Abstract
Publications on COVID-19’s impact on the global tuberculosis burden are from adult cohorts, pediatric data are lacking for inform decision. We compared the TB trends in southern Nigerian children in the pre-COVID-19 and COVID-19 era. This was a retrospective, cross-sectional study of early morning sputum/gastric washing or stool samples from children with presumptive TB evaluated using GeneXpert in a tertiary hospital from January 2016 to May 2022. Of the 20 589 persons screened for presumed TB in the pre-COVID-19 and the COVID-19 era, only 1104 (88.7%) of 1245 children had complete data for analysis. In the COVID era, a significantly higher number of children were presumed to have TB 755 (68.4%), P < .001. The overall incidence of MTB detected by Xpert MTB/RIF during the study period was 6.4% (71/1104). The incidence of MTB in the pre-COVID-19 era was 24/349 (6.9%), which was slightly higher than the COVID-19 era (47/755; 6.2%), P > .05). The annual trends of MTB detected peaked in 2019 [18/115; 15.7%] in the pre-COVID-19 era, then plummeted to 12/228 (5.3%) in 2020 in the COVID-19 era, and reached its all-time low of 6/160 (3.8%) in the first half of 2022, (P < .001). The overall incidence of Rifampicin-resistant TB (RR-TB) was 2.8% among the MTB detected cases and all occurred in the COVID-19 era. This study found a significant decline in MTB diagnosis and in the emergence of RR-TB in the COVID-19 era. This necessitates re-prioritizing worldwide efforts to manage childhood tuberculosis, including increased testing, if the aim of eliminating tuberculosis by 2035 is to be met.
Keywords: tuberculosis, rifampicin resistance, children, COVID-19, pre-COVID-19, COVID-19 era
Plane Language Summary
Publications on how COVID-19 affects global tuberculosis (TB) trends are mostly based on data from adults, and there is little information on how COVID-19 affects tuberculosis in children. In this study, we looked at how TB affected children in southern Nigeria both before and following the COVID-19 outbreak. According to this study, the number of TB diagnoses has been drastically reduced since COVID-19 started. The lowest number of diagnoses was observed in the first half of 2022. Before the COVID-19 pandemic, there were no known cases of rifampicin-resistant-TB in our study population. The number of cases of rifampicin-resistant tuberculosis has increased by 33.3% by 2022. This study emphasizes the urgent need to re-prioritize global efforts to control childhood tuberculosis if the goal of eliminating tuberculosis by 2035 is to be met.
Introduction
The coronavirus disease 2019 (COVID-19) was designated a global pandemic by the World Health Organization on March 11, 2020, its status appears to have evolved from a pandemic wave into an endemic disease with 554 290 112 confirmed cases and 6 351 801 deaths as of July 3, 20221,2 COVID-19 therefore may as well be on its way to being described as a “pan-endemic”; (a conjoint of pandemic and endemic) due to its ongoing and prolonged pervasion of human activities and toll on human existence. This may necessitates a paradigm shift and a reconsideration of emerging approaches, whether preventative or control strategies. At the height of the outbreak, the world’s attention was diverted from other diseases such as tuberculosis (TB) with a resultant reversal of long-achieved successes.3-5
Children, who are among the most vulnerable populations, benefited less from COVID-19 containment and vaccination programs.6 The COVID 19 intervention approaches such as school closures and 14-day parental self-isolation significantly increased the children exposure persons with infectious diseases like tuberculosis.7,8 In addition, children were at heightened risk of TB infection due to limited access to health-care institutions, unrestricted exposure to patients/caregivers who had failed first-line anti-TB medication, and the inability to access diagnose for both drug-sensitive and drug-resistant TB.7,9 Furthermore, the interruption in the immunization program, leading to missed BCG vaccine doses, the poor nutrition status of children at the peak of the outbreak because of food insecurity, potentially increase the risk of TB transmission and spread of TB among children.7,10,11
The diagnosis of tuberculosis in children has always been challenging, but the COVID-19 pandemic has made it even more difficult.7,9,12 The similarity of clinical symptoms, the paucibacillary nature of tuberculosis in children, and the lack of specific radiographic findings are all confounders that may delay diagnosis in children.13 As a result, children are at an increased risk of exposure while also being less likely to receive timely diagnosis and prompt treatment.
