Highlights
-
•
Measles, mumps, and rubella vaccine (MMR) may be given to adolescents and adults.
-
•
MMR safety data in adolescents and adults are limited.
-
•
Over 276,000 doses of MMR given to adolescents and adults were studied.
-
•
Clinically serious outcomes were rare.
-
•
Common non-serious outcomes included arthropathy, injection site reaction, and rash.
Keywords: MMR, Vaccine, Safety, Adolescents, Adults
Abbreviations: ACIP, Advisory Committee on Immunization Practices; CDC, Centers for Disease Control and Prevention; CI, confidence interval; ED, emergency department; GBS, Guillain-Barré syndrome; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; IQR, interquartile range; ITP, immune thrombocytopenia; MMR, measles, mumps, and rubella vaccine; MMRV, measles, mumps, rubella, and varicella vaccine; RR, relative risk; SCRI, self-controlled risk interval; VAERS, Vaccine Adverse Event Reporting System; VSD, Vaccine Safety Datalink
Abstract
Background
Measles, mumps, and rubella vaccine (MMR) is routinely administered to children; however, adolescents and adults may receive MMR for various reasons. Safety studies in adolescents and adults are limited. We report on safety of MMR in this age group in the Vaccine Safety Datalink.
Methods
We included adolescents (aged 9–17 years) and adults (aged ≥ 18 years) who received ≥ 1 dose of MMR from January 1, 2010–December 31, 2018. Pre-specified outcomes were identified by diagnosis codes. Clinically serious outcomes included anaphylaxis, encephalitis/myelitis, Guillain-Barré syndrome, immune thrombocytopenia, meningitis, and seizure. Non-serious outcomes were allergic reaction, arthropathy, fever, injection site reaction, lymphadenopathy, non-specific reaction, parotitis, rash, and syncope. All serious outcomes underwent medical record review. Outcome-specific incidence was calculated in pre-defined post-vaccination windows. A self-controlled risk interval design was used to determine the relative risk of each outcome in a risk window after vaccination compared to a more distal control window.
Results
During the study period, 276,327 MMR doses were administered to adolescents and adults. Mean age of vaccinees was 34.8 years; 65.8 % were female; 53.2 % of doses were administered simultaneously with ≥ 1 other vaccine. Serious outcomes were rare, with incidence ≤ 6 per 100,000 doses for each outcome assessed, and none had a significant elevation in incidence during the risk window compared to the control window. Incidence of non-serious outcomes per 100,000 doses ranged from 3.4 for parotitis to 263.0 for arthropathy. Other common outcomes included injection site reaction and rash (157.0 and 112.9 per 100,000 doses, respectively). Significantly more outcomes were observed during the risk window compared to the control window for all non-serious outcomes except parotitis. Some variability was observed by sex and age group.
Conclusion
Serious outcomes after MMR are rare in adolescents and adults, but vaccinees should be counseled regarding anticipated local and systemic non-serious adverse events.
Introduction
The U.S. Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination with two doses of measles, mumps, and rubella-containing vaccine (measles, mumps, and rubella vaccine [MMR] or measles, mumps, rubella, and varicella vaccine [MMRV]) in childhood, with the first dose at age 12–15 months and the second at age 4–6 years [1]. ACIP also recommends two-dose catch-up vaccination for children and adolescents through age 18 years and vaccination of adults born after 1956 with at least one dose. Many adults do not have documentation of past receipt of MMR-containing vaccines, and vaccination with 2 doses may be required or specifically recommended for certain situations such as college entry, international travel, and employment in healthcare. Additionally, women of childbearing age are often tested for immunity against rubella, and those who are seronegative may be vaccinated before pregnancy or after delivery. Adolescents and adults may also be vaccinated during outbreaks. Per ACIP, a second dose of MMR should be considered during measles outbreaks for adults who have previously only received one dose of MMR, and a third dose is recommended for adolescents and adults determined to be at increased risk for mumps during mumps outbreaks [1], [2].
Previous studies have found that MMR-containing vaccines are generally well tolerated [3], [4], [5]; however, these studies have primarily been conducted among young children. Data on the safety of MMR administered to adolescents or adults are mainly from vaccination campaigns during outbreaks [6], [7], [8], [9], [10] and an assessment of events reported to the U.S. Vaccine Adverse Event Reporting System (VAERS) [11]. These studies are limited by lack of denominators and passive reporting. A few previous studies have systematically assessed safety following MMR in adolescents and/or adults [12], [13], [14], [15]; nevertheless, these studies were small (each had < 1000 vaccinees) and therefore were unlikely to detect rare, serious outcomes. Additionally, prior studies have found that the frequency of some adverse events after MMR is variable by sex. For example, headache, lymphadenopathy, and arthralgia were more likely to be reported after MMR among females than males [14], [15]. Historically, the rate of adverse events was also variable by age group after monovalent rubella vaccine, with arthralgia and arthritis occurring more frequently in adults and rarely in children [16], [17]. It is therefore essential to assess the safety of MMR among adolescents and adults by age group and sex. We conducted a study in the Vaccine Safety Datalink (VSD) to systematically assess the safety of MMR in adolescents and adults. Specifically, we aimed to 1) describe the population of adolescents and adults that received MMR and 2) estimate the incidence and risk of medically-attended, pre-specified clinically serious and non-serious outcomes following MMR receipt, including by age group and sex for non-serious outcomes.
Methods
Study setting
VSD is a collaboration between nine integrated healthcare systems and the U.S. Centers for Disease Control and Prevention (CDC) that conducts vaccine safety surveillance and research [18]. For this study, seven sites contributed data: HealthPartners, Bloomington, MN; Kaiser Permanente Colorado, Denver, CO; Kaiser Permanente Northern California, Oakland, CA; Kaiser Permanente Northwest, Portland, OR; Kaiser Permanente Southern California, Pasadena, CA; Kaiser Permanente Washington, Seattle, WA; and Marshfield Clinic Health System, Marshfield, WI. All sites have access to comprehensive electronic medical records for their members, including information on demographics, immunizations, and diagnosis codes associated with outpatient, emergency department (ED), and hospital encounters. Additionally, sites have the ability to capture vaccines administered outside of the healthcare system through automated (e.g., weekly) or trigger-based (e.g., specific healthcare event) data exchanges with jurisdictional immunization information systems [19]; however, these processes were not in place at all sites when this study was conducted. This study was approved by the Institutional Review Boards at all participating sites, including a waiver of informed consent, and was conducted consistent with federal law and CDC policy.
Study design & population
This study used a retrospective, vaccinated-only cohort design. The study population included adolescents (aged 9–17 years) and adults (aged ≥ 18 years) who were members at participating sites (9.9 million members aged ≥ 9 years in 2018), and received at least one dose of MMR between January 1, 2010 and December 31, 2018. Continuous enrollment (excluding enrollment gaps < 31 days) was required from 60 days before vaccination through 100 days after vaccination to ensure capture of both events before vaccination and outcomes following vaccination. Uptake and safety of MMRV was not assessed since this vaccine is only licensed for persons through 12 years of age; however, doses of MMRV were captured in order to assess vaccination validity and history, as described below.
Exposure classification
MMR exposures were identified in electronic data using vaccine administered (CVX) codes. Valid doses of MMR were defined as those given at least 28 days after receipt of a previous MMR-containing vaccine (MMR or MMRV) or another live vaccine (e.g., varicella, zoster vaccine live, live attenuated influenza vaccine), the minimum allowable interval for live vaccines. Doses of MMR were excluded if vaccine type was unknown (i.e., MMR and MMRV recorded on the same date) or if administered in 2010 to Marshfield Clinic Health System patients as part of a research study. Additionally, individuals with a record of another live vaccine in the 28 days after MMR receipt or with multiple MMR doses within the follow-up period (30 days for non-serious outcomes and 92 days for serious outcomes) were excluded from safety analyses.
