Abstract
Anti-myelin oligodendrocyte glycoprotein (MOG)-immunoglobulin G (IgG) associated disorder (MOGAD) is an immune-mediated central nervous system (CNS) inflammatory demyelinating disorder that has been widely recognized in recent years. It is distinct from multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD), which are separate disease spectrums. Here we report the case of a 5-year-old boy who was admitted for 3 days with fever, headache, and vomiting. Magnetic resonance imaging revealed abnormal hyperintensity in the left thalamus and positive serum IgM for M. pneumoniae. After treatment with azithromycin, the headache gradually disappeared, but paralysis and urinary retention occurred on the 6th day after admission. MRI re-examination showed that the original abnormal signal in the left thalamus was significantly weakened, but new abnormal signals appeared in the brain and cerebrospinal cord, and the serum MOG-IgG was positive. After treatment, the child has fully recovered and is still receiving follow-up care. We believe that this is a case of MOGAD in a child with a biphasic ADEM phenotype secondary to M. pneumoniae infection, which has potential value in elucidating the pathophysiology of MOGAD.
Keywords: Anti-myelin oligodendrocyte glycoprotein-IgG associated disorders, Acute disseminated encephalomyelitis, Inflammatory demyelinating disease, M. pneumoniae infection, Case report
Abbreviations: MOGAD, Anti-myelin oligodendrocyte glycoprotein (MOG)-immunoglobulin G associated disorders; CNS, central nervous system; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; T2WI, T2 weighted images
Highlights
-
•
This is a case of MOGAD in a child with a biphasic ADEM phenotype.
-
•
The case may be secondary to M. pneumoniae infection.
-
•
The case has potential value in elucidating the pathophysiology of MOGAD.
1. Background
Myelin oligodendrocyte glycoprotein (MOG)-immunoglobulin G (IgG) associated disorder (MOGAD) is a newly named inflammatory demyelinating disease of central nervous system in 2018 [1], and its main symptom is optic neuritis (ON), myelitis, optic neuromyelitis, and acute disseminated encephalomyelitis (ADEM). In children, ADEM is a common clinical manifestation of MOGAD [2], and is thought to be related to previous stimuli such as a viral infection or immunity, with an annual incidence of approximately 0.3–0.6 per 100 000 children [3]. ADEM cases caused by M. pneumoniae infection have been confirmed, but there are few reports of anti-MOG -IgG-related ADEM caused by M. pneumoniae infection [4]. However, the pathogenesis of CNS demyelination induced by M. pneumoniae infection remains unclear. Some scholars believe that it may be related to the direct damage of nerve cells by M. pneumoniae. Most scientists believe that M. pneumoniae infection triggers an immune attack leading to demyelination of the central nervous system [5]. Here, we report a case of MOGAD secondary to M. pneumoniae infection in a child presenting clinically as ADEM.
2. Case presentation
The child is 5 years old. He was admitted to the pediatric emergency department with fever, headache, and vomiting for 3 days. The child's fever was irregular, with the highest body temperature of 38.7 °C. The headache was located in the front of the head, accompanied by non-projective vomiting 4–5 times. One week before the onset of fever, he had a mild cough that resolved spontaneously without treatment. Physical examination shows that the child was clear consciousness, listlessness, normal gait, neck resistance, lung auscultation, heart and abdominal examination were normal. Knee and Achilles tendon reflexes were normal. Babinski is negative, normal muscle strength and tone in the extremities. The emergency department performed head CT examination for the child, which indicated brainswelling. Blood routine examination: white blood cells 33.02 × 10^9/L, neutrophils 87.9%. C-reactive protein 39.12 mg/L. Therefore, the emergency department admitted the child with encephalitis.