Due to the unbalanced approach to COVID prevention strategies, an estimated increase of 6.3 million TB cases and 1.4 million TB deaths are expected within the next 5 years.9,14 A recent study in Spain found that the care given to TB patients changed significantly between the pre-COVID and COVID 19 eras.7 Access to TB treatment was restricted with fewer human capacity available to undertake such care.7 While a lower incidence of newly diagnosed TB was observed in various studies, an increase in cases of latent and active TB among children living in households with a TB patient was observed. This may be explained in part by the prolonged isolation of individuals with non-specific respiratory symptoms that could be TB and the prolonged contact with family members.7,9
Comparing the pre-COVID and COVID eras, Muhammad Dayyab et al found a significant increase in drug-resistant TB cases among 132 patients15 Whereas most of the studies that evaluated the impact of COVID-19 on TB are in adult populations, the trends and impact in the pediatric population remain largely unknown due to paucity of data. This study therefore compared the trends in tuberculosis diagnoses between the pre-COVID era (January 2016 to December 2019) and the COVID-19 era (January 2020 to May 2022) in a large cohort of presumed TB cases in southwestern Nigeria.
Material and Methods
Study Design and Location
This was a retrospective, cross-sectional, analytical study of early morning sputum/gastric washing or stool samples from children with presumptive tuberculosis TB at a referral and treatment center located in a tertiary mission hospital in Southwest Nigeria from January 2016 to May 2022.
Study Population and Eligibility Criteria
The study included all children with presumptive TB diagnosis at the hospital or referred from primary health care centers from neighboring towns and states. Xpert MTB/RIF was the primary diagnostic method for evaluating all children with presumed tuberculosis.
The subjects with indeterminate or incomplete results were excluded from the final analysis.
Sample Collection and Laboratory Analysis
Each caregiver of a child 10 years or older suspected of having tuberculosis was provided with a 25-ml leak-proof, wide-mouthed screw-cap cup to collect sputum for analysis. For children under the age of 10, a gastric washing sample was obtained in the ward for the Xpert MTB/RIF. To complete liquefaction for polymerase chain reaction, the sample was diluted 1:2 (v/v) with carbonic acid buffer, mixed thoroughly, and maintained at room temperature for 10 minutes (PCR). A 2 ml aliquot of the diluted sample was put through the supplied cartridge port into the GeneXpert machine (GeneXpert® Sunnyvale, CA, USA). During the ultrasonic lysis of the mycobacterium, the DNA was liberated. Using an integrated microfluidic system 910, the GeneXpert extracts, purifies, amplifies, and quantifies DNA using real-time PCR. A molecular beacon is also utilized to identify rpoB gene mutations, which have been linked to rifampin-resistant tuberculosis (RR-TB). Children with positive results were directed to the hospital’s TB treatment program, which was supported by the Damien Foundation. Utilizing Determine® Kits (Alere Medical, Matsuhidai, Matsuhidai-shi, Chiba, Japan) the HIV status of the child was determined for all presumptive cases of TB. Uni-Gold® HIV (Trinity Biotech, Wicklow, Ireland) was used for confirmation and STAT-PAK®, HIV-1 and HIV-2 (Chembio Diagnostic System, Medford NY, USA) as tie-break where conflicting results were obtained from the initial 2 tests.
Data Analysis
IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, N.Y., USA) was utilized to analyze the data. We summarized the demographic data (age groups and gender) and HIV status using the frequency table. Using the Xpert MTB/RIF, the proportion of individuals who tested positive for mycobacterium tuberculosis (MTB) and rifampicin-resistant tuberculosis (RR-TB) was assessed. We compared the pre-COVID period (January 2016 to December 2019) to the COVID-19 era using the chi-square test and odds ratio with a 95% confidence interval (from January 2020 to May 2022). Using line graphs, the percentage of MTB and RR-TB identified each year was presented. The P-value for statistical significance was set at <.05.
The Bowen University Health Research Ethics Committee (HREC) approved the study (Ethics clearance number BUTH-REC – 047) and study was conducted in accordance with the Helsinki Declaration.
