 (0.32–1.77) | 0.518 |
|
CD8 TILs in primary tumours High vs low |
0.39 (0.20–0.76) | 0.036 | 0.28 (0.09–0.93) | 0.038 |
|
CD8 TILs in paired metastases High vs low |
0.46 (0.25–0.85) | 0.031 | 0.97 (0.39–2.44) | 0.950 |
Note:
Univariate analysis of overall survival was performed using the log rank test. # Multivariate analysis of overall survival was performed using the Cox proportional hazard model. Bold indicates p < 0.05.
Fig. 2.
Kaplan-Meier curves for overall survival (OS) of metastatic colorectal cancer patients. (A) PD-L1 expression in primary tumours(P) and paired distant metastases(M) does not affect overall survival; (B) High CD8 lymphocytes infiltration in primary tumours(P) or paired distant metastases(M) predicts a good prognosis; (C) In high CD8 lymphocytes infiltration group, PD-L1 positive expression in primary tumours predicts a worse prognosis; (D) In low CD8 lymphocytes infiltration group, PD-L1 expression in primary tumours does not affect overall survival; (E) CD8 TIL infiltration in primary tumours were independently predictive factors for overall survival.
To further investigate the prognostic significance of PD-L1 expression in different immune microenvironments, patients were divided into four subgroups: PD-L1 negative/CD8 high (n = 10), PD-L1 positive/CD8 high (n = 10), PD-L1 negative/CD8 low (n = 54), and PD-L1 positive/CD8 low (n = 8). Interestingly, PD-L1 expression in primary tumours had a significant effect on prognosis exclusively in the high CD8 TIL density group (HR 0.20, 95% CI 0.05–0.85, P = 0.03) (Fig. 2c and d).
The prognostic significance of PD-L1 expression and CD8 TIL infiltration was also examined in different MMR/MSI status. In d MMR (MSI-H) subgroup, CD8 TIL density in primary tumours was significant prognostic factor (P = 0.038). In p MMR (MSI-L/MS-S) subgroup, CD8 TIL density in primary tumours and paired metastases were all significant prognostic factors (P = 0.036, P = 0.031). However, PD-L1 expression in primary tumours and paired metastases showed no prognostic value in both subgroups (Table 7).
Table 7.
Univariate analysis of overall survival in different MMR/MSI subgroups.
| Characteristic | d MMR (MSI-H) | p MMR (MSI-L/MS-S) | ||
|---|---|---|---|---|
| HR (95%CI) | P valuea | HR (95%CI) | P valuea | |
|
PD-L1 in primary tumours Positive vs negative |
0.23 (0.01–4.71) | 0.343 | 0.81 (0.39–1.67) | 0.590 |
|
PD-L1 in paired metastases Positive vs negative |
1.15 (0.07–18.58) | 0.919 | 0.65 (0.34–1.26) | 0.245 |
|
CD8 TILs in primary tumours High vs low |
0.10 (0.01–0.31) | 0.008 | 0.37 (0.15–0.92) | 0.026 |
|
CD8 TILs in paired metastases High vs low |
0.62 (0.04–10.68) | 0.728 | 0.47 (0.25–0.88) | 0.046 |
Note:
Univariate analysis of overall survival was performed using the log rank test. Bold indicates p < 0.05.
