Abstract
Rationale & Objective
Chronic kidney disease (CKD) is associated with an increased risk of cardiovascular (CV) mortality, but there are limited data on temporal trends disaggregated by sex, race, and urban/rural status in this population.
Study Design
Retrospective observational study.
Setting & Participants
The Centers for Disease Control and Prevention Wide-Ranging, Online Data for Epidemiologic Research database.
Exposure & Predictors
Patients with CKD and end-stage kidney disease (ESKD) stratified according to key demographic groups.
Outcomes
Etiologies of CKD- and ESKD-associated mortality between 1999 and 2000.
Analytical Approach
Presentation of age-adjusted mortality rates (per 100,000 people) characterized by CV categories, ethnicity, sex (male or female), age categories, state, and urban/rural status.
Results
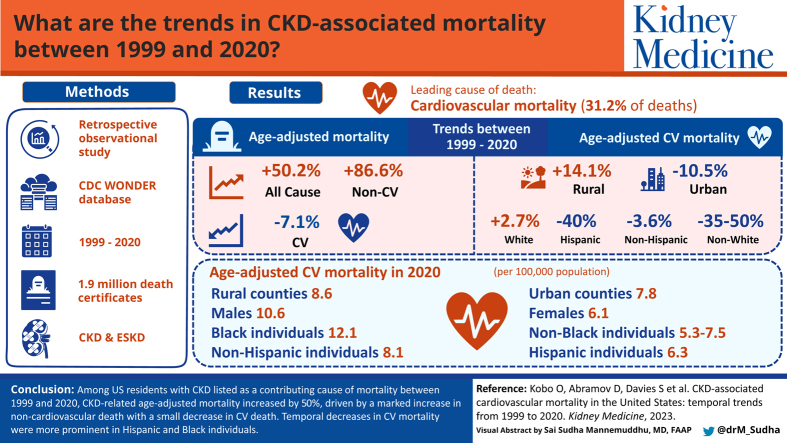
Between 1999 and 2020, we identified 1,938,505 death certificates with CKD (and ESKD) as an associated cause of mortality. Of all CKD-associated mortality, the most common etiology was CV, with 31.2% of cases. Between 1999 and 2020, CKD-related age-adjusted mortality increased by 50.2%, which was attributed to an 86.6% increase in non-CV mortality but a 7.1% decrease in CV mortality. Black patients had a higher rate of CV mortality throughout the study period, although Black patients experienced a 38.6% reduction in mortality whereas White patients saw a 2.7% increase. Hispanic patients experienced a greater reduction in CV mortality over the study period (40% reduction) compared to non-Hispanic patients (3.6% reduction). CV mortality was higher in urban areas in 1999 but in rural areas in 2020.
Limitations
Reliance on accurate characterization of causes of mortality in a large dataset.
Conclusions
Among patients with CKD-related mortality in the United States between 1999 and 2020, there was an increase in all-cause mortality though a small decrease in CV-related mortality. Overall, temporal decreases in CV mortality were more prominent in Hispanic versus non-Hispanic patients and Black patients versus White patients.
Index Words: Cardiovascular mortality, chronic kidney disease, race differences
Graphical abstract
Plain-Language Summary.
Chronic kidney disease (CKD) is associated with an increased risk of cardiovascular (CV) mortality. We wanted to further evaluate CV mortality in the CKD population, including how CV mortality varied over time based on sex, race, and ethnicity. We found that between 1999 and 2020, there was a reduction in CV mortality among all patients with CKD. The reduction in CV mortality was similar between men and women. Reduction in CV mortality was more prominent in patients of Hispanic ethnicity and Black race, although Black patients with CKD continued to have the highest CV mortality throughout the study period. These results suggest potential improvement in CV care for patients with CKD, but also note ongoing needs to address race-based differences in care.
Kidney failure and earlier stages of chronic kidney disease (CKD) are associated with increases in both cardiovascular (CV) and non-CV mortality.1,2 Patients with CKD have a risk of CV mortality that is similar to diabetes mellitus or a history of prior myocardial infarction,3 and have higher incidence of heart failure (HF)4 as well as coronary artery disease and stroke-associated mortality.5 Although non-CV mortality may be similar to CV mortality among patients with kidney failure and earlier stages of CKD, CV disease remains the single leading cause of death in these populations, accounting for 35%-40% of deaths.1,6,7 Public health interventions, as well as improved primary and secondary prevention, have contributed to significant decreases in CV mortality in the general population over the past 2 decades,8 though it is unclear whether patients with kidney failure and earlier stages of CKD have experienced similar improvement in CV mortality.
Furthermore, although it is established that there are significant sex and racial differences in CV-related mortality that have persisted over time,8,9 there are limited data on temporal changes in CV mortality in patients with CKD across sex, race, and geographic groups within the United States. An appreciation of these trends is important because of the rising prevalence of CKD and because of sex- and race-based risk differences for the development of CKD.10 Additionally, whether known sex and racial differences in CV mortality in the general population extend to or are magnified in the CKD population and whether such trends have narrowed or widened over time is unknown. Consequently, we sought to evaluate the trends in CV and non-CV mortality in patients with CKD and ESKD in the United States over a 20-year period with a focus on trends in key individual causes of CV mortality as well as trends in CV mortality based on age, sex, race, and urban status.