Missing Data
The TB treatment center’s registers were searched for missing data for the dataset. When data could not be linked to the outcome variable, they were omitted from the final analysis.
Results
Of the 20 589 persons screened for presumed TB in the pre-COVID-19 and the COVID-19 era, only 1104 (88.7%) of 1245 children had complete data for analysis. In the COVID-19 era (January 2020 to May 2022), a significantly higher number of children were presumed to have TB 755 (68.4%) compared with pre-COVID era (January 2016 to December 2019) (349: 31.6%; P < .001). The gender distribution was comparable (females 573; 51.9%). More than half of all presumptive cases of TB were Teenagers (≥13 years) (592/1104; 53.6%). Forty-one children (3.7%) tested positive for HIV, 426 (38.5%) were seronegative and the HIV status of the remaining cases was unknown (Table 1). There was a reversal of age at presentation of presumed TB cases, with older children (teenagers) 464 (78.4%) predominating in the COVID-19 era, as opposed to the pre-COVID-19 era, in which younger children 221(63.3%) dominated (P < .001). In the COVID-19 era, there were more cases of unknown HIV status and fewer cases of presumed HIV-TB co-infection (P < .001).
Table 1.
Patients’ Demographic and HIV Status of the Presumptive Tuberculosis.
| Variables | n = 1104 (%) | Pre-COVID-19a n = 349 (%) | COVID-19b n = 755 (%) | Σ2 | P |
|---|---|---|---|---|---|
| Age (years) | |||||
| 0-5 | 169 | 81 (47.9) | 88 (52.1) | ||
| 6-10 | 230 | 103 (44.8) | 127 (55.2) | 61.606 | <.001** |
| 11-15 | 472 | 114 (24.2) | 358 (75.8) | ||
| Above 15 | 233 | 51 (21.9) | 182 (78.1) | ||
| Sex | |||||
| Males | 531 | 181 (34.1) | 350 (65.9) | 2.897 | .089 |
| Females | 573 | 168 (29.3) | 405 (70.7) | ||
| HIV status | |||||
| Positive | 41 | 29 (70.7) | 12 (29.3) | 134.139 | <.001** |
| Negative | 426 | 204 (47.9) | 222 (52.1) | ||
| Unknown | 637 | 116 (18.2) | 521 (81.8) | ||
Pre Covid era (2016-2019).
COVID-19 era (2020 to May 2022).
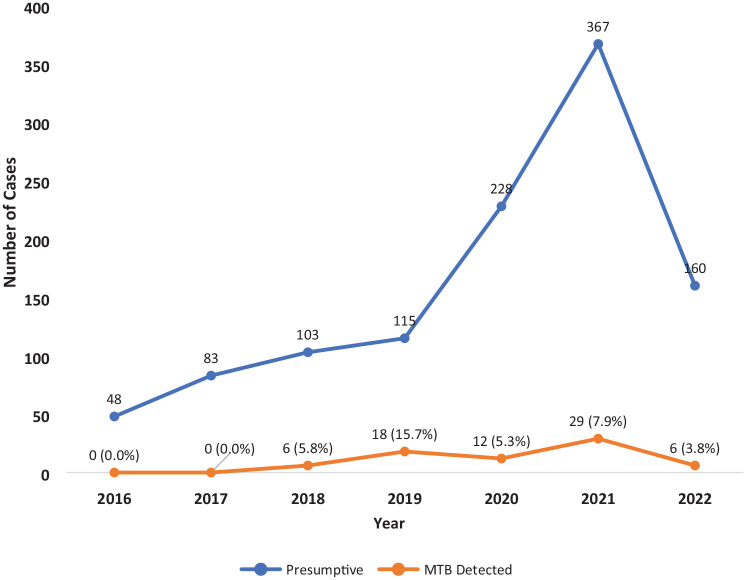
The overall incidence of MTB detected by Xpert MTB/RIF during the study period was 6.4% (71/1104). The incidence of MTB in the pre-COVID-19 era was 24/349 (6.9%), which was slightly higher though not significant when compared with COVID-19 era (47/755; 6.2%), P > .05), Figure 1. The annual trend of MTB cases peaked in 2019 [18/115; 15.7%] in the pre-COVID-19 era, then plummeted to 12/228 (5.3%) in 2020 in the COVID-19 era, and reached its all-time low of 6/160 (3.8%) in the first half of 2022, (P < .001), Figure 2.