4. Discussion
In recent years, immunotherapy has become a mainstay of treatment for gastrointestinal tumours and other types of solid tumours, including metastatic colorectal cancer. Searching for markers that predict response to immunotherapy as well as other combination therapeutics to enhance the effectiveness of immunotherapy has become an pressing challenge [[25], [26], [27], [28]]. As a target for immunotherapy in mCRC, better characterization of the PD-L1 expression pattern and extent of heterogeneity in primary and paired metastases is of great significance to clinical treatment. The heterogeneous features of PD-L1 expression in various tumours have been extensively described previously, hampering its predictive value for prognosis and immunotherapy efficacy [22,[29], [30], [31]]. Previous studies have reported that PD-L1 expression is enhanced in recurrent and metastatic tumours during colorectal cancer progression or metastasis [32]. Similar inconsistencies were also observed in a subgroup of mCRC patients undergoing simultaneous resection of primary and liver metastatic lesions [11]. In the present study, we observed discordant PD-L1 expression between primary tumours and paired metastases in 31.5% of patients. Therefore, in addition to primary tumours, it is also necessary to assess PD-L1 expression status in distant metastases of mCRC before immunotherapy. Additionally, in our study, we found that the heterogeneity of PD-L1 expression correlated with discordance of CD8 TIL density between primary tumours and paired metastases. It has been confirmed that the cytokines released by T lymphocytes may upregulate PD-L1 expression in tumour cells and thus promote immune escape [5]. Therefore, discrepant infiltration of CD8+ TILs might be partly responsible for the discordance in PD-L1 expression. Detailed mechanisms underlying the correlations need to be further explored.
Previous clinical trials have indicated that liver metastases of patients with mCRC are less sensitive to immunotherapy [20,21]. In the present study, we found that both CD4 and CD8 TIL densities in liver metastases were lower than those in lung metastases. Moreover, compared with corresponding colorectal tumours, liver metastases showed less CD8 TIL infiltration, whereas lung metastases exhibited more CD8 TIL infiltration. In contrast to colorectal tumours and lung metastases, liver metastases tend to exhibit a state of immunosuppression, which may partially explain the poor benefit from immunotherapy in mCRC with liver metastases in clinical trials. The low antigenicity of metastatic tumour cells and hepatic immune tolerance may collectively result in diminished CD4 and CD8 TIL infiltration in liver metastases. Due to the selection of local and systemic immune pressure during colorectal tumour metastasis, subclones with low immunogenicity tend to escape from the immune system and colonize the liver. Thus, liver metastases tend to have lower antigenicity, resulting in insufficient activation and lower infiltration of CD4+ and CD8+ TILs [33]. Hepatic immune tolerance can maintain homeostasis of the liver environment by preventing excessive immune responses to large amounts of antigens from the portal vein. Moreover, hepatic immune tolerance also mediates weaker antitumour immunity in liver metastases [34]. A previous study reported that tumour-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) were recruited in the vicinity of liver metastases to maintain a state of immunosuppression by restraining CD4 and CD8 lymphocyte cell-mediated adaptive immunity [35,36]. Therefore, breaking hepatic immune tolerance to increase the infiltration of effector T cells in liver metastases may be critical to improve the efficacy of immunotherapy for mCRC. In addition, we found relatively increased infiltration of CD4+ and CD8+ TILs in lung metastases. Frequent exposure to pathogens from the atmospheric environment leads to a high infiltration of immune cells in the basal state, which may shape a specific immune landscape in pulmonary metastases [37].
In the present study, we also found that radiotherapy or chemotherapy might promote CD8 TIL infiltration in colorectal tumours. Similar results have also been reported previously by Monjazeb et al. [38]. Preoperative chemo/radiotherapy may remodel the tumour immune microenvironment and turn the so-called “cold tumours” into “hot tumours”. Tumour cells damaged by radiotherapy or chemotherapy may generate new antigens that are presented to T lymphocytes and trigger the activation and infiltration of CD4+ and CD8+ TILs [39]. Therefore, chemo/radiotherapy and immune checkpoint inhibitors might have synergistic effects in treating mCRC, which still remains to be verified in more clinical trials. Additionally, in this study, CD8 TIL density in primary tumours and CD4, CD8 TIL density in paired metastases were all found higher in d MMR (MSI-H) group than p MMR (MSI-L/MS-S) subgroup. As has been reported previously, tumour cells with d MMR (MSI-H) signature have high overall mutation burden and present more neoantigen peptides through MHC class I molecules [26,28]. Thus, these tumours are more likely to be identified as non-self and highly infiltrated by immune cells, especially CD8+ cytotoxic T cells, CD4+ T helper 1(Th1) cells and macrophages [40]. This accounts for the fact that more than half of mCRC patients with d MMR (MSI-H) signature can benefit from immunotherapy [17].
