Abstract
Background
Although homeostasis of the cardiovascular system is regulated by the cerebral cortex via the autonomic nervous system, the role of abnormal brain functional connectivity (FC) networks in patients with cardiac dysfunction remains unclear. Here, we report thalamus-based FC alterations and their relationship with clinical characteristics in patients with coronary heart disease (CHD).
Methods
We employed resting-state functional magnetic resonance imaging (rs-fMRI) to acquire imaging data in twenty-six patients with CHD alongside sixteen healthy controls (HCs). Next, we performed a thalamus-based FC analysis to profile abnormal FC patterns in the whole brain. Subsequently, the mean time series of the brain regions that survived in the FC analysis were used to determine correlations with clinical parameters in patients with CHD.
Results
We found no statistically significant differences in demographic and clinical data between patients with CHD and HCs. Patients with CHD showed decreased FC patterns between bilateral thalami and left hemisphere, encompassing supplementary motor area, superior frontal gyrus, superior parietal gyrus, inferior parietal gyrus, middle cingulate cortex, lingual gyrus and calcarine sulcus.
Conclusions
These findings not only have implications in clarifying the relationship between cerebral functional imbalance and cardiovascular system, but also provide valuable insights to guide future evaluation and management of cardiac autonomic regulation via the brain-heart axis.
Keywords: Coronary heart disease, Functional connectivity analysis, Resting-state, Functional magnetic resonance imaging, Thalamus
Abbreviations: ANS, autonomic nervous system; CHD, coronary heart disease; CNS, central nervous system; CVD, cardiovascular disease; DMN, default mode network; ECN, executive control network; FC, functional connectivity; IPG, inferior parietal gyrus; MCC, middle cingulate cortex; MCI, myocardial ischemia; MoCA, Montreal Cognitive Assessment; Rs-fMRI, resting-state functional magnetic resonance imaging; SFG, superior frontal gyrus; SMA, supplementary motor area; SMN, sensorimotor network; SN, salient network; SNS, sympathetic nervous system; SPG, superior parietal gyrus
1. Introduction
Over the past three decades, the incidence of cardiovascular diseases (CVDs) has rapidly risen, from 271 million in 1990 to 523 million in 2019, thus becoming the leading cause of mortality and disability worldwide and causing a serious public health problem [1]. CVDs are a group of disorders affecting the heart and blood vessels, and coronary heart disease (CHD) is one of the most common CVDs in clinical practice. There has been increasing evidence that CHD is associated with structural and functional changes in the central nervous system (CNS) [2]. Accumulating clinical evidence suggests a close relationship between cerebral damage and cardiac dysfunction [3,4]. Additionally, studies have attributed a considerable proportion of post-stroke deaths to cardiovascular complications, which is only inferior to neurological damage [5]. Moreover, cardiac function is closely regulated by the autonomic nervous system (ANS), which is controlled by core regions in the cerebral cortex and brainstem. The ANS is divided into the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS), which work antagonistically to maintain cardiovascular homeostasis [6]. Based on these findings, it is plausible that CHD may have significant adverse effects on central nervous function. However, little is known regarding the neuropathological mechanism underlying the dysfunction of the cerebral cortex and its relationship with CHD.
Resting-state functional magnetic resonance imaging (rs-fMRI) is a non-invasive method for probing changes in cerebral neurofunction. Some studies have demonstrated that cardiogenic visceral inputs ascend to brainstem nuclei amygdala, insula and thalamus, and are subsequently relayed forward to the high-level cortex [6,7]. These cerebral regions have not only been confirmed to process cardiac activity, but also to construct some core functional networks, such as the default mode network (DMN), salient network (SN), executive control network (ECN), and limbic system [8]. Several longitudinal studies recently revealed that CHD are independent risk factors for cognitive impairment [9] and affective disorders [10], mainly manifesting decreased executive function and abnormal emotional response. Results from a previous fMRI study also demonstrated that patients with CHD had cognitive difficulties that were significantly associated with the right frontal cortex through a working memory task [11]. Moreover, the depressive symptom is the frequent affective comorbidity of CHD. There is a mutual relationship between psychiatric disorders and CHD. Previous studies have shown that patients with CHD may be more prone to developing depression, and vice versa [12,13]. Cognitive impairment and psychiatric disorders have an impact on many vital cerebral networks associated with regulating ANS activity and neuroendocrine function, as well as emotional stability.
