Highlights
-
•
This is the first WTP study for routine childhood vaccination in sub-Saharan Africa.
-
•
Almost all women place a positive value on the timely vaccination of their children.
-
•
The value of timely vaccination varies with vaccination attitudes and access.
-
•
System-level barriers, access, and attitudes appear to jointly impact vaccine uptake.
-
•
Most women prefer non-monetary over monetary incentives for timely vaccinations.
Keywords: Childhood vaccinations, Vaccination timeliness, Tanzania, Willingness to pay, Contingent valuation, Incentives
Abstract
Background
Globally, approximately 19.7 million children remain under-vaccinated; many more receive delayed vaccinations. Sustained progress towards global vaccination targets requires overcoming, or compensating for, incrementally greater barriers to vaccinating hard-to-reach and hard-to-vaccinate children. We prospectively assessed pregnant women’s valuations of routine childhood vaccinations and preferences for alternative incentives to inform interventions aiming to increase vaccination coverage and timeliness in southern Tanzania.
Methods
Between August and December 2017, 406 women in their last trimester of pregnancy were enrolled from health facilities and communities in the Mtwara region of Tanzania and asked contingent valuation questions about their willingness to vaccinate their child if they were (a) given an incentive, or (b) facing a cost for each vaccination. Interval censored regressions assessed correlates of women’s willingness to pay (WTP) for timely vaccinations. Participants were asked to rank monetary and non-monetary incentive options for the timely vaccination of their children.
Findings
All women expected to get their children vaccinated according to the recommended schedule, even without incentives. Nearly all women (393; 96.8 %) were willing to pay for vaccinations. The average WTP was Tanzania Shilling (Tsh) 3,066 (95 % confidence interval Tsh 2,523–3,610; 1 USD ∼ Tsh 2,200) for each vaccination. Women’s valuations of timely vaccinations varied significantly with vaccine-related knowledge and attitudes, economic status, and rural vs urban residence. Women tended to prefer non-monetary over monetary incentives for the timely vaccination of their children.
Interpretation
Women placed a high value on timely childhood vaccinations, suggesting that unexpected system-level barriers rather than individual-level demand factors are likely to be the primary drivers of missed vaccinations. Systematic variation in the value of vaccinations across women reflects variation in perceived benefits and opportunity costs. In this setting, nonmonetary incentives and other interventions to increase demand and compensate for system-level barriers hold significant potential for improving vaccination coverage and timeliness.
ClinicalTrials.gov Protocol
Nomenclature:
Abbreviations
- CI
Confidence Interval
- GDP
Gross domestic product
- NIMR
National Institute for Medical Research
- SSA
sub-Saharan Africa
- Tsh
Tanzanian Shilling
- WTA
Willingness to accept
- WTP
Willingness to pay
Background
Childhood vaccinations continue to be among the most cost-effective public health interventions to prevent under-5 mortality [1]. Since the rollout of routine immunization programs in the 1970s, large investments in vaccination infrastructure and programs resulted in substantial improvements in global vaccination coverage. Yet, regional inequities in coverage persist, with only one-third of countries successfully achieving the global vaccination target of 80 % vaccination coverage in every district [2]. Globally, approximately 19.7 million children remain under-vaccinated and many more received delayed vaccinations, i.e., vaccinations that are administered outside the recommended age range specified in the national vaccination schedules [3]. Deviations from the recommended national vaccination schedules, either due to vaccination inequities or due to vaccine hesitancy, can result in pockets of under-vaccination that are susceptible to vaccine-preventable outbreaks [2]. Among reasons for vaccine hesitancy, risk/benefit trade-offs related to vaccine safety and efficacy represent some of the most common concerns, followed by socio-cultural and economic barriers, and low knowledge or awareness [2]. System-level barriers resulting in vaccine inequity, such as service unreliability and vaccine stockouts, exacerbate these individual-level barriers to timely vaccinations [4]. Multicomponent interventions that jointly target individual- and system-level barriers may be needed to promote vaccinations.
Basic economic theory states that rational individuals will vaccinate their children if the expected benefits are greater than the expected costs. This framework applies in high-income settings, such as Western Europe or the United States, and in low-income settings, such as sub-Saharan Africa (SSA). The key benefit of childhood vaccinations is the immunity conferred against a host of diseases, such as polio, diphtheria, pertussis, and measles. When administered according to the recommended schedule, i.e., in a timely manner, vaccinations confer age-appropriate protection against vaccine-preventable diseases. Additionally, vaccinations provide nonspecific beneficial effects that reduce the probability of other diseases, and may increase cognition, educational attainment, and productivity [5]. Finally, vaccinations provide community-level protections by reducing disease transmission potential. While childhood vaccinations are available free of charge in most settings, there are offsets to these benefits (henceforth referred to as costs). These may include individual-level barriers (e.g., transport and opportunity costs, discomforts associated with the vaccination, risks of side effects) and system-level barriers (e.g., limited provider availability, vaccine stock-outs, or provider hesitancy to open multi-dose vials for small numbers of children) [4].
The decision of a parent to vaccinate their child reveals an implicit value of the vaccination (implicit willingness to pay; WTP) that exceeds the costs. Conversely, stagnant rates of vaccination coverage and low rates of vaccination timeliness [6] suggest that for many parents the value of a timely vaccination does not exceed the costs of going to get their children vaccinated, either due to a negative valuation of the vaccination itself, or due to individual- or system-level barriers that reduce the likelihood of parents acting on their intention. The negative net value of vaccinations to these individuals may be described by their willingness to accept (WTA) compensation in exchange for getting their child vaccinated on time. For these individuals, subsidies in the form of incentives may be used to align individual and social preferences by “tipping the scale” toward making timely vaccination a utility-maximizing choice [7].
Sustained progress beyond the 80 % coverage target in each of Tanzania's 169 districts (similar to U.S. counties), requires vaccinating incrementally harder-to-reach and incrementally harder-to-vaccinate children [6], [8], [9]. Vaccinating these children, in turn, requires addressing, or compensating for, incrementally greater barriers. While effective interventions can be designed to address most barriers, information on their relative importance for individuals’ vaccination decisions is needed to guide policy priorities regarding alternative intervention strategies (e.g., improving access, increasing vaccine-related knowledge) and their targeting toward specific populations (e.g., rural populations, populations with lower economic status, or populations with varying types or degrees of vaccine hesitancy). The systematic design and efficient targeting of interventions thus requires an understanding of the costs associated with different barriers, and the distribution of the net value of timely vaccination in the target population. WTP and WTA are standard metrics that can be used to characterize these costs and value distributions.
While there are a plethora of studies describing the benefits of routine vaccinations and the economic burden of vaccine-preventable illnesses to the society [1], [5], [10], [11], and a robust body of literature exists on individual valuations of diverse adult and adolescent vaccines in high-income countries, e.g., for influenza [12], Hepatitis [13], or HPV [14], [15], [16], we identified only two studies that touched on individuals’ WTP for routine childhood immunizations in SSA [17], [18]. Further, while incentives, motivated in behavioural economics [19], have been used to encourage diverse health related behaviours [20], [21], [22], [23], including vaccinations [24], [25], [26], evidence on their effectiveness is mixed [27]. It is also not clear whether monetary or non-monetary incentives, fixed or probabilistic incentives, or targeted or universal offers are most acceptable and effective in our Tanzania setting which is likely to be similar to many others in SSA [21], [28].