Outcomes
Outcomes were pre-specified to include events known to be associated or possibly associated with MMR receipt that are biologically plausible, clinically well-defined with relatively acute onset, and potentially severe enough to result in a medical encounter [1], [20], [21]. Outcomes were categorized into two groups based on clinical severity. Clinically serious outcomes included anaphylaxis, encephalitis/myelitis, Guillain-Barré syndrome (GBS), immune thrombocytopenia (ITP), meningitis, and seizure (febrile and afebrile). Clinically non-serious outcomes included allergic reaction (e.g., angioneurotic edema and urticaria), arthropathy (arthralgia or arthritis), fever, injection site reaction, lymphadenopathy, non-specific reaction (e.g., adverse effect of other viral vaccines), parotitis, rash, and syncope (including hypotension [i.e., presyncope]). Outcomes were identified in electronic data using the International Classification of Diseases, 9th or 10th Revision, Clinical Modification diagnosis codes (ICD-9-CM or ICD-10-CM) assigned during inpatient, outpatient, or ED encounters; for each outcome, specific post-vaccination exposure windows (risk and control) and diagnosis settings were designated (Table 1). Timing and length of the risk window varied by outcome based on prior studies. For example, the risk window was 1–42 days for most clinically serious outcomes, 0–2 days for anaphylaxis and allergic reactions, and 6–14 days for clinically non-serious outcomes such as fever, rash, and lymphadenopathy.
Table 1.
Pre-Specified Outcomes after MMR Administration.a
| Outcome | Diagnosis Setting | Risk Window | Control Windowb | ICD-9-CM Codesc | ICD-10-CM Codesd |
|---|---|---|---|---|---|
| Serious Outcomese | |||||
| Anaphylaxis | ED, IP | 0–2 days | 10–12 days | 995.0, 999.42 | T78.2*, T80.52*, T88.6* |
| Encephalitis/Myelitis | ED, IP | 1–42 days | 50–91 days | 055.0, 056.01, 072.2, 323.5*, 323.6*, 323.8*, 323.9, 348.30 | B05.0, B06.01, B26.2, G04.0*, G04.3*, G04.8*, G04.9*, G05.3, G05.4, G93.40 |
| GBS | ED, IP, OP | 1–42 days | 50–91 days | 357.0 | G61.0 |
| ITP | ED, IP, OP | 1–42 days | 50–91 days | 287.31 | D69.3 |
| Meningitis | ED, IP | 1–42 days | 50–91 days | 047.9, 055.79, 056.09, 072.1, 322.0 | A87.9, B05.1, B06.02, B26.1, G03.0 |
| Seizure | ED, IP | 0–42 days | 50–92 days | 345.80, 780.31, 780.32, 780.39 | G40.50*, G40.89, R56.0*, R56.9 |
| Non-Serious Outcomes | |||||
| Allergic Reaction | ED, IP, OP | 0–2 days | 10–12 days | 708.0, 708.1, 708.9, 995.1, 995.21, 995.27, 995.3 | L50.0, L50.1, L50.9, T78.3*, T78.4* |
| Arthropathy | ED, IP, OP | 6–14 days | 22–30 days | 716.4*, 716.5*, 716.6*, 716.9*, 719.4*, 719.5* | M12.9, M13.0, M13.1*, M25.5*, M25.6* |
| Fever | ED, IP, OP | 6–14 days | 22–30 days | 780.60, 780.61, 780.62, 780.63 | R50.81, R50.82, R50.83, R50.9 |
| Injection Site Reaction | ED, IP, OP | 1–6 days | 14–19 days | 680.3, 680.9, 682.3, 682.8, 682.9, 686.9, 729.5, 729.81 | L02.413, L02.414, L02.419, L02.423, L02.424, L02.429, L02.433, L02.434, L02.439, L02.9*, L03.113, L03.114, L03.119, L03.123, L03.124, L03.129, L03.818, L03.898, L03.9*, L08.9, M79.601, M79.602, M79.603, M79.609, M79.62*, M79.63*, M79.89 |
| Lymphadenopathy | ED, IP, OP | 6–14 days | 22–30 days | 289.3, 683, 785.6 | I88.8, I88.9, L04.2, L04.8, L04.9, R59.* |
| Non-Specific Reaction | ED, IP, OP | 0–6 days | 14–20 days | 979.4, 979.6, 979.9, 995.20, 995.29, 999.39, 999.9, E949.4, E949.6, E949.9 | T50.901*, T50.905*, T50.991*, T50.995*, T50.B91*, T50.B95*, T50.Z91*, T50.Z95*, T78.8*, T80.29*, T88.0*, T88.1*, T88.7*, T88.9* |
| Parotitis | ED, IP, OP | 6–14 days | 22–30 days | 527.2 | K11.20, K11.21 |
| Rash | ED, IP, OP | 6–14 days | 22–30 days | 782.1 | R21 |
| Syncope | ED, IP, OP | Day 0 | Day 8 | 458.29, 458.8, 458.9, 780.2 | I95.2, I95.8*, I95.9, R55 |
Abbreviations: MMR = measles, mumps, and rubella vaccine; ED = emergency department; IP = inpatient; OP = outpatient; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM = International Classification of Diseases, Tenth Revision, Clinical Modification; *=wildcard; GBS = Guillain-Barré syndrome; ITP = immune thrombocytopenia.
For all pre-specified outcomes, events were considered incident if the diagnosis code that appeared in the post-vaccination window was the first occurrence of a diagnosis code for that outcome in 60 days.
Control windows were designed to be the same length (i.e., number of days) as the risk windows, with a washout period of 7 days between the risk window and control window.
ICD-9-CM were used to identify outcomes prior to October 1, 2015.
ICD-10-CM were used to identify outcomes on or after October 1, 2015.
Medical record reviews were conducted for all serious outcomes to confirm electronic diagnoses.
All clinically serious outcomes (as defined above) that were identified via diagnosis codes underwent medical record review to confirm the diagnosis and ascertain additional information not readily available in electronic data, such as symptom onset. Trained medical record abstractors completed medical record reviews at each participating site, and information was entered into a centralized, secure REDCap database hosted at Marshfield Clinic Research Institute [22], [23]. An epidemiologist subsequently adjudicated records in consultation with a pediatric infectious disease physician. Only serious outcomes that were confirmed via medical record review and adjudication with onset occurring in the risk or control windows were included in analyses.
Vaccinee characteristics
Characteristics of vaccinees were extracted from electronic data and included age, sex, race/ethnicity, simultaneous vaccination, MMR/MMRV vaccination history, length of VSD enrollment, and postpartum status (females only). All prior valid MMR-containing vaccinations were counted to estimate the number of lifetime doses and calculate the time since the most recent dose; monovalent vaccines (e.g., measles vaccine) were not included. Prior vaccinations were considered valid if they were administered at least 28 days after a previous dose of MMR-containing vaccine, at age 6 months or older, and after the vaccine type became available (1971 for MMR and 2006 for MMRV). Postpartum status was defined as the eight week period following a live birth or stillbirth, as identified by diagnosis codes in the inpatient setting for “outcome of delivery” (ICD-9-CM: V27.* or ICD-10-CM: Z37.*).
Although reason for vaccination was not available in electronic data, several characteristics were used to identify three possible indications for MMR receipt: catch-up vaccination, rubella seronegative status, and international travel. To identify adolescents who likely received MMR as catch-up vaccination, we used MMR dose number (receipt of first or second dose) and simultaneous administration with vaccines typically administered to younger children, specifically varicella or polio vaccine. Postpartum status was used to identify women who likely received MMR due to their rubella seronegative status. Lastly, simultaneous administration of vaccines primarily indicated for international travel, i.e., cholera, Japanese encephalitis, typhoid, and yellow fever, was used to identify individuals who likely received MMR due to travel.