The thalamus is a vital structure of the CNS. It is located in the center of the brain from where it essentially acts as a relay to the vast majority of sensory inputs that span diverse cortical regions [[14], [15], [16]]. Notably, these regions have a wide range of different cognitive functions, including perception, attention, memory, and executive decision-making, via different modulatory types of thalamus-related circuits. Thus, dysfunctional neural pathways associated with the thalamus in CHD may lay a solid foundation for the incidence of neuropathological disorders. Nevertheless, thalamus-based dysfunctional connectivity with the whole brain, as well as its relationship with clinical characteristics of patients with CHD remain unknown.
Functional connectivity (FC) analysis, a descriptive measure of spatiotemporal correlations of spontaneous functional activity between distinct cerebral regions, has been widely used in neuropsychiatric disorders, such as schizophrenia, depression, and anxiety. The overarching aim of this study was to evaluate abnormal FC patterns between the thalamus and other cerebral cortex in patients with CHD. Considering the importance of the thalamic function and location, we chose bilateral thalami as seed regions for thalamus-based FC analysis and hypothesized that patients with CHD exhibit disrupted FC patterns of the thalamus, a phenomenon that might be associated with specific clinical characteristics. To the best of our knowledge, this is the first study reporting disrupted resting-state FC patterns of the thalamus in patients with CHD.
2. Methods
2.1. Participant selection criteria
Patients with CHD were prospectively recruited if they met the following criteria: (1) coronary stenosis greater than or equal to 50% in at least one main coronary artery determined through coronary angiography; (2) stable coronary heart disease [17]; (3) typical angina symptoms; and (4) without undergoing coronary interventional therapy prior to recruitment. Sixteen healthy controls (HCs) were also recruited. Subjects that met the following criteria were excluded from the study: (1) had a history of psychiatric or neurological disorder; (2) had a major head injury and dementia; (3) were pregnant or lactating; (4) exhibited severe important organ dysfunction; (5) had Montreal Cognitive Assessment (MoCA) scores less than 26 [18]; and (6) had MRI contraindications. Subjects in both groups were right-handed and matched in gender, age, and body mass index (BMI). Next, we adopted the clinical grading standard of hypertension to stratify all participants into normal, grade I, grade II, and grade III hypertension [19]. A summary of their demographic and clinical data is outlined in Table 1. This study was approved by the Ethics Committee of Nanjing Medical University. All participants voluntarily signed a written informed consent prior to enrollment in the study.
Table 1.
Demographic and clinical data of patients with CHD and HCs.
| CHD (n = 26) | HCs (n = 16) | t/z/χ | Effect size | p value | |
|---|---|---|---|---|---|
| Age (year) | 60.46 ± 8.85 | 57.25 ± 10.48 | 1.064 | 0.338a | 0.294c |
| Sex (male/female) | 13/13 | 5/11 | 1.422 | 0.184b | 0.233 |
| BMI | 24.25 ± 3.01 | 24.94 ± 3.93 | −0.181 | 0.205a | 0.856d |
| Hypertension classification (Normal/Grade I/II/III) | 10/9/5/2 | 9/5/2/0 | 1.852 | 0.220b | 0.699 |
| Total cholesterol (mmol/L) | 4.40 ± 1.12 | 3.86 ± 0.90 | 1.653 | 0.525a | 0.106c |
| Triglycerides (mmol/L) | 1.94 ± 1.14 | 1.49 ± 0.68 | −0.933 | 0.449a | 0.351d |
| Low-density lipoproteins (mmol/L) | 2.62 ± 1.03 | 2.29 ± 0.91 | 1.063 | 0.338a | 0.294c |
| Lipoprotein (a) (mg/L) | 186.25 ± 200.35 | 245.08 ± 277.41 | −0.195 | 0.253a | 0.846d |
| Uric acid (umol/L) | 335.69 ± 87.95 | 323.75 ± 95.27 | 0.414 | 0.132a | 0.681c |
| Ejection fraction (%) | 65.38 ± 4.06 | 65.38 ± 7.07 | −1.040 | 0.002a | 0.298d |
The continuous variables are expressed as the mean ± standard deviation (Mean ± SD). BMI: body mass index; CHD: coronary heart disease; HCs: healthy controls. Effect size: Cohen's d (a) and η2 (b); Statistical analyses: independent-sample t-test (c) and Mann-Whitney test (d).