Figure 1.
Prevalence of Mycobacterium tuberculosis detected by GeneXpert among the presumptive cases.
Figure 2.
Trends of prevalence of MTB detected during pre-COVID-19 (2016-2019) and COVID-19 era (2020-2022).
The overall incidence of RR-TB was 2.8% among the MTB detected cases. No single case of RR-TB was detected among children with presumed TB during pre-COVID-19 era (2016-2019). However, emerging cases of RR-TB were observed during the COVID-19 era specifically in 2022 with a cumulative incidence of 3.1%, which equals 33.3% rise in 2022 (Figures 3 and 4).
Figure 3.
Incidence of Rifampicin resistance (RR) among the patients with MTB detected.
Figure 4.
Trends of Rifampicin resistance (RIF) during pre-COVID-19 (2016-2018) and COVID-19 era (2019-2022).
Age, sex, HIV status, and era of the study were not associated with MTB detection on multivariable binary logistic regression (Table 2).
Table 2.
Factors Associated With MTB Detection Amongst the Presumptive Tuberculosis.
| Variable | Sub-categories | (MTB positive) n = 71 | uOR | 95% CI | aOR | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Below 13 years | 33 | - | - | - | - | - | |
| 13 years and above | 38 | 0.996 | 0.615-1.612 | 1.059 | 0.642-1.746 | .822 | |
| Sex | |||||||
| Male | 39 | - | - | - | - | - | |
| Female | 32 | 0.746 | 0.460 -1.210 | 0.763 | 0.468-1.244 | .279 | |
| HIV status | |||||||
| Positive | 3 | - | - | - | - | - | |
| Negative | 27 | 0.857 | 0.248-2.957 | 0.875 | 0.252-3.045 | .834 | |
| Unknown | 41 | 0.871 | 0.258 -2.943 | 0.910 | 0.260-3.183 | .883 | |
| Era | |||||||
| Pre-COVID-19 | 24 | - | - | - | - | - | |
| COVID-19 | 47 | 0.899 | 0.540-1.495 | 0.938 | 0.538-1.633 | .820 | |
Abbreviations: MTB, Mycobacterium tuberculosis; uOR, unadjusted odds ratio; aOR, unadjusted odds ratio; CI, confidence interval.
Discussion
Coronavirus disease 2019 (COVID-19) has been reported to have a negative impact on the global burden of tuberculosis (TB), primarily in adult cohorts, but data for the pediatric population is lacking. A comparison of TB trends in children in southwestern Nigeria from January 2016 to December 2019 and January 2020 to May 2022 found a concerning decline in cases of MTB detected by Xpert MTB/RIF in the COVID-19 era, reaching an all-time low in the first half of 2022. More concerning are the emerging trends in rifampicin-resistant tuberculosis, with a 33.3% incidence in the first half of 2022, compared to zero cases reported prior to the COVID era.
Despite substantial progress in recent years, tuberculosis remains endemic in low-income nations. The COVID-19 pandemic appears to have eroded and reversed the gains made over several years.16,17 The fear of COVID-19 having a negative impact on TB control and progress was raised by WHO and others, and has since been confirmed by some recent reports.16,17
The WHO 2021 TB report revealed a declining rate of TB detection since the start of the COVID-19 pandemic, which has been attributed to the measures adopted to combat COVID-19. These measures had resulted in inaccessibility to healthcare systems, a halt in routine childhood immunizations, and the closure of sections of health facilities due to a lack of manpower and a limited supply of personal protective equipment18 All the above observation may have contributed to the declining rate of MTB detected among the children with presumptive TB observed in this study. Quite concerning is the fact that the rate of detection of MTB appears to be declining in the first half of 2022, despite the fact that the country has lifted ban on restrictions with improved access to the health care system. This observation also called for urgent interventions especially at the community level to scale up evaluation of presumptive TB cases with emphasis on adults, who are the primary source of infection for the pediatric population. Our study also shows the incidence of RR-TB of 2.8%, with all cases detected during COVID-19 era. This value is slightly less than 3.7% mono-drug resistance reported in a systematic review and meta-analysis19 but comparable to 2.5% in 2020 from 2021 Global report.16 In the current study, however, all cases of RR-TB were observed during the COVID-19 era, as opposed to the trends reported in other studies.12,16 This finding affirmed the concern of experts on the possible rise in the resistance TB especially among children who are one of the most vulnerable groups. The rise in the RR-TB observed in this study may be a reflection of increase risk of contact of children with adults who have been reported to have increase drug resistance due to impact of COVID-19 on the access to TB diagnosis, treatment and preventive services.15,18 The findings may possibly be the result of delayed diagnosis and access to treatment as a result of the influence of COVID-19 on children, which highlights the difficulties in the diagnosis of childhood tuberculosis. This finding implies a worsening of childhood TB indicators and an accompanying increase in mortality. This therefore calls for various stakeholders to scale up the detection and evaluation of all children with TB for drug resistance. This will ensure early identification and adequate treatment of drug resistance TB that will ultimately result in improved outcomes.
