Abstract
Chronic obstructive pulmonary disease (COPD) is one of the common diseases of the respiratory system. As the disease recurs, damage to the airways and lung tissue gradually worsens, leading to a progressive decline in lung function, affecting the patient’s workforce and quality of life, and causing a huge social and economic burden. Diabetes is a common comorbidity of COPD and patients with COPD are at increased risk of developing diabetes, while hyperglycemia can also reduce lung function and contribute to the progression and poor prognosis of COPD. Glucagon-like peptide-1 receptor agonist (GLP-1RA) is a new type of hypoglycemic agent that has been shown to regulate blood glucose levels, reduce inflammatory responses and oxidative stress, and regulate lipid metabolism, among other effects. GLP-1RAs may benefit COPD patients by acting directly on the lung from mechanisms such as reducing the inflammatory response, improving oxidative stress, regulating protease/anti-protease imbalance, improving airway mucus homeostasis, and reducing airway remodeling. This study provides a review of the potential role of GLP-1RAs in COPD and offers new ideas for the prevention and treatment of COPD.
Keywords: glucagon-like peptide 1 receptor agonist, chronic obstructive pulmonary disease, clinical application
COPD is a prevalent respiratory disease with high prevalence, morbidity, and mortality. In a new retrospective study,1 the prevalence of COPD was shown to be as high as 10.3% worldwide in 2019 for approximately 392 million people aged 30–79 years, and COPD is now one of the top three causes of death worldwide.2 COPD is associated with a variety of comorbidities, such as diabetes,3,4 and the underlying mechanisms between the two are complex, multifactorial and as yet unclear.5 A prospective cohort study showed6 that the prevalence of diabetes in COPD patients was as high as 25.5% and that COPD patients with diabetes were at higher risk of severe exacerbations and death than COPD patients without diabetes. A retrospective study showed that the prevalence of diabetes was higher in COPD patients (18.7%) than in the general population (10.5%).5 GLP-1RA is a common hypoglycemic drug. With in-depth research in China and abroad, GLP-1RA has been found to play a protective role in the respiratory system. As a chronic progressive disease, the high prevalence and mortality of COPD and the economic pressures it places on the population have further prompted us to explore the treatment of this disease. Therefore, this article will provide a brief overview of the use of GLP-1RA in COPD patients to provide a basis for the management of COPD.
Introduction to Glucagon-Like Peptide 1 and Others
Glucagon-like peptide-1 (GLP-1) is an enter glucagon found in L-cells of the small intestinal mucosa,7 and the release of GLP-1 is increased in response to elevated blood glucose conditions such as feeding. Glucagon-like peptide-1 receptor (GLP-1R) belongs to the B family of G protein-coupled receptors (GPCRs).8 GLP-1R is expressed in model organisms and humans and is highly conserved and widely distributed in the body,9,10 such as the pancreatic islets, central nervous system, stomach, heart, lung, liver and kidney.11 GLP-1, when bound to GLP-1R, can affect blood glucose levels by stimulating insulin secretion, inhibiting glucagon secretion, suppressing gastric emptying and reducing food intake. GLP-1 is rapidly inactivated by dipeptidyl peptidase 4 (DDP-4) after being secreted into the bloodstream, limiting the action of GLP-1. GLP-1RA has similar effects to GLP-1, is not easily degraded by DDP-4 and can mimic the binding of natural GLP-1 to GLP-1R, activating the body’s signaling pathways and exerting corresponding effects. GLP-1RAs include dulaglutide, exenatide, liraglutide, lixisenatide, somalutide and others.12
Chronic Obstructive Pulmonary Disease
COPD is characterized by persistent respiratory symptoms and airflow limitation.13 The main clinical manifestations are chronic cough, sputum, shortness of breath or dyspnea,14 wheezing, chest tightness, and other symptoms. COPD is diagnosed when the ratio of forceful expiratory volume in the first second to all expiratory volume (FEV1/FVC) is <70%15 after inhalation of bronchodilators, indicating persistent airflow limitation, combined with the patient’s clinical presentation and the presence of high-risk factors such as smoking, dust or chemical irritation, infection, and the exclusion of other diseases. The common pathogenesis of COPD includes16,17 chronic inflammatory mechanisms involving neutrophils, macrophages and lymphocytes; protease and anti-protease imbalance caused by an increase in protein hydrolases and a decrease in anti-protease; oxidative stress mechanisms and other mechanisms that increase oxidation beyond the body’s antioxidant capacity, leading to an oxidative and antioxidant imbalance. The conventional treatment of COPD is to improve the patient’s poor ventilation, get rid of sputum, control symptoms such as infection, protect lung function, improve the patient’s quality of life, slow down the progression of the disease and reduce death.