Methods
Setting and Study Population
We used the Multiple Cause of Death database accessed through the Centers for Disease Control and Prevention Wide-Ranging, Online Data for Epidemiologic Research (CDC WONDER).11 This database comprises mortality for US residents and population count from all US counties between 1999 and 2020. Each death certificate contains a single underlying cause of mortality, and up to 20 additional contributing causes, along with demographic data. The underlying cause of mortality is selected from conditions entered by the physician on the cause of death section of the death certificate. Causes of mortality are recorded in accordance with the International Classification of Diseases, Tenth Revision. The number of deaths, crude mortality rates, and AAMRs can be obtained by cause of mortality, place of residence, age, race, sex, and year.
Analysis Sample
We included all decedents with CKD (N18) as a contributing cause of mortality. We categorized the main cause of mortality in decedents with any CKD and ESKD into CV (I∗), genitourinary (N∗), endocrine (E∗), malignancy (C∗, D00-D49), infections (A∗-B∗), and gastrointestinal (K∗). We further subcategorized CVs into ischemic heart disease (IHD, I20-I25), HF (I50, I42), hypertensive diseases (I10-I15), cerebrovascular disease (I60-I69), and ‘other’ CVs. Common categories of the genitourinary codes (N∗) include glomerular diseases, tubule-interstitial diseases, acute kidney failure, and other diseases of the kidney and urinary system.
Statistical Analysis
We calculated the percentage contribution of CV versus other causes of mortality in patients with CKD and ESKD between 1999 and 2020. CV-related mortality was stratified by CV categories, ethnicity, sex (male or female), age categories, state, and urban/rural status. We presented the percent change in crude and age-adjusted CV mortality rates between 2 time points (1999 and 2020). The age-adjusted mortality rates (AAMRs per 100,000 people) are provided by the CDC WONDER website and were calculated yearly using the direct standardization method based on the age group weights from the 2000 US population. Analysis was performed using Microsoft Excel 2021 and the web-based analysis tool available through CDC WONDER. Figures were made using the web tools available through CDC WONDER.
Results
Population Characteristics
Between 1999 and 2020, we identified a total of 1,938,505 death certificates with CKD (including ESKD) as a contributing cause of mortality, amounting to an AAMR of 26.3 per 100,000 (Fig S1). Of the overall CKD-related mortality, 52.7% (1,022,485) were male and 77.1% (1,494,601) were White individuals; 19.1% were Black or African American individuals, 2.9% were Asian or Pacific Islander individuals, and 0.8% were American Indian or Alaska Native individuals. Overall, the AAMR was higher in males (34.0 vs 21.2 per 100,000 in females), and in Black individuals (53.1 vs 31.3, 23.4, and 20.9 per 100,000 in American Indians or Alaska Natives, White, and Asian or Pacific Islander individuals, respectively). Among individuals with CKD-related mortality, 31% of mortality cases (605,384) were attributed primarily to CV deaths (AAMR of 8.2 per 100,000), which was the most common cause of mortality during the study period. ESKD was recorded in 846,211 death certificates as a contributing cause of mortality between 1999 and 2020, of which 24% (201,392 cases) were classified as CV deaths. White individuals accounted for 68.5% (579,446 cases, AAMR of 10.8 per 100,000) of the ESKD-related mortality cohort, followed by Black individuals (26.7%, 225,818 cases, AAMR of 24.6 per 100,000), Asian or Pacific Islander individuals (3.7%, 31,407 cases, AAMR of 8.4 per 100,000) and American Indian or Alaska Native individuals (1.1%, 9,520 cases, AAMR of 10.8 per 100,000).
Temporal Trends
Between 1999 and 2020, the CKD-related AAMR increased by 50% (AAMR 21.9 to 32.9 per 100,000), and the ESKD-related AAMR increased by 44% (9.4-13.6 per 100,000). In 1999, among the CKD-related mortality cohort, CV diseases were the most common underlying causes of mortality (AAMR of 8.5 per 100,000, 39% of all mortality causes), followed by genitourinary (AAMR of 5.2 per 100,000, 24% of all mortality), endocrine (AAMR of 3.2 per 100,000, 15% of all mortality), and malignancy-related mortality (AAMR of 1.4 per 100,000, 6% of all mortality). In 2020, genitourinary causes were the most common causes of mortality (29% of mortality, 82.7% increase in AAMR from 5.2 to 9.5 per 100,000), and CV causes were the second most common (24% of mortality, 7.1% decrease AAMR from 8.5 to 7.9 per 100,000), followed by malignancy (9% of mortality, 121.4% increase in AAMR from 1.4 to 3.1 per 100,000), respiratory (7% of mortality, 100% increase in AAMR from 1.1 to 2.2 per 100,000), and endocrine-related mortality (3% of mortality, 65.6% decrease in AAMR from 3.2 to 1.1 per 100,000) (Table 1, Fig 1A).