Statistical analyses
MMR doses were considered independent observations; thus, analyses were conducted by dose, not by individual. Incidence was calculated as the number of events per 100,000 MMR doses in pre-defined post-vaccination risk and control windows; 95 % confidence intervals (CIs) were calculated using the exact binomial method. Incidence was calculated separately for the risk and control windows for each outcome; and by age group (9–17 years [adolescents], 18–25 years [young adults], 26–44 years [mid adults], ≥45 years [older adults]) and sex for non-serious outcomes. The self-controlled risk interval (SCRI) method was used to determine the relative risk (RR) of outcome incidence in the risk window compared to a more distal control window for each outcome, and by age group and sex for non-serious outcomes [24]. RRs and corresponding 95 % CIs were estimated using fixed-effects Poisson regression [25]. All analyses were conducted using SAS 9.4 (Cary, NC) and all statistical tests were two-sided with an a priori level of significance of 0.05.
Results
Descriptive characteristics of MMR recipients
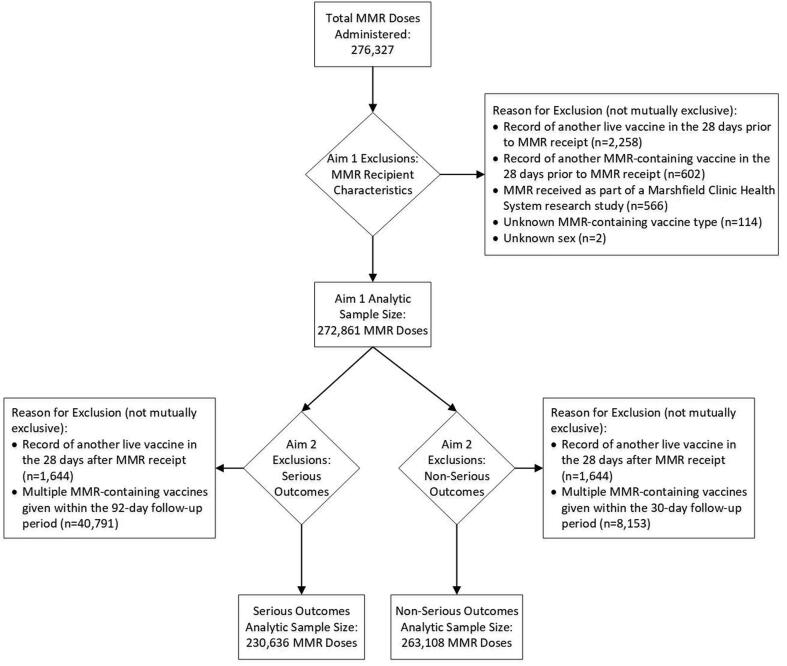
Between January 1, 2010, and December 31, 2018, 276,327 doses of MMR were administered to adolescents and adults in the VSD, of which 272,861 (98.8 %) doses were analyzed descriptively (Fig. 1). Vaccinees had a mean age of 34.8 years (standard deviation: 14.2 years), 65.8 % were female, and a plurality (38.1 %) were White, non-Hispanic (Table 2). Vaccinations occurred across the age spectrum, with 13.4 % of vaccinees classified as adolescents, 16.0 % as young adults, 45.6 % as mid adults, and 25.0 % as older adults (92.3 % were aged 45–64 years). More than half of all MMR doses (53.2 %) were administered simultaneously with ≥ 1 other vaccine; this was more common among adolescents (78.8 %) than among adult age groups (46.5 %-52.9 %). Most doses (64.4 %) of MMR administered during the study period were recorded as first doses in VSD data (range: first to ninth dose) (Table 2). Dose number varied by age group, with 52.4 % of adolescents receiving a second dose and the majority (≥75 %) of adults aged ≥ 26 years receiving a first dose, according to available data. Among those who had previously received at least 1 dose of MMR-containing vaccine, median time since most recent dose was 8.6 years (range: 28 days to 47 years). Vaccinees had been enrolled in VSD for a median of 3.9 years prior to MMR receipt (interquartile range: 1.5–9.4 years).
Fig. 1.
Analytic Sample Size and Exclusions by Study Aim. Abbreviation: MMR = measles, mumps, and rubella vaccine.
Table 2.
Descriptive Characteristics of Adolescent and Adult MMR Recipients, Vaccine Safety Datalink, 2010-2018.a
| Characteristicb | Overall (N = 272,861) | Age Groupc | |||
|---|---|---|---|---|---|
|
9–17 Years (N = 36,674) |
18–25 Years (N = 43,633) |
26–44 Years (N = 124,397) |
≥45 Years (N = 68,157) |
||
| Sex | |||||
| Female | 179,657 (65.8) | 18,274 (49.8) | 31,191 (71.5) | 88,039 (70.8) | 42,153 (61.9) |
| Male | 93,204 (34.2) | 18,400 (50.2) | 12,442 (28.5) | 36,358 (29.2) | 26,004 (38.2) |
| Race/Ethnicity | |||||
| White, Non-Hispanic | 104,060 (38.1) | 12,852 (35.0) | 14,066 (32.2) | 43,122 (34.7) | 34,020 (49.9) |
| Hispanic, Any Race | 70,468 (25.8) | 10,135 (27.6) | 14,199 (32.5) | 35,141 (28.3) | 10,993 (16.1) |
| Asian, Non-Hispanic | 52,446 (19.2) | 4,893 (13.3) | 8,067 (18.5) | 27,440 (22.1) | 12,046 (17.7) |
| Multiracial, Non-Hispanic | 24,093 (8.8) | 4,761 (13.0) | 4,099 (9.4) | 9,755 (7.8) | 5,478 (8.0) |
| Black, Non-Hispanic | 16,698 (6.1) | 3,102 (8.5) | 2,268 (5.2) | 6,701 (5.4) | 4,627 (6.8) |
| Other/Unknown | 5,096 (1.9) | 931 (2.5) | 934 (2.1) | 2,238 (1.8) | 993 (1.5) |
| Received ≥ 1 Simultaneous Vaccine | |||||
| Yes | 145,244 (53.2) | 28,910 (78.8) | 22,472 (51.5) | 57,816 (46.5) | 36,046 (52.9) |
| No | 127,617 (46.8) | 7,764 (21.2) | 21,161 (48.5) | 66,581 (53.5) | 32,111 (47.1) |
| Vaccination Year | |||||
| 2010 | 18,379 (6.7) | 4,686 (12.8) | 3,048 (7.0) | 7,606 (6.1) | 3,039 (4.5) |
| 2011 | 22,113 (8.1) | 6,019 (16.4) | 3,713 (8.5) | 8,555 (6.9) | 3,826 (5.6) |
| 2012 | 21,136 (7.8) | 4,864 (13.3) | 3,734 (8.6) | 8,858 (7.1) | 3,680 (5.4) |
| 2013 | 19,586 (7.2) | 3,185 (8.7) | 3,849 (8.8) | 8,681 (7.0) | 3,871 (5.7) |
| 2014 | 23,271 (8.5) | 3,222 (8.8) | 4,338 (9.9) | 10,734 (8.6) | 4,977 (7.3) |
| 2015 | 42,216 (15.5) | 4,409 (12.0) | 5,902 (13.5) | 18,813 (15.1) | 13,092 (19.2) |
| 2016 | 43,469 (15.9) | 3,675 (10.0) | 6,378 (14.6) | 21,079 (16.9) | 12,337 (18.1) |
| 2017 | 43,684 (16.0) | 3,711 (10.1) | 6,679 (15.3) | 20,931 (16.8) | 12,363 (18.1) |
| 2018 | 39,007 (14.3) | 2,903 (7.9) | 5,992 (13.7) | 19,140 (15.4) | 10,972 (16.1) |
| Dose Numberd | |||||
| 1 | 175,655 (64.4) | 11,229 (30.6) | 17,168 (39.4) | 93,733 (75.4) | 53,535 (78.5) |
| 2 | 68,555 (25.1) | 19,198 (52.4) | 9,546 (21.9) | 25,638 (20.6) | 14,173 (20.8) |
| 3 | 24,781 (9.1) | 5,875 (16.0) | 14,276 (32.7) | 4,225 (3.4) | 405 (0.6) |
| ≥4e | 3,870 (1.4) | 372 (1.0) | 2,643 (6.1) | 801 (0.6) | 54 (0.1) |
| Time Since Last Dose in Years, median (IQR)f | 8.6 (0.5–15.4) | 9.5 (4.7–12.2) | 15.6 (9.5–18.1) | 2.9 (0.1–16.9) | 0.4 (0.1–5.4) |
| Length of VSD Enrollmentg | |||||
| <1 Year | 47,531 (17.4) | 7,645 (20.9) | 8,500 (19.5) | 24,833 (20.0) | 6,553 (9.6) |
| 1–2 Years | 68,368 (25.1) | 10,367 (28.3) | 10,426 (23.9) | 36,781 (29.6) | 10,794 (15.8) |
| 3–4 Years | 39,661 (14.5) | 6,259 (17.1) | 5,545 (12.7) | 20,394 (16.4) | 7,463 (11.0) |
| 5–9 Years | 53,464 (19.6) | 8,152 (22.2) | 7,186 (16.5) | 25,182 (20.2) | 12,944 (19.0) |