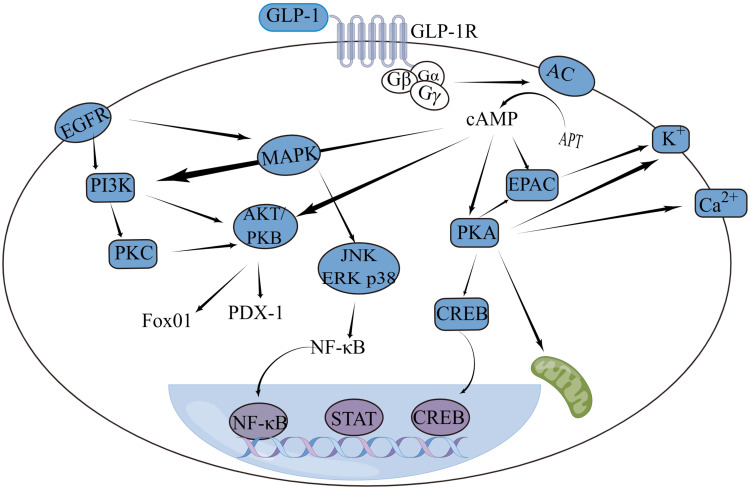
It was found that GLP-1 when bound to GLP-1R (Figure 1), can increase the concentration of cAMP by activating adenylate cyclase (AC) and stimulating the conversion of ATP to cyclic adenosine monophosphate (cAMP).18 The increased cAMP can further act on protein kinase A (PKA) and guanine nucleotide exchange protein (EPAC), and the activated PKA/EPAC can affect the activated PKA/EPAC can affect the concentration of K+ and Ca2+ ions inside and outside the cell,19 which in turn promotes insulin secretion. By activating the protein kinase C (PKC) signaling pathway, the cAMP/PKA pathway and the Ca2+ signaling pathway all increase islet glucagon gland glucagon gene transcription. GLP-1/GLP-1R enhances insulin-promoting factor-1 (PDX-1) responsiveness via the epidermal growth factor receptor (EGFR)/phosphoinositide-3-kinase (PI3K)/protein kinase B (AKT/PKB)/fork head box protein O1 (FOXO1) signaling pathway, thereby maintaining β-cell morphological function and promoting the conversion of non-β cells to β cells.20 The mechanism by which GLP-1 reduces oxidative stress can be mediated through the cAMP, PI3K and PKC pathways.21 The anti-apoptotic effect of GLP-1 is mediated by increasing levels of the anti-apoptotic proteins Bcl-2 and Bcl-xL and activating cAMP and PI3K as well as PKB and EGFR-PI3K signaling pathways to reduce cysteine-3 activity. GLP-1 and GLP-1RA exert anti-inflammatory and immunological properties through the mitogen-activated protein kinase (MAPK) signaling, transcriptional activator protein (STAT) and cAMP/PKA/nuclear factor-κB (NF-κB) signaling pathways.22
Figure 1.
The GLP-1/GLP-1R signaling pathway in cells.
Diabetes is a metabolic disease characterized by chronic hyperglycemia caused by a variety of etiologies. Although COPD and diabetes are two different diseases, studies have found that patients with COPD are more likely to develop diabetes23–27 and patients with diabetes are at increased risk of COPD.28 Possible common causal mechanisms for both are chronic systemic inflammation, oxidative stress, hypoxia, obesity and hyperglycaemia.29,30 Patients with COPD combined with diabetes have a significantly higher rate of hospitalization, and diabetes increases the risk of infection, and progression of disease course, leading to poor prognosis and increased mortality in COPD patients.31,32 Conversely, the inflammatory process, impaired lung function and/or the use of glucocorticoids in COPD patients may increase the risk of developing diabetes.24 Therefore, considering that GLP-1R is widely distributed in the body, that COPD and diabetes interact with each other and share common pathogenic mechanisms, and that GLP-1 and its receptor agonists have been widely used in diabetic patients and have demonstrated hypoglycemic, pro-inflammatory and anti-inflammatory factors, oxidative stress, neurogenesis and vascular protection,33 then GLP-1 and its receptor agonists also have a role to play in COPD patients.
The Role of GLP-1RAs in COPD
GLP-1RAs and Improvement of Airway Inflammation
Inflammatory cells and cytokines including neutrophils,34,35 macrophages,36 T-lymphocytes, eosinophils, C-reactive protein (CRP), tumors necrosis factor (TNF-α), interleukin-6 (IL-6), IL-10, IL-8, IL-17, etc. are collectively involved in the chronic inflammatory process in COPD. In contrast, improvement in the inflammatory process can be judged by improvements in the levels of markers such as neutrophils, macrophages, lymphocytes, eosinophils, CRP, TNF-α, IL-6 or changes in the production and expression of reactive oxygen species (ROS), and the clinical benefit that may be gained from these changes should be reflected in improvements in the final effects of chronic inflammation.37 Neutrophils cause a highly viscous state in the airways and destroy the lung parenchyma by releasing various bioactive substances such as neutrophil elastase (NE), histone G and oxidative substances.38 Macrophages have phagocytic, bactericidal and antigen-presenting functions. Tobacco stimulation and the chronic inflammatory response induced by COPD can increase the number of macrophages,39 but the function of macrophages is significantly impaired under the stimulus of injury. Activated macrophages release large amounts of inflammatory mediators such as IL-1, IL-6, IL-8, TNF-α, ROS and chemokines, leukotrienes and prostaglandins, matrix metalloproteinases (MMP), etc.40 which in turn chemotactic neutrophils and peripheral circulating macrophages and other inflammatory cells, further amplifying the inflammatory response, forming a vicious circle and aggravating the progression of COPD. Stimulated by a variety of cytokines, eosinophils cause an inflammatory response in the airways and fibroblast proliferation and collagen synthesis, resulting in airway damage, fibrosis and remodeling and contributing to the progression of COPD. In addition to neutrophils, macrophages and eosinophils, T lymphocytes are also involved in the chronic inflammatory process in COPD patients. CD4+ T lymphocytes can secrete cytokines such as interferon, IL-6, IL-17 and TNF-α, which further induce the production and release of MMP and the activation and aggregation of neutrophils in the body, thereby destroying lung tissue and activating elastase, ultimately leading to elastin hydrolysis and emphysema formation. CD8+ T lymphocytes can upregulate perforin expression and increase MMP activity, resulting in the airway and lung tissue destruction, as well as chemotactic activation of inflammatory cells through secretion of cytokines such as interferon, IL-12, TNF-α and chemokines, exacerbating the inflammatory response.