Table 1.
Overall and CV CKD-/ESKD-Related Mortality
| Crude Ratea |
Age-Adjusted Ratea |
|||||
|---|---|---|---|---|---|---|
| 1999 | 2020 | Percent Change | 1999 | 2020 | Percent Change | |
| Overall CKD-related mortality | 21.4 | 41.4 | +93.5% | 21.9 | 32.9 | +50.2% |
| CV mortality | 8.3 | 10.0 | +20.5% | 8.5 | 7.9 | -7.1% |
| Overall non-CV mortality | 13.1 | 31.4 | +139.7% | 13.4 | 25.0 | +86.6% |
| Genitourinary (including renal) | 5.0 | 11.9 | +138.0% | 5.2 | 9.5 | +82.7% |
| Endocrine and metabolic | 3.2 | 1.4 | -56.3% | 3.2 | 1.1 | -65.6% |
| Malignancy | 1.3 | 3.8 | +192.3% | 1.4 | 3.1 | +121.4% |
| Respiratory | 1.0 | 2.8 | +180.0% | 1.1 | 2.2 | +100% |
| Infectious | 0.8 | 1.2 | +50.0% | 0.8 | 0.9 | +12.5% |
| Overall ESKD-related mortality | 9.3 | 17.1 | +83.9% | 9.4 | 13.6 | +44.7% |
| CV mortality | 3.0 | 3.1 | +3.3% | 3.1 | 2.5 | -19.3% |
| Overall non-CV mortality | 6.3 | 14.0 | +122.2% | 6.3 | 11.1 | +76.2% |
| Genitourinary (including renal) | 2.5 | 7.7 | +208.0% | 2.5 | 6.1 | +144.0% |
| Endocrine and metabolic | 1.6 | 0.5 | -68.8% | 1.6 | 0.4 | -75.0% |
| Malignancy | 0.5 | 1.0 | +100% | 0.5 | 0.8 | +60.0% |
| Infectious | 0.6 | 0.7 | +16.7% | 0.6 | 0.5 | -16.7% |
| GI | 0.4 | 0.8 | +100% | 0.4 | 0.6 | +50.0% |
Abbreviations: CKD, chronic kidney disease; CV, cardiovascular; ESKD, end-stage kidney disease; GI, gastrointestinal.
Per 100,000 US population
Figure 1.
Main causes of mortality in patients with CKD and ESKD in 1999 and 2020. (A) Main causes of mortality in patients with CKD in 1999 (inner circle) vs 2020 (outer circle) expressed as a percent of total mortality. (B) Main causes of mortality in patients with ESKD in 1999 (inner circle) vs 2020 (outer circle) expressed as a percent of total mortality. Abbreviations: CKD, chronic kidney disease; ESKD, end-stage kidney disease; GI, gastrointestinal.
Among individuals with ESKD-related mortality, CV causes accounted for 32% of mortality in 1999 and were the most common causes of mortality (AAMR of 3.1). In 2020, CV causes were the second most common cause of mortality, accounting for 18% of mortality (AAMR of 2.5 per 100,000), and genitourinary mortality was the most common cause of death (45% of mortality and AAMR of 6.1 per 100,000, compared with 27% of mortality and AAMR of 2.5 per 100,000 in 1999) (Table 1, Fig 1B).
Table 2 presents the characteristics of CKD-related CV mortality, including patients with both CKD and ESKD. During the study period, the most common CV cause of mortality was IHD (AAMR of 5.2 in 1999 and 3.2 per 100,000 in 2020), followed by HF/cardiomyopathy (AAMR of 0.9 in 1999 and 1.5 per 100,000 in 2020), cerebrovascular disease (AAMR of 0.8 in 1999 and 0.7 per 100,000 in 2020), and hypertensive disease (AAMR of 0.4 in 1999 and 0.6 per 100,000 in 2020), as shown in Fig S2.
Table 2.