The prognostic significance of PD-L1 expression in mCRC is quite controversial in previous studies. Zhang et al. found that patients with higher PD-L1 expression in colorectal tumours tended to have a worse prognosis [13]. Liu et al. reported no association between PD-L1 expression in colorectal tumours and the overall survival of patients with mCRC [12]. Our data showed that PD-L1 expression in primary tumours exhibited prognostic value only in the high CD8 TIL density group, not in the whole cohort, indicating that high infiltration of CD8 lymphocytes in the tumour microenvironment might be a prerequisite for PD-L1 expression to affect overall survival. In addition, we found that CD8 TIL infiltration in primary tumours but not in paired metastases was an independent factor affecting prognosis, suggesting that CD8 TIL infiltration in primary tumours was more important for predicting overall survival in patients with mCRC.
There were some limitations in this study. First, cases with other sites of metastases, such as the peritoneum and brain, were too limited to achieve statistical analysis. Second, three representative areas for PD-L1 expression evaluation might not be representative of whole tissue sections. Third, only CD4 and CD8 TILs were evaluated without further subdivision of their subsets. Fourth, the infiltration of intratumoral and stromal TILs and their prognostic implications were not assessed separately, which deserves more attention in future investigations. Finally, this study is limited to a Chinese population with a relatively small sample size. More detailed studies remain to be conducted in the future.
5. Conclusion
In summary, heterogeneity in PD-L1 expression and CD8 TIL infiltration was found between primary tumours and paired metastases in mCRC. CD8 TIL infiltration in primary tumours was an independent factor affecting the overall survival of patients with mCRC.
Data availability
The data generated or analyzed in the present study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The study was approved by the Institutional Research Ethics Board of the Fourth Hospital of Hebei Medical University. All procedures performed in this study involving human participants were in accordance with ethical standards of institutional and/or national research committee and in compliance with the Declaration of Helsinki. Each enrolled patient signed an informed consent form to use their samples and records for scientific research.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation; took part in drafting, reversing or critically reviewing the article; have agreed on the journal to which the article will be submitted; and agreed to take responsibility for the contents of the article.
Funding
This work was supported by the Key Project of Precision Medical Joint Foundation of Hebei Province (No. H2020206485) and the Project of Central Government Guide Local Science and Technology Development Foundation of Hebei Province (No. 206Z7705G).
Disclosure
The authors declare they have no competing interests with other people or organizations.
Acknowledgments
We appreciate the team of Professor Yueping Liu (Department of Pathology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China) for their technical assistance in immunohistochemistry.
References