GLP-1R was detected in mouse and human macrophages, neutrophils, eosinophils, and lymphocytes.41–45 Studies have shown that GLP-1 and its receptor agonists not only inhibit the levels of total cells, neutrophils, macrophages, eosinophils, lymphocytes, TNF-α, IL-4, IL-5 and IL-13 in mouse alveolar lavage fluid46 but also mediate anti-inflammatory effects through stress-activated protein kinase (JNK) inhibition, signal transducer and activator of transcriptional activator protein 3 (STAT3) activation and cAMP/PKA/NF-κB signaling pathways, reducing the release of pro-inflammatory markers (iNOS, IL-1β, IL-6, TNF-α and monocyte chemotactic protein-1 (MCP-1), MMP-2, MMP-9, ROS) and increasing the release of anti-inflammatory markers (IL-10, mannose receptor-1 (MRC-1), arginine-1 (Arg-1) release as well as prostaglandin E2 (PGE2) and cyclooxygenase 2 (COX2) mRNA and COX2 protein levels,42,46 thereby achieving a reduced inflammatory response. GLP-1 reduced the number of CD3+ T lymphocytes, F4/80+ macrophages and CD11b+ macrophages in the blood of obese and COPD mice and reduced the size of emphysema in mice.47 Mitchell et al41 demonstrated through studies that GLP-1R was expressed on human eosinophils and neutrophils and that GLP-1 analogues significantly reduced the expression of eosinophil surface activation markers CD11b and CD69 and decreased eosinophil production of factors such as IL-4, IL-8 and IL-13. In a lipopolysaccharide (LPS)-induced mouse model, GLP-1 expression was reduced in peripheral blood and increased in alveolar lavage fluid and lung tissues, which was inhibited by liraglutide, a GLP-1RA, and was able to suppress LPS-induced lung injury in mice, inhibit polymorphonuclear neutrophil (PMN) extravasation, reduce the number of apoptotic cells in the lung, inhibit macrophage accumulation48 and Liraglutide also has anti-inflammatory and airway-protective effects by modulating the PKA/NF-κB signaling pathway to improve histopathological changes and airway inflammation in mice, inhibiting E-selectin overexpression and reducing mucus secretion and cupped cell proliferation.49 Liraglutide and somalutide can reduce the expression of pro-inflammatory factors such as TNF-α, interferon-γ (IFN-γ) and IL-6, thereby reducing the systemic inflammatory response.50 Exenatide increased IL-10 and Arg-1 levels and decreased TNF-a, IL-1b and iNOS expression, contributing to the conversion of the macrophage phenotype to an anti-inflammatory phenotype.43 Exenatide significantly decreased TNF-α, MCP-1 and ROS secretion, which may be associated with the activation of adenylate-activated protease (AMPK) and inhibited the inflammatory process through interaction with NF-κB, CCAAT/enhancer binding protein β (C/EBPβ) and mitogen-activated protein kinase (MAPK) (p38, ERK and JNK).51 Airflow restriction is a critical part of COPD pathogenesis and is closely related to the proliferation and migration of airway smooth muscle cells and the secretion of inflammatory factors. GLP-1R overexpression can inhibit the expression of inflammatory factors such as IL-6, IL-8, TNF-α and granulocyte and macrophage colony-stimulating factor (GM-CSF) by increasing the expression of adenosine triphosphate-binding cassette transporter A1 (ABCA1) and reduce the proliferation and migration of smooth muscle, indicating that GLP-1R can play a protective role in smooth muscle cells and COPD, thereby improving airway inflammation and airflow limitation in COPD patients, providing a basis for the application of GLP-1RA in COPD.51 Park et al52 found that empagliflozin, dulaglutide and a combination of both significantly attenuated the increase in cells, especially macrophages and neutrophils, in airway alveolar lavage fluid induced by a high-fat diet in mice, reduced the expression of IL-17 and transforming growth factor (TGF)-β1, and decreased the differentiation of Th cells to Th1 and Th17, thereby achieving a reduced inflammatory response.
GLP-1RAs and Oxidative Stress
Cigarette smoke and environmental pollution1,15 can bring a large number of exogenous chemicals, oxides, etc. activated inflammatory cells in the lungs can release endogenous oxides,53 when these substances exceed the body’s antioxidant capacity, the imbalance between oxidation and antioxidant causes oxidative stress, resulting in airway epithelial damage, reduced ciliary movement and release of NE, which in turn causes cellular dysfunction or apoptosis, inflammatory response, accumulation of mucus substances and their easy elimination, extracellular matrix destruction, protease/anti-protease imbalance, etc., leading to the development and progression of COPD and increased comorbidity.54
Stimuli such as smoke can cause airway inflammation and oxidation/antioxidation imbalance in rats with COPD, leading to lung function impairment and exacerbation of emphysema.55 ROS is a by-product of oxidative phosphorylation in the mitochondrial electron transport chain. It has been found that smooth muscle cells in COPD patients exhibit mitochondrial dysfunction, which in turn causes an increase in mitochondrial ROS, resulting in an oxidative-oxidative imbalance that contributes to the development and progression of COPD.44 Liraglutide reduces oxidative stress by reducing apoptosis and normalizing some of the mitochondrial dynamic imbalances.56 Somalutin and liraglutide alleviate chronic inflammatory responses, reduce lipid peroxidation, inhibit mitochondrial autophagic signaling pathways and reduce oxidative stress by reducing the accumulation of alpha-synuclein (alpha-syn) synthesis.57 Bułdak et al58 found that exenatide increased the antioxidant capacity of human monocytes/macrophages in vitro, reducing oxidative stress by decreasing the expression of ROS-producing NADPH oxidase, increasing the expression and activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), and decreasing ROS and malondialdehyde (MDA) levels. Liu et al59 found that compared with the insulin group, the liraglutide group could reduce serum MDA, MCP-1 and NF-κB levels and increase serum SOD levels, indicating that liraglutide could reduce oxidative stress and inhibit the inflammatory response of the body.
GLP-1RAs and Protease/Anti-Protease
The imbalance between proteases and anti-proteases caused by an increase in proteases and a decrease in anti-proteases can lead to the destruction of lung tissue structure and consequently to emphysema. Common proteases include NE and MMP, while anti-proteases include α1-antitrypsin and tissue matrix metalloproteinase inhibitors (TIMPs).