| ≥10 Years | 63,837 (23.4) | 4,251 (11.6) | 11,976 (27.5) | 17,207 (13.8) | 30,403 (44.6) |
| ≥1 Possible Indication Identifiedh | |||||
| Yes | 88,933 (32.6) | 32,481 (88.6) | 9,091 (20.8) | 36,182 (29.1) | 11,179 (16.4) |
| No | 183,928 (67.4) | 4,193 (11.4) | 34,542 (79.2) | 88,215 (70.9) | 56,978 (83.6) |
| Postpartumi | |||||
| Yes | 31,916 (17.8) | 330 (1.8) | 8,049 (25.8) | 23,488 (26.7) | 49 (0.1) |
| No | 147,741 (82.2) | 17,944 (98.2) | 23,142 (74.2) | 64,551 (73.3) | 42,104 (99.9) |
| ≥1 Travel Vaccine Simultaneously Administeredj | |||||
| Yes | 25,141 (9.2) | 268 (0.7) | 1,044 (2.4) | 12,699 (10.2) | 11,130 (16.3) |
| No | 247,720 (90.8) | 36,406 (99.3) | 42,589 (97.6) | 111,698 (89.8) | 57,027 (83.7) |
Aaabbreviations: MMR = measles, mumps, and rubella vaccine; IQR = interquartile range; VSD = Vaccine Safety Datalink.
Unit of analysis is vaccine dose not unique individual.
Characteristics are presented as number (column percentage) unless otherwise specified. Percentages may not add to 100.0% due to rounding.
Age group is based on age in years at the time of MMR administration.
Dose number is based on all available VSD electronic data on MMR-containing vaccines; full vaccination history may not be captured for all members, especially vaccines received prior to VSD enrollment.
Includes dose number 4–9. The vast majority of those classified as receiving ≥ 4 doses received a fourth dose (n = 3,474, 89.8 %).
Time since last dose of MMR-containing vaccine is missing for those that received a first dose during the study period.
Length of VSD enrollment is defined as the number of years of continuous VSD enrollment at the time of MMR administration.
Possible indication for MMR receipt identified from electronic data; indications include rubella seronegative status (females only), international travel, and catch-up vaccination (adolescents only).
Postpartum status is based on VSD electronic data and only applies to female vaccinees. Postpartum status is used as a proxy for rubella seronegative status.
Includes vaccines solely indicated for international travel: cholera, Japanese encephalitis, typhoid, and yellow fever.
Available data identified at least one possible indication for one-third of vaccinations, with differences by age group: 88.6 % for adolescents and 16.4–29.1 % for adults. A majority of adolescents (88.0 %) likely received MMR as part of catch-up vaccination, 17.8 % of female vaccinees were likely vaccinated postpartum due to their rubella serostatus (more common among young and mid adults, 25.8 % and 26.7 %, respectively), and 9.2 % of vaccinees were likely vaccinated due to international travel (more frequent among mid and older adults, 10.2 % and 16.3 %, respectively).
Incidence and risk of medically-attended, pre-specified outcomes after MMR receipt
Clinically serious outcomes
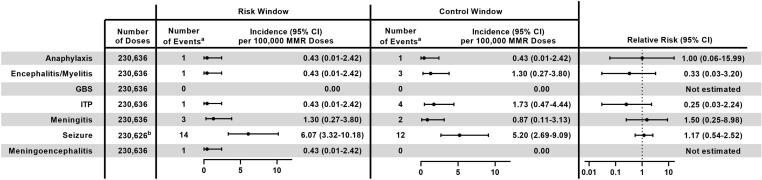
After excluding vaccinations that were followed by another live vaccine within 28 days or another MMR-containing vaccine during the 92-day follow-up period, clinically serious outcomes were assessed after 230,636 doses of MMR administered between 2010 and 2018 (Fig. 1). Across all ages, 21 serious outcomes were confirmed in the specific risk windows after MMR receipt: 14 seizures, 3 cases of meningitis, and 1 each anaphylaxis, encephalitis/myelitis, ITP, and meningoencephalitis; there were no cases of GBS. Meningoencephalitis was not a pre-specified outcome, but one individual with diagnosis codes for both encephalitis/myelitis and meningitis was determined upon medical record review to have a single condition, meningoencephalitis, so a new outcome was created post hoc. All seizures were afebrile, with onset from 3 to 42 days after MMR receipt. History of seizures was noted in 5 (36 %) patients, and a cause was identified for an additional 6 (43 %) patients upon medical record review; providers attributed new-onset seizures to alcohol withdrawal, metastatic cancer, brain malformations, postpartum eclampsia, hyperglycemia, and an unspecified underlying medication condition. The single instance of anaphylaxis occurred 10 min after co-administration of MMR and hepatitis B vaccine, and the episode was noted as attributable to vaccination in the medical record. None of the other serious outcomes were attributed to MMR receipt by the treating providers.
Incidence of clinically serious outcomes in the pre-specified post-vaccination risk window ranged from 0 per 100,000 doses of MMR for GBS to 6.07 per 100,000 doses for seizure (Fig. 2). For all serious outcomes, incidence in the risk window was similar to that in the control window, and the 95 % CIs for the RR included 1. RRs were 0.25 (95 % CI: 0.03–2.24) for ITP, 0.33 (95 % CI: 0.03–3.20) for encephalitis/myelitis, 1.00 (95 % CI: 0.06–15.99) for anaphylaxis, 1.17 (95 % CI: 0.54–2.52) for seizures, and 1.50 (95 % CI: 0.25–8.98) for meningitis; RRs were not estimated for meningoencephalitis and GBS because there were no events in the control window.
Fig. 2.
Incidence and Relative Risk of Clinically Serious Outcomes Following MMR Receipt in Adolescents and Adults (Age ≥ 9 Years), Vaccine Safety Datalink, 2010–2018. Abbreviations: 95 % CI = 95 % confidence interval; MMR = measles, mumps, and rubella vaccine; GBS = Guillain-Barré syndrome; ITP = immune thrombocytopenia. aEvents confirmed via medical record review. bThe number of doses is different for seizures because there were 10 potential cases of seizure identified in automated data that were unable to be confirmed or refuted via medical record review, 6 in the risk window and 4 in the control window. For analysis, these doses were excluded.