To inform the design of a digital health intervention for improving the timeliness of routine childhood vaccinations in Tanzania, we conducted a combined WTA/WTP study among pregnant women and asked them to rank alternative incentives for the timely vaccination of their children. Information on the WTA and WTP distributions in this population may be used to characterize variation in the balance between vaccine-related benefits and costs across individuals, and inform targeted, evidence-based policies for mitigating vaccine hesitancy and other barriers to timely childhood vaccinations.
Methods
This cross-sectional contingent valuation [29] study was part of a larger study that aimed to understand barriers to timely vaccinations in southern Tanzania and develop a digital health intervention to promote timely vaccine uptake. The protocol and key aspects of the parent study have been previously described [30]. Methods pertaining to this sub-study are described in concordance with the STROBE reporting guidelines for cross-sectional studies (Supplementary Table 1), and the statistical and analytical guidelines checklist for this journal.
Setting
The study was conducted in one urban district (Mtwara Municipality) and one rural district (Mtwara District Council) in Mtwara Region in southern Tanzania. The national Immunization and Vaccine Development program, which is under the Tanzania Ministry of Health, oversees the provision of routine childhood vaccinations in the area. While district level rates of vaccination coverage and timeliness are unknown, in 2015–16, coverage of all basic childhood vaccines as per national guidelines in the entire Mtwara Region (similar to a U.S. state) was estimated at 79 %, mirroring the national coverage rate of 75 % [31]. The timeliness of individual vaccine doses is estimated to range from 40 to 65 % [32].
Study participants and sampling strategy
Eligible study participants included women, ages 16 or older, in their third trimester of pregnancy, with access to a mobile phone. In 2017, 93 % of urban and 76 % of rural households in Tanzania owned a mobile phone [33], although only access to – not ownership of – a mobile phone was required to participate in the study. The target enrolment was 400 women, driven by the statistical power to detect a 10 to 15 percentage point increase in vaccination timeliness resulting from an effective intervention.[30] Participants were recruited from 9 urban and 10 rural health facilities that regularly provided childhood vaccinations and the surrounding communities. A combination of purposive and snowball sampling strategies was used for recruitment as follows: Eligible women presenting for antenatal care at participating facilities were approached by trained study personnel and offered enrolment in the study. To minimize biases from facility-based enrolment, participating women and local community leaders (balozis) were asked to identify other pregnant women in their communities, who, if eligible, were also offered enrolment in the study.
Survey
Surveys were administered by trained interviewers in Kiswahili, the language most commonly spoken in the study area. Surveys assessed women’s sociodemographic characteristics, parity, household characteristics, and knowledge of, and attitudes toward, childhood vaccinations. In addition, the survey included contingent valuation questions in a triple-bounded dichotomous choice format that assessed participants’ willingness to accept (WTA) or pay (WTP) for timely childhood vaccinations. To ensure that the survey did not adversely affect women’s actual willingness to vaccinate their children, WTA/WTP questions were prefaced with an introductory script:
“Now I will ask you some questions to understand how financial incentives influence the decision of women to vaccinate their children. The scenarios that are presented in the questions are hypothetical and for the purpose of research only.”
Following the introductory script, participants were asked:
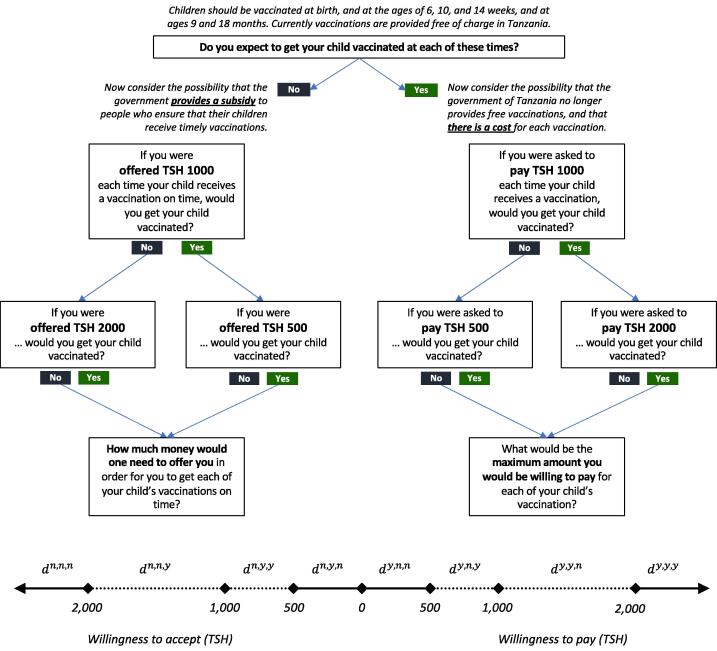
“Children should be vaccinated at birth, and at the ages of 6, 10, and 14 weeks and at ages 9 and 18 months. Currently vaccinations are provided free of charge in Tanzania. Do you expect to get your child vaccinated at each of these times?”
The answer to this dichotomous choice determined whether a woman was presented with WTA or WTP questions (Fig. 1).
Fig. 1.
Question format and potential response scenarios. Abbreviations: TSH – Tanzania shilling. Notes: denotes response scenarios, with ct describing the response (y = yes, n = no) to choice t (). Depending on the response scenario, a woman’s valuation of timely vaccinations could be captured exactly (solid line; point data) or be known to fall within some interval (dashed line; interval data). At the time of the study 1 US Dollar was worth approximately TSH 2,200.
WTA
Women answering “no” to the initial choice were told “Now consider the possibility that the government provides a subsidy to people, to ensure that their children receive timely vaccinations.” Next women were asked “If you were offered Tsh 1,000 each time your child receives a vaccination on time, would you get your child vaccinated?” Those who responded positively were asked “If you were offered Tsh 500 each time your child receives a vaccination on time, would you get your child vaccinated?”, while those who responded negatively were asked “If you were offered Tsh 2,000 each time your child receives a vaccination on time, would you get your child vaccinated?”. Women answering “yes” to both questions and women answering “no” to all questions were further asked “How much money would one need to offer you in order for you to get each of your child’s vaccinations on time?”.
WTP
Women answering “yes” to the initial choice were told “Now consider the possibility that the government of Tanzania no longer provides free vaccinations, and that there is a cost for each vaccination for your child.” Next women were asked “If you were asked to pay Tsh 1,000 each time your child receives a vaccination, would you get your child vaccinated?” Those who responded negatively were asked “If you were asked to pay Tsh 500 each time your child receives a vaccination, would you get your child vaccinated?”, while those who responded positively were asked “If you were asked to pay Tsh 2,000 each time your child receives a vaccination, would you get your child vaccinated?”. Women answering “no” to both questions and women answering “yes” to all questions, were further asked “What would be the maximum amount you would be willing to pay for each of your child’s vaccinations?”.