NE60 is a member of the neutrophil-derived serine protease family. When inflammation occurs, activation of neutrophils can release large amounts of NE,61 causing damage to the lung parenchyma and alveolar wall, and increasing mucin synthesis and mucus secretion by promoting inflammatory cell aggregation and secretion of pro-mucin secretagogues, aggravating the airway inflammatory response and airway obstruction in COPD and causing a decrease in ventilation function. MMP62 is mainly secreted by neutrophils and macrophages, and relies on cofactors such as zinc and calcium to actively degrade the extracellular matrix causing airway destruction and remodeling in COPD patients,63 and activates the nuclear factor-κB signaling pathway, Toll-like receptors and Wnt signaling pathway through a series of reactions, thereby increasing the secretion of MMP and the expression of intercellular adhesion molecules, promoting inflammatory cell aggregation and exacerbating COPD progression. Alpha 1-antitrypsin64 is synthesized by the liver and deposited in alveolar cells to inhibit the action of many tissue proteases, including elastase and trypsin, and to protect lung tissue from protease damage. TIMPs have an inhibitory effect on most MMP activity, thereby reducing lung tissue destruction and emphysema formation.
GLP-1RAs can reduce the number of inflammatory cells and are also able to cause a decrease in protease levels in lung tissue. GLP-1 has been reported to have a positive effect on hepatocytes,65 promoting the synthesis of alpha1-antitrypsin and increasing alpha1-antitrypsin deposition in the lungs. Thus, the ability of GLP-1 treatment to reduce the area of dilated alveolar tissue in emphysematous mice may be related to the protease mechanism that inhibits emphysema formation.47 Gallego-Colon et al66 found that exenatide could down-regulate the expression of protein hydrolases MMP-1, MMP-2 and MMP-9 and increase the protein concentration of TIMP-1 and TIMP-2 by inhibiting protein kinase B-Thr308 phosphorylation, PRAIS40 and S6 protein signaling pathways in smooth muscle. Garczorz et al67 found that GLP-1R agonists reduced endothelial cell MMP-1, MMP-2, and MMP-9 production and stimulated TIMP-1 and TIMP-2 synthesis by inhibiting TNF-α-induced activation of NF-κB, suggesting that GLP-1RAs may play a role in the treatment of COPD by altering protease/antiprotease synthesis.
GLP-1RAs and Other Mechanisms
According to the Global Burden of Disease 2019 data report,68 although age-standardized mortality from chronic respiratory diseases has shown a gradual decline globally from 1990 to 2019, COPD still has the highest age-standardized mortality rate of all chronic respiratory diseases and COPD is also the largest burden of death from chronic respiratory diseases, a situation that may remain difficult to improve in the future.
By Meta-analysis, Wei et al69 found that GLP-1RAs reduced the risk of nine respiratory diseases, including COPD, compared to placebo. Mortality and morbidity in COPD mice were significantly reduced following treatment with GLP-1RA liraglutide and exenatide in a COPD mouse model.10 GLP-1RA treatment significantly improved FEV1, FVC, MEF75 and MEF50 measurements and improved lung function in patients with diabetes.70 Liraglutide improves the defective GLP-1 receptor signaling in peripheral blood mononuclear cells of COPD patients, reduces the expression of programmed death protein 1 on T cells, improves abnormal T lymphocyte function and enhances the immunity of COPD patients.71 GLP-1R signaling can act through the cAMP pathway to regulate lymphocyte proliferation and maintain peripheral regulatory T cells,72 thus potentially preventing acute exacerbations of COPD. GLP-1R is expressed in the lung and is associated with the regulation of the surfactant lipid fraction. In a rat model of pulmonary dysplasia, liraglutide improved lung physiology and alveolar surfactant protein A and B production.73 Romaní-Pérez et al74 showed that GLP-1R expression was upregulated in newborn rats after birth and that administration of GLP-1RA exenatide or liraglutide in pregnant rats increased the expression of alveolar surfactant protein A and B mRNA and the amount of alveolar surfactant in amniotic fluid. In vitro studies revealed a dramatic increase in the number of cells expressing the intercellular adhesion molecule CD34 and an increase in the number of CD31+ cells with active esterase under the direct action of GLP-1, suggesting that endothelial progenitor cells expressing CD31 and CD34 endothelial progenitor cell markers may be targets of GLP-1 and participate in the regeneration process of damaged endothelium in emphysematous mice with heterotrophic insulin,47 and thus in airway remodeling. Altintas Dogan et al75 experiment found that liraglutide significantly reduced body weight, decreased inflammatory markers such as IL-6 and MCP-1, increased exertional spirometry (FVC) and carbon monoxide diffusion capacity, and improved COPD assessment test