Clinically non-serious outcomes
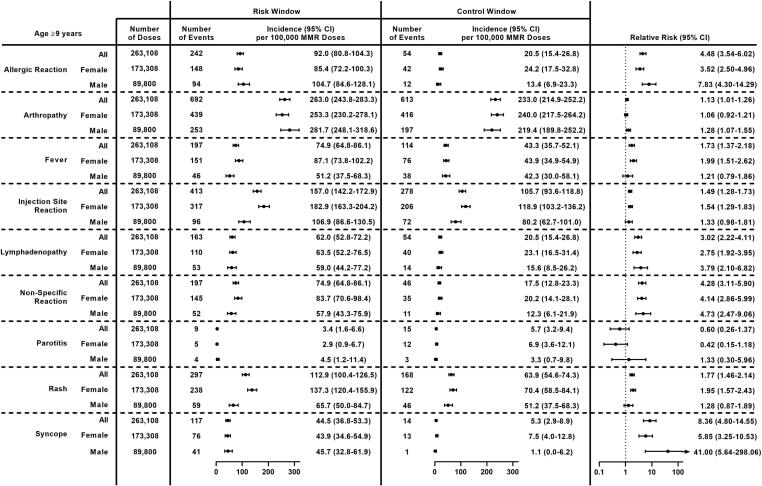
After excluding vaccinations that were followed by another live vaccine within 28 days or another MMR-containing vaccine during the 30-day follow-up period, clinically non-serious outcomes were assessed after 263,108 doses of MMR administered between 2010 and 2018 (Fig. 1). The most common non-serious outcomes during the outcome-specific risk window were arthropathy (692 events), injection site reaction (413 events), and rash (297 events). Incidence of clinically non-serious outcomes in the risk window ranged from 3.4 per 100,000 doses of MMR for parotitis to 263.0 per 100,000 doses for arthropathy (Fig. 3). Significantly higher incidence was observed during the post-vaccination risk window compared to the control window for 8 of 9 pre-specified non-serious outcomes; RRs ranged from 1.13 (95 % CI: 1.01–1.26) for arthropathy to 8.36 (95 % CI: 4.80–14.55) for syncope. No differences were observed for parotitis.
Fig. 3.
Incidence and Relative Risk of Clinically Non-Serious Outcomes Following MMR Receipt in Adolescents and Adults (Age ≥ 9 Years) Overall and by Sex, Vaccine Safety Datalink, 2010–2018. Abbreviations: 95 % CI = 95 % confidence interval; MMR = measles, mumps, and rubella vaccine.
Some variability in both incidence and RR was observed after stratifying by sex (Fig. 3). Incidence per 100,000 doses of MMR was higher among female vaccinees than male vaccinees for fever (87.1 vs 51.2, p = 0.001), injection site reaction (182.9 vs 106.9, p < 0.0001), non-specific reaction (83.7 vs 57.9, p = 0.02), and rash (137.3 vs 65.7, p < 0.0001). Among females, significantly higher incidence during the risk window compared to the control window was observed for 7 outcomes: allergic reaction, fever, injection site reaction, lymphadenopathy, non-specific reaction, rash, and syncope. Among males, significantly higher incidence during the risk window compared to the control window was observed for 5 outcomes: allergic reaction, arthropathy, lymphadenopathy, non-specific reaction, and syncope.
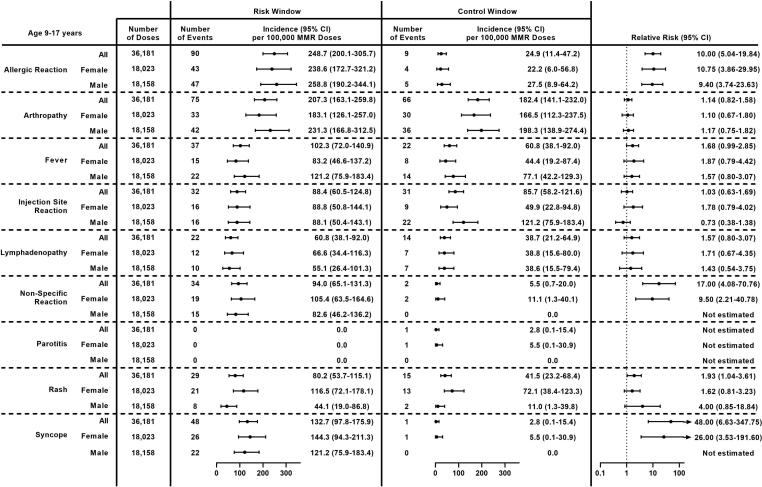
Adolescents
Among adolescents (age 9–17 years), the most common non-serious outcomes after MMR receipt were allergic reaction (248.7 per 100,000 doses), arthropathy (207.3 per 100,000 doses), syncope (132.7 per 100,000 doses), and fever (102.3 per 100,000 doses) (Fig. 4). The incidence of rash per 100,000 doses was variable by sex, with higher incidence observed among adolescent females than males (116.5 vs 44.1, respectively, p = 0.01). Significantly higher incidence was observed during the risk window compared to the control window for 4 outcomes: allergic reaction, non-specific reaction, rash, and syncope. RRs for allergic reaction, non-specific reaction, and syncope were large (RR ≥ 10). Results were similar after stratifying by sex. RRs could not be estimated for non-specific reaction or syncope among males because there were no events in the comparison window.
Fig. 4.
Incidence and Relative Risk of Clinically Non-Serious Outcomes Following MMR Receipt in Adolescents (Age 9–17 Years) Overall and by Sex, Vaccine Safety Datalink, 2010–2018. Abbreviations: 95 % CI = 95 % confidence interval; MMR = measles, mumps, and rubella vaccine.
Young adults
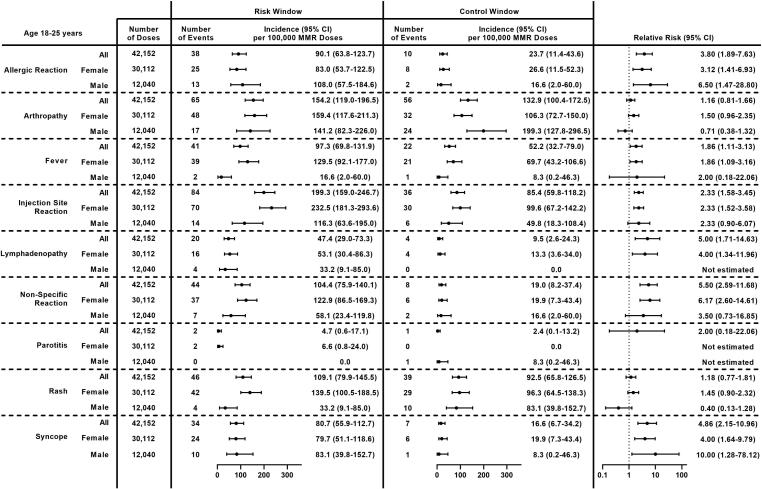
Among young adults (age 18–25 years), the most frequent non-serious outcomes following MMR receipt were injection site reaction (199.3 per 100,000 doses), arthropathy (154.2 per 100,000 doses), rash (109.1 per 100,000 doses), and non-specific reaction (104.4 per 100,000 doses) (Fig. 5). Incidence per 100,000 doses was higher among females compared to males for fever (129.5 vs 16.6, p = 0.008), injection site reaction (232.5 vs 116.3, p = 0.02), and rash (139.5 vs 33.2, p = 0.003). Significantly higher incidence was observed during the risk window compared to the control window for 6 of 9 pre-specified non-serious outcomes; RRs ranged from 1.86 (95 % CI: 1.11–3.13) for fever to 5.50 (95 % CI: 2.59–11.68) for non-specific reaction. No differences were observed for arthropathy, parotitis, or rash. Results were similar among females; among males, no differences were observed for fever, injection site reaction, or non-specific reaction, although CIs were wide. The RR for lymphadenopathy among males could not be estimated.
Fig. 5.
Incidence and Relative Risk of Clinically Non-Serious Outcomes Following MMR Receipt in Young Adults (Age 18–25 Years) Overall and by Sex, Vaccine Safety Datalink, 2010–2018. Abbreviations: 95 % CI = 95 % confidence interval; MMR = measles, mumps, and rubella vaccine.