2.2. Acquisition of imaging data
MRI data were acquired using a 3.0 T SIEMENS MAGNETOM Prisma with a 64- channel receiver array head coil. Summarily, participants were instructed to lie quietly with their eyes closed and think about nothing, but remain awake. To improve image quality, foam pads were placed on both sides of the participant's head to minimize head motion during data acquisition. High resolution T1-weighted three-dimensional anatomical images were acquired with a sagittal magnetization-prepared rapid gradient echo sequence, based on the following parameters: repetition time (TR) = 5000 ms, echo time (TE) = 2.98 ms, flip angle = 9°, matrix size = 256 × 256, field of view (FOV) = 230 × 230 mm, slice thickness = 1.0 mm, and slices = 176. The structural sequence took 8 min and 22 s. Whole-brain resting-state functional images were obtained axially using an echo-planar imaging sequence with the following parameters: TR = 2000 ms, TE = 30 ms, slices = 36, thickness = 4 mm, gap = 0 mm, FOV = 230 mm × 230 mm, matrix = 64 × 64, flip angle = 90°, and volumes = 230. The functional sequence took 7 min and 48 s. The sections were placed approximately parallel to the anterior commissure-posterior commissure line, and functional data were acquired using a parallel imaging technique with an acceleration factor of two.
2.3. Data preprocessing
Standard image data preprocessing and statistical analysis were performed using the Rs-fMRI Data Analysis Toolkit plus (RESTplus, http://restfmri.net/forum/). The first 10 time points from all subjects were discarded to avoid instability of the initial MRI signals. Thereafter, the remaining 220 vol were processed under the following steps: slice-timing adjustment, realignment, spatial normalization into Montreal Neurological Institute (MNI) (resampling voxel size = 3 × 3 × 3 mm), smoothing with a 6-mm Gaussian kernel, detrending, and filtering (0.01–0.08 Hz). Participants who exhibited head motion of less than 2.0 mm displacement or a 2.0° rotation in any direction were included. To control for non-neural noise in the time series, parameters for head motion, white matter signal, and cerebrospinal fluid signal were included as covariates in the linear regression.
Bilateral thalami were chosen as regions of interest (ROIs) and subsequently generated using the Automated Anatomical Labeling (AAL) atlas (http://www.gin.cnrs.fr/AAL). The mean time series for each thalamus was defined as the reference course. Next, we generated Pearson's correlation coefficients, which were subsequently used to correlate the mean time series of the thalamus with those of other whole-brain voxels. Finally, Fisher's z-transform was applied to normalize the correlation coefficients.
2.4. Statistical analysis
Differences in demographic and clinical data between the two groups were determined using a two-tailed t-test for continuous variables with a normal distribution or Mann–Whitney test for continuous variables with a non-normal distribution. Categorical covariables were analyzed using the Chi-square or Fisher's exact tests. Statistical analyses were performed using SPSS 24.0 software (Chicago, IL, USA). The difference (p < 0.05) was considered to be statistically significant. For fMRI data statistical analysis, the FC statistical differences between the two groups were performed in the statistical module of RESTplus (two-sample t-test analysis, uncorrected, p < 0.001) with age, sex, hypertension classification and BMI as control covariates. The minimum cluster size was set as 20.
3. Results
3.1. Demographic and clinical data
A total of fifty subjects were eligible to participate in the study. However, eight patients were excluded due to poor image quality. Details of the demographic and clinical characteristics among patients with CHD alongside HCs are summarized in Table 1. According to the results, there were no statistically significant differences between the two groups with regard to the aforementioned parameters (p > 0.05).
3.2. Thalamus-based FC patterns
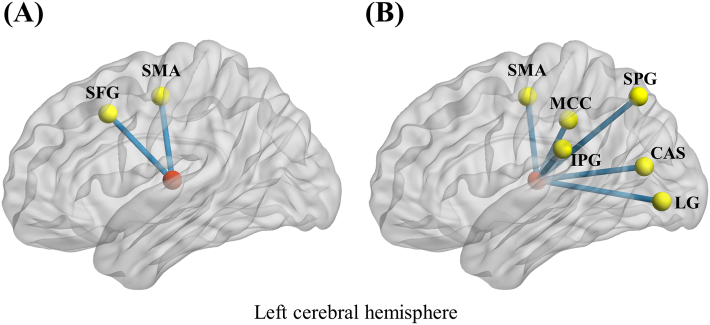
FC analysis of the left thalamus revealed that patients with CHD exhibited decreased FC patterns with the left supplementary motor area (SMA) and superior frontal gyrus (SFG), compared to HCs (Table 2; Fig. 1A). Moreover, for the right thalamus, we found decreased FC patterns with the left hemisphere, encompassing SMA, superior parietal gyrus (SPG), inferior parietal gyrus (IPG), middle cingulate cortex (MCC), lingual gyrus, and calcarine sulcus (Table 3; Fig. 1B).