scores, but no significant improvement in FEV1, FEV1/FVC, or 6-minute walk tests, suggesting the possibility that GLP-1RA may reduce inflammatory responses, improve lung function, and mitigate the degree of impaired health in patients with COPD. Fork head box protein A2 (FOXA2), a key transcriptional regulator of airway mucus homeostasis, is under-expressed in the airways of COPD patients, thereby inducing cupped cell proliferation and chemotaxis as well as mucus secretion. GLP-1R expression is reduced in the lungs of COPD patients. Exenatide restores GLP-1R expression and reduces mucin production in COPD airway cells by restoring FOXA2 function through activation of GLP-1R-peroxisome proliferator-activated receptor gamma (PPAR-γ)-phosphatase and tension homolog (PTEN) signaling, thereby increasing the ectopic and efficacy of other drugs in COPD and reducing the high morbidity and mortality induced by excess mucus.76
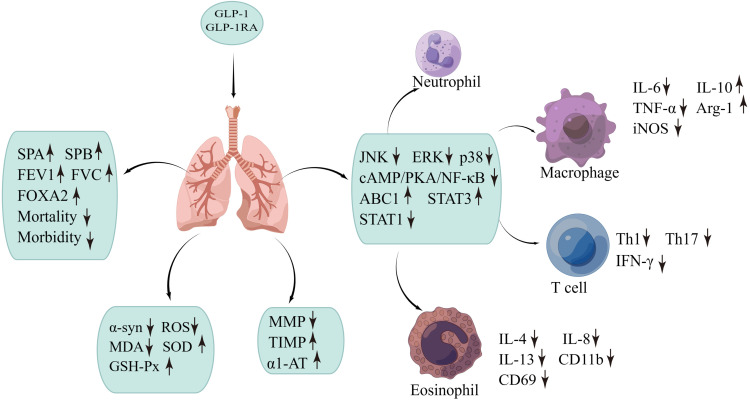
Clinical studies have shown that GLP-1 and its receptor agonist exenatide can improve human bronchial hyperresponsiveness (BHR) by inducing pulmonary vasorelaxation and phosphatidylcholine secretion through activation of the cAMP-PKA-dependent pathway,77 and by stimulating the secretion of phosphatidylcholine (a major component of surfactant) through cAMP-dependent PKA and cAMP-dependent PKC, thereby improving lung function.78 Exenatide restores FOXA2 expression and mucus homeostasis by activating the GLP-1R-PKA-PPAR-γ pathway to achieve improvement in COPD.76 And a population-based cohort study suggests79 that GLP-1RAs can reduce the risk of severe exacerbations in patients with COPD combined with diabetes by 30% compared to sulphonylureas. Overall, GLP-1 and GLP-1RAs (as shown in Figure 2) can improve lung function, and airway remodeling, reduce mucus secretion, reduce disease risk and mortality through a variety of pathways, and may hold potential promise in the treatment of airway disease,80 but GLP-1RAs are not approved for the treatment of COPD, so further studies are needed to confirm this.
Figure 2.
Effect of GLP-1/GLP-1RA on COPD through different pathways. ↑: indicates an increase ↓: indicates a decrease.
Conclusion
Diabetes, a comorbid condition in COPD patients,5 can affect lung function and hyperglycemia can cause increased oxidative stress in the respiratory system, changes in lung tissue structure and airflow exchange, increasing the probability of lung infection, increasing the risk of hospitalization and leading to a poor prognosis for COPD patients.
As a novel hypoglycemic agent, GLP-1RAs can not only control patients’ blood glucose and improve their diabetes-related symptoms but also reduce inflammatory cells and factors through multiple signaling pathways, increase the expression of anti-inflammatory factors and alleviate inflammatory responses; reduce the damage caused by oxidative stress by decreasing the expression of oxidants and increasing the level of antioxidants; improve protease/anti-protease imbalance by down-regulating protease secretion and It also improves the protease/anti-protease imbalance by down-regulating protease secretion and increasing anti-protease levels; and enhances immunity by restoring GLP-1R expression, improving lung function and increasing alveolar surfactant levels in COPD patients.
In conclusion, GLP-1RAs may effectively improve the clinical symptoms of COPD patients, alleviate airflow limitation, airway damage, fibrosis, and remodeling, shorten the hospital stay, reduce the economic burden of patients, and reduce the occurrence of COPD-related complications, improve the long-term prognosis of patients, thus playing a therapeutic and preventive role for COPD patients, and may provide a new target for the prevention and treatment of COPD. However, GLP-1RAs are mainly used in diabetic patients, and their application to the respiratory system (eg COPD) still needs to be explored in further clinical trials to provide more evidence for the prevention and treatment of COPD.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81400288, 81573244), Hubei Provincial Natural Science Foundation (2022CFB453), Foundation of Health Commission of Hubei (WJ2021M061), the Natural Science Foundation of the Bureau of Science and Technology of Shiyan City (grant no. 21Y71), the Faculty Development Grants from Hubei University of Medicine (2018QDJZR04), and Hubei Key Laboratory of Wudang Local Chinese Medicine Research (Hubei University of Medicine) (Grant No. WDCM2022007), and the Advantages discipline Group (Medicine) Project in Higher Education of Hubei Province (2021-2025) (Grant No. 2022XKQT4).
Data Sharing Statement
There is no raw data associated with this review.
Disclosure
The authors declare that there is no conflict of interest.
References
- 1.Adeloye D, Song P, Zhu Y, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10(5):447–458. doi: 10.1016/S2213-2600(21)00511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. GOLD website; 2022.