The series of dichotomous choice questions resulted in 8 possible response scenarios that for individual can be described by 8 binary indicator variables , where ct reflects the response (y = yes, n = no) to choice t ():
-
a)
“No” to the initial dichotomous choice ()
-
•
“Yes” to the subsidy of 1,000 and again “yes” to the subsidy of 500 ()
-
•
“Yes” to the subsidy of 1,000 and “no” to the subsidy of 500 ()
-
•
“No” to the subsidy of 1,000 and “yes” to the subsidy of 2,000 ()
-
•
“No” to the subsidy of 1,000 and again “no” to the subsidy of 2,000 ()
-
b)
“Yes” to the initial dichotomous choice ()
-
•
“Yes” to the cost of 1,000 and again “yes” to the cost of 2,000 ()
-
•
“Yes” to the cost of 1,000 and “no” to the cost of 2,000 ()
-
•
“No” to the cost of 1,000 and “yes” to the cost of 500 ()
-
•
“No” to the cost of 1,000 and again “no” to the cost of 500 ()
As the line at the bottom of Fig. 1 illustrates, depending on the response scenario, a woman’s valuation of timely vaccinations could be captured exactly, i.e., as point data, or be known to fall within some interval. Four of the eight response scenarios (, , , ), when combined with an open-ended follow-up question, elicited an exact valuation. The remaining four response scenarios (, , , ) elicited an interval that contains the woman’s (unobserved) valuation. The cost and incentive ranges were selected based on feasibility considerations. At the time of the study 1 US Dollar was worth approximately Tsh 2,200, thus, the values of Tsh 500, Tsh 1,000 and Tsh 2,000 corresponded to approximately $0.23, $0.45, and $0.90, respectively. The gross domestic product (GDP) per capita was approximately $2.75 per day.
Alternative incentive options for timely vaccination
Women were also asked to rank six incentive options for each timely vaccination of their children, including (a) mobile phone credit of Tsh 2,000, (b) a pharmacy voucher valued at Tsh 2,000, (c) a lottery ticket with the chance of winning Tsh 20,000, (d) a free health check for the mother, (e) a birth certificate, and (f) a mobile money payment of Tsh 2,000. Women’s odds of winning the lottery or the costs of health checks (estimated at Tsh 8,000 in 2019) or birth certificates (Tsh 3,500 in 2020) were not specified.
Model
We assume that individuals maximize utility and face a trade-off between vaccination and the level of consumption of all other goods, subject to resource constraints in terms of income and time. From a societal perspective, the value of a vaccination is positive because of the preventive benefits for the individual and potential “herd immunity” effects within communities. From the individual mother’s perspective, however, the value, i.e., the expected utility derived from the timely vaccination of her children, depends on perceived benefits and risks, knowledge and beliefs, and opportunity costs. To capture these trade-offs, the survey assessed several potential correlates of women’s valuation of vaccinations.
Most pertinently, the value of a vaccination is a function of women’s knowledge and beliefs regarding vaccinations as a means of protecting children against disease. The perceived benefits of vaccinations (vb) were captured by an indicator variable for women’s ability to name at least one vaccine-preventable disease. Similarly, an indicator variable for women’s ability to name at least one potential side effect of vaccinations (vs) was included as a measure of vaccine-related costs. Attitudes toward vaccinations were captured by a vaccine hesitancy score (vh) comprised of women’s answers to 15 questions about vaccine-related benefits and risks (Supplementary Table 2) [4]. Each question was scored 0 if not hesitant, 1 if not sure, and 2 if hesitant, with a potential range of 0, if the mother scored not hesitant for all items, to 30, if the mother scored hesitant on all 15 items. Preferences for vaccinations and perceived benefits and risks may depend on experiences with prior vaccinations (ex), thus an indicator variable for prior births was also included as a covariate.
Vaccinations are freely available in health facilities throughout the study area; therefore, the cost of vaccinations primarily consists of transportation cost and the opportunity cost of time. To approximate distance (d), we included rural vs urban residence and travel time (t) to the nearest health facility as covariates. Socioeconomic status, a proxy for health literacy and women’s opportunity cost of time, was captured by covariates for age (a), education (e), and a household asset index (ha). The 12-item asset index summarizes the presence or absence of electricity, radio, television, computer, refrigerator, iron, motorcycle, car, bank account, tap water, flush toilet, and finished flooring. Marital status (m) and household composition (hc) may affect both the benefits and costs of vaccinations. For instance, other adults in the household may provide financial or logistical support to women when taking the child for vaccinations but may also interfere with women’s independence when making healthcare decisions on behalf of the child.
Combining these factors, the demand for vaccinations () is a function of the positive or negative value or “price” of vaccinations, the price of all other goods (), a resource endowment (), and random factors () representing unmeasured preferences or measurement error. The individual utility function is expressed as . The value of vaccinations is a function of the characteristics discussed above, q = f(vb, vs vh, ex, d, t, a, e, ha, m, hc). A priori, the demand for vaccinations, q0, represents a utility-maximizing choice weighing the consumption of vaccination against the consumption of other goods. In our survey, the subsidies or costs specified in the WTA and WTP questions alter the hypothetical utility function, and demand for vaccination may change from to .
The prices of other goods () are constant in both scenarios and cancel out of the econometric model, so they are not included in the model or available on our survey. Income () is also not available, although it is captured indirectly through age, education, and assets.
Estimation
Women’s valuation of timely vaccinations was estimated in a regression framework. In the regression , represents an individual’s (observed or unobserved) WTA or WTP and represents a normally distributed error term that is uncorrelated across individuals. In some cases, we observe . In other cases, is known only to lie within an interval, e.g., yi ∊ [500, 999]. The log likelihood of observed responses across the 8 possible response scenarios and open-ended follow-up questions can be described as follows:
where the individuals’ binary choices are represented by the mutually exclusive indicator variables , , represents the cardinal responses to the open-ended follow-up questions, and is the cumulative standard normal distribution. The estimation of the interval censored regression models was implemented using the intreg command in Stata version 16.1 (StataCorp, College Station, TX, 2019).
Owing to the distribution of responses, the dependent variable was natural log-transformed after adding Tsh 1 to responses of Tsh 0. In alternative specifications, the x vector included the covariates described above, either individually (‘bivariable associations’) or jointly (‘multivariable associations’), or a constant only (in order to generate estimates of the sample average WTP). Bivariable associations may inform screening approaches by characterizing women with higher vs lower valuations of timely vaccinations, whereas multivariable associations better characterize the relative importance of different covariates, accounting for the fact that many of the characteristics evaluated may be correlated. Models were estimated for the entire sample and separately by rural vs urban residence.
Expected WTP values were calculated for each individual on the raw scale (i.e., antilog), incorporating Duan’s smearing factor, and averaged across individuals to provide sample averages of the estimated WTP [34].
Sample average marginal effects estimates were derived using the same approach, i.e., for each covariate, the sample average estimated WTP was calculated at alternate values of the respective covariate; the difference between the averages was interpreted as the sample average marginal effect.
Characteristics of participating mothers and preferences for alternative incentive options for timely vaccination were analysed descriptively. Student’s t-tests (for continuous variables) and chi-squared tests (for categorical variables) were used to assess the statistical significance of differences in the characteristics of rural vs urban women.
Results
Sample characteristics
Between August and December 2017, 406 pregnant women in their last trimester of pregnancy were enrolled in the study. Characteristics of the participating women are shown in Table 1. One in three women had less than primary school education, most were married, and three out of four women had previously given birth. Two out of three women were able to name at least one disease that could be prevented by vaccines; nearly half named at least one vaccine-related side effect. Compared to rural women, urban women reported higher education, more assets, greater vaccine knowledge, lower vaccine hesitancy, and shorter travel time to the nearest health facility.
Table 1.