Table 2.
Left thalamus-based FC patterns between patients with CHD and HCs.
| Brain regions | MNI coordinates |
Cluster size | T score | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Left supplementary motor area | −6 | −12 | 51 | 25 | −4.1161 |
| Left superior frontal gyrus | −9 | 15 | 42 | 22 | −3.6615 |
CHD: coronary heart disease; FC: functional connectivity; HCs: healthy controls; MNI: Montreal Neurological Institute. P < 0.001, uncorrected.
Fig. 1.
Left thalamus-based (A) and right thalamus-based (B) abnormal functional connectivity patterns with left hemisphere in patients with coronary heart disease. Red nodes mean bilateral thalami. CAL: calcarine sulcus; IPG: inferior parietal gyrus; LG: lingual gyrus; MCC: middle cingulate cortex; SFG: superior frontal gyrus; SMA: supplementary motor area; SPG: superior parietal gyrus. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
Right thalamus-based FC patterns between patients with CHD and HCs.
| Brain regions | MNI coordinates |
Cluster size | T score | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Left supplementary motor area | −9 | −12 | 51 | 26 | −4.3674 |
| Left superior parietal gyrus | −27 | −69 | 51 | 86 | −4.1766 |
| Left inferior parietal gyrus | −42 | −30 | 24 | 30 | −3.6629 |
| Left middle cingulate cortex | −9 | −33 | 39 | 29 | −3.8385 |
| Left lingual gyrus | −15 | −81 | −3 | 45 | −3.8120 |
| Left calcarine sulcus | −18 | −72 | 15 | 86 | −3.8195 |
CHD: coronary heart disease; FC: functional connectivity; HCs: healthy controls; MNI: Montreal Neurological Institute. P < 0.001, uncorrected.
4. Discussion
To the best of our knowledge, this is the first study employing FC analysis to investigate thalamus-based whole-brain FC in patients with CHD. The thalamus not only plays a crucial role in integrating multisensory information through cortico-cortical and cortical-subcortical transmissions but also in regulating brain-heart axis connection through the ANS. Although previous studies have shown that bilateral sections of ANS innervate separate cardiac areas [20] or regulate antagonistically sympathetic and parasympathetic effects on cardiovascular function, produced by brain lateralization [21,22], the precise neural mechanisms of brain lateralization and their effect on cardiac autonomic nervous function have not been fully investigated. The main finding from this study was that patients with CHD had a propensity to lateralization of the left hemisphere in bilateral thalamic FC patterns. Anatomically, the right vagus and left vagus mainly innervates the sinus node and atrioventricular node respectively, caused by brain lateralization [23]. This asymmetrical performance of the nervous system is considered to be modulated by the endogenous physiological factor. It has been hypothesized that the brain functions are in an asymmetrical model and speculated that imbalances of brain asymmetry (such as becoming symmetrical or more asymmetrical) might lead to neuropathological deviations in the modulation of the ANS and peripheral nervous system [24]. Thus, it seems that asymmetrical cerebral pathways may be considered potential neural mechanisms to mediate the interaction between the ANS and cardiovascular system.