After hospitalization, additional examinations were performed on the child. Cerebrospinal fluid pressure was 180 mmH2O, white blood cells were 28 × 10^6/L, mononuclear cells were 36%, and polymorphonuclear cells were 64%. Glucose, chloride, and protein were normal (Table 1). Brain MRI showed abnormal hyperintensity in the left dorsal thalamus on T2 weighted images (T2WI) (Fig. 1A–D). EEG results showed that the central area, parieto-occipital area, and temporal-middle-posterior area were mainly active at frequencies of 2–3 Hz and 4–7 Hz, and amplitudes of 50–140 μV, with poor background amplitude modulation and unstable baseline, suggesting impaired brain function. 1:32 Serum M. pneumoniae IgM test was positive. In addition, we performed cerebrospinal fluid and blood cultures, antibody testing for respiratory viruses (including respiratory syncytial virus, influenza, parainfluenza virus, and adenovirus), and interferon-gamma releasing assay for Mycobacterium tuberculosis, all of which were negative. According to the above medical history and examination results, we considered the possibility of meningoencephalitis caused by M. pneumoniae, and gave azithromycin (10 mg/kg/day) and mannitol to reduce brainswelling. After treatment, the mental state of the child improved, and symptoms such as headache and vomiting gradually disappeared. The child's fever symptoms still existed, but the body temperature dropped from a heat peak of 38.7 °C–37.5 °C.
Table 1.
Summary of notable serum and spinal fluid studies.
| Test | The 1st day after admission | The 6th day after admission | The 13th day after admission |
|---|---|---|---|
| CSF studies | |||
| White blood cell count (0–15 × 106/L) | 28 × 106/L | 214 × 106/L | 8 × 106/L |
| Mononuclear cells | 36.0% | 6.0% | 87.0% |
| Polymorphonuclear cells | 64.0% | 94.0% | 13.0% |
| Protein (0.15–0.45g/L) | 0.23g/L | 0.83g/L | 0.16g/L |
| Glucose (2.80–4.50mmol/L) | 4.18mmol/L | 4.21mmol/L | 3.05mmol/L |
| Chloride (120.0–132.0mmol/L) | 119.0mmol/L | 121.0mmol/L | 124.6mmol/L |
| IgG (0–0.04g/L) | 0.060g/L | 0.14g/L | 0.000g/L |
| IgA (0.0015–0.006g/L) | 0.000g/L | 0.000g/L | 0.000g/L |
| IgM (0–0.001g/L) | 0.000g/L | 0.000g/L | 0.000g/L |
| Lactate dehydrogenase (109–245U/L) | 15.1U/L | 31.9U/L | 26.1U/L |
| Adenosine deaminase (4–18U/L) | 0.6U/L | 0.0U/L | 0.6U/L |
| Serum studies | |||
| Serum Mycoplasma pneumoniae IgM | Positive (1:32 titer) | Not tested | Not tested |
| Serum MOG IgG | Not tested | Positive (1:10 titer) | Negative (2 months after illness) |
| Serum AQP4 IgG | Not tested | Negative | Not tested |
| Serum GFAP IgG | Not tested | Negative | Not tested |
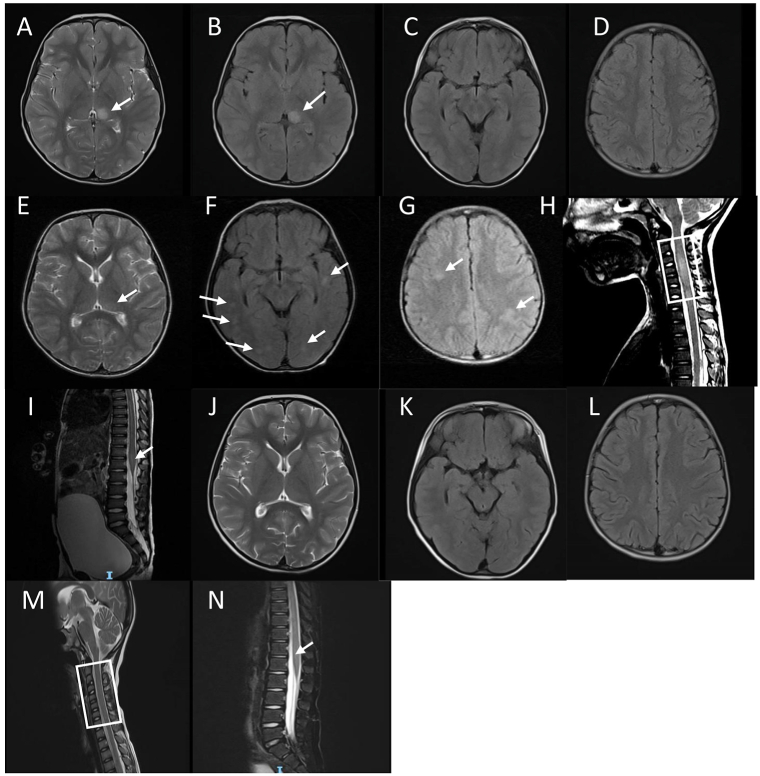
Fig. 1.