Characteristics of participating pregnant women in their last trimester of pregnancy (southern Tanzania, 2017, N = 406).
| All women | Rural women | Urban women | ||
|---|---|---|---|---|
| (N = 406) | (N = 194; 47.8 %) | (N = 212; 52.2 %) | p | |
| Age in years, mean (sd) | 27.9 (7.2) | 28.4 (7.9) | 27.5 (6.5) | 0.193 |
| Less than primary school education | 33.0 % | 50.0 % | 17.5 % | <0.001 |
| Married | 81.5 % | 80.9 % | 82.1 % | 0.799 |
| More than 2 adults in the household | 30.3 % | 26.8 % | 33.5 % | 0.160 |
| Asset score, mean (sd) | 2.5 (2.5) | 1.1 (1.4) | 3.7 (2.7) | <0.001 |
| Previously gave birth | 74.1 % | 75.3 % | 73.1 % | 0.651 |
| Vaccine hesitancy score, mean (sd) | 7.3 (3.3) | 7.7 (3.6) | 6.9 (2.9) | 0.015 |
| Names 1 + vaccine-preventable disease | 67.0 % | 58.2 % | 75.0 % | <0.001 |
| Names 1 + vaccine-related side effect | 47.5 % | 45.9 % | 49.1 % | 0.551 |
| Time to nearest health facility in minutes, mean (sd) | 19.9 (19.6) | 24.0 (24.2) | 16.1 (13.1) | <0.001 |
Notes: Abbreviations: sd – standard deviation.
The statistical significance of differences between rural and urban women was assessed using Student’s t-tests (for continuous variables) and chi-squared tests (for categorical variables).
Willingness to accept and willingness to pay for routine childhood vaccinations
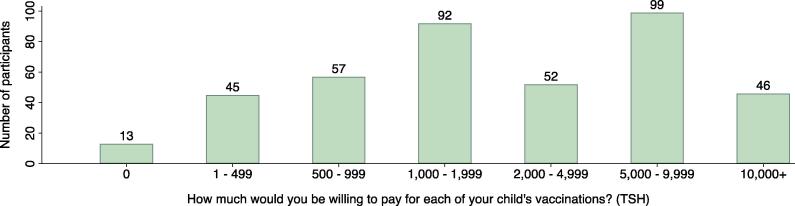
A graphical overview of the WTA and WTP questions is shown in Fig. 1; the distribution of responses is shown in Fig. 2. All women indicated that they expected to get their children vaccinated according to the recommended schedule, even without a monetary incentive, thus precluding an analysis of the distribution of potential subsidies needed to ensure the timely vaccination of children. Nearly all women (393; 96.8 %) were willing to pay some money for a vaccination; almost half (198; 48.9 %) were willing to pay Tsh 2,000 or more, and more than one in ten (46; 11.4 %) were willing to pay Tsh 10,000 or more for each vaccination.
Fig. 2.
Nearly universally positive willingness to pay for routine childhood vaccinations (southern Tanzania, 2017, N = 406). Notes: Abbreviations: Tsh. – Tanzania shilling. Groupings of responses in the ranges of 1 ≤ WTP ≤ 499 Tsh and ≥ 2000 Tsh are for visualization only. Exact values were used in the model estimation. At the time of the study 1 US Dollar was worth approximately Tsh 2,200. Two women answered yes to all 3 dichotomous choice questions and stated Tsh 1,000 as their maximum willingness to pay in the open-ended follow-up question; these two women are excluded from the bar chart.
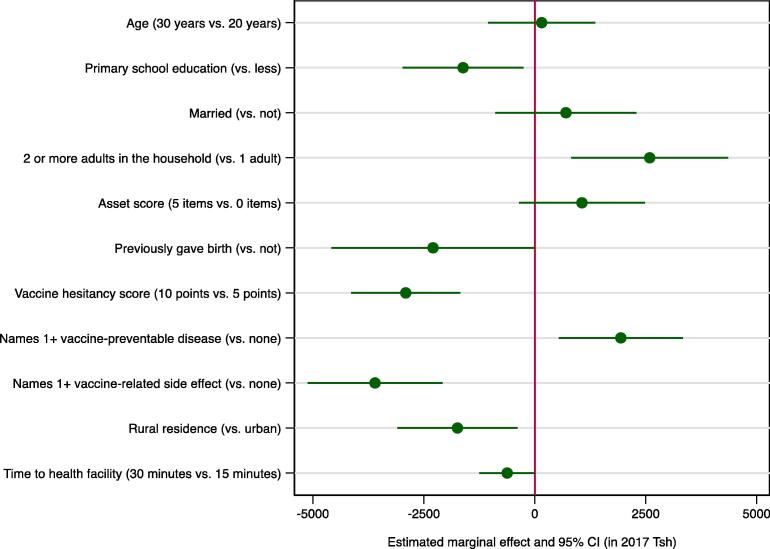
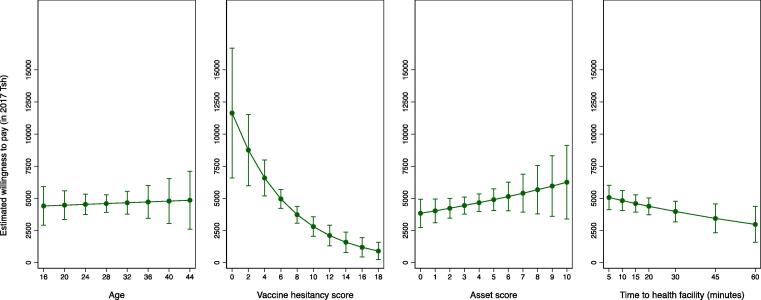
An interval censored regression model containing only a constant indicated that women’s average WTP was Tsh 3,066 (95 % CI:2,523–3,610). When the model was estimated separately for rural vs urban women, the average WTP for rural women was Tsh 1,872 (95 % CI:1,339–2,406) compared to Tsh 4,820 (95 % CI:3,862–5,780) for urban women (results not shown). In bivariable analyses there was significant variation in women’s WTP with education, household composition, household assets, parity, and knowledge and attitudes toward vaccines, as well as rural vs urban residence and travel time to the nearest health facility (Table 2). In multivariable analyses, with an indicator for rural vs urban residence in the model, the coefficient on travel time to the nearest health facility was only significant at the p < 0.10 level on a two-tailed test. Marginal effects estimates for the multivariable model in Table 2 are presented in Fig. 2, Fig. 3. The results show graphically the extent to which lower education and fewer household assets, a prior birth, and a residence in rural areas with greater travel times to the nearest health facility were associated with a significantly lower WTP. WTP was also lower for women with greater vaccine hesitancy scores, those unable to name at least one vaccine-preventable disease, and those familiar with vaccine-related side effects. Fig. 3 visualizes the variation in the estimated mean willingness to pay across the ranges of continuous explanatory variables, highlighting in particular the strong negative association of vaccine hesitancy with WTP. Notably, WTP estimates remained positive across the full ranges of age, household assets, travel time to the nearest health facility, and vaccine hesitancy scores.
Table 2.