- 3.Castañ-Abad MT, Godoy P, Bertran S, Montserrat-Capdevila J, Ortega M. Incidencia de exacerbación grave en pacientes codiagnosticados de diabetes y enfermedad pulmonar obstructiva crónica: estudio de cohorte [Incidence of severe exacerbation in patients diagnosed with diabetes and chronic obstructive pulmonary disease: Cohort study]. Aten Primaria. 2021;53(8):102074. doi: 10.1016/j.aprim.2021.102074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolahian S, Leiss V, Nürnberg B. Diabetic lung disease: fact or fiction? Rev Endocr Metab Disord. 2019;20(3):303–319. doi: 10.1007/s11154-019-09516-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cazzola M, Bettoncelli G, Sessa E, Cricelli C, Biscione G. Prevalence of comorbidities in patients with chronic obstructive pulmonary disease. Respiration. 2010;80(2):112–119. doi: 10.1159/000281880 [DOI] [PubMed] [Google Scholar]
- 6.Castañ-Abad MT, Montserrat-Capdevila J, Godoy P, et al. Diabetes as a risk factor for severe exacerbation and death in patients with COPD: a prospective cohort study. Eur J Public Health. 2020;30(4):822–827. doi: 10.1093/eurpub/ckz219 [DOI] [PubMed] [Google Scholar]
- 7.Cazzola M, Rogliani P, Calzetta L, Lauro D, Page C, Matera MG. Targeting mechanisms linking COPD to type 2 diabetes mellitus. Trends Pharmacol Sci. 2017;38(10):940–951. doi: 10.1016/j.tips.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 8.Graaf CD, Donnelly D, Wootten D, et al. Glucagon-like peptide-1 and its class B G protein–coupled receptors: a long march to therapeutic successes. Pharmacol Rev. 2016;68(4):954–1013. doi: 10.1124/pr.115.011395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korner M, Stockli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007;48(5):736–743. doi: 10.2967/jnumed.106.038679 [DOI] [PubMed] [Google Scholar]
- 10.Viby N, Isidor MS, Buggeskov KB, Poulsen SS, Hansen JB, Kissow H. Glucagon-Like Peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 2013;154(12):4503–4511. doi: 10.1210/en.2013-1666 [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Jun H. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. J Am Acad Physician Assist. 2020;33(S8):3–18. doi: 10.1097/01.JAA.0000669456.13763.bd [DOI] [PubMed] [Google Scholar]
- 13.Soriano JB, Polverino F, Cosio BG. What is early COPD and why is it important? Eur Respir J. 2018;52(6):1801448. doi: 10.1183/13993003.01448-2018 [DOI] [PubMed] [Google Scholar]
- 14.O Donnell DE, Milne KM, James MD, de Torres JP, Neder JA. Dyspnea in COPD: new mechanistic insights and management implications. Adv Ther. 2020;37(1):41–60. doi: 10.1007/s12325-019-01128-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Ye X, Zhang Y, Ling S. Global, regional, and national burden of chronic obstructive pulmonary disease from 1990 to 2019. Front Physiol. 2022;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo P, Li R, Piao TH, Wang CL, Wu XL, Cai HY. Pathological mechanism and targeted drugs of COPD. Int J Chron Obstruct. 2022;17:1565–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arellano-Orden E, Calero Acuña C, Sánchez-López V, et al. Cellular mechanisms involved in the pathogenesis of airway remodeling in chronic lung disease. Eur Clin Respir J. 2022;9(1). doi: 10.1080/20018525.2022.2097377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzook A, Tomas A, Jones B. The interplay of glucagon-like peptide-1 receptor trafficking and signaling in pancreatic beta cells. Front Endocrinol. 2021;12. doi: 10.3389/fendo.2021.678055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Wang M, Wen Z, et al. GLP-1 receptor agonists: beyond their pancreatic effects. Front Endocrinol. 2021;12. doi: 10.3389/fendo.2021.721135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joly E, Prentki M, Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia. 2004;47(5):806–815. doi: 10.1007/s00125-004-1379-6 [DOI] [PubMed] [Google Scholar]
- 21.Oh Y, Jun H. Effects of glucagon-like peptide-1 on oxidative stress and Nrf2 signaling. Int J Mol Sci. 2018;19(1):26. doi: 10.3390/ijms19010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bendotti G, Montefusco L, Lunati ME, et al. The anti-inflammatory and immunological properties of GLP-1 receptor agonists. Pharmacol Res. 2022;182:106320. doi: 10.1016/j.phrs.2022.106320 [DOI] [PubMed] [Google Scholar]
- 23.Gayle A, Dickinson S, Poole C, Pang M, Fauconnot O, Quint JK. Incidence of type II diabetes in chronic obstructive pulmonary disease: a nested case-control study. NPJ Prim Care Respir Med. 2019;29(1):26–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng Y, Zhong G, Wang L, et al. Chronic obstructive pulmonary disease, lung function and risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Bmc Pulm Med. 2020;20(1). doi: 10.1186/s12890-020-1178-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho T, Huang C, Ruan S, Tsai Y, Lai F, Yu C. Diabetes mellitus in patients with chronic obstructive pulmonary disease-the impact on mortality. PLoS One. 2017;12(4):e175794. doi: 10.1371/journal.pone.0175794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flattet Y, Garin N, Serratrice J, Perrier A, Stirnemann J, Carballo S. Determining prognosis in acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:467–475. doi: 10.2147/COPD.S122382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L, Shi J, Kang J, Wang Q. Analysis of prevalence and prognosis of type 2 diabetes mellitus in patients with acute exacerbation of COPD. Bmc Pulm Med. 2021;21(1). doi: 10.1186/s12890-020-01371-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu I, Lu C, Li C, et al. Population-based cohort study suggesting a significantly increased risk of developing chronic obstructive pulmonary disease in people with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;138:66–74. doi: 10.1016/j.diabres.2018.01.037 [DOI] [PubMed] [Google Scholar]
- 29.Katsiki N, Steiropoulos P, Papanas N, Mikhailidis DP. Diabetes mellitus and chronic obstructive pulmonary disease: an overview. Exp Clin Endocrinol Diabetes. 2021;129(10):699. doi: 10.1055/a-1038-3883 [DOI] [PubMed] [Google Scholar]