Thalamus-related circuits are extensively connected to many cortical and subcortical regions, such as the frontal cortex, insula, cingulate cortex, putamen and caudate nucleus, contributing to the cortical–striatal–thalamic–cortical (CSTC) circuits. Previous studies have suggested that dysfunction of CSTC circuits is associated with emotional disturbance, cognitive impairment and behavior modulation [25]. Findings from a study on cardiovascular rehabilitation program study involving patients with CHD revealed that cognitive modulation was significantly correlated with the frontoparietal cortex, including the prefrontal cortex, SMA and inferior parietal gyrus [26]. In addition, angina is a common clinical syndrome caused by acute and temporary myocardial hypoxia or infarction. Numerous studies have emphasized that aggressive treatment of angina might be a necessary need to improve the prognosis [27] and a cornerstone for managing patients with CHD [28]. Therefore, pain-related functional brain networks may also be involved in the neural mediation mechanisms of the brain-heart axis. For example, results from a task-state fMRI study [29], performed during distinct phases of the cardiac cycle, revealed that nociceptive perception was negatively associated with blood pressure, which was modulated by the CNS through baroreceptor-induced feedback. These cortical and subcortical brain areas include the prefrontal cortex, MCC, IPG, SPG, thalamus and visual cortex. Similarly, results from this study showed significantly reduced FC patterns of the thalamus with the left posterior frontal cortex, parietal cortex and visual cortex, especially in the SMA.
SMA, which forms part of the sensorimotor network (SMN), is located in the posterior third of the medial aspect of the superior frontal gyrus. This region was originally defined as a motor area. The striatum receives motor-related inputs from cortical areas and, via the thalamus, which it then projects to the prefrontal cortex, premotor cortex and SMA. Wu et al. demonstrated that self-initiated movement was correlated to the medial SMA, and externally triggered movement is associated with the lateral premotor area [30]. Results from the present study showed that dysfunction of the SMA may be attributed to the imbalance of homeostasis. Deeper previous research has stratified this SMA concept into two subareas, namely pre-SMA and SMA proper [31]. Especially, pre-SMA, a rostral area connected to the prefrontal cortex, plays a crucial role in movement planning. On the other hand, SMA proper is caudal and has a connection to the primary motor and premotor cortex, involved in executing movements. Previous studies have also demonstrated that a positive trend in cardiac output was associated with the activity of the SMA during a verbal working memory task in elderly patients with CHD who tended to be at an increased risk of contracting vascular cognitive impairments [32]. Results of the present study revealed that patients with CHD exhibited decreased FC patterns between the thalamus and SMA and posterior SPG, consistent with the above study. Therefore, impairments of executive function involved in SMA in patients with CHD seem to be underpinned by cardiac dysfunction.
Moreover, the parietal cortex, together with the prefrontal cortex, consists of an executive control network (ECN), which has been implicated in multiple cognitive and executive functions, including attention, working memory and cognitive control [33,34]. Results from a combined brain-heart study, based-on cerebral high-resolution positron emission tomography (HRPET) imaging and cardiac single-photon emission computed tomography (SPECT) imaging [35], showed that patients with CHD had increased activation in the frontal and parietal cortex under extrinsic mental stress-induced stimuli. On the other hand, patients with mental stress-induced myocardial ischemia (MCI) had higher activation during stress in the parietal cortex. However, we found an opposite trend in the present study, as evidenced by decreased FC patterns of the frontoparietal cortex in patients with CHD. The inconsistency might be attributed to variation in the methodological applications during task-state and resting-state processing. Alternatively, compared to the stable patients with CHD, the increased activation in patients with MCI during stress may be attributed to temporary compensatory regulation of ANS receiving visceral afferent signals. Collectively, these findings indicated that activation of the frontoparietal cortex is associated with stress response and autonomic regulation of the cardiovascular system. Therefore, decreased FC patterns in patients with CHD between the thalamus and frontoparietal network could be due to prolonged dysfunction of ECN to regulate inputs from the cardiovascular system, resulting in deficient ability to provide adequate executive control and reduce maladaptive responses. Altered thalamus-ECN connectivity patterns could represent a mechanism through which cardiac dysfunction leads to an increased risk of cerebral nervous system dysfunction.
Notably, we observed a disrupted FC pattern between the right thalamus and left MCC. The MCC, which is part of the limbic system, is primarily involved in emotional and affective regulation [36]. Previous studies have associated patients with CHD with depression and other emotional complications [2]. Another study implicated cardiovascular risk factors, such as high-density lipoprotein, low-density lipoprotein, and triglyceride levels in structural remodeling of the limbic system identified as early predictors of cognitive decline [37]. Therefore, the altered FC pattern of the MCC may partly explain the shared neural circuit mechanism between CVDs and depression. Moreover, results from a resting-state fMRI FC analysis [38], based on the whole brain, illustrated sympathetic- and parasympathetic-associated hypo-connected subnetworks supporting the brain-heart axis in Takotsubo syndrome. These networks contain ECN, default mode network, primary motor cortex, and limbic system including the MCC. These findings from the aforementioned study were similar to this study and suggested that integration of the ANS and limbic system may not only play an important role in the pathophysiology but also contribute to the understanding of the brain-heart axis concerning cardiac dysfunction.