Correlates of women’s willingness to pay for routine childhood vaccinations (southern Tanzania, 2017, N = 406).
| Bivariable estimates | Multivariable estimates | |||||
|---|---|---|---|---|---|---|
| Coefficient | Std. Err. | p | Coefficient | Std. Err. | p | |
| Age in years (range 16–45) | −0.022 | 0.012 | 0.080 | 0.003 | 0.014 | 0.803 |
| Less than primary school education | −1.121 | 0.184 | <0.001 | −0.401 | 0.190 | 0.035 |
| Married | 0.055 | 0.233 | 0.813 | 0.160 | 0.193 | 0.407 |
| More than 2 adults in the household | 0.470 | 0.196 | 0.016 | 0.519 | 0.159 | 0.001 |
| Asset score (range 0–10) | 0.204 | 0.035 | <0.001 | 0.049 | 0.035 | 0.160 |
| Previously gave birth | −0.619 | 0.204 | 0.002 | −0.462 | 0.214 | 0.031 |
| Vaccine hesitancy score (range 0–17) | −0.163 | 0.027 | <0.001 | −0.142 | 0.032 | <0.001 |
| Names 1 + vaccine-preventable disease | 0.655 | 0.190 | <0.001 | 0.472 | 0.181 | 0.009 |
| Names 1 + vaccine-related side effect | −0.707 | 0.178 | <0.001 | −0.831 | 0.171 | <0.001 |
| Rural residence | −0.945 | 0.175 | <0.001 | −0.405 | 0.165 | 0.014 |
| Time to nearest health facility in minutes (range 2–180) | −0.020 | 0.005 | <0.001 | −0.010 | 0.005 | 0.064 |
| Constant | 8.761 | 0.449 | <0.001 | |||
| RMSE | 1.553 | 0.107 | ||||
Abbreviations: WTP – willingness to pay; Tsh. – Tanzanian shilling; Std. Err. – Standard error; RMSE – root mean square error.
Notes: Estimates from bivariable and multivariable interval censored regression models predicting log(WTP) as a function of the respective covariate(s). Estimates in 2017 Tsh. At the time of the study 1 US Dollar was worth approximately Tsh 2,200.
Fig. 3.
Estimated marginal effects on women’s WTP for timely routine childhood vaccinations (southern Tanzania, 2017, N = 406). Notes: Abbreviations: WTP–willingness to pay; Tsh–Tanzania shilling; CI–confidence interval. Sample average marginal effects were calculated based on estimates from a multivariable interval censored regression model predicting log(WTP) as a function of the covariates shown in Table 2, (see Methods for details). Circles represent point estimates; error bars represent 95 % confidence intervals. Estimates in 2017 Tanzanian shilling (Tsh). At the time of the study 1 US Dollar was worth approximately Tsh 2,200. For binary variables, the estimated marginal effects show the sample average expected change in WTP for a discrete change in the variable from 0 to 1. For continuous variables, the estimated marginal effects show the change in WTP when the variable increases from the lower to the upper bound of the respective interval (see also Fig. 4).
Preferences for alternative incentives for the timely vaccination of children
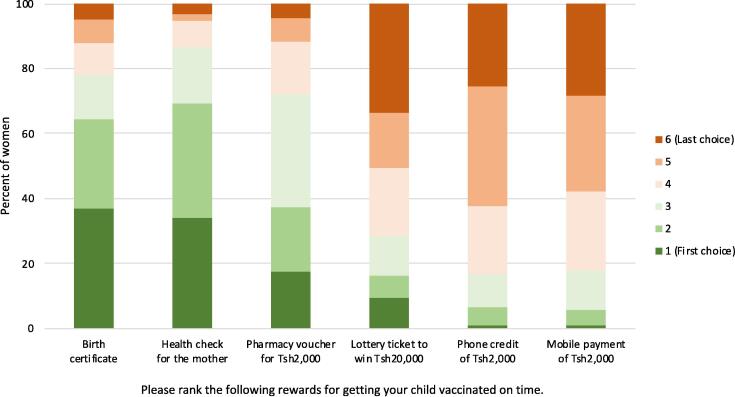
When asked to rank alternative incentives for getting their children vaccinated on time, women tended to prefer non-monetary incentives, such as a birth certificate, a maternal health check, or, to a lesser extent, a pharmacy voucher, over monetary incentives such as a lottery ticket with a monetary pay-out, a mobile money payment, or mobile phone credit (Fig. 5.)
Fig. 4.
Estimated marginal effects of women’s age, vaccination attitudes, household assets, and distance to care on their WTP for timely routine childhood vaccinations (southern Tanzania, 2017, N = 406). Notes: Abbreviations: WTP – willingness to pay; Tsh – Tanzania shilling. Sample average marginal effects were calculated based on estimates from a multivariable interval censored regression model predicting log(WTP) as a function of the covariates shown in Table 2, (see Methods for details). Circles represent point estimates; error bars represent 95 % confidence intervals. Estimates in 2017 Tanzanian shilling (Tsh). At the time of the study 1 US Dollar was worth approximately Tsh 2,200.
Fig. 5.
Women's rankings of alternative incentive options for timely vaccinations (southern Tanzania, 2017, N = 406). Notes: Abbreviations: Tsh – Tanzania shilling. Women were asked to rank six potential incentives for each timely vaccination of their children, including (a) mobile phone credit of Tsh 2,000, (b) a pharmacy voucher valued at Tsh 2,000, (c) a lottery ticket with the chance of winning Tsh 20,000, (d) a free health check for the mother, (e) a birth certificate, and (f) a mobile money payment of Tsh 2,000. Women’s odds of winning the lottery or the costs of health checks (estimated at Tsh 8,000 in 2019) or birth certificates (Tsh 3,500 in 2020) were not specified.
Discussion
This study aimed to characterize the value of timely routine childhood vaccinations to pregnant women in southern Tanzania. Using contingent valuation questions, the study sought to elicit the WTA and WTP distributions in this population and identify the potential role of incentives as a means of overcoming barriers to timely vaccinations. In a cohort of 406 pregnant women in their last trimester of pregnancy, all indicated that they expected to get their children vaccinated according to the recommended schedule, even without incentives. Nearly all women were willing to pay a positive amount of money for the timely vaccination of their children, suggesting that individual-level demand-side factors are not the primary drivers of non– or delayed vaccinations in this setting. WTP, interpreted as the value of a timely vaccination that summarizes women’s expected benefits and costs, varied systematically with socio-economic and access characteristics and with vaccine-related knowledge and attitudes. WTP was significantly lower among women with less education, fewer household assets, women living in rural areas with greater travel time to the nearest health facility, and those with greater vaccine hesitancy. For some of these women, additional, unexpected costs, such as those resulting from provider unavailability, stockouts, or rescheduled appointments [4], may plausibly exceed the value of a timely vaccination, leading to non– or delayed vaccinations. Incentives may compensate these women for such additional costs and ensure that timely vaccination remains a utility-maximizing choice. When given the choice of diverse incentives for the timely vaccination of their children, women tended to prefer non-monetary over monetary incentives.
This study is the first to use contingent valuation methods to elicit the value of routine childhood vaccinations to individuals in SSA. There is a plethora of studies characterizing the value of vaccinations to society [1], [5], [10]; and studies from high income settings [35], [36] and Asia [37] suggest a positive WTP for routine childhood vaccinations in those settings. However, we identified only two studies that touched on individuals’ WTP for routine childhood immunizations in SSA [17], [18]; neither used valuation methods. In 2002, in rural Tanzania, only 49 % of households with children ages 12–23 months were willing to contribute to the cost of kerosine for vaccine cold storage in case of emergency [18]; and in 2013, in southern Nigeria, only 55 % of clients presenting to rural and urban health facilities for their children’s vaccinations were willing to pay for vaccinations [17]. Our findings suggest that in 2017, in southern Tanzania, WTP for routine childhood vaccinations was higher, and more in line with WTP estimates for other vaccines. For example, in 2005, in urban Mozambique, the majority of citizens were willing to pay some money for a cholera vaccine [38], and positive WTP estimates have been obtained for HPV [39] and hypothetical Malaria [40], Ebola [41], or HIV [42] vaccines in diverse settings in SSA. Similarly, WTP studies for childhood vaccines from Asia suggest positive WTP for routine childhood vaccinations [37], as well as for cholera, typhoid, and pneumococcal vaccines, and to avoid episodes of shigellosis [43], [44], [45], [46], [47]; these studies also highlight substantial within-sample variation in WTP. The literature on WTP for childhood vaccinations in high income countries has focused primarily on the value of specific vaccine characteristics, such as reductions in the probability or severity of adverse events, side effects, or pain; reductions in the number of doses or injections; or increases in the number of diseases prevented by combination vaccines. Notable exceptions, namely studies of the WTP for varicella [35], rotavirus [36], and pneumococcal vaccines [48], and for vaccine-effected changes in the probabilities of outcomes or health states, also suggest a positive WTP, on average.