MRI of the head and spinal cord. (A–D) On the 2nd day after admission. (A) the T2WI sequence showed hyperintensity in the left thalamus; (B) the FLAIR sequence showed hyperintensity in the left thalamus; (C and D) the FLAIR sequence showed that there was no obvious abnormal signal in the temporal lobe and semi-oval centers; (E–I). On the 6th day after admission. (E) the T2WI sequence showed a significant decrease in left thalamus hyperintensity compared with A; (F) the FLAIR sequence showed multiple asymmetric abnormally hyperintensity in bilateral temporal and occipital lobes at the temporal lobe level; (G) the FLAIR sequence showed multiple asymmetric abnormally hyperintensity in bilateral semi-oval centers; (H) the T2WI sequence showed abnormally hyperintensity of C3–C7 in the cervical spinal cord; (I) the T2WI sequence showed abnormally hyperintense signals in the conus spinal cord; (J–N) 2 months after illness. (J) the T2WI sequence showed dissipation of the left thalamic lesion; (K) the axial FLAIR sequence showed bilateral temporal lobe level lesion dissipation; (L) the FLAIR sequence showed significant dissipation of bilateral semi-oval centers lesion; (M) the T2WI sequence showed that the cervical medullary lesion was basically dispersed. (N) the T2WI sequence showed that the conus lesions were basically dispersed.
However, on the 6th day after admission, he developed quadriparesis, hyperalgesia, dysuria, and his highest body temperature was 38.6 °C. Physical examination revealed that the child was conscious, listless, without cervical resistance, and had a distended abdomen. The abdominal button examination showed that the bladder area rose to 2 cm below the navel, and the physiological reflexes such as abdominal reflex and knee reflex are normal. Babinski was negative, his limb strength was grade 4, and he could not walk independently. Considering that the child has spinal cord lesions and autoimmune diseases cannot be ruled out, we re-examined the cerebrospinal fluid and head MRI, adding a spinal cord MRI scan. In addition, MOG-IgG, AQP4-IgG and GFAP-IgG were also tested in serum. Cerebrospinal fluid examination showed an increase in white blood cells from 28 × 10^6/L to 214 × 10^6/L with a predominance of multinucleated cells (94%), protein 0.83 g/L, and glucose and chloride normal (Table 1). Brain MRI T2WI showed that the original abnormal hyperintensity in the thalamus was significantly attenuated, but there were new abnormally asymmetrical hyperintensity with blurred borders in the bilateral frontal, parietal, and temporal lobes (Fig. 1E–I). In addition, the 3rd to 7th segment of the cervical spine, the 6th to 12th segment of the thoracic spine, and the medullary cone also had abnormally hyperintensity. According to the above clinical manifestations and examination results, the child was diagnosed as acute disseminated encephalomyelitis (ADEM), and given intravenous immunoglobulin (400 mg/kg/day, for 5 consecutive days) and methyl prednisolone treatment (20 mg/kg/day, for 3 consecutive days). After treatment with methyl prednisolone, oral prednisone was given as maintenance therapy. With the results of serum MOG-IgG positive at 1:10 and both AQP4-IgG and GFAP-IgG negative, we revised the diagnosis of this child to MOG-IgG-associated disease (MOGAD).