CKD- and ESKD-Related CV Mortality
| Crude Ratea |
Age-Adjusted Ratea |
|||||
|---|---|---|---|---|---|---|
| 1999 | 2020 | Percent Change | 1999 | 2020 | Percent Change | |
| Overall | 8.3 | 10.0 | +20.5% | 8.5 | 7.9 | -7.1% |
| Main causes of death | ||||||
| Ischemic heart diseases | 5.1 | 4.1 | -19.6% | 5.2 | 3.2 | -38.5% |
| Hypertensive diseases | 0.4 | 0.8 | +100.0% | 0.4 | 0.6 | +50.0% |
| Heart failure/cardiomyopathy | 0.8 | 1.9 | +137.5% | 0.9 | 1.5 | +66.7% |
| Cerebrovascular diseases | 0.8 | 0.8 | 0% | 0.8 | 0.7 | -12.5% |
| Sex | ||||||
| Male | 9.0 | 11.2 | +24.4% | 11.5 | 10.5 | -8.7% |
| Female | 7.6 | 8.8 | +15.8% | 6.6 | 6.1 | -7.6% |
| Age | ||||||
| Under 65 | 1.8 | 1.5 | -16.7% | 1.8 | 1.1 | -38.9% |
| 65-84 | 43.9 | 30.8 | -29.8% | 44.1 | 33.5 | -24.0% |
| 85 and over | 130.7 | 207.9 | +59.1% | N/A | ||
| Hispanic origin | ||||||
| Hispanic or Latino | 4.2 | 4.0 | -4.8% | 10.5 | 6.3 | -40.0% |
| Not Hispanic or Latino | 8.8 | 11.4 | +29.5% | 8.4 | 8.1 | -3.6% |
| Race | ||||||
| White | 7.7 | 10.4 | +35.1% | 7.3 | 7.5 | +2.7% |
| Black or African American | 13.0 | 10.7 | -17.7% | 19.7 | 12.1 | -38.6% |
| Asian or Pacific Islander | 5.7 | 5.1 | -10.5% | 10.6 | 5.3 | -50.0% |
| American Indian or Alaska Native | 5.0 | 5.2 | +4% | 11.4 | 7.4 | -35.1% |
| Region | ||||||
| Northeast | 8.9 | 9.3 | +4.5% | 8.2 | 6.7 | -18.3% |
| Midwest | 8.7 | 12.1 | +39.1% | 8.6 | 9.2 | +7.0% |
| South | 8.2 | 9.6 | +17.1% | 8.4 | 7.9 | -6.0% |
| West | 7.6 | 9.3 | +22.4% | 8.8 | 7.8 | -11.4% |
| Urbanization | ||||||
| Urban | 8.2 | 9.5 | +15.9% | 8.6 | 7.7 | -10.5% |
| Rural | 9.1 | 13.3 | +46.2% | 7.8 | 8.9 | +14.1% |
Abbreviations: CKD, chronic kidney disease; CV, cardiovascular; ESKD, end-stage kidney disease.
Per 100,000 population.
Regional Differences in CV Mortality in Patients with CKD and ESKD
Differences between urban and rural counties mortality trends were observed. In 1999, the CV AAMR was higher in urban compared to rural counties (8.6 vs 7.8 per 100,000). During the study period, the AAMR decreased in urban counties (-10.5%) and increased in rural counties (14.1%); as a result, in 2020, the AAMR in rural counties was higher than in urban counties (8.9 vs 7.7 per 100,000). Figure S3 presents AAMR between 1999 and 2000 by state, whereas Figure S4 presents the change in CV AAMR in individuals with CKD between 1999 and 2020 by state.
Sex Differences in CV Mortality in Patients with CKD and ESKD
During the study period, males accounted for 54% of the CV mortality in individuals with CKD (326,837 deaths), and the AAMR of males was higher than of females (11.0 vs 6.3). During the study period, both male and female patients demonstrated similar decreases in CV AAMR (8.7% reduction in males and 7.6% reduction in females). Male patients had higher CV AAMR than females both at the beginning (11.5 vs 6.6 per 100,000) and end (10.5 vs 6.1 per 100,000) of the study period (Table 2).
Racial or Ethnic Differences in CV Mortality in Patients with CKD and ESKD
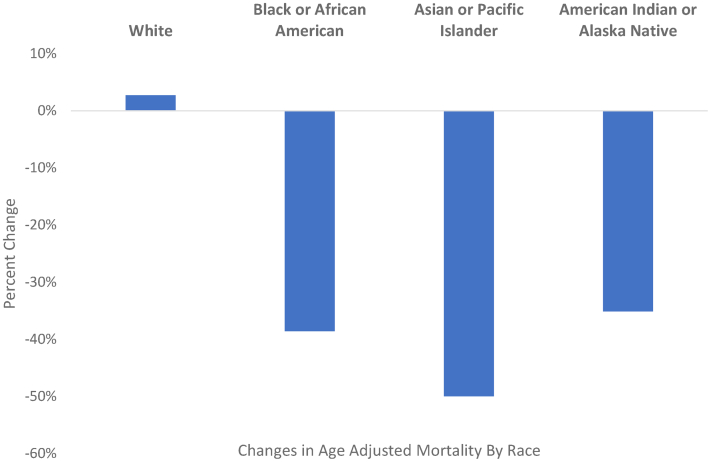
Although 79.2% of CV mortality in patients with CKD during the study period was observed in White individuals, the CV AAMR was highest in Black individuals (15.0 per 100,000) followed by American Indian or Alaska Native, White, and Asian or Pacific Islander individuals (8.0, 7.7, and 6.9 per 100,000, respectively). In 1999, the highest CV AAMR was observed in Black individuals (19.7 per 100,000) and the lowest was observed in White individuals (7.3 per 100,000). Between 1999 and 2020, we observed a decrease in AAMR among ethnic minorities (50%, 38.6%, and 35.1% among Asian or Pacific Islander, Black or African American, and American Indian or Alaska Native individuals, respectively), but not in White individuals (2.7% increase), representing a narrowing of racial differences in CV mortality over time. In 2020, the highest AAMR was still observed in Black patients (12.1 per 100,000), followed by White, American Indian or Alaska Native, and Asian or Pacific Islander individuals (7.5, 7.4, and 5.3 per 100,000, respectively) (Fig 2). Regarding patients of Hispanic ethnicity, we noted that CV AAMR was 10.5 per 100,000 in 1999 but dropped to 6.3 per 100,000 in 2020, a decline of 40.0%. CV AAMR among non-Hispanics dropped from 8.4 to 8.1 per 100,000 over the study period, representing a smaller 3.6% decline. Likewise, this suggests a narrowing in differences in the CV mortality of Hispanic patients, with Hispanics having a lower CV AAMR than non-Hispanics in 2000.