Our findings of a comparable incidence of MTB cases detected in the pre-COVID-19 era (6.9%) and the COVID-19 era (6.2%) contradicts an Indian report that indicated a lower MTB detection rate in the COVID19 era.20 The disparities could be related to the shorter research duration in the Indian report versus the current study, which spans 5.5 years.20
Furthermore, in the aftermath of the pandemic, Nigeria adopted a pragmatic approach to TB control programs, with regard to minimizing the impact of COVID-19. The country reported a modest rise in TB notification, which could explain the lack of a substantial difference in TB incidence trends between the 2 time periods. Regardless, the overall picture implies that access to children TB in Nigeria must be expanded, especially if the goal of eliminating TB by 2035 is to be met.
This study also shows overall detection rate of 6.4% and variables (age, sex, and HIV status) were not associated with MTB detection. The finding of a 6.4% MTB detection rate and the absence of a statistically significant association between MTB detection and the factors of age, gender, and HIV status compared favorably with a 7.6% MTB incidence reported in Ethiopia over a 5 years study period.21 In contrast to the findings of this study, a report in Indonesia found that gender and age group were factors related to MTB detection.22 Despite the adoption of the Xpert MTB/RIF test as the first line diagnostic, the poor detection rate for MTB may reflect the difficulty in diagnosing childhood TB. This also calls for greater study into the development of better diagnostic methods to improve case detection and prompt treatment for both drug-sensitive and drug-resistant tuberculosis in children.
Our study has some limitations. This was retrospective study, and some missing data were excluded from the final data set analyzed. However, the number of children excluded from this study was small and may not have affected the final results. Also, the details of household contact would have been useful in this study, but they were not available in the data source. In addition, these are findings of a single-center study and are less generalizable to the whole pediatric population in Nigeria.
Conclusion
This study shows a significant drop in MTB detection rates in the COVID-19 era, with the lowest rate occurring in the first half of 2022. Whereas there was no detectable case RR-TB prior to COVID-19 pandemic, there was sharp rise of RR-TB incidence in 2022. This necessitates re-prioritizing worldwide efforts to manage childhood tuberculosis, including increased testing, if the aim of eliminating tuberculosis by 2035 is to be met.
Acknowledgments
We appreciate all of the personnel who have worked tirelessly at the TB treatment center as well as the laboratory scientist who analyzed the samples. Most importantly, we wish to thank the patients who submitted the samples for the test.
Footnotes
Author Contributions: Michael Abel Alao: Substantially contributed to conception or design; contributed to acquisition, analysis, or interpretation of data; drafted the manuscript; critically revised the manuscript for important intellectual content; gave final approval; agree to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Olayinka Rasheed Ibrahim: Substantially contributed to the design, analysis, and interpretation of data; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. Babatunde Oluwatosin Ogunbosi: Critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: This study was a retrospective study, ethic approval was obtained from the Health Research Ethical Committee of the with approval number BUTH/REC/-047.
Informed Consent: Informed consent was not sought for the present study because this was a retrospective study.
Data Availability Statement: The data is available to editors, reviewers, and readers without unnecessary restriction wherever possible.