After the above treatment, the child's body temperature returned to normal on the 8th day after admission. On day 14, he felt much better, his limb strength returned to normal, and his dyuria disappeared. On the 15th day, the child was discharged from the hospital. He continued to receive prednisone maintenance therapy for 6 months after discharge. Two months after discharge, MRI was re-examined, and the original abnormal signals in the brain and spinal cord of the child were significantly weakened (Fig. 1J-N), and serum MOG-IgG was negative (Table 1). At present, the child is still under follow-up, and no special discomfort has been reported.
3. Discussion
MOGAD is a group of inflammatory demyelinating diseases of the central nervous system. According to the diagnostic criteria proposed by the research team of Sean J. Pittock research team in 2018 [1], the diagnosis of MOGAD needs to meet the following three conditions: a. Serum MOG-IgG positive based on cell method detection; b. Clinical manifestations are any of the following: 1).ADEM; 2).ON, including chronic recurrent inflammatory optic neuropathy; 3).Transverse myelitis, including long-segment transverse myelitis and short-segment transverse myelitis; 4).Brain or brainstem syndrome with demyelinating disease; 5).Any of the above Combination; c. Exclude other diagnoses. The patient had the clinical manifestations of quadriparesis, hyperalgesia, and urinary retention. MRI of the head showed multiple demyelinating lesions in the brain, and MRI of the spine showed long-segment myelopathy involving the cervical,thoracic and conus cords, consistent with the imaging features of ADEM. Combined with the positive results of serum MOG-IgG, the diagnosis of MOGAD was confirmed. ADEM occurs in children, usually as a result of early infection or vaccination. According to some researchers, the early infection with viruses is more common, such as influenza virus, Epstein-Barr virus, measles virus, enterovirus, and coronavirus. M. pneumoniae infection has also been reported, but it is relatively rare [6]. The MOGAD presenting as ADEM secondary to M. pneumoniae infection has only been reported by Bonagiri P et al. [4]. The case we report is unique because it represents a biphasic course. In the first stage, the clinical manifestations of the child were fever, headache, vomiting, positive meningeal irritation signs, mildly elevated white blood cells in the cerebrospinal fluid, and head MRI showed abnormal hyperintense left thalamus in the T2WI sequence. The EEG slow wave increased in multiple parts, and the serum M. pneumoniae antibody was positive at 1:32. After treatment with azithromycin, the child's clinical symptoms gradually improved, and the MRI review of the brain also showed that the original lesion in the left thalamus had subsided compared with before. All of these suggest that the first stage may be the course of mycoplasmal meningoencephalitis. However, we were not able to perform detection of M. pneumoniae in CSF such as high-throughput sequencing or PCR, which is a limitation in this case. In the second stage, the child develops persistent fever and new neurological symptoms such as quadriparesis and difficulty urinating. MRI showed multiple demyelinating lesions in the brain and spinal cord, and serum MOG-IgG was positive. The results indicated that the second stage of the ADEM phenotype induced by Mycoplasma meningoencephalitis is the MOGAD process.
There are two main views on the mechanism of central nervous system injury after M. pneumoniae infection. On the one hand, M. pneumoniae enters the brain through the blood-brain barrier and directly damages the central nervous system. The mechanism is based on the detection of mycoplasma DNA in the cerebrospinal fluid of patients. Another view is that M. pneumoniae damages the central nervous system through the principle of molecular simulation through immune mechanism, and the detection of corresponding pathogenic antibodies in serum and cerebrospinal fluid is the evidence of this mechanism [5,7]. However, the pathogenesis of M. pneumoniae causing MOGAD remains unclear. The first stage of this case we reported may be the course of mycoplasma meningoencephalitis, suggesting that the pathological process at this stage may be the direct damage of mycoplasma to the central nervous system. The second stage of the pathological process supports the immune-mediated damage of the CNS by mycoplasma. Therefore, the mechanism by which mycoplasma damages the central nervous system may be different in different stages of the disease.