Figure 2.
Percent change in age-adjusted total CKD-related CV death 1999-2020 by race. Abbreviations: AAMR, age-adjusted mortality rate; CKD, chronic kidney disease; CV, cardiovascular; ESKD, end-stage kidney disease.
Discussion
The current analysis demonstrates a decrease in CV mortality associated with CKD and ESKD between 1999 and 2020, which was more prominent in Black and Hispanic patients as well as in urban communities. We report that age-adjusted all-cause mortality where CKD was listed as a contributor increased by about 50% over the study period, which was because of an 86% increase in non-CV mortality that was offset by a 7% decrease in CV mortality. We identified persistent racial differences in CV mortality, which remained highest in Black patients throughout the study period, although race-related differences between Black and White patients in CV mortality narrowed. Likewise, Hispanic patients with CKD saw a greater reduction in CV mortality than non-Hispanic patients over the study period, with higher CV mortality among Hispanic patients in 1999 but lower CV mortality in 2020 compared with non-Hispanics. Although we report that urban CV mortality rates were higher than in rural communities at the start of the study period, these have declined but are offset by an increase in rural CV mortality rates, reflecting a growing burden of CV mortality in rural communities. Finally, among individual causes of CV mortality, there was a decline in mortality attributed to IHD and cerebrovascular disease, but an increase in mortality due to HF. Given the growing prevalence of CKD in the United States, these results may have important implications in prioritizing mortality reduction strategies to improve the care of the CKD population.
The results from this cohort expand on prior literature about the CV and non-CV mortality in patients with kidney failure and earlier stages of CKD. Among prior cohorts with CKD, CV-related mortality, particularly from IHD and cerebrovascular disease, was higher in those with lower glomerular filtration rates (GFRs), whereas patients with higher GFRs were more likely to have malignancy and other causes of death predominate.1,12 HF had previously been shown to be a less common cause of mortality in patients with CKD, although it was more predominant at lower GFRs.12 Similar causes of mortality were identified in patients who progressed to kidney failure, with infections and malignancy13 as common non-CV causes and myocardial infarction, cerebrovascular disease, and HF as common CV causes of mortality.7 The current study builds on this prior literature by both highlighting data from a more contemporary cohort of CKD- and ESKD-associated mortality as well as demonstrating trends in specific CV and non-CV etiologies of mortality over time. An appreciation of the mortality trends highlighted here may play an important role in selecting targets for both research and therapeutic interventions. Furthermore, such mortality trends help identify public health policy priorities to narrow racial differences and the growing chasm between CV outcomes among rural and urban populations.
Targeting morbidity and mortality in patients with kidney failure and earlier stages of CKD has been challenging for a variety of reasons.14 Despite lack of randomized evidence for certain CV mortality-reducing therapies, such as statins, in the advanced CKD (particularly dialysis) population,15 the noted reduction in CKD-associated IHD and cerebrovascular mortality suggests that currently instituted strategies for CV disease care have yielded benefit. Nevertheless, the relative decrease in CV mortality among patients with CKD over the study period may be smaller than the declines in CV mortality seen in the general US population over the same time period,8 a finding that has also been observed in other cohorts.16 This suggests that additional focus on reducing overall CV mortality in the CKD population as well as targeting individual components of CV mortality is warranted. For example, mortality from hypertensive disease and HF have increased over time, implying that additional focus on HF and common comorbid conditions contributing to HF, such as hypertension, should become important strategies in an effort to alter the current trends and potentially reduce system costs.17 Care of patients with CKD and HF has been complicated by traditional exclusion of patients with advanced CKD and kidney failure from HF trials,18 underdosing of HF evidence-based therapies,19 as well as potential difficulty in diagnosing HF in this population20 given overlapping symptomatology. Improving approaches to diagnosis and management of HF are therefore important treatment targets to reduce both absolute and relative increases in HF mortality in the CKD population. Further CV benefit in patients with CKD may also be expected with wider adoption of novel therapies including sodium/glucose cotransporter 2 inhibitors21,22 and selective nonsteroidal mineralocorticoid receptor antagonists,23 which have demonstrated favorable effects on CV outcomes among patients with CKD, although these therapies have not been studied in ESKD.