The results of our study have important policy implications. First, we demonstrate that, in the Tanzanian setting, women broadly recognize vaccinations as beneficial and, in the absence of barriers, may not need to be incentivized to vaccinate their children on time. Vaccine hesitancy per se does not appear to be the primary driver of sub-optimal vaccination coverage and timeliness. Instead, positive WTP estimates, coupled with vaccination rates below target levels, suggest that the implicit costs of vaccinations (e.g., a combination of system-level barriers, transport costs, and opportunity costs), combined with vaccine hesitancy, may be “tipping the scale” for a subset of women against the timely vaccination of their children. To the extent that women incorporated known barriers to timely vaccinations into their WTP responses, universally non-negative valuations suggest that unexpected system-level barriers are likely to be the primary drivers of missed or delayed vaccinations in this setting. Such barriers may include the complexity of the vaccination schedule (which dictates an order to vaccines and sometimes variable spacing between doses), providers’ communication and responsiveness to scheduled time-sensitive appointments for patients [49], and supply-side barriers such as facility closures, staffing shortages, or providers turning away children because they do not want to open a multi-dose vial for small numbers of children [4]. Significantly lower WTP estimates for women who previously gave birth, relative to nulliparous women (Table 2), are consistent with this interpretation. Additional research should focus on exploring means of reducing unexpected and implicit costs to mothers.
Second, systematic variation in WTP plausibly reflects variation in women’s valuations of benefits and costs and may inform the design and adaptation of interventions that target specific barriers to timely vaccinations. Lower WTP estimates were observed for rural women and for women with less education; rural women were also less likely than urban women to be able to name a vaccine-preventable disease. Owing to the strong associations of WTP with vaccine hesitancy (Fig. 3) and knowledge (Table 2), these observations are indicative of information deficits among pregnant women and suggest a role for antenatal care visits as a means of improving vaccine knowledge and pre-empting vaccine hesitancy. Similarly, the associations of WTP with household assets and travel time to the nearest health facility highlight potential access barriers and suggest a role for expanded hours, reduced waiting time, advance notification of stock-outs, and mobile vaccination clinics or other means of reducing transport barriers and opportunity costs.
Third, our exploratory analysis of women’s preferences for alternative incentive options for the timely vaccination of their children highlights preferences for non-monetary over monetary incentives. Notably, we observed substantial variation in the rankings of alternative incentive options across participants. While incentives, motivated in behavioural economics have been used to encourage diverse health related behaviours [22], [23], including vaccinations [25], [26], it is not clear which incentive mechanisms are most acceptable and effective in this setting, how combinations of incentive offers across vaccination appointments might impact vaccination decisions, or what budgetary impact incentives would have at the population level. Choices in our study may have been driven by actual or perceived values of non-monetary incentives (e.g., the cost of a health check for the mother may vary with insurance coverage; a birth certificate in Tanzania currently costs TSH 3,500), the locus of financial decision making within families (e.g., women may not be able to control how money is spent; phone credit may be applied to a shared phone), or women’s unmet needs for health services. Additional formative work is needed to characterize the mechanisms underlying these choices and inform the design of incentive structures and policies with maximum impact on parents’ vaccination decisions.
We acknowledge several limitations and ethical considerations that derive from our results. First, results are not based on a random sample of women making vaccination decisions. Sample selection thus represents a threat to validity. The findings should be considered exploratory, and the transferability of WTP estimates to other women, including women without access to mobile phones and women in other parts of Tanzania, needs further consideration.
Second, answers to questions about vaccination preferences during pregnancy may diverge from actual decisions after childbirth. WTP estimates based on stated preferences may substantively diverge from estimates obtained from revealed preferences when the underlying conditions characterizing the relevant contexts or incentive structures diverge; however, stated and revealed preferences are generally highly correlated and roughly of the same magnitude [50]. Answers may also have been affected by social desirability biases, i.e., participants responding normatively appropriately to questions about their intent to vaccinate their children. However, our finding of a universally non-negative WTP is consistent with the fact that the vast majority of women will eventually get their children vaccinated [32], thus social desirability bias is not likely a major factor driving our results.
Third, our WTP estimates could be improved by using larger sample sizes and more efficient experimental designs. While we used WTP as a monetary measure of a woman’s desire to vaccinating her child, WTP measures have well-known issues when a respondent has very limited resources because that measure is income constrained [51]. The usual difficulties of reliably measuring income make assessing the implications of this issue difficult.
Fourth, to inform the development of effective, contextually relevant interventions to improve vaccination uptake and timeliness, further research is needed to evaluate the extent to which women’s WTP values correlate with the WTP of expectant fathers and other individuals involved in vaccination decision making, and how WTP values were influenced by vaccination experiences after previous births and vaccination information received during antenatal care.
Finally, there are trade-offs with respect to the ethics and practicality of rewards, incentives, or payments for health services in low resource settings that have been explored by others [51]. While, at the population level, a positive WTP might indicate that user fees may be used to mitigate supply-side obstacles [52], they may have unintended consequences in terms of vaccination equity [53]. The costs, logistics, and unintended consequences of implementing and sustaining user fees or incentives must also be considered. There is evidence that extrinsic motivators hold potential to crowd out intrinsic motivators [54], and attention must be paid to potential adverse consequences of incentivizing behaviors that may already be governed by “social contracts” [55], e.g., expectations of herd immunity and other positive externalities resulting from timely vaccinations. Ethical and cost-effectiveness considerations at the population level, and the extent to which behavioral economics can inform the optimal design of incentive structures for mothers and providers, thus merit careful deliberation.
Conclusion
This study used a contingent valuation method to assess the value of routine childhood immunizations among pregnant women in a low-resource setting. The results suggest that women value vaccinations for their children, but also support differentiated interventions such as continued efforts to mitigate access barriers, information campaigns to highlight benefits and correct misperceptions about vaccinations, and non-monetary incentives to compensate for system-level barriers to timely vaccinations. The methods may be applied to other vaccines, populations, and settings, and may inform ongoing efforts to rapidly vaccinate large populations against SARS-CoV-2.
Funding
The research presented in this manuscript was supported by grants from the Fogarty International Center of the National Institutes of Health under Award Number R21TW010262, and the Maternal, Adolescent and Child Health (MACH) working group of the Duke Global Health Institute. LV received funding from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR002554. The funding bodies had no role in the design of the study, the collection, analysis, and interpretation of data, or writing of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors’ contributions
JO, EN, SM, JB and LV jointly conceived the larger study within which the WTA/WTP study was embedded and contributed to the study design. JO and LV conceived the WTA/WTP study. JO, EN, SM, and LV coordinated the study and oversaw its implementation. JO performed the statistical analyses with input from NH. JO and LV interpreted the data and drafted the initial version of manuscript. All authors read and approved the final manuscript.