Furthermore, we also identified decreased thalamic FC patterns with the calcarine sulcus and lingual gyrus, which are core structures of the primary visual cortex. Previous studies have shown that nociceptive- [29] and affective-related [38] brain networks including visual cortex, share neural mechanisms with brain-heart pathways and are involved in the neural regulation of ANS and cardiovascular function. Based on the above findings, not only do our results of asymmetrical FC patterns attribute to intrinsic lateralization of brain functions, but it also explains the potential interactions between the CNS and cardiovascular system. These findings are beneficial to enhance our understanding of the neuropathological mechanisms of the brain-heart axis and provide new insights into neuroimaging features to detect cardiac dysfunction in further preclinical work.
This study had some limitations. First, the cross-sectional study had a relatively small sample size, which may have affected the clear establishment of the causal relationship between thalamic FC changes and cardiac dysfunction in patients with CHD. Further longitudinal fMRI investigations, based on larger sample sizes, are required to validate our findings. Second, we exclusively selected the bilateral thalami as the seeds for the whole-brain FC analysis. It does not imply that other FC patterns are not important and requires more comprehensive investigations. Third, there is no neuropsychiatric inventory to assess neuropsychiatric and behavioral symptoms. Future studies are expected to ascertain whether changes in resting-state networks are related to any of these assessments. Fourth, the selected masks of bilateral thalami were taken from a pre-defined anatomical atlas, it would certainly be more accurate to investigate possible functional differences in future studies based on the data-driven segmentation of structural images. Finally, the credibility of our results is restricted owing to the relatively loose threshold value. A more stringent statistical threshold should be applied to further validate these functional correlations.
5. Conclusions
In conclusion, our results revealed a significant correlation between decreased functional lateralization of thalamic FC patterns with several subnetworks in the left hemisphere in patients with CHD. Most of the structures, including ECN, SMN, limbic system, and visual cortex, play important roles in regulating a variety of cognitive, emotional, and regulatory functions. It is possible that the underlying pathophysiology of patients with CHD is not only restricted to the cardiovascular system alone, but may also be due to the interaction between the brain and heart. Taken together, these findings might reflect a neurological substrate engaged in CHD, thereby reinforcing the existing concept of the bidirectional involvement of the brain-heart axis interaction.
Author contribution statement
Heng-Le Wei; Ming-Qiang Ao: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Meng-Yao Wang: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Gang-Ping Zhou: Contributed reagents, materials, analysis tools or data.
Yu-Sheng Yu: Analyzed and interpreted the data.
Qin Tao; Hong Zhang: Conceived and designed the experiments.
Funding statement
This study was supported by Science and Technology Development Project of Nanjing Jiangning Hospital, China [JNYYZXKY202034].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to all study participants.
Contributor Information
Qin Tao, Email: 88025822@qq.com.
Hong Zhang, Email: jnyyfsk@126.com.