- 30.Park SS, Perez Perez JL, Perez Gandara B, et al. Mechanisms linking COPD to type 1 and 2 diabetes mellitus: is there a relationship between diabetes and COPD? Medicina. 2022;58(8):1030. doi: 10.3390/medicina58081030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gläser S, Krüger S, Merkel M, Bramlage P, Herth FJF. Chronic obstructive pulmonary disease and diabetes mellitus: a systematic review of the literature. Respiration. 2015;89(3):253–264. doi: 10.1159/000369863 [DOI] [PubMed] [Google Scholar]
- 32.Khateeb J, Fuchs E, Khamaisi M. Diabetes and lung disease: an underestimated relationship. Rev Diabet Stud. 2019;15(1):1–15. doi: 10.1900/RDS.2019.15.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaribeygi H, Maleki M, Sathyapalan T, Jamialahmadi T, Sahebkar A. Anti-inflammatory potentials of incretin-based therapies used in the management of diabetes. Life Sci. 2020;241:117152. doi: 10.1016/j.lfs.2019.117152 [DOI] [PubMed] [Google Scholar]
- 34.Herrero-Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. 2022;19(2):177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamin JT, Plosa EJ, Sucre JMS, et al. Neutrophilic inflammation during lung development disrupts elastin assembly and predisposes adult mice to COPD. J Clin Invest. 2021;131(1). doi: 10.1172/JCI139481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N, Liu Y, Cai J. LncRNA MIR155HG regulates M1/M2 macrophage polarization in chronic obstructive pulmonary disease. Biomed Pharmacother. 2019;117:109015. doi: 10.1016/j.biopha.2019.109015 [DOI] [PubMed] [Google Scholar]
- 37.Hatwal A. Inflammation and incretins. Indian J Endocrinol Metab. 2012;16(8):239. doi: 10.4103/2230-8210.104049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genschmer KR, Russell DW, Lal C, et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. 2019;176(1–2):113–126. doi: 10.1016/j.cell.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finicelli M, Digilio FA, Galderisi U, Peluso G. The emerging role of macrophages in chronic obstructive pulmonary disease: the potential impact of oxidative stress and extracellular vesicle on macrophage polarization and function. Antioxidants. 2022;11(3):464. doi: 10.3390/antiox11030464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takiguchi H, Yang CX, Yang CWT, et al. Macrophages with reduced expressions of classical M1 and M2 surface markers in human bronchoalveolar lavage fluid exhibit pro-inflammatory gene signatures. Sci Rep. 2021;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell PD, Salter BM, Oliveria JP, et al. Glucagon-like peptide-1 receptor expression on human eosinophils and its regulation of eosinophil activation. Clin Exp Allergy. 2017;47(3):331–338. doi: 10.1111/cea.12860 [DOI] [PubMed] [Google Scholar]
- 42.Batty MJ, Chabrier G, Sheridan A, Gage MC. Metabolic hormones modulate macrophage inflammatory responses. Cancers. 2021;13(18):4661. doi: 10.3390/cancers13184661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bułdak Ł, Machnik G, Bułdak RJ, et al. Exenatide (a GLP-1 agonist) expresses anti-inflammatory properties in cultured human monocytes/macrophages in a protein kinase A and B/Akt manner. Pharmacol Rep. 2016;68(2):329–337. doi: 10.1016/j.pharep.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 44.Wiegman CH, Michaeloudes C, Haji G, et al. Oxidative stress–induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015;136(3):769–780. doi: 10.1016/j.jaci.2015.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Mei A, Liu X, et al. Glucagon-like peptide-1 receptor regulates macrophage migration in monosodium urate-induced peritoneal inflammation. Front Immunol. 2022;13:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo C, Huang T, Chen A, et al. Glucagon-like peptide 1 improves insulin resistance in vitro through anti-inflammation of macrophages. Braz J Med Biol Res. 2016;49(12). doi: 10.1590/1414-431x20165826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skurikhin E, Pershina O, Pakhomova A, et al. Endothelial progenitor cells as pathogenetic and diagnostic factors, and potential targets for GLP-1 in combination with metabolic syndrome and chronic obstructive pulmonary disease. Int J Mol Sci. 2019;20(5):1105. doi: 10.3390/ijms20051105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, Wei G, Wang J, et al. Glucagon-like peptide-1 receptor activation alleviates lipopolysaccharide-induced acute lung injury in mice via maintenance of endothelial barrier function. Lab Invest. 2019;99(4):577–587. doi: 10.1038/s41374-018-0170-0 [DOI] [PubMed] [Google Scholar]
- 49.Zhu T, Wu X, Zhang W, Xiao M. Glucagon Like Peptide-1 (GLP-1) modulates OVA-induced airway inflammation and mucus secretion involving a Protein Kinase A (PKA)-Dependent Nuclear Factor-κB (NF-κB) signaling pathway in mice. Int J Mol Sci. 2015;16(9):20195–20211. doi: 10.3390/ijms160920195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rakipovski G, Rolin B, Nøhr J, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE−/− and LDLr−/− mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3(6):844–857. doi: 10.1016/j.jacbts.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bułdak Ł, Machnik G, Bułdak RJ, Łabuzek K, Bołdys A, Okopień B. Exenatide and metformin express their anti-inflammatory effects on human monocytes/macrophages by the attenuation of MAPKs and NFκB signaling. Naunyn-Schmiedeb Arch Pharmacol. 2016;389(10):1103–1115. doi: 10.1007/s00210-016-1277-8 [DOI] [PubMed] [Google Scholar]