Ethics statement
The protocol was approved by the Institutional Review Boards at Duke University (Protocol 2017–0591-D0271) and University of South Carolina (facilitated review, Pro00051213), USA, and the National Institute for Medical Research (NIMR) in Tanzania (NIMR/HQ/R.8a/Vol. IX/2194). Informed consent was obtained from all study participants.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge the women who participated in the study as well as research assistants from the NIMR Muhimbili Research Centre who supported data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2023.100266.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sim S.Y., Watts E., Constenla D., Brenzel L., Patenaude B.N. Return On Investment From Immunization Against 10 Pathogens In 94 Low- And Middle-Income Countries, 2011–30. Health Aff (Millwood) 2020;39(8):1343–1353. doi: 10.1377/hlthaff.2020.00103. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald N., Mohsni E., Al-Mazrou Y., Kim Andrus J., Arora N., Elden S., et al. Global vaccine action plan lessons learned I: Recommendations for the next decade. Vaccine. 2020;38(33):5364–5371. doi: 10.1016/j.vaccine.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, Immunization coverage, 2020.https://www.who.int/news-room/fact-sheets/detail/immunization-coverage. (Accessed 2020-12-18).
- 4.Vasudevan L., Baumgartner J.N., Moses S., Ngadaya E., Mfinanga S.G., Ostermann J. Parental concerns and uptake of childhood vaccines in rural Tanzania - a mixed methods study. BMC Public Health. 2020;20(1):1573. doi: 10.1186/s12889-020-09598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deogaonkar R., Hutubessy R., van der Putten I., Evers S., Jit M. Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC Public Health. 2012;12:878. doi: 10.1186/1471-2458-12-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peck M., Gacic-Dobo M., Diallo M.S., Nedelec Y., Sodha S.V., Wallace A.S. Global Routine Vaccination Coverage, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(42):937–942. doi: 10.15585/mmwr.mm6842a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowles S., Polania-Reyes S. Economic incentives and social preferences: substitutes or complements? J Econ Lit. 2012;50(2):368–425. [Google Scholar]
- 8.Ozawa S., Yemeke T.T., Evans D.R., Pallas S.E., Wallace A.S., Lee B.Y. Defining hard-to-reach populations for vaccination. Vaccine. 2019;37(37):5525–5534. doi: 10.1016/j.vaccine.2019.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization, Immunization Agenda 2030: A Global Strategy to Leave No One Behind, 2020. https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/ia2030. (Accessed 2020-12-18).
- 10.Barnighausen T., Bloom D.E., Cafiero-Fonseca E.T., O'Brien J.C. Valuing vaccination. Proc Natl Acad Sci U S A. 2014;111(34):12313–12319. doi: 10.1073/pnas.1400475111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozawa S., Clark S., Portnoy A., Grewal S., Brenzel L., Walker D.G. Return On Investment From Childhood Immunization In Low- And Middle-Income Countries, 2011–20. Health Aff (Millwood) 2016;35(2):199–207. doi: 10.1377/hlthaff.2015.1086. [DOI] [PubMed] [Google Scholar]
- 12.Prosser L.A., Payne K., Rusinak D., Shi P., Uyeki T., Messonnier M. Valuing health across the lifespan: health state preferences for seasonal influenza illnesses in patients of different ages. Value Health. 2011;14(1):135–143. doi: 10.1016/j.jval.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs R.J., Moleski R.J., Meyerhoff A.S. Valuation of symptomatic hepatitis a in adults: estimates based on time trade-off and willingness-to-pay measurement. Pharmacoeconomics. 2002;20(11):739–747. doi: 10.2165/00019053-200220110-00003. [DOI] [PubMed] [Google Scholar]
- 14.Poulos C., Yang J.C., Levin C., Van Minh H., Giang K.B., Nguyen D. Mothers' preferences and willingness to pay for HPV vaccines in Vinh Long Province. Vietnam, Soc Sci Med. 2011;73(2):226–234. doi: 10.1016/j.socscimed.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Brown D.S., Johnson F.R., Poulos C., Messonnier M.L. Mothers' preferences and willingness to pay for vaccinating daughters against human papillomavirus. Vaccine. 2010;28(7):1702–1708. doi: 10.1016/j.vaccine.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y., Lin Z., He F., Chen H., Lin X., Zimet G.D., et al. HPV vaccination intent and willingness to pay for 2-,4-, and 9-valent HPV vaccines: A study of adult women aged 27–45 years in China. Vaccine. 2020;38(14):3021–3030. doi: 10.1016/j.vaccine.2020.02.042. [DOI] [PubMed] [Google Scholar]
- 17.Ossai E.N., Fatiregun A.A. Clients' Willingness to Pay for Immunization Services in the Urban and Rural Primary Health Centers of Enugu State, Nigeria. J Public Health Afr. 2015;6(1):480. doi: 10.4081/jphia.2015.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semali I.A., Tanner M., de Savigny D. Decentralizing EPI services and prospects for increasing coverage: the case of Tanzania. Int J Health Plann Manage. 2005;20(1):21–39. doi: 10.1002/hpm.794. [DOI] [PubMed] [Google Scholar]
- 19.Kamenica E. Behavioral Economics and Psychology of Incentives. Annual Review of Economics. 2012;4(1):427–452. [Google Scholar]
- 20.Gopalan S.S., Mutasa R., Friedman J., Das A. Health sector demand-side financial incentives in low- and middle-income countries: a systematic review on demand- and supply-side effects. Soc Sci Med. 2014;100:72–83. doi: 10.1016/j.socscimed.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Lagarde M., Haines A., Palmer N. The impact of conditional cash transfers on health outcomes and use of health services in low and middle income countries. Cochrane Database Syst Rev. 2009;4:CD008137. doi: 10.1002/14651858.CD008137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranganathan M., Lagarde M. Promoting healthy behaviours and improving health outcomes in low and middle income countries: a review of the impact of conditional cash transfer programmes. Prev Med. 2012;55(Suppl):S95–S105. doi: 10.1016/j.ypmed.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Bassani D.G., Arora P., Wazny K., Gaffey M.F., Lenters L., Bhutta Z.A. Financial incentives and coverage of child health interventions: a systematic review and meta-analysis. BMC Public Health. 2013;13(Suppl 3):S30. doi: 10.1186/1471-2458-13-S3-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandir S., Khan A.J., Hussain H., Usman H.R., Khowaja S., Halsey N.A., et al. Effect of food coupon incentives on timely completion of DTP immunization series in children from a low-income area in Karachi, Pakistan: a longitudinal intervention study. Vaccine. 2010;28(19):3473–3478. doi: 10.1016/j.vaccine.2010.02.061. [DOI] [PubMed] [Google Scholar]
- 25.Robertson L., Mushati P., Eaton J.W., Dumba L., Mavise G., Makoni J., et al. Effects of unconditional and conditional cash transfers on child health and development in Zimbabwe: a cluster-randomised trial. Lancet. 2013;381(9874):1283–1292. doi: 10.1016/S0140-6736(12)62168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakadha H., Chandir S., Were E.V., Rubin A., Obor D., Levine O.S., et al. The feasibility of using mobile-phone based SMS reminders and conditional cash transfers to improve timely immunization in rural Kenya. Vaccine. 2013;31(6):987–993. doi: 10.1016/j.vaccine.2012.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelstein E.A., Bilger M., Baid D. Effectiveness and cost-effectiveness of incentives as a tool for prevention of non-communicable diseases: A systematic review. Soc Sci Med. 2019;232:340–350. doi: 10.1016/j.socscimed.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Adams J., McNaughton R.J., Wigham S., Flynn D., Ternent L., Shucksmith J. Acceptability of Parental Financial Incentives and Quasi-Mandatory Interventions for Preschool Vaccinations: Triangulation of Findings from Three Linked Studies. PLoS One. 2016;11(6):e0156843. doi: 10.1371/journal.pone.0156843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carson R.T., Hanemann W.M. Handbook of Environmental Economics. Elsevier; 2006. pp. 821–936. [Google Scholar]
- 30.Ostermann J., Vasudevan L., Baumgartner J.N., Ngadaya E., Mfinanga S.G. Do mobile phone-based reminders and conditional financial transfers improve the timeliness of childhood vaccinations in Tanzania? Study protocol for a quasi-randomized controlled trial. Trials. 2019;20(1):397. doi: 10.1186/s13063-019-3430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The DHS Program, Demographic and Health Survey, and Malaria Indicator Survey, Tanzania, 2015-16. https://dhsprogram.com/pubs/pdf/FR321/FR321.pdf. (Accessed June 09 2017).