Fever is not a common symptom in patients with demyelinating central nervous system diseases such as multiple sclerosis, neuromyelitis Optica, and ADEM. However, fever is a common symptom in MOG-IgG-positive ADEM. Lampros A et al. reviewed 146 patients with MOGAD, 37% of whom developed fever, and 79% of whom had fever for more than 7 days. The cause of the fever is unknown, but it may be caused by encephalomyelitis and meningitis [8,9]. The biphasic course of this case may provide clues to explain the cause of fever in MOGAD cases. The fever in the first stage of the child improved after using azithromycin, suggesting that the fever in this stage may be caused by mycoplasma infection. The persistent fever in the second stage returned to normal after immunoglobulin and glucocorticoid treatment, suggesting that the cause of the fever in the second stage should be considered to be autoimmunity.
4. Conclusion
We believe that this is a case of MOGAD in a child with a biphasic ADEM phenotype secondary to M. pneumoniae infection, which has potential value in elucidating the pathophysiology of MOGAD.
Ethics approval and consent to participate
Ethical approval was obtained by the ethical committee of Liuzhou People's Hospital.
Consent for publication
Written informed consent for publication was obtained from patient.
Declarations
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article.
Funding statement
This study was supported by the National Natural Science Foundation of China (No. 82060393), and Guangxi Natural Science Foundation (No. 2020GXNSFAA159124), Guangxi Medical and health key cultivation discipline construction project, and Guangxi Zhuang Autonomous Region Health Committee self-raised scientific research project (Z-B20221301).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
None.
References
- 1.López-Chiriboga A.S., Majed M., Fryer J., Dubey D., McKeon A., Flanagan E.P., et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-associated disorders. JAMA Neurol. 2018;75(11):1355–1363. doi: 10.1001/jamaneurol.2018.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramanathan S., Mohammad S., Tantsis E., Nguyen T.K., Merheb V., Fung V.S.C., et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J. Neurol. Neurosurg. Psychiatry. 2018;89(2):127–137. doi: 10.1136/jnnp-2017-316880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray M.P., Gorelick M.H. Acute disseminated encephalomyelitis. Pediatr. Emerg. Care. 2016;32(6):395–400. doi: 10.1097/pec.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 4.Bonagiri P., Park D., Ingebritsen J., Christie L.J. Seropositive anti-MOG antibody-associated acute disseminated encephalomyelitis (ADEM): a sequelae of Mycoplasma pneumoniae infection. BMJ Case Rep. 2020;13(5) doi: 10.1136/bcr-2020-234565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer Sauteur P.M., Jacobs B.C., Spuesens E.B., Jacobs E., Nadal D., Vink C., et al. Antibody responses to Mycoplasma pneumoniae: role in pathogenesis and diagnosis of encephalitis? PLoS Pathog. 2014;10(6) doi: 10.1371/journal.ppat.1003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito S., Di Pietro G.M., Madini B., Mastrolia M.V., Rigante D. A spectrum of inflammation and demyelination in acute disseminated encephalomyelitis (ADEM) of children. Autoimmun. Rev. 2015;14(10):923–929. doi: 10.1016/j.autrev.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Zaidy S.A., MacGregor D., Mahant S., Richardson S.E., Bitnun A. Neurological complications of PCR-proven M. Pneumoniae infections in children: prodromal illness duration may reflect pathogenetic mechanism. Clin. Infect. Dis. 2015;61(7):1092–1098. doi: 10.1093/cid/civ473. [DOI] [PubMed] [Google Scholar]
- 8.Lampros A., De Broucker T., Bonnan M. Fever is a common onset feature of MOG-IgG associated disorders (MOGAD) Mult. Scler. Relat. Disord/ 2021;49 doi: 10.1016/j.msard.2021.102748. [DOI] [PubMed] [Google Scholar]
- 9.Armangue T., Olivé-Cirera G., Martínez-Hernandez E., Sepulveda M., Ruiz-Garcia R., Muñoz-Batista M., et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: a multicentre observational stud. Lancet Neurol. 2020;19(3):234–246. doi: 10.1016/s1474-4422(19)30488-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.