- 52.Park HJ, Han H, Oh E, et al. Empagliflozin and dulaglutide are effective against obesity-induced airway hyperresponsiveness and fibrosis in a murine model. Sci Rep. 2019;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mumby S, Adcock IM. Recent evidence from omic analysis for redox signaling and mitochondrial oxidative stress in COPD. J Inflamm. 2022;19(1). doi: 10.1186/s12950-022-00308-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnes PJ. Oxidative stress in chronic obstructive pulmonary disease. Antioxidants. 2022;11(5):965. doi: 10.3390/antiox11050965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020;33:101544. doi: 10.1016/j.redox.2020.101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin T, Lin K, Lin H, et al. Glucagon-Like Peptide-1 receptor agonist ameliorates 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) neurotoxicity through enhancing mitophagy flux and reducing α-synuclein and oxidative stress. Front Mol Neurosci. 2021;14. doi: 10.3389/fnmol.2021.697440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L, Zhang L, Li L, Hölscher C. Semaglutide is neuroprotective and reduces α-synuclein levels in the chronic MPTP mouse model of parkinson’s disease. J Parkinsons Dis. 2019;9(1):157–171. doi: 10.3233/JPD-181503 [DOI] [PubMed] [Google Scholar]
- 58.Bułdak Ł, Łabuzek K, Bułdak RJ, Machnik G, Bołdys A, Okopień B. Exenatide (a GLP-1 agonist) improves the antioxidative potential of in vitro cultured human monocytes/macrophages. Naunyn-Schmiedeb Arch Pharmacol. 2015;388(9):905–919. doi: 10.1007/s00210-015-1124-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Huang J, Li J, Mao Q, He J. Effects of liraglutide combined with insulin on oxidative stress and serum MCP-1 and NF-κB levels in type 2 diabetes. J Coll Physicians Surg Pak. 2019;29(3):218–221. doi: 10.29271/jcpsp.2019.03.218 [DOI] [PubMed] [Google Scholar]
- 60.Voynow JA, Shinbashi M. Neutrophil elastase and chronic lung disease. Biomolecules. 2021;11(8):1065. doi: 10.3390/biom11081065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thulborn SJ, Mistry V, Brightling CE, Moffitt KL, Ribeiro D, Bafadhel M. Neutrophil elastase as a biomarker for bacterial infection in COPD. Respir Res. 2019;20(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gharib SA, Manicone AM, Parks WC. Matrix metalloproteinases in emphysema. Matrix Biol. 2018;73:34–51. doi: 10.1016/j.matbio.2018.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wells JM, Parker MM, Oster RA, et al. Elevated circulating MMP-9 is linked to increased COPD exacerbation risk in SPIROMICS and COPDGene. JCI Insight. 2018;3(22). doi: 10.1172/jci.insight.123614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet. 2005;365(9478):2225–2236. doi: 10.1016/S0140-6736(05)66781-5 [DOI] [PubMed] [Google Scholar]
- 65.Armstrong MJ, Hull D, Guo K, et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol. 2016;64(2):399–408. doi: 10.1016/j.jhep.2015.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallego-Colon E, Klych-Ratuszny A, Kosowska A, et al. Exenatide modulates metalloproteinase expression in human cardiac smooth muscle cells via the inhibition of Akt signaling pathway. Pharmacol Rep. 2018;70(1):178–183. doi: 10.1016/j.pharep.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 67.Garczorz W, Gallego-Colon E, Kosowska A, et al. Exenatide exhibits anti-inflammatory properties and modulates endothelial response to tumor necrosis factor α-mediated activation. Cardiovasc Ther. 2018;36(2):e12317. doi: 10.1111/1755-5922.12317 [DOI] [PubMed] [Google Scholar]
- 68.Gan H, Hou X, Zhu Z, et al. Smoking: a leading factor for the death of chronic respiratory diseases derived from global burden of disease study 2019. BMC Pulm Med. 2022;22(1). doi: 10.1186/s12890-022-01944-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei J-P, Yang C-L, Leng W-H, Ding -L-L, Zhao G-H. Use of GLP1RAs and occurrence of respiratory disorders: a meta-analysis of large randomized trials of GLP1RAs. Clin Respir J. 2021;15(7):847–850. doi: 10.1111/crj.13372 [DOI] [PubMed] [Google Scholar]
- 70.Rogliani P, Matera MG, Calzetta L, et al. Long-term observational study on the impact of GLP-1R agonists on lung function in diabetic patients. Resp Med. 2019;154:86–92. doi: 10.1016/j.rmed.2019.06.015 [DOI] [PubMed] [Google Scholar]
- 71.Huang J, Yi H, Zhao C, et al. Glucagon-like peptide-1 receptor (GLP-1R) signaling ameliorates dysfunctional immunity in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:3191–3202. doi: 10.2147/COPD.S175145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. Glucagon-like peptide-1 receptor signaling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia. 2010;53(4):730–740. doi: 10.1007/s00125-009-1643-x [DOI] [PubMed] [Google Scholar]
- 73.Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM, et al. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156(10):3559–3569. doi: 10.1210/en.2014-1685 [DOI] [PubMed] [Google Scholar]
- 74.Romaní-Pérez M, Outeiriño-Iglesias V, Gil-Lozano M, González-Matías LC, Mallo F, Vigo E. Pulmonary GLP-1 receptor increases at birth and exogenous GLP-1 receptor agonists augmented surfactant-protein levels in litters from normal and nitrofen-treated pregnant rats. Endocrinology. 2013;154(3):1144–1155. doi: 10.1210/en.2012-1786 [DOI] [PubMed] [Google Scholar]
- 75.Altintas Dogan AD, Hilberg O, Hess S, Jensen TT, Bladbjerg E, Juhl CB. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int J Chron Obstruct. 2022;17:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi W, Choe S, Lin J, et al. Exendin-4 restores airway mucus homeostasis through the GLP1R-PKA-PPARγ-FOXA2-phosphatase signaling. Mucosal Immunol. 2020;13(4):637–651. doi: 10.1038/s41385-020-0262-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rogliani P, Calzetta L, Capuani B, et al. Glucagon-like peptide 1 receptor: a novel pharmacological target for treating human bronchial hyperresponsiveness. Am J Respir Cell Mol. 2016;55(6):804–814. doi: 10.1165/rcmb.2015-0311OC [DOI] [PubMed] [Google Scholar]
- 78.Figat M, Kardas G, Kuna P, Panek MG. Beneficial influence of exendin-4 on specific organs and mechanisms favourable for the elderly with concomitant obstructive lung diseases. Brain Sci. 2022;12(8):1090. doi: 10.3390/brainsci12081090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pradhan R, Lu S, Yin H, et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ. 2022;2022:e71380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN. Asthma exacerbations in patients with type 2 diabetes and asthma on glucagon-like peptide-1 receptor agonists. Am J Respir Crit Care. 2021;203(7):831–840. doi: 10.1164/rccm.202004-0993OC [DOI] [PMC free article] [PubMed] [Google Scholar]