The current study also highlights the trends in sex- and race-based CV mortality in patients with CKD over time. CKD-related excess in mortality has previously been shown to be similar or only marginally higher among male compared to female patients,13,24 and the current study demonstrates that CV mortality improved similarly for both sexes. Regarding race, prior studies have demonstrated variable effects of race on outcomes of patients with CKD and ESKD, with studies demonstrating similar, lower, and higher all-cause mortality among Black compared to White patients.25, 26, 27, 28, 29 Prior data on the effects of race on specifically CV mortality is limited. CV mortality among patients with CKD was noted to be higher among Black than White patients when adjusted for age and sex but not when concomitantly adjusted for CV risk factors or socioeconomic status.27 These results may be explained by data suggesting that Black patients have a higher risk for HF30 and a higher burden of comorbid conditions27 including hypertension, diabetes, obesity, and cigarette smoking, which lead to higher IHD risk and mortality.31 The current findings add to this prior literature around the association between Black race and CV mortality. Despite more prominent improvements over time, current nationwide data suggests that Black patients continued to have the highest age-adjusted mortality in 2020. Although absolute and relative improvements in mortality among Black patients are encouraging and suggest that racial differences in CV care may be decreasing over time,32,33 such differences remain prevalent.9 Additional focus on improving care of comorbid conditions, disease progression, and addressing socioeconomic differences are important to improve higher CV mortality rates observed in Black patients.
This study also demonstrates temporal trends in CV mortality rates among Hispanic versus non-Hispanic patients with CKD, which is important as Hispanic patients are the largest ethnic minority group in the United States. Hispanic patients experienced greater improvement in mortality rates over the study period and had lower CV mortality compared to non-Hispanic patients in 2020. Prior data on all-cause and CV mortality risk among Hispanic patients with CKD has been mixed, with studies demonstrating similar to lower mortality risk in the Hispanic population.27,34 Various explanations may explain the propensity for lower CV mortality risk among the Hispanic population, including social factors such as family cohesion,34 equivalent to lower risk for atherosclerotic events,34 as well as lower rates of CKD progression.35 Although differences in care for the Hispanic community remain prominent,34 the favorable trends in CV mortality suggests that these differences may be narrowing over time similar to what is seen among Black patients with CKD.
Another important finding of the current study is differential trends in CKD-associated CV mortality between urban and rural areas of the United States, with a decrease in mortality in urban regions but an increase in mortality in rural regions over the study period. Patients residing in rural areas may have poorer access to care, which may increase CKD-related events in this population.36 Additionally, the urban population in the United States may have different age and racial makeup compared to rural areas, with younger and more non-White individuals,37 both groups that saw prominent mortality reductions over time in the current cohort. Divergent trends between urban and rural CV mortality in patients with CKD deserve further evaluation, but these findings are concordant with all-cause38 and CV39 mortality trends in the United States, which demonstrate relatively worse outcomes among rural compared with urban populations.
The current study has limitations. Analyses of the CDC dataset are dependent on the accuracy of the entered data. Causes of death may be multifactorial and individual contributors may be difficult to determine with clinician variability in assigning causes. This may affect classification of mortality into CV, genitourinary, or other categories, and significant discrepancies among causes of mortality between datasets and patient records among patients with CKD have been described.40 Additionally, it is possible that CKD or ESKD was not listed as a contributor to mortality among some patients with CKD or ESKD. Other important data including severity of CKD based on estimated glomerular filtration rate and duration of CKD or ESKD are not available. Presence, severity, and treatment of CV and non-CV comorbid conditions are also not available in this dataset, and therefore, outcomes cannot be adjusted for differences in comorbid burden. Diagnosis of CKD may have varied over the long study period, including due to increased attention to estimated glomerular filtration rate as well as use of variable formulas for estimating glomerular filtration rate over time.41 Higher rates of diagnosis over time may lead to greater likelihood that a death would be classified as being related to CKD, and this may explain rising mortality rates during the study. However, demonstration of similar trends in the ESKD cohort suggests that ascertainment bias in CKD diagnosis may not be sufficient to explain the current findings, although incorrect diagnosis or coding of CKD and ESKD remains possible. Care of patients in this cohort may vary because of insurance coverage, and patients with ESKD may have greater access to care because of universal coverage through Medicare. Patients with ESKD who underwent kidney transplant are not specifically included in this analysis. The last year of the data includes mortality that may be related to the COVID pandemic, and the effects of COVID on CKD- and ESKD-related CV mortality will require further evaluation. This manuscript focuses primarily on mortality causes classified as CV, and changes in other causes of mortality based on sex, race, ethnicity, or urbanization status are not evaluated in this manuscript.
In conclusion, the current analysis of mortality data between 1999 and 2020 demonstrates an increase in overall CKD- and ESKD-associated mortality, although there was a decrease in mortality associated with CV causes. Overall declines in CV mortality were similar based on sex but were more prominent in Hispanic and Black patients, although the overall absolute burden remains greater in Black patients. Finally, we report that there are growing differences in CV mortality between urban and rural communities. Further evaluation on contributors to mortality and strategies to reduce CV- and non-CV–associated mortality are needed in the CKD population at the policy level.