ORCID iD: Michael Abel Alao  https://orcid.org/0000-0003-0109-4435
https://orcid.org/0000-0003-0109-4435
References
- 1. World Health Organization (WHO). Coronavirus (COVID-19) Dashboard. 2022. Accessed February 28, 2022. https://covid19.who.int/
- 2. Burke JF, Chan AK, Mummaneni V, et al. In reply: the coronavirus disease 2019 global pandemic: a neurosurgical treatment algorithm. Neurosurg. 2020;87:E407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torkington S. COVID-19: What you need to know about the coronavirus pandemic. World Economic Forum. 2022. Accessed June 15, 2022. https://www.weforum.org/agenda/2022/06/covid-19-what-you-need-to-know-about-the-coronavirus-pandemic-on-13-june/
- 4. Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(9):e1132-e1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiang C-Y, Islam T, Xu C, et al. The impact of COVID-19 and the restoration of tuberculosis services in the western Pacific region. Eur Respir J. 2020;56(4):2003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brusa M, Barilan YM. Voluntary COVID-19 vaccination of children: a social responsibility. J Med Ethics. 2021;47(8):543-546. [DOI] [PubMed] [Google Scholar]
- 7. Aznar ML, Espinosa-Pereiro J, Saborit N, et al. Impact of the COVID-19 pandemic on tuberculosis management in Spain. Int J Infect Dis. 2021;108:300-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim S, Wu X, Hughes MD, et al. High prevalence of tuberculosis infection and disease in child household contacts of adults with rifampin-resistant tuberculosis. J Pediatr Infect Dis. 2022;41(5):e194-e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coker M, Folayan MO, Michelow IC, Oladokun RE, Torbunde N, Sam-Agudu NA. Things must not fall apart: the ripple effects of the COVID-19 pandemic on children in sub-Saharan Africa. Pediatr Res. 2021;89(5):1078-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JD. The social determinants of tuberculosis: from evidence to action. Am J Public Health. 2011;101(4):654-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wingfield T, Cuevas LE, MacPherson P, Millington KA, Squire SB. Tackling two pandemics: a plea on World Tuberculosis Day. Lancet Respir Med. 2020;8(6):536-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howell P, Achar J, Huang GKL, Mariandyshev A, Schaaf HS, Garcia-Prats AJ. Treatment of rifampicin-resistant tuberculosis disease and infection in children: key updates, challenges and opportunities. Pathogens. 2022;11(4):381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas TA. Tuberculosis in children. Thorac Surg Clin. 2019;29(1):109-121. [DOI] [PubMed] [Google Scholar]
- 14. Dookie N, Padayatchi N, Naidoo K. Tuberculosis Elimination in the Era of Coronavirus Disease 2019 (COVID-19): A Moving Target, Vol. 74. Oxford University Press US; 2022:509-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muhammad Dayyab F, Iliyasu G, Garba Ahmad B, et al. Emerging threat of drug-resistant tuberculosis and trends in the era of COVID-19: a descriptive study from northwestern Nigeria. J Clin Tuberc Other Mycobact Dis. 2022;28:100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization (WHO). Global Tuberculosis Report 2021. World Health Organization; 2021. [Google Scholar]
- 17. Chakaya J, Petersen E, Nantanda R, et al. The WHO Global Tuberculosis 2021 report – not so good news and turning the tide back to end TB. Int J Infect Dis. 2022;124:S26-S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McQuaid CF, McCreesh N, Read JM, et al. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur Respir J. 2020;56(2):2001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song W-M, Li Y-F, Liu Y-X, et al. Drug-resistant tuberculosis among children: a systematic review and meta-analysis. Front Public Health. 2021;9:721817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Golandaj JA. Pediatric TB detection in the era of COVID-19. Indian J Tuberc. 2022;69(1):104-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diriba K, Awulachew E, Churiso G. The magnitude of MTB and rifampicin resistance MTB using Xpert-MTB/RIF assay among tuberculosis suspected patients in Gedeo Zone, southern Ethiopia. Infect Drug Resist. 2021;14:3961-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agustina B, Kartasasmita C, Hilmanto D. Comparison of GeneXpert MTB to Mycobacterium tuberculosis culture in children with tuberculosis. Paediatr Indones. 2019;59(3):113-118. [Google Scholar]