- 1.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., Jemal A., Yu X.Q., He J. Cancer statistics in China, 2015. CA A Cancer J. Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Holch J.W., Demmer M., Lamersdorf C., Michl M., Schulz C., von Einem J.C., Modest D.P., Heinemann V. Pattern and dynamics of distant metastases in metastatic colorectal cancer. Vis. Med. 2017;33:70–75. doi: 10.1159/000454687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franke A.J., Skelton W.P., Starr J.S., Parekh H., Lee J.J., Overman M.J., Allegra C., George T.J. Immunotherapy for colorectal cancer: a review of current and novel therapeutic approaches. J. Natl. Cancer Inst. 2019;111:1131–1141. doi: 10.1093/jnci/djz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez H., Hagerling C., Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S., Crabill G.A., Pritchard T.S., McMiller T.L., Wei P., Pardoll D.M., Pan F., Topalian S.L. Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother. Cancer. 2019;7:305. doi: 10.1186/s40425-019-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha J.H., Chan L.C., Li C.W., Hsu J.L., Hung M.C. Mechanisms controlling PD-L1 expression in cancer. Mol. Cell. 2019;76:359–370. doi: 10.1016/j.molcel.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun C., Mezzadra R., Schumacher T.N. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manson Q.F., Schrijver W., Ter Hoeve N.D., Moelans C.B., van Diest P.J. Frequent discordance in PD-1 and PD-L1 expression between primary breast tumors and their matched distant metastases. Clin. Exp. Metastasis. 2019;36:29–37. doi: 10.1007/s10585-018-9950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engerud H., Berg H.F., Myrvold M., Halle M.K., Bjorge L., Haldorsen I.S., Hoivik E.A., Trovik J., Krakstad C. High degree of heterogeneity of PD-L1 and PD-1 from primary to metastatic endometrial cancer. Gynecol. Oncol. 2020;157:260–267. doi: 10.1016/j.ygyno.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Callea M., Albiges L., Gupta M., Cheng S.C., Genega E.M., Fay A.P., Song J., Carvo I., Bhatt R.S., Atkins M.B., Hodi F.S., Choueiri T.K., McDermott D.F., Freeman G.J., Signoretti S. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol. Res. 2015;3:1158–1164. doi: 10.1158/2326-6066.cir-15-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei X.L., Luo X., Sheng H., Wang Y., Chen D.L., Li J.N., Wang F.H., Xu R.H. PD-L1 expression in liver metastasis: its clinical significance and discordance with primary tumor in colorectal cancer. J. Transl. Med. 2020;18:475. doi: 10.1186/s12967-020-02636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu R., Peng K., Yu Y., Liang L., Xu X., Li W., Yu S., Liu T. Prognostic value of immunoscore and PD-L1 expression in metastatic colorectal cancer patients with different RAS status after palliative operation. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/5920608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W., Acuna-Villaorduna A., Kuan K., Gupta S., Hu S., Ohaegbulam K., Albanese J., Kaumaya M., Levy R., Hwang R.R., Zang X., Lin J., Liu Q., Maitra R., Goel S. B7-H3 and PD-L1 expression are prognostic biomarkers in a multi-racial cohort of patients with colorectal cancer. Clin. Colorectal Cancer. 2021;20:161–169. doi: 10.1016/j.clcc.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Berraondo P., Teijeira A., Melero I. Cancer immunosurveillance caught in the act. Immunity. 2016;44:525–526. doi: 10.1016/j.immuni.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Teng M.W., Ngiow S.F., Ribas A., Smyth M.J. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.can-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angell H., Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr. Opin. Immunol. 2013;25:261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., Goldberg M.V., Cao Z.A., Ledeine J.M., Maglinte G.A., Kopetz S., André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/s1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalabi M., Fanchi L.F., Dijkstra K.K., Van den Berg J.G., Aalbers A.G., Sikorska K., Lopez-Yurda M., Grootscholten C., Beets G.L., Snaebjornsson P., Maas M., Mertz M., Veninga V., Bounova G., Broeks A., Beets-Tan R.G., de Wijkerslooth T.R., van Lent A.U., Marsman H.A., Nuijten E., Kok N.F., Kuiper M., Verbeek W.H., Kok M., Van Leerdam M.E., Schumacher T.N., Voest E.E., Haanen J.B. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020;26:566–576. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 19.Tumeh P.C., Hellmann M.D., Hamid O., Tsai K.K., Loo K.L., Gubens M.A., Rosenblum M., Harview C.L., Taube J.M., Handley N., Khurana N., Nosrati A., Krummel M.F., Tucker A., Sosa E.V., Sanchez P.J., Banayan N., Osorio J.C., Nguyen-Kim D.L., Chang J., Shintaku I.P., Boasberg P.D., Taylor E.J., Munster P.N., Algazi A.P., Chmielowski B., Dummer R., Grogan T.R., Elashoff D., Hwang J., Goldinger S.M., Garon E.B., Pierce R.H., Daud A. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol. Res. 2017;5:417–424. doi: 10.1158/2326-6066.cir-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuoka S., Hara H., Takahashi N., Kojima T., Kawazoe A., Asayama M., Yoshii T., Kotani D., Tamura H., Mikamoto Y., Hirano N., Wakabayashi M., Nomura S., Sato A., Kuwata T., Togashi Y., Nishikawa H., Shitara K. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603) J. Clin. Oncol. 2020;38:2053–2061. doi: 10.1200/jco.19.03296. [DOI] [PubMed] [Google Scholar]
- 21.Wang C., Sandhu J., Ouyang C., Ye J., Lee P.P., Fakih M. Clinical response to immunotherapy targeting programmed cell death receptor 1/programmed cell death ligand 1 in patients with treatment-resistant microsatellite stable colorectal cancer with and without liver metastases. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.18416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou K.I., Peterson B., Serritella A., Thomas J., Reizine N., Moya S., Tan C., Wang Y., Catenacci D.V.T. Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin. Cancer Res. 2020;26:6453–6463. doi: 10.1158/1078-0432.ccr-20-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salgado R., Denkert C., Demaria S., Sirtaine N., Klauschen F., Pruneri G., Wienert S., Van den Eynden G., Baehner F.L., Penault-Llorca F., Perez E.A., Thompson E.A., Symmans W.F., Richardson A.L., Brock J., Criscitiello C., Bailey H., Ignatiadis M., Floris G., Sparano J., Kos Z., Nielsen T., Rimm D.L., Allison K.H., Reis-Filho J.S., Loibl S., Sotiriou C., Viale G., Badve S., Adams S., Willard-Gallo K., Loi S. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gataa I., Mezquita L., Rossoni C., Auclin E., Kossai M., Aboubakar F., Le Moulec S., Massé J., Masson M., Radosevic-Robin N., Alemany P., Rouanne M., Bluthgen V., Hendriks L., Caramella C., Gazzah A., Planchard D., Pignon J.P., Besse B., Adam J. Tumour-infiltrating lymphocyte density is associated with favourable outcome in patients with advanced non-small cell lung cancer treated with immunotherapy. Eur. J. Cancer. 2021;145:221–229. doi: 10.1016/j.ejca.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Ricci A.D., Rizzo A., Brandi G. The DNA damage repair (DDR) pathway in biliary tract cancer (BTC): a new Pandora's box? ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzo A., Ricci A.D. Biomarkers for breast cancer immunotherapy: PD-L1, TILs, and beyond. Expet Opin. Invest. Drugs. 2022;31:549–555. doi: 10.1080/13543784.2022.2008354. [DOI] [PubMed] [Google Scholar]
- 27.Mollica V., Santoni M., Matrana M.R., Basso U., De Giorgi U., Rizzo A., Maruzzo M., Marchetti A., Rosellini M., Bleve S., Maslov D., Tawagi K., Philon E., Blake Z., Massari F. Concomitant proton pump inhibitors and outcome of patients treated with nivolumab alone or plus ipilimumab for advanced renal cell carcinoma. Targeted Oncol. 2022;17:61–68. doi: 10.1007/s11523-021-00861-y. [DOI] [PubMed] [Google Scholar]
- 28.Rosellini M., Marchetti A., Mollica V., Rizzo A., Santoni M., Massari F. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat. Rev. Urol. 2022 doi: 10.1038/s41585-022-00676-0. [DOI] [PubMed] [Google Scholar]