- 32.Yelverton V., Hair N., Vasudevan L., Baumgartner J.N., Ngadaya E., Mfinanga S.G., Ostermann J. Beyond coverage: Rural-urban disparities in the timeliness of childhood vaccinations in Tanzania. Vaccine. 2022 Sep 2;40(37):5483–5493. doi: 10.1016/j.vaccine.2022.07.020. Epub 2022 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ministry of Health Community Development Gender Elderly and Children (MoHCDGEC) [Tanzania Mainland], Ministry of Health (MoH) [Zanzibar], National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), ICF, TANZANIA. Malaria Indicator Survey 2017, 2017. https://www.dhsprogram.com/pubs/pdf/MIS31/MIS31.pdf. (Accessed April 29, 2019).
- 34.Duan N. Smearing Estimate: A Nonparametric Retransformation Method. J Am Stat Assoc. 1983;78(383):605–610. [Google Scholar]
- 35.Hall J., Kenny P., King M., Louviere J., Viney R., Yeoh A. Using stated preference discrete choice modelling to evaluate the introduction of varicella vaccination. Health Econ. 2002;11(5):457–465. doi: 10.1002/hec.694. [DOI] [PubMed] [Google Scholar]
- 36.Sansom S.L., Barker L., Corso P.S., Brown C., Deuson R. Rotavirus vaccine and intussusception: how much risk will parents in the United States accept to obtain vaccine benefits? Am J Epidemiol. 2001;154(11):1077–1085. doi: 10.1093/aje/154.11.1077. [DOI] [PubMed] [Google Scholar]
- 37.Rezaei S., Woldemichael A., Mirzaei M., Mohammadi S., Karami Matin B. Mothers' willingness to accept and pay for vaccines to their children in western Iran: a contingent valuation study. BMC Pediatr. 2020;20(1):307. doi: 10.1186/s12887-020-02208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucas M.E., Jeuland M., Deen J., Lazaro N., MacMahon M., Nyamete A., et al. Private demand for cholera vaccines in Beira. Mozambique, Vaccine. 2007;25(14):2599–2609. doi: 10.1016/j.vaccine.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 39.Umeh I.B., Nduka S.O., Ekwunife O.I. Mothers' willingness to pay for HPV vaccines in Anambra state, Nigeria: a cross sectional contingent valuation study. Cost Eff Resour Alloc. 2016;14:8. doi: 10.1186/s12962-016-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cropper M.L. The Demand for a Malaria Vaccine: Evidence from Ethiopia. J Dev Econ. 2004;75(1):303–318. [Google Scholar]
- 41.Ughasoro M.D., Esangbedo D.O., Tagbo B.N., Mejeha I.C. Acceptability and Willingness-to-Pay for a Hypothetical Ebola Virus Vaccine in Nigeria. PLoS Negl Trop Dis. 2015;9(6):e0003838. doi: 10.1371/journal.pntd.0003838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishai D., Pariyo G., Ainsworth M., Hill K. Determinants of personal demand for an AIDS vaccine in Uganda: contingent valuation survey. Bull World Health Organ. 2004;82(9):652–660. [PMC free article] [PubMed] [Google Scholar]
- 43.Do G.C., Whittington D., Le T.K., Utomo N., Nguyen T.H., Poulos C., et al. Household demand for typhoid fever vaccines in Hue. Vietnam, Health Policy Plan. 2006;21(3):241–255. doi: 10.1093/heapol/czl009. [DOI] [PubMed] [Google Scholar]
- 44.Guh S., Xingbao C., Poulos C., Qi Z., Jianwen C., von Seidlein L., et al. Comparison of cost-of-illness with willingness-to-pay estimates to avoid shigellosis: evidence from China. Health Policy Plan. 2008;23(2):125–136. doi: 10.1093/heapol/czm047. [DOI] [PubMed] [Google Scholar]
- 45.Heinzen R.R., Bridges J.F. Comparison of four contingent valuation methods to estimate the economic value of a pneumococcal vaccine in Bangladesh. Int J Technol Assess Health Care. 2008;24(4):481–487. doi: 10.1017/S026646230808063X. [DOI] [PubMed] [Google Scholar]
- 46.Islam Z., Maskery B., Nyamete A., Horowitz M.S., Yunus M., Whittington D. Private demand for cholera vaccines in rural Matlab. Bangladesh, Health Policy. 2008;85(2):184–195. doi: 10.1016/j.healthpol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Kim D., Canh D.G., Poulos C., Thoa L.T., Cook J., Hoa N.T., et al. Private demand for cholera vaccines in Hue. Vietnam, Value Health. 2008;11(1):119–128. doi: 10.1111/j.1524-4733.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 48.Lieu T.A., Finkelstein J.A., Adams M.M., Miroshnik I.L., Lett S.M., Palfrey S., et al. Pediatricians' views on financial barriers and values for pneumococcal vaccine for children. Ambul Pediatr. 2002;2(5):358–366. doi: 10.1367/1539-4409(2002)002<0358:pvofba>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.Baumgartner J.N., Morroni C., Mlobeli R.D., Otterness C., Myer L., Janowitz B., et al. Timeliness of contraceptive reinjections in South Africa and its relation to unintentional discontinuation. Int Fam Plan Perspect. 2007;33(2):66–74. doi: 10.1363/3306607. [DOI] [PubMed] [Google Scholar]
- 50.Carson R., Flores N., Martin K.M., Wright J.L. Contingent Valuation and Revealed Preference Methodologies: Comparing the Estimates for Quasi-Public Goods. Land Econ. 1996;72(1):80–99. [Google Scholar]
- 51.Dow W.H., White J.S. Incentivizing use of health care. United Nations Population Division Publications. 2013 [Google Scholar]
- 52.Cook J. Using Private Demand Studies to Calculate Socially Optimal Vaccine Subsidies in Developing Countries. J Policy Anal Manage. 2009;28(1):6–28. doi: 10.1002/pam.20401. [DOI] [PubMed] [Google Scholar]
- 53.Kessing S.G., Nuscheler R. Monopoly Pricing with Negative Network Effects: The Case of Vaccines. Eur Econ Rev. 2006;50(4):1061–1069. [Google Scholar]
- 54.Deci E.L., Koestner R., Ryan R.M. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627–628. doi: 10.1037/0033-2909.125.6.627. discussion 692–700. [DOI] [PubMed] [Google Scholar]
- 55.Uri Gneezy B., Rustichini A. A Fine Is a Price. J Leg Stud. 2000;29(1):1–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.