Article Information
Authors’ Full Names and Academic Degrees
Ofer Kobo, MD, MHA∗, Dmitry Abramov, MD∗, Simon Davies, MBBS, Sofia B. Ahmed, MD, Louise Y. Sun, MD, Jennifer H. Mieres, MD, Purvi Parwani, MBBS, Zbigniew Siudak, MD, PhD, Harriette G.C. Van Spall, MD, MPH, and Mamas A Mamas, DPhil
Authors’ Contributions
Research idea and study design: OK, MAM; data acquisition: OK; data analysis/interpretation: OK, DA, SD, SBA, LYS, JHM, PP, ZS, HGCVS, MAM; statistical analysis: OK, MAM; supervision or mentorship: MAM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received August 13, 2022. Evaluated by 1 external peer reviewer, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form December 4, 2022.
Footnotes
Complete author and article information provided before references.
Figure S1: Flow diagram of study population
Figure S2: CV mortality causes in patients with CKD/ESKD in 1999 (A) and 2020 (B), presented as AAMR per 100,000 population
Figure S3: Age-adjusted CV mortality rate in patients with CKD 1999-2020, by state
Figure S4: Percent change in CV age-adjusted mortality in patients with CKD, 1999-2020; by state
Supplementary Material
Fig S1-S4.
References
- 1.Navaneethan S.D., Schold J.D., Arrigain S., Jolly S.E., Nally J.V. Cause-specific deaths in non–dialysis-dependent CKD. J Am Soc Nephrol. 2015;26(10):2512–2520. doi: 10.1681/asn.2014101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marks A., Macleod C., McAteer A., et al. Chronic kidney disease, a useful trigger for proactive primary care? Mortality results from a large U.K. cohort. Fam Pract. 2013;30(3):282–289. doi: 10.1093/fampra/cms079. [DOI] [PubMed] [Google Scholar]
- 3.Rashidi A., Sehgal A.R., Rahman M., O’Connor A.S. The case for chronic kidney disease, diabetes mellitus, and myocardial infarction being equivalent risk factors for cardiovascular mortality in patients older than 65 years. Am J Cardiol. 2008;102(12):1668–1673. doi: 10.1016/j.amjcard.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 4.Dhingra R., Gaziano J.M., Djoussé L. Chronic kidney disease and the risk of heart failure in men. Circ Heart Fail. 2011;4(2):138–144. doi: 10.1161/circheartfailure.109.899070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Angelantonio E., Chowdhury R., Sarwar N., Aspelund T., Danesh J., Gudnason V. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ. 2010;341:c4986. doi: 10.1136/bmj.c4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried L.F., Katz R., Sarnak M.J., et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16(12):3728–3735. doi: 10.1681/asn.2005040384. [DOI] [PubMed] [Google Scholar]
- 7.Carrero J.J., de Jager D.J., Verduijn M., et al. Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol. 2011;6(7):1722–1730. doi: 10.2215/CJN.11331210. [DOI] [PubMed] [Google Scholar]
- 8.Kyalwazi A.N., Loccoh E.C., Brewer L.C., et al. Disparities in cardiovascular mortality between black and white adults in the United States, 1999 to 2019. Circulation. 2022;146(3):211–228. doi: 10.1161/CIRCULATIONAHA.122.060199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Javed Z., Haisum Maqsood M., Yahya T., et al. Race, racism, and cardiovascular health: applying a social determinants of health framework to racial/ethnic disparities in cardiovascular disease. Circ Cardiovasc Qual Outcomes. 2022;15(1) doi: 10.1161/CIRCOUTCOMES.121.007917. [DOI] [PubMed] [Google Scholar]
- 10.Grams M.E., Chow E.K.H., Segev D.L., Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62(2):245–252. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention CDC WONDER. About underlying cause of death, 1999-2020. http://wonder.cdc.gov/ucd-icd10.html
- 12.Thompson S., James M., Wiebe N., et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26(10):2504–2511. doi: 10.1681/asn.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jager D.J., Grootendorst D.C., Jager K.J., et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302(16):1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 14.Luyckx V.A., Tuttle K.R., Garcia-Garcia G., et al. Reducing major risk factors for chronic kidney disease. Kidney Int Suppl (2011) 2017;7(2):71–87. doi: 10.1016/j.kisu.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jager K.J., Lindholm B., Goldsmith D., et al. Cardiovascular and non-cardiovascular mortality in dialysis patients: where is the link? Kidney Int Suppl (2011) 2011;1(1):21–23. doi: 10.1038/kisup.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts M.A., Polkinghorne K.R., McDonald S.P., Ierino F.L. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis. 2011;58(1):64–72. doi: 10.1053/j.ajkd.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Kwok C.S., Abramov D., Parwani P., et al. Cost of inpatient heart failure care and 30-day readmissions in the United States. Int J Cardiol. 2021;329:115–122. doi: 10.1016/j.ijcard.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Beldhuis I.E., Lam C.S.P., Testani J.M., et al. Evidence-based medical therapy in patients with heart failure with reduced ejection fraction and chronic kidney disease. Circulation. 2022;145(9):693–712. doi: 10.1161/CIRCULATIONAHA.121.052792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel R.B., Fonarow G.C., Greene S.J., et al. Kidney function and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2021;78(4):330–343. doi: 10.1016/j.jacc.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abramov D., Kittleson M.M. The universal definition of heart failure: strengths and opportunities. J Card Fail. 2021;27(6):622–624. doi: 10.1016/j.cardfail.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 21.EMPA-KIDNEY Collaborative Group. Herrington W.G., Staplin N., et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. Published online November 4. 2022 doi: 10.1056/NEJMoa2204233. [DOI] [Google Scholar]