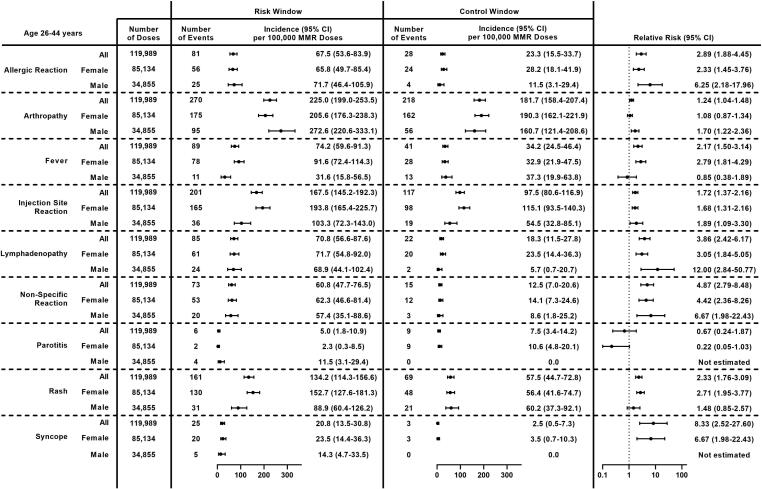
Mid adults
Among mid adults (age 26–44 years), the most common non-serious outcomes after MMR receipt were arthropathy (225.0 per 100,000 doses), injection site reaction (167.5 per 100,000 doses), and rash (134.2 per 100,000 doses) (Fig. 6). Incidence per 100,000 doses was higher among females compared to males for fever (91.6 vs 31.6, p = 0.0005), injection site reaction (193.8 vs 103.3, p = 0.0005), and rash (152.7 vs 88.9, p = 0.006). Conversely, incidence of arthropathy was higher among males compared to females (272.6 vs 205.6, p = 0.03). Significantly higher incidence was observed during the post-vaccination risk window compared to the control window for all non-serious outcomes except parotitis; RRs ranged from 1.24 (95 % CI: 1.04–1.48) for arthropathy to 8.33 (95 % CI: 2.52–27.60) for syncope. Results were similar after stratifying by sex; the RR could not be estimated for syncope among males because there were no events in the comparison window, and no significant difference was observed for fever or rash among males nor arthropathy among females.
Fig. 6.
Incidence and Relative Risk of Clinically Non-Serious Outcomes Following MMR Receipt in Mid Adults (Age 26–44 Years) Overall and by Sex, Vaccine Safety Datalink, 2010–2018. Abbreviations: 95 % CI = 95 % confidence interval; MMR = measles, mumps, and rubella vaccine.
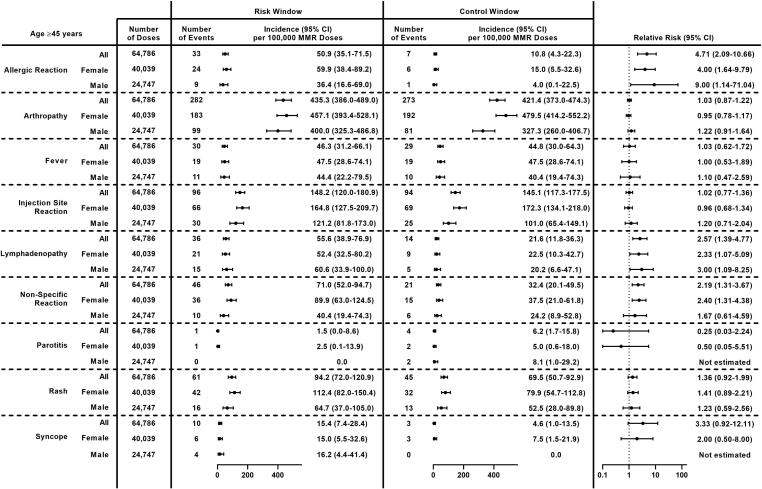
Older adults
Among older adults (age ≥ 45 years), the most common non-serious outcomes after MMR receipt were arthropathy (435.3 per 100,000 doses) and injection site reaction (148.2 per 100,000 doses) (Fig. 7). The incidence of non-specific reaction was higher among females compared to males (89.9 vs 40.4, p = 0.02). Significantly higher incidence was observed during the risk window compared to the control window for 3 outcomes, allergic reaction (RR: 4.71, 95 % CI: 2.09–10.66), lymphadenopathy (RR: 2.57, 95 % CI: 1.39–4.77), and non-specific reaction (RR: 2.19, 95 % CI: 1.31–3.67). Despite high incidence in the risk window, no difference between the risk window and control window was observed for arthropathy (RR: 1.03, 95 % CI: 0.87–1.22). Results were similar among females. Among males, no difference was observed for non-specific reaction, and the RR for allergic reaction was 9.00 (95 % CI: 1.14–71.04).
Fig. 7.
Incidence and Relative Risk of Clinically Non-Serious Outcomes Following MMR Receipt in Older Adults (Age ≥ 45 Years) Overall and by Sex, Vaccine Safety Datalink, 2010–2018. Abbreviations: 95 % CI = 95 % confidence interval; MMR = measles, mumps, and rubella vaccine.
Discussion
We conducted a large observational study on the safety of MMR in adolescents and adults that included over 276,000 doses administered to individuals aged ≥ 9 years over a nine-year period. Clinically serious outcomes were rare following MMR receipt in adolescents and adults, with incidence of ≤ 6 per 100,000 doses for each serious outcome assessed: anaphylaxis, encephalitis/myelitis, GBS, ITP, meningitis, meningoencephalitis, and seizures. Additionally, self-controlled risk interval analyses revealed that incidence in the post-vaccination risk window was similar to incidence in the more distal control window for all serious outcomes for which the RR could be estimated. This suggests that serious outcomes observed in the risk window are unlikely to be associated with MMR receipt; however, among all serious outcomes identified, one case of anaphylaxis was attributed to vaccination in the medical record. Although CIs were wide for several serious outcomes, including meningitis and anaphylaxis, these findings are reassuring and consistent with smaller studies of MMR safety in adolescents and young adults [10], [13], as well as studies in young children [3], [26].
The incidence of medically-attended, clinically non-serious outcomes was ≤ 263 per 100,000 doses for each outcome assessed. The most frequent non-serious outcomes were arthropathy, injection site reaction, and rash, all of which are known vaccine-associated adverse events and included on the MMR Vaccine Information Sheet [21]. We confirmed an increased risk of several non-serious outcomes, including syncope, allergic reaction, arthropathy, non-specific reaction, lymphadenopathy, fever, rash, and injection site reaction. The incidence of each one was significantly higher in the post-vaccination risk window compared to the control window, with RRs ranging from 1.1 (arthropathy) to 8.4 (syncope).
Several non-serious outcomes, including fever, injection site reaction, non-specific reaction, and rash, were more common among female vaccinees than male vaccinees. This finding may be driven by differences in healthcare-seeking behavior by sex (i.e., confounding) rather than a true biological sex difference in adverse event profiles. Nevertheless, sex differences have been observed in other MMR safety studies that solicited adverse events by diary or survey, including a recent study among adult travelers from Israel [14], suggesting that females may, in fact be more likely to experience certain adverse events after MMR. A systematic review of sex differences related to seasonal influenza vaccine in older adults similarly found higher rates of adverse events following immunization among females [27]. The large VSD study population allowed us to analyze the incidence of non-serious outcomes by age group and the trends were more variable. Some outcomes (e.g., syncope) were more frequent in adolescents, and other outcomes (e.g., arthropathy) were more frequent in older adults. More research is needed for MMR and other vaccines to better describe the anticipated adverse event profile by sex and age group.