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J. Am. Coll. Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu W., Zhang X., Wu Z., Huang K., Yang C., Yang L. Brain–heart communication in health and diseases. Brain Res. Bull. 2022;183:27–37. doi: 10.1016/j.brainresbull.2022.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Xu C., Zheng A., He T., Cao Z. Brain–heart axis and biomarkers of cardiac damage and dysfunction after stroke: a systematic review and meta-analysis. Int. J. Mol. Sci. 2020;21(7):2347. doi: 10.3390/ijms21072347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Méloux A., Béjot Y., Rochette L., Cottin Y., Vergely C. Brain-heart interactions during ischemic processes. Stroke. 2020;51(2):679–686. doi: 10.1161/STROKEAHA.119.027732. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura S., Toyoda K., Ohara T., Nagasawa H., Ohtani N., Kuwashiro T., et al. Takotsubo cardiomyopathy in acute ischemic stroke. Ann. Neurol. 2008;64(5):547–554. doi: 10.1002/ana.21459. [DOI] [PubMed] [Google Scholar]
- 6.Palma J.A., Benarroch E.E. Neural control of the heart: recent concepts and clinical correlations. Neurology. 2014;83(3):261–271. doi: 10.1212/WNL.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 7.Silvani A., Calandra-Buonaura G., Dampney R.A.L., Cortelli P. Brain–heart interactions: physiology and clinical implications. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2016;374(2067) doi: 10.1098/rsta.2015.0181. [DOI] [PubMed] [Google Scholar]
- 8.Tumati S., Paulus M.P., Northoff G. Out-of-step: brain-heart desynchronization in anxiety disorders. Mol. Psychiatr. 2021;26(6):1726–1737. doi: 10.1038/s41380-021-01029-w. [DOI] [PubMed] [Google Scholar]
- 9.Tamashiro-Duran J.H., Squarzoni P., de Souza Duran F.L., Curiati P.K., Vallada H.P., Buchpiguel C.A., et al. Cardiovascular risk in cognitively preserved elderlies is associated with glucose hypometabolism in the posterior cingulate cortex and precuneus regardless of brain atrophy and apolipoprotein gene variations. Age. 2013;35(3):777–792. doi: 10.1007/s11357-012-9413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tawakol A., Ishai A., Takx R.A., Figueroa A.L., Ali A., Kaiser Y., et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389(10071):834–845. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haley A.P., Eagan D.E., Gonzales M.M., Biney F.O., Cooper R.A. Functional magnetic resonance imaging of working memory reveals frontal hypoactivation in middle-aged adults with cognitive complaints. J. Int. Neuropsychol. Soc. 2011;17(5):915–924. doi: 10.1017/S1355617711000956. [DOI] [PubMed] [Google Scholar]
- 12.Lichtman J.H., Froelicher E.S., Blumenthal J.A., Carney R.M., Doering L.V., Frasure-Smith N., et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations. Circulation. 2014;129(12):1350–1369. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein B.I., Carnethon M.R., Matthews K.A., Mcintyre R.S., Miller G.E., Raghuveer G., et al. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease. Circulation. 2015;132(10):965–986. doi: 10.1161/CIR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 14.Cassel J., Pereira De Vasconcelos A. Routes of the thalamus through the history of neuroanatomy. Neurosci. Biobehav. Rev. 2021;125:442–465. doi: 10.1016/j.neubiorev.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Boelens Keun J.T., van Heese E.M., Laansma M.A., Weeland C.J., de Joode N.T., van den Heuvel O.A., et al. Structural assessment of thalamus morphology in brain disorders: a review and recommendation of thalamic nucleus segmentation and shape analysis. Neurosci. Biobehav. Rev. 2021;131:466–478. doi: 10.1016/j.neubiorev.2021.09.044. [DOI] [PubMed] [Google Scholar]
- 16.Parnaudeau S., Bolkan S.S., Kellendonk C. The mediodorsal thalamus: an essential partner of the prefrontal cortex for cognition. Biol. Psychiatr. 2018;83(8):648–656. doi: 10.1016/j.biopsych.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart R.A.H., Held C., Hadziosmanovic N., Armstrong P.W., Cannon C.P., Granger C.B., et al. Physical activity and mortality in patients with stable coronary heart disease. J. Am. Coll. Cardiol. 2017;70(14):1689–1700. doi: 10.1016/j.jacc.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Nasreddine Z.S., Phillips N.A., Bã Dirian V.R., Charbonneau S., Whitehead V., Collin I., et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 19.Brouwers S., Sudano I., Kokubo Y., Sulaica E.M. Arterial hypertension. Lancet. 2021;398(10296):249–261. doi: 10.1016/S0140-6736(21)00221-X. [DOI] [PubMed] [Google Scholar]