- 22.Vogt L. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2021;384(4):388–389. doi: 10.1056/nejmc2032809. [DOI] [PubMed] [Google Scholar]
- 23.Pitt B., Filippatos G., Agarwal R., et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 24.Shajahan S., Amin J., Phillips J.K., Hildreth C.M. Relationship between sex and cardiovascular mortality in chronic kidney disease: a systematic review and meta-analysis. PLOS ONE. 2021;16(7) doi: 10.1371/journal.pone.0254554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson B.M., Joffe M.M., Pisoni R.L., Port F.K., Feldman H.I. Revisiting survival differences by race and ethnicity among hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol. 2006;17(10):2910–2918. doi: 10.1681/asn.2005101078. [DOI] [PubMed] [Google Scholar]
- 26.Jolly S.E., Burrows N.R., Chen S.C., et al. Racial and ethnic differences in mortality among individuals with chronic kidney disease: results from the Kidney Early Evaluation Program (KEEP) Clin J Am Soc Nephrol. 2011;6(8):1858–1865. doi: 10.2215/CJN.00500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehrotra R., Kermah D., Fried L., Adler S., Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19(7):1403–1410. doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovesdy C.P., Quarles L.D., Lott E.H., et al. Survival advantage in black versus white men with CKD: effect of estimated GFR and case mix. Am J Kidney Dis. 2013;62(2):228–235. doi: 10.1053/j.ajkd.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fedewa S.A., McClellan W.M., Judd S., Gutiérrez O.M., Crews D.C. The association between race and income on risk of mortality in patients with moderate chronic kidney disease. BMC Nephrol. 2014;15:136. doi: 10.1186/1471-2369-15-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bibbins-Domingo K., Chertow G.M., Fried L.F., et al. Renal function and heart failure risk in older black and white individuals: the Health, Aging, and Body Composition Study. Arch Intern Med. 2006;166(13):1396–1402. doi: 10.1001/archinte.166.13.1396. [DOI] [PubMed] [Google Scholar]
- 31.Muntner P., He J., Hamm L., Loria C., Whelton P.K. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13(3):745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 32.Brown A.F., Liang L.J., Vassar S.D., et al. Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Ann Intern Med. 2018;168(8):541–549. doi: 10.7326/M17-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewey J., Choudhry N.K. The current state of ethnic and racial disparities in cardiovascular care: lessons from the past and opportunities for the future. Curr Cardiol Rep. 2014;16(10):530. doi: 10.1007/s11886-014-0530-3. [DOI] [PubMed] [Google Scholar]
- 34.Desai N., Lora C.M., Lash J.P., Ricardo A.C. CKD and ESRD in US Hispanics. Am J Kidney Dis. 2019;73(1):102–111. doi: 10.1053/j.ajkd.2018.02.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer M.J., Hsu J.Y., Lora C.M., et al. CKD progression and mortality among Hispanics and non-Hispanics. J Am Soc Nephrol. 2016;27(11):3488–3497. doi: 10.1681/ASN.2015050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan J., Mehrotra A., Nadkarni G.N., et al. Telenephrology: providing healthcare to remotely located patients with chronic kidney disease. Am J Nephrol. 2018;47(3):200–207. doi: 10.1159/000488004. [DOI] [PubMed] [Google Scholar]
- 37.Hall Y.N., Choi A.I., Chertow G.M., Bindman A.B. Chronic kidney disease in the urban poor. Clin J Am Soc Nephrol. 2010;5(5):828–835. doi: 10.2215/cjn.09011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtin S., Spencer M.R. Trends in death rates in urban and rural areas: United States, 1999-2019. NCHS Data Brief. 2021;(417):1–8. [PubMed] [Google Scholar]
- 39.Cross S.H., Mehra M.R., Bhatt D.L., et al. Rural-urban differences in cardiovascular mortality in the US, 1999-2017. JAMA. 2020;323(18):1852–1854. doi: 10.1001/jama.2020.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhandari S.K., Zhou H., Shaw S.F., et al. Causes of death in end-stage kidney disease: comparison between the United States renal data system and a large integrated health care system. Am J Nephrol. 2022;53(1):32–40. doi: 10.1159/000520466. [DOI] [PubMed] [Google Scholar]
- 41.Matsushita K., Mahmoodi B.K., Woodward M., et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1-S4.