Comparison of results of non-serious outcomes among adolescents and adults to those experienced by younger children is challenging. Most data on safety of MMR in children are derived from clinical trials and other studies in which adverse events were prospectively solicited and did not require seeking medical attention [3], [5]. It is therefore difficult to conclude whether or not non-serious outcomes after MMR, namely local and systemic reactogenicity, are less common in adolescents and adults than children. However, clinically serious outcomes after MMR receipt may be rarer in adolescents and adults than in young children. Several instances of seizures were identified in this study within the six weeks after MMR receipt, but none of them were febrile (although simple febrile seizures typically occur in children < 6 years of age). Additionally, only one case of ITP was identified in the six weeks after MMR receipt, corresponding to an incidence of 0.43 per 100,000 doses. Prior VSD studies have found associations between MMR and both febrile seizures in children < 7 years and ITP in children aged 12–23 months with an attributable risk of 25–34 per 100,000 for febrile seizures and 1 per 40,000 doses (2.5 per 100,000 doses) for ITP, respectively [28], [29]. Based on the number of doses observed in this study, we had sufficient power to detect a risk of similar magnitude for febrile seizures in adolescents and adults, but the sample size was not large enough to make a similar conclusion for ITP. Nevertheless, we have 95 % confidence that the estimated incidence of ITP after MMR receipt in adolescents and adults is < 2.4 per 100,000 doses.
The median duration of VSD enrollment prior to MMR administration during the study period was only 4 years, therefore it is unlikely the full vaccination history (i.e., MMR-containing vaccines received prior to VSD enrollment) was captured for all individuals, especially for adults. Therefore, we did not attempt to assess incidence or risk by MMR dose number. This was also a limitation of the previous VAERS study on MMR safety in adults [11]. Nevertheless, at least one third of MMR doses administered in our population were second or third doses of MMR-containing vaccines and about half of MMR doses were administered simultaneously with at least one other vaccine, providing reassurance that clinically serious outcomes are rare in these situations. Additional studies could be considered in populations or systems with better capture of childhood vaccination history in adults.
Strengths & limitations
Strengths of this analysis include systematic capture of vaccines and outcomes in a large, well-defined population over nine years, inclusion of vaccinees from various racial/ethnic groups across a wide age spectrum, medical record confirmation of serious outcomes, and estimation of risk by age group and sex using SCRI analyses. The SCRI approach minimizes the potential for residual confounding by factors that are stable over time.
There are several important limitations to our analysis. MMR dose number may be inaccurate in VSD data due to incomplete capture of historical vaccines, especially childhood vaccines for adults. Not enough doses of MMR were observed to detect very rare adverse events such as those occurring at a rate of 1 per 1,000,000 doses and confidence intervals were wide for some estimates. Our study was observational in nature and unmeasured confounding, especially by time-varying factors, may not have been accounted for in analyses. Individuals who experience non-serious outcomes, especially those known to be associated with vaccination such as fever or injection site pain, may not seek medical attention for their symptoms. Incidence and risk of non-serious outcomes may therefore be underestimated in this study since we relied on diagnosis codes assigned during healthcare encounters. Diagnosis codes assigned on the day of MMR administration (i.e., day 0) were included for allergic reaction, non-specific reaction and syncope; however, these diagnoses may reflect unrelated prevalent conditions rather than incident postvaccination adverse events, and their inclusion may have conversely resulted in the overestimation of incidence and risk of several non-serious outcomes. The SCRI design was used to estimate risk of medically-attended outcomes after MMR receipt; however, this design is not ideal for exposure-driven outcomes such as anaphylaxis and injection site reaction. Nevertheless, the unique nature of MMR recommendations for adults precluded the use of other study designs such as comparison to another vaccine or to unvaccinated persons eligible for MMR. Lastly, findings for clinically serious outcomes may not be generalizable to individuals that receive 2 doses of MMR within a short interval (i.e., 1–3 months), as the use of the SCRI design with a control window extending to 92 days post-vaccination precluded inclusion of such individuals in analysis.
Conclusion
Although MMR is typically considered a vaccine of childhood, hundreds of thousands of adolescents and adults receive MMR each year in the United States. Our large systematic study confirmed that clinically serious outcomes such as anaphylaxis are rare following MMR receipt among adolescents and adults. Previously reported clinically non-serious outcomes that resulted in medical care were observed after MMR receipt, with some variability by sex and age group. MMR recipients should be counseled about expected local and systemic adverse events and observed post-vaccination for syncope and anaphylaxis, as is best practice for all vaccines.
CRediT authorship contribution statement
Kayla E. Hanson: Conceptualization, Methodology, Software, Formal analysis, Data curation, Writing – original draft, Visualization, Project administration. Mona Marin: Conceptualization, Writing – review & editing. Matthew F. Daley: Conceptualization, Writing – review & editing. Holly C. Groom: Conceptualization, Writing – review & editing. Lisa A. Jackson: Conceptualization, Writing – review & editing. Lina S. Sy: Conceptualization, Writing – review & editing. Nicola P. Klein: Conceptualization, Writing – review & editing. Malini B. DeSilva: Conceptualization, Writing – review & editing. Lakshmi Panagiotakopoulos: Conceptualization, Writing – review & editing. Eric Weintraub: Conceptualization, Writing – review & editing. Edward A. Belongia: Conceptualization, Writing – review & editing, Funding acquisition. Huong Q. McLean: Conceptualization, Methodology, Software, Writing – original draft, Visualization, Supervision, Funding acquisition.
Acknowledgments
Acknowledgements
The authors gratefully acknowledge investigators, data managers, project managers, and medical record abstractors at participating VSD sites for their contributions to this research including: Jim Donahue, Linda Heeren, Burney Kieke, Erik Kronholm, Dave McClure, Scott Olson, and Erica Scotty (Marshfield Clinic Research Institute); Dianne Eggen and Laurie VanArman (HealthPartners Institute); Ruth Bedoy, Kate Burniece, and Jo Ann Shoup (Kaiser Permanente Colorado); Ned Lewis, Margarita Magallon, Virginia Robinson, and Pat Ross (Kaiser Permanente Northern California); Stacy Harsh and Mara Kalter (Kaiser Permanente Northwest); Bernadine Dizon, Joy Gelfond, Sungching Glenn, Vennis Hong, Runxin Huang, Steve Jacobsen, Zhuoxin Li, Kerresa Morrissette, Jose Pio, Denison Ryan, and Karen Schenk (Kaiser Permanente Southern California); and Erika Kiniry and Jennifer Covey (Kaiser Permanente Washington).
Declarations of Interest
Dr. Klein reports research support from Merck, GlaxoSmithKline, Sanofi Pasteur, Pfizer, and Protein Science (now Sanofi Pasteur). Ms. Sy reports research support unrelated to this study from Moderna, GlaxoSmithKline, Dynavax, and Seqirus. None of the other authors have known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
This work was supported by the Centers for Disease Control and Prevention (CDC) [200-2012-53587-0009 and 200-2022-15422-0001]. CDC scientists participated in interpretation of the data, and preparation, review, and approval of the manuscript for publication.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of Centers for Disease Control and Prevention.
Data Statement
The data that support the study conclusions are unavailable for public access because a waiver of informed consent to share said data (beyond the research team) was not obtained.