- 20.Guidolin D., Anderlini D., Marcoli M., Cortelli P., Calandra-Buonaura G., Woods A.S., et al. A new integrative theory of brain-body-ecosystem medicine: from the hippocratic holistic view of medicine to our modern society. Int. J. Environ. Res. Publ. Health. 2019;16(17):3136. doi: 10.3390/ijerph16173136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer S., Strittmatter M., Fischer C., Georg T., Schmitz B. Lateralization in autononic dysfunction in ischemic stroke involving the insular cortex. Neuroreport. 2004;15(2):357–361. doi: 10.1097/00001756-200402090-00029. [DOI] [PubMed] [Google Scholar]
- 22.Nagai M., Hoshide S., Kario K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J. Am. Soc. Hypertens. 2010;4(4):174–182. doi: 10.1016/j.jash.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Guidolin D., Anderlini D., Marcoli M., Cortelli P., Calandra-Buonaura G., Woods A.S., et al. A new integrative theory of brain-body-ecosystem medicine: from the hippocratic holistic view of medicine to our modern society. Int. J. Environ. Res. Publ. Health. 2019;16(17):3136. doi: 10.3390/ijerph16173136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez M., Prieto I., Vives F., de Gasparo M., Alba F. Neuropeptides, neuropeptidases and brain asymmetry. Curr. Protein Pept. Sci. 2004;5(6):497–506. doi: 10.2174/1389203043379350. [DOI] [PubMed] [Google Scholar]
- 25.Cyr M., Pagliaccio D., Yanes-Lukin P., Fontaine M., Rynn M.A., Marsh R. Altered network connectivity predicts response to cognitive-behavioral therapy in pediatric obsessive–compulsive disorder. Neuropsychopharmacology. 2020;45(7):1232–1240. doi: 10.1038/s41386-020-0613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anazodo U.C., Shoemaker J.K., Suskin N., St Lawrence K.S. An investigation of changes in regional gray matter volume in cardiovascular disease patients, pre and post cardiovascular rehabilitation. Neuroimage: Clin. 2013;3:388–395. doi: 10.1016/j.nicl.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X., Luo D., Zhang J., Du L. Distribution and relative expression of vasoactive receptors on arteries. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-72352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qintar M., Spertus J.A., Gosch K.L., Beltrame J., Kureshi F., Shafiq A., et al. Effect of angina under-recognition on treatment in outpatients with stable ischaemic heart disease. Eur. Heart J. Qual. Care Clin. Outcomes. 2016;2(3):208–214. doi: 10.1093/ehjqcco/qcw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottaviani C., Fagioli S., Mattei E., Censi F., Edwards L., Macaluso E., et al. Brain–heart pathways to blood pressure-related hypoalgesia. Psychosom. Med. 2018;80(9):845–852. doi: 10.1097/PSY.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 30.Wu T., Wang L., Hallett M., Chen Y., Li K., Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. Neuroimage. 2011;55(1):204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]
- 31.Cona G., Semenza C. Supplementary motor area as key structure for domain-general sequence processing: a unified account. Neurosci. Biobehav. Rev. 2017;72:28–42. doi: 10.1016/j.neubiorev.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 32.Irani F., Sweet L.H., Haley A.P., Gunstad J.J., Jerskey B.A., Mulligan R.C., et al. A fMRI study of verbal working memory, cardiac output, and ejection fraction in elderly patients with cardiovascular disease. Brain Imaging Behav. 2009;3(4):350–357. doi: 10.1007/s11682-009-9077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander A.S., Tung J.C., Chapman G.W., Conner A.M., Shelley L.E., Hasselmo M.E., et al. Adaptive integration of self-motion and goals in posterior parietal cortex. Cell Rep. 2022;38(10) doi: 10.1016/j.celrep.2022.110504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krönke K., Wolff M., Shi Y., Kräplin A., Smolka M.N., Bühringer G., et al. Functional connectivity in a triple-network saliency model is associated with real-life self-control. Neuropsychologia. 2020;149 doi: 10.1016/j.neuropsychologia.2020.107667. [DOI] [PubMed] [Google Scholar]
- 35.Bremner J.D., Campanella C., Khan Z., Shah M., Hammadah M., Wilmot K., et al. Brain correlates of mental stress-induced myocardial ischemia. Psychosom. Med. 2018;80(6):515–525. doi: 10.1097/PSY.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palomero-Gallagher N., Amunts K. A short review on emotion processing: a lateralized network of neuronal networks. Brain Struct. Funct. 2022;227(2):673–684. doi: 10.1007/s00429-021-02331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasa R.N., Rossetti H.C., Gupta M.K., Rosenberg R.N., Weiner M.F., Peshock R.M., et al. Cardiovascular risk factors associated with smaller brain volumes in regions identified as early predictors of cognitive decline. Radiology. 2016;278(1):198–204. doi: 10.1148/radiol.2015142488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Templin C., Hänggi J., Klein C., Topka M.S., Hiestand T., Levinson R.A., et al. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur. Heart J. 2019;40(15):1183–1187. doi: 10.1093/eurheartj/ehz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.