- 29.Li M., Li A., Zhou S., Xu Y., Xiao Y., Bi R., Yang W. Heterogeneity of PD-L1 expression in primary tumors and paired lymph node metastases of triple negative breast cancer. BMC Cancer. 2018;18:4. doi: 10.1186/s12885-017-3916-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan C., Liu Z., Yu Q., Wang X., Bian M., Yu Z., Yu J. Expression of PD-1/PD-L1 in primary breast tumours and metastatic axillary lymph nodes and its correlation with clinicopathological parameters. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-50898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stovgaard E.S., Bokharaey M., List-Jensen K., Roslind A., Kümler I., Høgdall E., Nielsen D., Balslev E. PD-L1 diagnostics in the neoadjuvant setting: implications of intratumoral heterogeneity of PD-L1 expression in triple negative breast cancer for assessment in small biopsies. Breast Cancer Res. Treat. 2020;181:553–560. doi: 10.1007/s10549-020-05655-w. [DOI] [PubMed] [Google Scholar]
- 32.Wang H.B., Yao H., Li C.S., Liang L.X., Zhang Y., Chen Y.X., Fang J.Y., Xu J. Rise of PD-L1 expression during metastasis of colorectal cancer: implications for immunotherapy. J. Dig. Dis. 2017;18:574–581. doi: 10.1111/1751-2980.12538. [DOI] [PubMed] [Google Scholar]
- 33.Angelova M., Mlecnik B., Vasaturo A., Bindea G., Fredriksen T., Lafontaine L., Buttard B., Morgand E., Bruni D., Jouret-Mourin A., Hubert C., Kartheuser A., Humblet Y., Ceccarelli M., Syed N., Marincola F.M., Bedognetti D., Van den Eynde M., Galon J. Evolution of metastases in space and time under immune selection. Cell. 2018;175:751–765. doi: 10.1016/j.cell.2018.09.018. e716. [DOI] [PubMed] [Google Scholar]
- 34.Doherty D.G. Immunity, tolerance and autoimmunity in the liver: a comprehensive review. J. Autoimmun. 2016;66:60–75. doi: 10.1016/j.jaut.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Yu J., Green M.D., Li S., Sun Y., Journey S.N., Choi J.E., Rizvi S.M., Qin A., Waninger J.J., Lang X., Chopra Z., El Naqa I., Zhou J., Bian Y., Jiang L., Tezel A., Skvarce J., Achar R.K., Sitto M., Rosen B.S., Su F., Narayanan S.P., Cao X., Wei S., Szeliga W., Vatan L., Mayo C., Morgan M.A., Schonewolf C.A., Cuneo K., Kryczek I., Ma V.T., Lao C.D., Lawrence T.S., Ramnath N., Wen F., Chinnaiyan A.M., Cieslik M., Alva A., Zou W. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciner A.T., Jones K., Muschel R.J., Brodt P. The unique immune microenvironment of liver metastases: challenges and opportunities. Semin. Cancer Biol. 2021;71:143–156. doi: 10.1016/j.semcancer.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Altorki N.K., Markowitz G.J., Gao D., Port J.L., Saxena A., Stiles B., McGraw T., Mittal V. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer. 2019;19:9–31. doi: 10.1038/s41568-018-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monjazeb A.M., Giobbie-Hurder A., Lako A., Thrash E.M., Brennick R.C., Kao K.Z., Manuszak C., Gentzler R.D., Tesfaye A., Jabbour S.K., Alese O.B., Rahma O.E., Cleary J.M., Sharon E., Mamon H.J., Cho M., Streicher H., Chen H.X., Ahmed M.M., Mariño-Enríquez A., Kim-Schulze S., Gnjatic S., Maverakis E., Marusina A.I., Merleev A.A., Severgnini M., Pfaff K.L., Lindsay J., Weirather J.L., Ranasinghe S., Spektor A., Rodig S.J., Hodi S.F., Schoenfeld J.D. A randomized trial of combined PD-L1 and CTLA-4 inhibition with targeted low-dose or hypofractionated radiation for patients with metastatic colorectal cancer. Clin. Cancer Res. 2021;27:2470–2480. doi: 10.1158/1078-0432.ccr-20-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajamanickam V., Ballesteros-Merino C., Samson K., Ross D., Bernard B., Fox B.A., Tran E., Newell P., Duhen T. Robust antitumor immunity in a patient with metastatic colorectal cancer treated with cytotoxic regimens. Cancer Immunol. Res. 2021;9:602–611. doi: 10.1158/2326-6066.cir-20-1024. [DOI] [PubMed] [Google Scholar]
- 40.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B., Shia J., Segal N.H., Diaz L.A., Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated or analyzed in the present study are available from the corresponding author upon reasonable request.