References
- 1.McLean H.Q., Fiebelkorn A.P., Temte J.L., Wallace G.S. Prevention of Measles, Rubella, Congenital Rubella Syndrome, and Mumps, 2013: Summary Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2013;62:1–34. [PubMed] [Google Scholar]
- 2.Marin M., Marlow M., Moore K.L., Patel M. Recommendation of the Advisory Committee on Immunization Practices for Use of a Third Dose of Mumps Virus-Containing Vaccine in Persons at Increased Risk for Mumps During an Outbreak. MMWR Morb Mortal Wkly Rep. 2018;67:33–38. doi: 10.15585/mmwr.mm6701a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyaku M., Richardson E., Martinon-Torres F., Kuter B.J. Evaluation of the Safety and Immunogenicity of M-M-RII (Combination Measles-Mumps-Rubella Vaccine): Clinical Trials of Healthy Children and Adults Published Between 2010 and 2019. Pediatr Infect Dis J. 2021;40:1046–1054. doi: 10.1097/INF.0000000000003273. [DOI] [PubMed] [Google Scholar]
- 4.Pawaskar M, Schmidt E, Marshall GS, Fergie J, Richardson E, Saldutti LP, et al. Use of M-M-R II Outside of the Routinely Recommended Age Range - A Systematic Literature Review. Hum Vaccin Immunother. 2021:1-7. https://doi.org/10.1080/21645515.2021.1933874. [DOI] [PMC free article] [PubMed]
- 5.Di Pietrantonj C., Rivetti A., Marchione P., Debalini M.G., Demicheli V. Vaccines for Measles, Mumps, Rubella, and Varicella in Children. Cochrane Database Syst Rev. 2021;11:CD004407. doi: 10.1002/14651858.CD004407.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Nationwide Campaign for Vaccination of Adults Against Rubella and Measles --- Costa Rica, 2001. MMWR Morb Mort Wkly Report. 2001;50:976-9. [PubMed]
- 7.Chen R.T., Moses J.M., Markowitz L.E., Orenstein W.A. Adverse Events Following Measles-Mumps-Rubella and Measles Vaccinations in College Students. Vaccine. 1991;9:297–299. doi: 10.1016/0264-410x(91)90053-9. [DOI] [PubMed] [Google Scholar]
- 8.Yalcin S.S., Kanra G., Pehlivan T. Outbreak of Measles in Medical Students and Determination of Immune Status to Measles-Mumps-Rubella Viruses. Int J Adolesc Med Health. 2006;18:615–622. doi: 10.1515/ijamh.2006.18.4.615. [DOI] [PubMed] [Google Scholar]
- 9.Nelson G.E., Aguon A., Valencia E., Oliva R., Guerrero M.L., Reyes R., et al. Epidemiology of a Mumps Outbreak in a Highly Vaccinated Island Population and Use of a Third Dose of Measles-Mumps-Rubella Vaccine for Outbreak Control-Guam 2009 to 2010. Pediatr Infect Dis J. 2013;32:374–380. doi: 10.1097/INF.0b013e318279f593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abedi G.R., Mutuc J.D., Lawler J., Leroy Z.C., Hudson J.M., Blog D.S., et al. Adverse Events Following a Third Dose of Measles, Mumps, and Rubella Vaccine in a Mumps Outbreak. Vaccine. 2012;30:7052–7058. doi: 10.1016/j.vaccine.2012.09.053. [DOI] [PubMed] [Google Scholar]
- 11.Sukumaran L., McNeil M.M., Moro P.L., Lewis P.W., Winiecki S.K., Shimabukuro T.T. Adverse Events Following Measles, Mumps, and Rubella Vaccine in Adults Reported to the Vaccine Adverse Event Reporting System (VAERS), 2003–2013. Clin Infect Dis. 2015;60:e58–e65. doi: 10.1093/cid/civ061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeBaron C.W., Bi D., Sullivan B.J., Beck C., Gargiullo P. Evaluation of Potentially Common Adverse Events Associated with the First and Second Doses of Measles-Mumps-Rubella Vaccine. Pediatrics. 2006;118:1422–1430. doi: 10.1542/peds.2006-0678. [DOI] [PubMed] [Google Scholar]
- 13.Kaaijk P., Wijmenga-Monsuur A.J., van Houten M.A., Veldhuijzen I.K., Ten Hulscher H.I., Kerkhof J., et al. A Third Dose of Measles-Mumps-Rubella Vaccine to Improve Immunity Against Mumps in Young Adults. J Infect Dis. 2020;221:902–909. doi: 10.1093/infdis/jiz188. [DOI] [PubMed] [Google Scholar]
- 14.Ami N., Eyal N., Asaf B., Chen A., Adi B., Drorit A., et al. Safety of Measles, Rubella and Mumps Vaccines in Adults: A Prospective Cohort Study. J Travel Med. 2021:28. doi: 10.1093/jtm/taab071. [DOI] [PubMed] [Google Scholar]
- 15.Marin M., Fiebelkorn A.P., Bi D., Coleman L.A., Routh J., Curns A.T., et al. Adverse Events Among Young Adults Following a Third Dose of Measles-Mumps-Rubella Vaccine. Clin Infect Dis. 2021;73:e1546–e1553. doi: 10.1093/cid/ciaa1090. [DOI] [PubMed] [Google Scholar]
- 16.Dudgeon J.A., Marshall W.C., Peckham C.S. Rubella Vaccine Trials in Adults and Children: Comparison of Three Attenuated Vaccines. Am J Dis Child. 1969;118:237–243. doi: 10.1001/archpedi.1969.02100040239015. [DOI] [PubMed] [Google Scholar]
- 17.Cooper L.Z., Ziring P.R., Weiss H.J., Matters B.A., Krugman S. Transient Arthritis After Rubella Vaccination. Am J Dis Child. 1969;118:218–225. doi: 10.1001/archpedi.1969.02100040220011. [DOI] [PubMed] [Google Scholar]
- 18.Baggs J., Gee J., Lewis E., Fowler G., Benson P., Lieu T., et al. The Vaccine Safety Datalink: A Model for Monitoring Immunization Safety. Pediatrics. 2011;127(Suppl 1):S45–S53. doi: 10.1542/peds.2010-1722H. [DOI] [PubMed] [Google Scholar]
- 19.Groom H.C., Crane B., Naleway A.L., Weintraub E., Daley M.F., Wain K., et al. Monitoring Vaccine Safety using the Vaccine Safety Datalink: Assessing Capacity to Integrate Data from Immunization Information Systems. Vaccine. 2022;40:752–756. doi: 10.1016/j.vaccine.2021.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institute of Medicine. Mealses, Mumps, and Rubella Vaccine. In: Stratton K, Ford A, Rusch E, Clayton EW, editors. Adverse Effects of Vaccines: Evidence and Causality. Washington (DC): The National Academies Press; 2011. p. 103-237. [PubMed]
- 21.Centers for Disease Control and Prevention. Vaccine Information Statement: MMR Vaccine, https://www.cdc.gov/vaccines/hcp/vis/vis-statements/mmr.html; 2021 [accessed 11 Jan 2022].
- 22.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research Electronic Data Capture (REDCap) - A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li R., Stewart B., Weintraub E. Evaluating Efficiency and Statistical Power of Self-Controlled Case Series and Self-Controlled Risk Interval Designs in Vaccine Safety. J Biopharm Stat. 2016;26:686–693. doi: 10.1080/10543406.2015.1052819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu S., Zeng C., Newcomer S., Nelson J., Glanz J. Use of Fixed Effects Models to Analyze Self-Controlled Case Series Data in Vaccine Safety Studies. J Biom Biostat. 2012;Suppl 7:006. doi: 10.4172/2155-6180.s7-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein N.P., Lewis E., Fireman B., Hambidge S.J., Naleway A., Nelson J.C., et al. Safety of Measles-Containing Vaccines in 1-Year-Old Children. Pediatrics. 2015;135:e321–e329. doi: 10.1542/peds.2014-1822. [DOI] [PubMed] [Google Scholar]
- 27.Tadount F., Doyon-Plourde P., Rafferty E., MacDonald S., Sadarangani M., Quach C. Is There a Difference in the Immune Response, Efficacy, Effectiveness and Safety of Seasonal Influenza Vaccine in Males and Females? - A Systematic Review. Vaccine. 2020;38:444–459. doi: 10.1016/j.vaccine.2019.10.091. [DOI] [PubMed] [Google Scholar]
- 28.Barlow W.E., Davis R.L., Glasser J.W., Rhodes P.H., Thompson R.S., Mullooly J.P., et al. The Risk of Seizures after Receipt of Whole-Cell Pertussis or Measles, Mumps, and Rubella Vaccine. N Engl J Med. 2001;345:656–661. doi: 10.1056/NEJMoa003077. [DOI] [PubMed] [Google Scholar]
- 29.France E.K., Glanz J., Xu S., Hambidge S., Yamasaki K., Black S.B., et al. Risk of Immune Thrombocytopenic Purpura after Measles-Mumps-Rubella Immunization in Children. Pediatrics. 2008;121:e687–e692. doi: 10.1542/peds.2007-1578. [DOI] [PubMed] [Google Scholar]